User login
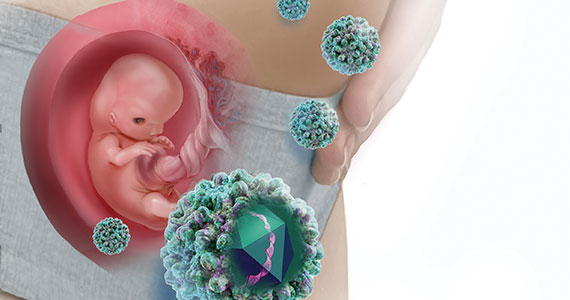
Hepatitis B is one of the more common infections encountered in the daily practice of obstetrics. It is responsible for 40% to 45% of all cases of viral hepatitis.1,2 Hepatitis B may cause serious complications in both the infected mother and neonate.
In this article, I review the virology, epidemiology, and clinical presentation of hepatitis B and then discuss the key diagnostic tests and, subsequently, the clinical management for both the mother and neonate. I focus particular attention on relatively new information about the value of specific antiviral medication to enhance the protective effect of conventional neonatal immunoprophylaxis.
To set the framework for the discussion, consider the following 2 case studies.
CASE 1 Undetectable level of hepatitis B surface antibody in a pregnant woman
A 25-year-old healthy primigravid woman at 10 weeks’ gestation had a series of laboratory studies that included a test for hepatitis B surface antigen (HBsAg) and hepatitis B surface antibody (HBsAb). The test for the surface antigen was negative. The test for the surface antibody was below the level of detection. Upon questioning, the patient indicates that she received the 3-dose hepatitis B vaccine when she was age 13 years.
- What treatment, if any, is indicated for this patient?
- What treatment is indicated for her neonate?
CASE 2 Pregnant woman tests positive for hepatitis B surface antigen
A 31-year-old woman (G3P2002) at 12 weeks’ gestation tested positive for HBsAg. She indicates that she never has had symptomatic hepatitis and that she considers herself to be in excellent health.
- What additional laboratory tests are indicated at this time?
- What additional laboratory test should be performed at the end of the second trimester?
- What treatment is indicated for the mother and neonate?
Virology and epidemiology of hepatitis B
Hepatitis B is caused by a double-stranded, enveloped DNA virus. The virus has 10 genotypes and 24 subtypes.3 The organism contains 3 major antigens. Detection of these antigens and their corresponding antibodies is an essential step in the diagnostic workup of patients who may be infected.
The surface antigen (HBsAg) confers infectivity and is the most valuable serologic marker of infection. The e antigen (HBeAg) is not present in every infected patient. It is secreted from infected cells, but it is not incorporated into the viral particle. When present, it denotes a high level of viral replication and exceptionally high infectivity. The core antigen (HBcAg) is a valuable serologic marker for distinguishing between acute and chronic infection.1-3
Hepatitis B is highly infectious, much more so than HIV or hepatitis C. The virus has an incubation period of 4 weeks to 6 months, and the duration of incubation is inversely related to the size of the viral inoculum. The virus is transmitted in 3 principal ways: sexual contact with contaminated genital tract secretions, contact with infected blood from sharing contaminated drug-injecting paraphernalia or from receiving a blood transfusion (extremely rare today), and transmission from an infected mother to her neonate. Perinatal transmission occurs primarily during the delivery process as opposed to transplacental infection. Transmission also can occur by more casual household contact, such as sharing eating utensils, kissing, and handling an infant.1,2,4,5
Worldwide, more than 400 million people have chronic hepatitis B infection. In the United States, approximately 1.25 to 1.5 million individuals are infected. Several groups are at particularly high risk for being infected, including1-3:
- Asians
- Alaska Natives
- sub-Saharan Africans
- sex workers
- intravenous drug users
- individuals with hemophilia
- international travelers
- staff and residents of long-term care facilities
- tattoo recipients.
Continue to: Clinical presentation...
Clinical presentation
Approximately 90% of adult patients who contract hepatitis B, either symptomatically or asymptomatically, will develop protective levels of antibody and clear the virus from their system. They will then have lifelong immunity to reinfection. Approximately 10% of patients will fail to develop protective levels of antibody and will become chronically infected, posing a risk to their household members, sexual contacts, and their fetus if they become pregnant. Persistence of the surface antigen in the patient’s serum for more than 6 months denotes chronic infection. A very small number of individuals—less than 1%—will develop acute liver failure and experience a fatal outcome.1-3,5
In the United States, the prevalence of acute hepatitis B in pregnancy is 1 to 2 per 1,000. Clinical manifestations typically include anorexia, nausea, low-grade fever, right upper quadrant pain and tenderness, passage of clay-colored stools, and jaundice.
The prevalence of chronic infection in pregnancy is significantly higher, approximately 5 to 15 per 1,000. Over the long term, patients with chronic infection are at risk for progressive liver injury, including cirrhosis and even hepatocellular carcinoma. These serious sequelae are particularly likely to occur when the patient is co-infected with hepatitis C, D, or both. The overall risk of progression to chronic cirrhosis is approximately 15% to 30%. In patients who progress to cirrhosis, the annual incidence of hepatocellular carcinoma is 10%.1-3
Diagnosis of hepatitis B infection
Patients with acute hepatitis B will test positive for HBsAg and immunoglobulin M (IgM) antibody to the core antigen. Some patients will also test positive for HBeAg. Assessment of the patient’s serum by polymerase chain reaction (PCR) allows quantitation of the viral load, which often is expressed as viral copies per milliliter. Alternatively, the quantitative hepatitis B DNA concentration may be expressed as international units per milliliter (IU/mL). The World Health Organization recommends this latter quantitative method. Multiplying the DNA in IU/mL by 5.6 provides the conversion to viral copies per milliliter.
Patients with chronic hepatitis B infection will test positive for the HBsAg and for immunoglobulin G (IgG) antibody to the core antigen. They may also have a positive test for the HBeAg, and PCR may be used to quantify the viral load.1-3
Managing hepatitis B infection in pregnancy
General supportive measures. All pregnant patients should be tested for the HBsAg and HBsAb at the time of the first prenatal appointment. The tests should be repeated at the beginning of the third trimester in high-risk patients. Seropositive patients should have a hepatitis B genotype, a test for the e antigen, and tests for other sexually transmissible infections (gonorrhea, chlamydia, syphilis, HIV) and for hepatitis C and D. Liver function tests should be performed to assess for elevations in the alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels. Patients with elevated transaminase enzymes should have a coagulation profile to be certain they are not at risk for a coagulopathy.
At the end of the second trimester, patients should have a PCR assessment to determine the viral load. This assessment will be important for deciding if specific antiviral therapy is indicated during the third trimester to enhance the effects of neonatal immunoprophylaxis (see below). Of note, patients who are positive for the e antigen may have a very high viral load and yet have normal or near-normal transaminase levels. This seemingly paradoxical finding reflects the non-cytopathic nature of hepatitis B.
