User login
EVIDENCE SUMMARY
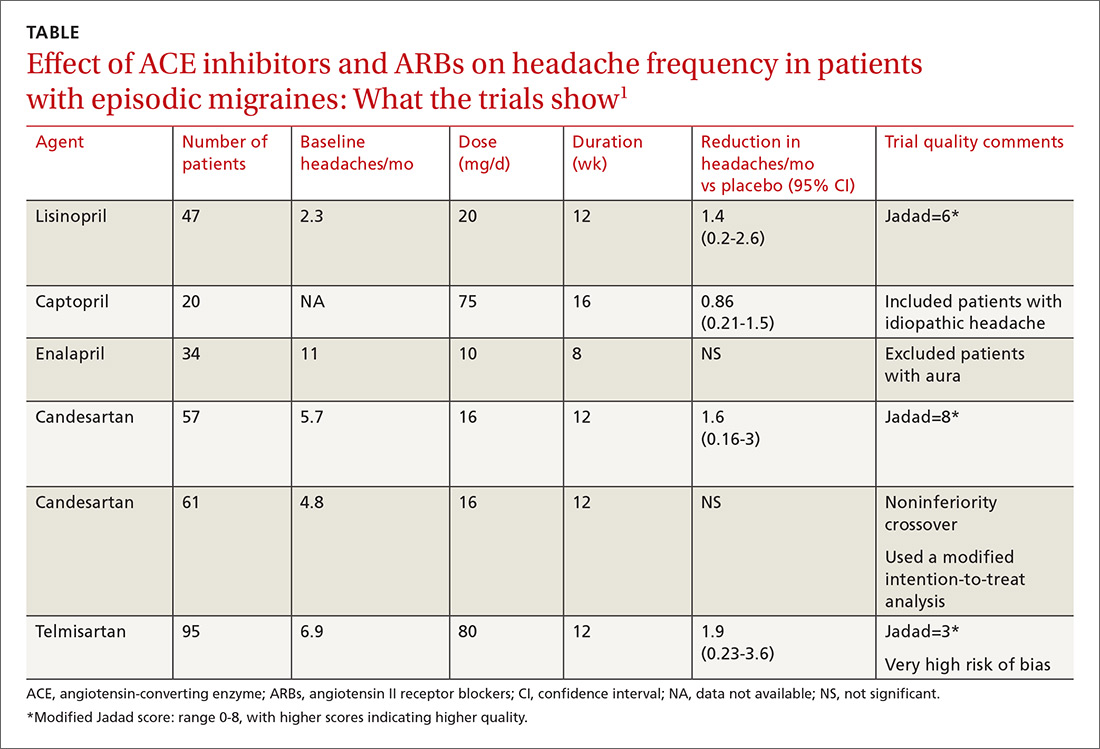
A network meta-analysis of 179 placebo-controlled trials of medications to treat migraine1 headache identified 3 trials involving ACE inhibitors and 3 involving ARBs (TABLE1). The authors of the meta-analysis gave 2 trials (one of lisinopril and one of candesartan) relatively high scores for methodologic quality.
Lisinopril reduces hours, days with headache and days with migraine
The first, a placebo-controlled lisinopril crossover trial, included 60 patients, 19 to 59 years of age, who experienced migraines with or without auras 2 to 6 times per month.2 Thirty patients received lisinopril 10 mg once daily for 1 week followed by 20 mg once daily (using 10-mg tablets) for 11 weeks. The other 30 patients received a similarly titrated placebo for 12 weeks. After a 2-week washout period, the groups were given the other therapy. Patients took triptan medications and analgesics as needed. Primary outcomes, extracted from headache diaries, included the number of hours and days with headache (of any type) and number of days with migraine specifically.
Out of the initial 60 participants, 47 completed the study. Using intention-to-treat analysis, lisinopril therapy resulted in fewer hours with headache (162 vs 138, a 15% difference; 95% confidence interval [CI], 0-30), fewer days with headache (25 vs 21, a 16% difference; 95% CI, 5-27), and fewer days with migraine (19 vs 15, a 22% change; 95% CI, 11-33), compared with placebo. Three patients discontinued lisinopril because of adverse events. Mean blood pressure reduction with lisinopril was 7 mm Hg systolic and 5 mm Hg diastolic more than placebo (P<.0001 for both comparisons).
