User login
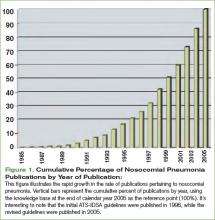
In February 2005, the American Thoracic Society (ATS) and the Infectious Disease Society of America (IDSA) released their joint guidelines for the management of nosocomial pneumonia.1 This impressive document was an extensive revision of guidelines released in 1996.2 These revised guidelines were widely anticipated because during the intervening nine-year span two trends had evolved. First, academic interest in nosocomial pneumonias grew dramatically. This is best illustrated by the exponential growth in the rate of scholarly publications regarding nosocomial pneumonia. A simple PubMed search of “nosocomial pneumonia” shows a six-fold increase in the number of publications devoted to this topic during the time that elapsed between the two sets of guidelines. (See Figure 1, p. 28.) Second, antimicrobial susceptibility to antibiotic therapy rapidly worsened, with many facilities reporting routine resistance in their intensive care units.
Given the growth in related scholarly activity, the 2005 guidelines are both comprehensive and lengthy—some 28 pages in total. The authors meticulously reviewed the nosocomial pneumonia literature, dividing it into manageable and clinically relevant subtopics: epidemiology, pathogenesis, modifiable risk factors, diagnostic testing, diagnostic strategies, antibiotic treatment, and response to therapy. The obvious strengths of the guidelines include that they were written by a collection of authoritative experts representing collaboration between the ATS and the IDSA and that they result in a collection of practical, evidence-based recommendations.
One of the most significant aspects of these guidelines is that they defined a “new” entity: healthcare-associated pneumonia (HCAP). The authors recognize HCAP patients as a unique population because they are at increased risk of harboring resistant pathogens. As defined by the guidelines, HCAP patients include those admitted for more than 48 hours in the past 90 days, those who reside in a nursing home or receive home infusion therapy or wound care, those who are on chronic hemodialysis, or patients who have a family member with a multidrug-resistant pathogen. Although not explicitly stated by the guidelines, it’s worth noting that the recommendations provided were intended to apply only to hospitalized patients with pneumonia.
Looking at the ATS-IDSA definition of HCAP, it is clear that the largest subset of HCAP patients is nursing home residents with pneumonia. Although these patients have historically been treated with agents recommended in community-acquired pneumonia (CAP) guidelines, they more closely resemble hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP) patients in terms of their comorbidities, the presence of catheters and lines, and their prior exposure to antibiotics. Because these patients live longer and have more comorbid disease their care has become increasingly complex, often involving frequent transfers to hospitals or providers’ offices. Not surprisingly, long-term-care facility residents have become a reservoir for multidrug-resistant pathogens.3,4 This patient population will become increasingly important; the number of geriatric Americans is projected to continue to grow for the next 20 years.
Because increased mortality in pneumonia is strongly linked to initially inappropriate antibiotic therapy, the guidelines recommend much broader antibiotic coverage for the newly defined HCAP population.5,6 In the recent past, these patients have been most frequently treated with a third-generation cephalosporin and macrolide combination or a respiratory fluoroquinolone as monotherapy. Given the risk of multidrug-resistant pathogens in HCAP, these regimens are deemed inadequate by the current guidelines, which recommend a combination of:
- An antipseudomonal cephalosporin, an antipseudomonal carbapenem, or a ß-lactam/ß-lactamase inhibitor combination; plus
- An antipseudomonal fluoroquinolone or an aminoglycoside; plus
- Vancomycin or linezolid. (See Table 1, above.)
Controversies Surrounding the Guidelines
Although the revised guidelines were generally greeted with enthusiasm, several authors quickly pointed out that the evidence base supporting HCAP has significant limitations.7-9 Most importantly, studies dealing specifically with HCAP as it is defined by the guidelines are lacking. A review of the ATS-IDSA guidelines’ references reveals that only seven publications deal specifically with HCAP, and all of these focus on nursing home-acquired pneumonia.10-16 Also note that these seven studies use widely discrepant definitions of pneumonia, have varying microbiologic criteria, and include varying numbers of hospitalized and non-hospitalized patients. In addition to these limitations, much of the epidemiology of HCAP pathogens has been extrapolated from diseases other than pneumonia.
Given the paucity of supporting data, it is unclear how similar the subgroups lumped together under the HCAP umbrella really are. Intuitively all of these patients are at increased risk for colonization with resistant pathogens, given their frequent contact with the healthcare system and/or its extenders. The repeated observation that the presence of any one of the factors defining HCAP—recent admission, nursing-home-residence, renal failure, and so on—is associated with increased mortality in patients with nosocomial infections strengthens this assumption.17-19
There are data that challenge the conclusion that such nosocomial pathogens are a frequent enough cause of pneumonia in these patients to merit routine empiric therapy, however. In one large retrospective database review, hemodialysis patients admitted for pneumonia had a spectrum of causative pathogens that was a hybrid of community- and nosocomial-acquired microbes.20 Similarly, in a large systematic review of nursing-home acquired pneumonia studies published between 1978-1994, the causative pathogens were a diverse blend of community- and nosocomial-acquired organisms.21 Both of these studies were retrospective analyses that were limited by the availability and quality of the previously collected microbiology data.
The only study looking specifically at the epidemiology of HCAP as defined by the ATS-IDSA guidelines was published in December 2005—10 months after the guidelines themselves.22 This study raises the possibility that earlier epidemiology studies of pneumonia may suffer from obsolescence, given recent trends in worsening microbial resistance and an increasingly complex medical system.
The authors of this large (n=4,453), retrospective, multi-center database analysis concluded that HCAP was justified as a new category of pneumonia based on the observation that the pathogens causing HCAP were more similar to HAP and VAP than they were to CAP. For instance, in HCAP, Staphylococcus and Pseudomonas species were isolated in 46.7% and 25.3% of patients, while in CAP patients, these organisms were recovered in 25.5% and 17.1%, respectively. Conversely, Streptococcus pneumoniae and Haemophilus species predominated in CAP (16.6% and 16.6%) but were less common in HCAP (5.5% and 5.8%), HAP (3.1% and 5.6%), and VAP (5.8% and 12.2%). Compared with CAP HCAP was also associated with more severe disease, a higher mortality rate, a prolonged length of stay, and significantly increased costs.
