User login
Lentigo maligna (LM) and LM melanoma (LMM) represent diagnostic and therapeutic challenges due to their heterogeneous nature and location on cosmetically sensitive areas. Newer ancillary technologies such as reflectance confocal microscopy (RCM) have helped improve diagnosis and management of these challenging lesions.1,2
Reflectance confocal microscopy is a noninvasive laser system that provides real-time imaging of the epidermis and dermis with cellular resolution and improves diagnostic accuracy of melanocytic lesions.2,3 Normal melanocytes appear as round bright structures on RCM that are similar in size to surrounding keratinocytes located in the basal layer and regularly distributed around the dermal papillae (junctional nevi) or form regular dense nests in the dermis (intradermal nevi).4,5 In LM/LMM, there may be widespread infiltration of atypical melanocytes invading hair follicles; large, round, pagetoid melanocytes (larger than surrounding keratinocytes); sheets of large atypical cells at the dermoepidermal junction (DEJ); loss of contour in the dermal papillae; and atypical melanocytes invading the dermal papillae.2 Indeed, RCM has good correlation with the degree of histologic atypia and is useful to distinguish between benign nevi, atypical nevi, and melanoma.6 By combining lateral mosaics with vertical stacks, RCM allows 3-dimensional approximation of tumor margins and monitoring of nonsurgical therapies.7,8 The advent of handheld RCM (HRCM) has allowed assessment of large lesions as well as those presenting in difficult locations.9 Furthermore, the generation of videomosaics overcomes the limited field of view of traditional RCM and allows for accurate assessment of large lesions.10
Traditional and handheld RCM have been used to diagnose and map primary LM.1,2,11 Guitera et al2 developed an algorithm using traditional RCM to distinguish benign facial macules and LM. In their training set, they found that when their score resulted in 2 or more points, the sensitivity and specificity to diagnose LM was 85% and 76%, respectively, with an odds ratio of 18.6 for LM. They later applied the algorithm in a test set of 44 benign facial macules and 29 LM and obtained an odds ratio of 60.7 for LM, with sensitivity and specificity rates of 93% and 82%, respectively.2 This algorithm also was tested by Menge et al11 using the HRCM. They found 100% sensitivity and 71% specificity for LM when evaluating 63 equivocal facial lesions. Although these results suggest that RCM can accurately distinguish LM from benign lesions in the primary setting, few reports have studied the impact of HRCM in the recurrent setting and its impact in monitoring treatment of LM.12,13
Herein, we present 5 cases in which HRCM was used to manage complex facial LM/LMM, highlighting its versatility and potential for use in the clinical setting (eTable).
Case Series
Following institutional review board approval, cases of facial LM/LMM presenting for assessment and treatment from January 2014 to December 2015 were retrospectively reviewed. Initially, the clinical margins of the lesions were determined using Wood lamp and/or dermoscopy. Using HRCM, vertical stacks were taken at the 12-, 3-, 6-, and 9-o'clock positions, and videos were captured along the peripheral margins at the DEJ. To create videomosaics, HRCM video frames were extracted and later stitched using a computer algorithm written in a fourth-generation programming language based on prior studies.10,14 An example HRCM video that was captured and turned into a videomosaic accompanies this article online (http://bit.ly/2oDYS6k). Additional stacks were taken in suspicious areas. We considered an area positive for LM under HRCM when the LM score developed by Guitera et al2 was 2 or more. The algorithm scoring includes 2 major criteria--nonedged papillae and round large pagetoid cells--which score 2 points, and 4 minor criteria, including 3 positive criteria--atypical cells at the DEJ, follicular invasion, nucleated cells in the papillae--which each score 1 point, and 1 negative criterion--broadened honeycomb pattern--which scores -1 point.2
RELATED VIDEO: RCM Videomosaic of Melanoma In Situ
Patient 1
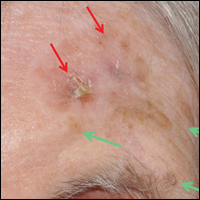
An 82-year-old woman was referred to us for management of an LMM on the left side of the forehead (Figure 1A). Handheld RCM from the biopsy site showed large atypical cells in the epidermis, DEJ, and papillary dermis. Superiorly, HRCM showed large dendritic processes but did not reveal LM features in 3 additional clinically worrisome areas. Biopsies showed LMM at the prior biopsy site, LM superiorly, and actinic keratosis in the remaining 3 areas, supporting the HRCM findings. Due to upstaging, the patient was referred for head and neck surgery. To aid in resection, HRCM was performed intraoperatively in a multidisciplinary approach (Figure 1B). Due to the large size of the lesion, surgical margins were taken right outside the HRCM border. Pathology showed LMM extending focally into the margins that were reexcised, achieving clearance.

Patient 2
An 88-year-old woman presented with a slightly pigmented, 2.5×2.3-cm LMM on the left cheek. Because of her age and comorbidities (eg, osteoporosis, deep vein thrombosis in both lower legs requiring anticoagulation therapy, presence of an inferior vena cava filter, bilateral lymphedema of the legs, irritable bowel syndrome, hyperparathyroidism), she was treated with imiquimod cream 5% achieving partial response. The lesion was subsequently excised showing LMM extending to the margins. Not wanting to undergo further surgery, she opted for radiation therapy. Handheld RCM was performed to guide the radiation field, showing pagetoid cells within 1 cm of the scar and clear margins beyond 2 cm. She underwent radiation therapy followed by treatment with imiquimod. On 6-month follow-up, no clinical lesion was apparent, but HRCM showed atypical cells. Biopsies revealed an atypical intraepidermal melanocytic proliferation, but due to patient's comorbidities, close observation was decided.
Patient 3
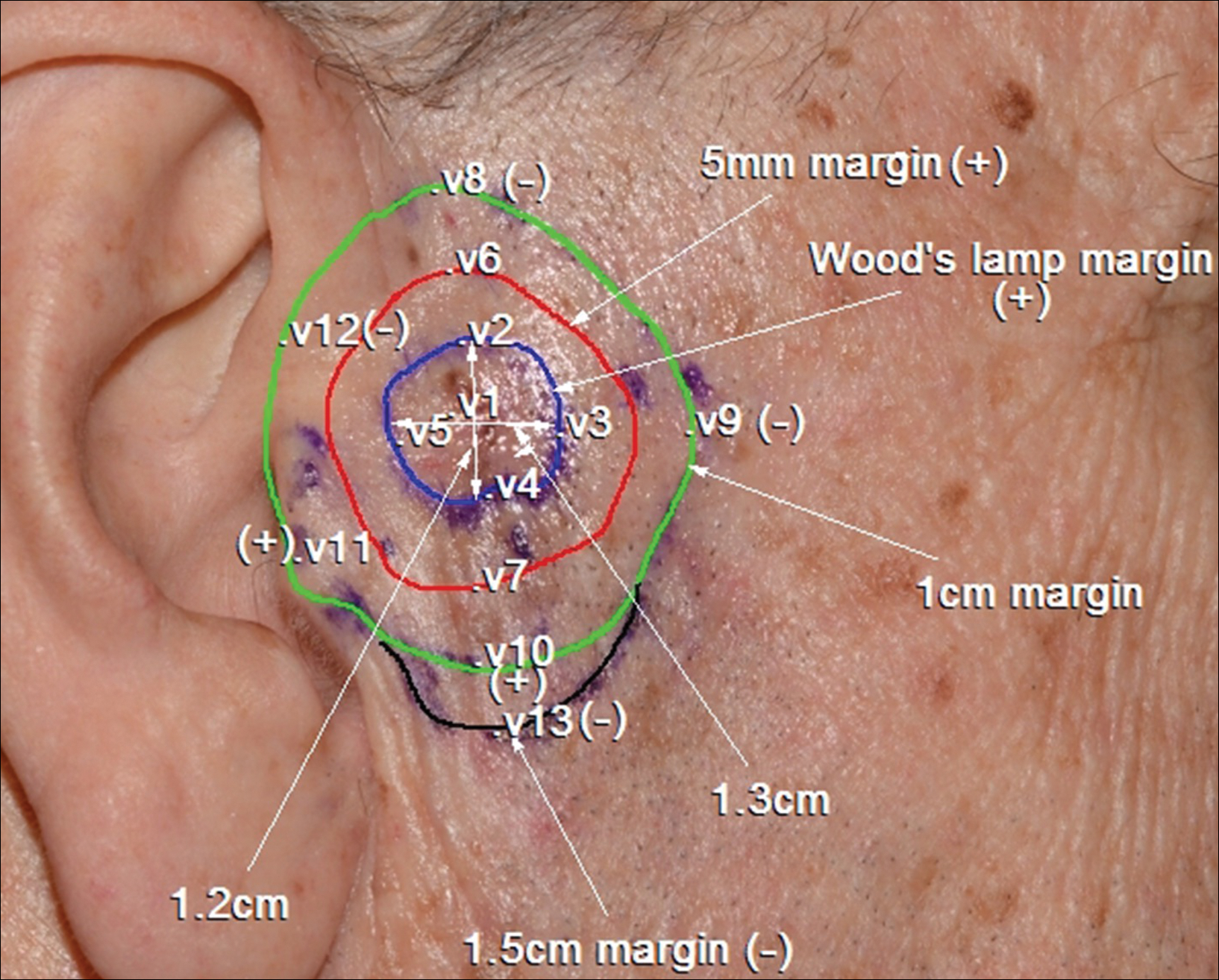
A 78-year-old man presented with an LMM on the right preauricular area. Handheld RCM demonstrated pleomorphic pagetoid cells along and beyond the clinical margins. Wide excision with sentinel lymph node biopsy was planned, and to aid surgery a confocal map was created (Figure 2). Margins were clear at 1 cm, except inferiorly where they extended to 1.5 cm. Using this preoperative HRCM map, all intraoperative sections were clear. Final pathology confirmed clear margins throughout.