Candesartan also decreases headaches and migraine
The other study given a high methodologic quality score by the network-meta-analysis authors was a placebo-controlled candesartan crossover trial.3 It enrolled 60 patients, 18 to 65 years of age, who experienced migraines with or without auras 2 to 6 times per month.
Thirty patients received 16 mg candesartan daily for 12 weeks, followed by a 4-week washout period before taking a placebo tablet daily for 12 weeks. The other 30 received placebo followed by candesartan. Patients took triptan medications and analgesics as needed. The primary outcome measure was days with headache, recorded by patients using daily diaries. Three patients didn’t complete the study.
Using intention-to-treat analysis, the mean number of days with headache was 18.5 with placebo and 13.6 with candesartan (P=.001). Secondary end points that also favored candesartan were hours with migraine (92 vs 59; P<.001), hours with headache (139 vs 95; P<.001), days with migraine (13 vs 9; P<.001), and days of sick leave (3.9 vs 1.4; P=.01). Adverse events, including dizziness, were similar with candesartan and placebo. Mean blood pressure reduction with candesartan was 11 mm Hg systolic and 7 mm Hg diastolic over placebo (P<.001 for both comparisons).
Continue to: Overall both drugs have a significant effect on number of headaches
Overall both drugs have a significant effect on number of headaches
Among all ACE inhibitor and ARB trials in the review, a network meta-analysis (designed to compare interventions never studied head-to-head) could be performed only on candesartan, which had a small effect size on headache frequency relative to placebo (2 trials, 118 patients; standardized mean difference [SMD]= −0.33; 95% CI, −0.59 to −0.7).1 (An SMD of 0.2 is considered small, 0.6 moderate, and 1.2 large). Combining data from all ACE inhibitor and ARB trials together in a standard meta-analysis yielded a large effect size on number of headaches per month compared with placebo (6 trials, 351 patients; SMD= −1.12; 95% CI, −1.97 to −0.27).1
RECOMMENDATIONS
In 2012, the American Academy of Neurology and the American Headache Society published guidelines on pharmacologic treatment for episodic migraine prevention in adults.4 The guidelines stated that lisinopril and candesartan were “possibly effective” for migraine prevention (level C recommendation based on a single lower-quality randomized clinical trial). They further advised clinicians to be “mindful of comorbid and coexistent conditions in patients with migraine to maximize potential treatment efficacy.”
1. Jackson JL, Cogbil E, Santana-Davila R, et al. A comparative effectiveness meta-analysis of drugs for the prophylaxis of migraine headache. PloS One. 2015;10:e0130733.
2. Schrader H, Stovner LJ, Helde G, et al. Prophylactic treatment of migraine with angiotensin converting enzyme inhibitor (lisinopril): randomized, placebo controlled, crossover study. BMJ. 2001;322:19-22.
3. Tronvik E, Stovner LJ, Helde G, et al. Prophylactic treatment of migraine with an angiotensin II receptor blocker: a randomized controlled trial. JAMA. 2003;289:65-69.
4. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
EVIDENCE SUMMARY
A network meta-analysis of 179 placebo-controlled trials of medications to treat migraine1 headache identified 3 trials involving ACE inhibitors and 3 involving ARBs (TABLE1). The authors of the meta-analysis gave 2 trials (one of lisinopril and one of candesartan) relatively high scores for methodologic quality.
Lisinopril reduces hours, days with headache and days with migraine
The first, a placebo-controlled lisinopril crossover trial, included 60 patients, 19 to 59 years of age, who experienced migraines with or without auras 2 to 6 times per month.2 Thirty patients received lisinopril 10 mg once daily for 1 week followed by 20 mg once daily (using 10-mg tablets) for 11 weeks. The other 30 patients received a similarly titrated placebo for 12 weeks. After a 2-week washout period, the groups were given the other therapy. Patients took triptan medications and analgesics as needed. Primary outcomes, extracted from headache diaries, included the number of hours and days with headache (of any type) and number of days with migraine specifically.
Out of the initial 60 participants, 47 completed the study. Using intention-to-treat analysis, lisinopril therapy resulted in fewer hours with headache (162 vs 138, a 15% difference; 95% confidence interval [CI], 0-30), fewer days with headache (25 vs 21, a 16% difference; 95% CI, 5-27), and fewer days with migraine (19 vs 15, a 22% change; 95% CI, 11-33), compared with placebo. Three patients discontinued lisinopril because of adverse events. Mean blood pressure reduction with lisinopril was 7 mm Hg systolic and 5 mm Hg diastolic more than placebo (P<.0001 for both comparisons).
