User login
The author has no financial relationships relevant to this article.
CASE: Inadvertent ureteral transection
A gynecologic surgeon operates via Pfannenstiel incision to remove a 12-cm complex left adnexal mass from a 36-year-old obese woman. When she discovers that the mass is densely adherent to the pelvic peritoneum, the surgeon incises the peritoneum lateral to the mass and opens the retroperitoneal space. However, the size and relative immobility of the mass, coupled with the low transverse incision, impair visualization of retroperitoneal structures.
The surgeon clamps and divides the ovarian vessels above the mass but, afterward, suspects that the ureter has been transected and that its ends are included within the clamps. She separates the ovarian vessels above the clamp and ligates them, at which time transection of the ureter is confirmed.
How should she proceed?
The ureter is intimately associated with the female internal genitalia in a way that challenges the gynecologic surgeon to avoid it. In a small percentage of cases involving surgical extirpation in a woman who has severe pelvic pathology, ureteral injury may be inevitable.
Several variables predispose a patient to ureteral injury, including limited exposure, as in the opening case. Others include distorted anatomy of the urinary tract relative to internal genitalia and operations that require extensive resection of pelvic tissues.
This article describes:
- prevention and intraoperative recognition of ureteral injury during gynecologic surgery
- management of intraoperatively recognized ureteral injury.
Maintain a high index of suspicion
The surgeon in the opening case has already taken the first and most important step in ensuring a good outcome: She suspected ureteral injury. In high-risk situations, intraoperative recognition of ureteral injury is more likely when the operative field is inspected thoroughly during and at the conclusion of the surgical procedure.
In a high-risk case, the combined use of intravenous indigo carmine, careful inspection of the operative field, cystoscopy, and ureteral dissection is recommended and should be routine.
Common sites of injury
During gynecologic surgery, the ureter is susceptible to injury along its entire course through the pelvis (see “The ureter takes a course fraught with hazard,”).
During adnexectomy, the gonadal vessels are generally ligated 2 to 3 cm above the adnexa. The ureter lies in close proximity to these vessels and may inadvertently be included in the ligation.
During hysterectomy, the ureter is susceptible to injury as it passes through the parametrium a short distance from the uterus and vaginal fornix.
Sutures placed in the posterior lateral cul de sac during prolapse surgery lie near the midpelvic ureter, and sutures placed during vaginal cuff closure, anterior colporrhaphy, and retropubic urethropexy are in close proximity to the trigonal portion of the ureter.
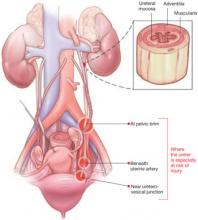
The ureter extends from the renal pelvis to the bladder, with a length that ranges from 25 to 30 cm, depending on the patient’s height. It crosses the pelvic brim near the bifurcation of the common iliac artery, where it becomes the “pelvic” ureter. The abdominal and pelvic portions of the ureter are approximately equal in length.
ILLUSTRATIONS BY ROB FLEWELL FOR OBG MANAGEMENT
The blood supply of the ureter derives from branches of the major arterial system of the lower abdomen and pelvis. These branches reach the medial aspect of the abdominal ureter and the lateral side of the pelvic ureter to form an anastomotic vascular network protected by an adventitial layer surrounding the ureter.
The ureter is attached to the posterior lateral pelvic peritoneum running dorsal to ovarian vessels. At the midpelvis, it separates from the peritoneum to pierce the base of the broad ligament underneath the uterine artery. At this point, the ureter is about 1.5 to 2 cm lateral to the uterus and curves medially and ventrally, tunneling through the cardinal and vesicovaginal ligaments to enter the bladder trigone.
Risky procedures
In gynecologic surgery, ureteral injury occurs most often during abdominal hysterectomy—probably because of how frequently this operation is performed and the range of pathology managed. The incidence of ureteral injury is much higher during abdominal hysterectomy than vaginal hysterectomy.1-4
Laparoscopic hysterectomy also has been associated with a higher incidence of ureteral injury, especially in the early phase of training.5,6 Possible explanations include:
- greater difficulty identifying the ureter
- a steeper learning curve
- more frequent use of energy to hemostatically divide pedicles, with the potential for thermal injury
- less traction–countertraction, resulting in dissection closer to the ureter
- management of complex pathology.
Although the overall incidence of ureteral injury during adnexectomy is low, it is probably much higher in women undergoing this procedure after a previous hysterectomy or in the presence of complex adnexal pathology.
When injury is likely
Compromised exposure, distorted anatomy, and certain procedures can heighten the risk of ureteral injury. Large tumors may limit the ability of the surgeon to visualize or palpate the ureter (FIGURE 1). Extensive adhesions may cause similar difficulties, and a small incision or obesity may hinder identification of pelvic sidewall structures.
A number of pathologic conditions can distort the anatomy of the ureter, especially as it relates to the female genital tract:
- Malignancies such as ovarian cancer often encroach on and occasionally encase the ureter
- Pelvic inflammatory disease, endometriosis, and a history of surgery or pelvic radiotherapy can retract and encase the ureter toward the gynecologic tract
- Some masses expand against the lower ureter, such as cervical or broad-ligament leiomyomata or placenta previa with accreta
- During vaginal hysterectomy for complete uterine prolapse, the ureters frequently extend beyond the introitus well within the operative field
- Congenital anomalies of the ureter or hydroureter can also cause distortion.
Even in the presence of relatively normal anatomy, certain procedures predispose the ureter to injury. For example, radical hysterectomy involves the almost complete separation of the pelvic ureter from the gynecologic tract and its surrounding soft tissue. When pelvic pathology is significant, the plane of dissection will always be near the ureter.
FIGURE 1 Access to the ureter is obstructed, putting it in jeopardy
Large tumors may limit the ability of the surgeon to visualize or palpate the ureter.
Prevention is the best strategy
At least 50% of ureteral injuries reported during gynecologic surgery have occurred in the absence of a recognizable risk factor.2,7 Nevertheless, knowledge of anatomy and the ability to recognize situations in which there is an elevated risk for ureteral injury will best enable the surgeon to prevent such injury.
When a high-risk situation is encountered, critical preventive steps include:
- adequate exposure
- competent assistance
- exposure of the path of the ureter through the planned course of dissection. Dissecting the ureter beyond this area is usually unnecessary and may itself cause injury.
Skip preoperative IVP in most cases
The vast majority of women who undergo gynecologic surgery do not benefit from preoperative intravenous pyelography (IVP). This measure does not appear to reduce the likelihood of ureteral injury, even in the face of obvious gynecologic disease. However, preoperative identification of obvious ureteral involvement by the disease process is useful. In such cases, the plane of dissection will probably lie closer to the ureter. One of the goals of surgery will then be to clear the urinary tract from the affected area.