Patient 4
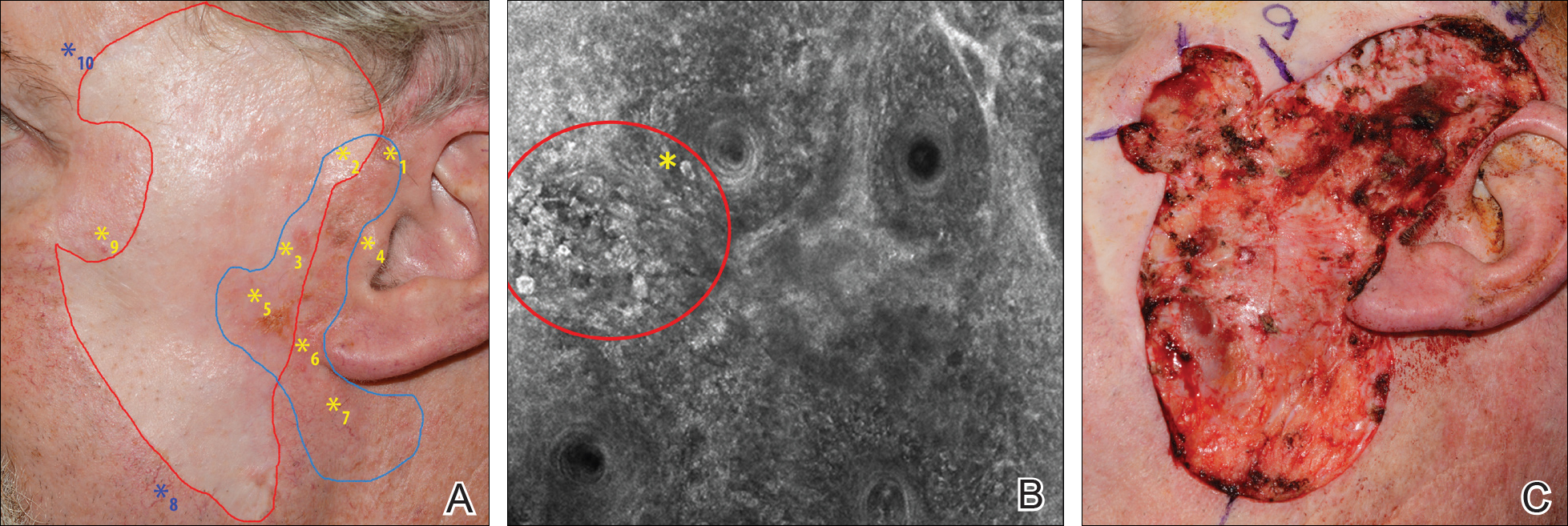
A 62-year-old man presented with hyperpigmentation and bleeding on the left cheek where an LMM was previously removed 8 times over 18 years. Handheld RCM showed pleomorphic cells along the graft border and interestingly within the graft. Ten biopsies were taken, 8 at sites with confocal features that were worrisome for LM (Figures 3A and 3B) and 2 at clinically suspicious sites. The former revealed melanomas (2 that were invasive to 0.3 mm), and the latter revealed solar lentigines. The patient underwent staged excision guided by HRCM (Figure 3C), achieving clear histologic margins except for a focus in the helix. This area was RCM positive but was intentionally not resected due to reconstructive difficulties; imiquimod was indicated in this area.
Patient 5
An 85-year-old woman with 6 prior melanomas over 15 years presented with ill-defined light brown patches on the left cheek at the site where an LM was previously excised 15 years prior. Biopsies showed LM, and due to the patient's age, health, and personal preference to avoid extensive surgery, treatment with imiquimod cream 5% was decided. Over a period of 6 to 12 months, she developed multiple erythematous macules with 2 faintly pigmented areas. Handheld RCM demonstrated atypical cells within the papillae in previously biopsied sites that were rebiopsied, revealing LMM (Breslow depth, 0.2 mm). Staged excision achieved clear margins, but after 8 months HRCM showed LM features. Histology confirmed the diagnosis and imiquimod was reapplied.
Comment
Diagnosis and choice of treatment modality for cases of facial LM is a challenge, and there are a number of factors that may create even more of a clinical dilemma. Surgical excision is the treatment of choice for LM/LMM, and better results are achieved when using histologically controlled surgical procedures such as Mohs micrographic surgery, staged excision, or the "spaghetti technique."15-17 However, advanced patient age, multiple comorbidities (eg, coronary artery disease, deep vein thrombosis, other conditions requiring anticoagulation therapy), large lesion size in functionally or aesthetically sensitive areas, and indiscriminate borders on photodamaged skin may make surgical excision complicated or not feasible. Additionally, prior treatments to the affected area may further obscure clinical borders, complicating the diagnosis of recurrence/persistence when observed with the naked eye, dermoscopy, or Wood lamp. Because RCM can detect small amounts of melanin and has cellular resolution, it has been suggested as a great diagnostic tool to be combined with dermoscopy when evaluating lightly pigmented/amelanotic facial lesions arising on sun-damaged skin.18,19 In this case series, we highlighted these difficulties and showed how HRCM can be useful in a variety of scenarios, both pretreatment and posttreatment in complex LM/LMM cases.
Pretreatment Evaluation
Blind mapping biopsies of LM are prone to sample bias and depend greatly on biopsy technique; however, HRCM can guide mapping biopsies by detecting features of LM in vivo with high sensitivity.11 Due to the cosmetically sensitive nature of the lesions, many physicians are discouraged to do multiple mapping biopsies, making it difficult to assess the breadth of the lesion and occult invasion. Multiple studies have shown that occult invasion was not apparent until complete lesion excision was done.15,20,21 Agarwal-Antal et al20 reported 92 cases of LM, of which 16% (15/92) had unsuspected invasion on final excisional pathology. A long-standing disadvantage of treating LM with nonsurgical modalities has been the inability to detect occult invasion or multifocal invasion within the lesion. As described in patients 1, 4, and 5 in the current case series, utilizing real-time video imaging of the DEJ at the margins and within the lesion has allowed for the detection of deep atypical melanocytes suspicious for perifollicular infiltration and invasion. Knowing the depth of invasion before treatment is essential for not only counseling the patient about disease risk but also for choosing an appropriate treatment modality. Therefore, prospective studies evaluating the performance of RCM to identify invasion are crucial to improve sampling error and avoid unnecessary biopsies.
