User login
How to safeguard the ureter and repair surgical injury
The author has no financial relationships relevant to this article.
CASE: Inadvertent ureteral transection
A gynecologic surgeon operates via Pfannenstiel incision to remove a 12-cm complex left adnexal mass from a 36-year-old obese woman. When she discovers that the mass is densely adherent to the pelvic peritoneum, the surgeon incises the peritoneum lateral to the mass and opens the retroperitoneal space. However, the size and relative immobility of the mass, coupled with the low transverse incision, impair visualization of retroperitoneal structures.
The surgeon clamps and divides the ovarian vessels above the mass but, afterward, suspects that the ureter has been transected and that its ends are included within the clamps. She separates the ovarian vessels above the clamp and ligates them, at which time transection of the ureter is confirmed.
How should she proceed?
The ureter is intimately associated with the female internal genitalia in a way that challenges the gynecologic surgeon to avoid it. In a small percentage of cases involving surgical extirpation in a woman who has severe pelvic pathology, ureteral injury may be inevitable.
Several variables predispose a patient to ureteral injury, including limited exposure, as in the opening case. Others include distorted anatomy of the urinary tract relative to internal genitalia and operations that require extensive resection of pelvic tissues.
This article describes:
- prevention and intraoperative recognition of ureteral injury during gynecologic surgery
- management of intraoperatively recognized ureteral injury.
Maintain a high index of suspicion
The surgeon in the opening case has already taken the first and most important step in ensuring a good outcome: She suspected ureteral injury. In high-risk situations, intraoperative recognition of ureteral injury is more likely when the operative field is inspected thoroughly during and at the conclusion of the surgical procedure.
In a high-risk case, the combined use of intravenous indigo carmine, careful inspection of the operative field, cystoscopy, and ureteral dissection is recommended and should be routine.
Common sites of injury
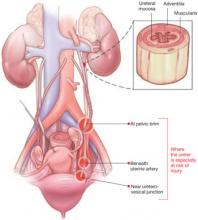
During gynecologic surgery, the ureter is susceptible to injury along its entire course through the pelvis (see “The ureter takes a course fraught with hazard,”).
During adnexectomy, the gonadal vessels are generally ligated 2 to 3 cm above the adnexa. The ureter lies in close proximity to these vessels and may inadvertently be included in the ligation.
During hysterectomy, the ureter is susceptible to injury as it passes through the parametrium a short distance from the uterus and vaginal fornix.
Sutures placed in the posterior lateral cul de sac during prolapse surgery lie near the midpelvic ureter, and sutures placed during vaginal cuff closure, anterior colporrhaphy, and retropubic urethropexy are in close proximity to the trigonal portion of the ureter.
The ureter extends from the renal pelvis to the bladder, with a length that ranges from 25 to 30 cm, depending on the patient’s height. It crosses the pelvic brim near the bifurcation of the common iliac artery, where it becomes the “pelvic” ureter. The abdominal and pelvic portions of the ureter are approximately equal in length.
ILLUSTRATIONS BY ROB FLEWELL FOR OBG MANAGEMENT
The blood supply of the ureter derives from branches of the major arterial system of the lower abdomen and pelvis. These branches reach the medial aspect of the abdominal ureter and the lateral side of the pelvic ureter to form an anastomotic vascular network protected by an adventitial layer surrounding the ureter.
The ureter is attached to the posterior lateral pelvic peritoneum running dorsal to ovarian vessels. At the midpelvis, it separates from the peritoneum to pierce the base of the broad ligament underneath the uterine artery. At this point, the ureter is about 1.5 to 2 cm lateral to the uterus and curves medially and ventrally, tunneling through the cardinal and vesicovaginal ligaments to enter the bladder trigone.
Risky procedures
In gynecologic surgery, ureteral injury occurs most often during abdominal hysterectomy—probably because of how frequently this operation is performed and the range of pathology managed. The incidence of ureteral injury is much higher during abdominal hysterectomy than vaginal hysterectomy.1-4
Laparoscopic hysterectomy also has been associated with a higher incidence of ureteral injury, especially in the early phase of training.5,6 Possible explanations include:
- greater difficulty identifying the ureter
- a steeper learning curve
- more frequent use of energy to hemostatically divide pedicles, with the potential for thermal injury
- less traction–countertraction, resulting in dissection closer to the ureter
- management of complex pathology.
Although the overall incidence of ureteral injury during adnexectomy is low, it is probably much higher in women undergoing this procedure after a previous hysterectomy or in the presence of complex adnexal pathology.
When injury is likely
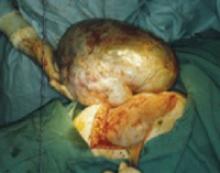
Compromised exposure, distorted anatomy, and certain procedures can heighten the risk of ureteral injury. Large tumors may limit the ability of the surgeon to visualize or palpate the ureter (FIGURE 1). Extensive adhesions may cause similar difficulties, and a small incision or obesity may hinder identification of pelvic sidewall structures.
A number of pathologic conditions can distort the anatomy of the ureter, especially as it relates to the female genital tract:
- Malignancies such as ovarian cancer often encroach on and occasionally encase the ureter
- Pelvic inflammatory disease, endometriosis, and a history of surgery or pelvic radiotherapy can retract and encase the ureter toward the gynecologic tract
- Some masses expand against the lower ureter, such as cervical or broad-ligament leiomyomata or placenta previa with accreta
- During vaginal hysterectomy for complete uterine prolapse, the ureters frequently extend beyond the introitus well within the operative field
- Congenital anomalies of the ureter or hydroureter can also cause distortion.
Even in the presence of relatively normal anatomy, certain procedures predispose the ureter to injury. For example, radical hysterectomy involves the almost complete separation of the pelvic ureter from the gynecologic tract and its surrounding soft tissue. When pelvic pathology is significant, the plane of dissection will always be near the ureter.
FIGURE 1 Access to the ureter is obstructed, putting it in jeopardy
Large tumors may limit the ability of the surgeon to visualize or palpate the ureter.
Prevention is the best strategy
At least 50% of ureteral injuries reported during gynecologic surgery have occurred in the absence of a recognizable risk factor.2,7 Nevertheless, knowledge of anatomy and the ability to recognize situations in which there is an elevated risk for ureteral injury will best enable the surgeon to prevent such injury.
When a high-risk situation is encountered, critical preventive steps include:
- adequate exposure
- competent assistance
- exposure of the path of the ureter through the planned course of dissection. Dissecting the ureter beyond this area is usually unnecessary and may itself cause injury.
Skip preoperative IVP in most cases
The vast majority of women who undergo gynecologic surgery do not benefit from preoperative intravenous pyelography (IVP). This measure does not appear to reduce the likelihood of ureteral injury, even in the face of obvious gynecologic disease. However, preoperative identification of obvious ureteral involvement by the disease process is useful. In such cases, the plane of dissection will probably lie closer to the ureter. One of the goals of surgery will then be to clear the urinary tract from the affected area.
When there is a high index of suspicion of an abnormality such as obstruction, intrinsic ureteral endometriosis, or congenital anomaly, preoperative IVP is indicated.
A stent may be helpful in some cases
Ureteral stents are sometimes placed in order to aid in identification and dissection of the ureters during surgery. Some authors of reports on this topic, including Hoffman, believe that stents are useful in certain situations, such as excision of an ovarian remnant, radical vaginal hysterectomy, and when pelvic organs are encased by malignant ovarian tumors. However, stents do not clearly reduce the risk of injury and, in some cases, may increase the risk by providing a false sense of security and predisposing the ureter to adventitial injury during difficult dissection.
Anticipate the effects of disease
The surgeon must have a thorough knowledge of the gynecologic disease process as it relates to surgery involving the urinary tract. For example, an ovarian remnant will almost always be somewhat densely adherent to the pelvic ureter. When severe endometriosis involves the posterior leaf of the broad ligament, the ureter will often be fibrotically retracted toward the operative field.
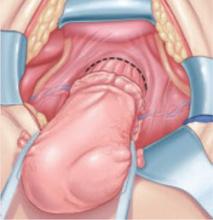
Certain procedures have special challenges. During resection of adnexa, for example, it is important that the ureter be identified in the retroperitoneum before the ovarian vessels are ligated. During hysterectomy, soft tissues that contain the bladder and ureters should be mobilized caudally and laterally, respectively, creating a U-shaped region (“U” for urinary tract, FIGURE 2) to which the surgeon must limit dissection.
FIGURE 2 During hysterectomy, mobilize the bladder and ureter
Mobilize the soft tissues that contain the bladder and ureters caudally and laterally, respectively, creating a U-shaped region. During division of the paracervical tissues, the surgeon must remain within this region.
Intraoperative detection
Two main types of ureteral injury occur during gynecologic surgery: transection and destruction. The latter includes ligation, crushing, devascularization, and thermal injury.
Intraoperative detection of ureteral injury is more likely when the surgeon recognizes at the outset that the operation places the ureter at increased risk. When dissection has been difficult or complicated for any reason, be concerned about possible injury.
In general, ureteral injury is first recognized by careful inspection of the surgical field. Begin by instilling 5 ml of indigo carmine intravenously. Once the dye begins to appear in the Foley catheter, inspect the area of dissection under a small amount of irrigation fluid, looking for extravasation of dye that indicates partial or complete transection.
If no injury is identified, cystoscopy is the next step. I perform all major abdominal operations with the patient in the low lithotomy position, which provides easy access to the perineum. Cystoscopic identification of urine jetting from both ureteral orifices confirms patency. When only wisps of dye are observed, it is likely that the ureter in question has been partially occluded (e.g., by acute angulation). Failure of any urine to appear from one of the orifices highly suggests injury to that ureter.
During inspection of the operative field, attempt to pass a ureteral stent into the affected orifice. If the stent passes easily and dyed urine is seen to drip freely from it, look for possible angulation of the ureter. If you find none, remove the stent and inspect the orifice again for jetting urine.
If the ureteral stent will move only a few centimeters into the ureteral orifice, ligation (with or without transection) is likely. In this case, leave the stent in place. If the operative site is readily accessible, dissect the applicable area to identify the problem. Depending on the circumstances, you may wish to infuse dye through the stent to aid in operative identification or radiographic evaluation.
Intraoperative IVP may be useful, especially when cystoscopy is unavailable.
Fundamentals of repair
Repair of major injury to the pelvic ureter is generally best accomplished by ureteroneocystostomy or, in selected cases involving injury to the proximal pelvic ureter, by ureteroureterostomy.
When intraoperatively recognized injury to the pelvic ureter appears to be minor, it can be managed by placing a ureteral stent and a closed-suction pelvic drain. Also consider wrapping the injured area with vascularized tissue such as perivesical fat. Minor lacerations can be closed perpendicular to the axis of the ureter using interrupted 4-0 delayed absorbable suture.
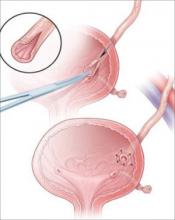
Most injuries to the pelvic ureter are optimally managed by ureteroneocystostomy (FIGURE 3). When a significant portion of the pelvic ureter has been lost, ureteroneocystostomy usually requires a combination of:
- extensive mobilization of the bladder
- conservative mobilization of the ureter
- elongation of the bladder
- psoas hitch.
When necessary, mobilization of the kidney with suturing of the caudal perinephric fascia to the psoas muscle will bridge an additional 2- to 3-cm gap.
Major injury to the distal half of the pelvic ureter is repaired using straightforward ureteroneocystostomy.
When there is no significant pelvic disease and the distal ureter is healthy, injury to the proximal pelvic ureter during division of the ovarian vessels may be repaired via ureteroureterostomy. If the ureteral ends will be anastomosed on tension or there is any question about the integrity of the distal portion of the ureter, as when extensive distal ureterolysis has been necessary, consider ureteroneocystostomy.
FIGURE 3 When the distal ureter is injured
Most injuries to the pelvic ureter are managed optimally by ureteroneocystostomy.
Ureter injured during emergent hysterectomy
A 37-year-old woman, para 4, undergoes her fourth repeat cesarean section. When the OB attempts to manually extract the placenta, the patient begins to hemorrhage profusely. Conservative measures fail to stop the bleeding, and the patient becomes hypotensive. The physician performs emergent hysterectomy, taking large pedicles of tissue. Although the patient stabilizes, the doctor worries that the ureters may have been injured.
Resolution: Cystoscopy is performed to check for injury. Because indigo carmine does not spill from the left ureteral orifice, the physician passes a stent with the abdomen still open, and it stops within the most distal ligamentous pedicle. Upon deligation, indigo carmine begins to drain from the stent, which then passes easily.
The stent is withdrawn to below the site of injury, and dilute methylene blue is instilled through it while the ureter is observed under irrigation. No extravasation is noted. Because the ligature had been around a block of tissue that was thought to have acutely angulated rather than incorporated the ureter, the physician concludes that severe damage is unlikely. He places a 6 French double-J stent, wraps the damaged portion of the distal ureter in perivesical fat, and places a closed-suction pelvic drain. Healing is uneventful.
Obstruction is confirmed. Now the surgeon must find it
A 45-year-old woman, para 3, who has a symptomatic 14-weeks’ size myomatous uterus, undergoes vaginal hysterectomy. The surgeon ligates and divides the uterine vessel pedicles before beginning morcellation. At the completion of the procedure, during cystoscopy, indigo carmine fails to spill from the right ureteral orifice, suggesting injury to that ureter. The surgeon passes a stent into the ureter, and it stops approximately 6 cm from the orifice. A retrograde pyelogram confirms complete obstruction.
Resolution: With the stent left in place, the surgeon performs a midline laparotomy, tracing the ureter to the uterine artery pedicle in which it has been incorporated and transected. The distal ureter with the stent is found within soft tissue lateral to the cardinal ligament pedicle, and the transected end is securely ligated using 2–0 silk suture. After the bladder is mobilized, a ureteroneocystostomy is performed. The patient recovers fully.
Postoperative management
After repair of a ureteral injury, leave a closed-suction pelvic drain in place for 2 to 3 days so that any major urinary leak can be detected; it also enhances spontaneous closure and helps prevent potentially infected fluid from accumulating in the region of anastomosis.
The cystotomy performed during ureteroneocystostomy generally heals quickly with a low risk of complications.
Leave a large-bore (20 or 22 French) urethral Foley catheter in place for 2 weeks.
I recommend that a 6 French double-J ureteral stent be left in place for 6 weeks. Potential benefits of the stent include:
- prevention of stricture
- stabilization and immobilization of the ureter during healing
- reduced risk of extravasation of urine
- reduced risk of angulation of the ureter
- isolation of the repair from infection, retroperitoneal fibrosis, and cancer.
I perform IVP approximately 1 week after stent removal to ensure ureteral patency.
CASE RESOLVED
Exposure is improved by widening the incision and dividing the tendonous insertions of the rectus abdominus muscles. The surgeon then removes the mass, preserving the distal ureter, which is estimated to be 12 cm in length and to have intact adventitia.
The surgeon performs a double-spatulated end-to-end ureteroureterostomy over a 6 French double-J ureteral stent that has been passed proximally into the renal pelvis and distally into the bladder. The stent is removed 6 weeks postoperatively, and an IVP the following week demonstrates excellent patency.
The majority of payers consider ureterolysis integral to good surgical technique, but there can be exceptions when documentation supports existing codes. Three CPT codes describe this procedure:
50715 Ureterolysis, with or without repositioning of ureter for retroperitoneal fibrosis
50722 Ureterolysis for ovarian vein syndrome
50725 Ureterolysis for retrocaval ureter, with reanastomosis of upper urinary tract or vena cava
The key to getting paid will be to document the existence of the condition indicated by each of the codes.
The ICD-9 code for both retroperitoneal fibrosis and ovarian vein syndrome is the same, 593.4 (Other ureteric obstruction). If the patient requires ureterolysis for a retrocaval ureter, the code 753.4 (Other specified anomalies of ureter) would be reported instead. Note, however, that these procedure codes cannot be reported if the ureterolysis is performed laparoscopically. In that case, the most appropriate code is 50949 (Unlisted laparoscopy procedure, ureter).
When repair is necessary, you have several codes to choose from, but the supporting diagnosis code 998.2 (Accidental puncture or laceration during a procedure) must be indicated. If a Medicare patient is involved, the surgeon who created the injury would not be paid additionally for repair.
50780 Ureteroneocystostomy; anastomosis of single ureter to bladder
50782 Ureteroneocystostomy; anastomosis of duplicated ureter to bladder
50783 Ureteroneocystostomy; with extensive ureteral tailoring
50785 Ureteroneocystostomy; with vesico-psoas hitch or bladder flap
50760 Ureteroureterostomy; fusion of ureters
50770 Transureteroureterostomy, anastomosis of ureter to contralateral ureter—MELANIE WITT, RN, CPC-OBGYN, MA
1. St. Lezin MA, Stoller ML. Surgical ureteral injuries. Urology. 1991;38:497-506.
2. Liapis A, Bakas P, Giannopoulos V, Creatsas G. Ureteral injuries during gynecological surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:391-394.
3. Vakili B, Chesson RR, Kyle BL, et al. The incidence of urinary tract injury during hysterectomy: a prospective analysis based on universal cystoscopy. Am J Obstet Gynecol. 2005;192:1599-1604.
4. Sakellariou P, Protopapas AG, Voulgaris Z, et al. Management of ureteric injuries during gynecological operations: 10 years experience. Eur J Obstet Gynecol Reprod Biol. 2002;101:179-184.
5. Assimos DG, Patterson LC, Taylor CL. Changing incidence and etiology of iatrogenic ureteral injuries. J Urol. 1994;152:2240-2246.
6. Härkki-Sirén P, Sjöberg J, Titinen A. Urinary tract injuries after hysterectomy. Obstet Gynecol. 1998;92:113-118.
7. Chan JK, Morrow J, Manetta A. Prevention of ureteral injuries in gynecologic surgery. Am J Obstet Gynecol. 2003;188:1273-1277.
The author has no financial relationships relevant to this article.
CASE: Inadvertent ureteral transection
A gynecologic surgeon operates via Pfannenstiel incision to remove a 12-cm complex left adnexal mass from a 36-year-old obese woman. When she discovers that the mass is densely adherent to the pelvic peritoneum, the surgeon incises the peritoneum lateral to the mass and opens the retroperitoneal space. However, the size and relative immobility of the mass, coupled with the low transverse incision, impair visualization of retroperitoneal structures.
The surgeon clamps and divides the ovarian vessels above the mass but, afterward, suspects that the ureter has been transected and that its ends are included within the clamps. She separates the ovarian vessels above the clamp and ligates them, at which time transection of the ureter is confirmed.
How should she proceed?
The ureter is intimately associated with the female internal genitalia in a way that challenges the gynecologic surgeon to avoid it. In a small percentage of cases involving surgical extirpation in a woman who has severe pelvic pathology, ureteral injury may be inevitable.
Several variables predispose a patient to ureteral injury, including limited exposure, as in the opening case. Others include distorted anatomy of the urinary tract relative to internal genitalia and operations that require extensive resection of pelvic tissues.
This article describes:
- prevention and intraoperative recognition of ureteral injury during gynecologic surgery
- management of intraoperatively recognized ureteral injury.
Maintain a high index of suspicion
The surgeon in the opening case has already taken the first and most important step in ensuring a good outcome: She suspected ureteral injury. In high-risk situations, intraoperative recognition of ureteral injury is more likely when the operative field is inspected thoroughly during and at the conclusion of the surgical procedure.
In a high-risk case, the combined use of intravenous indigo carmine, careful inspection of the operative field, cystoscopy, and ureteral dissection is recommended and should be routine.
Common sites of injury
During gynecologic surgery, the ureter is susceptible to injury along its entire course through the pelvis (see “The ureter takes a course fraught with hazard,”).
During adnexectomy, the gonadal vessels are generally ligated 2 to 3 cm above the adnexa. The ureter lies in close proximity to these vessels and may inadvertently be included in the ligation.
During hysterectomy, the ureter is susceptible to injury as it passes through the parametrium a short distance from the uterus and vaginal fornix.
Sutures placed in the posterior lateral cul de sac during prolapse surgery lie near the midpelvic ureter, and sutures placed during vaginal cuff closure, anterior colporrhaphy, and retropubic urethropexy are in close proximity to the trigonal portion of the ureter.
The ureter extends from the renal pelvis to the bladder, with a length that ranges from 25 to 30 cm, depending on the patient’s height. It crosses the pelvic brim near the bifurcation of the common iliac artery, where it becomes the “pelvic” ureter. The abdominal and pelvic portions of the ureter are approximately equal in length.
ILLUSTRATIONS BY ROB FLEWELL FOR OBG MANAGEMENT
The blood supply of the ureter derives from branches of the major arterial system of the lower abdomen and pelvis. These branches reach the medial aspect of the abdominal ureter and the lateral side of the pelvic ureter to form an anastomotic vascular network protected by an adventitial layer surrounding the ureter.
The ureter is attached to the posterior lateral pelvic peritoneum running dorsal to ovarian vessels. At the midpelvis, it separates from the peritoneum to pierce the base of the broad ligament underneath the uterine artery. At this point, the ureter is about 1.5 to 2 cm lateral to the uterus and curves medially and ventrally, tunneling through the cardinal and vesicovaginal ligaments to enter the bladder trigone.
Risky procedures
In gynecologic surgery, ureteral injury occurs most often during abdominal hysterectomy—probably because of how frequently this operation is performed and the range of pathology managed. The incidence of ureteral injury is much higher during abdominal hysterectomy than vaginal hysterectomy.1-4
Laparoscopic hysterectomy also has been associated with a higher incidence of ureteral injury, especially in the early phase of training.5,6 Possible explanations include:
- greater difficulty identifying the ureter
- a steeper learning curve
- more frequent use of energy to hemostatically divide pedicles, with the potential for thermal injury
- less traction–countertraction, resulting in dissection closer to the ureter
- management of complex pathology.
Although the overall incidence of ureteral injury during adnexectomy is low, it is probably much higher in women undergoing this procedure after a previous hysterectomy or in the presence of complex adnexal pathology.
When injury is likely
Compromised exposure, distorted anatomy, and certain procedures can heighten the risk of ureteral injury. Large tumors may limit the ability of the surgeon to visualize or palpate the ureter (FIGURE 1). Extensive adhesions may cause similar difficulties, and a small incision or obesity may hinder identification of pelvic sidewall structures.
A number of pathologic conditions can distort the anatomy of the ureter, especially as it relates to the female genital tract:
- Malignancies such as ovarian cancer often encroach on and occasionally encase the ureter
- Pelvic inflammatory disease, endometriosis, and a history of surgery or pelvic radiotherapy can retract and encase the ureter toward the gynecologic tract
- Some masses expand against the lower ureter, such as cervical or broad-ligament leiomyomata or placenta previa with accreta
- During vaginal hysterectomy for complete uterine prolapse, the ureters frequently extend beyond the introitus well within the operative field
- Congenital anomalies of the ureter or hydroureter can also cause distortion.
Even in the presence of relatively normal anatomy, certain procedures predispose the ureter to injury. For example, radical hysterectomy involves the almost complete separation of the pelvic ureter from the gynecologic tract and its surrounding soft tissue. When pelvic pathology is significant, the plane of dissection will always be near the ureter.
FIGURE 1 Access to the ureter is obstructed, putting it in jeopardy
Large tumors may limit the ability of the surgeon to visualize or palpate the ureter.
Prevention is the best strategy
At least 50% of ureteral injuries reported during gynecologic surgery have occurred in the absence of a recognizable risk factor.2,7 Nevertheless, knowledge of anatomy and the ability to recognize situations in which there is an elevated risk for ureteral injury will best enable the surgeon to prevent such injury.
When a high-risk situation is encountered, critical preventive steps include:
- adequate exposure
- competent assistance
- exposure of the path of the ureter through the planned course of dissection. Dissecting the ureter beyond this area is usually unnecessary and may itself cause injury.
Skip preoperative IVP in most cases
The vast majority of women who undergo gynecologic surgery do not benefit from preoperative intravenous pyelography (IVP). This measure does not appear to reduce the likelihood of ureteral injury, even in the face of obvious gynecologic disease. However, preoperative identification of obvious ureteral involvement by the disease process is useful. In such cases, the plane of dissection will probably lie closer to the ureter. One of the goals of surgery will then be to clear the urinary tract from the affected area.
When there is a high index of suspicion of an abnormality such as obstruction, intrinsic ureteral endometriosis, or congenital anomaly, preoperative IVP is indicated.
A stent may be helpful in some cases
Ureteral stents are sometimes placed in order to aid in identification and dissection of the ureters during surgery. Some authors of reports on this topic, including Hoffman, believe that stents are useful in certain situations, such as excision of an ovarian remnant, radical vaginal hysterectomy, and when pelvic organs are encased by malignant ovarian tumors. However, stents do not clearly reduce the risk of injury and, in some cases, may increase the risk by providing a false sense of security and predisposing the ureter to adventitial injury during difficult dissection.
Anticipate the effects of disease
The surgeon must have a thorough knowledge of the gynecologic disease process as it relates to surgery involving the urinary tract. For example, an ovarian remnant will almost always be somewhat densely adherent to the pelvic ureter. When severe endometriosis involves the posterior leaf of the broad ligament, the ureter will often be fibrotically retracted toward the operative field.
Certain procedures have special challenges. During resection of adnexa, for example, it is important that the ureter be identified in the retroperitoneum before the ovarian vessels are ligated. During hysterectomy, soft tissues that contain the bladder and ureters should be mobilized caudally and laterally, respectively, creating a U-shaped region (“U” for urinary tract, FIGURE 2) to which the surgeon must limit dissection.