Candesartan also decreases headaches and migraine
The other study given a high methodologic quality score by the network-meta-analysis authors was a placebo-controlled candesartan crossover trial.3 It enrolled 60 patients, 18 to 65 years of age, who experienced migraines with or without auras 2 to 6 times per month.
Thirty patients received 16 mg candesartan daily for 12 weeks, followed by a 4-week washout period before taking a placebo tablet daily for 12 weeks. The other 30 received placebo followed by candesartan. Patients took triptan medications and analgesics as needed. The primary outcome measure was days with headache, recorded by patients using daily diaries. Three patients didn’t complete the study.
Using intention-to-treat analysis, the mean number of days with headache was 18.5 with placebo and 13.6 with candesartan (P=.001). Secondary end points that also favored candesartan were hours with migraine (92 vs 59; P<.001), hours with headache (139 vs 95; P<.001), days with migraine (13 vs 9; P<.001), and days of sick leave (3.9 vs 1.4; P=.01). Adverse events, including dizziness, were similar with candesartan and placebo. Mean blood pressure reduction with candesartan was 11 mm Hg systolic and 7 mm Hg diastolic over placebo (P<.001 for both comparisons).
Continue to: Overall both drugs have a significant effect on number of headaches
Overall both drugs have a significant effect on number of headaches
Among all ACE inhibitor and ARB trials in the review, a network meta-analysis (designed to compare interventions never studied head-to-head) could be performed only on candesartan, which had a small effect size on headache frequency relative to placebo (2 trials, 118 patients; standardized mean difference [SMD]= −0.33; 95% CI, −0.59 to −0.7).1 (An SMD of 0.2 is considered small, 0.6 moderate, and 1.2 large). Combining data from all ACE inhibitor and ARB trials together in a standard meta-analysis yielded a large effect size on number of headaches per month compared with placebo (6 trials, 351 patients; SMD= −1.12; 95% CI, −1.97 to −0.27).1
RECOMMENDATIONS
In 2012, the American Academy of Neurology and the American Headache Society published guidelines on pharmacologic treatment for episodic migraine prevention in adults.4 The guidelines stated that lisinopril and candesartan were “possibly effective” for migraine prevention (level C recommendation based on a single lower-quality randomized clinical trial). They further advised clinicians to be “mindful of comorbid and coexistent conditions in patients with migraine to maximize potential treatment efficacy.”
EVIDENCE SUMMARY
A network meta-analysis of 179 placebo-controlled trials of medications to treat migraine1 headache identified 3 trials involving ACE inhibitors and 3 involving ARBs (TABLE1). The authors of the meta-analysis gave 2 trials (one of lisinopril and one of candesartan) relatively high scores for methodologic quality.
Lisinopril reduces hours, days with headache and days with migraine
The first, a placebo-controlled lisinopril crossover trial, included 60 patients, 19 to 59 years of age, who experienced migraines with or without auras 2 to 6 times per month.2 Thirty patients received lisinopril 10 mg once daily for 1 week followed by 20 mg once daily (using 10-mg tablets) for 11 weeks. The other 30 patients received a similarly titrated placebo for 12 weeks. After a 2-week washout period, the groups were given the other therapy. Patients took triptan medications and analgesics as needed. Primary outcomes, extracted from headache diaries, included the number of hours and days with headache (of any type) and number of days with migraine specifically.
Out of the initial 60 participants, 47 completed the study. Using intention-to-treat analysis, lisinopril therapy resulted in fewer hours with headache (162 vs 138, a 15% difference; 95% confidence interval [CI], 0-30), fewer days with headache (25 vs 21, a 16% difference; 95% CI, 5-27), and fewer days with migraine (19 vs 15, a 22% change; 95% CI, 11-33), compared with placebo. Three patients discontinued lisinopril because of adverse events. Mean blood pressure reduction with lisinopril was 7 mm Hg systolic and 5 mm Hg diastolic more than placebo (P<.0001 for both comparisons).