Surgical Treatment
Although surgery is the first-line treatment option for facial LM, it is not without associated morbidity, and LM is known to have histological subclinical extension, which makes margin assessment difficult. Wide surgical margins on the face are not always possible and become further complicated when trying to maintain adequate functional and cosmetic outcomes. Additionally, the margin for surgical clearance may not be straightforward for facial lesions. Hazan et al15 showed the mean total surgical margins required for excision of LM and LMM was 7.1 and 10.3 mm, respectively; of the 91 tumors initially diagnosed as LM on biopsy, 16% (15/91) had unsuspected invasion. Guitera et al2 reported that the presence of atypical cells within the dermal papillae might be a sign of invasion, which occasionally is not detected histologically due to sampling bias. Handheld RCM offers the advantage of a rapid real-time assessment in areas that may not have been amenable to previous iterations of the device, and it also provides a larger field of view that would be time consuming if performed using conventional RCM. Compared to prior RCM devices that were not handheld, the use of the HRCM does not need to attach a ring to the skin and is less bulky, permitting its use at the bedside of the patient or even intraoperatively.13 In our experience, HRCM has helped to better characterize subclinical spread of LM during the initial consultation and better counsel patients about the extent of the lesion. Handheld RCM also has been used to guide the spaghetti technique in patients with LM/LMM with good correlation between HRCM and histology.22 In our case series, HRCM was used in complex LM/LMM to delineate surgical margins, though in some cases the histologic margins were too close or affected, suggesting HRCM underestimation. Lentigo maligna margin assessment with RCM uses an algorithm that evaluates confocal features in the center of the lesion.1,2 Therefore, further studies using HRCM should evaluate minor confocal features in the margins as potential markers of positivity to accurately delineate surgical margins.
Nonsurgical Treatment Options
For patients unable or unwilling to pursue surgical treatment, therapies such as imiquimod or radiation have been suggested.23,24 However, the lack of histological confirmation and possibility for invasive spread has limited these modalities. Lentigo malignas treated with radiation have a 5% recurrence rate, with a median follow-up time of 3 years.23 Recurrence often can be difficult to detect clinically, as it may manifest as an amelanotic lesion, or postradiation changes can hinder detection. Handheld RCM allows for a cellular-level observation of the irradiated field and can identify radiation-induced changes in LM lesions, including superficial necrosis, apoptotic cells, dilated vessels, and increased inflammatory cells.25 Handheld RCM has previously been used to assess LM treated with radiation and, as in patient 2, can help define the radiation field and detect treatment failure or recurrence.12,25
Similarly, as described in patient 5, HRCM was utilized to monitor treatment with imiquimod. Many reports use imiquimod for treatment of LM, but application and response vary greatly. Reflectance confocal microscopy has been shown to be useful in monitoring LM treated with imiquimod,8 which is important because clinical findings such as inflammation and erythema do not correlate well with response to therapy. Thus, RCM is an appealing noninvasive modality to monitor response to treatment and assess the need for longer treatment duration. Moreover, similar to postradiation changes, treatment with imiquimod may cause an alteration of the clinically apparent pigment. Therefore, it is difficult to assess treatment success by clinical inspection alone. The use of RCM before, during, and after treatment provides a longitudinal assessment of the lesion and has augmented dermatologists' ability to determine treatment success or failure; however, prospective studies evaluating the usefulness of HRCM in the recurrent setting are needed to validate these results.
Limitations
Limitations of this technology include the time needed to image large areas; technology cost; and associated learning curve, which may take from 6 months to 1 year based on our experience. Others have reported the training required for accurate RCM interpretation to be less than that of dermoscopy.26 It has been shown that key RCM diagnostic criteria for lesions including melanoma and basal cell carcinoma are reproducibly recognized among RCM users and that diagnostic accuracy increases with experience.27 These limitations can be overcome with advances in videomosaicing that may streamline the imaging as well as an eventual decrease in cost with greater user adoption and the development of training platforms that enable a faster learning of RCM.28
Conclusion
The use of HRCM can help in the diagnosis and management of facial LMs. Handheld RCM provides longitudinal assessment of LM/LMM that may help determine treatment success or failure and has proven to be useful in detecting the presence of recurrence/persistence in cases that were clinically poorly evident. Moreover, HRCM is a notable ancillary tool, as it can be performed at the bedside of the patient or even intraoperatively and provides a faster approach than conventional RCM in cases where large areas need to be mapped.
In summary, HRCM may eventually be a useful screening tool to guide scouting biopsies to diagnose de novo LM; guide surgical and nonsurgical therapies; and evaluate the presence of recurrence/persistence, especially in large, complex, amelanotic or poorly pigmented lesions. A more standardized use of HRCM in mapping surgical and nonsurgical approaches needs to be evaluated in further studies to provide a fast and reliable complement to histology in such complex cases; therefore, larger studies need to be performed to validate this technique in such complex cases.
- Guitera P, Moloney FJ, Menzies SW, et al. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatol. 2013;149:692-698.
- Guitera P, Pellacani G, Crotty KA, et al. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face. J Invest Dermatol. 2010;130:2080-2091.
- Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-2765.
- Segura S, Puig S, Carrera C, et al. Development of a two-step method for the diagnosis of melanoma by reflectance confocal microscopy. J Am Acad Dermatol. 2009;61:216-229.
- Hofmann-Wellenhof R, Pellacani G, Malvehy J, et al. Reflectance Confocal Microscopy for Skin Diseases. New York, NY: Springer; 2012.
- Pellacani G, Farnetani F, Gonzalez S, et al. In vivo confocal microscopy for detection and grading of dysplastic nevi: a pilot study. J Am Acad Dermatol. 2012;66:E109-E121.
- Nadiminti H, Scope A, Marghoob AA, et al. Use of reflectance confocal microscopy to monitor response of lentigo maligna to nonsurgical treatment. Dermatol Surg. 2010;36:177-184.
- Alarcon I, Carrera C, Alos L, et al. In vivo reflectance confocal microscopy to monitor the response of lentigo maligna to imiquimod. J Am Acad Dermatol. 2014;71:49-55.
- Fraga-Braghiroli NA, Stephens A, Grossman D, et al. Use of handheld reflectance confocal microscopy for in vivo diagnosis of solitary facial papules: a case series. J Eur Acad Dermatol Venereol. 2014;28:933-942.
- Kose K, Cordova M, Duffy M, et al. Video-mosaicing of reflectance confocal images for examination of extended areas of skin in vivo. Br J Dermatol. 2014;171:1239-1241.
- Menge TD, Hibler BP, Cordova MA, et al. Concordance of handheld reflectance confocal microscopy (RCM) with histopathology in the diagnosis of lentigo maligna (LM): a prospective study [published online January 27, 2016]. J Am Acad Dermatol. 2016;74:1114-1120.
- Hibler BP, Connolly KL, Cordova M, et al. Radiation therapy for synchronous basal cell carcinoma and lentigo maligna of the nose: response assessment by clinical examination and reflectance confocal microscopy. Pract Radiat Oncol. 2015;5:E543-E547.
- Hibler BP, Cordova M, Wong RJ, et al. Intraoperative real-time reflectance confocal microscopy for guiding surgical margins of lentigo maligna melanoma. Dermatol Surg. 2015;41:980-983.
- Kose K, Gou M, Yelamos O, et al. Video-mosaicking of in vivo reflectance confocal microscopy images for noninvasive examination of skin lesions [published February 6, 2017]. Proceedings of SPIE Photonics West. doi:10.1117/12.2253085.
- Hazan C, Dusza SW, Delgado R, et al. Staged excision for lentigo maligna and lentigo maligna melanoma: a retrospective analysis of 117 cases. J Am Acad Dermatol. 2008;58:142-148.
- Etzkorn JR, Sobanko JF, Elenitsas R, et al. Low recurrence rates for in situ and invasive melanomas using Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining: tissue processing methodology to optimize pathologic staging and margin assessment. J Am Acad Dermatol. 2015;72:840-850.
- Gaudy-Marqueste C, Perchenet AS, Tasei AM, et al. The "spaghetti technique": an alternative to Mohs surgery or staged surgery for problematic lentiginous melanoma (lentigo maligna and acral lentiginous melanoma). J Am Acad Dermatol. 2011;64:113-118.
- Guitera P, Menzies SW, Argenziano G, et al. Dermoscopy and in vivo confocal microscopy are complementary techniques for diagnosis of difficult amelanotic and light-coloured skin lesions [published online October 12, 2016]. Br J Dermatol. 2016;175:1311-1319.
- Borsari S, Pampena R, Lallas A, et al. Clinical indications for use of reflectance confocal microscopy for skin cancer diagnosis. JAMA Dermatol. 2016;152:1093-1098.
- Agarwal-Antal N, Bowen GM, Gerwels JW. Histologic evaluation of lentigo maligna with permanent sections: implications regarding current guidelines. J Am Acad Dermatol. 2002;47:743-748.
- Gardner KH, Hill DE, Wright AC, et al. Upstaging from melanoma in situ to invasive melanoma on the head and neck after complete surgical resection. Dermatol Surg. 2015;41:1122-1125.