When there is a high index of suspicion of an abnormality such as obstruction, intrinsic ureteral endometriosis, or congenital anomaly, preoperative IVP is indicated.
A stent may be helpful in some cases
Ureteral stents are sometimes placed in order to aid in identification and dissection of the ureters during surgery. Some authors of reports on this topic, including Hoffman, believe that stents are useful in certain situations, such as excision of an ovarian remnant, radical vaginal hysterectomy, and when pelvic organs are encased by malignant ovarian tumors. However, stents do not clearly reduce the risk of injury and, in some cases, may increase the risk by providing a false sense of security and predisposing the ureter to adventitial injury during difficult dissection.
Anticipate the effects of disease
The surgeon must have a thorough knowledge of the gynecologic disease process as it relates to surgery involving the urinary tract. For example, an ovarian remnant will almost always be somewhat densely adherent to the pelvic ureter. When severe endometriosis involves the posterior leaf of the broad ligament, the ureter will often be fibrotically retracted toward the operative field.
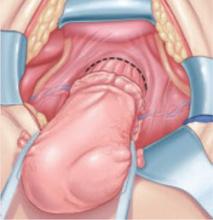
Certain procedures have special challenges. During resection of adnexa, for example, it is important that the ureter be identified in the retroperitoneum before the ovarian vessels are ligated. During hysterectomy, soft tissues that contain the bladder and ureters should be mobilized caudally and laterally, respectively, creating a U-shaped region (“U” for urinary tract, FIGURE 2) to which the surgeon must limit dissection.
FIGURE 2 During hysterectomy, mobilize the bladder and ureter
Mobilize the soft tissues that contain the bladder and ureters caudally and laterally, respectively, creating a U-shaped region. During division of the paracervical tissues, the surgeon must remain within this region.
Intraoperative detection
Two main types of ureteral injury occur during gynecologic surgery: transection and destruction. The latter includes ligation, crushing, devascularization, and thermal injury.
Intraoperative detection of ureteral injury is more likely when the surgeon recognizes at the outset that the operation places the ureter at increased risk. When dissection has been difficult or complicated for any reason, be concerned about possible injury.
In general, ureteral injury is first recognized by careful inspection of the surgical field. Begin by instilling 5 ml of indigo carmine intravenously. Once the dye begins to appear in the Foley catheter, inspect the area of dissection under a small amount of irrigation fluid, looking for extravasation of dye that indicates partial or complete transection.
If no injury is identified, cystoscopy is the next step. I perform all major abdominal operations with the patient in the low lithotomy position, which provides easy access to the perineum. Cystoscopic identification of urine jetting from both ureteral orifices confirms patency. When only wisps of dye are observed, it is likely that the ureter in question has been partially occluded (e.g., by acute angulation). Failure of any urine to appear from one of the orifices highly suggests injury to that ureter.
During inspection of the operative field, attempt to pass a ureteral stent into the affected orifice. If the stent passes easily and dyed urine is seen to drip freely from it, look for possible angulation of the ureter. If you find none, remove the stent and inspect the orifice again for jetting urine.
If the ureteral stent will move only a few centimeters into the ureteral orifice, ligation (with or without transection) is likely. In this case, leave the stent in place. If the operative site is readily accessible, dissect the applicable area to identify the problem. Depending on the circumstances, you may wish to infuse dye through the stent to aid in operative identification or radiographic evaluation.
Intraoperative IVP may be useful, especially when cystoscopy is unavailable.
Fundamentals of repair
Repair of major injury to the pelvic ureter is generally best accomplished by ureteroneocystostomy or, in selected cases involving injury to the proximal pelvic ureter, by ureteroureterostomy.
When intraoperatively recognized injury to the pelvic ureter appears to be minor, it can be managed by placing a ureteral stent and a closed-suction pelvic drain. Also consider wrapping the injured area with vascularized tissue such as perivesical fat. Minor lacerations can be closed perpendicular to the axis of the ureter using interrupted 4-0 delayed absorbable suture.
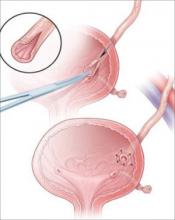
Most injuries to the pelvic ureter are optimally managed by ureteroneocystostomy (FIGURE 3). When a significant portion of the pelvic ureter has been lost, ureteroneocystostomy usually requires a combination of:
- extensive mobilization of the bladder
- conservative mobilization of the ureter
- elongation of the bladder
- psoas hitch.
When necessary, mobilization of the kidney with suturing of the caudal perinephric fascia to the psoas muscle will bridge an additional 2- to 3-cm gap.
Major injury to the distal half of the pelvic ureter is repaired using straightforward ureteroneocystostomy.
When there is no significant pelvic disease and the distal ureter is healthy, injury to the proximal pelvic ureter during division of the ovarian vessels may be repaired via ureteroureterostomy. If the ureteral ends will be anastomosed on tension or there is any question about the integrity of the distal portion of the ureter, as when extensive distal ureterolysis has been necessary, consider ureteroneocystostomy.
FIGURE 3 When the distal ureter is injured
Most injuries to the pelvic ureter are managed optimally by ureteroneocystostomy.
Ureter injured during emergent hysterectomy
A 37-year-old woman, para 4, undergoes her fourth repeat cesarean section. When the OB attempts to manually extract the placenta, the patient begins to hemorrhage profusely. Conservative measures fail to stop the bleeding, and the patient becomes hypotensive. The physician performs emergent hysterectomy, taking large pedicles of tissue. Although the patient stabilizes, the doctor worries that the ureters may have been injured.
Resolution: Cystoscopy is performed to check for injury. Because indigo carmine does not spill from the left ureteral orifice, the physician passes a stent with the abdomen still open, and it stops within the most distal ligamentous pedicle. Upon deligation, indigo carmine begins to drain from the stent, which then passes easily.
The stent is withdrawn to below the site of injury, and dilute methylene blue is instilled through it while the ureter is observed under irrigation. No extravasation is noted. Because the ligature had been around a block of tissue that was thought to have acutely angulated rather than incorporated the ureter, the physician concludes that severe damage is unlikely. He places a 6 French double-J stent, wraps the damaged portion of the distal ureter in perivesical fat, and places a closed-suction pelvic drain. Healing is uneventful.
Obstruction is confirmed. Now the surgeon must find it
A 45-year-old woman, para 3, who has a symptomatic 14-weeks’ size myomatous uterus, undergoes vaginal hysterectomy. The surgeon ligates and divides the uterine vessel pedicles before beginning morcellation. At the completion of the procedure, during cystoscopy, indigo carmine fails to spill from the right ureteral orifice, suggesting injury to that ureter. The surgeon passes a stent into the ureter, and it stops approximately 6 cm from the orifice. A retrograde pyelogram confirms complete obstruction.