Although not particularly germane to hospitalists, these recommendations were also found to be problematic for patients who were not hospitalized. Even for individuals residing in chronic-care facilities, the use of multiple broad-spectrum agents being given by IV would pose a logistic disaster that would quickly overwhelm staff. Safety issues would also likely abound, given the need for IV lines, pumps, and tubing in a patient population that would have a high density of cognitive deficits and a relatively unfavorable nurse-to-resident ratio. Further, the concept of empiric broad-spectrum therapy mandates subsequent de-escalation based on microbiology specimens obtained at the time of initiating antibiotics. It has been repeatedly illustrated that obtaining high-quality specimens outside the hospital setting is difficult. As a result, most non-hospitalized patients would be started on broad-spectrum therapy without any realistic possibility for de-escalation, a situation that would only accelerate the selection pressure on resistant organisms.
The guidelines are also vague regarding whether Legionella should be empirically treated in patients with HCAP. While this pathogen has long been accepted as a common and under-diagnosed cause of community-acquired pneumonia, the epidemiology of Legionella pneumophila (Legionnaires’ disease) in nosocomial pneumonia is more controversial. Although it is likely a relatively common cause of endemic nosocomial infections, the initial reports of Legionnaires’ disease have left the misperception that this pathogen is only a cause of epidemic disease.23-26 In the 2005 ATS-IDSA guidelines, Legionella is listed as a “potential pathogen.” Yet it would only be treated if a macrolide (e.g., azithromycin) or a fluoroquinolone (e.g., ciprofloxacin or levofloxacin) were prescribed.
The current guidelines do not specifically comment on the optimal duration of HCAP therapy or the assessment of HCAP patients not responding to therapy, likely because these areas have not been studied. Regardless, there is ever increasing support for shortening the duration of antibiotic therapies in clinically responding patients regardless of the subset of nosocomial pneumonia. As such, the duration of therapy should likely be closer to seven days than the conventional 14- to 21-day courses of therapy. In the absence of evidence to the contrary, the 2005 guidelines imply that the evaluation of HCAP patients who are not responding to therapy should be the same as for patients with other nosocomial pneumonias not responding to antibiotic therapy.
Although only briefly addressed in the guidelines, the importance of incorporating local microbiologic data cannot be overemphasized. Just as antimicrobial sensitivities vary within institutions, there is likely to be comparable variability between settings in which each subset of HCAP patients comes into contact with the healthcare system.27,28 Although, ideally, every long-term care facility would have its own, frequently updated, antibiogram, this is not feasible either logistically or financially. Due to these limitations, hospital antibiograms are typically employed as a surrogate whenever these patients are hospitalized. Given the similarity of HCAP pathogens to nosocomial organisms, individualized intensive care unit-specific antibiograms will likely better reflect the resistant pathogens of highest concern when prescribing empiric therapy.22,27,28
The implication of the severity of illness in prescribing initial antibiotic therapy is another poorly studied and controversial topic. Because HCAP likely includes a broad spectrum of potential pathogens—both community-acquired and nosocomial—it has been proposed that the presenting severity of illness may be a surrogate marker for the etiologic organisms. This philosophy proposes that less severely ill patients are more likely to have community-acquired organisms that may be successfully treated with more narrow spectrum agents, while those with a higher severity of illness are more likely to have nosocomial pathogens that should be empirically given broad spectrum agents. This topic is not addressed by the current ATS-IDSA guidelines.
Conclusions
The ATS-IDSA nosocomial pneumonia guidelines’ recent definition of HCAP and the attendant recommendations regarding therapies are welcome additions for clinicians who have long struggled with how best to treat these patients. It is important to realize the limitations behind the assertions made in this important document, however. First and foremost, the knowledge base on this topic is limited, and many conclusions are based on expert opinion or extrapolation of concepts relating to other nosocomial infections. What little knowledge is available comes almost exclusively from nursing-home-acquired pneumonia. This is problematic because these studies are plagued with issues regarding their limited or poor-quality microbiologic data.
In several areas, including the need for Legionella therapy, the implications of severity of illness, and the assessment of response to therapy, the data are entirely lacking. As a result, the recommendations for hospital-acquired pneumonia are generally followed in these instances. These facts highlight the need for increased epidemiologic HCAP data to optimize antibiograms, to promote adequate empiric antibiotic therapy, to assess the obsolescence of older trials, and to investigate the similarities and differences among the heterogeneous groups of patients included in the present definition. TH
Drs. Morrow and Malesker both work at Creighton University Medical Center, Omaha, Neb.
References
- American Thoracic Society. Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005 Feb 15;171(4):388-416. Comment in Am J Respir Crit Care Med. 2006 Jan 1;173(1):131-3; author reply 133.
- American Thoracic Society. Hospital-acquired pneumonia in adults: diagnosis, assessment of severity, initial antimicrobial therapy, and preventive strategies. A consensus statement, November 1995. Am J Respir Crit Care Med. 1996 May;153(5):1711-1725.
- Pop-Vicas AE, D’Agata EM. The rising influx of multidrug-resistant gram-negative bacilli into a tertiary care hospital. Clin Infect Dis. 2005 Jun 15;40(12):1792-1798. Epub 2005 May 6.
- Pop-Vicas AE. Debilitated elderly in long-term care facility (LTCF)—a major reservoir of colonization with multidrug-resistant gram-negative (MDRGN) pathogens. Paper presented at: 44th Annual Meeting of the Infectious Diseases Society of America. October 2006. Toronto, Ontario, Canada.
- Alvarez-Lerma F. Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. ICU-Acquired Pneumonia Study Group. Intensive Care Med. 1996 May;22(5):387-394.
- Celis R, Torres A, Gatell JM, et al. Nosocomial pneumonia. A multivariate analysis of risk and prognosis. Chest. 1988 Feb;93(2):318-324.
- Hiramatsu K, Niederman MS. Health-care-associated pneumonia: a new therapeutic paradigm. Chest. 2005 Dec;128(6):3784-3787.