- Champin J, Perrot JL, Cinotti E, et al. In vivo reflectance confocal microscopy to optimize the spaghetti technique for defining surgical margins of lentigo maligna. Dermatolog Surg. 2014;40:247-256.
- Fogarty GB, Hong A, Scolyer RA, et al. Radiotherapy for lentigo maligna: a literature review and recommendations for treatment. Br J Dermatol. 2014;170:52-58.
- Swetter SM, Chen FW, Kim DD, et al. Imiquimod 5% cream as primary or adjuvant therapy for melanoma in situ, lentigo maligna type. J Am Acad Dermatol. 2015;72:1047-1053.
- Richtig E, Arzberger E, Hofmann-Wellenhof R, et al. Assessment of changes in lentigo maligna during radiotherapy by in-vivo reflectance confocal microscopy--a pilot study. Br J Dermatol. 2015;172:81-87.
- Gerger A, Koller S, Kern T, et al. Diagnostic applicability of in vivo confocal laser scanning microscopy in melanocytic skin tumors. J Invest Dermatol. 2005;124:493-498.
- Farnetani F, Scope A, Braun RP, et al. Skin cancer diagnosis with reflectance confocal microscopy: reproducibility of feature recognition and accuracy of diagnosis. JAMA Dermatol. 2015;151:1075-1080.
- Rajadhyaksha M, Marghoob A, Rossi A, et al. Reflectance confocal microscopy of skin in vivo: from bench to bedside [published online October 27, 2016]. Lasers Surg Med. 2017;49:7-19.
Lentigo maligna (LM) and LM melanoma (LMM) represent diagnostic and therapeutic challenges due to their heterogeneous nature and location on cosmetically sensitive areas. Newer ancillary technologies such as reflectance confocal microscopy (RCM) have helped improve diagnosis and management of these challenging lesions.1,2
Reflectance confocal microscopy is a noninvasive laser system that provides real-time imaging of the epidermis and dermis with cellular resolution and improves diagnostic accuracy of melanocytic lesions.2,3 Normal melanocytes appear as round bright structures on RCM that are similar in size to surrounding keratinocytes located in the basal layer and regularly distributed around the dermal papillae (junctional nevi) or form regular dense nests in the dermis (intradermal nevi).4,5 In LM/LMM, there may be widespread infiltration of atypical melanocytes invading hair follicles; large, round, pagetoid melanocytes (larger than surrounding keratinocytes); sheets of large atypical cells at the dermoepidermal junction (DEJ); loss of contour in the dermal papillae; and atypical melanocytes invading the dermal papillae.2 Indeed, RCM has good correlation with the degree of histologic atypia and is useful to distinguish between benign nevi, atypical nevi, and melanoma.6 By combining lateral mosaics with vertical stacks, RCM allows 3-dimensional approximation of tumor margins and monitoring of nonsurgical therapies.7,8 The advent of handheld RCM (HRCM) has allowed assessment of large lesions as well as those presenting in difficult locations.9 Furthermore, the generation of videomosaics overcomes the limited field of view of traditional RCM and allows for accurate assessment of large lesions.10
Traditional and handheld RCM have been used to diagnose and map primary LM.1,2,11 Guitera et al2 developed an algorithm using traditional RCM to distinguish benign facial macules and LM. In their training set, they found that when their score resulted in 2 or more points, the sensitivity and specificity to diagnose LM was 85% and 76%, respectively, with an odds ratio of 18.6 for LM. They later applied the algorithm in a test set of 44 benign facial macules and 29 LM and obtained an odds ratio of 60.7 for LM, with sensitivity and specificity rates of 93% and 82%, respectively.2 This algorithm also was tested by Menge et al11 using the HRCM. They found 100% sensitivity and 71% specificity for LM when evaluating 63 equivocal facial lesions. Although these results suggest that RCM can accurately distinguish LM from benign lesions in the primary setting, few reports have studied the impact of HRCM in the recurrent setting and its impact in monitoring treatment of LM.12,13
Herein, we present 5 cases in which HRCM was used to manage complex facial LM/LMM, highlighting its versatility and potential for use in the clinical setting (eTable).
Case Series
Following institutional review board approval, cases of facial LM/LMM presenting for assessment and treatment from January 2014 to December 2015 were retrospectively reviewed. Initially, the clinical margins of the lesions were determined using Wood lamp and/or dermoscopy. Using HRCM, vertical stacks were taken at the 12-, 3-, 6-, and 9-o'clock positions, and videos were captured along the peripheral margins at the DEJ. To create videomosaics, HRCM video frames were extracted and later stitched using a computer algorithm written in a fourth-generation programming language based on prior studies.10,14 An example HRCM video that was captured and turned into a videomosaic accompanies this article online (http://bit.ly/2oDYS6k). Additional stacks were taken in suspicious areas. We considered an area positive for LM under HRCM when the LM score developed by Guitera et al2 was 2 or more. The algorithm scoring includes 2 major criteria--nonedged papillae and round large pagetoid cells--which score 2 points, and 4 minor criteria, including 3 positive criteria--atypical cells at the DEJ, follicular invasion, nucleated cells in the papillae--which each score 1 point, and 1 negative criterion--broadened honeycomb pattern--which scores -1 point.2
RELATED VIDEO: RCM Videomosaic of Melanoma In Situ
Patient 1
An 82-year-old woman was referred to us for management of an LMM on the left side of the forehead (Figure 1A). Handheld RCM from the biopsy site showed large atypical cells in the epidermis, DEJ, and papillary dermis. Superiorly, HRCM showed large dendritic processes but did not reveal LM features in 3 additional clinically worrisome areas. Biopsies showed LMM at the prior biopsy site, LM superiorly, and actinic keratosis in the remaining 3 areas, supporting the HRCM findings. Due to upstaging, the patient was referred for head and neck surgery. To aid in resection, HRCM was performed intraoperatively in a multidisciplinary approach (Figure 1B). Due to the large size of the lesion, surgical margins were taken right outside the HRCM border. Pathology showed LMM extending focally into the margins that were reexcised, achieving clearance.

Patient 2
An 88-year-old woman presented with a slightly pigmented, 2.5×2.3-cm LMM on the left cheek. Because of her age and comorbidities (eg, osteoporosis, deep vein thrombosis in both lower legs requiring anticoagulation therapy, presence of an inferior vena cava filter, bilateral lymphedema of the legs, irritable bowel syndrome, hyperparathyroidism), she was treated with imiquimod cream 5% achieving partial response. The lesion was subsequently excised showing LMM extending to the margins. Not wanting to undergo further surgery, she opted for radiation therapy. Handheld RCM was performed to guide the radiation field, showing pagetoid cells within 1 cm of the scar and clear margins beyond 2 cm. She underwent radiation therapy followed by treatment with imiquimod. On 6-month follow-up, no clinical lesion was apparent, but HRCM showed atypical cells. Biopsies revealed an atypical intraepidermal melanocytic proliferation, but due to patient's comorbidities, close observation was decided.
Patient 3
A 78-year-old man presented with an LMM on the right preauricular area. Handheld RCM demonstrated pleomorphic pagetoid cells along and beyond the clinical margins. Wide excision with sentinel lymph node biopsy was planned, and to aid surgery a confocal map was created (Figure 2). Margins were clear at 1 cm, except inferiorly where they extended to 1.5 cm. Using this preoperative HRCM map, all intraoperative sections were clear. Final pathology confirmed clear margins throughout.

Patient 4
A 62-year-old man presented with hyperpigmentation and bleeding on the left cheek where an LMM was previously removed 8 times over 18 years. Handheld RCM showed pleomorphic cells along the graft border and interestingly within the graft. Ten biopsies were taken, 8 at sites with confocal features that were worrisome for LM (Figures 3A and 3B) and 2 at clinically suspicious sites. The former revealed melanomas (2 that were invasive to 0.3 mm), and the latter revealed solar lentigines. The patient underwent staged excision guided by HRCM (Figure 3C), achieving clear histologic margins except for a focus in the helix. This area was RCM positive but was intentionally not resected due to reconstructive difficulties; imiquimod was indicated in this area.

Patient 5
An 85-year-old woman with 6 prior melanomas over 15 years presented with ill-defined light brown patches on the left cheek at the site where an LM was previously excised 15 years prior. Biopsies showed LM, and due to the patient's age, health, and personal preference to avoid extensive surgery, treatment with imiquimod cream 5% was decided. Over a period of 6 to 12 months, she developed multiple erythematous macules with 2 faintly pigmented areas. Handheld RCM demonstrated atypical cells within the papillae in previously biopsied sites that were rebiopsied, revealing LMM (Breslow depth, 0.2 mm). Staged excision achieved clear margins, but after 8 months HRCM showed LM features. Histology confirmed the diagnosis and imiquimod was reapplied.