Resolution: With the stent left in place, the surgeon performs a midline laparotomy, tracing the ureter to the uterine artery pedicle in which it has been incorporated and transected. The distal ureter with the stent is found within soft tissue lateral to the cardinal ligament pedicle, and the transected end is securely ligated using 2–0 silk suture. After the bladder is mobilized, a ureteroneocystostomy is performed. The patient recovers fully.
Postoperative management
After repair of a ureteral injury, leave a closed-suction pelvic drain in place for 2 to 3 days so that any major urinary leak can be detected; it also enhances spontaneous closure and helps prevent potentially infected fluid from accumulating in the region of anastomosis.
The cystotomy performed during ureteroneocystostomy generally heals quickly with a low risk of complications.
Leave a large-bore (20 or 22 French) urethral Foley catheter in place for 2 weeks.
I recommend that a 6 French double-J ureteral stent be left in place for 6 weeks. Potential benefits of the stent include:
- prevention of stricture
- stabilization and immobilization of the ureter during healing
- reduced risk of extravasation of urine
- reduced risk of angulation of the ureter
- isolation of the repair from infection, retroperitoneal fibrosis, and cancer.
I perform IVP approximately 1 week after stent removal to ensure ureteral patency.
CASE RESOLVED
Exposure is improved by widening the incision and dividing the tendonous insertions of the rectus abdominus muscles. The surgeon then removes the mass, preserving the distal ureter, which is estimated to be 12 cm in length and to have intact adventitia.
The surgeon performs a double-spatulated end-to-end ureteroureterostomy over a 6 French double-J ureteral stent that has been passed proximally into the renal pelvis and distally into the bladder. The stent is removed 6 weeks postoperatively, and an IVP the following week demonstrates excellent patency.
The majority of payers consider ureterolysis integral to good surgical technique, but there can be exceptions when documentation supports existing codes. Three CPT codes describe this procedure:
50715 Ureterolysis, with or without repositioning of ureter for retroperitoneal fibrosis
50722 Ureterolysis for ovarian vein syndrome
50725 Ureterolysis for retrocaval ureter, with reanastomosis of upper urinary tract or vena cava
The key to getting paid will be to document the existence of the condition indicated by each of the codes.
The ICD-9 code for both retroperitoneal fibrosis and ovarian vein syndrome is the same, 593.4 (Other ureteric obstruction). If the patient requires ureterolysis for a retrocaval ureter, the code 753.4 (Other specified anomalies of ureter) would be reported instead. Note, however, that these procedure codes cannot be reported if the ureterolysis is performed laparoscopically. In that case, the most appropriate code is 50949 (Unlisted laparoscopy procedure, ureter).
When repair is necessary, you have several codes to choose from, but the supporting diagnosis code 998.2 (Accidental puncture or laceration during a procedure) must be indicated. If a Medicare patient is involved, the surgeon who created the injury would not be paid additionally for repair.
50780 Ureteroneocystostomy; anastomosis of single ureter to bladder
50782 Ureteroneocystostomy; anastomosis of duplicated ureter to bladder
50783 Ureteroneocystostomy; with extensive ureteral tailoring
50785 Ureteroneocystostomy; with vesico-psoas hitch or bladder flap
50760 Ureteroureterostomy; fusion of ureters
50770 Transureteroureterostomy, anastomosis of ureter to contralateral ureter—MELANIE WITT, RN, CPC-OBGYN, MA
1. St. Lezin MA, Stoller ML. Surgical ureteral injuries. Urology. 1991;38:497-506.
2. Liapis A, Bakas P, Giannopoulos V, Creatsas G. Ureteral injuries during gynecological surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:391-394.
3. Vakili B, Chesson RR, Kyle BL, et al. The incidence of urinary tract injury during hysterectomy: a prospective analysis based on universal cystoscopy. Am J Obstet Gynecol. 2005;192:1599-1604.
4. Sakellariou P, Protopapas AG, Voulgaris Z, et al. Management of ureteric injuries during gynecological operations: 10 years experience. Eur J Obstet Gynecol Reprod Biol. 2002;101:179-184.
5. Assimos DG, Patterson LC, Taylor CL. Changing incidence and etiology of iatrogenic ureteral injuries. J Urol. 1994;152:2240-2246.
6. Härkki-Sirén P, Sjöberg J, Titinen A. Urinary tract injuries after hysterectomy. Obstet Gynecol. 1998;92:113-118.
7. Chan JK, Morrow J, Manetta A. Prevention of ureteral injuries in gynecologic surgery. Am J Obstet Gynecol. 2003;188:1273-1277.
The author has no financial relationships relevant to this article.
CASE: Inadvertent ureteral transection
A gynecologic surgeon operates via Pfannenstiel incision to remove a 12-cm complex left adnexal mass from a 36-year-old obese woman. When she discovers that the mass is densely adherent to the pelvic peritoneum, the surgeon incises the peritoneum lateral to the mass and opens the retroperitoneal space. However, the size and relative immobility of the mass, coupled with the low transverse incision, impair visualization of retroperitoneal structures.
The surgeon clamps and divides the ovarian vessels above the mass but, afterward, suspects that the ureter has been transected and that its ends are included within the clamps. She separates the ovarian vessels above the clamp and ligates them, at which time transection of the ureter is confirmed.
How should she proceed?
The ureter is intimately associated with the female internal genitalia in a way that challenges the gynecologic surgeon to avoid it. In a small percentage of cases involving surgical extirpation in a woman who has severe pelvic pathology, ureteral injury may be inevitable.
Several variables predispose a patient to ureteral injury, including limited exposure, as in the opening case. Others include distorted anatomy of the urinary tract relative to internal genitalia and operations that require extensive resection of pelvic tissues.
This article describes:
- prevention and intraoperative recognition of ureteral injury during gynecologic surgery
- management of intraoperatively recognized ureteral injury.
Maintain a high index of suspicion
The surgeon in the opening case has already taken the first and most important step in ensuring a good outcome: She suspected ureteral injury. In high-risk situations, intraoperative recognition of ureteral injury is more likely when the operative field is inspected thoroughly during and at the conclusion of the surgical procedure.
In a high-risk case, the combined use of intravenous indigo carmine, careful inspection of the operative field, cystoscopy, and ureteral dissection is recommended and should be routine.
Common sites of injury
During gynecologic surgery, the ureter is susceptible to injury along its entire course through the pelvis (see “The ureter takes a course fraught with hazard,”).