The patient should optimize her nutrition and sleep. She should avoid, or at least minimize, medications such as acetaminophen that could cause further liver injury. Without question, she should refrain from consuming even small amounts of alcohol. She should be tested for immunity to hepatitis A; if found to be susceptible, she should be vaccinated with the hepatitis A vaccine. This agent is an inactivated vaccine and is safe for administration at any time in pregnancy.1,2,5
Household contacts. In addition to the measures outlined above, the patient’s household contacts, particularly her sexual partner(s), should be tested for immunity to hepatitis B. If they do not have immunity by virtue of natural infection or previous vaccination, they should receive the hepatitis B vaccine series. It is also prudent to provide the sexual partner(s) with an initial dose of hepatitis B immune globulin (HBIG) to provide a temporary level of passive immunity.
Postdelivery care. After delivery, the patient should be referred to an infectious disease specialist or hepatologist for consideration of long-term treatment with antiviral agents, such as interferon alfa, pegylated interferon alfa, lamivudine, adefovir, entecavir, telbivudine, or tenofovir.6 The principal candidates for treatment are those who have cirrhosis and detectable levels of hepatitis B DNA. The ultimate goal of treatment is to reduce the serum hepatitis B DNA concentration to an undetectable level. Once the surface antigenemia is cleared, treatment can be stopped. A cure is defined when the absence of hepa-titis B DNA in the serum is sustained.
- Hepatitis B is a DNA virus that is transmitted via sexual contact, exposure to infected blood, and from an infected mother to her fetus.
- Most patients in our practice will most likely have chronic, asymptomatic infection, and the diagnosis will be established by detection of HBsAg in the patient’s serum.
- All obstetric patients should be tested for both HBsAg and HBsAb.
- Patients who are positive for the surface antigen should be tested for HIV infection and hepatitis C and D. They also should have a determination of the hepatitis B genotype and viral load and assessment of liver function (ALT, AST).
- Patients who are chronically infected with hepatitis B should be vaccinated against hepatitis A to prevent further liver injury. They also should avoid medications that might cause hepatic injury.
- Patients who have a viral DNA concentration greater than 200,000 IU/mL or a viral load greater than 1,120,000 million copies/mL should be treated with tenofovir, 300 mg daily, from week 28 until 4 to 8 weeks after delivery.
- Infants delivered to infected mothers should receive HBIG within 12 hours of birth and then begin the 3-dose hepatitis B vaccine series. The first dose should be administered prior to hospital discharge.
- Infants delivered to mothers who are negative for the surface antigen should begin the hepatitis B vaccine series prior to discharge from the hospital.
- Mothers who test negative for HBsAb should be questioned about prior vaccination. If they have never been vaccinated, they should receive the 3-dose vaccine series. If they have been vaccinated, they should receive a single hepatitis B vaccine booster. The vaccine is safe for administration at any time during pregnancy.
- Infected mothers may breastfeed as long as they do not have cracked or bleeding nipples or exudative skin lesions near the nipple(s).
Neonatal immunoprophylaxis
The Centers for Disease Control and Prevention recommends universal hepatitis B vaccination for all newborns. The first dose of the vaccine should be administered prior to hospital discharge. The second and third doses should be administered 1 and 6 months later.1,2,5 There are few, if any, medical contraindications to neonatal vaccination. For the vast majority of infants, the immunity induced by vaccination is lifelong. For a small number, immunity may wane over time. Thus, reassessment of the HBsAb concentration is indicated in selected situations, for example, acute high-risk exposure to an infected person, development of an immunosuppressive disorder, or pregnancy.
Infants delivered to mothers who are infected with hepatitis B also should receive HBIG in addition to the vaccine. HBIG provides passive immunization to counteract the high viral inoculum encountered by the neonate during delivery. This preparation should be administered within 12 hours of birth.1,2,5
In the absence of immunoprophylaxis, a neonate delivered to a mother who is seropositive for HBsAg has a 20% to 30% probability of becoming chronically infected. If the mother is positive for both the surface antigen and the e antigen, the risk of chronic infection increases to almost 90%. Approximately 90% of infants who are infected in the perinatal period subsequently develop chronic infection. However, with appropriate immunoprophylaxis in the neonatal period, the risk of perinatal transmission is reduced by 85% to 95%.1,2,5
Cesarean delivery offers no additional protection beyond that provided by immunoprophylaxis. Moreover, because immunoprophylaxis is so effective, infected mothers may breastfeed without fear of transmitting infection to their infant. Shi and colleagues published a systematic review and meta-analysis of the risk associated with breastfeeding in hepatitis B–infected mothers.7 Infants who breastfed did not have a higher rate of mother-to-child transmission, regardless of whether they received combined immunoprophylaxis or only hepatitis B vaccine and regardless of whether the HBsAg was detected in the mother’s breast milk. The only precaution is the need to avoid breastfeeding if the nipples are cracked or bleeding or if exudative lesions are present on the skin near the nipple.
Continue to: Maternal antiviral therapy...
Maternal antiviral therapy
As noted above, neonatal immunoprophylaxis is 85% to 95% effective in preventing perinatal transmission of hepatitis B infection. Failures of prophylaxis are primarily due to antenatal transmission in patients who have exceptionally high viral loads. Several cutoffs have been used to define “high viral load,” including greater than 1 to 2 million copies/mL and a hepatitis B DNA concentration greater than 200,000 IU/mL. There is not a perfect consensus on the appropriate cutoff.
In essence, 2 different approaches have been tried to further reduce the risk of perinatal transmission in these high-risk patients.8 The first major initiative was administration of HBIG (100–200 IU) intramuscularly to the patient at 28, 32, and 36 weeks. The outcomes with this approach have been inconsistent, due, at least in part, to varying doses of the agent and various cutoffs for defining “high risk,” and this intervention is no longer recommended.1,2
The second major approach is administration of specific antiviral drugs to the mother during the third trimester. The first agent widely used in clinical practice was lamivudine. In a systematic review and meta-analysis, Shi and colleagues reported that, in infants whose mothers received lamivudine plus conventional neonatal immunuprophylaxis, the risk of perinatal infection was significantly reduced compared with infants who received only immunoprophylaxis.9
Although lamivudine is effective, there is considerable concern about the rapid development of viral resistance to the medication. Accordingly, most attention today is focused on the use of tenofovir to prevent perinatal transmission.
In an important early investigation, Pan and colleagues reported the results of a randomized controlled trial conducted in China in women with a hepatitis B DNA concentration greater than 200,000 IU/mL (viral load > 1,120,000 copies/mL).10 Patients also were positive for the e antigen. Ninety-two patients were assigned to tenofovir disoproxil fumarate (TDF), 300 mg daily, from 30 to 32 weeks until postpartum week 4 plus conventional neonatal immunoprophylaxis, and 100 patients were assigned to immunoprophylaxis alone. In the intention-to-treat analysis, 18 neonates in the control group were infected compared with 5 in the treatment group (P = .007). In the per-protocol analysis, 7 neonates in the control group were infected compared with 0 in the treatment group (P = .01). No clinically significant adverse maternal or neonatal effects occurred in the treatment group.
Subsequently, Jourdain and colleagues reported a multicenter, double-blind trial conducted in 17 public health hospitals in Thailand.11 TDF (300 mg daily) or placebo was administered from 28 weeks’ gestation until 8 weeks postpartum. Patients in both arms of the study were positive for the e antigen; 87% to 90% of the patients had a serum hepatitis B DNA concentration greater than 200,000 IU/mL.Following birth, infants in both groups received an injection of HBIG and then 4 doses of hepatitis B vaccine (0, 1, 2, 4, and 6 months). Both the HBIG and hepatitis B vaccine were administered very promptly after birth (median time, 1.2–1.3 hours).