Comment
Diagnosis and choice of treatment modality for cases of facial LM is a challenge, and there are a number of factors that may create even more of a clinical dilemma. Surgical excision is the treatment of choice for LM/LMM, and better results are achieved when using histologically controlled surgical procedures such as Mohs micrographic surgery, staged excision, or the "spaghetti technique."15-17 However, advanced patient age, multiple comorbidities (eg, coronary artery disease, deep vein thrombosis, other conditions requiring anticoagulation therapy), large lesion size in functionally or aesthetically sensitive areas, and indiscriminate borders on photodamaged skin may make surgical excision complicated or not feasible. Additionally, prior treatments to the affected area may further obscure clinical borders, complicating the diagnosis of recurrence/persistence when observed with the naked eye, dermoscopy, or Wood lamp. Because RCM can detect small amounts of melanin and has cellular resolution, it has been suggested as a great diagnostic tool to be combined with dermoscopy when evaluating lightly pigmented/amelanotic facial lesions arising on sun-damaged skin.18,19 In this case series, we highlighted these difficulties and showed how HRCM can be useful in a variety of scenarios, both pretreatment and posttreatment in complex LM/LMM cases.
Pretreatment Evaluation
Blind mapping biopsies of LM are prone to sample bias and depend greatly on biopsy technique; however, HRCM can guide mapping biopsies by detecting features of LM in vivo with high sensitivity.11 Due to the cosmetically sensitive nature of the lesions, many physicians are discouraged to do multiple mapping biopsies, making it difficult to assess the breadth of the lesion and occult invasion. Multiple studies have shown that occult invasion was not apparent until complete lesion excision was done.15,20,21 Agarwal-Antal et al20 reported 92 cases of LM, of which 16% (15/92) had unsuspected invasion on final excisional pathology. A long-standing disadvantage of treating LM with nonsurgical modalities has been the inability to detect occult invasion or multifocal invasion within the lesion. As described in patients 1, 4, and 5 in the current case series, utilizing real-time video imaging of the DEJ at the margins and within the lesion has allowed for the detection of deep atypical melanocytes suspicious for perifollicular infiltration and invasion. Knowing the depth of invasion before treatment is essential for not only counseling the patient about disease risk but also for choosing an appropriate treatment modality. Therefore, prospective studies evaluating the performance of RCM to identify invasion are crucial to improve sampling error and avoid unnecessary biopsies.
Surgical Treatment
Although surgery is the first-line treatment option for facial LM, it is not without associated morbidity, and LM is known to have histological subclinical extension, which makes margin assessment difficult. Wide surgical margins on the face are not always possible and become further complicated when trying to maintain adequate functional and cosmetic outcomes. Additionally, the margin for surgical clearance may not be straightforward for facial lesions. Hazan et al15 showed the mean total surgical margins required for excision of LM and LMM was 7.1 and 10.3 mm, respectively; of the 91 tumors initially diagnosed as LM on biopsy, 16% (15/91) had unsuspected invasion. Guitera et al2 reported that the presence of atypical cells within the dermal papillae might be a sign of invasion, which occasionally is not detected histologically due to sampling bias. Handheld RCM offers the advantage of a rapid real-time assessment in areas that may not have been amenable to previous iterations of the device, and it also provides a larger field of view that would be time consuming if performed using conventional RCM. Compared to prior RCM devices that were not handheld, the use of the HRCM does not need to attach a ring to the skin and is less bulky, permitting its use at the bedside of the patient or even intraoperatively.13 In our experience, HRCM has helped to better characterize subclinical spread of LM during the initial consultation and better counsel patients about the extent of the lesion. Handheld RCM also has been used to guide the spaghetti technique in patients with LM/LMM with good correlation between HRCM and histology.22 In our case series, HRCM was used in complex LM/LMM to delineate surgical margins, though in some cases the histologic margins were too close or affected, suggesting HRCM underestimation. Lentigo maligna margin assessment with RCM uses an algorithm that evaluates confocal features in the center of the lesion.1,2 Therefore, further studies using HRCM should evaluate minor confocal features in the margins as potential markers of positivity to accurately delineate surgical margins.
Nonsurgical Treatment Options
For patients unable or unwilling to pursue surgical treatment, therapies such as imiquimod or radiation have been suggested.23,24 However, the lack of histological confirmation and possibility for invasive spread has limited these modalities. Lentigo malignas treated with radiation have a 5% recurrence rate, with a median follow-up time of 3 years.23 Recurrence often can be difficult to detect clinically, as it may manifest as an amelanotic lesion, or postradiation changes can hinder detection. Handheld RCM allows for a cellular-level observation of the irradiated field and can identify radiation-induced changes in LM lesions, including superficial necrosis, apoptotic cells, dilated vessels, and increased inflammatory cells.25 Handheld RCM has previously been used to assess LM treated with radiation and, as in patient 2, can help define the radiation field and detect treatment failure or recurrence.12,25
Similarly, as described in patient 5, HRCM was utilized to monitor treatment with imiquimod. Many reports use imiquimod for treatment of LM, but application and response vary greatly. Reflectance confocal microscopy has been shown to be useful in monitoring LM treated with imiquimod,8 which is important because clinical findings such as inflammation and erythema do not correlate well with response to therapy. Thus, RCM is an appealing noninvasive modality to monitor response to treatment and assess the need for longer treatment duration. Moreover, similar to postradiation changes, treatment with imiquimod may cause an alteration of the clinically apparent pigment. Therefore, it is difficult to assess treatment success by clinical inspection alone. The use of RCM before, during, and after treatment provides a longitudinal assessment of the lesion and has augmented dermatologists' ability to determine treatment success or failure; however, prospective studies evaluating the usefulness of HRCM in the recurrent setting are needed to validate these results.
Limitations
Limitations of this technology include the time needed to image large areas; technology cost; and associated learning curve, which may take from 6 months to 1 year based on our experience. Others have reported the training required for accurate RCM interpretation to be less than that of dermoscopy.26 It has been shown that key RCM diagnostic criteria for lesions including melanoma and basal cell carcinoma are reproducibly recognized among RCM users and that diagnostic accuracy increases with experience.27 These limitations can be overcome with advances in videomosaicing that may streamline the imaging as well as an eventual decrease in cost with greater user adoption and the development of training platforms that enable a faster learning of RCM.28
Conclusion
The use of HRCM can help in the diagnosis and management of facial LMs. Handheld RCM provides longitudinal assessment of LM/LMM that may help determine treatment success or failure and has proven to be useful in detecting the presence of recurrence/persistence in cases that were clinically poorly evident. Moreover, HRCM is a notable ancillary tool, as it can be performed at the bedside of the patient or even intraoperatively and provides a faster approach than conventional RCM in cases where large areas need to be mapped.
In summary, HRCM may eventually be a useful screening tool to guide scouting biopsies to diagnose de novo LM; guide surgical and nonsurgical therapies; and evaluate the presence of recurrence/persistence, especially in large, complex, amelanotic or poorly pigmented lesions. A more standardized use of HRCM in mapping surgical and nonsurgical approaches needs to be evaluated in further studies to provide a fast and reliable complement to histology in such complex cases; therefore, larger studies need to be performed to validate this technique in such complex cases.