- Fujitani S, Yu VL. A new category – healthcare-associated pneumonia: a good idea, but problems with its execution. Eur J Clin Microbiol Infect Dis. 2006 Oct;25(10):627-631.
- Guay DR. Guidelines for the management of adults with health care-associated pneumonia: implications for nursing facility residents. Consult Pharm. 2006 Sep;21(9):719-725.
- Hutt E, Kramer AM. Evidence-based guidelines for management of nursing home-acquired pneumonia. J Fam Pract. 2002 Aug;51(8):709-716.
- Mylotte JM. Nursing-home acquired pneumonia. Clin Infect Dis. 2002 Nov 15;35(10):1205-1211. Epub 2002 Oct 28. Comment in Clin Infect Dis. 2003 Jul 1; 37(1):148-9; author reply 149-150.
- El-Solh AA, Sikka P, Ramadan F, et al. Etiology of severe pneumonia in the very elderly. Am J Respir Crit Care Med. 2001 Mar;163(3 Pt 1):645-651.
- El-Solh AA, Aquilina AT, Dhillon RS, et al. Impact of invasive strategy on management of antimicrobial treatment failure in institutionalized older people with severe pneumonia. Am J Respir Crit Care Med. 2002 Oct 15;166(8):1038-1043.
- Lim WS, Macfarlane JT. A prospective comparison of nursing home acquired pneumonia with community acquired pneumonia. Eur Respir J. 2001Aug;18(2):362-368.
- Patriarca PA, Weber JA, Parker RA, et al. Efficacy of influenza vaccine in nursing homes. Reduction in illness and complications during an influenza A (H3N2) epidemic. JAMA. 1985 Feb 22;253(8):1136-1139.
- Lee C, Loeb M, Phillips A, et al. Zanamivir use during transmission of amantadine-resistant influenza A in a nursing home. Infect Control Hosp Epidemiol. 2000 Nov;21(11):700-704.
- Gaynes R. Health care-associated bloodstream infections: a change in thinking. Ann Intern Med. 2002 Nov 19;137(10):850-851.
- Friedman ND, Kaye KS, Stout JE, et al. Healthcare-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002 Nov 19;137(10):791-797.
- Trouillet JL, Chastre J, Vuagnat A, et al. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am J Respir Crit Care Med. 1998 Feb;157(2):531-539.
- Slinin Y, Foley RN, Collins AJ. Clinical epidemiology of pneumonia in hemodialysis patients: the USRDS waves 1, 3, and 4 study. Kidney Int. 2006 Sep;70(6):1135-1141.
- Muder RR. Pneumonia in residents of long-term care facilities: epidemiology, etiology, management, and prevention. Am J Med. 1998 Oct;105(4):319-330.
- Kollef MH, Shorr A, Tabak YP, et al. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest. 2005 Dec;128(6):3854-3862. Erratum in Chest. 2006 Mar; 129(3): 831. Comment in Chest. 2005 Dec; 128(6):3784-3787; Chest. 2006 Aug; 130(2):623.
- Fiore AE, Butler JC, Emori TG, et al. A survey of methods used to detect nosocomial legionellosis among participants in the National Nosocomial Infections Surveillance System. Infect Control Hosp Epidemiol. 1999 Jun;20(6):412-416.
- Korvick J, Yu VL, Fang GD. Legionella species as hospital-acquired respiratory pathogens. Semin Respir Infect. 1987 Mar;2(1):34-47.
- Haley CE, Cohen ML, Halter J, et al. Nosocomial Legionnaires’ disease: a continuing common-source epidemic at Wadsworth Medical Center. Ann Intern Med. 1979 Apr;90(4):583-586.
- Dondero TJ Jr, Rendtorff RC, Mallison GF, et al. An outbreak of Legionnaires’ disease associated with a contaminated air-conditioning cooling tower. N Engl J Med. 1980 Feb 14;302(7):365-370.
- Rello J, Sa-Borges M, Correa H. Variations in etiology of ventilator-associated pneumonia across four treatment sites: implications for antimicrobial prescribing practices. Am J Respir Crit Care Med. 1999 Aug;160(2):608-613.
- Namias N, Samiian L, Nino D, et al. Incidence and susceptibility of pathogenic bacteria vary between intensive care units within a single hospital: implications for empiric antibiotic strategies. J Trauma. 2000 Oct;49(4):638-645.
In February 2005, the American Thoracic Society (ATS) and the Infectious Disease Society of America (IDSA) released their joint guidelines for the management of nosocomial pneumonia.1 This impressive document was an extensive revision of guidelines released in 1996.2 These revised guidelines were widely anticipated because during the intervening nine-year span two trends had evolved. First, academic interest in nosocomial pneumonias grew dramatically. This is best illustrated by the exponential growth in the rate of scholarly publications regarding nosocomial pneumonia. A simple PubMed search of “nosocomial pneumonia” shows a six-fold increase in the number of publications devoted to this topic during the time that elapsed between the two sets of guidelines. (See Figure 1, p. 28.) Second, antimicrobial susceptibility to antibiotic therapy rapidly worsened, with many facilities reporting routine resistance in their intensive care units.
Given the growth in related scholarly activity, the 2005 guidelines are both comprehensive and lengthy—some 28 pages in total. The authors meticulously reviewed the nosocomial pneumonia literature, dividing it into manageable and clinically relevant subtopics: epidemiology, pathogenesis, modifiable risk factors, diagnostic testing, diagnostic strategies, antibiotic treatment, and response to therapy. The obvious strengths of the guidelines include that they were written by a collection of authoritative experts representing collaboration between the ATS and the IDSA and that they result in a collection of practical, evidence-based recommendations.
One of the most significant aspects of these guidelines is that they defined a “new” entity: healthcare-associated pneumonia (HCAP). The authors recognize HCAP patients as a unique population because they are at increased risk of harboring resistant pathogens. As defined by the guidelines, HCAP patients include those admitted for more than 48 hours in the past 90 days, those who reside in a nursing home or receive home infusion therapy or wound care, those who are on chronic hemodialysis, or patients who have a family member with a multidrug-resistant pathogen. Although not explicitly stated by the guidelines, it’s worth noting that the recommendations provided were intended to apply only to hospitalized patients with pneumonia.