At 6 months after delivery, 2% of infants in the placebo group (3 of 147) were HBsAg-positive compared with none of the infants in the treatment arm.11 No serious adverse effects occurred in infants in the TDF group. This difference in outcome was not statistically significant, but the overall rate of infection was so low in both groups that the sample size was definitely too small to exclude a type 2 statistical error. Moreover, the fourth dose of neonatal hepatitis B vaccine may have contributed to the surprisingly low rate of perinatal transmission. Of note, the serum hepatitis B DNA concentration in the TDF group declined from a mean of 7.6 log10 IU/mL to a mean of 4.0 log10 IU/mL at delivery.
In the most recent report, Wang and colleagues reported the results of a prospective cohort study in patients with a hepatitis B virus DNA concentration greater than 200,000 IU/mL.12 Beginning at either 24 or 32 weeks, patients were assigned to treatment with either oral TDF (300 mg daily) or oral telbivudine (LdT, 600 mg daily). The medications were continued for 4 weeks postpartum. In the intention-to-treat analysis, the rates of perinatal transmission were comparable, 1.5% versus 1.8%. In the per-protocol analysis, no infants in either group were infected. However, the predelivery decline in hepatitis Bvirus DNA concentration was greater in the TDF group. The ALT elevation rate was also lower in the TDF group. Patients in the LdT group had fewer problems with anorexia but more instances of arthralgia compared with those in the TDF group.
Based primarily on these 3 investigations, I recommend that all infected patients with a hepatitis B DNA concentration greater than 200,000 IU/mL or a viral load greater than 1,120,000 million copies/mL receive oral TDF, 300 mg daily, from 28 weeks until at least 4 to 8 weeks postpartum. The decision about duration of postpartum treatment should be made in consultation with an infectious disease specialist or hepatologist.
Case studies resolved
CASE 1 No protective level of surface antibody
This patient should promptly receive a single booster dose of the hepatitis B vaccine. The vaccine is an inactivated agent and is safe for administration at any time in pregnancy. Following delivery and prior to discharge from the hospital, the neonate should receive the first dose of the hepatitis B vaccine. A second dose should be administered 1 month later, and a third dose should be administered 6 months after the first dose.
CASE 2 Mother is seropositive for HBsAg
This patient should be tested immediately for HIV infection and hepatitis C and D. The hepatitis B viral genotype should be determined. She also should have a panel of liver function tests. If any of these tests are abnormal, a coagulation profile should be obtained to be certain that the patient is not at risk for a coagulopathy. Near the end of the second trimester, a hepatitis B viral load should be obtained. If the viral DNA concentration is greater than 200,000 IU/mLor a viral load greater than 1,120,000 million copies/mL, the patient should be treated with tenofovir, 300 mg daily, from week 28 until at least 4 weeks after delivery. The neonate should receive an injection of HBIG within 12 hours of birth and the first dose of the hepatitis B vaccine prior to discharge from the hospital. Two additional doses of the vaccine should be administered 1 and 6 months later. ●
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862.
- Bernstein HB, Lee MJ. Maternal and perinatal infection in pregnancy: viral. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics. Normal and Problem Pregnancies. 8th ed. Elsevier; 2021;1092.
- Dusheiko G, Agarwal K, Maini MK. New approaches to chronic hepatitis B. N Engl J Med. 2023;388:55-69.
- Ma L, Alla NR, Li X, et al. Mother to child transmission of HBV: review of current clinical management and prevention strategies. Rev Med Virol. 2014; 24: 396-406.
- Society for Maternal-Fetal Medicine; Dionne-Odom J, Tita ATN, Silverman NS. SMFM consult: preventing vertical transmission of hepatitis B. Contemporary OB/GYN. September 22, 2015. Accessed August 21, 2023. https://www .contemporaryobgyn.net/view/smfm-consult-preventing -vertical-transmission-hepatitis-b
- Lok ASF. The maze of treatments for hepatitis B. N Engl J Med. 2005;352:2743-2746.
- Shi Z, Yang Y, Wang H, et al. Breastfeeding of newborns by mothers carrying hepatitis B virus: a meta-analysis and systematic review. Arch Pediatr Adolesc Med. 2011;165:837-846.
- Dusheiko G. A shift in thinking to reduce mother-to-infant transmission of hepatitis B. N Engl J Med. 2018;378:952-953.
- Shi Z, Yang Y, Ma L, et al. Lamivudine in late pregnancy to interrupt in utero transmission of hepatitis B virus: a systematic review and meta-analysis. Obstet Gynecol. 2010;116:147-159.
- Pan C, Duan Z, Dai E, et al; China Study Group for the Motherto-Child Transmission of Hepatitis B. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374:2324-2334.
- Jourdain G, Ngo-Giang-Huong N, Harrison L, et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N Engl J Med. 2018;378:911-923.
- Wang M, Ran R, Zhu Y, et al. Comparison of tenofovir disoproxil fumarate and telbivudine in preventing hepatitis B transmission in mothers with high viral load. Int J Gynaecol Obstet. 2023:160:646-652.

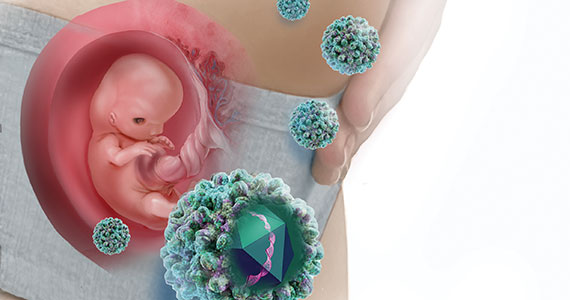
Hepatitis B is one of the more common infections encountered in the daily practice of obstetrics. It is responsible for 40% to 45% of all cases of viral hepatitis.1,2 Hepatitis B may cause serious complications in both the infected mother and neonate.
In this article, I review the virology, epidemiology, and clinical presentation of hepatitis B and then discuss the key diagnostic tests and, subsequently, the clinical management for both the mother and neonate. I focus particular attention on relatively new information about the value of specific antiviral medication to enhance the protective effect of conventional neonatal immunoprophylaxis.
To set the framework for the discussion, consider the following 2 case studies.
CASE 1 Undetectable level of hepatitis B surface antibody in a pregnant woman
A 25-year-old healthy primigravid woman at 10 weeks’ gestation had a series of laboratory studies that included a test for hepatitis B surface antigen (HBsAg) and hepatitis B surface antibody (HBsAb). The test for the surface antigen was negative. The test for the surface antibody was below the level of detection. Upon questioning, the patient indicates that she received the 3-dose hepatitis B vaccine when she was age 13 years.
- What treatment, if any, is indicated for this patient?
- What treatment is indicated for her neonate?
CASE 2 Pregnant woman tests positive for hepatitis B surface antigen
A 31-year-old woman (G3P2002) at 12 weeks’ gestation tested positive for HBsAg. She indicates that she never has had symptomatic hepatitis and that she considers herself to be in excellent health.
- What additional laboratory tests are indicated at this time?
- What additional laboratory test should be performed at the end of the second trimester?
- What treatment is indicated for the mother and neonate?