Lentigo maligna (LM) and LM melanoma (LMM) represent diagnostic and therapeutic challenges due to their heterogeneous nature and location on cosmetically sensitive areas. Newer ancillary technologies such as reflectance confocal microscopy (RCM) have helped improve diagnosis and management of these challenging lesions.1,2
Reflectance confocal microscopy is a noninvasive laser system that provides real-time imaging of the epidermis and dermis with cellular resolution and improves diagnostic accuracy of melanocytic lesions.2,3 Normal melanocytes appear as round bright structures on RCM that are similar in size to surrounding keratinocytes located in the basal layer and regularly distributed around the dermal papillae (junctional nevi) or form regular dense nests in the dermis (intradermal nevi).4,5 In LM/LMM, there may be widespread infiltration of atypical melanocytes invading hair follicles; large, round, pagetoid melanocytes (larger than surrounding keratinocytes); sheets of large atypical cells at the dermoepidermal junction (DEJ); loss of contour in the dermal papillae; and atypical melanocytes invading the dermal papillae.2 Indeed, RCM has good correlation with the degree of histologic atypia and is useful to distinguish between benign nevi, atypical nevi, and melanoma.6 By combining lateral mosaics with vertical stacks, RCM allows 3-dimensional approximation of tumor margins and monitoring of nonsurgical therapies.7,8 The advent of handheld RCM (HRCM) has allowed assessment of large lesions as well as those presenting in difficult locations.9 Furthermore, the generation of videomosaics overcomes the limited field of view of traditional RCM and allows for accurate assessment of large lesions.10
Traditional and handheld RCM have been used to diagnose and map primary LM.1,2,11 Guitera et al2 developed an algorithm using traditional RCM to distinguish benign facial macules and LM. In their training set, they found that when their score resulted in 2 or more points, the sensitivity and specificity to diagnose LM was 85% and 76%, respectively, with an odds ratio of 18.6 for LM. They later applied the algorithm in a test set of 44 benign facial macules and 29 LM and obtained an odds ratio of 60.7 for LM, with sensitivity and specificity rates of 93% and 82%, respectively.2 This algorithm also was tested by Menge et al11 using the HRCM. They found 100% sensitivity and 71% specificity for LM when evaluating 63 equivocal facial lesions. Although these results suggest that RCM can accurately distinguish LM from benign lesions in the primary setting, few reports have studied the impact of HRCM in the recurrent setting and its impact in monitoring treatment of LM.12,13
Herein, we present 5 cases in which HRCM was used to manage complex facial LM/LMM, highlighting its versatility and potential for use in the clinical setting (eTable).
Case Series
Following institutional review board approval, cases of facial LM/LMM presenting for assessment and treatment from January 2014 to December 2015 were retrospectively reviewed. Initially, the clinical margins of the lesions were determined using Wood lamp and/or dermoscopy. Using HRCM, vertical stacks were taken at the 12-, 3-, 6-, and 9-o'clock positions, and videos were captured along the peripheral margins at the DEJ. To create videomosaics, HRCM video frames were extracted and later stitched using a computer algorithm written in a fourth-generation programming language based on prior studies.10,14 An example HRCM video that was captured and turned into a videomosaic accompanies this article online (http://bit.ly/2oDYS6k). Additional stacks were taken in suspicious areas. We considered an area positive for LM under HRCM when the LM score developed by Guitera et al2 was 2 or more. The algorithm scoring includes 2 major criteria--nonedged papillae and round large pagetoid cells--which score 2 points, and 4 minor criteria, including 3 positive criteria--atypical cells at the DEJ, follicular invasion, nucleated cells in the papillae--which each score 1 point, and 1 negative criterion--broadened honeycomb pattern--which scores -1 point.2
RELATED VIDEO: RCM Videomosaic of Melanoma In Situ
Patient 1
An 82-year-old woman was referred to us for management of an LMM on the left side of the forehead (Figure 1A). Handheld RCM from the biopsy site showed large atypical cells in the epidermis, DEJ, and papillary dermis. Superiorly, HRCM showed large dendritic processes but did not reveal LM features in 3 additional clinically worrisome areas. Biopsies showed LMM at the prior biopsy site, LM superiorly, and actinic keratosis in the remaining 3 areas, supporting the HRCM findings. Due to upstaging, the patient was referred for head and neck surgery. To aid in resection, HRCM was performed intraoperatively in a multidisciplinary approach (Figure 1B). Due to the large size of the lesion, surgical margins were taken right outside the HRCM border. Pathology showed LMM extending focally into the margins that were reexcised, achieving clearance.

Patient 2
An 88-year-old woman presented with a slightly pigmented, 2.5×2.3-cm LMM on the left cheek. Because of her age and comorbidities (eg, osteoporosis, deep vein thrombosis in both lower legs requiring anticoagulation therapy, presence of an inferior vena cava filter, bilateral lymphedema of the legs, irritable bowel syndrome, hyperparathyroidism), she was treated with imiquimod cream 5% achieving partial response. The lesion was subsequently excised showing LMM extending to the margins. Not wanting to undergo further surgery, she opted for radiation therapy. Handheld RCM was performed to guide the radiation field, showing pagetoid cells within 1 cm of the scar and clear margins beyond 2 cm. She underwent radiation therapy followed by treatment with imiquimod. On 6-month follow-up, no clinical lesion was apparent, but HRCM showed atypical cells. Biopsies revealed an atypical intraepidermal melanocytic proliferation, but due to patient's comorbidities, close observation was decided.
Patient 3
A 78-year-old man presented with an LMM on the right preauricular area. Handheld RCM demonstrated pleomorphic pagetoid cells along and beyond the clinical margins. Wide excision with sentinel lymph node biopsy was planned, and to aid surgery a confocal map was created (Figure 2). Margins were clear at 1 cm, except inferiorly where they extended to 1.5 cm. Using this preoperative HRCM map, all intraoperative sections were clear. Final pathology confirmed clear margins throughout.

Patient 4
A 62-year-old man presented with hyperpigmentation and bleeding on the left cheek where an LMM was previously removed 8 times over 18 years. Handheld RCM showed pleomorphic cells along the graft border and interestingly within the graft. Ten biopsies were taken, 8 at sites with confocal features that were worrisome for LM (Figures 3A and 3B) and 2 at clinically suspicious sites. The former revealed melanomas (2 that were invasive to 0.3 mm), and the latter revealed solar lentigines. The patient underwent staged excision guided by HRCM (Figure 3C), achieving clear histologic margins except for a focus in the helix. This area was RCM positive but was intentionally not resected due to reconstructive difficulties; imiquimod was indicated in this area.

Patient 5
An 85-year-old woman with 6 prior melanomas over 15 years presented with ill-defined light brown patches on the left cheek at the site where an LM was previously excised 15 years prior. Biopsies showed LM, and due to the patient's age, health, and personal preference to avoid extensive surgery, treatment with imiquimod cream 5% was decided. Over a period of 6 to 12 months, she developed multiple erythematous macules with 2 faintly pigmented areas. Handheld RCM demonstrated atypical cells within the papillae in previously biopsied sites that were rebiopsied, revealing LMM (Breslow depth, 0.2 mm). Staged excision achieved clear margins, but after 8 months HRCM showed LM features. Histology confirmed the diagnosis and imiquimod was reapplied.
Comment
Diagnosis and choice of treatment modality for cases of facial LM is a challenge, and there are a number of factors that may create even more of a clinical dilemma. Surgical excision is the treatment of choice for LM/LMM, and better results are achieved when using histologically controlled surgical procedures such as Mohs micrographic surgery, staged excision, or the "spaghetti technique."15-17 However, advanced patient age, multiple comorbidities (eg, coronary artery disease, deep vein thrombosis, other conditions requiring anticoagulation therapy), large lesion size in functionally or aesthetically sensitive areas, and indiscriminate borders on photodamaged skin may make surgical excision complicated or not feasible. Additionally, prior treatments to the affected area may further obscure clinical borders, complicating the diagnosis of recurrence/persistence when observed with the naked eye, dermoscopy, or Wood lamp. Because RCM can detect small amounts of melanin and has cellular resolution, it has been suggested as a great diagnostic tool to be combined with dermoscopy when evaluating lightly pigmented/amelanotic facial lesions arising on sun-damaged skin.18,19 In this case series, we highlighted these difficulties and showed how HRCM can be useful in a variety of scenarios, both pretreatment and posttreatment in complex LM/LMM cases.
Pretreatment Evaluation
Blind mapping biopsies of LM are prone to sample bias and depend greatly on biopsy technique; however, HRCM can guide mapping biopsies by detecting features of LM in vivo with high sensitivity.11 Due to the cosmetically sensitive nature of the lesions, many physicians are discouraged to do multiple mapping biopsies, making it difficult to assess the breadth of the lesion and occult invasion. Multiple studies have shown that occult invasion was not apparent until complete lesion excision was done.15,20,21 Agarwal-Antal et al20 reported 92 cases of LM, of which 16% (15/92) had unsuspected invasion on final excisional pathology. A long-standing disadvantage of treating LM with nonsurgical modalities has been the inability to detect occult invasion or multifocal invasion within the lesion. As described in patients 1, 4, and 5 in the current case series, utilizing real-time video imaging of the DEJ at the margins and within the lesion has allowed for the detection of deep atypical melanocytes suspicious for perifollicular infiltration and invasion. Knowing the depth of invasion before treatment is essential for not only counseling the patient about disease risk but also for choosing an appropriate treatment modality. Therefore, prospective studies evaluating the performance of RCM to identify invasion are crucial to improve sampling error and avoid unnecessary biopsies.