FIGURE 2 During hysterectomy, mobilize the bladder and ureter
Mobilize the soft tissues that contain the bladder and ureters caudally and laterally, respectively, creating a U-shaped region. During division of the paracervical tissues, the surgeon must remain within this region.
Intraoperative detection
Two main types of ureteral injury occur during gynecologic surgery: transection and destruction. The latter includes ligation, crushing, devascularization, and thermal injury.
Intraoperative detection of ureteral injury is more likely when the surgeon recognizes at the outset that the operation places the ureter at increased risk. When dissection has been difficult or complicated for any reason, be concerned about possible injury.
In general, ureteral injury is first recognized by careful inspection of the surgical field. Begin by instilling 5 ml of indigo carmine intravenously. Once the dye begins to appear in the Foley catheter, inspect the area of dissection under a small amount of irrigation fluid, looking for extravasation of dye that indicates partial or complete transection.
If no injury is identified, cystoscopy is the next step. I perform all major abdominal operations with the patient in the low lithotomy position, which provides easy access to the perineum. Cystoscopic identification of urine jetting from both ureteral orifices confirms patency. When only wisps of dye are observed, it is likely that the ureter in question has been partially occluded (e.g., by acute angulation). Failure of any urine to appear from one of the orifices highly suggests injury to that ureter.
During inspection of the operative field, attempt to pass a ureteral stent into the affected orifice. If the stent passes easily and dyed urine is seen to drip freely from it, look for possible angulation of the ureter. If you find none, remove the stent and inspect the orifice again for jetting urine.
If the ureteral stent will move only a few centimeters into the ureteral orifice, ligation (with or without transection) is likely. In this case, leave the stent in place. If the operative site is readily accessible, dissect the applicable area to identify the problem. Depending on the circumstances, you may wish to infuse dye through the stent to aid in operative identification or radiographic evaluation.
Intraoperative IVP may be useful, especially when cystoscopy is unavailable.
Fundamentals of repair
Repair of major injury to the pelvic ureter is generally best accomplished by ureteroneocystostomy or, in selected cases involving injury to the proximal pelvic ureter, by ureteroureterostomy.
When intraoperatively recognized injury to the pelvic ureter appears to be minor, it can be managed by placing a ureteral stent and a closed-suction pelvic drain. Also consider wrapping the injured area with vascularized tissue such as perivesical fat. Minor lacerations can be closed perpendicular to the axis of the ureter using interrupted 4-0 delayed absorbable suture.
Most injuries to the pelvic ureter are optimally managed by ureteroneocystostomy (FIGURE 3). When a significant portion of the pelvic ureter has been lost, ureteroneocystostomy usually requires a combination of:
- extensive mobilization of the bladder
- conservative mobilization of the ureter
- elongation of the bladder
- psoas hitch.
When necessary, mobilization of the kidney with suturing of the caudal perinephric fascia to the psoas muscle will bridge an additional 2- to 3-cm gap.
Major injury to the distal half of the pelvic ureter is repaired using straightforward ureteroneocystostomy.
When there is no significant pelvic disease and the distal ureter is healthy, injury to the proximal pelvic ureter during division of the ovarian vessels may be repaired via ureteroureterostomy. If the ureteral ends will be anastomosed on tension or there is any question about the integrity of the distal portion of the ureter, as when extensive distal ureterolysis has been necessary, consider ureteroneocystostomy.
FIGURE 3 When the distal ureter is injured
Most injuries to the pelvic ureter are managed optimally by ureteroneocystostomy.
Ureter injured during emergent hysterectomy
A 37-year-old woman, para 4, undergoes her fourth repeat cesarean section. When the OB attempts to manually extract the placenta, the patient begins to hemorrhage profusely. Conservative measures fail to stop the bleeding, and the patient becomes hypotensive. The physician performs emergent hysterectomy, taking large pedicles of tissue. Although the patient stabilizes, the doctor worries that the ureters may have been injured.
Resolution: Cystoscopy is performed to check for injury. Because indigo carmine does not spill from the left ureteral orifice, the physician passes a stent with the abdomen still open, and it stops within the most distal ligamentous pedicle. Upon deligation, indigo carmine begins to drain from the stent, which then passes easily.
The stent is withdrawn to below the site of injury, and dilute methylene blue is instilled through it while the ureter is observed under irrigation. No extravasation is noted. Because the ligature had been around a block of tissue that was thought to have acutely angulated rather than incorporated the ureter, the physician concludes that severe damage is unlikely. He places a 6 French double-J stent, wraps the damaged portion of the distal ureter in perivesical fat, and places a closed-suction pelvic drain. Healing is uneventful.
Obstruction is confirmed. Now the surgeon must find it
A 45-year-old woman, para 3, who has a symptomatic 14-weeks’ size myomatous uterus, undergoes vaginal hysterectomy. The surgeon ligates and divides the uterine vessel pedicles before beginning morcellation. At the completion of the procedure, during cystoscopy, indigo carmine fails to spill from the right ureteral orifice, suggesting injury to that ureter. The surgeon passes a stent into the ureter, and it stops approximately 6 cm from the orifice. A retrograde pyelogram confirms complete obstruction.
Resolution: With the stent left in place, the surgeon performs a midline laparotomy, tracing the ureter to the uterine artery pedicle in which it has been incorporated and transected. The distal ureter with the stent is found within soft tissue lateral to the cardinal ligament pedicle, and the transected end is securely ligated using 2–0 silk suture. After the bladder is mobilized, a ureteroneocystostomy is performed. The patient recovers fully.
Postoperative management
After repair of a ureteral injury, leave a closed-suction pelvic drain in place for 2 to 3 days so that any major urinary leak can be detected; it also enhances spontaneous closure and helps prevent potentially infected fluid from accumulating in the region of anastomosis.
The cystotomy performed during ureteroneocystostomy generally heals quickly with a low risk of complications.
Leave a large-bore (20 or 22 French) urethral Foley catheter in place for 2 weeks.
I recommend that a 6 French double-J ureteral stent be left in place for 6 weeks. Potential benefits of the stent include:
- prevention of stricture
- stabilization and immobilization of the ureter during healing
- reduced risk of extravasation of urine
- reduced risk of angulation of the ureter
- isolation of the repair from infection, retroperitoneal fibrosis, and cancer.
I perform IVP approximately 1 week after stent removal to ensure ureteral patency.
CASE RESOLVED
Exposure is improved by widening the incision and dividing the tendonous insertions of the rectus abdominus muscles. The surgeon then removes the mass, preserving the distal ureter, which is estimated to be 12 cm in length and to have intact adventitia.
The surgeon performs a double-spatulated end-to-end ureteroureterostomy over a 6 French double-J ureteral stent that has been passed proximally into the renal pelvis and distally into the bladder. The stent is removed 6 weeks postoperatively, and an IVP the following week demonstrates excellent patency.
The majority of payers consider ureterolysis integral to good surgical technique, but there can be exceptions when documentation supports existing codes. Three CPT codes describe this procedure:
50715 Ureterolysis, with or without repositioning of ureter for retroperitoneal fibrosis
50722 Ureterolysis for ovarian vein syndrome
50725 Ureterolysis for retrocaval ureter, with reanastomosis of upper urinary tract or vena cava
The key to getting paid will be to document the existence of the condition indicated by each of the codes.
The ICD-9 code for both retroperitoneal fibrosis and ovarian vein syndrome is the same, 593.4 (Other ureteric obstruction). If the patient requires ureterolysis for a retrocaval ureter, the code 753.4 (Other specified anomalies of ureter) would be reported instead. Note, however, that these procedure codes cannot be reported if the ureterolysis is performed laparoscopically. In that case, the most appropriate code is 50949 (Unlisted laparoscopy procedure, ureter).
When repair is necessary, you have several codes to choose from, but the supporting diagnosis code 998.2 (Accidental puncture or laceration during a procedure) must be indicated. If a Medicare patient is involved, the surgeon who created the injury would not be paid additionally for repair.
50780 Ureteroneocystostomy; anastomosis of single ureter to bladder
50782 Ureteroneocystostomy; anastomosis of duplicated ureter to bladder
50783 Ureteroneocystostomy; with extensive ureteral tailoring
50785 Ureteroneocystostomy; with vesico-psoas hitch or bladder flap
50760 Ureteroureterostomy; fusion of ureters
50770 Transureteroureterostomy, anastomosis of ureter to contralateral ureter—MELANIE WITT, RN, CPC-OBGYN, MA
The author has no financial relationships relevant to this article.
CASE: Inadvertent ureteral transection
A gynecologic surgeon operates via Pfannenstiel incision to remove a 12-cm complex left adnexal mass from a 36-year-old obese woman. When she discovers that the mass is densely adherent to the pelvic peritoneum, the surgeon incises the peritoneum lateral to the mass and opens the retroperitoneal space. However, the size and relative immobility of the mass, coupled with the low transverse incision, impair visualization of retroperitoneal structures.
The surgeon clamps and divides the ovarian vessels above the mass but, afterward, suspects that the ureter has been transected and that its ends are included within the clamps. She separates the ovarian vessels above the clamp and ligates them, at which time transection of the ureter is confirmed.
How should she proceed?
The ureter is intimately associated with the female internal genitalia in a way that challenges the gynecologic surgeon to avoid it. In a small percentage of cases involving surgical extirpation in a woman who has severe pelvic pathology, ureteral injury may be inevitable.
Several variables predispose a patient to ureteral injury, including limited exposure, as in the opening case. Others include distorted anatomy of the urinary tract relative to internal genitalia and operations that require extensive resection of pelvic tissues.
This article describes:
- prevention and intraoperative recognition of ureteral injury during gynecologic surgery
- management of intraoperatively recognized ureteral injury.
Maintain a high index of suspicion
The surgeon in the opening case has already taken the first and most important step in ensuring a good outcome: She suspected ureteral injury. In high-risk situations, intraoperative recognition of ureteral injury is more likely when the operative field is inspected thoroughly during and at the conclusion of the surgical procedure.
In a high-risk case, the combined use of intravenous indigo carmine, careful inspection of the operative field, cystoscopy, and ureteral dissection is recommended and should be routine.
Common sites of injury
During gynecologic surgery, the ureter is susceptible to injury along its entire course through the pelvis (see “The ureter takes a course fraught with hazard,”).
During adnexectomy, the gonadal vessels are generally ligated 2 to 3 cm above the adnexa. The ureter lies in close proximity to these vessels and may inadvertently be included in the ligation.
During hysterectomy, the ureter is susceptible to injury as it passes through the parametrium a short distance from the uterus and vaginal fornix.
Sutures placed in the posterior lateral cul de sac during prolapse surgery lie near the midpelvic ureter, and sutures placed during vaginal cuff closure, anterior colporrhaphy, and retropubic urethropexy are in close proximity to the trigonal portion of the ureter.
The ureter extends from the renal pelvis to the bladder, with a length that ranges from 25 to 30 cm, depending on the patient’s height. It crosses the pelvic brim near the bifurcation of the common iliac artery, where it becomes the “pelvic” ureter. The abdominal and pelvic portions of the ureter are approximately equal in length.
ILLUSTRATIONS BY ROB FLEWELL FOR OBG MANAGEMENT
The blood supply of the ureter derives from branches of the major arterial system of the lower abdomen and pelvis. These branches reach the medial aspect of the abdominal ureter and the lateral side of the pelvic ureter to form an anastomotic vascular network protected by an adventitial layer surrounding the ureter.
The ureter is attached to the posterior lateral pelvic peritoneum running dorsal to ovarian vessels. At the midpelvis, it separates from the peritoneum to pierce the base of the broad ligament underneath the uterine artery. At this point, the ureter is about 1.5 to 2 cm lateral to the uterus and curves medially and ventrally, tunneling through the cardinal and vesicovaginal ligaments to enter the bladder trigone.
Risky procedures
In gynecologic surgery, ureteral injury occurs most often during abdominal hysterectomy—probably because of how frequently this operation is performed and the range of pathology managed. The incidence of ureteral injury is much higher during abdominal hysterectomy than vaginal hysterectomy.1-4
Laparoscopic hysterectomy also has been associated with a higher incidence of ureteral injury, especially in the early phase of training.5,6 Possible explanations include:
- greater difficulty identifying the ureter
- a steeper learning curve
- more frequent use of energy to hemostatically divide pedicles, with the potential for thermal injury
- less traction–countertraction, resulting in dissection closer to the ureter
- management of complex pathology.
Although the overall incidence of ureteral injury during adnexectomy is low, it is probably much higher in women undergoing this procedure after a previous hysterectomy or in the presence of complex adnexal pathology.
When injury is likely
Compromised exposure, distorted anatomy, and certain procedures can heighten the risk of ureteral injury. Large tumors may limit the ability of the surgeon to visualize or palpate the ureter (FIGURE 1). Extensive adhesions may cause similar difficulties, and a small incision or obesity may hinder identification of pelvic sidewall structures.
A number of pathologic conditions can distort the anatomy of the ureter, especially as it relates to the female genital tract:
- Malignancies such as ovarian cancer often encroach on and occasionally encase the ureter
- Pelvic inflammatory disease, endometriosis, and a history of surgery or pelvic radiotherapy can retract and encase the ureter toward the gynecologic tract
- Some masses expand against the lower ureter, such as cervical or broad-ligament leiomyomata or placenta previa with accreta
- During vaginal hysterectomy for complete uterine prolapse, the ureters frequently extend beyond the introitus well within the operative field
- Congenital anomalies of the ureter or hydroureter can also cause distortion.
Even in the presence of relatively normal anatomy, certain procedures predispose the ureter to injury. For example, radical hysterectomy involves the almost complete separation of the pelvic ureter from the gynecologic tract and its surrounding soft tissue. When pelvic pathology is significant, the plane of dissection will always be near the ureter.
FIGURE 1 Access to the ureter is obstructed, putting it in jeopardy
Large tumors may limit the ability of the surgeon to visualize or palpate the ureter.
Prevention is the best strategy
At least 50% of ureteral injuries reported during gynecologic surgery have occurred in the absence of a recognizable risk factor.2,7 Nevertheless, knowledge of anatomy and the ability to recognize situations in which there is an elevated risk for ureteral injury will best enable the surgeon to prevent such injury.
When a high-risk situation is encountered, critical preventive steps include:
- adequate exposure
- competent assistance
- exposure of the path of the ureter through the planned course of dissection. Dissecting the ureter beyond this area is usually unnecessary and may itself cause injury.
Skip preoperative IVP in most cases
The vast majority of women who undergo gynecologic surgery do not benefit from preoperative intravenous pyelography (IVP). This measure does not appear to reduce the likelihood of ureteral injury, even in the face of obvious gynecologic disease. However, preoperative identification of obvious ureteral involvement by the disease process is useful. In such cases, the plane of dissection will probably lie closer to the ureter. One of the goals of surgery will then be to clear the urinary tract from the affected area.
When there is a high index of suspicion of an abnormality such as obstruction, intrinsic ureteral endometriosis, or congenital anomaly, preoperative IVP is indicated.
A stent may be helpful in some cases
Ureteral stents are sometimes placed in order to aid in identification and dissection of the ureters during surgery. Some authors of reports on this topic, including Hoffman, believe that stents are useful in certain situations, such as excision of an ovarian remnant, radical vaginal hysterectomy, and when pelvic organs are encased by malignant ovarian tumors. However, stents do not clearly reduce the risk of injury and, in some cases, may increase the risk by providing a false sense of security and predisposing the ureter to adventitial injury during difficult dissection.
Anticipate the effects of disease
The surgeon must have a thorough knowledge of the gynecologic disease process as it relates to surgery involving the urinary tract. For example, an ovarian remnant will almost always be somewhat densely adherent to the pelvic ureter. When severe endometriosis involves the posterior leaf of the broad ligament, the ureter will often be fibrotically retracted toward the operative field.
Certain procedures have special challenges. During resection of adnexa, for example, it is important that the ureter be identified in the retroperitoneum before the ovarian vessels are ligated. During hysterectomy, soft tissues that contain the bladder and ureters should be mobilized caudally and laterally, respectively, creating a U-shaped region (“U” for urinary tract, FIGURE 2) to which the surgeon must limit dissection.
FIGURE 2 During hysterectomy, mobilize the bladder and ureter
Mobilize the soft tissues that contain the bladder and ureters caudally and laterally, respectively, creating a U-shaped region. During division of the paracervical tissues, the surgeon must remain within this region.
Intraoperative detection
Two main types of ureteral injury occur during gynecologic surgery: transection and destruction. The latter includes ligation, crushing, devascularization, and thermal injury.
Intraoperative detection of ureteral injury is more likely when the surgeon recognizes at the outset that the operation places the ureter at increased risk. When dissection has been difficult or complicated for any reason, be concerned about possible injury.
In general, ureteral injury is first recognized by careful inspection of the surgical field. Begin by instilling 5 ml of indigo carmine intravenously. Once the dye begins to appear in the Foley catheter, inspect the area of dissection under a small amount of irrigation fluid, looking for extravasation of dye that indicates partial or complete transection.
If no injury is identified, cystoscopy is the next step. I perform all major abdominal operations with the patient in the low lithotomy position, which provides easy access to the perineum. Cystoscopic identification of urine jetting from both ureteral orifices confirms patency. When only wisps of dye are observed, it is likely that the ureter in question has been partially occluded (e.g., by acute angulation). Failure of any urine to appear from one of the orifices highly suggests injury to that ureter.
During inspection of the operative field, attempt to pass a ureteral stent into the affected orifice. If the stent passes easily and dyed urine is seen to drip freely from it, look for possible angulation of the ureter. If you find none, remove the stent and inspect the orifice again for jetting urine.
If the ureteral stent will move only a few centimeters into the ureteral orifice, ligation (with or without transection) is likely. In this case, leave the stent in place. If the operative site is readily accessible, dissect the applicable area to identify the problem. Depending on the circumstances, you may wish to infuse dye through the stent to aid in operative identification or radiographic evaluation.
Intraoperative IVP may be useful, especially when cystoscopy is unavailable.
Fundamentals of repair
Repair of major injury to the pelvic ureter is generally best accomplished by ureteroneocystostomy or, in selected cases involving injury to the proximal pelvic ureter, by ureteroureterostomy.
When intraoperatively recognized injury to the pelvic ureter appears to be minor, it can be managed by placing a ureteral stent and a closed-suction pelvic drain. Also consider wrapping the injured area with vascularized tissue such as perivesical fat. Minor lacerations can be closed perpendicular to the axis of the ureter using interrupted 4-0 delayed absorbable suture.
Most injuries to the pelvic ureter are optimally managed by ureteroneocystostomy (FIGURE 3). When a significant portion of the pelvic ureter has been lost, ureteroneocystostomy usually requires a combination of:
- extensive mobilization of the bladder
- conservative mobilization of the ureter
- elongation of the bladder
- psoas hitch.
When necessary, mobilization of the kidney with suturing of the caudal perinephric fascia to the psoas muscle will bridge an additional 2- to 3-cm gap.
Major injury to the distal half of the pelvic ureter is repaired using straightforward ureteroneocystostomy.
When there is no significant pelvic disease and the distal ureter is healthy, injury to the proximal pelvic ureter during division of the ovarian vessels may be repaired via ureteroureterostomy. If the ureteral ends will be anastomosed on tension or there is any question about the integrity of the distal portion of the ureter, as when extensive distal ureterolysis has been necessary, consider ureteroneocystostomy.
FIGURE 3 When the distal ureter is injured
Most injuries to the pelvic ureter are managed optimally by ureteroneocystostomy.
Ureter injured during emergent hysterectomy
A 37-year-old woman, para 4, undergoes her fourth repeat cesarean section. When the OB attempts to manually extract the placenta, the patient begins to hemorrhage profusely. Conservative measures fail to stop the bleeding, and the patient becomes hypotensive. The physician performs emergent hysterectomy, taking large pedicles of tissue. Although the patient stabilizes, the doctor worries that the ureters may have been injured.
Resolution: Cystoscopy is performed to check for injury. Because indigo carmine does not spill from the left ureteral orifice, the physician passes a stent with the abdomen still open, and it stops within the most distal ligamentous pedicle. Upon deligation, indigo carmine begins to drain from the stent, which then passes easily.
The stent is withdrawn to below the site of injury, and dilute methylene blue is instilled through it while the ureter is observed under irrigation. No extravasation is noted. Because the ligature had been around a block of tissue that was thought to have acutely angulated rather than incorporated the ureter, the physician concludes that severe damage is unlikely. He places a 6 French double-J stent, wraps the damaged portion of the distal ureter in perivesical fat, and places a closed-suction pelvic drain. Healing is uneventful.
Obstruction is confirmed. Now the surgeon must find it
A 45-year-old woman, para 3, who has a symptomatic 14-weeks’ size myomatous uterus, undergoes vaginal hysterectomy. The surgeon ligates and divides the uterine vessel pedicles before beginning morcellation. At the completion of the procedure, during cystoscopy, indigo carmine fails to spill from the right ureteral orifice, suggesting injury to that ureter. The surgeon passes a stent into the ureter, and it stops approximately 6 cm from the orifice. A retrograde pyelogram confirms complete obstruction.
Resolution: With the stent left in place, the surgeon performs a midline laparotomy, tracing the ureter to the uterine artery pedicle in which it has been incorporated and transected. The distal ureter with the stent is found within soft tissue lateral to the cardinal ligament pedicle, and the transected end is securely ligated using 2–0 silk suture. After the bladder is mobilized, a ureteroneocystostomy is performed. The patient recovers fully.
Postoperative management
After repair of a ureteral injury, leave a closed-suction pelvic drain in place for 2 to 3 days so that any major urinary leak can be detected; it also enhances spontaneous closure and helps prevent potentially infected fluid from accumulating in the region of anastomosis.
The cystotomy performed during ureteroneocystostomy generally heals quickly with a low risk of complications.
Leave a large-bore (20 or 22 French) urethral Foley catheter in place for 2 weeks.
I recommend that a 6 French double-J ureteral stent be left in place for 6 weeks. Potential benefits of the stent include:
- prevention of stricture
- stabilization and immobilization of the ureter during healing
- reduced risk of extravasation of urine
- reduced risk of angulation of the ureter
- isolation of the repair from infection, retroperitoneal fibrosis, and cancer.
I perform IVP approximately 1 week after stent removal to ensure ureteral patency.
CASE RESOLVED
Exposure is improved by widening the incision and dividing the tendonous insertions of the rectus abdominus muscles. The surgeon then removes the mass, preserving the distal ureter, which is estimated to be 12 cm in length and to have intact adventitia.
The surgeon performs a double-spatulated end-to-end ureteroureterostomy over a 6 French double-J ureteral stent that has been passed proximally into the renal pelvis and distally into the bladder. The stent is removed 6 weeks postoperatively, and an IVP the following week demonstrates excellent patency.
The majority of payers consider ureterolysis integral to good surgical technique, but there can be exceptions when documentation supports existing codes. Three CPT codes describe this procedure:
50715 Ureterolysis, with or without repositioning of ureter for retroperitoneal fibrosis
50722 Ureterolysis for ovarian vein syndrome
50725 Ureterolysis for retrocaval ureter, with reanastomosis of upper urinary tract or vena cava
The key to getting paid will be to document the existence of the condition indicated by each of the codes.
The ICD-9 code for both retroperitoneal fibrosis and ovarian vein syndrome is the same, 593.4 (Other ureteric obstruction). If the patient requires ureterolysis for a retrocaval ureter, the code 753.4 (Other specified anomalies of ureter) would be reported instead. Note, however, that these procedure codes cannot be reported if the ureterolysis is performed laparoscopically. In that case, the most appropriate code is 50949 (Unlisted laparoscopy procedure, ureter).
When repair is necessary, you have several codes to choose from, but the supporting diagnosis code 998.2 (Accidental puncture or laceration during a procedure) must be indicated. If a Medicare patient is involved, the surgeon who created the injury would not be paid additionally for repair.
50780 Ureteroneocystostomy; anastomosis of single ureter to bladder
50782 Ureteroneocystostomy; anastomosis of duplicated ureter to bladder
50783 Ureteroneocystostomy; with extensive ureteral tailoring
50785 Ureteroneocystostomy; with vesico-psoas hitch or bladder flap
50760 Ureteroureterostomy; fusion of ureters
50770 Transureteroureterostomy, anastomosis of ureter to contralateral ureter—MELANIE WITT, RN, CPC-OBGYN, MA
1. St. Lezin MA, Stoller ML. Surgical ureteral injuries. Urology. 1991;38:497-506.
2. Liapis A, Bakas P, Giannopoulos V, Creatsas G. Ureteral injuries during gynecological surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:391-394.
3. Vakili B, Chesson RR, Kyle BL, et al. The incidence of urinary tract injury during hysterectomy: a prospective analysis based on universal cystoscopy. Am J Obstet Gynecol. 2005;192:1599-1604.
4. Sakellariou P, Protopapas AG, Voulgaris Z, et al. Management of ureteric injuries during gynecological operations: 10 years experience. Eur J Obstet Gynecol Reprod Biol. 2002;101:179-184.
5. Assimos DG, Patterson LC, Taylor CL. Changing incidence and etiology of iatrogenic ureteral injuries. J Urol. 1994;152:2240-2246.
6. Härkki-Sirén P, Sjöberg J, Titinen A. Urinary tract injuries after hysterectomy. Obstet Gynecol. 1998;92:113-118.
7. Chan JK, Morrow J, Manetta A. Prevention of ureteral injuries in gynecologic surgery. Am J Obstet Gynecol. 2003;188:1273-1277.
1. St. Lezin MA, Stoller ML. Surgical ureteral injuries. Urology. 1991;38:497-506.
2. Liapis A, Bakas P, Giannopoulos V, Creatsas G. Ureteral injuries during gynecological surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:391-394.