Virology and epidemiology of hepatitis B
Hepatitis B is caused by a double-stranded, enveloped DNA virus. The virus has 10 genotypes and 24 subtypes.3 The organism contains 3 major antigens. Detection of these antigens and their corresponding antibodies is an essential step in the diagnostic workup of patients who may be infected.
The surface antigen (HBsAg) confers infectivity and is the most valuable serologic marker of infection. The e antigen (HBeAg) is not present in every infected patient. It is secreted from infected cells, but it is not incorporated into the viral particle. When present, it denotes a high level of viral replication and exceptionally high infectivity. The core antigen (HBcAg) is a valuable serologic marker for distinguishing between acute and chronic infection.1-3
Hepatitis B is highly infectious, much more so than HIV or hepatitis C. The virus has an incubation period of 4 weeks to 6 months, and the duration of incubation is inversely related to the size of the viral inoculum. The virus is transmitted in 3 principal ways: sexual contact with contaminated genital tract secretions, contact with infected blood from sharing contaminated drug-injecting paraphernalia or from receiving a blood transfusion (extremely rare today), and transmission from an infected mother to her neonate. Perinatal transmission occurs primarily during the delivery process as opposed to transplacental infection. Transmission also can occur by more casual household contact, such as sharing eating utensils, kissing, and handling an infant.1,2,4,5
Worldwide, more than 400 million people have chronic hepatitis B infection. In the United States, approximately 1.25 to 1.5 million individuals are infected. Several groups are at particularly high risk for being infected, including1-3:
- Asians
- Alaska Natives
- sub-Saharan Africans
- sex workers
- intravenous drug users
- individuals with hemophilia
- international travelers
- staff and residents of long-term care facilities
- tattoo recipients.
Continue to: Clinical presentation...
Clinical presentation
Approximately 90% of adult patients who contract hepatitis B, either symptomatically or asymptomatically, will develop protective levels of antibody and clear the virus from their system. They will then have lifelong immunity to reinfection. Approximately 10% of patients will fail to develop protective levels of antibody and will become chronically infected, posing a risk to their household members, sexual contacts, and their fetus if they become pregnant. Persistence of the surface antigen in the patient’s serum for more than 6 months denotes chronic infection. A very small number of individuals—less than 1%—will develop acute liver failure and experience a fatal outcome.1-3,5
In the United States, the prevalence of acute hepatitis B in pregnancy is 1 to 2 per 1,000. Clinical manifestations typically include anorexia, nausea, low-grade fever, right upper quadrant pain and tenderness, passage of clay-colored stools, and jaundice.
The prevalence of chronic infection in pregnancy is significantly higher, approximately 5 to 15 per 1,000. Over the long term, patients with chronic infection are at risk for progressive liver injury, including cirrhosis and even hepatocellular carcinoma. These serious sequelae are particularly likely to occur when the patient is co-infected with hepatitis C, D, or both. The overall risk of progression to chronic cirrhosis is approximately 15% to 30%. In patients who progress to cirrhosis, the annual incidence of hepatocellular carcinoma is 10%.1-3
Diagnosis of hepatitis B infection
Patients with acute hepatitis B will test positive for HBsAg and immunoglobulin M (IgM) antibody to the core antigen. Some patients will also test positive for HBeAg. Assessment of the patient’s serum by polymerase chain reaction (PCR) allows quantitation of the viral load, which often is expressed as viral copies per milliliter. Alternatively, the quantitative hepatitis B DNA concentration may be expressed as international units per milliliter (IU/mL). The World Health Organization recommends this latter quantitative method. Multiplying the DNA in IU/mL by 5.6 provides the conversion to viral copies per milliliter.
Patients with chronic hepatitis B infection will test positive for the HBsAg and for immunoglobulin G (IgG) antibody to the core antigen. They may also have a positive test for the HBeAg, and PCR may be used to quantify the viral load.1-3
Managing hepatitis B infection in pregnancy
General supportive measures. All pregnant patients should be tested for the HBsAg and HBsAb at the time of the first prenatal appointment. The tests should be repeated at the beginning of the third trimester in high-risk patients. Seropositive patients should have a hepatitis B genotype, a test for the e antigen, and tests for other sexually transmissible infections (gonorrhea, chlamydia, syphilis, HIV) and for hepatitis C and D. Liver function tests should be performed to assess for elevations in the alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels. Patients with elevated transaminase enzymes should have a coagulation profile to be certain they are not at risk for a coagulopathy.
At the end of the second trimester, patients should have a PCR assessment to determine the viral load. This assessment will be important for deciding if specific antiviral therapy is indicated during the third trimester to enhance the effects of neonatal immunoprophylaxis (see below). Of note, patients who are positive for the e antigen may have a very high viral load and yet have normal or near-normal transaminase levels. This seemingly paradoxical finding reflects the non-cytopathic nature of hepatitis B.
The patient should optimize her nutrition and sleep. She should avoid, or at least minimize, medications such as acetaminophen that could cause further liver injury. Without question, she should refrain from consuming even small amounts of alcohol. She should be tested for immunity to hepatitis A; if found to be susceptible, she should be vaccinated with the hepatitis A vaccine. This agent is an inactivated vaccine and is safe for administration at any time in pregnancy.1,2,5
Household contacts. In addition to the measures outlined above, the patient’s household contacts, particularly her sexual partner(s), should be tested for immunity to hepatitis B. If they do not have immunity by virtue of natural infection or previous vaccination, they should receive the hepatitis B vaccine series. It is also prudent to provide the sexual partner(s) with an initial dose of hepatitis B immune globulin (HBIG) to provide a temporary level of passive immunity.
Postdelivery care. After delivery, the patient should be referred to an infectious disease specialist or hepatologist for consideration of long-term treatment with antiviral agents, such as interferon alfa, pegylated interferon alfa, lamivudine, adefovir, entecavir, telbivudine, or tenofovir.6 The principal candidates for treatment are those who have cirrhosis and detectable levels of hepatitis B DNA. The ultimate goal of treatment is to reduce the serum hepatitis B DNA concentration to an undetectable level. Once the surface antigenemia is cleared, treatment can be stopped. A cure is defined when the absence of hepa-titis B DNA in the serum is sustained.
- Hepatitis B is a DNA virus that is transmitted via sexual contact, exposure to infected blood, and from an infected mother to her fetus.
- Most patients in our practice will most likely have chronic, asymptomatic infection, and the diagnosis will be established by detection of HBsAg in the patient’s serum.
- All obstetric patients should be tested for both HBsAg and HBsAb.
- Patients who are positive for the surface antigen should be tested for HIV infection and hepatitis C and D. They also should have a determination of the hepatitis B genotype and viral load and assessment of liver function (ALT, AST).
- Patients who are chronically infected with hepatitis B should be vaccinated against hepatitis A to prevent further liver injury. They also should avoid medications that might cause hepatic injury.
- Patients who have a viral DNA concentration greater than 200,000 IU/mL or a viral load greater than 1,120,000 million copies/mL should be treated with tenofovir, 300 mg daily, from week 28 until 4 to 8 weeks after delivery.
- Infants delivered to infected mothers should receive HBIG within 12 hours of birth and then begin the 3-dose hepatitis B vaccine series. The first dose should be administered prior to hospital discharge.
- Infants delivered to mothers who are negative for the surface antigen should begin the hepatitis B vaccine series prior to discharge from the hospital.