During adnexectomy, the gonadal vessels are generally ligated 2 to 3 cm above the adnexa. The ureter lies in close proximity to these vessels and may inadvertently be included in the ligation.
During hysterectomy, the ureter is susceptible to injury as it passes through the parametrium a short distance from the uterus and vaginal fornix.
Sutures placed in the posterior lateral cul de sac during prolapse surgery lie near the midpelvic ureter, and sutures placed during vaginal cuff closure, anterior colporrhaphy, and retropubic urethropexy are in close proximity to the trigonal portion of the ureter.
The ureter extends from the renal pelvis to the bladder, with a length that ranges from 25 to 30 cm, depending on the patient’s height. It crosses the pelvic brim near the bifurcation of the common iliac artery, where it becomes the “pelvic” ureter. The abdominal and pelvic portions of the ureter are approximately equal in length.
ILLUSTRATIONS BY ROB FLEWELL FOR OBG MANAGEMENT
The blood supply of the ureter derives from branches of the major arterial system of the lower abdomen and pelvis. These branches reach the medial aspect of the abdominal ureter and the lateral side of the pelvic ureter to form an anastomotic vascular network protected by an adventitial layer surrounding the ureter.
The ureter is attached to the posterior lateral pelvic peritoneum running dorsal to ovarian vessels. At the midpelvis, it separates from the peritoneum to pierce the base of the broad ligament underneath the uterine artery. At this point, the ureter is about 1.5 to 2 cm lateral to the uterus and curves medially and ventrally, tunneling through the cardinal and vesicovaginal ligaments to enter the bladder trigone.
Risky procedures
In gynecologic surgery, ureteral injury occurs most often during abdominal hysterectomy—probably because of how frequently this operation is performed and the range of pathology managed. The incidence of ureteral injury is much higher during abdominal hysterectomy than vaginal hysterectomy.1-4
Laparoscopic hysterectomy also has been associated with a higher incidence of ureteral injury, especially in the early phase of training.5,6 Possible explanations include:
- greater difficulty identifying the ureter
- a steeper learning curve
- more frequent use of energy to hemostatically divide pedicles, with the potential for thermal injury
- less traction–countertraction, resulting in dissection closer to the ureter
- management of complex pathology.
Although the overall incidence of ureteral injury during adnexectomy is low, it is probably much higher in women undergoing this procedure after a previous hysterectomy or in the presence of complex adnexal pathology.
When injury is likely
Compromised exposure, distorted anatomy, and certain procedures can heighten the risk of ureteral injury. Large tumors may limit the ability of the surgeon to visualize or palpate the ureter (FIGURE 1). Extensive adhesions may cause similar difficulties, and a small incision or obesity may hinder identification of pelvic sidewall structures.
A number of pathologic conditions can distort the anatomy of the ureter, especially as it relates to the female genital tract:
- Malignancies such as ovarian cancer often encroach on and occasionally encase the ureter
- Pelvic inflammatory disease, endometriosis, and a history of surgery or pelvic radiotherapy can retract and encase the ureter toward the gynecologic tract
- Some masses expand against the lower ureter, such as cervical or broad-ligament leiomyomata or placenta previa with accreta
- During vaginal hysterectomy for complete uterine prolapse, the ureters frequently extend beyond the introitus well within the operative field
- Congenital anomalies of the ureter or hydroureter can also cause distortion.
Even in the presence of relatively normal anatomy, certain procedures predispose the ureter to injury. For example, radical hysterectomy involves the almost complete separation of the pelvic ureter from the gynecologic tract and its surrounding soft tissue. When pelvic pathology is significant, the plane of dissection will always be near the ureter.
FIGURE 1 Access to the ureter is obstructed, putting it in jeopardy
Large tumors may limit the ability of the surgeon to visualize or palpate the ureter.
Prevention is the best strategy
At least 50% of ureteral injuries reported during gynecologic surgery have occurred in the absence of a recognizable risk factor.2,7 Nevertheless, knowledge of anatomy and the ability to recognize situations in which there is an elevated risk for ureteral injury will best enable the surgeon to prevent such injury.
When a high-risk situation is encountered, critical preventive steps include:
- adequate exposure
- competent assistance
- exposure of the path of the ureter through the planned course of dissection. Dissecting the ureter beyond this area is usually unnecessary and may itself cause injury.
Skip preoperative IVP in most cases
The vast majority of women who undergo gynecologic surgery do not benefit from preoperative intravenous pyelography (IVP). This measure does not appear to reduce the likelihood of ureteral injury, even in the face of obvious gynecologic disease. However, preoperative identification of obvious ureteral involvement by the disease process is useful. In such cases, the plane of dissection will probably lie closer to the ureter. One of the goals of surgery will then be to clear the urinary tract from the affected area.
When there is a high index of suspicion of an abnormality such as obstruction, intrinsic ureteral endometriosis, or congenital anomaly, preoperative IVP is indicated.
A stent may be helpful in some cases
Ureteral stents are sometimes placed in order to aid in identification and dissection of the ureters during surgery. Some authors of reports on this topic, including Hoffman, believe that stents are useful in certain situations, such as excision of an ovarian remnant, radical vaginal hysterectomy, and when pelvic organs are encased by malignant ovarian tumors. However, stents do not clearly reduce the risk of injury and, in some cases, may increase the risk by providing a false sense of security and predisposing the ureter to adventitial injury during difficult dissection.
Anticipate the effects of disease
The surgeon must have a thorough knowledge of the gynecologic disease process as it relates to surgery involving the urinary tract. For example, an ovarian remnant will almost always be somewhat densely adherent to the pelvic ureter. When severe endometriosis involves the posterior leaf of the broad ligament, the ureter will often be fibrotically retracted toward the operative field.
Certain procedures have special challenges. During resection of adnexa, for example, it is important that the ureter be identified in the retroperitoneum before the ovarian vessels are ligated. During hysterectomy, soft tissues that contain the bladder and ureters should be mobilized caudally and laterally, respectively, creating a U-shaped region (“U” for urinary tract, FIGURE 2) to which the surgeon must limit dissection.
FIGURE 2 During hysterectomy, mobilize the bladder and ureter
Mobilize the soft tissues that contain the bladder and ureters caudally and laterally, respectively, creating a U-shaped region. During division of the paracervical tissues, the surgeon must remain within this region.
Intraoperative detection
Two main types of ureteral injury occur during gynecologic surgery: transection and destruction. The latter includes ligation, crushing, devascularization, and thermal injury.
Intraoperative detection of ureteral injury is more likely when the surgeon recognizes at the outset that the operation places the ureter at increased risk. When dissection has been difficult or complicated for any reason, be concerned about possible injury.