3. Vakili B, Chesson RR, Kyle BL, et al. The incidence of urinary tract injury during hysterectomy: a prospective analysis based on universal cystoscopy. Am J Obstet Gynecol. 2005;192:1599-1604.
4. Sakellariou P, Protopapas AG, Voulgaris Z, et al. Management of ureteric injuries during gynecological operations: 10 years experience. Eur J Obstet Gynecol Reprod Biol. 2002;101:179-184.
5. Assimos DG, Patterson LC, Taylor CL. Changing incidence and etiology of iatrogenic ureteral injuries. J Urol. 1994;152:2240-2246.
6. Härkki-Sirén P, Sjöberg J, Titinen A. Urinary tract injuries after hysterectomy. Obstet Gynecol. 1998;92:113-118.
7. Chan JK, Morrow J, Manetta A. Prevention of ureteral injuries in gynecologic surgery. Am J Obstet Gynecol. 2003;188:1273-1277.
A guide to management: Adnexal masses in pregnancy
CASE 1 An enlarging cystic tumor
A 20-year-old gravida 3 para 1011 visits the emergency department with persistent right flank pain. Although ultrasonography (US) shows a 21-week gestation, the patient has had no prenatal care. Imaging also reveals a right-sided ovarian tumor, 14×11×8 cm, that is mainly cystic with some internal echogenicity.
At 30 weeks’ gestation, a gynecologic oncologist is consulted. Repeat US reveals the mass to be about 20 cm in diameter and cystic, without internal papillation. The patient’s CA-125 level is 12 U/mL. Based on this information, the physicians decide the likely finding is a benign ovarian cystadenoma.
How should they proceed?
The discovery of an adnexal mass during pregnancy isn’t as rare as you might think—depending on when and how closely you look, it occurs in about 1 in 100 gestations. In most cases, we have found, the mass is clearly benign (TABLE 1), warranting only observation.
TABLE 1
Adnexal masses removed during pregnancy: Histologic profile
| HISTOLOGIC TYPE | NUMBER (%) |
|---|---|
| Cystadenoma | 549 (33) |
| Dermoid | 451 (27) |
| Paraovarian/paratubal | 204 (12) |
| Functional | 237 (14) |
| Endometrioma | 55 (3) |
| Benign stromal | 28 (2) |
| Leiomyoma | 23 (1.5) |
| Luteoma | 8 (0.5) |
| Miscellaneous | 55 (3) |
| Malignant | 68 (4) |
| Total | 1,678 |
| Data supplied by the authors from surgical experience | |
In the case described above, the physicians followed the patient and removed the mass at term because it was cystic with no other indications of malignancy. At 37 weeks’ gestation, a cesarean section was performed through a midline laparotomy incision, followed by removal of the ovarian tumor, which was benign. The pathologist measured the tumor at 16×12×4 cm and determined that it was a corpus luteum cyst.
Presence of mass raises questions
Despite the rarity of malignancy, the discovery of an ovarian mass during pregnancy prompts several important questions:
How should the mass be assessed? How can the likelihood of malignancy be determined as quickly and efficiently as possible, without jeopardy to the pregnancy?
When is surgical intervention warranted? And when can it be postponed? Specifically, is elective operative intervention for a tumor that is probably benign appropriate during pregnancy?
When is the best time to operate? And what is the optimal surgical route?
In this article, we address these questions with a focus on intervention. As we’ll explain, only a small percentage of gravidas who have an adnexal mass require surgery during pregnancy. When surgery is necessary, it is usually indicated for an emergent problem or suspicion of malignancy. Even when ovarian cancer is confirmed, we have found that it is usually in its early stages and therefore has a favorable prognosis (TABLE 2).
TABLE 2
Malignant adnexal masses removed during pregnancy
| HISTOLOGIC TYPE | NUMBER (%) |
|---|---|
| Epithelial | 101 (28) |
| Borderline epithelial | 147 (40) |
| Germ-cell dysgerminoma | 47 (13) |
| Other | 34 (9) |
| Stromal | 24 (7) |
| Undifferentiated | 5 (1.4) |
| Sarcoma | 2 (0.5) |
| Metastatic | 4 (1.1) |
| Total | 364 |
| Data supplied by the authors from surgical experience | |
How should a mass be assessed?
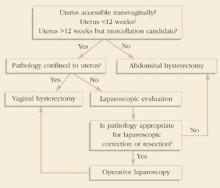
Ultrasonography and other imaging often reveal the presence of a mass and help determine whether it is benign or malignant. In fact, most adnexal masses discovered during pregnancy are incidental findings at the time of routine prenatal US. (see the most commonly found tumors.) Operative intervention is required in 3 situations:
- malignancy is suspected
- an acute complication develops
- the sheer size of the tumor is likely to cause difficulty.
Corpus luteum
A persistent corpus luteum is a normal component of pregnancy. Although it usually appears as a small cystic structure on ultrasonographic imaging, the corpus luteum of pregnancy can reach 10 cm in size. Other types of “functional” ovarian cysts may also be found during pregnancy. Most functional cysts resolve by the early second trimester.4,6 In rare cases, a cyst may develop complications such as torsion or rupture, causing acute pain or hemorrhage. Otherwise, a cystic tumor identified in the first trimester should be characterized and followed using ultrasonography (US).
Benign neoplasm
An adnexal mass that persists beyond the first trimester is more likely to be a neoplasm.3-5,10,11,22 Such a mass is generally considered clinically significant if it exceeds 5 cm in diameter and has a complex sonographic appearance. Usually such a neoplasm will be a benign cystadenoma or cystic teratoma.5,10-13,19,23,24
Benign cystic teratoma
This tumor can be identified with a fairly high degree of specificity using a variety of imaging techniques, with management based on the presumptive diagnosis. This tumor is unlikely to grow substantially during pregnancy. When it is smaller than 6 cm, such a tumor can simply be observed.14 A larger tumor can occasionally rupture or lead to torsion or obstruction of labor, but such occurrences are rare.
Benign cystadenoma
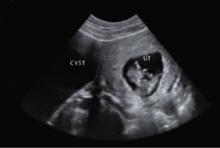
In an asymptomatic patient with imaging that suggests a benign cystadenoma (see sonogram), benign cystic teratoma, or other benign tumor, observation is reasonable in most cases.4,6,7,9-11,14,19 Operative intervention is required when there is less certainty regarding the benign nature of the tumor, an acute complication develops, or the tumor is expected to pose problems because of its large size alone.
Uterine leiomyoma
It is rare for an ovarian tumor detected during pregnancy to have a solid appearance on US. When it does, it may be a uterine leiomyoma mimicking an adnexal tumor (see intraoperative photograph). It should be reevaluated with more detailed US or magnetic resonance imaging.25
Malignancy
About 10% of adnexal masses that persist during pregnancy are malignant, according to recent series.4,5,7-10,12,13,24,26
Most of the ovarian cancers diagnosed during pregnancy are epithelial, and a substantial portion of these are low-malignant-potential (LMP) tumors.5,10,11,13,19,23,24,26,27 This ratio is in keeping with the age of these women, which also explains the stage distribution (most are stage 1) and the large percentage of germ-cell tumors detected. The majority of ovarian cancers discovered in pregnant women have a favorable prognosis.
Benign-appearing cystadenoma
A morphologically benign-appearing, large, cystic adnexal mass can be seen in association with an 11-week gestation.
Leiomyoma mimics an ovarian tumor
This 17-week gestation was marked by a large pedunculated leiomyoma that at fist appeared to be a right adnexal tumor.
Appearance of adnexal masses on US
A functional cyst such as a follicular cyst, corpus luteum cyst, or theca lutein cyst usually has smooth borders and a fluid center. Other cysts may sometimes contain debris, such as clotted blood, that suggests endometriosis or a simple cyst with bleeding into it.
A benign cystic teratoma often has multiple tissue lines, evidence of calcification, and layering of fat and fluid contents.
A benign cystadenoma usually has the appearance of a simple cyst without large septates, whereas a cystadenocarcinoma often contains septates, abnormal blood flow, increased vascularity, or all of these. However, it is impossible to definitively distinguish a cystadenoma from a cystadenocarcinoma using US imaging alone.
Functional cysts usually resolve by the second trimester. A cyst warrants closer scrutiny when it persists, is larger than 5 cm in diameter, or has a complex appearance on US.
CA-125 may be useful after the first trimester
The serum CA-125 level is typically elevated during the first trimester, but may be useful during later assessment or for follow-up of a malignancy.1
A markedly elevated serum level of alpha-fetoprotein (fractionated in some cases) has been reported in some gravidas with an endodermal sinus or mixed germ-cell ovarian tumor.2 Alpha-fetoprotein should be measured when there is suspicion for a germ-cell tumor based on clinical or US findings.
When a mass is discovered during cesarean section
Occasionally, an adnexal mass is detected at the time of cesarean section (FIGURE 1).3 This phenomenon is increasingly common, given the large number of cesarean deliveries in the United States. To eliminate the need for future surgery and avoid a delay in the diagnosis of an ovarian malignancy, inspect the adnexa routinely after closing the uterine incision in all women who deliver by cesarean section.
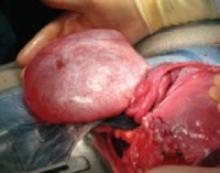
FIGURE 1 Mass discovered at cesarean section
This cystic tumor was discovered at cesarean section that was undertaken for obstetric indications.
CASE 2 LMP tumor is suspected
A 36-year-old gravida 3 para 1011 makes a prenatal visit during the first trimester. Her previous delivery was a cesarean section through a Pfannenstiel incision for a breech presentation. US imaging reveals a 6-week, 5-day fetus and a complex left adnexal mass, 4.5×3.9×4.1 cm. Imaging is repeated 1 month later at a tertiary-care center and shows an 11-week viable fetus, a right ovary with a corpus luteum cyst, and a left ovary with a 6.6×4 cm cystic mass with extensive vascular surface papillations that is suspicious for a low-malignant-potential (LMP) tumor. In several sonograms prior to the pregnancy, this mass appeared to be solid and was 3 cm in size.
When is surgery warranted?
Surgery is indicated when physical examination or imaging of a pregnant woman reveals an adnexal mass that is suspicious for malignancy, but the physician must weigh the benefit of prompt surgery against the risk to the pregnancy. This equation can be complicated in several ways. For example, surgical staging of clinically early ovarian cancer is more difficult due to the pregnant uterus, which is more extensively manipulated during these procedures. In addition, an optimal operation sometimes necessitates removal of the uterus.
At 13 weeks’ gestation, the patient described in case 2 underwent laparoscopy with peritoneal washings and left salpingo-oophorectomy, but the tumor ruptured during removal. Final pathology showed it to be a serous LMP tumor involving the surface of the left ovary. Washings were in line with this diagnosis.
The pregnancy continued uneventfully, and a repeat cesarean section was performed at 37 weeks through the Pfannenstiel scar, followed by limited surgical staging. Exploration and all biopsies were negative, and the final diagnosis was a stage 1C serous LMP tumor of the ovary.
The patient articulated a desire to preserve her fertility and was monitored with US imaging of the remaining ovary every 6 months.
Does ‘indolent’ behavior of malignancy justify watchful waiting?
LMP tumors comprise a relatively large percentage of ovarian “cancers” encountered during pregnancy. Some authors report the accurate identification of these tumors prospectively, based on ultrasonographic characteristics.4,5 When an LMP tumor is the likely diagnosis, serial observation during pregnancy may be appropriate because of the indolent nature of the tumor. Further studies are needed to refine preoperative diagnosis and determine the overall safety of this approach.
When the problem is acute
In rare cases, a pregnant patient will have (or develop during observation) an acute problem due to torsion or rupture of an adnexal mass. Some ovarian cancers may present acutely, such as a rapidly growing malignant germ-cell tumor or a ruptured and hemorrhaging granulosecell tumor. Emergent surgery is necessary to manage the acute adnexal disease and reduce the likelihood of pregnancy loss. These events are infrequent, occurring in less than 10% of women with a known, persistent adnexal mass during pregnancy.4-14 Furthermore, recent studies have not found a substantial pregnancy complication rate associated with such emergency surgeries.
CASE 3 Suspicious mass, ascites signal need for surgery
A 19-year-old gravida 1 para 0 seeks prenatal care at 17 weeks’ gestation, complaining of rapidly enlarging abdominal girth. The physical examination estimates gestational size to be considerably greater than dates, but US is consistent with a 17-week intrauterine pregnancy. Imaging also reveals a 12-cm heterogenous left adnexal mass and a large amount of ascites.
Surgery is clearly warranted, but how extensive should it be?
When a malignancy is detected, a thorough staging procedure may be justified, depending on gestational age, exposure, desires of the patient, and operative findings. A midline incision is preferred.
Pregnant and nonpregnant women with stage 1A or 1C epithelial ovarian cancer who undergo fertility-preserving surgery (with chemotherapy in selected patients) have a good prognosis and a high likelihood of achieving a subsequent normal pregnancy.15 The same is true for women with a malignant germ-cell tumor of the ovary, even when disease is advanced.16 However, careful surgical staging is necessary.
The most important consideration when deciding whether to continue the pregnancy is the need for adjuvant chemotherapy. Depending on the gestational age and diagnosis, a short delay (4 to 6 weeks) may be appropriate to allow the pregnancy to progress beyond the first trimester or to maturity.
In case 3, a laparotomy was performed at 19 weeks’ gestation via a midline incision, and approximately 5.3 L of ascites was evacuated. A large, nonadherent left ovarian tumor was removed. The right ovary appeared to be normal, as did the gravid uterus, which was minimally manipulated. The rest of the surgical exploration was normal, and the distal portion of the omentum was excised. The frozen-section diagnosis was a malignant stromal tumor. Final pathology showed an 18×13.5×8.8 cm, poorly differentiated, SertoliLeydig-cell tumor with heterologous elements in the form of mucinous epithelium. The omentum was negative for tumor.
Chemotherapy was initiated in the third trimester, based on the limited data available, with intravenous etoposide and platinum administered every 21 days. The patient received 3 cycles of chemotherapy prior to delivery.
At 37 weeks’ gestation, labor was successfully induced. After delivery, bleomycin was added to the chemotherapy regimen, and 3 additional courses with all 3 agents were administered. The patient was lost to follow-up shortly after completing chemotherapy.
Clearly, an informed discussion of the options with the patient is imperative before any surgery, especially when chemotherapy may be delayed. Pregnancy does not appear to alter the prognosis for the patient with an ovarian malignancy, and ovarian cancer has not been reported to metastasize to the fetus.
Pregnant women have a very low rate of ovarian cancer
Leiserowitz GS, Xing G, Cress R, Brahmbhatt B, Dalrymple JL, Smith LH. Adnexal masses in pregnancy: how often are they malignant? Gynecol Oncol. 2006;101:315–321.
Ovarian malignancies are rare during pregnancy. When they do occur, they are likely to be early stage and to have a favorable outcome, according to this recent population-based study.
Using 3 large databases containing records on 4,846,505 California obstetric patients between 1991 and 1999, Leiserowitz and colleagues identified 9,375 women who had an ovarian mass associated with pregnancy. Of these, 87 had ovarian cancer and 115 had a low-malignant-potential (LMP) tumor, for a cancer occurrence rate of 0.93%, or 0.0179 per 1,000 deliveries. Thirty-four of the 87 cancers were germ-cell tumors.
Of the 87 ovarian cancers, 65.5% were localized, 6.9% regional, 23% remote, and 4.6% of unknown stage. The respective rates for LMP tumors were 81.7%, 7.8%, 4.4%, and 6.1%.
Women with malignant tumors were more likely than pregnant controls without cancer to undergo cesarean delivery, hysterectomy, transfusion, and prolonged hospitalization. These women did not, however, have a higher rate of adverse neonatal outcomes.
When cancer is advanced
Few data shed light on whether a pregnancy should continue when ovarian cancer is advanced.17 The definitive surgical approach must be highly individualized.
It is not always possible to make an accurate diagnosis based on a frozen section. In such a case, the pregnancy should be preserved until the time of definitive diagnosis. As always, the patient’s wishes and gestational age must be considered.
How factors besides malignancy can influence care
Most persistent adnexal masses move well out of the pelvis as pregnancy progresses. Occasionally, however, an ovarian tumor may be located in the posterior cul-de-sac even at term, a fact easily confirmed by examination or US.4,7 A tumor in the posterior cul-de-sac can obstruct delivery or rupture. When it has a benign cystic appearance on US, it may be decompressed via transvaginal aspiration. Otherwise, the best approach is cesarean section and concomitant management of the mass.
When size alone is the problem
Some ovarian tumors are so large they seem incompatible with an advancing pregnancy. Tumors up to 20 cm in diameter have been removed intact at the time of cesarean section (FIGURE 2).18 The tumor may accommodate in shape and become less problematic as it is gradually pushed into the upper abdomen (FIGURE 3).
The ability of the peritoneal cavity to accommodate a tumor varies greatly among women. As pregnancy advances, the likelihood that a large cystic mass will rupture tends to increase. Depending on the circumstances, percutaneous aspiration7,18 or removal of a benign-appearing cystic tumor may be appropriate.
FIGURE 2 Even a very large tumor may coexist with advancing pregnancy
This benign serous cystadenoma was exteriorized at the time of cesarean section at term.
FIGURE 3 Large ovarian tumor has accommodated to the pregnancy
Laparotomy—performed at term for cesarean section and to manage this large tumor—revealed that the tumor had accommodated in shape between the enlarging pregnant uterus and the abdominal wall.
When is the best time to operate?
Surgery is generally not recommended during the first trimester.5-11 Among the reasons are the high likelihood of a corpus luteum cyst, the low likelihood of an invasive malignancy, the low risk of adnexal complications associated with observation, and the potential for pregnancy loss or teratogenicity. However, as pregnancy progresses beyond the first trimester, surgery poses other problems: Operative exposure diminishes and the need to manipulate the pregnant uterus increases.
Can surgery be delayed when a mass is detected?
Schmeler KM, Mayo-Smith WW, Peipert JF, Weitzen S, Manuel MD, Gordinier ME. Adnexal masses in pregnancy: surgery compared with observation. Obstet Gynecol. 2005;105:1098–1103.
Close observation is a reasonable alternative to operative intervention during pregnancy, unless a malignancy is suspected.
Schmeler and colleagues reviewed the cases of 59 women who had an adnexal mass larger than 5 cm in diameter detected during pregnancy, out of a total of 127,177 deliveries at a single institution between 1990 and 2003. Antepartum surgery was performed in 17 women (29%). Of these, 13 cases had ultrasonographic findings suggesting malignancy, and 4 had ovarian torsion. The remaining women were observed, with surgery delayed until the time of cesarean section or later.
Twenty-five of the 59 masses (42%) were dermoid cysts. Cancer was diagnosed in 4 patients (6.8%), and 1 patient (1.7%) had an LMP tumor. All 5 cases (100%) involving a malignancy had a suspicious US appearance and were identified during antepartum surgery, whereas only 12 patients with a benign tumor (22%) underwent surgery prior to delivery.
Surgery poses risks to the pregnancy
Elective surgery for an adnexal mass any time during pregnancy increases the risk of pregnancy loss and the likelihood of intrauterine growth restriction (IUGR) and preterm delivery.5,7,10,13,19 A 1989 study from Sweden20 defined a cohort of 5,405 women (from 720,000 births) who were known to have a nonobstetric operation while pregnant, with the following results:
- Congenital malformation and stillbirth were not increased in the women undergoing surgery
- The number of very-low- and low-birth-weight infants did rise, however—the result of both prematurity and IUGR
- Also elevated was the incidence of infants born alive but dying within 168 hours; these risks increased regardless of trimester
- No specific type of anesthesia or operation was associated with adverse reproductive outcomes, and the cause of those adverse outcomes was not determined.
Some recent data suggest that adnexal surgery during the late second or early third trimester poses the greatest risk of preterm delivery or IUGR, or both.13
Window of opportunity: early to mid- second trimester
During this time frame, elective surgery for an adnexal mass still affords some pelvic exposure without the need for significant uterine manipulation and has been associated with a lower risk of pregnancy complications.
The other window for operation is at the time of cesarean section. An elective cesarean section is sometimes performed specifically to manage a persistent adnexal mass. Among the factors that warrant consideration when contemplating this approach are the elective uterine incision (with its attendant implications for future pregnancies), the higher risks associated with cesarean delivery in general, the type of skin incision (a vertical incision is appropriate in the event of ovarian malignancy), the potential for better exposure or laparoscopy at a later date, the increased difficulty of ovarian cystectomy at the time of cesarean section, and the patient’s wishes.
The data on laparoscopy during the first and second trimesters of pregnancy indicate that it is as safe as laparotomy. A 1997 Swedish study21 identified cohorts of 2,181 women undergoing laparoscopy and 1,522 women undergoing laparotomy (from a total of 2,015,000 deliveries) between the fourth and 20th weeks of pregnancy. In both groups there was an increased risk for the infant to weigh less than 2,500 g, to be delivered before 37 weeks, and to have IUGR. There were no differences between the 2 groups for these and other adverse outcomes.
Small series of laparoscopic procedures to manage an adnexal mass during pregnancy suggest that this approach is most applicable during the first (for highly selected emergent cases) or early second trimester to manage masses less than 10 cm in diameter, particularly when adnexectomy is planned.
Laparoscopy may be considered “minimally invasive” because it reduces manipulation of the pregnant uterus during adnexal surgery. However, it is more difficult to assess and remove ovarian cysts laparoscopically, although an early ovarian malignancy could be staged via laparoscopy by an experienced surgeon.
Considerations during laparotomy
When performing a laparotomy or cesarean section for an adnexal mass, the surgeon must take into account a number of variables when selecting the type of incision (ie, vertical vs transverse). In general, if malignancy is suspected, or if uterine manipulation is to be minimized, a vertical incision is best. Other considerations include a prior scar, body habitus, obstetric issues, and the patient’s wishes.
The author reports no financial relationships relevant to this article.
1. Spitzer M, Kaushal N, Benjamin F. Maternal CA-125 levels in pregnancy and the puerperium. J Reprod Med. 1998;43:387-392.
2. Aoki Y, Higashino M, Ishii S, Tanaka K. Yolk sac tumor of the ovary during pregnancy: a case report. Gynecol Oncol. 2005;99:497-499.
3. Koonings PP, Platt LD, Wallace R. Incidental adnexal neoplasms at cesarean section. Obstet Gynecol. 1988;72:767-769.
4. Zanetta G, Mariani E, Lissoni A, et al. A prospective study of the role of ultrasound in the management of adnexal masses in pregnancy. BJOG. 2003;110:578-583.
5. Sherard GB, 3rd, Hodson CA, Williams HJ, et al. Adnexal masses and pregnancy: a 12 year experience. Am J Obstet Gynecol. 2003;189:358-363.
6. Bernhard LM, Klebba PK, Gray DL, Mutch DG. Predictors of persistence of adnexal masses in pregnancy. Obstet Gynecol. 1999;93:585-589.
7. Platek DN, Henderson CE, Goldberg GL. The management of a persistent adnexal mass in pregnancy. Am J Obstet Gynecol. 1995;173:1236-1240.
8. Bromley B, Benacerraf B. Adnexal masses during pregnancy: accuracy of sonographic diagnosis and outcome. J Ultrasound Med. 1997;16:447-452.
9. Hill LM, Connors-Beatty DJ, Nowak A, Tush B. The role of ultrasonography in the detection and management of adnexal mass during the second and third trimesters of pregnancy. Am J Obstet Gynecol. 1998;179:703-707.
10. Agarwal N, Parul, Kriplani A, Bhatla N, Gupta A. Management and outcome of pregnancies complicated with adnexal masses. Arch Gynecol Obstet. 2003;267:148-152.
11. Schmeler KM, Mayo-Smith WW, Peipert JF, Weitzen S, Manuel MD, Gordinier ME. Adnexal masses in pregnancy: surgery compared with observation. Obstet Gynecol. 2005;105:1098-1103.
12. Coenen VH, Dunton C, Cardonick E, Berghella V. Persistent adnexal masses during pregnancy. Obstet Gynecol. 1999;93:66S.-
13. Whitecar P, Turner S, Higby K. Adnexal masses in pregnancy: a review of 130 cases undergoing surgical management. Am J Obstet Gynecol. 1999;181:19-24.
14. Caspi B, Levi R, Appelman Z, Rabinerson D, Goldman G, Hagay Z. Conservative management of ovarian cystic teratoma during pregnancy and labor. Am J Obstet Gynecol. 2000;182:503-505.
15. Schilder JM, Thompson AM, DePriest PD, et al. Outcome of reproductive age women with stage IA or IC invasive epithelial ovarian cancer treated with fertility-sparing therapy. Gynecol Oncol. 2002;87:1-7.
16. Tangir J, Zelterman D, Ma W, Schwartz PE. Reproductive function after conservative surgery and chemotherapy for malignant germ cell tumors of the ovary. Obstet Gynecol. 2003;101:251-257.
17. Ferrandina G, Distefano M, Testa A, De Vincenzo R, Scambia G. Management of an advanced ovarian cancer at 15 weeks of gestation: case report and literature review. Gynecol Oncol. 2005;97:693-696.
18. Caspi B, Ben-Arie A, Appelman Z, Or Y, Hagay Z. Aspiration of simple pelvic cysts during pregnancy. Gynecol Obstet Invest. 2000;49:102-105.
19. Usui R, Minakami H, Kosuge S, et al. A retrospective survey of clinical, pathologic, and prognostic features of adnexal masses operated on during pregnancy. J Obstet Gynaecol Res. 2000;26(2):89-93.