- Mothers who test negative for HBsAb should be questioned about prior vaccination. If they have never been vaccinated, they should receive the 3-dose vaccine series. If they have been vaccinated, they should receive a single hepatitis B vaccine booster. The vaccine is safe for administration at any time during pregnancy.
- Infected mothers may breastfeed as long as they do not have cracked or bleeding nipples or exudative skin lesions near the nipple(s).
Neonatal immunoprophylaxis
The Centers for Disease Control and Prevention recommends universal hepatitis B vaccination for all newborns. The first dose of the vaccine should be administered prior to hospital discharge. The second and third doses should be administered 1 and 6 months later.1,2,5 There are few, if any, medical contraindications to neonatal vaccination. For the vast majority of infants, the immunity induced by vaccination is lifelong. For a small number, immunity may wane over time. Thus, reassessment of the HBsAb concentration is indicated in selected situations, for example, acute high-risk exposure to an infected person, development of an immunosuppressive disorder, or pregnancy.
Infants delivered to mothers who are infected with hepatitis B also should receive HBIG in addition to the vaccine. HBIG provides passive immunization to counteract the high viral inoculum encountered by the neonate during delivery. This preparation should be administered within 12 hours of birth.1,2,5
In the absence of immunoprophylaxis, a neonate delivered to a mother who is seropositive for HBsAg has a 20% to 30% probability of becoming chronically infected. If the mother is positive for both the surface antigen and the e antigen, the risk of chronic infection increases to almost 90%. Approximately 90% of infants who are infected in the perinatal period subsequently develop chronic infection. However, with appropriate immunoprophylaxis in the neonatal period, the risk of perinatal transmission is reduced by 85% to 95%.1,2,5
Cesarean delivery offers no additional protection beyond that provided by immunoprophylaxis. Moreover, because immunoprophylaxis is so effective, infected mothers may breastfeed without fear of transmitting infection to their infant. Shi and colleagues published a systematic review and meta-analysis of the risk associated with breastfeeding in hepatitis B–infected mothers.7 Infants who breastfed did not have a higher rate of mother-to-child transmission, regardless of whether they received combined immunoprophylaxis or only hepatitis B vaccine and regardless of whether the HBsAg was detected in the mother’s breast milk. The only precaution is the need to avoid breastfeeding if the nipples are cracked or bleeding or if exudative lesions are present on the skin near the nipple.
Continue to: Maternal antiviral therapy...
Maternal antiviral therapy
As noted above, neonatal immunoprophylaxis is 85% to 95% effective in preventing perinatal transmission of hepatitis B infection. Failures of prophylaxis are primarily due to antenatal transmission in patients who have exceptionally high viral loads. Several cutoffs have been used to define “high viral load,” including greater than 1 to 2 million copies/mL and a hepatitis B DNA concentration greater than 200,000 IU/mL. There is not a perfect consensus on the appropriate cutoff.
In essence, 2 different approaches have been tried to further reduce the risk of perinatal transmission in these high-risk patients.8 The first major initiative was administration of HBIG (100–200 IU) intramuscularly to the patient at 28, 32, and 36 weeks. The outcomes with this approach have been inconsistent, due, at least in part, to varying doses of the agent and various cutoffs for defining “high risk,” and this intervention is no longer recommended.1,2
The second major approach is administration of specific antiviral drugs to the mother during the third trimester. The first agent widely used in clinical practice was lamivudine. In a systematic review and meta-analysis, Shi and colleagues reported that, in infants whose mothers received lamivudine plus conventional neonatal immunuprophylaxis, the risk of perinatal infection was significantly reduced compared with infants who received only immunoprophylaxis.9
Although lamivudine is effective, there is considerable concern about the rapid development of viral resistance to the medication. Accordingly, most attention today is focused on the use of tenofovir to prevent perinatal transmission.
In an important early investigation, Pan and colleagues reported the results of a randomized controlled trial conducted in China in women with a hepatitis B DNA concentration greater than 200,000 IU/mL (viral load > 1,120,000 copies/mL).10 Patients also were positive for the e antigen. Ninety-two patients were assigned to tenofovir disoproxil fumarate (TDF), 300 mg daily, from 30 to 32 weeks until postpartum week 4 plus conventional neonatal immunoprophylaxis, and 100 patients were assigned to immunoprophylaxis alone. In the intention-to-treat analysis, 18 neonates in the control group were infected compared with 5 in the treatment group (P = .007). In the per-protocol analysis, 7 neonates in the control group were infected compared with 0 in the treatment group (P = .01). No clinically significant adverse maternal or neonatal effects occurred in the treatment group.
Subsequently, Jourdain and colleagues reported a multicenter, double-blind trial conducted in 17 public health hospitals in Thailand.11 TDF (300 mg daily) or placebo was administered from 28 weeks’ gestation until 8 weeks postpartum. Patients in both arms of the study were positive for the e antigen; 87% to 90% of the patients had a serum hepatitis B DNA concentration greater than 200,000 IU/mL.Following birth, infants in both groups received an injection of HBIG and then 4 doses of hepatitis B vaccine (0, 1, 2, 4, and 6 months). Both the HBIG and hepatitis B vaccine were administered very promptly after birth (median time, 1.2–1.3 hours).
At 6 months after delivery, 2% of infants in the placebo group (3 of 147) were HBsAg-positive compared with none of the infants in the treatment arm.11 No serious adverse effects occurred in infants in the TDF group. This difference in outcome was not statistically significant, but the overall rate of infection was so low in both groups that the sample size was definitely too small to exclude a type 2 statistical error. Moreover, the fourth dose of neonatal hepatitis B vaccine may have contributed to the surprisingly low rate of perinatal transmission. Of note, the serum hepatitis B DNA concentration in the TDF group declined from a mean of 7.6 log10 IU/mL to a mean of 4.0 log10 IU/mL at delivery.
In the most recent report, Wang and colleagues reported the results of a prospective cohort study in patients with a hepatitis B virus DNA concentration greater than 200,000 IU/mL.12 Beginning at either 24 or 32 weeks, patients were assigned to treatment with either oral TDF (300 mg daily) or oral telbivudine (LdT, 600 mg daily). The medications were continued for 4 weeks postpartum. In the intention-to-treat analysis, the rates of perinatal transmission were comparable, 1.5% versus 1.8%. In the per-protocol analysis, no infants in either group were infected. However, the predelivery decline in hepatitis Bvirus DNA concentration was greater in the TDF group. The ALT elevation rate was also lower in the TDF group. Patients in the LdT group had fewer problems with anorexia but more instances of arthralgia compared with those in the TDF group.
Based primarily on these 3 investigations, I recommend that all infected patients with a hepatitis B DNA concentration greater than 200,000 IU/mL or a viral load greater than 1,120,000 million copies/mL receive oral TDF, 300 mg daily, from 28 weeks until at least 4 to 8 weeks postpartum. The decision about duration of postpartum treatment should be made in consultation with an infectious disease specialist or hepatologist.
Case studies resolved
CASE 1 No protective level of surface antibody
This patient should promptly receive a single booster dose of the hepatitis B vaccine. The vaccine is an inactivated agent and is safe for administration at any time in pregnancy. Following delivery and prior to discharge from the hospital, the neonate should receive the first dose of the hepatitis B vaccine. A second dose should be administered 1 month later, and a third dose should be administered 6 months after the first dose.