Surgical Treatment
Although surgery is the first-line treatment option for facial LM, it is not without associated morbidity, and LM is known to have histological subclinical extension, which makes margin assessment difficult. Wide surgical margins on the face are not always possible and become further complicated when trying to maintain adequate functional and cosmetic outcomes. Additionally, the margin for surgical clearance may not be straightforward for facial lesions. Hazan et al15 showed the mean total surgical margins required for excision of LM and LMM was 7.1 and 10.3 mm, respectively; of the 91 tumors initially diagnosed as LM on biopsy, 16% (15/91) had unsuspected invasion. Guitera et al2 reported that the presence of atypical cells within the dermal papillae might be a sign of invasion, which occasionally is not detected histologically due to sampling bias. Handheld RCM offers the advantage of a rapid real-time assessment in areas that may not have been amenable to previous iterations of the device, and it also provides a larger field of view that would be time consuming if performed using conventional RCM. Compared to prior RCM devices that were not handheld, the use of the HRCM does not need to attach a ring to the skin and is less bulky, permitting its use at the bedside of the patient or even intraoperatively.13 In our experience, HRCM has helped to better characterize subclinical spread of LM during the initial consultation and better counsel patients about the extent of the lesion. Handheld RCM also has been used to guide the spaghetti technique in patients with LM/LMM with good correlation between HRCM and histology.22 In our case series, HRCM was used in complex LM/LMM to delineate surgical margins, though in some cases the histologic margins were too close or affected, suggesting HRCM underestimation. Lentigo maligna margin assessment with RCM uses an algorithm that evaluates confocal features in the center of the lesion.1,2 Therefore, further studies using HRCM should evaluate minor confocal features in the margins as potential markers of positivity to accurately delineate surgical margins.
Nonsurgical Treatment Options
For patients unable or unwilling to pursue surgical treatment, therapies such as imiquimod or radiation have been suggested.23,24 However, the lack of histological confirmation and possibility for invasive spread has limited these modalities. Lentigo malignas treated with radiation have a 5% recurrence rate, with a median follow-up time of 3 years.23 Recurrence often can be difficult to detect clinically, as it may manifest as an amelanotic lesion, or postradiation changes can hinder detection. Handheld RCM allows for a cellular-level observation of the irradiated field and can identify radiation-induced changes in LM lesions, including superficial necrosis, apoptotic cells, dilated vessels, and increased inflammatory cells.25 Handheld RCM has previously been used to assess LM treated with radiation and, as in patient 2, can help define the radiation field and detect treatment failure or recurrence.12,25
Similarly, as described in patient 5, HRCM was utilized to monitor treatment with imiquimod. Many reports use imiquimod for treatment of LM, but application and response vary greatly. Reflectance confocal microscopy has been shown to be useful in monitoring LM treated with imiquimod,8 which is important because clinical findings such as inflammation and erythema do not correlate well with response to therapy. Thus, RCM is an appealing noninvasive modality to monitor response to treatment and assess the need for longer treatment duration. Moreover, similar to postradiation changes, treatment with imiquimod may cause an alteration of the clinically apparent pigment. Therefore, it is difficult to assess treatment success by clinical inspection alone. The use of RCM before, during, and after treatment provides a longitudinal assessment of the lesion and has augmented dermatologists' ability to determine treatment success or failure; however, prospective studies evaluating the usefulness of HRCM in the recurrent setting are needed to validate these results.
Limitations
Limitations of this technology include the time needed to image large areas; technology cost; and associated learning curve, which may take from 6 months to 1 year based on our experience. Others have reported the training required for accurate RCM interpretation to be less than that of dermoscopy.26 It has been shown that key RCM diagnostic criteria for lesions including melanoma and basal cell carcinoma are reproducibly recognized among RCM users and that diagnostic accuracy increases with experience.27 These limitations can be overcome with advances in videomosaicing that may streamline the imaging as well as an eventual decrease in cost with greater user adoption and the development of training platforms that enable a faster learning of RCM.28
Conclusion
The use of HRCM can help in the diagnosis and management of facial LMs. Handheld RCM provides longitudinal assessment of LM/LMM that may help determine treatment success or failure and has proven to be useful in detecting the presence of recurrence/persistence in cases that were clinically poorly evident. Moreover, HRCM is a notable ancillary tool, as it can be performed at the bedside of the patient or even intraoperatively and provides a faster approach than conventional RCM in cases where large areas need to be mapped.
In summary, HRCM may eventually be a useful screening tool to guide scouting biopsies to diagnose de novo LM; guide surgical and nonsurgical therapies; and evaluate the presence of recurrence/persistence, especially in large, complex, amelanotic or poorly pigmented lesions. A more standardized use of HRCM in mapping surgical and nonsurgical approaches needs to be evaluated in further studies to provide a fast and reliable complement to histology in such complex cases; therefore, larger studies need to be performed to validate this technique in such complex cases.
- Guitera P, Moloney FJ, Menzies SW, et al. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatol. 2013;149:692-698.
- Guitera P, Pellacani G, Crotty KA, et al. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face. J Invest Dermatol. 2010;130:2080-2091.
- Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-2765.
- Segura S, Puig S, Carrera C, et al. Development of a two-step method for the diagnosis of melanoma by reflectance confocal microscopy. J Am Acad Dermatol. 2009;61:216-229.
- Hofmann-Wellenhof R, Pellacani G, Malvehy J, et al. Reflectance Confocal Microscopy for Skin Diseases. New York, NY: Springer; 2012.
- Pellacani G, Farnetani F, Gonzalez S, et al. In vivo confocal microscopy for detection and grading of dysplastic nevi: a pilot study. J Am Acad Dermatol. 2012;66:E109-E121.
- Nadiminti H, Scope A, Marghoob AA, et al. Use of reflectance confocal microscopy to monitor response of lentigo maligna to nonsurgical treatment. Dermatol Surg. 2010;36:177-184.
- Alarcon I, Carrera C, Alos L, et al. In vivo reflectance confocal microscopy to monitor the response of lentigo maligna to imiquimod. J Am Acad Dermatol. 2014;71:49-55.
- Fraga-Braghiroli NA, Stephens A, Grossman D, et al. Use of handheld reflectance confocal microscopy for in vivo diagnosis of solitary facial papules: a case series. J Eur Acad Dermatol Venereol. 2014;28:933-942.
- Kose K, Cordova M, Duffy M, et al. Video-mosaicing of reflectance confocal images for examination of extended areas of skin in vivo. Br J Dermatol. 2014;171:1239-1241.
- Menge TD, Hibler BP, Cordova MA, et al. Concordance of handheld reflectance confocal microscopy (RCM) with histopathology in the diagnosis of lentigo maligna (LM): a prospective study [published online January 27, 2016]. J Am Acad Dermatol. 2016;74:1114-1120.
- Hibler BP, Connolly KL, Cordova M, et al. Radiation therapy for synchronous basal cell carcinoma and lentigo maligna of the nose: response assessment by clinical examination and reflectance confocal microscopy. Pract Radiat Oncol. 2015;5:E543-E547.
- Hibler BP, Cordova M, Wong RJ, et al. Intraoperative real-time reflectance confocal microscopy for guiding surgical margins of lentigo maligna melanoma. Dermatol Surg. 2015;41:980-983.
- Kose K, Gou M, Yelamos O, et al. Video-mosaicking of in vivo reflectance confocal microscopy images for noninvasive examination of skin lesions [published February 6, 2017]. Proceedings of SPIE Photonics West. doi:10.1117/12.2253085.
- Hazan C, Dusza SW, Delgado R, et al. Staged excision for lentigo maligna and lentigo maligna melanoma: a retrospective analysis of 117 cases. J Am Acad Dermatol. 2008;58:142-148.
- Etzkorn JR, Sobanko JF, Elenitsas R, et al. Low recurrence rates for in situ and invasive melanomas using Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining: tissue processing methodology to optimize pathologic staging and margin assessment. J Am Acad Dermatol. 2015;72:840-850.
- Gaudy-Marqueste C, Perchenet AS, Tasei AM, et al. The "spaghetti technique": an alternative to Mohs surgery or staged surgery for problematic lentiginous melanoma (lentigo maligna and acral lentiginous melanoma). J Am Acad Dermatol. 2011;64:113-118.
- Guitera P, Menzies SW, Argenziano G, et al. Dermoscopy and in vivo confocal microscopy are complementary techniques for diagnosis of difficult amelanotic and light-coloured skin lesions [published online October 12, 2016]. Br J Dermatol. 2016;175:1311-1319.