In general, ureteral injury is first recognized by careful inspection of the surgical field. Begin by instilling 5 ml of indigo carmine intravenously. Once the dye begins to appear in the Foley catheter, inspect the area of dissection under a small amount of irrigation fluid, looking for extravasation of dye that indicates partial or complete transection.
If no injury is identified, cystoscopy is the next step. I perform all major abdominal operations with the patient in the low lithotomy position, which provides easy access to the perineum. Cystoscopic identification of urine jetting from both ureteral orifices confirms patency. When only wisps of dye are observed, it is likely that the ureter in question has been partially occluded (e.g., by acute angulation). Failure of any urine to appear from one of the orifices highly suggests injury to that ureter.
During inspection of the operative field, attempt to pass a ureteral stent into the affected orifice. If the stent passes easily and dyed urine is seen to drip freely from it, look for possible angulation of the ureter. If you find none, remove the stent and inspect the orifice again for jetting urine.
If the ureteral stent will move only a few centimeters into the ureteral orifice, ligation (with or without transection) is likely. In this case, leave the stent in place. If the operative site is readily accessible, dissect the applicable area to identify the problem. Depending on the circumstances, you may wish to infuse dye through the stent to aid in operative identification or radiographic evaluation.
Intraoperative IVP may be useful, especially when cystoscopy is unavailable.
Fundamentals of repair
Repair of major injury to the pelvic ureter is generally best accomplished by ureteroneocystostomy or, in selected cases involving injury to the proximal pelvic ureter, by ureteroureterostomy.
When intraoperatively recognized injury to the pelvic ureter appears to be minor, it can be managed by placing a ureteral stent and a closed-suction pelvic drain. Also consider wrapping the injured area with vascularized tissue such as perivesical fat. Minor lacerations can be closed perpendicular to the axis of the ureter using interrupted 4-0 delayed absorbable suture.
Most injuries to the pelvic ureter are optimally managed by ureteroneocystostomy (FIGURE 3). When a significant portion of the pelvic ureter has been lost, ureteroneocystostomy usually requires a combination of:
- extensive mobilization of the bladder
- conservative mobilization of the ureter
- elongation of the bladder
- psoas hitch.
When necessary, mobilization of the kidney with suturing of the caudal perinephric fascia to the psoas muscle will bridge an additional 2- to 3-cm gap.
Major injury to the distal half of the pelvic ureter is repaired using straightforward ureteroneocystostomy.
When there is no significant pelvic disease and the distal ureter is healthy, injury to the proximal pelvic ureter during division of the ovarian vessels may be repaired via ureteroureterostomy. If the ureteral ends will be anastomosed on tension or there is any question about the integrity of the distal portion of the ureter, as when extensive distal ureterolysis has been necessary, consider ureteroneocystostomy.
FIGURE 3 When the distal ureter is injured
Most injuries to the pelvic ureter are managed optimally by ureteroneocystostomy.
Ureter injured during emergent hysterectomy
A 37-year-old woman, para 4, undergoes her fourth repeat cesarean section. When the OB attempts to manually extract the placenta, the patient begins to hemorrhage profusely. Conservative measures fail to stop the bleeding, and the patient becomes hypotensive. The physician performs emergent hysterectomy, taking large pedicles of tissue. Although the patient stabilizes, the doctor worries that the ureters may have been injured.
Resolution: Cystoscopy is performed to check for injury. Because indigo carmine does not spill from the left ureteral orifice, the physician passes a stent with the abdomen still open, and it stops within the most distal ligamentous pedicle. Upon deligation, indigo carmine begins to drain from the stent, which then passes easily.
The stent is withdrawn to below the site of injury, and dilute methylene blue is instilled through it while the ureter is observed under irrigation. No extravasation is noted. Because the ligature had been around a block of tissue that was thought to have acutely angulated rather than incorporated the ureter, the physician concludes that severe damage is unlikely. He places a 6 French double-J stent, wraps the damaged portion of the distal ureter in perivesical fat, and places a closed-suction pelvic drain. Healing is uneventful.
Obstruction is confirmed. Now the surgeon must find it
A 45-year-old woman, para 3, who has a symptomatic 14-weeks’ size myomatous uterus, undergoes vaginal hysterectomy. The surgeon ligates and divides the uterine vessel pedicles before beginning morcellation. At the completion of the procedure, during cystoscopy, indigo carmine fails to spill from the right ureteral orifice, suggesting injury to that ureter. The surgeon passes a stent into the ureter, and it stops approximately 6 cm from the orifice. A retrograde pyelogram confirms complete obstruction.
Resolution: With the stent left in place, the surgeon performs a midline laparotomy, tracing the ureter to the uterine artery pedicle in which it has been incorporated and transected. The distal ureter with the stent is found within soft tissue lateral to the cardinal ligament pedicle, and the transected end is securely ligated using 2–0 silk suture. After the bladder is mobilized, a ureteroneocystostomy is performed. The patient recovers fully.
Postoperative management
After repair of a ureteral injury, leave a closed-suction pelvic drain in place for 2 to 3 days so that any major urinary leak can be detected; it also enhances spontaneous closure and helps prevent potentially infected fluid from accumulating in the region of anastomosis.
The cystotomy performed during ureteroneocystostomy generally heals quickly with a low risk of complications.
Leave a large-bore (20 or 22 French) urethral Foley catheter in place for 2 weeks.
I recommend that a 6 French double-J ureteral stent be left in place for 6 weeks. Potential benefits of the stent include:
- prevention of stricture
- stabilization and immobilization of the ureter during healing
- reduced risk of extravasation of urine
- reduced risk of angulation of the ureter
- isolation of the repair from infection, retroperitoneal fibrosis, and cancer.
I perform IVP approximately 1 week after stent removal to ensure ureteral patency.
CASE RESOLVED
Exposure is improved by widening the incision and dividing the tendonous insertions of the rectus abdominus muscles. The surgeon then removes the mass, preserving the distal ureter, which is estimated to be 12 cm in length and to have intact adventitia.
The surgeon performs a double-spatulated end-to-end ureteroureterostomy over a 6 French double-J ureteral stent that has been passed proximally into the renal pelvis and distally into the bladder. The stent is removed 6 weeks postoperatively, and an IVP the following week demonstrates excellent patency.
The majority of payers consider ureterolysis integral to good surgical technique, but there can be exceptions when documentation supports existing codes. Three CPT codes describe this procedure:
50715 Ureterolysis, with or without repositioning of ureter for retroperitoneal fibrosis
50722 Ureterolysis for ovarian vein syndrome
50725 Ureterolysis for retrocaval ureter, with reanastomosis of upper urinary tract or vena cava
The key to getting paid will be to document the existence of the condition indicated by each of the codes.