CASE 2 Mother is seropositive for HBsAg
This patient should be tested immediately for HIV infection and hepatitis C and D. The hepatitis B viral genotype should be determined. She also should have a panel of liver function tests. If any of these tests are abnormal, a coagulation profile should be obtained to be certain that the patient is not at risk for a coagulopathy. Near the end of the second trimester, a hepatitis B viral load should be obtained. If the viral DNA concentration is greater than 200,000 IU/mLor a viral load greater than 1,120,000 million copies/mL, the patient should be treated with tenofovir, 300 mg daily, from week 28 until at least 4 weeks after delivery. The neonate should receive an injection of HBIG within 12 hours of birth and the first dose of the hepatitis B vaccine prior to discharge from the hospital. Two additional doses of the vaccine should be administered 1 and 6 months later. ●

Hepatitis B is one of the more common infections encountered in the daily practice of obstetrics. It is responsible for 40% to 45% of all cases of viral hepatitis.1,2 Hepatitis B may cause serious complications in both the infected mother and neonate.
In this article, I review the virology, epidemiology, and clinical presentation of hepatitis B and then discuss the key diagnostic tests and, subsequently, the clinical management for both the mother and neonate. I focus particular attention on relatively new information about the value of specific antiviral medication to enhance the protective effect of conventional neonatal immunoprophylaxis.
To set the framework for the discussion, consider the following 2 case studies.
CASE 1 Undetectable level of hepatitis B surface antibody in a pregnant woman
A 25-year-old healthy primigravid woman at 10 weeks’ gestation had a series of laboratory studies that included a test for hepatitis B surface antigen (HBsAg) and hepatitis B surface antibody (HBsAb). The test for the surface antigen was negative. The test for the surface antibody was below the level of detection. Upon questioning, the patient indicates that she received the 3-dose hepatitis B vaccine when she was age 13 years.
- What treatment, if any, is indicated for this patient?
- What treatment is indicated for her neonate?
CASE 2 Pregnant woman tests positive for hepatitis B surface antigen
A 31-year-old woman (G3P2002) at 12 weeks’ gestation tested positive for HBsAg. She indicates that she never has had symptomatic hepatitis and that she considers herself to be in excellent health.
- What additional laboratory tests are indicated at this time?
- What additional laboratory test should be performed at the end of the second trimester?
- What treatment is indicated for the mother and neonate?
Virology and epidemiology of hepatitis B
Hepatitis B is caused by a double-stranded, enveloped DNA virus. The virus has 10 genotypes and 24 subtypes.3 The organism contains 3 major antigens. Detection of these antigens and their corresponding antibodies is an essential step in the diagnostic workup of patients who may be infected.
The surface antigen (HBsAg) confers infectivity and is the most valuable serologic marker of infection. The e antigen (HBeAg) is not present in every infected patient. It is secreted from infected cells, but it is not incorporated into the viral particle. When present, it denotes a high level of viral replication and exceptionally high infectivity. The core antigen (HBcAg) is a valuable serologic marker for distinguishing between acute and chronic infection.1-3
Hepatitis B is highly infectious, much more so than HIV or hepatitis C. The virus has an incubation period of 4 weeks to 6 months, and the duration of incubation is inversely related to the size of the viral inoculum. The virus is transmitted in 3 principal ways: sexual contact with contaminated genital tract secretions, contact with infected blood from sharing contaminated drug-injecting paraphernalia or from receiving a blood transfusion (extremely rare today), and transmission from an infected mother to her neonate. Perinatal transmission occurs primarily during the delivery process as opposed to transplacental infection. Transmission also can occur by more casual household contact, such as sharing eating utensils, kissing, and handling an infant.1,2,4,5
Worldwide, more than 400 million people have chronic hepatitis B infection. In the United States, approximately 1.25 to 1.5 million individuals are infected. Several groups are at particularly high risk for being infected, including1-3:
- Asians
- Alaska Natives
- sub-Saharan Africans
- sex workers
- intravenous drug users
- individuals with hemophilia
- international travelers
- staff and residents of long-term care facilities
- tattoo recipients.
Continue to: Clinical presentation...
Clinical presentation
Approximately 90% of adult patients who contract hepatitis B, either symptomatically or asymptomatically, will develop protective levels of antibody and clear the virus from their system. They will then have lifelong immunity to reinfection. Approximately 10% of patients will fail to develop protective levels of antibody and will become chronically infected, posing a risk to their household members, sexual contacts, and their fetus if they become pregnant. Persistence of the surface antigen in the patient’s serum for more than 6 months denotes chronic infection. A very small number of individuals—less than 1%—will develop acute liver failure and experience a fatal outcome.1-3,5
In the United States, the prevalence of acute hepatitis B in pregnancy is 1 to 2 per 1,000. Clinical manifestations typically include anorexia, nausea, low-grade fever, right upper quadrant pain and tenderness, passage of clay-colored stools, and jaundice.
The prevalence of chronic infection in pregnancy is significantly higher, approximately 5 to 15 per 1,000. Over the long term, patients with chronic infection are at risk for progressive liver injury, including cirrhosis and even hepatocellular carcinoma. These serious sequelae are particularly likely to occur when the patient is co-infected with hepatitis C, D, or both. The overall risk of progression to chronic cirrhosis is approximately 15% to 30%. In patients who progress to cirrhosis, the annual incidence of hepatocellular carcinoma is 10%.1-3
Diagnosis of hepatitis B infection
Patients with acute hepatitis B will test positive for HBsAg and immunoglobulin M (IgM) antibody to the core antigen. Some patients will also test positive for HBeAg. Assessment of the patient’s serum by polymerase chain reaction (PCR) allows quantitation of the viral load, which often is expressed as viral copies per milliliter. Alternatively, the quantitative hepatitis B DNA concentration may be expressed as international units per milliliter (IU/mL). The World Health Organization recommends this latter quantitative method. Multiplying the DNA in IU/mL by 5.6 provides the conversion to viral copies per milliliter.
Patients with chronic hepatitis B infection will test positive for the HBsAg and for immunoglobulin G (IgG) antibody to the core antigen. They may also have a positive test for the HBeAg, and PCR may be used to quantify the viral load.1-3
Managing hepatitis B infection in pregnancy
General supportive measures. All pregnant patients should be tested for the HBsAg and HBsAb at the time of the first prenatal appointment. The tests should be repeated at the beginning of the third trimester in high-risk patients. Seropositive patients should have a hepatitis B genotype, a test for the e antigen, and tests for other sexually transmissible infections (gonorrhea, chlamydia, syphilis, HIV) and for hepatitis C and D. Liver function tests should be performed to assess for elevations in the alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels. Patients with elevated transaminase enzymes should have a coagulation profile to be certain they are not at risk for a coagulopathy.
At the end of the second trimester, patients should have a PCR assessment to determine the viral load. This assessment will be important for deciding if specific antiviral therapy is indicated during the third trimester to enhance the effects of neonatal immunoprophylaxis (see below). Of note, patients who are positive for the e antigen may have a very high viral load and yet have normal or near-normal transaminase levels. This seemingly paradoxical finding reflects the non-cytopathic nature of hepatitis B.