20. Mazze RI, Källén B. Reproductive outcome after anesthesia and operation during pregnancy; a registry study of 5,405 cases. Am J Obstet Gynecol. 1989;161:1178-1185.
21. Reedy MB, Källén B, Kuehl TJ. Laparoscopy during pregnancy: a study of five fetal outcome parameters with use of the Swedish health registry. Am J Obstet Gynecol. 1997;177:673-679.
22. Hermans RHM, Fischer D-C, van der Putten HWHM, et al. Adnexal masses in pregnancy. Onkologie. 2003;26:167-172.
23. Hoffman MS. Primary ovarian carcinoma during pregnancy. Clin Consul Obstet Gynecol. 1995;7:237-241.
24. Ueda M, Ueki M. Ovarian tumors associated with pregnancy. Int J Obstet Gynaecol. 1996;55:59-65.
25. Curtis M, Hopkins MP, Zarlingo T, et al. Magnetic resonance imaging to avoid laparotomy in pregnancy. Obstet Gynecol. 1993;82:833-836.
26. Leiserowitz GS, Xing G, Cress R, et al. Adnexal masses in pregnancy; how often are they malignant? Gynecol Oncol. 2006;101:315-321.
27. Rahman MS, Al-Sibai MH, Rahman J, et al. Ovarian carcinoma associated with pregnancy. A review of 9 cases. Acta Obstet Gynecol Scand. 2002;81:260-264.
CASE 1 An enlarging cystic tumor
A 20-year-old gravida 3 para 1011 visits the emergency department with persistent right flank pain. Although ultrasonography (US) shows a 21-week gestation, the patient has had no prenatal care. Imaging also reveals a right-sided ovarian tumor, 14×11×8 cm, that is mainly cystic with some internal echogenicity.
At 30 weeks’ gestation, a gynecologic oncologist is consulted. Repeat US reveals the mass to be about 20 cm in diameter and cystic, without internal papillation. The patient’s CA-125 level is 12 U/mL. Based on this information, the physicians decide the likely finding is a benign ovarian cystadenoma.
How should they proceed?
The discovery of an adnexal mass during pregnancy isn’t as rare as you might think—depending on when and how closely you look, it occurs in about 1 in 100 gestations. In most cases, we have found, the mass is clearly benign (TABLE 1), warranting only observation.
TABLE 1
Adnexal masses removed during pregnancy: Histologic profile
| HISTOLOGIC TYPE | NUMBER (%) |
|---|---|
| Cystadenoma | 549 (33) |
| Dermoid | 451 (27) |
| Paraovarian/paratubal | 204 (12) |
| Functional | 237 (14) |
| Endometrioma | 55 (3) |
| Benign stromal | 28 (2) |
| Leiomyoma | 23 (1.5) |
| Luteoma | 8 (0.5) |
| Miscellaneous | 55 (3) |
| Malignant | 68 (4) |
| Total | 1,678 |
| Data supplied by the authors from surgical experience | |
In the case described above, the physicians followed the patient and removed the mass at term because it was cystic with no other indications of malignancy. At 37 weeks’ gestation, a cesarean section was performed through a midline laparotomy incision, followed by removal of the ovarian tumor, which was benign. The pathologist measured the tumor at 16×12×4 cm and determined that it was a corpus luteum cyst.
Presence of mass raises questions
Despite the rarity of malignancy, the discovery of an ovarian mass during pregnancy prompts several important questions:
How should the mass be assessed? How can the likelihood of malignancy be determined as quickly and efficiently as possible, without jeopardy to the pregnancy?
When is surgical intervention warranted? And when can it be postponed? Specifically, is elective operative intervention for a tumor that is probably benign appropriate during pregnancy?
When is the best time to operate? And what is the optimal surgical route?
In this article, we address these questions with a focus on intervention. As we’ll explain, only a small percentage of gravidas who have an adnexal mass require surgery during pregnancy. When surgery is necessary, it is usually indicated for an emergent problem or suspicion of malignancy. Even when ovarian cancer is confirmed, we have found that it is usually in its early stages and therefore has a favorable prognosis (TABLE 2).
TABLE 2
Malignant adnexal masses removed during pregnancy
| HISTOLOGIC TYPE | NUMBER (%) |
|---|---|
| Epithelial | 101 (28) |
| Borderline epithelial | 147 (40) |
| Germ-cell dysgerminoma | 47 (13) |
| Other | 34 (9) |
| Stromal | 24 (7) |
| Undifferentiated | 5 (1.4) |
| Sarcoma | 2 (0.5) |
| Metastatic | 4 (1.1) |
| Total | 364 |
| Data supplied by the authors from surgical experience | |
How should a mass be assessed?
Ultrasonography and other imaging often reveal the presence of a mass and help determine whether it is benign or malignant. In fact, most adnexal masses discovered during pregnancy are incidental findings at the time of routine prenatal US. (see the most commonly found tumors.) Operative intervention is required in 3 situations:
- malignancy is suspected
- an acute complication develops
- the sheer size of the tumor is likely to cause difficulty.
Corpus luteum
A persistent corpus luteum is a normal component of pregnancy. Although it usually appears as a small cystic structure on ultrasonographic imaging, the corpus luteum of pregnancy can reach 10 cm in size. Other types of “functional” ovarian cysts may also be found during pregnancy. Most functional cysts resolve by the early second trimester.4,6 In rare cases, a cyst may develop complications such as torsion or rupture, causing acute pain or hemorrhage. Otherwise, a cystic tumor identified in the first trimester should be characterized and followed using ultrasonography (US).
Benign neoplasm
An adnexal mass that persists beyond the first trimester is more likely to be a neoplasm.3-5,10,11,22 Such a mass is generally considered clinically significant if it exceeds 5 cm in diameter and has a complex sonographic appearance. Usually such a neoplasm will be a benign cystadenoma or cystic teratoma.5,10-13,19,23,24
Benign cystic teratoma
This tumor can be identified with a fairly high degree of specificity using a variety of imaging techniques, with management based on the presumptive diagnosis. This tumor is unlikely to grow substantially during pregnancy. When it is smaller than 6 cm, such a tumor can simply be observed.14 A larger tumor can occasionally rupture or lead to torsion or obstruction of labor, but such occurrences are rare.
Benign cystadenoma
In an asymptomatic patient with imaging that suggests a benign cystadenoma (see sonogram), benign cystic teratoma, or other benign tumor, observation is reasonable in most cases.4,6,7,9-11,14,19 Operative intervention is required when there is less certainty regarding the benign nature of the tumor, an acute complication develops, or the tumor is expected to pose problems because of its large size alone.
Uterine leiomyoma
It is rare for an ovarian tumor detected during pregnancy to have a solid appearance on US. When it does, it may be a uterine leiomyoma mimicking an adnexal tumor (see intraoperative photograph). It should be reevaluated with more detailed US or magnetic resonance imaging.25
Malignancy
About 10% of adnexal masses that persist during pregnancy are malignant, according to recent series.4,5,7-10,12,13,24,26
Most of the ovarian cancers diagnosed during pregnancy are epithelial, and a substantial portion of these are low-malignant-potential (LMP) tumors.5,10,11,13,19,23,24,26,27 This ratio is in keeping with the age of these women, which also explains the stage distribution (most are stage 1) and the large percentage of germ-cell tumors detected. The majority of ovarian cancers discovered in pregnant women have a favorable prognosis.
Benign-appearing cystadenoma
A morphologically benign-appearing, large, cystic adnexal mass can be seen in association with an 11-week gestation.
Leiomyoma mimics an ovarian tumor
This 17-week gestation was marked by a large pedunculated leiomyoma that at fist appeared to be a right adnexal tumor.
Appearance of adnexal masses on US
A functional cyst such as a follicular cyst, corpus luteum cyst, or theca lutein cyst usually has smooth borders and a fluid center. Other cysts may sometimes contain debris, such as clotted blood, that suggests endometriosis or a simple cyst with bleeding into it.
A benign cystic teratoma often has multiple tissue lines, evidence of calcification, and layering of fat and fluid contents.
A benign cystadenoma usually has the appearance of a simple cyst without large septates, whereas a cystadenocarcinoma often contains septates, abnormal blood flow, increased vascularity, or all of these. However, it is impossible to definitively distinguish a cystadenoma from a cystadenocarcinoma using US imaging alone.
Functional cysts usually resolve by the second trimester. A cyst warrants closer scrutiny when it persists, is larger than 5 cm in diameter, or has a complex appearance on US.
CA-125 may be useful after the first trimester
The serum CA-125 level is typically elevated during the first trimester, but may be useful during later assessment or for follow-up of a malignancy.1
A markedly elevated serum level of alpha-fetoprotein (fractionated in some cases) has been reported in some gravidas with an endodermal sinus or mixed germ-cell ovarian tumor.2 Alpha-fetoprotein should be measured when there is suspicion for a germ-cell tumor based on clinical or US findings.
When a mass is discovered during cesarean section
Occasionally, an adnexal mass is detected at the time of cesarean section (FIGURE 1).3 This phenomenon is increasingly common, given the large number of cesarean deliveries in the United States. To eliminate the need for future surgery and avoid a delay in the diagnosis of an ovarian malignancy, inspect the adnexa routinely after closing the uterine incision in all women who deliver by cesarean section.
FIGURE 1 Mass discovered at cesarean section
This cystic tumor was discovered at cesarean section that was undertaken for obstetric indications.
CASE 2 LMP tumor is suspected
A 36-year-old gravida 3 para 1011 makes a prenatal visit during the first trimester. Her previous delivery was a cesarean section through a Pfannenstiel incision for a breech presentation. US imaging reveals a 6-week, 5-day fetus and a complex left adnexal mass, 4.5×3.9×4.1 cm. Imaging is repeated 1 month later at a tertiary-care center and shows an 11-week viable fetus, a right ovary with a corpus luteum cyst, and a left ovary with a 6.6×4 cm cystic mass with extensive vascular surface papillations that is suspicious for a low-malignant-potential (LMP) tumor. In several sonograms prior to the pregnancy, this mass appeared to be solid and was 3 cm in size.
When is surgery warranted?
Surgery is indicated when physical examination or imaging of a pregnant woman reveals an adnexal mass that is suspicious for malignancy, but the physician must weigh the benefit of prompt surgery against the risk to the pregnancy. This equation can be complicated in several ways. For example, surgical staging of clinically early ovarian cancer is more difficult due to the pregnant uterus, which is more extensively manipulated during these procedures. In addition, an optimal operation sometimes necessitates removal of the uterus.
At 13 weeks’ gestation, the patient described in case 2 underwent laparoscopy with peritoneal washings and left salpingo-oophorectomy, but the tumor ruptured during removal. Final pathology showed it to be a serous LMP tumor involving the surface of the left ovary. Washings were in line with this diagnosis.
The pregnancy continued uneventfully, and a repeat cesarean section was performed at 37 weeks through the Pfannenstiel scar, followed by limited surgical staging. Exploration and all biopsies were negative, and the final diagnosis was a stage 1C serous LMP tumor of the ovary.
The patient articulated a desire to preserve her fertility and was monitored with US imaging of the remaining ovary every 6 months.
Does ‘indolent’ behavior of malignancy justify watchful waiting?
LMP tumors comprise a relatively large percentage of ovarian “cancers” encountered during pregnancy. Some authors report the accurate identification of these tumors prospectively, based on ultrasonographic characteristics.4,5 When an LMP tumor is the likely diagnosis, serial observation during pregnancy may be appropriate because of the indolent nature of the tumor. Further studies are needed to refine preoperative diagnosis and determine the overall safety of this approach.
When the problem is acute
In rare cases, a pregnant patient will have (or develop during observation) an acute problem due to torsion or rupture of an adnexal mass. Some ovarian cancers may present acutely, such as a rapidly growing malignant germ-cell tumor or a ruptured and hemorrhaging granulosecell tumor. Emergent surgery is necessary to manage the acute adnexal disease and reduce the likelihood of pregnancy loss. These events are infrequent, occurring in less than 10% of women with a known, persistent adnexal mass during pregnancy.4-14 Furthermore, recent studies have not found a substantial pregnancy complication rate associated with such emergency surgeries.
CASE 3 Suspicious mass, ascites signal need for surgery
A 19-year-old gravida 1 para 0 seeks prenatal care at 17 weeks’ gestation, complaining of rapidly enlarging abdominal girth. The physical examination estimates gestational size to be considerably greater than dates, but US is consistent with a 17-week intrauterine pregnancy. Imaging also reveals a 12-cm heterogenous left adnexal mass and a large amount of ascites.
Surgery is clearly warranted, but how extensive should it be?
When a malignancy is detected, a thorough staging procedure may be justified, depending on gestational age, exposure, desires of the patient, and operative findings. A midline incision is preferred.
Pregnant and nonpregnant women with stage 1A or 1C epithelial ovarian cancer who undergo fertility-preserving surgery (with chemotherapy in selected patients) have a good prognosis and a high likelihood of achieving a subsequent normal pregnancy.15 The same is true for women with a malignant germ-cell tumor of the ovary, even when disease is advanced.16 However, careful surgical staging is necessary.
The most important consideration when deciding whether to continue the pregnancy is the need for adjuvant chemotherapy. Depending on the gestational age and diagnosis, a short delay (4 to 6 weeks) may be appropriate to allow the pregnancy to progress beyond the first trimester or to maturity.
In case 3, a laparotomy was performed at 19 weeks’ gestation via a midline incision, and approximately 5.3 L of ascites was evacuated. A large, nonadherent left ovarian tumor was removed. The right ovary appeared to be normal, as did the gravid uterus, which was minimally manipulated. The rest of the surgical exploration was normal, and the distal portion of the omentum was excised. The frozen-section diagnosis was a malignant stromal tumor. Final pathology showed an 18×13.5×8.8 cm, poorly differentiated, SertoliLeydig-cell tumor with heterologous elements in the form of mucinous epithelium. The omentum was negative for tumor.
Chemotherapy was initiated in the third trimester, based on the limited data available, with intravenous etoposide and platinum administered every 21 days. The patient received 3 cycles of chemotherapy prior to delivery.
At 37 weeks’ gestation, labor was successfully induced. After delivery, bleomycin was added to the chemotherapy regimen, and 3 additional courses with all 3 agents were administered. The patient was lost to follow-up shortly after completing chemotherapy.
Clearly, an informed discussion of the options with the patient is imperative before any surgery, especially when chemotherapy may be delayed. Pregnancy does not appear to alter the prognosis for the patient with an ovarian malignancy, and ovarian cancer has not been reported to metastasize to the fetus.
Pregnant women have a very low rate of ovarian cancer
Leiserowitz GS, Xing G, Cress R, Brahmbhatt B, Dalrymple JL, Smith LH. Adnexal masses in pregnancy: how often are they malignant? Gynecol Oncol. 2006;101:315–321.
Ovarian malignancies are rare during pregnancy. When they do occur, they are likely to be early stage and to have a favorable outcome, according to this recent population-based study.
Using 3 large databases containing records on 4,846,505 California obstetric patients between 1991 and 1999, Leiserowitz and colleagues identified 9,375 women who had an ovarian mass associated with pregnancy. Of these, 87 had ovarian cancer and 115 had a low-malignant-potential (LMP) tumor, for a cancer occurrence rate of 0.93%, or 0.0179 per 1,000 deliveries. Thirty-four of the 87 cancers were germ-cell tumors.
Of the 87 ovarian cancers, 65.5% were localized, 6.9% regional, 23% remote, and 4.6% of unknown stage. The respective rates for LMP tumors were 81.7%, 7.8%, 4.4%, and 6.1%.
Women with malignant tumors were more likely than pregnant controls without cancer to undergo cesarean delivery, hysterectomy, transfusion, and prolonged hospitalization. These women did not, however, have a higher rate of adverse neonatal outcomes.
When cancer is advanced
Few data shed light on whether a pregnancy should continue when ovarian cancer is advanced.17 The definitive surgical approach must be highly individualized.
It is not always possible to make an accurate diagnosis based on a frozen section. In such a case, the pregnancy should be preserved until the time of definitive diagnosis. As always, the patient’s wishes and gestational age must be considered.
How factors besides malignancy can influence care
Most persistent adnexal masses move well out of the pelvis as pregnancy progresses. Occasionally, however, an ovarian tumor may be located in the posterior cul-de-sac even at term, a fact easily confirmed by examination or US.4,7 A tumor in the posterior cul-de-sac can obstruct delivery or rupture. When it has a benign cystic appearance on US, it may be decompressed via transvaginal aspiration. Otherwise, the best approach is cesarean section and concomitant management of the mass.
When size alone is the problem
Some ovarian tumors are so large they seem incompatible with an advancing pregnancy. Tumors up to 20 cm in diameter have been removed intact at the time of cesarean section (FIGURE 2).18 The tumor may accommodate in shape and become less problematic as it is gradually pushed into the upper abdomen (FIGURE 3).
The ability of the peritoneal cavity to accommodate a tumor varies greatly among women. As pregnancy advances, the likelihood that a large cystic mass will rupture tends to increase. Depending on the circumstances, percutaneous aspiration7,18 or removal of a benign-appearing cystic tumor may be appropriate.
FIGURE 2 Even a very large tumor may coexist with advancing pregnancy
This benign serous cystadenoma was exteriorized at the time of cesarean section at term.
FIGURE 3 Large ovarian tumor has accommodated to the pregnancy
Laparotomy—performed at term for cesarean section and to manage this large tumor—revealed that the tumor had accommodated in shape between the enlarging pregnant uterus and the abdominal wall.
When is the best time to operate?
Surgery is generally not recommended during the first trimester.5-11 Among the reasons are the high likelihood of a corpus luteum cyst, the low likelihood of an invasive malignancy, the low risk of adnexal complications associated with observation, and the potential for pregnancy loss or teratogenicity. However, as pregnancy progresses beyond the first trimester, surgery poses other problems: Operative exposure diminishes and the need to manipulate the pregnant uterus increases.
Can surgery be delayed when a mass is detected?
Schmeler KM, Mayo-Smith WW, Peipert JF, Weitzen S, Manuel MD, Gordinier ME. Adnexal masses in pregnancy: surgery compared with observation. Obstet Gynecol. 2005;105:1098–1103.
Close observation is a reasonable alternative to operative intervention during pregnancy, unless a malignancy is suspected.
Schmeler and colleagues reviewed the cases of 59 women who had an adnexal mass larger than 5 cm in diameter detected during pregnancy, out of a total of 127,177 deliveries at a single institution between 1990 and 2003. Antepartum surgery was performed in 17 women (29%). Of these, 13 cases had ultrasonographic findings suggesting malignancy, and 4 had ovarian torsion. The remaining women were observed, with surgery delayed until the time of cesarean section or later.
Twenty-five of the 59 masses (42%) were dermoid cysts. Cancer was diagnosed in 4 patients (6.8%), and 1 patient (1.7%) had an LMP tumor. All 5 cases (100%) involving a malignancy had a suspicious US appearance and were identified during antepartum surgery, whereas only 12 patients with a benign tumor (22%) underwent surgery prior to delivery.
Surgery poses risks to the pregnancy
Elective surgery for an adnexal mass any time during pregnancy increases the risk of pregnancy loss and the likelihood of intrauterine growth restriction (IUGR) and preterm delivery.5,7,10,13,19 A 1989 study from Sweden20 defined a cohort of 5,405 women (from 720,000 births) who were known to have a nonobstetric operation while pregnant, with the following results:
- Congenital malformation and stillbirth were not increased in the women undergoing surgery
- The number of very-low- and low-birth-weight infants did rise, however—the result of both prematurity and IUGR
- Also elevated was the incidence of infants born alive but dying within 168 hours; these risks increased regardless of trimester
- No specific type of anesthesia or operation was associated with adverse reproductive outcomes, and the cause of those adverse outcomes was not determined.
Some recent data suggest that adnexal surgery during the late second or early third trimester poses the greatest risk of preterm delivery or IUGR, or both.13
Window of opportunity: early to mid- second trimester
During this time frame, elective surgery for an adnexal mass still affords some pelvic exposure without the need for significant uterine manipulation and has been associated with a lower risk of pregnancy complications.
The other window for operation is at the time of cesarean section. An elective cesarean section is sometimes performed specifically to manage a persistent adnexal mass. Among the factors that warrant consideration when contemplating this approach are the elective uterine incision (with its attendant implications for future pregnancies), the higher risks associated with cesarean delivery in general, the type of skin incision (a vertical incision is appropriate in the event of ovarian malignancy), the potential for better exposure or laparoscopy at a later date, the increased difficulty of ovarian cystectomy at the time of cesarean section, and the patient’s wishes.
The data on laparoscopy during the first and second trimesters of pregnancy indicate that it is as safe as laparotomy. A 1997 Swedish study21 identified cohorts of 2,181 women undergoing laparoscopy and 1,522 women undergoing laparotomy (from a total of 2,015,000 deliveries) between the fourth and 20th weeks of pregnancy. In both groups there was an increased risk for the infant to weigh less than 2,500 g, to be delivered before 37 weeks, and to have IUGR. There were no differences between the 2 groups for these and other adverse outcomes.
Small series of laparoscopic procedures to manage an adnexal mass during pregnancy suggest that this approach is most applicable during the first (for highly selected emergent cases) or early second trimester to manage masses less than 10 cm in diameter, particularly when adnexectomy is planned.
Laparoscopy may be considered “minimally invasive” because it reduces manipulation of the pregnant uterus during adnexal surgery. However, it is more difficult to assess and remove ovarian cysts laparoscopically, although an early ovarian malignancy could be staged via laparoscopy by an experienced surgeon.
Considerations during laparotomy
When performing a laparotomy or cesarean section for an adnexal mass, the surgeon must take into account a number of variables when selecting the type of incision (ie, vertical vs transverse). In general, if malignancy is suspected, or if uterine manipulation is to be minimized, a vertical incision is best. Other considerations include a prior scar, body habitus, obstetric issues, and the patient’s wishes.
The author reports no financial relationships relevant to this article.
CASE 1 An enlarging cystic tumor
A 20-year-old gravida 3 para 1011 visits the emergency department with persistent right flank pain. Although ultrasonography (US) shows a 21-week gestation, the patient has had no prenatal care. Imaging also reveals a right-sided ovarian tumor, 14×11×8 cm, that is mainly cystic with some internal echogenicity.
At 30 weeks’ gestation, a gynecologic oncologist is consulted. Repeat US reveals the mass to be about 20 cm in diameter and cystic, without internal papillation. The patient’s CA-125 level is 12 U/mL. Based on this information, the physicians decide the likely finding is a benign ovarian cystadenoma.
How should they proceed?
The discovery of an adnexal mass during pregnancy isn’t as rare as you might think—depending on when and how closely you look, it occurs in about 1 in 100 gestations. In most cases, we have found, the mass is clearly benign (TABLE 1), warranting only observation.
TABLE 1
Adnexal masses removed during pregnancy: Histologic profile
| HISTOLOGIC TYPE | NUMBER (%) |
|---|---|
| Cystadenoma | 549 (33) |
| Dermoid | 451 (27) |
| Paraovarian/paratubal | 204 (12) |
| Functional | 237 (14) |
| Endometrioma | 55 (3) |
| Benign stromal | 28 (2) |
| Leiomyoma | 23 (1.5) |
| Luteoma | 8 (0.5) |
| Miscellaneous | 55 (3) |
| Malignant | 68 (4) |
| Total | 1,678 |
| Data supplied by the authors from surgical experience | |
In the case described above, the physicians followed the patient and removed the mass at term because it was cystic with no other indications of malignancy. At 37 weeks’ gestation, a cesarean section was performed through a midline laparotomy incision, followed by removal of the ovarian tumor, which was benign. The pathologist measured the tumor at 16×12×4 cm and determined that it was a corpus luteum cyst.
Presence of mass raises questions
Despite the rarity of malignancy, the discovery of an ovarian mass during pregnancy prompts several important questions:
How should the mass be assessed? How can the likelihood of malignancy be determined as quickly and efficiently as possible, without jeopardy to the pregnancy?
When is surgical intervention warranted? And when can it be postponed? Specifically, is elective operative intervention for a tumor that is probably benign appropriate during pregnancy?
When is the best time to operate? And what is the optimal surgical route?
In this article, we address these questions with a focus on intervention. As we’ll explain, only a small percentage of gravidas who have an adnexal mass require surgery during pregnancy. When surgery is necessary, it is usually indicated for an emergent problem or suspicion of malignancy. Even when ovarian cancer is confirmed, we have found that it is usually in its early stages and therefore has a favorable prognosis (TABLE 2).
TABLE 2
Malignant adnexal masses removed during pregnancy
| HISTOLOGIC TYPE | NUMBER (%) |
|---|---|
| Epithelial | 101 (28) |
| Borderline epithelial | 147 (40) |
| Germ-cell dysgerminoma | 47 (13) |
| Other | 34 (9) |
| Stromal | 24 (7) |
| Undifferentiated | 5 (1.4) |
| Sarcoma | 2 (0.5) |
| Metastatic | 4 (1.1) |
| Total | 364 |
| Data supplied by the authors from surgical experience | |
How should a mass be assessed?
Ultrasonography and other imaging often reveal the presence of a mass and help determine whether it is benign or malignant. In fact, most adnexal masses discovered during pregnancy are incidental findings at the time of routine prenatal US. (see the most commonly found tumors.) Operative intervention is required in 3 situations:
- malignancy is suspected
- an acute complication develops
- the sheer size of the tumor is likely to cause difficulty.
Corpus luteum
A persistent corpus luteum is a normal component of pregnancy. Although it usually appears as a small cystic structure on ultrasonographic imaging, the corpus luteum of pregnancy can reach 10 cm in size. Other types of “functional” ovarian cysts may also be found during pregnancy. Most functional cysts resolve by the early second trimester.4,6 In rare cases, a cyst may develop complications such as torsion or rupture, causing acute pain or hemorrhage. Otherwise, a cystic tumor identified in the first trimester should be characterized and followed using ultrasonography (US).