Candesartan also decreases headaches and migraine
The other study given a high methodologic quality score by the network-meta-analysis authors was a placebo-controlled candesartan crossover trial.3 It enrolled 60 patients, 18 to 65 years of age, who experienced migraines with or without auras 2 to 6 times per month.
Thirty patients received 16 mg candesartan daily for 12 weeks, followed by a 4-week washout period before taking a placebo tablet daily for 12 weeks. The other 30 received placebo followed by candesartan. Patients took triptan medications and analgesics as needed. The primary outcome measure was days with headache, recorded by patients using daily diaries. Three patients didn’t complete the study.
Using intention-to-treat analysis, the mean number of days with headache was 18.5 with placebo and 13.6 with candesartan (P=.001). Secondary end points that also favored candesartan were hours with migraine (92 vs 59; P<.001), hours with headache (139 vs 95; P<.001), days with migraine (13 vs 9; P<.001), and days of sick leave (3.9 vs 1.4; P=.01). Adverse events, including dizziness, were similar with candesartan and placebo. Mean blood pressure reduction with candesartan was 11 mm Hg systolic and 7 mm Hg diastolic over placebo (P<.001 for both comparisons).
Continue to: Overall both drugs have a significant effect on number of headaches
Overall both drugs have a significant effect on number of headaches
Among all ACE inhibitor and ARB trials in the review, a network meta-analysis (designed to compare interventions never studied head-to-head) could be performed only on candesartan, which had a small effect size on headache frequency relative to placebo (2 trials, 118 patients; standardized mean difference [SMD]= −0.33; 95% CI, −0.59 to −0.7).1 (An SMD of 0.2 is considered small, 0.6 moderate, and 1.2 large). Combining data from all ACE inhibitor and ARB trials together in a standard meta-analysis yielded a large effect size on number of headaches per month compared with placebo (6 trials, 351 patients; SMD= −1.12; 95% CI, −1.97 to −0.27).1
RECOMMENDATIONS
In 2012, the American Academy of Neurology and the American Headache Society published guidelines on pharmacologic treatment for episodic migraine prevention in adults.4 The guidelines stated that lisinopril and candesartan were “possibly effective” for migraine prevention (level C recommendation based on a single lower-quality randomized clinical trial). They further advised clinicians to be “mindful of comorbid and coexistent conditions in patients with migraine to maximize potential treatment efficacy.”
1. Jackson JL, Cogbil E, Santana-Davila R, et al. A comparative effectiveness meta-analysis of drugs for the prophylaxis of migraine headache. PloS One. 2015;10:e0130733.
2. Schrader H, Stovner LJ, Helde G, et al. Prophylactic treatment of migraine with angiotensin converting enzyme inhibitor (lisinopril): randomized, placebo controlled, crossover study. BMJ. 2001;322:19-22.
3. Tronvik E, Stovner LJ, Helde G, et al. Prophylactic treatment of migraine with an angiotensin II receptor blocker: a randomized controlled trial. JAMA. 2003;289:65-69.
4. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
1. Jackson JL, Cogbil E, Santana-Davila R, et al. A comparative effectiveness meta-analysis of drugs for the prophylaxis of migraine headache. PloS One. 2015;10:e0130733.
2. Schrader H, Stovner LJ, Helde G, et al. Prophylactic treatment of migraine with angiotensin converting enzyme inhibitor (lisinopril): randomized, placebo controlled, crossover study. BMJ. 2001;322:19-22.
3. Tronvik E, Stovner LJ, Helde G, et al. Prophylactic treatment of migraine with an angiotensin II receptor blocker: a randomized controlled trial. JAMA. 2003;289:65-69.
4. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
EVIDENCE-BASED ANSWER:
The angiotensin-converting enzyme (ACE) inhibitor lisinopril reduces the number of migraines by about 1.5 per month in patients experiencing 2 to 6 migraines monthly (strength of recommendation [SOR]: B, small crossover trial); the angiotensin II receptor blocker (ARB) candesartan may produce a similar reduction (SOR: C, conflicting crossover trials).
Considered as a group, ACE inhibitors and ARBs have a moderate to large effect on the frequency of migraine headaches (SOR: B, meta-analysis of small clinical trials), although only lisinopril and candesartan show fair to good evidence of efficacy.
Providers may consider lisinopril or candesartan for migraine prevention, taking into account their effect on other medical conditions (SOR: C, expert opinion).