The ICD-9 code for both retroperitoneal fibrosis and ovarian vein syndrome is the same, 593.4 (Other ureteric obstruction). If the patient requires ureterolysis for a retrocaval ureter, the code 753.4 (Other specified anomalies of ureter) would be reported instead. Note, however, that these procedure codes cannot be reported if the ureterolysis is performed laparoscopically. In that case, the most appropriate code is 50949 (Unlisted laparoscopy procedure, ureter).
When repair is necessary, you have several codes to choose from, but the supporting diagnosis code 998.2 (Accidental puncture or laceration during a procedure) must be indicated. If a Medicare patient is involved, the surgeon who created the injury would not be paid additionally for repair.
50780 Ureteroneocystostomy; anastomosis of single ureter to bladder
50782 Ureteroneocystostomy; anastomosis of duplicated ureter to bladder
50783 Ureteroneocystostomy; with extensive ureteral tailoring
50785 Ureteroneocystostomy; with vesico-psoas hitch or bladder flap
50760 Ureteroureterostomy; fusion of ureters
50770 Transureteroureterostomy, anastomosis of ureter to contralateral ureter—MELANIE WITT, RN, CPC-OBGYN, MA
The author has no financial relationships relevant to this article.
CASE: Inadvertent ureteral transection
A gynecologic surgeon operates via Pfannenstiel incision to remove a 12-cm complex left adnexal mass from a 36-year-old obese woman. When she discovers that the mass is densely adherent to the pelvic peritoneum, the surgeon incises the peritoneum lateral to the mass and opens the retroperitoneal space. However, the size and relative immobility of the mass, coupled with the low transverse incision, impair visualization of retroperitoneal structures.
The surgeon clamps and divides the ovarian vessels above the mass but, afterward, suspects that the ureter has been transected and that its ends are included within the clamps. She separates the ovarian vessels above the clamp and ligates them, at which time transection of the ureter is confirmed.
How should she proceed?
The ureter is intimately associated with the female internal genitalia in a way that challenges the gynecologic surgeon to avoid it. In a small percentage of cases involving surgical extirpation in a woman who has severe pelvic pathology, ureteral injury may be inevitable.
Several variables predispose a patient to ureteral injury, including limited exposure, as in the opening case. Others include distorted anatomy of the urinary tract relative to internal genitalia and operations that require extensive resection of pelvic tissues.
This article describes:
- prevention and intraoperative recognition of ureteral injury during gynecologic surgery
- management of intraoperatively recognized ureteral injury.
Maintain a high index of suspicion
The surgeon in the opening case has already taken the first and most important step in ensuring a good outcome: She suspected ureteral injury. In high-risk situations, intraoperative recognition of ureteral injury is more likely when the operative field is inspected thoroughly during and at the conclusion of the surgical procedure.
In a high-risk case, the combined use of intravenous indigo carmine, careful inspection of the operative field, cystoscopy, and ureteral dissection is recommended and should be routine.
Common sites of injury
During gynecologic surgery, the ureter is susceptible to injury along its entire course through the pelvis (see “The ureter takes a course fraught with hazard,”).
During adnexectomy, the gonadal vessels are generally ligated 2 to 3 cm above the adnexa. The ureter lies in close proximity to these vessels and may inadvertently be included in the ligation.
During hysterectomy, the ureter is susceptible to injury as it passes through the parametrium a short distance from the uterus and vaginal fornix.
Sutures placed in the posterior lateral cul de sac during prolapse surgery lie near the midpelvic ureter, and sutures placed during vaginal cuff closure, anterior colporrhaphy, and retropubic urethropexy are in close proximity to the trigonal portion of the ureter.
The ureter extends from the renal pelvis to the bladder, with a length that ranges from 25 to 30 cm, depending on the patient’s height. It crosses the pelvic brim near the bifurcation of the common iliac artery, where it becomes the “pelvic” ureter. The abdominal and pelvic portions of the ureter are approximately equal in length.
ILLUSTRATIONS BY ROB FLEWELL FOR OBG MANAGEMENT
The blood supply of the ureter derives from branches of the major arterial system of the lower abdomen and pelvis. These branches reach the medial aspect of the abdominal ureter and the lateral side of the pelvic ureter to form an anastomotic vascular network protected by an adventitial layer surrounding the ureter.
The ureter is attached to the posterior lateral pelvic peritoneum running dorsal to ovarian vessels. At the midpelvis, it separates from the peritoneum to pierce the base of the broad ligament underneath the uterine artery. At this point, the ureter is about 1.5 to 2 cm lateral to the uterus and curves medially and ventrally, tunneling through the cardinal and vesicovaginal ligaments to enter the bladder trigone.
Risky procedures
In gynecologic surgery, ureteral injury occurs most often during abdominal hysterectomy—probably because of how frequently this operation is performed and the range of pathology managed. The incidence of ureteral injury is much higher during abdominal hysterectomy than vaginal hysterectomy.1-4
Laparoscopic hysterectomy also has been associated with a higher incidence of ureteral injury, especially in the early phase of training.5,6 Possible explanations include:
- greater difficulty identifying the ureter
- a steeper learning curve
- more frequent use of energy to hemostatically divide pedicles, with the potential for thermal injury
- less traction–countertraction, resulting in dissection closer to the ureter
- management of complex pathology.
Although the overall incidence of ureteral injury during adnexectomy is low, it is probably much higher in women undergoing this procedure after a previous hysterectomy or in the presence of complex adnexal pathology.
When injury is likely
Compromised exposure, distorted anatomy, and certain procedures can heighten the risk of ureteral injury. Large tumors may limit the ability of the surgeon to visualize or palpate the ureter (FIGURE 1). Extensive adhesions may cause similar difficulties, and a small incision or obesity may hinder identification of pelvic sidewall structures.
A number of pathologic conditions can distort the anatomy of the ureter, especially as it relates to the female genital tract:
- Malignancies such as ovarian cancer often encroach on and occasionally encase the ureter
- Pelvic inflammatory disease, endometriosis, and a history of surgery or pelvic radiotherapy can retract and encase the ureter toward the gynecologic tract
- Some masses expand against the lower ureter, such as cervical or broad-ligament leiomyomata or placenta previa with accreta
- During vaginal hysterectomy for complete uterine prolapse, the ureters frequently extend beyond the introitus well within the operative field
- Congenital anomalies of the ureter or hydroureter can also cause distortion.
Even in the presence of relatively normal anatomy, certain procedures predispose the ureter to injury. For example, radical hysterectomy involves the almost complete separation of the pelvic ureter from the gynecologic tract and its surrounding soft tissue. When pelvic pathology is significant, the plane of dissection will always be near the ureter.