Benign neoplasm
An adnexal mass that persists beyond the first trimester is more likely to be a neoplasm.3-5,10,11,22 Such a mass is generally considered clinically significant if it exceeds 5 cm in diameter and has a complex sonographic appearance. Usually such a neoplasm will be a benign cystadenoma or cystic teratoma.5,10-13,19,23,24
Benign cystic teratoma
This tumor can be identified with a fairly high degree of specificity using a variety of imaging techniques, with management based on the presumptive diagnosis. This tumor is unlikely to grow substantially during pregnancy. When it is smaller than 6 cm, such a tumor can simply be observed.14 A larger tumor can occasionally rupture or lead to torsion or obstruction of labor, but such occurrences are rare.
Benign cystadenoma
In an asymptomatic patient with imaging that suggests a benign cystadenoma (see sonogram), benign cystic teratoma, or other benign tumor, observation is reasonable in most cases.4,6,7,9-11,14,19 Operative intervention is required when there is less certainty regarding the benign nature of the tumor, an acute complication develops, or the tumor is expected to pose problems because of its large size alone.
Uterine leiomyoma
It is rare for an ovarian tumor detected during pregnancy to have a solid appearance on US. When it does, it may be a uterine leiomyoma mimicking an adnexal tumor (see intraoperative photograph). It should be reevaluated with more detailed US or magnetic resonance imaging.25
Malignancy
About 10% of adnexal masses that persist during pregnancy are malignant, according to recent series.4,5,7-10,12,13,24,26
Most of the ovarian cancers diagnosed during pregnancy are epithelial, and a substantial portion of these are low-malignant-potential (LMP) tumors.5,10,11,13,19,23,24,26,27 This ratio is in keeping with the age of these women, which also explains the stage distribution (most are stage 1) and the large percentage of germ-cell tumors detected. The majority of ovarian cancers discovered in pregnant women have a favorable prognosis.
Benign-appearing cystadenoma
A morphologically benign-appearing, large, cystic adnexal mass can be seen in association with an 11-week gestation.
Leiomyoma mimics an ovarian tumor
This 17-week gestation was marked by a large pedunculated leiomyoma that at fist appeared to be a right adnexal tumor.
Appearance of adnexal masses on US
A functional cyst such as a follicular cyst, corpus luteum cyst, or theca lutein cyst usually has smooth borders and a fluid center. Other cysts may sometimes contain debris, such as clotted blood, that suggests endometriosis or a simple cyst with bleeding into it.
A benign cystic teratoma often has multiple tissue lines, evidence of calcification, and layering of fat and fluid contents.
A benign cystadenoma usually has the appearance of a simple cyst without large septates, whereas a cystadenocarcinoma often contains septates, abnormal blood flow, increased vascularity, or all of these. However, it is impossible to definitively distinguish a cystadenoma from a cystadenocarcinoma using US imaging alone.
Functional cysts usually resolve by the second trimester. A cyst warrants closer scrutiny when it persists, is larger than 5 cm in diameter, or has a complex appearance on US.
CA-125 may be useful after the first trimester
The serum CA-125 level is typically elevated during the first trimester, but may be useful during later assessment or for follow-up of a malignancy.1
A markedly elevated serum level of alpha-fetoprotein (fractionated in some cases) has been reported in some gravidas with an endodermal sinus or mixed germ-cell ovarian tumor.2 Alpha-fetoprotein should be measured when there is suspicion for a germ-cell tumor based on clinical or US findings.
When a mass is discovered during cesarean section
Occasionally, an adnexal mass is detected at the time of cesarean section (FIGURE 1).3 This phenomenon is increasingly common, given the large number of cesarean deliveries in the United States. To eliminate the need for future surgery and avoid a delay in the diagnosis of an ovarian malignancy, inspect the adnexa routinely after closing the uterine incision in all women who deliver by cesarean section.
FIGURE 1 Mass discovered at cesarean section
This cystic tumor was discovered at cesarean section that was undertaken for obstetric indications.
CASE 2 LMP tumor is suspected
A 36-year-old gravida 3 para 1011 makes a prenatal visit during the first trimester. Her previous delivery was a cesarean section through a Pfannenstiel incision for a breech presentation. US imaging reveals a 6-week, 5-day fetus and a complex left adnexal mass, 4.5×3.9×4.1 cm. Imaging is repeated 1 month later at a tertiary-care center and shows an 11-week viable fetus, a right ovary with a corpus luteum cyst, and a left ovary with a 6.6×4 cm cystic mass with extensive vascular surface papillations that is suspicious for a low-malignant-potential (LMP) tumor. In several sonograms prior to the pregnancy, this mass appeared to be solid and was 3 cm in size.
When is surgery warranted?
Surgery is indicated when physical examination or imaging of a pregnant woman reveals an adnexal mass that is suspicious for malignancy, but the physician must weigh the benefit of prompt surgery against the risk to the pregnancy. This equation can be complicated in several ways. For example, surgical staging of clinically early ovarian cancer is more difficult due to the pregnant uterus, which is more extensively manipulated during these procedures. In addition, an optimal operation sometimes necessitates removal of the uterus.
At 13 weeks’ gestation, the patient described in case 2 underwent laparoscopy with peritoneal washings and left salpingo-oophorectomy, but the tumor ruptured during removal. Final pathology showed it to be a serous LMP tumor involving the surface of the left ovary. Washings were in line with this diagnosis.
The pregnancy continued uneventfully, and a repeat cesarean section was performed at 37 weeks through the Pfannenstiel scar, followed by limited surgical staging. Exploration and all biopsies were negative, and the final diagnosis was a stage 1C serous LMP tumor of the ovary.
The patient articulated a desire to preserve her fertility and was monitored with US imaging of the remaining ovary every 6 months.
Does ‘indolent’ behavior of malignancy justify watchful waiting?
LMP tumors comprise a relatively large percentage of ovarian “cancers” encountered during pregnancy. Some authors report the accurate identification of these tumors prospectively, based on ultrasonographic characteristics.4,5 When an LMP tumor is the likely diagnosis, serial observation during pregnancy may be appropriate because of the indolent nature of the tumor. Further studies are needed to refine preoperative diagnosis and determine the overall safety of this approach.
When the problem is acute
In rare cases, a pregnant patient will have (or develop during observation) an acute problem due to torsion or rupture of an adnexal mass. Some ovarian cancers may present acutely, such as a rapidly growing malignant germ-cell tumor or a ruptured and hemorrhaging granulosecell tumor. Emergent surgery is necessary to manage the acute adnexal disease and reduce the likelihood of pregnancy loss. These events are infrequent, occurring in less than 10% of women with a known, persistent adnexal mass during pregnancy.4-14 Furthermore, recent studies have not found a substantial pregnancy complication rate associated with such emergency surgeries.
CASE 3 Suspicious mass, ascites signal need for surgery
A 19-year-old gravida 1 para 0 seeks prenatal care at 17 weeks’ gestation, complaining of rapidly enlarging abdominal girth. The physical examination estimates gestational size to be considerably greater than dates, but US is consistent with a 17-week intrauterine pregnancy. Imaging also reveals a 12-cm heterogenous left adnexal mass and a large amount of ascites.
Surgery is clearly warranted, but how extensive should it be?
When a malignancy is detected, a thorough staging procedure may be justified, depending on gestational age, exposure, desires of the patient, and operative findings. A midline incision is preferred.
Pregnant and nonpregnant women with stage 1A or 1C epithelial ovarian cancer who undergo fertility-preserving surgery (with chemotherapy in selected patients) have a good prognosis and a high likelihood of achieving a subsequent normal pregnancy.15 The same is true for women with a malignant germ-cell tumor of the ovary, even when disease is advanced.16 However, careful surgical staging is necessary.
The most important consideration when deciding whether to continue the pregnancy is the need for adjuvant chemotherapy. Depending on the gestational age and diagnosis, a short delay (4 to 6 weeks) may be appropriate to allow the pregnancy to progress beyond the first trimester or to maturity.
In case 3, a laparotomy was performed at 19 weeks’ gestation via a midline incision, and approximately 5.3 L of ascites was evacuated. A large, nonadherent left ovarian tumor was removed. The right ovary appeared to be normal, as did the gravid uterus, which was minimally manipulated. The rest of the surgical exploration was normal, and the distal portion of the omentum was excised. The frozen-section diagnosis was a malignant stromal tumor. Final pathology showed an 18×13.5×8.8 cm, poorly differentiated, SertoliLeydig-cell tumor with heterologous elements in the form of mucinous epithelium. The omentum was negative for tumor.
Chemotherapy was initiated in the third trimester, based on the limited data available, with intravenous etoposide and platinum administered every 21 days. The patient received 3 cycles of chemotherapy prior to delivery.
At 37 weeks’ gestation, labor was successfully induced. After delivery, bleomycin was added to the chemotherapy regimen, and 3 additional courses with all 3 agents were administered. The patient was lost to follow-up shortly after completing chemotherapy.
Clearly, an informed discussion of the options with the patient is imperative before any surgery, especially when chemotherapy may be delayed. Pregnancy does not appear to alter the prognosis for the patient with an ovarian malignancy, and ovarian cancer has not been reported to metastasize to the fetus.
Pregnant women have a very low rate of ovarian cancer
Leiserowitz GS, Xing G, Cress R, Brahmbhatt B, Dalrymple JL, Smith LH. Adnexal masses in pregnancy: how often are they malignant? Gynecol Oncol. 2006;101:315–321.
Ovarian malignancies are rare during pregnancy. When they do occur, they are likely to be early stage and to have a favorable outcome, according to this recent population-based study.
Using 3 large databases containing records on 4,846,505 California obstetric patients between 1991 and 1999, Leiserowitz and colleagues identified 9,375 women who had an ovarian mass associated with pregnancy. Of these, 87 had ovarian cancer and 115 had a low-malignant-potential (LMP) tumor, for a cancer occurrence rate of 0.93%, or 0.0179 per 1,000 deliveries. Thirty-four of the 87 cancers were germ-cell tumors.
Of the 87 ovarian cancers, 65.5% were localized, 6.9% regional, 23% remote, and 4.6% of unknown stage. The respective rates for LMP tumors were 81.7%, 7.8%, 4.4%, and 6.1%.
Women with malignant tumors were more likely than pregnant controls without cancer to undergo cesarean delivery, hysterectomy, transfusion, and prolonged hospitalization. These women did not, however, have a higher rate of adverse neonatal outcomes.
When cancer is advanced
Few data shed light on whether a pregnancy should continue when ovarian cancer is advanced.17 The definitive surgical approach must be highly individualized.
It is not always possible to make an accurate diagnosis based on a frozen section. In such a case, the pregnancy should be preserved until the time of definitive diagnosis. As always, the patient’s wishes and gestational age must be considered.
How factors besides malignancy can influence care
Most persistent adnexal masses move well out of the pelvis as pregnancy progresses. Occasionally, however, an ovarian tumor may be located in the posterior cul-de-sac even at term, a fact easily confirmed by examination or US.4,7 A tumor in the posterior cul-de-sac can obstruct delivery or rupture. When it has a benign cystic appearance on US, it may be decompressed via transvaginal aspiration. Otherwise, the best approach is cesarean section and concomitant management of the mass.
When size alone is the problem
Some ovarian tumors are so large they seem incompatible with an advancing pregnancy. Tumors up to 20 cm in diameter have been removed intact at the time of cesarean section (FIGURE 2).18 The tumor may accommodate in shape and become less problematic as it is gradually pushed into the upper abdomen (FIGURE 3).
The ability of the peritoneal cavity to accommodate a tumor varies greatly among women. As pregnancy advances, the likelihood that a large cystic mass will rupture tends to increase. Depending on the circumstances, percutaneous aspiration7,18 or removal of a benign-appearing cystic tumor may be appropriate.
FIGURE 2 Even a very large tumor may coexist with advancing pregnancy
This benign serous cystadenoma was exteriorized at the time of cesarean section at term.
FIGURE 3 Large ovarian tumor has accommodated to the pregnancy
Laparotomy—performed at term for cesarean section and to manage this large tumor—revealed that the tumor had accommodated in shape between the enlarging pregnant uterus and the abdominal wall.
When is the best time to operate?
Surgery is generally not recommended during the first trimester.5-11 Among the reasons are the high likelihood of a corpus luteum cyst, the low likelihood of an invasive malignancy, the low risk of adnexal complications associated with observation, and the potential for pregnancy loss or teratogenicity. However, as pregnancy progresses beyond the first trimester, surgery poses other problems: Operative exposure diminishes and the need to manipulate the pregnant uterus increases.
Can surgery be delayed when a mass is detected?
Schmeler KM, Mayo-Smith WW, Peipert JF, Weitzen S, Manuel MD, Gordinier ME. Adnexal masses in pregnancy: surgery compared with observation. Obstet Gynecol. 2005;105:1098–1103.
Close observation is a reasonable alternative to operative intervention during pregnancy, unless a malignancy is suspected.
Schmeler and colleagues reviewed the cases of 59 women who had an adnexal mass larger than 5 cm in diameter detected during pregnancy, out of a total of 127,177 deliveries at a single institution between 1990 and 2003. Antepartum surgery was performed in 17 women (29%). Of these, 13 cases had ultrasonographic findings suggesting malignancy, and 4 had ovarian torsion. The remaining women were observed, with surgery delayed until the time of cesarean section or later.
Twenty-five of the 59 masses (42%) were dermoid cysts. Cancer was diagnosed in 4 patients (6.8%), and 1 patient (1.7%) had an LMP tumor. All 5 cases (100%) involving a malignancy had a suspicious US appearance and were identified during antepartum surgery, whereas only 12 patients with a benign tumor (22%) underwent surgery prior to delivery.
Surgery poses risks to the pregnancy
Elective surgery for an adnexal mass any time during pregnancy increases the risk of pregnancy loss and the likelihood of intrauterine growth restriction (IUGR) and preterm delivery.5,7,10,13,19 A 1989 study from Sweden20 defined a cohort of 5,405 women (from 720,000 births) who were known to have a nonobstetric operation while pregnant, with the following results:
- Congenital malformation and stillbirth were not increased in the women undergoing surgery
- The number of very-low- and low-birth-weight infants did rise, however—the result of both prematurity and IUGR
- Also elevated was the incidence of infants born alive but dying within 168 hours; these risks increased regardless of trimester
- No specific type of anesthesia or operation was associated with adverse reproductive outcomes, and the cause of those adverse outcomes was not determined.
Some recent data suggest that adnexal surgery during the late second or early third trimester poses the greatest risk of preterm delivery or IUGR, or both.13
Window of opportunity: early to mid- second trimester
During this time frame, elective surgery for an adnexal mass still affords some pelvic exposure without the need for significant uterine manipulation and has been associated with a lower risk of pregnancy complications.
The other window for operation is at the time of cesarean section. An elective cesarean section is sometimes performed specifically to manage a persistent adnexal mass. Among the factors that warrant consideration when contemplating this approach are the elective uterine incision (with its attendant implications for future pregnancies), the higher risks associated with cesarean delivery in general, the type of skin incision (a vertical incision is appropriate in the event of ovarian malignancy), the potential for better exposure or laparoscopy at a later date, the increased difficulty of ovarian cystectomy at the time of cesarean section, and the patient’s wishes.
The data on laparoscopy during the first and second trimesters of pregnancy indicate that it is as safe as laparotomy. A 1997 Swedish study21 identified cohorts of 2,181 women undergoing laparoscopy and 1,522 women undergoing laparotomy (from a total of 2,015,000 deliveries) between the fourth and 20th weeks of pregnancy. In both groups there was an increased risk for the infant to weigh less than 2,500 g, to be delivered before 37 weeks, and to have IUGR. There were no differences between the 2 groups for these and other adverse outcomes.
Small series of laparoscopic procedures to manage an adnexal mass during pregnancy suggest that this approach is most applicable during the first (for highly selected emergent cases) or early second trimester to manage masses less than 10 cm in diameter, particularly when adnexectomy is planned.
Laparoscopy may be considered “minimally invasive” because it reduces manipulation of the pregnant uterus during adnexal surgery. However, it is more difficult to assess and remove ovarian cysts laparoscopically, although an early ovarian malignancy could be staged via laparoscopy by an experienced surgeon.
Considerations during laparotomy
When performing a laparotomy or cesarean section for an adnexal mass, the surgeon must take into account a number of variables when selecting the type of incision (ie, vertical vs transverse). In general, if malignancy is suspected, or if uterine manipulation is to be minimized, a vertical incision is best. Other considerations include a prior scar, body habitus, obstetric issues, and the patient’s wishes.
The author reports no financial relationships relevant to this article.
1. Spitzer M, Kaushal N, Benjamin F. Maternal CA-125 levels in pregnancy and the puerperium. J Reprod Med. 1998;43:387-392.
2. Aoki Y, Higashino M, Ishii S, Tanaka K. Yolk sac tumor of the ovary during pregnancy: a case report. Gynecol Oncol. 2005;99:497-499.
3. Koonings PP, Platt LD, Wallace R. Incidental adnexal neoplasms at cesarean section. Obstet Gynecol. 1988;72:767-769.
4. Zanetta G, Mariani E, Lissoni A, et al. A prospective study of the role of ultrasound in the management of adnexal masses in pregnancy. BJOG. 2003;110:578-583.
5. Sherard GB, 3rd, Hodson CA, Williams HJ, et al. Adnexal masses and pregnancy: a 12 year experience. Am J Obstet Gynecol. 2003;189:358-363.
6. Bernhard LM, Klebba PK, Gray DL, Mutch DG. Predictors of persistence of adnexal masses in pregnancy. Obstet Gynecol. 1999;93:585-589.
7. Platek DN, Henderson CE, Goldberg GL. The management of a persistent adnexal mass in pregnancy. Am J Obstet Gynecol. 1995;173:1236-1240.
8. Bromley B, Benacerraf B. Adnexal masses during pregnancy: accuracy of sonographic diagnosis and outcome. J Ultrasound Med. 1997;16:447-452.
9. Hill LM, Connors-Beatty DJ, Nowak A, Tush B. The role of ultrasonography in the detection and management of adnexal mass during the second and third trimesters of pregnancy. Am J Obstet Gynecol. 1998;179:703-707.
10. Agarwal N, Parul, Kriplani A, Bhatla N, Gupta A. Management and outcome of pregnancies complicated with adnexal masses. Arch Gynecol Obstet. 2003;267:148-152.
11. Schmeler KM, Mayo-Smith WW, Peipert JF, Weitzen S, Manuel MD, Gordinier ME. Adnexal masses in pregnancy: surgery compared with observation. Obstet Gynecol. 2005;105:1098-1103.
12. Coenen VH, Dunton C, Cardonick E, Berghella V. Persistent adnexal masses during pregnancy. Obstet Gynecol. 1999;93:66S.-
13. Whitecar P, Turner S, Higby K. Adnexal masses in pregnancy: a review of 130 cases undergoing surgical management. Am J Obstet Gynecol. 1999;181:19-24.
14. Caspi B, Levi R, Appelman Z, Rabinerson D, Goldman G, Hagay Z. Conservative management of ovarian cystic teratoma during pregnancy and labor. Am J Obstet Gynecol. 2000;182:503-505.
15. Schilder JM, Thompson AM, DePriest PD, et al. Outcome of reproductive age women with stage IA or IC invasive epithelial ovarian cancer treated with fertility-sparing therapy. Gynecol Oncol. 2002;87:1-7.
16. Tangir J, Zelterman D, Ma W, Schwartz PE. Reproductive function after conservative surgery and chemotherapy for malignant germ cell tumors of the ovary. Obstet Gynecol. 2003;101:251-257.
17. Ferrandina G, Distefano M, Testa A, De Vincenzo R, Scambia G. Management of an advanced ovarian cancer at 15 weeks of gestation: case report and literature review. Gynecol Oncol. 2005;97:693-696.
18. Caspi B, Ben-Arie A, Appelman Z, Or Y, Hagay Z. Aspiration of simple pelvic cysts during pregnancy. Gynecol Obstet Invest. 2000;49:102-105.
19. Usui R, Minakami H, Kosuge S, et al. A retrospective survey of clinical, pathologic, and prognostic features of adnexal masses operated on during pregnancy. J Obstet Gynaecol Res. 2000;26(2):89-93.
20. Mazze RI, Källén B. Reproductive outcome after anesthesia and operation during pregnancy; a registry study of 5,405 cases. Am J Obstet Gynecol. 1989;161:1178-1185.
21. Reedy MB, Källén B, Kuehl TJ. Laparoscopy during pregnancy: a study of five fetal outcome parameters with use of the Swedish health registry. Am J Obstet Gynecol. 1997;177:673-679.
22. Hermans RHM, Fischer D-C, van der Putten HWHM, et al. Adnexal masses in pregnancy. Onkologie. 2003;26:167-172.
23. Hoffman MS. Primary ovarian carcinoma during pregnancy. Clin Consul Obstet Gynecol. 1995;7:237-241.
24. Ueda M, Ueki M. Ovarian tumors associated with pregnancy. Int J Obstet Gynaecol. 1996;55:59-65.
25. Curtis M, Hopkins MP, Zarlingo T, et al. Magnetic resonance imaging to avoid laparotomy in pregnancy. Obstet Gynecol. 1993;82:833-836.
26. Leiserowitz GS, Xing G, Cress R, et al. Adnexal masses in pregnancy; how often are they malignant? Gynecol Oncol. 2006;101:315-321.
27. Rahman MS, Al-Sibai MH, Rahman J, et al. Ovarian carcinoma associated with pregnancy. A review of 9 cases. Acta Obstet Gynecol Scand. 2002;81:260-264.
1. Spitzer M, Kaushal N, Benjamin F. Maternal CA-125 levels in pregnancy and the puerperium. J Reprod Med. 1998;43:387-392.
2. Aoki Y, Higashino M, Ishii S, Tanaka K. Yolk sac tumor of the ovary during pregnancy: a case report. Gynecol Oncol. 2005;99:497-499.
3. Koonings PP, Platt LD, Wallace R. Incidental adnexal neoplasms at cesarean section. Obstet Gynecol. 1988;72:767-769.
4. Zanetta G, Mariani E, Lissoni A, et al. A prospective study of the role of ultrasound in the management of adnexal masses in pregnancy. BJOG. 2003;110:578-583.
5. Sherard GB, 3rd, Hodson CA, Williams HJ, et al. Adnexal masses and pregnancy: a 12 year experience. Am J Obstet Gynecol. 2003;189:358-363.
6. Bernhard LM, Klebba PK, Gray DL, Mutch DG. Predictors of persistence of adnexal masses in pregnancy. Obstet Gynecol. 1999;93:585-589.
7. Platek DN, Henderson CE, Goldberg GL. The management of a persistent adnexal mass in pregnancy. Am J Obstet Gynecol. 1995;173:1236-1240.
8. Bromley B, Benacerraf B. Adnexal masses during pregnancy: accuracy of sonographic diagnosis and outcome. J Ultrasound Med. 1997;16:447-452.
9. Hill LM, Connors-Beatty DJ, Nowak A, Tush B. The role of ultrasonography in the detection and management of adnexal mass during the second and third trimesters of pregnancy. Am J Obstet Gynecol. 1998;179:703-707.
10. Agarwal N, Parul, Kriplani A, Bhatla N, Gupta A. Management and outcome of pregnancies complicated with adnexal masses. Arch Gynecol Obstet. 2003;267:148-152.
11. Schmeler KM, Mayo-Smith WW, Peipert JF, Weitzen S, Manuel MD, Gordinier ME. Adnexal masses in pregnancy: surgery compared with observation. Obstet Gynecol. 2005;105:1098-1103.
12. Coenen VH, Dunton C, Cardonick E, Berghella V. Persistent adnexal masses during pregnancy. Obstet Gynecol. 1999;93:66S.-
13. Whitecar P, Turner S, Higby K. Adnexal masses in pregnancy: a review of 130 cases undergoing surgical management. Am J Obstet Gynecol. 1999;181:19-24.
14. Caspi B, Levi R, Appelman Z, Rabinerson D, Goldman G, Hagay Z. Conservative management of ovarian cystic teratoma during pregnancy and labor. Am J Obstet Gynecol. 2000;182:503-505.
15. Schilder JM, Thompson AM, DePriest PD, et al. Outcome of reproductive age women with stage IA or IC invasive epithelial ovarian cancer treated with fertility-sparing therapy. Gynecol Oncol. 2002;87:1-7.
16. Tangir J, Zelterman D, Ma W, Schwartz PE. Reproductive function after conservative surgery and chemotherapy for malignant germ cell tumors of the ovary. Obstet Gynecol. 2003;101:251-257.
17. Ferrandina G, Distefano M, Testa A, De Vincenzo R, Scambia G. Management of an advanced ovarian cancer at 15 weeks of gestation: case report and literature review. Gynecol Oncol. 2005;97:693-696.
18. Caspi B, Ben-Arie A, Appelman Z, Or Y, Hagay Z. Aspiration of simple pelvic cysts during pregnancy. Gynecol Obstet Invest. 2000;49:102-105.
19. Usui R, Minakami H, Kosuge S, et al. A retrospective survey of clinical, pathologic, and prognostic features of adnexal masses operated on during pregnancy. J Obstet Gynaecol Res. 2000;26(2):89-93.
20. Mazze RI, Källén B. Reproductive outcome after anesthesia and operation during pregnancy; a registry study of 5,405 cases. Am J Obstet Gynecol. 1989;161:1178-1185.