Looking at the ATS-IDSA definition of HCAP, it is clear that the largest subset of HCAP patients is nursing home residents with pneumonia. Although these patients have historically been treated with agents recommended in community-acquired pneumonia (CAP) guidelines, they more closely resemble hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP) patients in terms of their comorbidities, the presence of catheters and lines, and their prior exposure to antibiotics. Because these patients live longer and have more comorbid disease their care has become increasingly complex, often involving frequent transfers to hospitals or providers’ offices. Not surprisingly, long-term-care facility residents have become a reservoir for multidrug-resistant pathogens.3,4 This patient population will become increasingly important; the number of geriatric Americans is projected to continue to grow for the next 20 years.
Because increased mortality in pneumonia is strongly linked to initially inappropriate antibiotic therapy, the guidelines recommend much broader antibiotic coverage for the newly defined HCAP population.5,6 In the recent past, these patients have been most frequently treated with a third-generation cephalosporin and macrolide combination or a respiratory fluoroquinolone as monotherapy. Given the risk of multidrug-resistant pathogens in HCAP, these regimens are deemed inadequate by the current guidelines, which recommend a combination of:
- An antipseudomonal cephalosporin, an antipseudomonal carbapenem, or a ß-lactam/ß-lactamase inhibitor combination; plus
- An antipseudomonal fluoroquinolone or an aminoglycoside; plus
- Vancomycin or linezolid. (See Table 1, above.)
Controversies Surrounding the Guidelines
Although the revised guidelines were generally greeted with enthusiasm, several authors quickly pointed out that the evidence base supporting HCAP has significant limitations.7-9 Most importantly, studies dealing specifically with HCAP as it is defined by the guidelines are lacking. A review of the ATS-IDSA guidelines’ references reveals that only seven publications deal specifically with HCAP, and all of these focus on nursing home-acquired pneumonia.10-16 Also note that these seven studies use widely discrepant definitions of pneumonia, have varying microbiologic criteria, and include varying numbers of hospitalized and non-hospitalized patients. In addition to these limitations, much of the epidemiology of HCAP pathogens has been extrapolated from diseases other than pneumonia.
Given the paucity of supporting data, it is unclear how similar the subgroups lumped together under the HCAP umbrella really are. Intuitively all of these patients are at increased risk for colonization with resistant pathogens, given their frequent contact with the healthcare system and/or its extenders. The repeated observation that the presence of any one of the factors defining HCAP—recent admission, nursing-home-residence, renal failure, and so on—is associated with increased mortality in patients with nosocomial infections strengthens this assumption.17-19
There are data that challenge the conclusion that such nosocomial pathogens are a frequent enough cause of pneumonia in these patients to merit routine empiric therapy, however. In one large retrospective database review, hemodialysis patients admitted for pneumonia had a spectrum of causative pathogens that was a hybrid of community- and nosocomial-acquired microbes.20 Similarly, in a large systematic review of nursing-home acquired pneumonia studies published between 1978-1994, the causative pathogens were a diverse blend of community- and nosocomial-acquired organisms.21 Both of these studies were retrospective analyses that were limited by the availability and quality of the previously collected microbiology data.
The only study looking specifically at the epidemiology of HCAP as defined by the ATS-IDSA guidelines was published in December 2005—10 months after the guidelines themselves.22 This study raises the possibility that earlier epidemiology studies of pneumonia may suffer from obsolescence, given recent trends in worsening microbial resistance and an increasingly complex medical system.
The authors of this large (n=4,453), retrospective, multi-center database analysis concluded that HCAP was justified as a new category of pneumonia based on the observation that the pathogens causing HCAP were more similar to HAP and VAP than they were to CAP. For instance, in HCAP, Staphylococcus and Pseudomonas species were isolated in 46.7% and 25.3% of patients, while in CAP patients, these organisms were recovered in 25.5% and 17.1%, respectively. Conversely, Streptococcus pneumoniae and Haemophilus species predominated in CAP (16.6% and 16.6%) but were less common in HCAP (5.5% and 5.8%), HAP (3.1% and 5.6%), and VAP (5.8% and 12.2%). Compared with CAP HCAP was also associated with more severe disease, a higher mortality rate, a prolonged length of stay, and significantly increased costs.
Although not particularly germane to hospitalists, these recommendations were also found to be problematic for patients who were not hospitalized. Even for individuals residing in chronic-care facilities, the use of multiple broad-spectrum agents being given by IV would pose a logistic disaster that would quickly overwhelm staff. Safety issues would also likely abound, given the need for IV lines, pumps, and tubing in a patient population that would have a high density of cognitive deficits and a relatively unfavorable nurse-to-resident ratio. Further, the concept of empiric broad-spectrum therapy mandates subsequent de-escalation based on microbiology specimens obtained at the time of initiating antibiotics. It has been repeatedly illustrated that obtaining high-quality specimens outside the hospital setting is difficult. As a result, most non-hospitalized patients would be started on broad-spectrum therapy without any realistic possibility for de-escalation, a situation that would only accelerate the selection pressure on resistant organisms.
The guidelines are also vague regarding whether Legionella should be empirically treated in patients with HCAP. While this pathogen has long been accepted as a common and under-diagnosed cause of community-acquired pneumonia, the epidemiology of Legionella pneumophila (Legionnaires’ disease) in nosocomial pneumonia is more controversial. Although it is likely a relatively common cause of endemic nosocomial infections, the initial reports of Legionnaires’ disease have left the misperception that this pathogen is only a cause of epidemic disease.23-26 In the 2005 ATS-IDSA guidelines, Legionella is listed as a “potential pathogen.” Yet it would only be treated if a macrolide (e.g., azithromycin) or a fluoroquinolone (e.g., ciprofloxacin or levofloxacin) were prescribed.
The current guidelines do not specifically comment on the optimal duration of HCAP therapy or the assessment of HCAP patients not responding to therapy, likely because these areas have not been studied. Regardless, there is ever increasing support for shortening the duration of antibiotic therapies in clinically responding patients regardless of the subset of nosocomial pneumonia. As such, the duration of therapy should likely be closer to seven days than the conventional 14- to 21-day courses of therapy. In the absence of evidence to the contrary, the 2005 guidelines imply that the evaluation of HCAP patients who are not responding to therapy should be the same as for patients with other nosocomial pneumonias not responding to antibiotic therapy.