FIGURE 1 Access to the ureter is obstructed, putting it in jeopardy
Large tumors may limit the ability of the surgeon to visualize or palpate the ureter.
Prevention is the best strategy
At least 50% of ureteral injuries reported during gynecologic surgery have occurred in the absence of a recognizable risk factor.2,7 Nevertheless, knowledge of anatomy and the ability to recognize situations in which there is an elevated risk for ureteral injury will best enable the surgeon to prevent such injury.
When a high-risk situation is encountered, critical preventive steps include:
- adequate exposure
- competent assistance
- exposure of the path of the ureter through the planned course of dissection. Dissecting the ureter beyond this area is usually unnecessary and may itself cause injury.
Skip preoperative IVP in most cases
The vast majority of women who undergo gynecologic surgery do not benefit from preoperative intravenous pyelography (IVP). This measure does not appear to reduce the likelihood of ureteral injury, even in the face of obvious gynecologic disease. However, preoperative identification of obvious ureteral involvement by the disease process is useful. In such cases, the plane of dissection will probably lie closer to the ureter. One of the goals of surgery will then be to clear the urinary tract from the affected area.
When there is a high index of suspicion of an abnormality such as obstruction, intrinsic ureteral endometriosis, or congenital anomaly, preoperative IVP is indicated.
A stent may be helpful in some cases
Ureteral stents are sometimes placed in order to aid in identification and dissection of the ureters during surgery. Some authors of reports on this topic, including Hoffman, believe that stents are useful in certain situations, such as excision of an ovarian remnant, radical vaginal hysterectomy, and when pelvic organs are encased by malignant ovarian tumors. However, stents do not clearly reduce the risk of injury and, in some cases, may increase the risk by providing a false sense of security and predisposing the ureter to adventitial injury during difficult dissection.
Anticipate the effects of disease
The surgeon must have a thorough knowledge of the gynecologic disease process as it relates to surgery involving the urinary tract. For example, an ovarian remnant will almost always be somewhat densely adherent to the pelvic ureter. When severe endometriosis involves the posterior leaf of the broad ligament, the ureter will often be fibrotically retracted toward the operative field.
Certain procedures have special challenges. During resection of adnexa, for example, it is important that the ureter be identified in the retroperitoneum before the ovarian vessels are ligated. During hysterectomy, soft tissues that contain the bladder and ureters should be mobilized caudally and laterally, respectively, creating a U-shaped region (“U” for urinary tract, FIGURE 2) to which the surgeon must limit dissection.
FIGURE 2 During hysterectomy, mobilize the bladder and ureter
Mobilize the soft tissues that contain the bladder and ureters caudally and laterally, respectively, creating a U-shaped region. During division of the paracervical tissues, the surgeon must remain within this region.
Intraoperative detection
Two main types of ureteral injury occur during gynecologic surgery: transection and destruction. The latter includes ligation, crushing, devascularization, and thermal injury.
Intraoperative detection of ureteral injury is more likely when the surgeon recognizes at the outset that the operation places the ureter at increased risk. When dissection has been difficult or complicated for any reason, be concerned about possible injury.
In general, ureteral injury is first recognized by careful inspection of the surgical field. Begin by instilling 5 ml of indigo carmine intravenously. Once the dye begins to appear in the Foley catheter, inspect the area of dissection under a small amount of irrigation fluid, looking for extravasation of dye that indicates partial or complete transection.
If no injury is identified, cystoscopy is the next step. I perform all major abdominal operations with the patient in the low lithotomy position, which provides easy access to the perineum. Cystoscopic identification of urine jetting from both ureteral orifices confirms patency. When only wisps of dye are observed, it is likely that the ureter in question has been partially occluded (e.g., by acute angulation). Failure of any urine to appear from one of the orifices highly suggests injury to that ureter.
During inspection of the operative field, attempt to pass a ureteral stent into the affected orifice. If the stent passes easily and dyed urine is seen to drip freely from it, look for possible angulation of the ureter. If you find none, remove the stent and inspect the orifice again for jetting urine.
If the ureteral stent will move only a few centimeters into the ureteral orifice, ligation (with or without transection) is likely. In this case, leave the stent in place. If the operative site is readily accessible, dissect the applicable area to identify the problem. Depending on the circumstances, you may wish to infuse dye through the stent to aid in operative identification or radiographic evaluation.
Intraoperative IVP may be useful, especially when cystoscopy is unavailable.
Fundamentals of repair
Repair of major injury to the pelvic ureter is generally best accomplished by ureteroneocystostomy or, in selected cases involving injury to the proximal pelvic ureter, by ureteroureterostomy.
When intraoperatively recognized injury to the pelvic ureter appears to be minor, it can be managed by placing a ureteral stent and a closed-suction pelvic drain. Also consider wrapping the injured area with vascularized tissue such as perivesical fat. Minor lacerations can be closed perpendicular to the axis of the ureter using interrupted 4-0 delayed absorbable suture.
Most injuries to the pelvic ureter are optimally managed by ureteroneocystostomy (FIGURE 3). When a significant portion of the pelvic ureter has been lost, ureteroneocystostomy usually requires a combination of:
- extensive mobilization of the bladder
- conservative mobilization of the ureter
- elongation of the bladder
- psoas hitch.
When necessary, mobilization of the kidney with suturing of the caudal perinephric fascia to the psoas muscle will bridge an additional 2- to 3-cm gap.
Major injury to the distal half of the pelvic ureter is repaired using straightforward ureteroneocystostomy.
When there is no significant pelvic disease and the distal ureter is healthy, injury to the proximal pelvic ureter during division of the ovarian vessels may be repaired via ureteroureterostomy. If the ureteral ends will be anastomosed on tension or there is any question about the integrity of the distal portion of the ureter, as when extensive distal ureterolysis has been necessary, consider ureteroneocystostomy.
FIGURE 3 When the distal ureter is injured
Most injuries to the pelvic ureter are managed optimally by ureteroneocystostomy.
Ureter injured during emergent hysterectomy
A 37-year-old woman, para 4, undergoes her fourth repeat cesarean section. When the OB attempts to manually extract the placenta, the patient begins to hemorrhage profusely. Conservative measures fail to stop the bleeding, and the patient becomes hypotensive. The physician performs emergent hysterectomy, taking large pedicles of tissue. Although the patient stabilizes, the doctor worries that the ureters may have been injured.
Resolution: Cystoscopy is performed to check for injury. Because indigo carmine does not spill from the left ureteral orifice, the physician passes a stent with the abdomen still open, and it stops within the most distal ligamentous pedicle. Upon deligation, indigo carmine begins to drain from the stent, which then passes easily.