The patient should optimize her nutrition and sleep. She should avoid, or at least minimize, medications such as acetaminophen that could cause further liver injury. Without question, she should refrain from consuming even small amounts of alcohol. She should be tested for immunity to hepatitis A; if found to be susceptible, she should be vaccinated with the hepatitis A vaccine. This agent is an inactivated vaccine and is safe for administration at any time in pregnancy.1,2,5
Household contacts. In addition to the measures outlined above, the patient’s household contacts, particularly her sexual partner(s), should be tested for immunity to hepatitis B. If they do not have immunity by virtue of natural infection or previous vaccination, they should receive the hepatitis B vaccine series. It is also prudent to provide the sexual partner(s) with an initial dose of hepatitis B immune globulin (HBIG) to provide a temporary level of passive immunity.
Postdelivery care. After delivery, the patient should be referred to an infectious disease specialist or hepatologist for consideration of long-term treatment with antiviral agents, such as interferon alfa, pegylated interferon alfa, lamivudine, adefovir, entecavir, telbivudine, or tenofovir.6 The principal candidates for treatment are those who have cirrhosis and detectable levels of hepatitis B DNA. The ultimate goal of treatment is to reduce the serum hepatitis B DNA concentration to an undetectable level. Once the surface antigenemia is cleared, treatment can be stopped. A cure is defined when the absence of hepa-titis B DNA in the serum is sustained.
- Hepatitis B is a DNA virus that is transmitted via sexual contact, exposure to infected blood, and from an infected mother to her fetus.
- Most patients in our practice will most likely have chronic, asymptomatic infection, and the diagnosis will be established by detection of HBsAg in the patient’s serum.
- All obstetric patients should be tested for both HBsAg and HBsAb.
- Patients who are positive for the surface antigen should be tested for HIV infection and hepatitis C and D. They also should have a determination of the hepatitis B genotype and viral load and assessment of liver function (ALT, AST).
- Patients who are chronically infected with hepatitis B should be vaccinated against hepatitis A to prevent further liver injury. They also should avoid medications that might cause hepatic injury.
- Patients who have a viral DNA concentration greater than 200,000 IU/mL or a viral load greater than 1,120,000 million copies/mL should be treated with tenofovir, 300 mg daily, from week 28 until 4 to 8 weeks after delivery.
- Infants delivered to infected mothers should receive HBIG within 12 hours of birth and then begin the 3-dose hepatitis B vaccine series. The first dose should be administered prior to hospital discharge.
- Infants delivered to mothers who are negative for the surface antigen should begin the hepatitis B vaccine series prior to discharge from the hospital.
- Mothers who test negative for HBsAb should be questioned about prior vaccination. If they have never been vaccinated, they should receive the 3-dose vaccine series. If they have been vaccinated, they should receive a single hepatitis B vaccine booster. The vaccine is safe for administration at any time during pregnancy.
- Infected mothers may breastfeed as long as they do not have cracked or bleeding nipples or exudative skin lesions near the nipple(s).
Neonatal immunoprophylaxis
The Centers for Disease Control and Prevention recommends universal hepatitis B vaccination for all newborns. The first dose of the vaccine should be administered prior to hospital discharge. The second and third doses should be administered 1 and 6 months later.1,2,5 There are few, if any, medical contraindications to neonatal vaccination. For the vast majority of infants, the immunity induced by vaccination is lifelong. For a small number, immunity may wane over time. Thus, reassessment of the HBsAb concentration is indicated in selected situations, for example, acute high-risk exposure to an infected person, development of an immunosuppressive disorder, or pregnancy.
Infants delivered to mothers who are infected with hepatitis B also should receive HBIG in addition to the vaccine. HBIG provides passive immunization to counteract the high viral inoculum encountered by the neonate during delivery. This preparation should be administered within 12 hours of birth.1,2,5
In the absence of immunoprophylaxis, a neonate delivered to a mother who is seropositive for HBsAg has a 20% to 30% probability of becoming chronically infected. If the mother is positive for both the surface antigen and the e antigen, the risk of chronic infection increases to almost 90%. Approximately 90% of infants who are infected in the perinatal period subsequently develop chronic infection. However, with appropriate immunoprophylaxis in the neonatal period, the risk of perinatal transmission is reduced by 85% to 95%.1,2,5
Cesarean delivery offers no additional protection beyond that provided by immunoprophylaxis. Moreover, because immunoprophylaxis is so effective, infected mothers may breastfeed without fear of transmitting infection to their infant. Shi and colleagues published a systematic review and meta-analysis of the risk associated with breastfeeding in hepatitis B–infected mothers.7 Infants who breastfed did not have a higher rate of mother-to-child transmission, regardless of whether they received combined immunoprophylaxis or only hepatitis B vaccine and regardless of whether the HBsAg was detected in the mother’s breast milk. The only precaution is the need to avoid breastfeeding if the nipples are cracked or bleeding or if exudative lesions are present on the skin near the nipple.
Continue to: Maternal antiviral therapy...
Maternal antiviral therapy
As noted above, neonatal immunoprophylaxis is 85% to 95% effective in preventing perinatal transmission of hepatitis B infection. Failures of prophylaxis are primarily due to antenatal transmission in patients who have exceptionally high viral loads. Several cutoffs have been used to define “high viral load,” including greater than 1 to 2 million copies/mL and a hepatitis B DNA concentration greater than 200,000 IU/mL. There is not a perfect consensus on the appropriate cutoff.
In essence, 2 different approaches have been tried to further reduce the risk of perinatal transmission in these high-risk patients.8 The first major initiative was administration of HBIG (100–200 IU) intramuscularly to the patient at 28, 32, and 36 weeks. The outcomes with this approach have been inconsistent, due, at least in part, to varying doses of the agent and various cutoffs for defining “high risk,” and this intervention is no longer recommended.1,2
The second major approach is administration of specific antiviral drugs to the mother during the third trimester. The first agent widely used in clinical practice was lamivudine. In a systematic review and meta-analysis, Shi and colleagues reported that, in infants whose mothers received lamivudine plus conventional neonatal immunuprophylaxis, the risk of perinatal infection was significantly reduced compared with infants who received only immunoprophylaxis.9
Although lamivudine is effective, there is considerable concern about the rapid development of viral resistance to the medication. Accordingly, most attention today is focused on the use of tenofovir to prevent perinatal transmission.
In an important early investigation, Pan and colleagues reported the results of a randomized controlled trial conducted in China in women with a hepatitis B DNA concentration greater than 200,000 IU/mL (viral load > 1,120,000 copies/mL).10 Patients also were positive for the e antigen. Ninety-two patients were assigned to tenofovir disoproxil fumarate (TDF), 300 mg daily, from 30 to 32 weeks until postpartum week 4 plus conventional neonatal immunoprophylaxis, and 100 patients were assigned to immunoprophylaxis alone. In the intention-to-treat analysis, 18 neonates in the control group were infected compared with 5 in the treatment group (P = .007). In the per-protocol analysis, 7 neonates in the control group were infected compared with 0 in the treatment group (P = .01). No clinically significant adverse maternal or neonatal effects occurred in the treatment group.
Subsequently, Jourdain and colleagues reported a multicenter, double-blind trial conducted in 17 public health hospitals in Thailand.11 TDF (300 mg daily) or placebo was administered from 28 weeks’ gestation until 8 weeks postpartum. Patients in both arms of the study were positive for the e antigen; 87% to 90% of the patients had a serum hepatitis B DNA concentration greater than 200,000 IU/mL.Following birth, infants in both groups received an injection of HBIG and then 4 doses of hepatitis B vaccine (0, 1, 2, 4, and 6 months). Both the HBIG and hepatitis B vaccine were administered very promptly after birth (median time, 1.2–1.3 hours).