Although only briefly addressed in the guidelines, the importance of incorporating local microbiologic data cannot be overemphasized. Just as antimicrobial sensitivities vary within institutions, there is likely to be comparable variability between settings in which each subset of HCAP patients comes into contact with the healthcare system.27,28 Although, ideally, every long-term care facility would have its own, frequently updated, antibiogram, this is not feasible either logistically or financially. Due to these limitations, hospital antibiograms are typically employed as a surrogate whenever these patients are hospitalized. Given the similarity of HCAP pathogens to nosocomial organisms, individualized intensive care unit-specific antibiograms will likely better reflect the resistant pathogens of highest concern when prescribing empiric therapy.22,27,28
The implication of the severity of illness in prescribing initial antibiotic therapy is another poorly studied and controversial topic. Because HCAP likely includes a broad spectrum of potential pathogens—both community-acquired and nosocomial—it has been proposed that the presenting severity of illness may be a surrogate marker for the etiologic organisms. This philosophy proposes that less severely ill patients are more likely to have community-acquired organisms that may be successfully treated with more narrow spectrum agents, while those with a higher severity of illness are more likely to have nosocomial pathogens that should be empirically given broad spectrum agents. This topic is not addressed by the current ATS-IDSA guidelines.
Conclusions
The ATS-IDSA nosocomial pneumonia guidelines’ recent definition of HCAP and the attendant recommendations regarding therapies are welcome additions for clinicians who have long struggled with how best to treat these patients. It is important to realize the limitations behind the assertions made in this important document, however. First and foremost, the knowledge base on this topic is limited, and many conclusions are based on expert opinion or extrapolation of concepts relating to other nosocomial infections. What little knowledge is available comes almost exclusively from nursing-home-acquired pneumonia. This is problematic because these studies are plagued with issues regarding their limited or poor-quality microbiologic data.
In several areas, including the need for Legionella therapy, the implications of severity of illness, and the assessment of response to therapy, the data are entirely lacking. As a result, the recommendations for hospital-acquired pneumonia are generally followed in these instances. These facts highlight the need for increased epidemiologic HCAP data to optimize antibiograms, to promote adequate empiric antibiotic therapy, to assess the obsolescence of older trials, and to investigate the similarities and differences among the heterogeneous groups of patients included in the present definition. TH
Drs. Morrow and Malesker both work at Creighton University Medical Center, Omaha, Neb.
References
- American Thoracic Society. Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005 Feb 15;171(4):388-416. Comment in Am J Respir Crit Care Med. 2006 Jan 1;173(1):131-3; author reply 133.
- American Thoracic Society. Hospital-acquired pneumonia in adults: diagnosis, assessment of severity, initial antimicrobial therapy, and preventive strategies. A consensus statement, November 1995. Am J Respir Crit Care Med. 1996 May;153(5):1711-1725.
- Pop-Vicas AE, D’Agata EM. The rising influx of multidrug-resistant gram-negative bacilli into a tertiary care hospital. Clin Infect Dis. 2005 Jun 15;40(12):1792-1798. Epub 2005 May 6.
- Pop-Vicas AE. Debilitated elderly in long-term care facility (LTCF)—a major reservoir of colonization with multidrug-resistant gram-negative (MDRGN) pathogens. Paper presented at: 44th Annual Meeting of the Infectious Diseases Society of America. October 2006. Toronto, Ontario, Canada.
- Alvarez-Lerma F. Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. ICU-Acquired Pneumonia Study Group. Intensive Care Med. 1996 May;22(5):387-394.
- Celis R, Torres A, Gatell JM, et al. Nosocomial pneumonia. A multivariate analysis of risk and prognosis. Chest. 1988 Feb;93(2):318-324.
- Hiramatsu K, Niederman MS. Health-care-associated pneumonia: a new therapeutic paradigm. Chest. 2005 Dec;128(6):3784-3787.
- Fujitani S, Yu VL. A new category – healthcare-associated pneumonia: a good idea, but problems with its execution. Eur J Clin Microbiol Infect Dis. 2006 Oct;25(10):627-631.
- Guay DR. Guidelines for the management of adults with health care-associated pneumonia: implications for nursing facility residents. Consult Pharm. 2006 Sep;21(9):719-725.
- Hutt E, Kramer AM. Evidence-based guidelines for management of nursing home-acquired pneumonia. J Fam Pract. 2002 Aug;51(8):709-716.
- Mylotte JM. Nursing-home acquired pneumonia. Clin Infect Dis. 2002 Nov 15;35(10):1205-1211. Epub 2002 Oct 28. Comment in Clin Infect Dis. 2003 Jul 1; 37(1):148-9; author reply 149-150.
- El-Solh AA, Sikka P, Ramadan F, et al. Etiology of severe pneumonia in the very elderly. Am J Respir Crit Care Med. 2001 Mar;163(3 Pt 1):645-651.
- El-Solh AA, Aquilina AT, Dhillon RS, et al. Impact of invasive strategy on management of antimicrobial treatment failure in institutionalized older people with severe pneumonia. Am J Respir Crit Care Med. 2002 Oct 15;166(8):1038-1043.
- Lim WS, Macfarlane JT. A prospective comparison of nursing home acquired pneumonia with community acquired pneumonia. Eur Respir J. 2001Aug;18(2):362-368.
- Patriarca PA, Weber JA, Parker RA, et al. Efficacy of influenza vaccine in nursing homes. Reduction in illness and complications during an influenza A (H3N2) epidemic. JAMA. 1985 Feb 22;253(8):1136-1139.
- Lee C, Loeb M, Phillips A, et al. Zanamivir use during transmission of amantadine-resistant influenza A in a nursing home. Infect Control Hosp Epidemiol. 2000 Nov;21(11):700-704.
- Gaynes R. Health care-associated bloodstream infections: a change in thinking. Ann Intern Med. 2002 Nov 19;137(10):850-851.
- Friedman ND, Kaye KS, Stout JE, et al. Healthcare-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002 Nov 19;137(10):791-797.