21. Reedy MB, Källén B, Kuehl TJ. Laparoscopy during pregnancy: a study of five fetal outcome parameters with use of the Swedish health registry. Am J Obstet Gynecol. 1997;177:673-679.
22. Hermans RHM, Fischer D-C, van der Putten HWHM, et al. Adnexal masses in pregnancy. Onkologie. 2003;26:167-172.
23. Hoffman MS. Primary ovarian carcinoma during pregnancy. Clin Consul Obstet Gynecol. 1995;7:237-241.
24. Ueda M, Ueki M. Ovarian tumors associated with pregnancy. Int J Obstet Gynaecol. 1996;55:59-65.
25. Curtis M, Hopkins MP, Zarlingo T, et al. Magnetic resonance imaging to avoid laparotomy in pregnancy. Obstet Gynecol. 1993;82:833-836.
26. Leiserowitz GS, Xing G, Cress R, et al. Adnexal masses in pregnancy; how often are they malignant? Gynecol Oncol. 2006;101:315-321.
27. Rahman MS, Al-Sibai MH, Rahman J, et al. Ovarian carcinoma associated with pregnancy. A review of 9 cases. Acta Obstet Gynecol Scand. 2002;81:260-264.
Vaginal intraepithelial neoplasia: Risky and underrecognized
- Most women diagnosed with vaginal intraepithelial neoplasia (VAIN) have a history of cervical intraepithelial neoplasia.
- Compelling clinical and laboratory data indicate a causal relationship between human papillomavirus and VAIN.
- Like its cervical counterpart, VAIN 3 is thought to have substantial potential to progress to invasive cancer.
- Diagnosis includes careful gross and colposcopic inspection of the entire vagina (with mapping of involved areas), representative colposcopically directed biopsies, and careful palpation of the vaginal walls, especially the vaginal cuff scar.
- Important factors to consider when selecting appropriate treatment for women with VAIN include prior hysterectomy, prior radiation therapy, age, whether she is sexually active, comorbidities, vaginal anatomy, and prior treatments.
We can easily identify vulvar intraepithelial neoplasia (VIN): The patient complains of itching and has a visible lesion. We find cervical intraepithelial neoplasia (CIN) by investigating an abnormal Pap test. But what about vaginal intraepithelial neoplasia (VAIN)? It does not itch and is invisible to the naked eye. A Pap test sometimes catches it, although this test is used mainly to screen for CIN, not VAIN.
VAIN just does not grab our attention. It is uncommon, and invasive vaginal cancer is rare. But before you slip this article into the “obscure disease” file, consider the following:
- VAIN is difficult to diagnose, but some women are at increased risk.
- It is difficult to manage, but understanding the treatment options is important to success.
- The potential for VAIN to evolve into invasive cancer is probably substantial.
- Treatment of invasive vaginal cancer has a high rate of complications and is often unsuccessful.
This article describes the epidemiology, natural history, diagnosis, and treatment of VAIN, focusing primarily on management.
A range of risk factors
As with many uncommon diseases that are difficult to diagnose, good data on the incidence of VAIN are not available. Women diagnosed with VAIN can be in their late teens or senior citizens; mean age is about 50 years.1-5 Race is not mentioned in most studies.
CIN. Although concomitant or subsequent VAIN is very unusual in the approximately 600,000 women identified with CIN each year in the United States, most patients diagnosed with VAIN have a history of CIN. The small number who still have a cervix and are diagnosed with VAIN have a high incidence of concomitant CIN.1 Of women who have had a hysterectomy for CIN, only 1% to 5% are subsequently diagnosed with VAIN.6,7
Since most women who develop vaginal intraepithelial neoplasia have a prior or current history of cervical neoplasia, the “field effect” also renders them at risk for vulvar neoplasia.
HPV. Compelling data indicate a causal relationship between human papillomavirus (HPV) and CIN; the same is true for VAIN.8
Tobacco use. A history of tobacco use is frequent among women diagnosed with VAIN.1
Pelvic radiotherapy is commonly reported in case series.1,3-5 Generally, malignancy is potentially radiation-related if it develops at least 5 to 10 years after treatment.
We do not know whether radiotherapy induces neoplastic transformation in the vagina, but women with a history of radiation warrant long-term follow-up, as they seem to be at increased risk and diagnosis may be difficult.4,5
Chronically immunocompromised women are at particular risk for multifocal lower genital tract neoplasia.9,10
Natural history
The limited data available do suggest that vaginal intraepithelial neoplasia is a premalignant condition.11,12 Unfortunately, little is known about the relationship between severity of the intraepithelial neoplastic process and degree of risk. Natural history studies of VAIN 3 are even more limited than those of CIN 3.
Like its cervical counterpart, VAIN 3 is thought to have substantial potential for progression to invasive cancer.1-4,10-13
EVALUATIONDiagnosis entails inspection, palpation, and directed biopsies
VAIN is most commonly diagnosed after investigation of an abnormal Pap test taken from the vaginal cuff of a woman who has undergone a prior hysterectomy for cervical neoplasia. Occasionally, the disease is identified during colposcopy as extension of a cervical lesion. In either case, VAIN usually involves the upper third of the vagina.1-5,11 A minority of patients will be found to have diffuse multifocal lesions along the vaginal walls.
Adequate diagnosis mandates:
- careful gross and colposcopic inspection of the entire vagina (with mapping of involved areas),
- representative colposcopically directed biopsies,
- careful palpation of the vaginal walls, especially the vaginal cuff scar, and
- in some cases, excision of the vaginal cuff scar.
VAIN is often readily visualized with a colposcope, and the appearance may be more prominent than that of a comparable cervical lesion (FIGURE 1). The lesions are sometimes hyperkeratotic and grossly visible. However, colposcopy of the vagina is more difficult than that of the cervix due to vaginal folding, a larger surface area, and vaginal cuff irregularities.
Vaginal atrophy also creates diagnostic difficulties related to colposcopic assessment, and to overreading of vaginal cytology (eg, when a lesion is interpreted as high-grade dysplasia or suspicious). Reassessment after 4 to 6 weeks of estrogen therapy helps resolve these issues.
FIGURE 1 Colposcopic view of VAIN 3
Note the hyperkeratotic, prominent appearance of the VAIN lesions.
TREATMENT
Because women with VAIN are a heterogeneous group, treatment must be individualized. Important factors to consider when selecting appropriate treatment include prior hysterectomy, prior radiation therapy, age, whether the woman is sexually active, comorbidities, vaginal anatomy, and prior treatments.
Prior hysterectomy
Most women diagnosed with VAIN have previously undergone hysterectomy, usually because of cervical neoplasia.2-5,10 In these women, VAIN is generally confined to the vaginal apex (FIGURE 2).
Watch for occult neoplasia. Buried epithelium within the vaginal cuff scar may harbor occult neoplasia. Patients with VAIN at the vaginal apex5,10,11,13 and a vaginal cuff from a hysterectomy for cervical neoplasia are analogous to women with CIN and an unsatisfactory colposcopy.
Excision is usual management. Most of these women are appropriately managed with excision of the involved vaginal apex, including the cuff scar.1,3-5,11,14 Problematic vaginal shortening is uncommon following this procedure. The status of the resection margin is predictive of the likelihood of recurrence.3
With more extensive vaginal epithelial involvement, consider cuff excision in selected cases to eliminate the potential for occult disease. The remainder of the involved vagina can be treated by other means outlined below.
FIGURE 2 VAIN 3 at vaginal apex
VAIN usually is confined to the apex in women hysterectomized for cervical neoplasia.
When CIN extends onto the vaginal fornix
This is an uncommon scenario, although its underrecognition may explain why some VAIN is diagnosed shortly after hysterectomy for CIN.
Management is simpler than for disease involving the posthysterectomy apex because there is no scarring, distortion, or buried epithelium, and traction on the cervix generally provides good exposure.
Laser vaporization is an option. For many such patients, this is one of the few good indications remaining for laser vaporization.5,15,16
If hysterectomy is indicated, remove the affected portion of the upper vagina along with the cervix.5,17
Multifocal or diffuse pattern: Typical of immunocompromise
Multifocal or diffuse manifestation is likewise uncommon, often involving low-grade neoplasia and condylomatous changes.5,18 This is the pattern typically seen in chronically immunocompromised women.5,10
The natural history of VAIN—especially the malignant potential—is less well understood for women with this disease pattern. When managing such patients, keep in mind the potential for occult neoplasia in the vaginal cuff scar. However, broaden the focus of treatment to encompass the entire vagina. The various management options are described below:
- If the patient has cervical intraepithelial neoplasia 2 or 3, treat with laser, cryotherapy, or large loop excision of the transformation zone at least 2 weeks prior to beginning 5-fluorouracil (5-FU).
- Treat vulvar, anal, or urethral lesions with a laser or local excision prior to 5-FU.
- Treat vulvovaginitis prior to 5-FU.
- Have the patient administer 1/2 vaginal applicator of 5% 5-FU (Efudex; 2.5 g) deep in the vagina weekly at bedtime for 10 weeks. Instruct her to place a tampon or cotton balls into the outer vagina, unless the distal vagina is involved. Also have her apply petroleum jelly to all vulvar tissues, around the urethra, and to the anus. In the morning, she should remove the tampon, wash the vulva with soap and water, and dry carefully. This should be followed by another application of petroleum jelly (sparingly) to vulvar tissues. A mini napkin may help prevent staining of garments.
- When 5-FU treatment ends, have the patient administer 1/3 applicator of vaginal estrogen cream nightly for 2 weeks beginning 3 weeks prior to reevaluation.
- Evaluate with colposcopy and cytology 8 weeks after completing 5-FU therapy.
Observation only is particularly suited for women who have low-grade disease or who are severely debilitated and chronically immunocompromised.
5-fluorouracil (5-FU) cream is a very good treatment when VAIN involves the upper half to two thirds of the vagina.2,9 However, the cream does not reach buried epithelium in the vaginal cuff scar and probably does not consistently treat the lower vagina when applied with the standard vaginal applicator. Applying the cream directly to the lower vagina may be more effective when that region is involved (see “6-step vaginal fluorouracil therapy for intraepithelial neoplasia,” above).
Laser vaporization is well accepted and commonly used.2,9,15,19,20 Advantages are versatility in treatment of multifocal disease without sacrificing vaginal epithelium, and low likelihood of complications. Disadvantages include inability to treat buried vaginal cuff epithelium; technical difficulties in applying the laser to a folding and often distorted (cuff) surface within a confined space; and the expensive equipment, technical support, and surgical expertise required.1,4,5,11,15,16,20
Planned combined treatment using laser vaporization followed by 5-FU cream has been reported efficacious in the treatment of diffuse vaginal condylomata.21 Selected patients with VAIN also may benefit,22 such as those with plaque-like disease (where a thick layer of keratin can reduce penetration of the 5-FU cream), diffuse/multifocal disease where laser vaporization is likely to be incomplete, or vaginal anatomy that makes it difficult to accomplish complete laser vaporization.
Vaginectomy is definitive management for selected patients with extensive VAIN. The operation is done transperineally, although hysterovaginectomy may require a combined approach.23 Leaving the distal third of the vagina intact (when disease distribution allows) makes the operation easier and may help avert iatrogenic urinary incontinence. Follow-up examination—and treatment, if necessary—of the remaining short vaginal stump is fairly easy.
Removal of the vagina is technically demanding in some women. A Schuchardt incision is useful in such instances.
The other obvious disadvantage of vaginectomy is loss of coital function, although placement of a skin graft is an option.
Brachytherapy is another option for treating extensive VAIN in highly selected patients.24,25
A cylindrical apparatus placed in the vagina delivers radiotherapy to the vaginal epithelium; the likelihood of significant morbidity is low. This method is most applicable to poor surgical candidates with extensive VAIN. Disadvantages include fibrosis of the vagina, limited data on efficacy (and particular concern about inadequate dosing to buried or distorted vaginal cuff epithelium), and potential difficulties with follow-up and treatment of recurrence.3,5
“Chemosurgery,” specifically 5-FU cream followed by surgical removal of the then-partially-detached VAIN, followed by additional 5-FU cream, has been used effectively at 1 center.10 Other reported-but-less-investigated methods include cryotherapy, electrocautery, loop electrosurgical excision, and cavitational ultrasonic surgical aspiration (CUSA).3-5
VAIN in a radiated vagina
Most women who develop VAIN in this scenario received radiation therapy many years earlier for carcinoma of the cervix.5,11
The most common sites are the upper third to upper half of the remaining vagina, where radiation changes are prominent.
Diagnosis often is problematic due to:
- difficulties with interpreting cytologic preparations in such patients;
- radiation changes in the vagina (pale and fibrotic with telangiectasis), which largely obscure colposcopic findings;
- the difficulty and potential hazards of biopsy of a thin, fibrotic upper vagina; and
- obliterative coaptation of the upper third to half of the vagina.
Significantly abnormal cytology in the absence of a colposcopically identified lesion (or palpable abnormality) is of particular concern. In such patients, view the abnormal cytologic interpretation with caution and consider further initial evaluation, including outside review of the cytology slides, treatment of the vagina with estrogen, and repeat cytologic and colposcopic evaluation.
When there is clear cytologic evidence of a severe abnormality, consider the possibility of occult neoplasia within the coapted upper vagina and/or cervix.
Management of VAIN in a radiated vagina.
Take into account the anatomic distortion of the upper vagina, the thin and fibrotic nature of the epithelium, and the potential for fistula formation with excisional procedures or other treatments that produce injury beneath the surface of the epithelium. Among the options:
- 5-FU cream or laser vaporization. VAIN that is completely visualized within the remaining vagina is probably best treated with one of these modalities, provided the physician is experienced in managing such patients.5,9
- Excision of the upper vagina,5 including the coapted portion (and sometimes the residual cervix with or without the uterus) is appropriate for highly selected cases, but only after careful consideration of:
- the likelihood of finding significant neoplasia,
- the anatomic feasibility (it is desirable to perform the procedure transvaginally), and
- the overall risk versus benefit. Such procedures should be performed only by an experienced physician.
The chronically immunocompromised
These patients often have undergone organ transplants or are human immunodeficiency virus (HIV)-positive. Diffuse HPV infection of the lower genital tract is pervasive in these women,5,10,26 and diffuse/multifocal lower genital tract intraepithelial neoplasia is often present as well.
These women may be at increased risk for progression to invasive cancer.
Eradication may not be possible. Attempts to eliminate intraepithelial disease are usually unsuccessful.
Management. Many of these women are severely debilitated, with other, more significant medical problems and a short life expectancy. Vigilance is required, as other lower genital tract (and anal) sites are frequently involved. Since eradication of diffuse/multifocal intraepithelial disease is not a realistic goal, treatment followed by chronic suppressive therapy (such as a low intermittent dose of 5-FU) is reasonable.9,10,22
Another approach is close observation, including frequent examinations, with prompt intervention when invasive disease is suspected.
FOLLOW-UP
After treatment for VAIN, follow-up is similar to that for a comparable cervical lesion. Once the vagina has healed, see the patient every 3 to 6 months for 2 years and annually thereafter. In addition to obtaining vaginal cytology, carefully inspect and palpate the vagina (including the vaginal cuff scar).
Effects of 5-FU. Following a course of 5-FU cream, vaginal mucosal ulcerations may persist for several weeks. Occasionally, these may lead to partial coaptations.27 In addition, subsequent islands of columnar epithelium have been described.28 Keep these factors in mind during the follow-up of women who have been treated with transvaginal 5-FU cream.
Long-term annual follow-up is reasonable, since these women are probably at increased risk for developing a second primary vaginal lesion and/or lower anogenital tract neoplasia at other sites.
When extra vigilance is warranted. Some patients merit closer follow-up, such as the chronically immunosuppressed women described; also, women who have been treated for vaginal apical disease without resection of the vaginal cuff scar, previously radiated patients, and women whose VAIN was treated with brachytherapy.
Dr. Hoffman reports no financial relationships relevant to this article.
1. Dodge JA, Eltabbakh GH, Mount SL, Walker RP, Morgan A. Clinical features and risk of recurrence among patients with vaginal intraepithelial neoplasia. Gynecol Oncol. 2001;83:363-369.
2. Petrilli ES, Townsend DE, Morrow CP, Nakao CY. Vaginal intraepithelial neoplasia: biologic aspects and treatment with topical 5-fluorouracil and the carbon dioxide laser. Am J Obstet Gynecol. 1980;138:321-328.
3. Benedet JL, Sanders BH. Carcinoma in situ of the vagina. Am J Obstet Gynecol. 1984;148:695-700.
4. Lenehan PM, Meffe F, Lickrish GM. Vaginal intraepithelial neoplasia: biologic aspects and management. Obstet Gynecol. 1986;68:333-337.
5. Audet-Lapointe P, Body G, Vauclair R, Drouin P, Ayoub J. Vaginal intraepithelial neoplasia. Gynecol Oncol. 1990;36:232-239.
6. Gallup DG, Morley GW. Carcinoma in situ of the vagina—a study and review. Obstet Gynecol. 1975;46:334-440.
7. Woodruff JD. Treatment of recurrent carcinoma in situ in the lower genital canal. Clin Obstet Gynecol. 1965;8:757-770.
8. McCance DJ, Clarkson PK, Dyson JL, Walker PG, Singer A. Human papillomavirus types 6 and 16 in multifocal intraepithelial neoplasias of the female lower genital tract. Br J Obstet Gynaecol. 1985;92:1093-1100.
9. Krebs HB. Treatment of vaginal intraepithelial neoplasia with laser and topical 5-fluorouracil. Obstet Gynecol. 1989;73:657-660.
10. Sillman FH, Fruchter RG, Chen YS, Camilien L, Sedlis A, McTigue E. Vaginal intraepithelial neoplasia: risk factors for persistence, recurrence, and invasion and its management. Am J Obstet Gynecol. 1997;176:93-99.
11. Woodman CBJ, Jordan JA, Wade-Evans T. The management of vaginal intraepithelial neoplasia after hysterectomy. Br J Obstet Gynaecol. 1984;91:103-105.
12. Aho M, Vesterinen E, Meyer B, Purola E, Paavonen J. Natural history of vaginal intraepithelial neoplasia. Cancer. 1991;68:195-197.
13. Hoffman MS, Roberts WS, LaPolla JP, Sterghos S, Jr, Cavanagh D. Neoplasia in vaginal cuff epithelial inclusion cysts after hysterectomy. J Reprod Med. 1989;34:412-416.
14. Hoffman MS, DeCesare SL, Roberts WS, Fiorica JV, Finan MA, Cavanagh D. Upper vaginectomy for in situ and occult, superficially invasive carcinoma of the vagina. Am J Obstet Gynecol. 1992;166:30-33.
15. Curtin JP, Twiggs LB, Julian TM. Treatment of vaginal intraepithelial neoplasia with the CO2laser. J Reprod Med. 1985;30:942-944.
16. Hoffman MS, Roberts WS, LaPolla JP, Fiorica JV, Cavanagh D. Laser vaporization of grade 3 vaginal intraepithelial neoplasia. Am J Obstet Gynecol. 1991;165:1342-1344.
17. Hoffman MS, Spellacy WN. The Difficult Vaginal Hysterectomy. New York, NY: Springer-Verlag; 1995;117-119.
18. Reid R. Human papillomaviral infection. The key to rational triage of cervical neoplasia. Obstet Gynecol Clin North Am. 1987;14:407-429.
19. Townsend DE, Levine RU, Crum CP, Richart RM. Treatment of vaginal carcinoma in situ with the carbon dioxide laser. Am J Obstet Gynecol. 1982;143:565-568.
20. Sherman AI. Laser therapy for vaginal intraepithelial neoplasia after hysterectomy. J Reprod Med. 1990;35—941-944.
21. Krebs HB. Combination of laser plus 5-fluorouracil for the treatment of extensive genital condylomata acuminata. Lasers Surg Med. 1988;8:135-138.
22. Krebs HB. Prophylactic topical 5-fluorouracil following treatment of human papillomavirus-associated lesions of the vulva and vagina. Obstet Gynecol. 1986;68:837-841.
23. Hoffman MS, Bomalaski JJ, Lockhart JL, Greenwald DP. Total removal of the normal non-prolapsed vagina. Technique, results and anatomic observations. J Pelvic Surg. 2002;8:89-94.
24. MacLeod C, Fowler A, Dalrymple C, Atkinson K, Elliott P, Carter J. High-dose-rate brachytherapy in the management of high-grade intraepithelial neoplasia of the vagina. Gynecol Oncol. 1997;65:74-77.
25. Teruya Y, Sakumoto K, Moromizato H, et al. High dose-rate intracavitary brachytherapy for carcinoma in situ of the vagina occurring after hysterectomy: a rational prescription of radiation dose. Am J Obstet Gynecol. 2002;187:360-364.
26. Sillman FH, Sedlis A. Anogenital HPV/neoplasia in immunosuppressed women: an update. Dermatol Clin North Am. 1991;9:353-369.
27. Krebs HB, Helmkamp BF. Chronic ulcerations following topical therapy with 5-fluorouracil for vaginal human papillomavirus-associated lesions. Obstet Gynecol. 1991;78:205-212.
28. Dungar CF, Wilkinson EJ. Vaginal columnar cell metaplasia: an acquired adenosis associated with topical 5-FU therapy. J Reprod Med. 1995;40:361-366.
- Most women diagnosed with vaginal intraepithelial neoplasia (VAIN) have a history of cervical intraepithelial neoplasia.
- Compelling clinical and laboratory data indicate a causal relationship between human papillomavirus and VAIN.
- Like its cervical counterpart, VAIN 3 is thought to have substantial potential to progress to invasive cancer.
- Diagnosis includes careful gross and colposcopic inspection of the entire vagina (with mapping of involved areas), representative colposcopically directed biopsies, and careful palpation of the vaginal walls, especially the vaginal cuff scar.
- Important factors to consider when selecting appropriate treatment for women with VAIN include prior hysterectomy, prior radiation therapy, age, whether she is sexually active, comorbidities, vaginal anatomy, and prior treatments.
We can easily identify vulvar intraepithelial neoplasia (VIN): The patient complains of itching and has a visible lesion. We find cervical intraepithelial neoplasia (CIN) by investigating an abnormal Pap test. But what about vaginal intraepithelial neoplasia (VAIN)? It does not itch and is invisible to the naked eye. A Pap test sometimes catches it, although this test is used mainly to screen for CIN, not VAIN.
VAIN just does not grab our attention. It is uncommon, and invasive vaginal cancer is rare. But before you slip this article into the “obscure disease” file, consider the following:
- VAIN is difficult to diagnose, but some women are at increased risk.
- It is difficult to manage, but understanding the treatment options is important to success.
- The potential for VAIN to evolve into invasive cancer is probably substantial.
- Treatment of invasive vaginal cancer has a high rate of complications and is often unsuccessful.
This article describes the epidemiology, natural history, diagnosis, and treatment of VAIN, focusing primarily on management.
A range of risk factors
As with many uncommon diseases that are difficult to diagnose, good data on the incidence of VAIN are not available. Women diagnosed with VAIN can be in their late teens or senior citizens; mean age is about 50 years.1-5 Race is not mentioned in most studies.
CIN. Although concomitant or subsequent VAIN is very unusual in the approximately 600,000 women identified with CIN each year in the United States, most patients diagnosed with VAIN have a history of CIN. The small number who still have a cervix and are diagnosed with VAIN have a high incidence of concomitant CIN.1 Of women who have had a hysterectomy for CIN, only 1% to 5% are subsequently diagnosed with VAIN.6,7
Since most women who develop vaginal intraepithelial neoplasia have a prior or current history of cervical neoplasia, the “field effect” also renders them at risk for vulvar neoplasia.
HPV. Compelling data indicate a causal relationship between human papillomavirus (HPV) and CIN; the same is true for VAIN.8
Tobacco use. A history of tobacco use is frequent among women diagnosed with VAIN.1
Pelvic radiotherapy is commonly reported in case series.1,3-5 Generally, malignancy is potentially radiation-related if it develops at least 5 to 10 years after treatment.
We do not know whether radiotherapy induces neoplastic transformation in the vagina, but women with a history of radiation warrant long-term follow-up, as they seem to be at increased risk and diagnosis may be difficult.4,5
Chronically immunocompromised women are at particular risk for multifocal lower genital tract neoplasia.9,10
Natural history
The limited data available do suggest that vaginal intraepithelial neoplasia is a premalignant condition.11,12 Unfortunately, little is known about the relationship between severity of the intraepithelial neoplastic process and degree of risk. Natural history studies of VAIN 3 are even more limited than those of CIN 3.
Like its cervical counterpart, VAIN 3 is thought to have substantial potential for progression to invasive cancer.1-4,10-13
EVALUATIONDiagnosis entails inspection, palpation, and directed biopsies
VAIN is most commonly diagnosed after investigation of an abnormal Pap test taken from the vaginal cuff of a woman who has undergone a prior hysterectomy for cervical neoplasia. Occasionally, the disease is identified during colposcopy as extension of a cervical lesion. In either case, VAIN usually involves the upper third of the vagina.1-5,11 A minority of patients will be found to have diffuse multifocal lesions along the vaginal walls.
Adequate diagnosis mandates:
- careful gross and colposcopic inspection of the entire vagina (with mapping of involved areas),
- representative colposcopically directed biopsies,
- careful palpation of the vaginal walls, especially the vaginal cuff scar, and
- in some cases, excision of the vaginal cuff scar.
VAIN is often readily visualized with a colposcope, and the appearance may be more prominent than that of a comparable cervical lesion (FIGURE 1). The lesions are sometimes hyperkeratotic and grossly visible. However, colposcopy of the vagina is more difficult than that of the cervix due to vaginal folding, a larger surface area, and vaginal cuff irregularities.