At 6 months after delivery, 2% of infants in the placebo group (3 of 147) were HBsAg-positive compared with none of the infants in the treatment arm.11 No serious adverse effects occurred in infants in the TDF group. This difference in outcome was not statistically significant, but the overall rate of infection was so low in both groups that the sample size was definitely too small to exclude a type 2 statistical error. Moreover, the fourth dose of neonatal hepatitis B vaccine may have contributed to the surprisingly low rate of perinatal transmission. Of note, the serum hepatitis B DNA concentration in the TDF group declined from a mean of 7.6 log10 IU/mL to a mean of 4.0 log10 IU/mL at delivery.
In the most recent report, Wang and colleagues reported the results of a prospective cohort study in patients with a hepatitis B virus DNA concentration greater than 200,000 IU/mL.12 Beginning at either 24 or 32 weeks, patients were assigned to treatment with either oral TDF (300 mg daily) or oral telbivudine (LdT, 600 mg daily). The medications were continued for 4 weeks postpartum. In the intention-to-treat analysis, the rates of perinatal transmission were comparable, 1.5% versus 1.8%. In the per-protocol analysis, no infants in either group were infected. However, the predelivery decline in hepatitis Bvirus DNA concentration was greater in the TDF group. The ALT elevation rate was also lower in the TDF group. Patients in the LdT group had fewer problems with anorexia but more instances of arthralgia compared with those in the TDF group.
Based primarily on these 3 investigations, I recommend that all infected patients with a hepatitis B DNA concentration greater than 200,000 IU/mL or a viral load greater than 1,120,000 million copies/mL receive oral TDF, 300 mg daily, from 28 weeks until at least 4 to 8 weeks postpartum. The decision about duration of postpartum treatment should be made in consultation with an infectious disease specialist or hepatologist.
Case studies resolved
CASE 1 No protective level of surface antibody
This patient should promptly receive a single booster dose of the hepatitis B vaccine. The vaccine is an inactivated agent and is safe for administration at any time in pregnancy. Following delivery and prior to discharge from the hospital, the neonate should receive the first dose of the hepatitis B vaccine. A second dose should be administered 1 month later, and a third dose should be administered 6 months after the first dose.
CASE 2 Mother is seropositive for HBsAg
This patient should be tested immediately for HIV infection and hepatitis C and D. The hepatitis B viral genotype should be determined. She also should have a panel of liver function tests. If any of these tests are abnormal, a coagulation profile should be obtained to be certain that the patient is not at risk for a coagulopathy. Near the end of the second trimester, a hepatitis B viral load should be obtained. If the viral DNA concentration is greater than 200,000 IU/mLor a viral load greater than 1,120,000 million copies/mL, the patient should be treated with tenofovir, 300 mg daily, from week 28 until at least 4 weeks after delivery. The neonate should receive an injection of HBIG within 12 hours of birth and the first dose of the hepatitis B vaccine prior to discharge from the hospital. Two additional doses of the vaccine should be administered 1 and 6 months later. ●
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862.
- Bernstein HB, Lee MJ. Maternal and perinatal infection in pregnancy: viral. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics. Normal and Problem Pregnancies. 8th ed. Elsevier; 2021;1092.
- Dusheiko G, Agarwal K, Maini MK. New approaches to chronic hepatitis B. N Engl J Med. 2023;388:55-69.
- Ma L, Alla NR, Li X, et al. Mother to child transmission of HBV: review of current clinical management and prevention strategies. Rev Med Virol. 2014; 24: 396-406.
- Society for Maternal-Fetal Medicine; Dionne-Odom J, Tita ATN, Silverman NS. SMFM consult: preventing vertical transmission of hepatitis B. Contemporary OB/GYN. September 22, 2015. Accessed August 21, 2023. https://www .contemporaryobgyn.net/view/smfm-consult-preventing -vertical-transmission-hepatitis-b
- Lok ASF. The maze of treatments for hepatitis B. N Engl J Med. 2005;352:2743-2746.
- Shi Z, Yang Y, Wang H, et al. Breastfeeding of newborns by mothers carrying hepatitis B virus: a meta-analysis and systematic review. Arch Pediatr Adolesc Med. 2011;165:837-846.
- Dusheiko G. A shift in thinking to reduce mother-to-infant transmission of hepatitis B. N Engl J Med. 2018;378:952-953.
- Shi Z, Yang Y, Ma L, et al. Lamivudine in late pregnancy to interrupt in utero transmission of hepatitis B virus: a systematic review and meta-analysis. Obstet Gynecol. 2010;116:147-159.
- Pan C, Duan Z, Dai E, et al; China Study Group for the Motherto-Child Transmission of Hepatitis B. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374:2324-2334.
- Jourdain G, Ngo-Giang-Huong N, Harrison L, et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N Engl J Med. 2018;378:911-923.
- Wang M, Ran R, Zhu Y, et al. Comparison of tenofovir disoproxil fumarate and telbivudine in preventing hepatitis B transmission in mothers with high viral load. Int J Gynaecol Obstet. 2023:160:646-652.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862.
- Bernstein HB, Lee MJ. Maternal and perinatal infection in pregnancy: viral. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics. Normal and Problem Pregnancies. 8th ed. Elsevier; 2021;1092.
- Dusheiko G, Agarwal K, Maini MK. New approaches to chronic hepatitis B. N Engl J Med. 2023;388:55-69.
- Ma L, Alla NR, Li X, et al. Mother to child transmission of HBV: review of current clinical management and prevention strategies. Rev Med Virol. 2014; 24: 396-406.
- Society for Maternal-Fetal Medicine; Dionne-Odom J, Tita ATN, Silverman NS. SMFM consult: preventing vertical transmission of hepatitis B. Contemporary OB/GYN. September 22, 2015. Accessed August 21, 2023. https://www .contemporaryobgyn.net/view/smfm-consult-preventing -vertical-transmission-hepatitis-b
- Lok ASF. The maze of treatments for hepatitis B. N Engl J Med. 2005;352:2743-2746.
- Shi Z, Yang Y, Wang H, et al. Breastfeeding of newborns by mothers carrying hepatitis B virus: a meta-analysis and systematic review. Arch Pediatr Adolesc Med. 2011;165:837-846.
- Dusheiko G. A shift in thinking to reduce mother-to-infant transmission of hepatitis B. N Engl J Med. 2018;378:952-953.
- Shi Z, Yang Y, Ma L, et al. Lamivudine in late pregnancy to interrupt in utero transmission of hepatitis B virus: a systematic review and meta-analysis. Obstet Gynecol. 2010;116:147-159.
- Pan C, Duan Z, Dai E, et al; China Study Group for the Motherto-Child Transmission of Hepatitis B. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374:2324-2334.
- Jourdain G, Ngo-Giang-Huong N, Harrison L, et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N Engl J Med. 2018;378:911-923.
- Wang M, Ran R, Zhu Y, et al. Comparison of tenofovir disoproxil fumarate and telbivudine in preventing hepatitis B transmission in mothers with high viral load. Int J Gynaecol Obstet. 2023:160:646-652.