- Trouillet JL, Chastre J, Vuagnat A, et al. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am J Respir Crit Care Med. 1998 Feb;157(2):531-539.
- Slinin Y, Foley RN, Collins AJ. Clinical epidemiology of pneumonia in hemodialysis patients: the USRDS waves 1, 3, and 4 study. Kidney Int. 2006 Sep;70(6):1135-1141.
- Muder RR. Pneumonia in residents of long-term care facilities: epidemiology, etiology, management, and prevention. Am J Med. 1998 Oct;105(4):319-330.
- Kollef MH, Shorr A, Tabak YP, et al. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest. 2005 Dec;128(6):3854-3862. Erratum in Chest. 2006 Mar; 129(3): 831. Comment in Chest. 2005 Dec; 128(6):3784-3787; Chest. 2006 Aug; 130(2):623.
- Fiore AE, Butler JC, Emori TG, et al. A survey of methods used to detect nosocomial legionellosis among participants in the National Nosocomial Infections Surveillance System. Infect Control Hosp Epidemiol. 1999 Jun;20(6):412-416.
- Korvick J, Yu VL, Fang GD. Legionella species as hospital-acquired respiratory pathogens. Semin Respir Infect. 1987 Mar;2(1):34-47.
- Haley CE, Cohen ML, Halter J, et al. Nosocomial Legionnaires’ disease: a continuing common-source epidemic at Wadsworth Medical Center. Ann Intern Med. 1979 Apr;90(4):583-586.
- Dondero TJ Jr, Rendtorff RC, Mallison GF, et al. An outbreak of Legionnaires’ disease associated with a contaminated air-conditioning cooling tower. N Engl J Med. 1980 Feb 14;302(7):365-370.
- Rello J, Sa-Borges M, Correa H. Variations in etiology of ventilator-associated pneumonia across four treatment sites: implications for antimicrobial prescribing practices. Am J Respir Crit Care Med. 1999 Aug;160(2):608-613.
- Namias N, Samiian L, Nino D, et al. Incidence and susceptibility of pathogenic bacteria vary between intensive care units within a single hospital: implications for empiric antibiotic strategies. J Trauma. 2000 Oct;49(4):638-645.
In February 2005, the American Thoracic Society (ATS) and the Infectious Disease Society of America (IDSA) released their joint guidelines for the management of nosocomial pneumonia.1 This impressive document was an extensive revision of guidelines released in 1996.2 These revised guidelines were widely anticipated because during the intervening nine-year span two trends had evolved. First, academic interest in nosocomial pneumonias grew dramatically. This is best illustrated by the exponential growth in the rate of scholarly publications regarding nosocomial pneumonia. A simple PubMed search of “nosocomial pneumonia” shows a six-fold increase in the number of publications devoted to this topic during the time that elapsed between the two sets of guidelines. (See Figure 1, p. 28.) Second, antimicrobial susceptibility to antibiotic therapy rapidly worsened, with many facilities reporting routine resistance in their intensive care units.
Given the growth in related scholarly activity, the 2005 guidelines are both comprehensive and lengthy—some 28 pages in total. The authors meticulously reviewed the nosocomial pneumonia literature, dividing it into manageable and clinically relevant subtopics: epidemiology, pathogenesis, modifiable risk factors, diagnostic testing, diagnostic strategies, antibiotic treatment, and response to therapy. The obvious strengths of the guidelines include that they were written by a collection of authoritative experts representing collaboration between the ATS and the IDSA and that they result in a collection of practical, evidence-based recommendations.
One of the most significant aspects of these guidelines is that they defined a “new” entity: healthcare-associated pneumonia (HCAP). The authors recognize HCAP patients as a unique population because they are at increased risk of harboring resistant pathogens. As defined by the guidelines, HCAP patients include those admitted for more than 48 hours in the past 90 days, those who reside in a nursing home or receive home infusion therapy or wound care, those who are on chronic hemodialysis, or patients who have a family member with a multidrug-resistant pathogen. Although not explicitly stated by the guidelines, it’s worth noting that the recommendations provided were intended to apply only to hospitalized patients with pneumonia.
Looking at the ATS-IDSA definition of HCAP, it is clear that the largest subset of HCAP patients is nursing home residents with pneumonia. Although these patients have historically been treated with agents recommended in community-acquired pneumonia (CAP) guidelines, they more closely resemble hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP) patients in terms of their comorbidities, the presence of catheters and lines, and their prior exposure to antibiotics. Because these patients live longer and have more comorbid disease their care has become increasingly complex, often involving frequent transfers to hospitals or providers’ offices. Not surprisingly, long-term-care facility residents have become a reservoir for multidrug-resistant pathogens.3,4 This patient population will become increasingly important; the number of geriatric Americans is projected to continue to grow for the next 20 years.
Because increased mortality in pneumonia is strongly linked to initially inappropriate antibiotic therapy, the guidelines recommend much broader antibiotic coverage for the newly defined HCAP population.5,6 In the recent past, these patients have been most frequently treated with a third-generation cephalosporin and macrolide combination or a respiratory fluoroquinolone as monotherapy. Given the risk of multidrug-resistant pathogens in HCAP, these regimens are deemed inadequate by the current guidelines, which recommend a combination of:
- An antipseudomonal cephalosporin, an antipseudomonal carbapenem, or a ß-lactam/ß-lactamase inhibitor combination; plus
- An antipseudomonal fluoroquinolone or an aminoglycoside; plus
- Vancomycin or linezolid. (See Table 1, above.)
Controversies Surrounding the Guidelines
Although the revised guidelines were generally greeted with enthusiasm, several authors quickly pointed out that the evidence base supporting HCAP has significant limitations.7-9 Most importantly, studies dealing specifically with HCAP as it is defined by the guidelines are lacking. A review of the ATS-IDSA guidelines’ references reveals that only seven publications deal specifically with HCAP, and all of these focus on nursing home-acquired pneumonia.10-16 Also note that these seven studies use widely discrepant definitions of pneumonia, have varying microbiologic criteria, and include varying numbers of hospitalized and non-hospitalized patients. In addition to these limitations, much of the epidemiology of HCAP pathogens has been extrapolated from diseases other than pneumonia.