Vaginal atrophy also creates diagnostic difficulties related to colposcopic assessment, and to overreading of vaginal cytology (eg, when a lesion is interpreted as high-grade dysplasia or suspicious). Reassessment after 4 to 6 weeks of estrogen therapy helps resolve these issues.
FIGURE 1 Colposcopic view of VAIN 3
Note the hyperkeratotic, prominent appearance of the VAIN lesions.
TREATMENT
Because women with VAIN are a heterogeneous group, treatment must be individualized. Important factors to consider when selecting appropriate treatment include prior hysterectomy, prior radiation therapy, age, whether the woman is sexually active, comorbidities, vaginal anatomy, and prior treatments.
Prior hysterectomy
Most women diagnosed with VAIN have previously undergone hysterectomy, usually because of cervical neoplasia.2-5,10 In these women, VAIN is generally confined to the vaginal apex (FIGURE 2).
Watch for occult neoplasia. Buried epithelium within the vaginal cuff scar may harbor occult neoplasia. Patients with VAIN at the vaginal apex5,10,11,13 and a vaginal cuff from a hysterectomy for cervical neoplasia are analogous to women with CIN and an unsatisfactory colposcopy.
Excision is usual management. Most of these women are appropriately managed with excision of the involved vaginal apex, including the cuff scar.1,3-5,11,14 Problematic vaginal shortening is uncommon following this procedure. The status of the resection margin is predictive of the likelihood of recurrence.3
With more extensive vaginal epithelial involvement, consider cuff excision in selected cases to eliminate the potential for occult disease. The remainder of the involved vagina can be treated by other means outlined below.
FIGURE 2 VAIN 3 at vaginal apex
VAIN usually is confined to the apex in women hysterectomized for cervical neoplasia.
When CIN extends onto the vaginal fornix
This is an uncommon scenario, although its underrecognition may explain why some VAIN is diagnosed shortly after hysterectomy for CIN.
Management is simpler than for disease involving the posthysterectomy apex because there is no scarring, distortion, or buried epithelium, and traction on the cervix generally provides good exposure.
Laser vaporization is an option. For many such patients, this is one of the few good indications remaining for laser vaporization.5,15,16
If hysterectomy is indicated, remove the affected portion of the upper vagina along with the cervix.5,17
Multifocal or diffuse pattern: Typical of immunocompromise
Multifocal or diffuse manifestation is likewise uncommon, often involving low-grade neoplasia and condylomatous changes.5,18 This is the pattern typically seen in chronically immunocompromised women.5,10
The natural history of VAIN—especially the malignant potential—is less well understood for women with this disease pattern. When managing such patients, keep in mind the potential for occult neoplasia in the vaginal cuff scar. However, broaden the focus of treatment to encompass the entire vagina. The various management options are described below:
- If the patient has cervical intraepithelial neoplasia 2 or 3, treat with laser, cryotherapy, or large loop excision of the transformation zone at least 2 weeks prior to beginning 5-fluorouracil (5-FU).
- Treat vulvar, anal, or urethral lesions with a laser or local excision prior to 5-FU.
- Treat vulvovaginitis prior to 5-FU.
- Have the patient administer 1/2 vaginal applicator of 5% 5-FU (Efudex; 2.5 g) deep in the vagina weekly at bedtime for 10 weeks. Instruct her to place a tampon or cotton balls into the outer vagina, unless the distal vagina is involved. Also have her apply petroleum jelly to all vulvar tissues, around the urethra, and to the anus. In the morning, she should remove the tampon, wash the vulva with soap and water, and dry carefully. This should be followed by another application of petroleum jelly (sparingly) to vulvar tissues. A mini napkin may help prevent staining of garments.
- When 5-FU treatment ends, have the patient administer 1/3 applicator of vaginal estrogen cream nightly for 2 weeks beginning 3 weeks prior to reevaluation.
- Evaluate with colposcopy and cytology 8 weeks after completing 5-FU therapy.
Observation only is particularly suited for women who have low-grade disease or who are severely debilitated and chronically immunocompromised.
5-fluorouracil (5-FU) cream is a very good treatment when VAIN involves the upper half to two thirds of the vagina.2,9 However, the cream does not reach buried epithelium in the vaginal cuff scar and probably does not consistently treat the lower vagina when applied with the standard vaginal applicator. Applying the cream directly to the lower vagina may be more effective when that region is involved (see “6-step vaginal fluorouracil therapy for intraepithelial neoplasia,” above).
Laser vaporization is well accepted and commonly used.2,9,15,19,20 Advantages are versatility in treatment of multifocal disease without sacrificing vaginal epithelium, and low likelihood of complications. Disadvantages include inability to treat buried vaginal cuff epithelium; technical difficulties in applying the laser to a folding and often distorted (cuff) surface within a confined space; and the expensive equipment, technical support, and surgical expertise required.1,4,5,11,15,16,20
Planned combined treatment using laser vaporization followed by 5-FU cream has been reported efficacious in the treatment of diffuse vaginal condylomata.21 Selected patients with VAIN also may benefit,22 such as those with plaque-like disease (where a thick layer of keratin can reduce penetration of the 5-FU cream), diffuse/multifocal disease where laser vaporization is likely to be incomplete, or vaginal anatomy that makes it difficult to accomplish complete laser vaporization.
Vaginectomy is definitive management for selected patients with extensive VAIN. The operation is done transperineally, although hysterovaginectomy may require a combined approach.23 Leaving the distal third of the vagina intact (when disease distribution allows) makes the operation easier and may help avert iatrogenic urinary incontinence. Follow-up examination—and treatment, if necessary—of the remaining short vaginal stump is fairly easy.
Removal of the vagina is technically demanding in some women. A Schuchardt incision is useful in such instances.
The other obvious disadvantage of vaginectomy is loss of coital function, although placement of a skin graft is an option.
Brachytherapy is another option for treating extensive VAIN in highly selected patients.24,25
A cylindrical apparatus placed in the vagina delivers radiotherapy to the vaginal epithelium; the likelihood of significant morbidity is low. This method is most applicable to poor surgical candidates with extensive VAIN. Disadvantages include fibrosis of the vagina, limited data on efficacy (and particular concern about inadequate dosing to buried or distorted vaginal cuff epithelium), and potential difficulties with follow-up and treatment of recurrence.3,5
“Chemosurgery,” specifically 5-FU cream followed by surgical removal of the then-partially-detached VAIN, followed by additional 5-FU cream, has been used effectively at 1 center.10 Other reported-but-less-investigated methods include cryotherapy, electrocautery, loop electrosurgical excision, and cavitational ultrasonic surgical aspiration (CUSA).3-5
VAIN in a radiated vagina
Most women who develop VAIN in this scenario received radiation therapy many years earlier for carcinoma of the cervix.5,11
The most common sites are the upper third to upper half of the remaining vagina, where radiation changes are prominent.
Diagnosis often is problematic due to:
- difficulties with interpreting cytologic preparations in such patients;
- radiation changes in the vagina (pale and fibrotic with telangiectasis), which largely obscure colposcopic findings;
- the difficulty and potential hazards of biopsy of a thin, fibrotic upper vagina; and
- obliterative coaptation of the upper third to half of the vagina.
Significantly abnormal cytology in the absence of a colposcopically identified lesion (or palpable abnormality) is of particular concern. In such patients, view the abnormal cytologic interpretation with caution and consider further initial evaluation, including outside review of the cytology slides, treatment of the vagina with estrogen, and repeat cytologic and colposcopic evaluation.
When there is clear cytologic evidence of a severe abnormality, consider the possibility of occult neoplasia within the coapted upper vagina and/or cervix.
Management of VAIN in a radiated vagina.
Take into account the anatomic distortion of the upper vagina, the thin and fibrotic nature of the epithelium, and the potential for fistula formation with excisional procedures or other treatments that produce injury beneath the surface of the epithelium. Among the options:
- 5-FU cream or laser vaporization. VAIN that is completely visualized within the remaining vagina is probably best treated with one of these modalities, provided the physician is experienced in managing such patients.5,9
- Excision of the upper vagina,5 including the coapted portion (and sometimes the residual cervix with or without the uterus) is appropriate for highly selected cases, but only after careful consideration of:
- the likelihood of finding significant neoplasia,
- the anatomic feasibility (it is desirable to perform the procedure transvaginally), and
- the overall risk versus benefit. Such procedures should be performed only by an experienced physician.
The chronically immunocompromised
These patients often have undergone organ transplants or are human immunodeficiency virus (HIV)-positive. Diffuse HPV infection of the lower genital tract is pervasive in these women,5,10,26 and diffuse/multifocal lower genital tract intraepithelial neoplasia is often present as well.
These women may be at increased risk for progression to invasive cancer.
Eradication may not be possible. Attempts to eliminate intraepithelial disease are usually unsuccessful.
Management. Many of these women are severely debilitated, with other, more significant medical problems and a short life expectancy. Vigilance is required, as other lower genital tract (and anal) sites are frequently involved. Since eradication of diffuse/multifocal intraepithelial disease is not a realistic goal, treatment followed by chronic suppressive therapy (such as a low intermittent dose of 5-FU) is reasonable.9,10,22
Another approach is close observation, including frequent examinations, with prompt intervention when invasive disease is suspected.
FOLLOW-UP
After treatment for VAIN, follow-up is similar to that for a comparable cervical lesion. Once the vagina has healed, see the patient every 3 to 6 months for 2 years and annually thereafter. In addition to obtaining vaginal cytology, carefully inspect and palpate the vagina (including the vaginal cuff scar).
Effects of 5-FU. Following a course of 5-FU cream, vaginal mucosal ulcerations may persist for several weeks. Occasionally, these may lead to partial coaptations.27 In addition, subsequent islands of columnar epithelium have been described.28 Keep these factors in mind during the follow-up of women who have been treated with transvaginal 5-FU cream.
Long-term annual follow-up is reasonable, since these women are probably at increased risk for developing a second primary vaginal lesion and/or lower anogenital tract neoplasia at other sites.
When extra vigilance is warranted. Some patients merit closer follow-up, such as the chronically immunosuppressed women described; also, women who have been treated for vaginal apical disease without resection of the vaginal cuff scar, previously radiated patients, and women whose VAIN was treated with brachytherapy.
Dr. Hoffman reports no financial relationships relevant to this article.
- Most women diagnosed with vaginal intraepithelial neoplasia (VAIN) have a history of cervical intraepithelial neoplasia.
- Compelling clinical and laboratory data indicate a causal relationship between human papillomavirus and VAIN.
- Like its cervical counterpart, VAIN 3 is thought to have substantial potential to progress to invasive cancer.
- Diagnosis includes careful gross and colposcopic inspection of the entire vagina (with mapping of involved areas), representative colposcopically directed biopsies, and careful palpation of the vaginal walls, especially the vaginal cuff scar.
- Important factors to consider when selecting appropriate treatment for women with VAIN include prior hysterectomy, prior radiation therapy, age, whether she is sexually active, comorbidities, vaginal anatomy, and prior treatments.
We can easily identify vulvar intraepithelial neoplasia (VIN): The patient complains of itching and has a visible lesion. We find cervical intraepithelial neoplasia (CIN) by investigating an abnormal Pap test. But what about vaginal intraepithelial neoplasia (VAIN)? It does not itch and is invisible to the naked eye. A Pap test sometimes catches it, although this test is used mainly to screen for CIN, not VAIN.
VAIN just does not grab our attention. It is uncommon, and invasive vaginal cancer is rare. But before you slip this article into the “obscure disease” file, consider the following:
- VAIN is difficult to diagnose, but some women are at increased risk.
- It is difficult to manage, but understanding the treatment options is important to success.
- The potential for VAIN to evolve into invasive cancer is probably substantial.
- Treatment of invasive vaginal cancer has a high rate of complications and is often unsuccessful.
This article describes the epidemiology, natural history, diagnosis, and treatment of VAIN, focusing primarily on management.
A range of risk factors
As with many uncommon diseases that are difficult to diagnose, good data on the incidence of VAIN are not available. Women diagnosed with VAIN can be in their late teens or senior citizens; mean age is about 50 years.1-5 Race is not mentioned in most studies.
CIN. Although concomitant or subsequent VAIN is very unusual in the approximately 600,000 women identified with CIN each year in the United States, most patients diagnosed with VAIN have a history of CIN. The small number who still have a cervix and are diagnosed with VAIN have a high incidence of concomitant CIN.1 Of women who have had a hysterectomy for CIN, only 1% to 5% are subsequently diagnosed with VAIN.6,7
Since most women who develop vaginal intraepithelial neoplasia have a prior or current history of cervical neoplasia, the “field effect” also renders them at risk for vulvar neoplasia.
HPV. Compelling data indicate a causal relationship between human papillomavirus (HPV) and CIN; the same is true for VAIN.8
Tobacco use. A history of tobacco use is frequent among women diagnosed with VAIN.1
Pelvic radiotherapy is commonly reported in case series.1,3-5 Generally, malignancy is potentially radiation-related if it develops at least 5 to 10 years after treatment.
We do not know whether radiotherapy induces neoplastic transformation in the vagina, but women with a history of radiation warrant long-term follow-up, as they seem to be at increased risk and diagnosis may be difficult.4,5
Chronically immunocompromised women are at particular risk for multifocal lower genital tract neoplasia.9,10
Natural history
The limited data available do suggest that vaginal intraepithelial neoplasia is a premalignant condition.11,12 Unfortunately, little is known about the relationship between severity of the intraepithelial neoplastic process and degree of risk. Natural history studies of VAIN 3 are even more limited than those of CIN 3.
Like its cervical counterpart, VAIN 3 is thought to have substantial potential for progression to invasive cancer.1-4,10-13
EVALUATIONDiagnosis entails inspection, palpation, and directed biopsies
VAIN is most commonly diagnosed after investigation of an abnormal Pap test taken from the vaginal cuff of a woman who has undergone a prior hysterectomy for cervical neoplasia. Occasionally, the disease is identified during colposcopy as extension of a cervical lesion. In either case, VAIN usually involves the upper third of the vagina.1-5,11 A minority of patients will be found to have diffuse multifocal lesions along the vaginal walls.
Adequate diagnosis mandates:
- careful gross and colposcopic inspection of the entire vagina (with mapping of involved areas),
- representative colposcopically directed biopsies,
- careful palpation of the vaginal walls, especially the vaginal cuff scar, and
- in some cases, excision of the vaginal cuff scar.
VAIN is often readily visualized with a colposcope, and the appearance may be more prominent than that of a comparable cervical lesion (FIGURE 1). The lesions are sometimes hyperkeratotic and grossly visible. However, colposcopy of the vagina is more difficult than that of the cervix due to vaginal folding, a larger surface area, and vaginal cuff irregularities.
Vaginal atrophy also creates diagnostic difficulties related to colposcopic assessment, and to overreading of vaginal cytology (eg, when a lesion is interpreted as high-grade dysplasia or suspicious). Reassessment after 4 to 6 weeks of estrogen therapy helps resolve these issues.
FIGURE 1 Colposcopic view of VAIN 3
Note the hyperkeratotic, prominent appearance of the VAIN lesions.
TREATMENT
Because women with VAIN are a heterogeneous group, treatment must be individualized. Important factors to consider when selecting appropriate treatment include prior hysterectomy, prior radiation therapy, age, whether the woman is sexually active, comorbidities, vaginal anatomy, and prior treatments.
Prior hysterectomy
Most women diagnosed with VAIN have previously undergone hysterectomy, usually because of cervical neoplasia.2-5,10 In these women, VAIN is generally confined to the vaginal apex (FIGURE 2).
Watch for occult neoplasia. Buried epithelium within the vaginal cuff scar may harbor occult neoplasia. Patients with VAIN at the vaginal apex5,10,11,13 and a vaginal cuff from a hysterectomy for cervical neoplasia are analogous to women with CIN and an unsatisfactory colposcopy.
Excision is usual management. Most of these women are appropriately managed with excision of the involved vaginal apex, including the cuff scar.1,3-5,11,14 Problematic vaginal shortening is uncommon following this procedure. The status of the resection margin is predictive of the likelihood of recurrence.3
With more extensive vaginal epithelial involvement, consider cuff excision in selected cases to eliminate the potential for occult disease. The remainder of the involved vagina can be treated by other means outlined below.
FIGURE 2 VAIN 3 at vaginal apex
VAIN usually is confined to the apex in women hysterectomized for cervical neoplasia.
When CIN extends onto the vaginal fornix
This is an uncommon scenario, although its underrecognition may explain why some VAIN is diagnosed shortly after hysterectomy for CIN.
Management is simpler than for disease involving the posthysterectomy apex because there is no scarring, distortion, or buried epithelium, and traction on the cervix generally provides good exposure.
Laser vaporization is an option. For many such patients, this is one of the few good indications remaining for laser vaporization.5,15,16
If hysterectomy is indicated, remove the affected portion of the upper vagina along with the cervix.5,17
Multifocal or diffuse pattern: Typical of immunocompromise
Multifocal or diffuse manifestation is likewise uncommon, often involving low-grade neoplasia and condylomatous changes.5,18 This is the pattern typically seen in chronically immunocompromised women.5,10
The natural history of VAIN—especially the malignant potential—is less well understood for women with this disease pattern. When managing such patients, keep in mind the potential for occult neoplasia in the vaginal cuff scar. However, broaden the focus of treatment to encompass the entire vagina. The various management options are described below:
- If the patient has cervical intraepithelial neoplasia 2 or 3, treat with laser, cryotherapy, or large loop excision of the transformation zone at least 2 weeks prior to beginning 5-fluorouracil (5-FU).
- Treat vulvar, anal, or urethral lesions with a laser or local excision prior to 5-FU.
- Treat vulvovaginitis prior to 5-FU.
- Have the patient administer 1/2 vaginal applicator of 5% 5-FU (Efudex; 2.5 g) deep in the vagina weekly at bedtime for 10 weeks. Instruct her to place a tampon or cotton balls into the outer vagina, unless the distal vagina is involved. Also have her apply petroleum jelly to all vulvar tissues, around the urethra, and to the anus. In the morning, she should remove the tampon, wash the vulva with soap and water, and dry carefully. This should be followed by another application of petroleum jelly (sparingly) to vulvar tissues. A mini napkin may help prevent staining of garments.
- When 5-FU treatment ends, have the patient administer 1/3 applicator of vaginal estrogen cream nightly for 2 weeks beginning 3 weeks prior to reevaluation.
- Evaluate with colposcopy and cytology 8 weeks after completing 5-FU therapy.
Observation only is particularly suited for women who have low-grade disease or who are severely debilitated and chronically immunocompromised.
5-fluorouracil (5-FU) cream is a very good treatment when VAIN involves the upper half to two thirds of the vagina.2,9 However, the cream does not reach buried epithelium in the vaginal cuff scar and probably does not consistently treat the lower vagina when applied with the standard vaginal applicator. Applying the cream directly to the lower vagina may be more effective when that region is involved (see “6-step vaginal fluorouracil therapy for intraepithelial neoplasia,” above).
Laser vaporization is well accepted and commonly used.2,9,15,19,20 Advantages are versatility in treatment of multifocal disease without sacrificing vaginal epithelium, and low likelihood of complications. Disadvantages include inability to treat buried vaginal cuff epithelium; technical difficulties in applying the laser to a folding and often distorted (cuff) surface within a confined space; and the expensive equipment, technical support, and surgical expertise required.1,4,5,11,15,16,20
Planned combined treatment using laser vaporization followed by 5-FU cream has been reported efficacious in the treatment of diffuse vaginal condylomata.21 Selected patients with VAIN also may benefit,22 such as those with plaque-like disease (where a thick layer of keratin can reduce penetration of the 5-FU cream), diffuse/multifocal disease where laser vaporization is likely to be incomplete, or vaginal anatomy that makes it difficult to accomplish complete laser vaporization.
Vaginectomy is definitive management for selected patients with extensive VAIN. The operation is done transperineally, although hysterovaginectomy may require a combined approach.23 Leaving the distal third of the vagina intact (when disease distribution allows) makes the operation easier and may help avert iatrogenic urinary incontinence. Follow-up examination—and treatment, if necessary—of the remaining short vaginal stump is fairly easy.
Removal of the vagina is technically demanding in some women. A Schuchardt incision is useful in such instances.
The other obvious disadvantage of vaginectomy is loss of coital function, although placement of a skin graft is an option.
Brachytherapy is another option for treating extensive VAIN in highly selected patients.24,25
A cylindrical apparatus placed in the vagina delivers radiotherapy to the vaginal epithelium; the likelihood of significant morbidity is low. This method is most applicable to poor surgical candidates with extensive VAIN. Disadvantages include fibrosis of the vagina, limited data on efficacy (and particular concern about inadequate dosing to buried or distorted vaginal cuff epithelium), and potential difficulties with follow-up and treatment of recurrence.3,5
“Chemosurgery,” specifically 5-FU cream followed by surgical removal of the then-partially-detached VAIN, followed by additional 5-FU cream, has been used effectively at 1 center.10 Other reported-but-less-investigated methods include cryotherapy, electrocautery, loop electrosurgical excision, and cavitational ultrasonic surgical aspiration (CUSA).3-5
VAIN in a radiated vagina
Most women who develop VAIN in this scenario received radiation therapy many years earlier for carcinoma of the cervix.5,11
The most common sites are the upper third to upper half of the remaining vagina, where radiation changes are prominent.
Diagnosis often is problematic due to:
- difficulties with interpreting cytologic preparations in such patients;
- radiation changes in the vagina (pale and fibrotic with telangiectasis), which largely obscure colposcopic findings;
- the difficulty and potential hazards of biopsy of a thin, fibrotic upper vagina; and
- obliterative coaptation of the upper third to half of the vagina.
Significantly abnormal cytology in the absence of a colposcopically identified lesion (or palpable abnormality) is of particular concern. In such patients, view the abnormal cytologic interpretation with caution and consider further initial evaluation, including outside review of the cytology slides, treatment of the vagina with estrogen, and repeat cytologic and colposcopic evaluation.
When there is clear cytologic evidence of a severe abnormality, consider the possibility of occult neoplasia within the coapted upper vagina and/or cervix.
Management of VAIN in a radiated vagina.
Take into account the anatomic distortion of the upper vagina, the thin and fibrotic nature of the epithelium, and the potential for fistula formation with excisional procedures or other treatments that produce injury beneath the surface of the epithelium. Among the options:
- 5-FU cream or laser vaporization. VAIN that is completely visualized within the remaining vagina is probably best treated with one of these modalities, provided the physician is experienced in managing such patients.5,9
- Excision of the upper vagina,5 including the coapted portion (and sometimes the residual cervix with or without the uterus) is appropriate for highly selected cases, but only after careful consideration of:
- the likelihood of finding significant neoplasia,
- the anatomic feasibility (it is desirable to perform the procedure transvaginally), and
- the overall risk versus benefit. Such procedures should be performed only by an experienced physician.
The chronically immunocompromised
These patients often have undergone organ transplants or are human immunodeficiency virus (HIV)-positive. Diffuse HPV infection of the lower genital tract is pervasive in these women,5,10,26 and diffuse/multifocal lower genital tract intraepithelial neoplasia is often present as well.
These women may be at increased risk for progression to invasive cancer.
Eradication may not be possible. Attempts to eliminate intraepithelial disease are usually unsuccessful.
Management. Many of these women are severely debilitated, with other, more significant medical problems and a short life expectancy. Vigilance is required, as other lower genital tract (and anal) sites are frequently involved. Since eradication of diffuse/multifocal intraepithelial disease is not a realistic goal, treatment followed by chronic suppressive therapy (such as a low intermittent dose of 5-FU) is reasonable.9,10,22
Another approach is close observation, including frequent examinations, with prompt intervention when invasive disease is suspected.
FOLLOW-UP
After treatment for VAIN, follow-up is similar to that for a comparable cervical lesion. Once the vagina has healed, see the patient every 3 to 6 months for 2 years and annually thereafter. In addition to obtaining vaginal cytology, carefully inspect and palpate the vagina (including the vaginal cuff scar).
Effects of 5-FU. Following a course of 5-FU cream, vaginal mucosal ulcerations may persist for several weeks. Occasionally, these may lead to partial coaptations.27 In addition, subsequent islands of columnar epithelium have been described.28 Keep these factors in mind during the follow-up of women who have been treated with transvaginal 5-FU cream.
Long-term annual follow-up is reasonable, since these women are probably at increased risk for developing a second primary vaginal lesion and/or lower anogenital tract neoplasia at other sites.
When extra vigilance is warranted. Some patients merit closer follow-up, such as the chronically immunosuppressed women described; also, women who have been treated for vaginal apical disease without resection of the vaginal cuff scar, previously radiated patients, and women whose VAIN was treated with brachytherapy.
Dr. Hoffman reports no financial relationships relevant to this article.
1. Dodge JA, Eltabbakh GH, Mount SL, Walker RP, Morgan A. Clinical features and risk of recurrence among patients with vaginal intraepithelial neoplasia. Gynecol Oncol. 2001;83:363-369.
2. Petrilli ES, Townsend DE, Morrow CP, Nakao CY. Vaginal intraepithelial neoplasia: biologic aspects and treatment with topical 5-fluorouracil and the carbon dioxide laser. Am J Obstet Gynecol. 1980;138:321-328.
3. Benedet JL, Sanders BH. Carcinoma in situ of the vagina. Am J Obstet Gynecol. 1984;148:695-700.
4. Lenehan PM, Meffe F, Lickrish GM. Vaginal intraepithelial neoplasia: biologic aspects and management. Obstet Gynecol. 1986;68:333-337.
5. Audet-Lapointe P, Body G, Vauclair R, Drouin P, Ayoub J. Vaginal intraepithelial neoplasia. Gynecol Oncol. 1990;36:232-239.