- Borsari S, Pampena R, Lallas A, et al. Clinical indications for use of reflectance confocal microscopy for skin cancer diagnosis. JAMA Dermatol. 2016;152:1093-1098.
- Agarwal-Antal N, Bowen GM, Gerwels JW. Histologic evaluation of lentigo maligna with permanent sections: implications regarding current guidelines. J Am Acad Dermatol. 2002;47:743-748.
- Gardner KH, Hill DE, Wright AC, et al. Upstaging from melanoma in situ to invasive melanoma on the head and neck after complete surgical resection. Dermatol Surg. 2015;41:1122-1125.
- Champin J, Perrot JL, Cinotti E, et al. In vivo reflectance confocal microscopy to optimize the spaghetti technique for defining surgical margins of lentigo maligna. Dermatolog Surg. 2014;40:247-256.
- Fogarty GB, Hong A, Scolyer RA, et al. Radiotherapy for lentigo maligna: a literature review and recommendations for treatment. Br J Dermatol. 2014;170:52-58.
- Swetter SM, Chen FW, Kim DD, et al. Imiquimod 5% cream as primary or adjuvant therapy for melanoma in situ, lentigo maligna type. J Am Acad Dermatol. 2015;72:1047-1053.
- Richtig E, Arzberger E, Hofmann-Wellenhof R, et al. Assessment of changes in lentigo maligna during radiotherapy by in-vivo reflectance confocal microscopy--a pilot study. Br J Dermatol. 2015;172:81-87.
- Gerger A, Koller S, Kern T, et al. Diagnostic applicability of in vivo confocal laser scanning microscopy in melanocytic skin tumors. J Invest Dermatol. 2005;124:493-498.
- Farnetani F, Scope A, Braun RP, et al. Skin cancer diagnosis with reflectance confocal microscopy: reproducibility of feature recognition and accuracy of diagnosis. JAMA Dermatol. 2015;151:1075-1080.
- Rajadhyaksha M, Marghoob A, Rossi A, et al. Reflectance confocal microscopy of skin in vivo: from bench to bedside [published online October 27, 2016]. Lasers Surg Med. 2017;49:7-19.
- Guitera P, Moloney FJ, Menzies SW, et al. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatol. 2013;149:692-698.
- Guitera P, Pellacani G, Crotty KA, et al. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face. J Invest Dermatol. 2010;130:2080-2091.
- Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-2765.
- Segura S, Puig S, Carrera C, et al. Development of a two-step method for the diagnosis of melanoma by reflectance confocal microscopy. J Am Acad Dermatol. 2009;61:216-229.
- Hofmann-Wellenhof R, Pellacani G, Malvehy J, et al. Reflectance Confocal Microscopy for Skin Diseases. New York, NY: Springer; 2012.
- Pellacani G, Farnetani F, Gonzalez S, et al. In vivo confocal microscopy for detection and grading of dysplastic nevi: a pilot study. J Am Acad Dermatol. 2012;66:E109-E121.
- Nadiminti H, Scope A, Marghoob AA, et al. Use of reflectance confocal microscopy to monitor response of lentigo maligna to nonsurgical treatment. Dermatol Surg. 2010;36:177-184.
- Alarcon I, Carrera C, Alos L, et al. In vivo reflectance confocal microscopy to monitor the response of lentigo maligna to imiquimod. J Am Acad Dermatol. 2014;71:49-55.
- Fraga-Braghiroli NA, Stephens A, Grossman D, et al. Use of handheld reflectance confocal microscopy for in vivo diagnosis of solitary facial papules: a case series. J Eur Acad Dermatol Venereol. 2014;28:933-942.
- Kose K, Cordova M, Duffy M, et al. Video-mosaicing of reflectance confocal images for examination of extended areas of skin in vivo. Br J Dermatol. 2014;171:1239-1241.
- Menge TD, Hibler BP, Cordova MA, et al. Concordance of handheld reflectance confocal microscopy (RCM) with histopathology in the diagnosis of lentigo maligna (LM): a prospective study [published online January 27, 2016]. J Am Acad Dermatol. 2016;74:1114-1120.
- Hibler BP, Connolly KL, Cordova M, et al. Radiation therapy for synchronous basal cell carcinoma and lentigo maligna of the nose: response assessment by clinical examination and reflectance confocal microscopy. Pract Radiat Oncol. 2015;5:E543-E547.
- Hibler BP, Cordova M, Wong RJ, et al. Intraoperative real-time reflectance confocal microscopy for guiding surgical margins of lentigo maligna melanoma. Dermatol Surg. 2015;41:980-983.
- Kose K, Gou M, Yelamos O, et al. Video-mosaicking of in vivo reflectance confocal microscopy images for noninvasive examination of skin lesions [published February 6, 2017]. Proceedings of SPIE Photonics West. doi:10.1117/12.2253085.
- Hazan C, Dusza SW, Delgado R, et al. Staged excision for lentigo maligna and lentigo maligna melanoma: a retrospective analysis of 117 cases. J Am Acad Dermatol. 2008;58:142-148.
- Etzkorn JR, Sobanko JF, Elenitsas R, et al. Low recurrence rates for in situ and invasive melanomas using Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining: tissue processing methodology to optimize pathologic staging and margin assessment. J Am Acad Dermatol. 2015;72:840-850.
- Gaudy-Marqueste C, Perchenet AS, Tasei AM, et al. The "spaghetti technique": an alternative to Mohs surgery or staged surgery for problematic lentiginous melanoma (lentigo maligna and acral lentiginous melanoma). J Am Acad Dermatol. 2011;64:113-118.
- Guitera P, Menzies SW, Argenziano G, et al. Dermoscopy and in vivo confocal microscopy are complementary techniques for diagnosis of difficult amelanotic and light-coloured skin lesions [published online October 12, 2016]. Br J Dermatol. 2016;175:1311-1319.
- Borsari S, Pampena R, Lallas A, et al. Clinical indications for use of reflectance confocal microscopy for skin cancer diagnosis. JAMA Dermatol. 2016;152:1093-1098.
- Agarwal-Antal N, Bowen GM, Gerwels JW. Histologic evaluation of lentigo maligna with permanent sections: implications regarding current guidelines. J Am Acad Dermatol. 2002;47:743-748.
- Gardner KH, Hill DE, Wright AC, et al. Upstaging from melanoma in situ to invasive melanoma on the head and neck after complete surgical resection. Dermatol Surg. 2015;41:1122-1125.
- Champin J, Perrot JL, Cinotti E, et al. In vivo reflectance confocal microscopy to optimize the spaghetti technique for defining surgical margins of lentigo maligna. Dermatolog Surg. 2014;40:247-256.
- Fogarty GB, Hong A, Scolyer RA, et al. Radiotherapy for lentigo maligna: a literature review and recommendations for treatment. Br J Dermatol. 2014;170:52-58.
- Swetter SM, Chen FW, Kim DD, et al. Imiquimod 5% cream as primary or adjuvant therapy for melanoma in situ, lentigo maligna type. J Am Acad Dermatol. 2015;72:1047-1053.
- Richtig E, Arzberger E, Hofmann-Wellenhof R, et al. Assessment of changes in lentigo maligna during radiotherapy by in-vivo reflectance confocal microscopy--a pilot study. Br J Dermatol. 2015;172:81-87.
- Gerger A, Koller S, Kern T, et al. Diagnostic applicability of in vivo confocal laser scanning microscopy in melanocytic skin tumors. J Invest Dermatol. 2005;124:493-498.
- Farnetani F, Scope A, Braun RP, et al. Skin cancer diagnosis with reflectance confocal microscopy: reproducibility of feature recognition and accuracy of diagnosis. JAMA Dermatol. 2015;151:1075-1080.
- Rajadhyaksha M, Marghoob A, Rossi A, et al. Reflectance confocal microscopy of skin in vivo: from bench to bedside [published online October 27, 2016]. Lasers Surg Med. 2017;49:7-19.
Practice Points
- Diagnosis and management of lentigo maligna (LM) and LM melanoma (LMM) is challenging due to their ill-defined margins and location mainly on the head and neck.
- Handheld reflectance confocal microscopy (RCM) has high diagnostic accuracy for LM/LMM and can be used in curved locations to assess large lesions.
- Handheld RCM can be a versatile tool in pretreatment decision-making, intraoperative surgical mapping, and posttreatment monitoring of both surgical and nonsurgical therapies for complex facial LM/LMM.