Given the paucity of supporting data, it is unclear how similar the subgroups lumped together under the HCAP umbrella really are. Intuitively all of these patients are at increased risk for colonization with resistant pathogens, given their frequent contact with the healthcare system and/or its extenders. The repeated observation that the presence of any one of the factors defining HCAP—recent admission, nursing-home-residence, renal failure, and so on—is associated with increased mortality in patients with nosocomial infections strengthens this assumption.17-19
There are data that challenge the conclusion that such nosocomial pathogens are a frequent enough cause of pneumonia in these patients to merit routine empiric therapy, however. In one large retrospective database review, hemodialysis patients admitted for pneumonia had a spectrum of causative pathogens that was a hybrid of community- and nosocomial-acquired microbes.20 Similarly, in a large systematic review of nursing-home acquired pneumonia studies published between 1978-1994, the causative pathogens were a diverse blend of community- and nosocomial-acquired organisms.21 Both of these studies were retrospective analyses that were limited by the availability and quality of the previously collected microbiology data.
The only study looking specifically at the epidemiology of HCAP as defined by the ATS-IDSA guidelines was published in December 2005—10 months after the guidelines themselves.22 This study raises the possibility that earlier epidemiology studies of pneumonia may suffer from obsolescence, given recent trends in worsening microbial resistance and an increasingly complex medical system.
The authors of this large (n=4,453), retrospective, multi-center database analysis concluded that HCAP was justified as a new category of pneumonia based on the observation that the pathogens causing HCAP were more similar to HAP and VAP than they were to CAP. For instance, in HCAP, Staphylococcus and Pseudomonas species were isolated in 46.7% and 25.3% of patients, while in CAP patients, these organisms were recovered in 25.5% and 17.1%, respectively. Conversely, Streptococcus pneumoniae and Haemophilus species predominated in CAP (16.6% and 16.6%) but were less common in HCAP (5.5% and 5.8%), HAP (3.1% and 5.6%), and VAP (5.8% and 12.2%). Compared with CAP HCAP was also associated with more severe disease, a higher mortality rate, a prolonged length of stay, and significantly increased costs.
Although not particularly germane to hospitalists, these recommendations were also found to be problematic for patients who were not hospitalized. Even for individuals residing in chronic-care facilities, the use of multiple broad-spectrum agents being given by IV would pose a logistic disaster that would quickly overwhelm staff. Safety issues would also likely abound, given the need for IV lines, pumps, and tubing in a patient population that would have a high density of cognitive deficits and a relatively unfavorable nurse-to-resident ratio. Further, the concept of empiric broad-spectrum therapy mandates subsequent de-escalation based on microbiology specimens obtained at the time of initiating antibiotics. It has been repeatedly illustrated that obtaining high-quality specimens outside the hospital setting is difficult. As a result, most non-hospitalized patients would be started on broad-spectrum therapy without any realistic possibility for de-escalation, a situation that would only accelerate the selection pressure on resistant organisms.
The guidelines are also vague regarding whether Legionella should be empirically treated in patients with HCAP. While this pathogen has long been accepted as a common and under-diagnosed cause of community-acquired pneumonia, the epidemiology of Legionella pneumophila (Legionnaires’ disease) in nosocomial pneumonia is more controversial. Although it is likely a relatively common cause of endemic nosocomial infections, the initial reports of Legionnaires’ disease have left the misperception that this pathogen is only a cause of epidemic disease.23-26 In the 2005 ATS-IDSA guidelines, Legionella is listed as a “potential pathogen.” Yet it would only be treated if a macrolide (e.g., azithromycin) or a fluoroquinolone (e.g., ciprofloxacin or levofloxacin) were prescribed.
The current guidelines do not specifically comment on the optimal duration of HCAP therapy or the assessment of HCAP patients not responding to therapy, likely because these areas have not been studied. Regardless, there is ever increasing support for shortening the duration of antibiotic therapies in clinically responding patients regardless of the subset of nosocomial pneumonia. As such, the duration of therapy should likely be closer to seven days than the conventional 14- to 21-day courses of therapy. In the absence of evidence to the contrary, the 2005 guidelines imply that the evaluation of HCAP patients who are not responding to therapy should be the same as for patients with other nosocomial pneumonias not responding to antibiotic therapy.
Although only briefly addressed in the guidelines, the importance of incorporating local microbiologic data cannot be overemphasized. Just as antimicrobial sensitivities vary within institutions, there is likely to be comparable variability between settings in which each subset of HCAP patients comes into contact with the healthcare system.27,28 Although, ideally, every long-term care facility would have its own, frequently updated, antibiogram, this is not feasible either logistically or financially. Due to these limitations, hospital antibiograms are typically employed as a surrogate whenever these patients are hospitalized. Given the similarity of HCAP pathogens to nosocomial organisms, individualized intensive care unit-specific antibiograms will likely better reflect the resistant pathogens of highest concern when prescribing empiric therapy.22,27,28
The implication of the severity of illness in prescribing initial antibiotic therapy is another poorly studied and controversial topic. Because HCAP likely includes a broad spectrum of potential pathogens—both community-acquired and nosocomial—it has been proposed that the presenting severity of illness may be a surrogate marker for the etiologic organisms. This philosophy proposes that less severely ill patients are more likely to have community-acquired organisms that may be successfully treated with more narrow spectrum agents, while those with a higher severity of illness are more likely to have nosocomial pathogens that should be empirically given broad spectrum agents. This topic is not addressed by the current ATS-IDSA guidelines.
Conclusions
The ATS-IDSA nosocomial pneumonia guidelines’ recent definition of HCAP and the attendant recommendations regarding therapies are welcome additions for clinicians who have long struggled with how best to treat these patients. It is important to realize the limitations behind the assertions made in this important document, however. First and foremost, the knowledge base on this topic is limited, and many conclusions are based on expert opinion or extrapolation of concepts relating to other nosocomial infections. What little knowledge is available comes almost exclusively from nursing-home-acquired pneumonia. This is problematic because these studies are plagued with issues regarding their limited or poor-quality microbiologic data.