The stent is withdrawn to below the site of injury, and dilute methylene blue is instilled through it while the ureter is observed under irrigation. No extravasation is noted. Because the ligature had been around a block of tissue that was thought to have acutely angulated rather than incorporated the ureter, the physician concludes that severe damage is unlikely. He places a 6 French double-J stent, wraps the damaged portion of the distal ureter in perivesical fat, and places a closed-suction pelvic drain. Healing is uneventful.
Obstruction is confirmed. Now the surgeon must find it
A 45-year-old woman, para 3, who has a symptomatic 14-weeks’ size myomatous uterus, undergoes vaginal hysterectomy. The surgeon ligates and divides the uterine vessel pedicles before beginning morcellation. At the completion of the procedure, during cystoscopy, indigo carmine fails to spill from the right ureteral orifice, suggesting injury to that ureter. The surgeon passes a stent into the ureter, and it stops approximately 6 cm from the orifice. A retrograde pyelogram confirms complete obstruction.
Resolution: With the stent left in place, the surgeon performs a midline laparotomy, tracing the ureter to the uterine artery pedicle in which it has been incorporated and transected. The distal ureter with the stent is found within soft tissue lateral to the cardinal ligament pedicle, and the transected end is securely ligated using 2–0 silk suture. After the bladder is mobilized, a ureteroneocystostomy is performed. The patient recovers fully.
Postoperative management
After repair of a ureteral injury, leave a closed-suction pelvic drain in place for 2 to 3 days so that any major urinary leak can be detected; it also enhances spontaneous closure and helps prevent potentially infected fluid from accumulating in the region of anastomosis.
The cystotomy performed during ureteroneocystostomy generally heals quickly with a low risk of complications.
Leave a large-bore (20 or 22 French) urethral Foley catheter in place for 2 weeks.
I recommend that a 6 French double-J ureteral stent be left in place for 6 weeks. Potential benefits of the stent include:
- prevention of stricture
- stabilization and immobilization of the ureter during healing
- reduced risk of extravasation of urine
- reduced risk of angulation of the ureter
- isolation of the repair from infection, retroperitoneal fibrosis, and cancer.
I perform IVP approximately 1 week after stent removal to ensure ureteral patency.
CASE RESOLVED
Exposure is improved by widening the incision and dividing the tendonous insertions of the rectus abdominus muscles. The surgeon then removes the mass, preserving the distal ureter, which is estimated to be 12 cm in length and to have intact adventitia.
The surgeon performs a double-spatulated end-to-end ureteroureterostomy over a 6 French double-J ureteral stent that has been passed proximally into the renal pelvis and distally into the bladder. The stent is removed 6 weeks postoperatively, and an IVP the following week demonstrates excellent patency.
The majority of payers consider ureterolysis integral to good surgical technique, but there can be exceptions when documentation supports existing codes. Three CPT codes describe this procedure:
50715 Ureterolysis, with or without repositioning of ureter for retroperitoneal fibrosis
50722 Ureterolysis for ovarian vein syndrome
50725 Ureterolysis for retrocaval ureter, with reanastomosis of upper urinary tract or vena cava
The key to getting paid will be to document the existence of the condition indicated by each of the codes.
The ICD-9 code for both retroperitoneal fibrosis and ovarian vein syndrome is the same, 593.4 (Other ureteric obstruction). If the patient requires ureterolysis for a retrocaval ureter, the code 753.4 (Other specified anomalies of ureter) would be reported instead. Note, however, that these procedure codes cannot be reported if the ureterolysis is performed laparoscopically. In that case, the most appropriate code is 50949 (Unlisted laparoscopy procedure, ureter).
When repair is necessary, you have several codes to choose from, but the supporting diagnosis code 998.2 (Accidental puncture or laceration during a procedure) must be indicated. If a Medicare patient is involved, the surgeon who created the injury would not be paid additionally for repair.
50780 Ureteroneocystostomy; anastomosis of single ureter to bladder
50782 Ureteroneocystostomy; anastomosis of duplicated ureter to bladder
50783 Ureteroneocystostomy; with extensive ureteral tailoring
50785 Ureteroneocystostomy; with vesico-psoas hitch or bladder flap
50760 Ureteroureterostomy; fusion of ureters
50770 Transureteroureterostomy, anastomosis of ureter to contralateral ureter—MELANIE WITT, RN, CPC-OBGYN, MA
1. St. Lezin MA, Stoller ML. Surgical ureteral injuries. Urology. 1991;38:497-506.
2. Liapis A, Bakas P, Giannopoulos V, Creatsas G. Ureteral injuries during gynecological surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:391-394.
3. Vakili B, Chesson RR, Kyle BL, et al. The incidence of urinary tract injury during hysterectomy: a prospective analysis based on universal cystoscopy. Am J Obstet Gynecol. 2005;192:1599-1604.
4. Sakellariou P, Protopapas AG, Voulgaris Z, et al. Management of ureteric injuries during gynecological operations: 10 years experience. Eur J Obstet Gynecol Reprod Biol. 2002;101:179-184.
5. Assimos DG, Patterson LC, Taylor CL. Changing incidence and etiology of iatrogenic ureteral injuries. J Urol. 1994;152:2240-2246.
6. Härkki-Sirén P, Sjöberg J, Titinen A. Urinary tract injuries after hysterectomy. Obstet Gynecol. 1998;92:113-118.
7. Chan JK, Morrow J, Manetta A. Prevention of ureteral injuries in gynecologic surgery. Am J Obstet Gynecol. 2003;188:1273-1277.
1. St. Lezin MA, Stoller ML. Surgical ureteral injuries. Urology. 1991;38:497-506.
2. Liapis A, Bakas P, Giannopoulos V, Creatsas G. Ureteral injuries during gynecological surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:391-394.
3. Vakili B, Chesson RR, Kyle BL, et al. The incidence of urinary tract injury during hysterectomy: a prospective analysis based on universal cystoscopy. Am J Obstet Gynecol. 2005;192:1599-1604.
4. Sakellariou P, Protopapas AG, Voulgaris Z, et al. Management of ureteric injuries during gynecological operations: 10 years experience. Eur J Obstet Gynecol Reprod Biol. 2002;101:179-184.
5. Assimos DG, Patterson LC, Taylor CL. Changing incidence and etiology of iatrogenic ureteral injuries. J Urol. 1994;152:2240-2246.
6. Härkki-Sirén P, Sjöberg J, Titinen A. Urinary tract injuries after hysterectomy. Obstet Gynecol. 1998;92:113-118.
7. Chan JK, Morrow J, Manetta A. Prevention of ureteral injuries in gynecologic surgery. Am J Obstet Gynecol. 2003;188:1273-1277.