In several areas, including the need for Legionella therapy, the implications of severity of illness, and the assessment of response to therapy, the data are entirely lacking. As a result, the recommendations for hospital-acquired pneumonia are generally followed in these instances. These facts highlight the need for increased epidemiologic HCAP data to optimize antibiograms, to promote adequate empiric antibiotic therapy, to assess the obsolescence of older trials, and to investigate the similarities and differences among the heterogeneous groups of patients included in the present definition. TH
Drs. Morrow and Malesker both work at Creighton University Medical Center, Omaha, Neb.
References
- American Thoracic Society. Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005 Feb 15;171(4):388-416. Comment in Am J Respir Crit Care Med. 2006 Jan 1;173(1):131-3; author reply 133.
- American Thoracic Society. Hospital-acquired pneumonia in adults: diagnosis, assessment of severity, initial antimicrobial therapy, and preventive strategies. A consensus statement, November 1995. Am J Respir Crit Care Med. 1996 May;153(5):1711-1725.
- Pop-Vicas AE, D’Agata EM. The rising influx of multidrug-resistant gram-negative bacilli into a tertiary care hospital. Clin Infect Dis. 2005 Jun 15;40(12):1792-1798. Epub 2005 May 6.
- Pop-Vicas AE. Debilitated elderly in long-term care facility (LTCF)—a major reservoir of colonization with multidrug-resistant gram-negative (MDRGN) pathogens. Paper presented at: 44th Annual Meeting of the Infectious Diseases Society of America. October 2006. Toronto, Ontario, Canada.
- Alvarez-Lerma F. Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. ICU-Acquired Pneumonia Study Group. Intensive Care Med. 1996 May;22(5):387-394.
- Celis R, Torres A, Gatell JM, et al. Nosocomial pneumonia. A multivariate analysis of risk and prognosis. Chest. 1988 Feb;93(2):318-324.
- Hiramatsu K, Niederman MS. Health-care-associated pneumonia: a new therapeutic paradigm. Chest. 2005 Dec;128(6):3784-3787.
- Fujitani S, Yu VL. A new category – healthcare-associated pneumonia: a good idea, but problems with its execution. Eur J Clin Microbiol Infect Dis. 2006 Oct;25(10):627-631.
- Guay DR. Guidelines for the management of adults with health care-associated pneumonia: implications for nursing facility residents. Consult Pharm. 2006 Sep;21(9):719-725.
- Hutt E, Kramer AM. Evidence-based guidelines for management of nursing home-acquired pneumonia. J Fam Pract. 2002 Aug;51(8):709-716.
- Mylotte JM. Nursing-home acquired pneumonia. Clin Infect Dis. 2002 Nov 15;35(10):1205-1211. Epub 2002 Oct 28. Comment in Clin Infect Dis. 2003 Jul 1; 37(1):148-9; author reply 149-150.
- El-Solh AA, Sikka P, Ramadan F, et al. Etiology of severe pneumonia in the very elderly. Am J Respir Crit Care Med. 2001 Mar;163(3 Pt 1):645-651.
- El-Solh AA, Aquilina AT, Dhillon RS, et al. Impact of invasive strategy on management of antimicrobial treatment failure in institutionalized older people with severe pneumonia. Am J Respir Crit Care Med. 2002 Oct 15;166(8):1038-1043.
- Lim WS, Macfarlane JT. A prospective comparison of nursing home acquired pneumonia with community acquired pneumonia. Eur Respir J. 2001Aug;18(2):362-368.
- Patriarca PA, Weber JA, Parker RA, et al. Efficacy of influenza vaccine in nursing homes. Reduction in illness and complications during an influenza A (H3N2) epidemic. JAMA. 1985 Feb 22;253(8):1136-1139.
- Lee C, Loeb M, Phillips A, et al. Zanamivir use during transmission of amantadine-resistant influenza A in a nursing home. Infect Control Hosp Epidemiol. 2000 Nov;21(11):700-704.
- Gaynes R. Health care-associated bloodstream infections: a change in thinking. Ann Intern Med. 2002 Nov 19;137(10):850-851.
- Friedman ND, Kaye KS, Stout JE, et al. Healthcare-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002 Nov 19;137(10):791-797.
- Trouillet JL, Chastre J, Vuagnat A, et al. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am J Respir Crit Care Med. 1998 Feb;157(2):531-539.
- Slinin Y, Foley RN, Collins AJ. Clinical epidemiology of pneumonia in hemodialysis patients: the USRDS waves 1, 3, and 4 study. Kidney Int. 2006 Sep;70(6):1135-1141.
- Muder RR. Pneumonia in residents of long-term care facilities: epidemiology, etiology, management, and prevention. Am J Med. 1998 Oct;105(4):319-330.
- Kollef MH, Shorr A, Tabak YP, et al. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest. 2005 Dec;128(6):3854-3862. Erratum in Chest. 2006 Mar; 129(3): 831. Comment in Chest. 2005 Dec; 128(6):3784-3787; Chest. 2006 Aug; 130(2):623.
- Fiore AE, Butler JC, Emori TG, et al. A survey of methods used to detect nosocomial legionellosis among participants in the National Nosocomial Infections Surveillance System. Infect Control Hosp Epidemiol. 1999 Jun;20(6):412-416.
- Korvick J, Yu VL, Fang GD. Legionella species as hospital-acquired respiratory pathogens. Semin Respir Infect. 1987 Mar;2(1):34-47.
- Haley CE, Cohen ML, Halter J, et al. Nosocomial Legionnaires’ disease: a continuing common-source epidemic at Wadsworth Medical Center. Ann Intern Med. 1979 Apr;90(4):583-586.
- Dondero TJ Jr, Rendtorff RC, Mallison GF, et al. An outbreak of Legionnaires’ disease associated with a contaminated air-conditioning cooling tower. N Engl J Med. 1980 Feb 14;302(7):365-370.
- Rello J, Sa-Borges M, Correa H. Variations in etiology of ventilator-associated pneumonia across four treatment sites: implications for antimicrobial prescribing practices. Am J Respir Crit Care Med. 1999 Aug;160(2):608-613.
- Namias N, Samiian L, Nino D, et al. Incidence and susceptibility of pathogenic bacteria vary between intensive care units within a single hospital: implications for empiric antibiotic strategies. J Trauma. 2000 Oct;49(4):638-645.