6. Gallup DG, Morley GW. Carcinoma in situ of the vagina—a study and review. Obstet Gynecol. 1975;46:334-440.
7. Woodruff JD. Treatment of recurrent carcinoma in situ in the lower genital canal. Clin Obstet Gynecol. 1965;8:757-770.
8. McCance DJ, Clarkson PK, Dyson JL, Walker PG, Singer A. Human papillomavirus types 6 and 16 in multifocal intraepithelial neoplasias of the female lower genital tract. Br J Obstet Gynaecol. 1985;92:1093-1100.
9. Krebs HB. Treatment of vaginal intraepithelial neoplasia with laser and topical 5-fluorouracil. Obstet Gynecol. 1989;73:657-660.
10. Sillman FH, Fruchter RG, Chen YS, Camilien L, Sedlis A, McTigue E. Vaginal intraepithelial neoplasia: risk factors for persistence, recurrence, and invasion and its management. Am J Obstet Gynecol. 1997;176:93-99.
11. Woodman CBJ, Jordan JA, Wade-Evans T. The management of vaginal intraepithelial neoplasia after hysterectomy. Br J Obstet Gynaecol. 1984;91:103-105.
12. Aho M, Vesterinen E, Meyer B, Purola E, Paavonen J. Natural history of vaginal intraepithelial neoplasia. Cancer. 1991;68:195-197.
13. Hoffman MS, Roberts WS, LaPolla JP, Sterghos S, Jr, Cavanagh D. Neoplasia in vaginal cuff epithelial inclusion cysts after hysterectomy. J Reprod Med. 1989;34:412-416.
14. Hoffman MS, DeCesare SL, Roberts WS, Fiorica JV, Finan MA, Cavanagh D. Upper vaginectomy for in situ and occult, superficially invasive carcinoma of the vagina. Am J Obstet Gynecol. 1992;166:30-33.
15. Curtin JP, Twiggs LB, Julian TM. Treatment of vaginal intraepithelial neoplasia with the CO2laser. J Reprod Med. 1985;30:942-944.
16. Hoffman MS, Roberts WS, LaPolla JP, Fiorica JV, Cavanagh D. Laser vaporization of grade 3 vaginal intraepithelial neoplasia. Am J Obstet Gynecol. 1991;165:1342-1344.
17. Hoffman MS, Spellacy WN. The Difficult Vaginal Hysterectomy. New York, NY: Springer-Verlag; 1995;117-119.
18. Reid R. Human papillomaviral infection. The key to rational triage of cervical neoplasia. Obstet Gynecol Clin North Am. 1987;14:407-429.
19. Townsend DE, Levine RU, Crum CP, Richart RM. Treatment of vaginal carcinoma in situ with the carbon dioxide laser. Am J Obstet Gynecol. 1982;143:565-568.
20. Sherman AI. Laser therapy for vaginal intraepithelial neoplasia after hysterectomy. J Reprod Med. 1990;35—941-944.
21. Krebs HB. Combination of laser plus 5-fluorouracil for the treatment of extensive genital condylomata acuminata. Lasers Surg Med. 1988;8:135-138.
22. Krebs HB. Prophylactic topical 5-fluorouracil following treatment of human papillomavirus-associated lesions of the vulva and vagina. Obstet Gynecol. 1986;68:837-841.
23. Hoffman MS, Bomalaski JJ, Lockhart JL, Greenwald DP. Total removal of the normal non-prolapsed vagina. Technique, results and anatomic observations. J Pelvic Surg. 2002;8:89-94.
24. MacLeod C, Fowler A, Dalrymple C, Atkinson K, Elliott P, Carter J. High-dose-rate brachytherapy in the management of high-grade intraepithelial neoplasia of the vagina. Gynecol Oncol. 1997;65:74-77.
25. Teruya Y, Sakumoto K, Moromizato H, et al. High dose-rate intracavitary brachytherapy for carcinoma in situ of the vagina occurring after hysterectomy: a rational prescription of radiation dose. Am J Obstet Gynecol. 2002;187:360-364.
26. Sillman FH, Sedlis A. Anogenital HPV/neoplasia in immunosuppressed women: an update. Dermatol Clin North Am. 1991;9:353-369.
27. Krebs HB, Helmkamp BF. Chronic ulcerations following topical therapy with 5-fluorouracil for vaginal human papillomavirus-associated lesions. Obstet Gynecol. 1991;78:205-212.
28. Dungar CF, Wilkinson EJ. Vaginal columnar cell metaplasia: an acquired adenosis associated with topical 5-FU therapy. J Reprod Med. 1995;40:361-366.
1. Dodge JA, Eltabbakh GH, Mount SL, Walker RP, Morgan A. Clinical features and risk of recurrence among patients with vaginal intraepithelial neoplasia. Gynecol Oncol. 2001;83:363-369.
2. Petrilli ES, Townsend DE, Morrow CP, Nakao CY. Vaginal intraepithelial neoplasia: biologic aspects and treatment with topical 5-fluorouracil and the carbon dioxide laser. Am J Obstet Gynecol. 1980;138:321-328.
3. Benedet JL, Sanders BH. Carcinoma in situ of the vagina. Am J Obstet Gynecol. 1984;148:695-700.
4. Lenehan PM, Meffe F, Lickrish GM. Vaginal intraepithelial neoplasia: biologic aspects and management. Obstet Gynecol. 1986;68:333-337.
5. Audet-Lapointe P, Body G, Vauclair R, Drouin P, Ayoub J. Vaginal intraepithelial neoplasia. Gynecol Oncol. 1990;36:232-239.
6. Gallup DG, Morley GW. Carcinoma in situ of the vagina—a study and review. Obstet Gynecol. 1975;46:334-440.
7. Woodruff JD. Treatment of recurrent carcinoma in situ in the lower genital canal. Clin Obstet Gynecol. 1965;8:757-770.
8. McCance DJ, Clarkson PK, Dyson JL, Walker PG, Singer A. Human papillomavirus types 6 and 16 in multifocal intraepithelial neoplasias of the female lower genital tract. Br J Obstet Gynaecol. 1985;92:1093-1100.
9. Krebs HB. Treatment of vaginal intraepithelial neoplasia with laser and topical 5-fluorouracil. Obstet Gynecol. 1989;73:657-660.
10. Sillman FH, Fruchter RG, Chen YS, Camilien L, Sedlis A, McTigue E. Vaginal intraepithelial neoplasia: risk factors for persistence, recurrence, and invasion and its management. Am J Obstet Gynecol. 1997;176:93-99.
11. Woodman CBJ, Jordan JA, Wade-Evans T. The management of vaginal intraepithelial neoplasia after hysterectomy. Br J Obstet Gynaecol. 1984;91:103-105.
12. Aho M, Vesterinen E, Meyer B, Purola E, Paavonen J. Natural history of vaginal intraepithelial neoplasia. Cancer. 1991;68:195-197.
13. Hoffman MS, Roberts WS, LaPolla JP, Sterghos S, Jr, Cavanagh D. Neoplasia in vaginal cuff epithelial inclusion cysts after hysterectomy. J Reprod Med. 1989;34:412-416.
14. Hoffman MS, DeCesare SL, Roberts WS, Fiorica JV, Finan MA, Cavanagh D. Upper vaginectomy for in situ and occult, superficially invasive carcinoma of the vagina. Am J Obstet Gynecol. 1992;166:30-33.
15. Curtin JP, Twiggs LB, Julian TM. Treatment of vaginal intraepithelial neoplasia with the CO2laser. J Reprod Med. 1985;30:942-944.
16. Hoffman MS, Roberts WS, LaPolla JP, Fiorica JV, Cavanagh D. Laser vaporization of grade 3 vaginal intraepithelial neoplasia. Am J Obstet Gynecol. 1991;165:1342-1344.
17. Hoffman MS, Spellacy WN. The Difficult Vaginal Hysterectomy. New York, NY: Springer-Verlag; 1995;117-119.
18. Reid R. Human papillomaviral infection. The key to rational triage of cervical neoplasia. Obstet Gynecol Clin North Am. 1987;14:407-429.
19. Townsend DE, Levine RU, Crum CP, Richart RM. Treatment of vaginal carcinoma in situ with the carbon dioxide laser. Am J Obstet Gynecol. 1982;143:565-568.
20. Sherman AI. Laser therapy for vaginal intraepithelial neoplasia after hysterectomy. J Reprod Med. 1990;35—941-944.
21. Krebs HB. Combination of laser plus 5-fluorouracil for the treatment of extensive genital condylomata acuminata. Lasers Surg Med. 1988;8:135-138.
22. Krebs HB. Prophylactic topical 5-fluorouracil following treatment of human papillomavirus-associated lesions of the vulva and vagina. Obstet Gynecol. 1986;68:837-841.
23. Hoffman MS, Bomalaski JJ, Lockhart JL, Greenwald DP. Total removal of the normal non-prolapsed vagina. Technique, results and anatomic observations. J Pelvic Surg. 2002;8:89-94.
24. MacLeod C, Fowler A, Dalrymple C, Atkinson K, Elliott P, Carter J. High-dose-rate brachytherapy in the management of high-grade intraepithelial neoplasia of the vagina. Gynecol Oncol. 1997;65:74-77.
25. Teruya Y, Sakumoto K, Moromizato H, et al. High dose-rate intracavitary brachytherapy for carcinoma in situ of the vagina occurring after hysterectomy: a rational prescription of radiation dose. Am J Obstet Gynecol. 2002;187:360-364.
26. Sillman FH, Sedlis A. Anogenital HPV/neoplasia in immunosuppressed women: an update. Dermatol Clin North Am. 1991;9:353-369.
27. Krebs HB, Helmkamp BF. Chronic ulcerations following topical therapy with 5-fluorouracil for vaginal human papillomavirus-associated lesions. Obstet Gynecol. 1991;78:205-212.
28. Dungar CF, Wilkinson EJ. Vaginal columnar cell metaplasia: an acquired adenosis associated with topical 5-FU therapy. J Reprod Med. 1995;40:361-366.
Determining the best route for hysterectomy
- Choose the abdominal route when extensive intraperitoneal surgery and/or exploration are required in addition to the hysterectomy, i.e., in cases of pelvic organ carcinoma.
- Use a combination of uterine morcellation techniques to accomplish a vaginal hysterectomy, as researchers have found morcellation of an enlarged uterus to be safer than removing it abdominally.
- For laparoscopic-assisted vaginal hysterectomy, use 3 ports and avoid most disposable instruments by using cautery on vascular pedicles to help minimize costs.
- Seek alternatives to abdominal hysterectomy given its less favorable outcome in terms of morbidity and recovery.
While hysterectomy is one of the most frequently performed operations in gynecology, how to perform it—abdominally, vaginally, or laparoscopically—is less evident. Numerous studies have been published in an attempt to shed some light on this controversy.
Individualize the approach for each patient rather than rely on a dogmatic assignment of technique.
Prior to the introduction of the laparoscopic-assisted vaginal hysterectomy (LAVH) by Reich et al in 1989,1 several large studies were published that compared the abdominal and vaginal routes for hysterectomy. The largest was the Collaborative Review of Sterilization (CREST) study conducted by the Centers for Disease Control (CDC).2 This report included 1,856 women aged 15 to 44 who underwent non-emergency, non-radical hysterectomies at 9 institutions between 1978 and 1981. Fewer complications were associated with vaginal hysterectomy (VH) than abdominal hysterectomy (AH) (Table 1).
Now, several trials have included LAVH in the comparison of hysterectomy routes. In the most comprehensive study to date, Johns et al reviewed 2,563 hysterectomies performed for nonmalignant indications by 37 private gynecologists from a single institution.3 The researchers found that bowel, bladder, and ureteral injuries were uncommon, and the rates of each were similar among LAVH, abdominal hysterectomy, and vaginal hysterectomy (Table 2). In addition, a review of the literature between 1989 and 1995 revealed that LAVH is associated with a shorter hospital stay, decreased recovery time, and less analgesia compared with AH.4
However, since most of the data on route for hysterectomy are from retrospective and uncontrolled trials, one must interpret the findings carefully. For example, many studies do not control for additional procedures performed at the time of hysterectomy (e.g., enterocele, rectocele, and cystocele repairs). In addition, information on how researchers categorized unsuccessful attempts at VH or LAVH—which then had to be converted to AH—often is excluded. Also, physicians usually select the technique based on personal preference, practice style, and traditional dogma such as uterine size rather than a standard protocol.5,6 Therefore, the increased incidence of postoperative morbidity associated with AH is difficult to decipher. Is it due to the increased number of obese and nulliparous women undergoing AH, the surgeon’s experience, pelvic pathology or operative indication, or is it related to the actual opening of the abdomen and intraperitoneal manipulation? Most likely, it is a combination of these factors.
Overall, hysterectomy is a relatively safe procedure with a mortality rate of 1 to 2 per 1,000.7 Morbidity, however, remains high. Fortunately, most complications are minor and easily remedied with little clinical consequence. Since certain aspects of postoperative morbidity are related to the route for hysterectomy, the surgeon must individualize the approach for each patient and not rely on a dogmatic assignment of technique. Here, we will review the patient selection for and provide pearls on abdominal, vaginal, and laparoscopic-assisted vaginal hysterectomy, as well as look at the advantages and disadvantages of each method.
TABLE 1
CREST*study results
| VH | AH | |
|---|---|---|
| Mean age (yrs) | 34.4 | 35.8 |
| Nulliparous (%) | 1.4 | 13.3 |
| Prior cesarean section (%) | 4.8 | 10.1 |
| Obese** (%) | 38.0 | 44.7 |
| Febrile morbidity (%) | 15.3 | 32.3 |
| Required transfusion (%) | 8.3 | 15.4 |
| Death (%) | 0.2 | 0.1 |
| *The Collaborative Review of Sterilization | ||
| **Greater than 120% ideal body weight | ||
| VH=vaginal hysterectomy | ||
| AH=abdominal hysterectomy | ||
| Source: Dicker RC, Greenspan JR, Strauss LT, Cowart MR, Scally MJ, et al. Complications of abdominal and vaginal hysterectomy among women of reproductive age in the United States. Am J Obstet Gynecol. 1982;144:841-848. | ||
TABLE 2
Complications at hysterectomy
| AH | VH | LAVH | |
|---|---|---|---|
| Operating time (minutes) | 82 | 63 | 102 |
| Uterine weight (grams) | 216 | 113 | 129 |
| Febrile morbidity (%) | 9.1 | 3.2 | 2.0 |
| Required transfusion (%) | 2.5 | 1.0 | 0.06 |
| Bowel, bladder, or ureteral injury (%) | 1.0 | 0.9 | 1.1 |
| Death (%) | 0 | 0.2 | 0 |
| AH=abdominal hysterectomy | |||
| VH=vaginal hysterectomy | |||
| LAVH=laparoscopic-assisted vaginal hysterectomy | |||
| Source: Johns DA, et al. The medical and economic impact of laparoscopically assisted vaginal hysterectomy in a large, metropolitan, not-for-profit hospital. Am J Obstet Gynecol. 1995;172:1709-1719. | |||
Abdominal hysterectomy
Patient selection. Choose the abdominal route when extensive intraperitoneal surgery and/or exploration are required in addition to the hysterectomy, i.e., in cases of pelvic organ carcinoma. Severe pelvic adhesive disease from documented severe endometriosis, salpingitis, or significant adnexal pathology and a considerably enlarged uterus also are best approached abdominally (Figure 1). In addition, use the abdominal route for obstetric emergencies such as postpartum hemorrhage.
Technique. First, determine the type of incision based on the following factors: Which one will allow completion of the procedure in a safe and efficient manner, minimize complications, expedite recovery, and offer a favorable cosmetic result? A vertical midline incision is the most adaptable to unsuspected pathology, especially if found in the upper abdomen. It is the quickest to perform and is associated with the least blood loss, an important advantage when operating emergently on an anemic or hemorrhaging patient. However, bear in mind that vertical incisions that have been extended into the upper abdomen are accompanied by increased postoperative pulmonary morbidity, pain, and risk of hernia formation.8,9
Transverse incisions such as the commonly used Pfannenstiel, Cherney, and Maylard compromise upper abdominal exposure but are more cosmetically appealing than vertical incisions. Each of these involves a lower abdominal transverse skin incision and a transverse fascial incision. With the Pfannenstiel technique, the rectus fascia is separated from the rectus muscles, and the muscles are split vertically in the midline, allowing access to the peritoneal cavity. This is the quickest transverse incision to make but provides the least exposure.
If a Pfannenstiel incision has been made and additional lateral pelvic exposure is needed, convert the incision to a Cherney. Both the Cherney and Maylard incisions provide additional exposure to the pelvis, especially laterally, which is advantageous when complex pathology is limited to the pelvis. The Cherney incision involves dividing the rectus muscles from the pubic symphysis, whereas the Maylard incision entails horizontal transection of the rectus muscles at the level of the fascial incision. With the Maylard technique, the rectus fascia is not separated from the underlying muscles as it is in the Cherney incision. If upper abdominal exposure becomes necessary, all of these incisions can be extended upward from their lateral margin, creating a “J.” Alternatively, a second incision can be made in the upper abdomen.
Another option is mini-laparotomy (an incision less than 6 cm). Hoffman and Lynch reported success with this technique for hysterectomy in select patients.10 Most of the procedures were completed with minimal use of retractors by exteriorizing the uterus via one of the aforementioned incisions. They found this approach to be safe and effective in nonobese women in whom a vaginal approach was precluded due to anatomy.
Use the mini-laparotomy approach when there is a suspicious adnexal mass that can be removed through a small abdominal incision.11 In addition, a large benign-appearing cystic adnexal mass can be drained, and the procedure then completed through this incision. Proceed cautiously, however, since an occasional unanticipated carcinoma will be encountered, and the act of drainage will result in the need for chemotherapy in a woman with otherwise early-stage disease. If cancer is suspected, consider a vertical minilaparotomy, which allows easy extension into the upper abdomen should surgical staging or debulking become necessary.
Advantages and disadvantages. The abdominal approach offers the best exposure of the pelvic and upper abdominal cavity but is associated with a high rate of complications, including fever and excessive blood loss. We surmise that postoperative recovery with a mini-laparotomy is likely to be improved compared with the traditional abdominal technique.
Figure 1
Opt for abdominal hysterectomy when a considerably enlarged uterus due to numerous fibroids is encountered.
Vaginal hysterectomy
Patient selection. Choose the vaginal route when pelvic support defects are present (Figure 2), but bear in mind that the course of the ureter changes with worsening degrees of uterine prolapse. We have found the ureter to be palpable in the bladder pillar in most women undergoing VH for prolapse, which helps avoid intraoperative ureteral injury.
The ovaries can be safely removed through the vagina in a large percentage of patients.
Obese, elderly, and otherwise medically debilitated patients also will benefit from the vaginal approach. Avoiding an abdominal wound in these women has obvious advantages. Also, vaginal surgery is associated with a reduction in postoperative pulmonary complications when compared with AH.6,12
Vaginal accessibility inevitably influences the decision to proceed vaginally with a hysterectomy. An inadequate bony pelvis is reason to forgo a VH. Orthopedic conditions and muscular contractures of the lower extremities, which prevent safe positioning, also inhibit this approach. Some surgeons also consider prior abdominal or pelvic surgery a reason to avoid vaginal hysterectomy, while others find this route a means of eluding potential adhesions.
We are strong advocates of vaginal surgery and do not consider nulliparity, lack of uterine descent, or prior abdominopelvic surgery to be strict contraindications for VH. A Schuchardt incision may overcome a small introitus, and a mobile uterus often will descend as the uterosacral and cardinal ligaments are divided.
In women with a prior cesarean delivery, it may be easier to enter the vesicouterine space by approaching it from the less-scarred vaginal side. In a study of more than 200 patients with a previous cesarean who underwent VH, Sheth and Malpani found no increase in complications and concluded that VH is the route of choice in this patient population.13 When unsuspected pelvic adhesions are encountered, carefully dissect them transvaginally and complete the procedure.
Besides accessibility, uterine size must be considered. However, size alone should present a dilemma in only about 15% of patients since most hysterectomy specimens are 12 weeks’ gestational size or smaller.14
Technique. Large uteri can be removed vaginally, depending on the surgeon’s technical ability and experience. A familiarity with the various methods of morcellation—hemisection, posterior fundal morcellation, intramyometrial coring, and myomectomy—is mandatory. It often is beneficial to use a combination of these techniques to accomplish the procedure vaginally, as opposed to a single means of morcellation. With any morcellation procedure, maintain a midline orientation to avoid dissection into the broad ligament. Continued caudal traction also is helpful in eliminating excessive blood loss during this prolonged procedure. Do not morcellate a uterus when endometrial carcinoma is suspected.
In a prospective observational study comparing vaginal morcellation to AH, Hoffman et al15 found the former to be safer. Other researchers also have demonstrated the procedure’s safety in retrospective reports16-18 and as subgroups in other series.14 Alternatively, preoperative treatment with a gonadotropin-releasing hor-Surgical Techniques mone (GnRH) agonist can reduce uterine size and result in a technically less challenging VH in some women.19,20
Also, whether or not the ovaries will be resected may influence the decision to perform a VH. While some surgeons believe that oophorectomy precludes the vaginal approach, the ovaries can be safely removed through the vagina in a large percentage of patients.21-23 In fact, Sheth24 and Kovac14 have published 95% and 97% success rates in 2 separate series in which vaginal oophorectomy was attempted in 740 and 142 women, respectively. However, do not attempt adnexal removal without adequate transvaginal access and visibility, as it increases the risk of hemorrhage and ureteral injury. In these cases, LAVH or mini-laparotomy AH may be preferable if oophorectomy is mandatory, i.e., in women who are predisposed to ovarian malignancies because of genetic mutations. However, when patients are appropriately selected, there are essentially no disadvantages to VH.
Advantages and disadvantages.VH has many advantages over AH. Minimal intraperitoneal manipulation and the avoidance of an abdominal wound lead to a shorter hospital stay and decreased recovery time, which is coupled with less cost. Patients also find the lack of an abdominal scar to be an attractive feature of VH. A disadvantage is reduced operative exposure, making it difficult to manipulate pelvic pathology and resect the adnexa.
Figure 2
Choose the vaginal route when pelvic support defects are present, but bear in mind that the course of the ureter changes due to prolapse.
Laparoscopic-assisted vaginal hysterectomy
Patient selection. When in doubt about which approach to use, turn to LAVH. The laparoscope allows the surgeon to thoroughly assess intra-abdominal pathology, which aids in the appropriate selection of a hysterectomy route. For example, if cancer is suspected based on the visual inspection, opt for an AH. On the other hand, if only extensive adhesions are noted, laparoscopic adhesiolysis can be performed to allow the hysterectomy to be completed vaginally. As previously noted, LAVH is appropriate in patients undergoing hysterectomy with prophylactic oophorectomy due to genetic or familial risk factors.
Technique. Bear in mind that the laparoscope does not permit all patients to undergo a VH. In fact, about 10% of attempted LAVHs will be unsuccessful.25,26 Some authors have reported failure during the laparoscopic portion,25,26 while others have attributed the lack of success to the vaginal portion of the procedure.27 Thus, it is important to be skilled at both laparoscopic and vaginal surgery in order to be successful with an LAVH.
LAVH requires three to five 5-to 10-mm abdominal incisions for port sites. An umbilical port is commonly used for the camera, and the remaining ports are used for operating. We typically use 3 ports and avoid most disposable instruments by using cautery on vascular pedicles, which helps minimize costs.3 Other alternatives include the use of endoscopic staplers, the Harmonic Scalpel (Ethicon Endo-Surgery, a Johnson & Johnson company, Cincinnati, Ohio), and suture ligatures with extra-or intracorporeal knot tying. Whichever instrument is used, familiarize yourself with all available equipment given the occasional malfunction encountered during laparoscopy.
Advantages and disadvantages. LAVH requires special equipment and expertise beyond that needed for an abdominal or vaginal hysterectomy. Additionally, this procedure increases operative time, cost, and morbidity (depending on the surgeon’s experience),4,27,28 while the postoperative recovery is similar to that of VH.
Although we use the laparoscope infrequently and believe there is little need to perform excessive laparoscopy at the time of routine hysterectomy, we do not wish to understate the role of the laparoscope. Its use in specific instances can certainly avoid a laparotomy or help determine when an AH is more appropriate.
Conclusion
Emergent situations and patients with excessively enlarged uteri, significant pelvic pathology, or cancer are obvious candidates for AH. On the other hand, VH is frequently chosen for the small uterus in a multiparous woman with a large pelvis and no prior pelvic inflammatory disease or surgery. The dilemma arises when determining the approach for those patients with moderately enlarged uteri or presumptive risk factors for serious pelvic disease.
Kovac reported a standard protocol for selecting the route for hysterectomy. Uterine size, other pelvic pathology (endometriosis, adnexal disease, chronic pain, etc.), and uterine and adnexal accessibility (bony architecture, uterine support, and vaginal diameter) were each considered in the decision-making. A simplification of Kovac’s guidelines applicable to women undergoing hysterectomy for benign indications is summarized in Figure 3. Using these guidelines, Kovac reported a 99% (608/611) success rate for women assigned to VH or LAVH. The laparoscope was deemed necessary in only 19% of those assigned to the LAVH group, and ultimately only 9 patients required AH, yielding a 1:68 ratio for AH to VH.14
Gynecologists should seek alternatives to AH given its less favorable outcome in terms of morbidity and recovery. However, the surgeon who is competent with AH better serves the patient by performing the procedure via this route than by attempting an alternative procedure without the necessary proficiency. In other words, pelvic surgeons must be cognizant of their abilities and practice within that realm. Ultimately, the final selection of hysterectomy route should be based on the surgeon’s experience, the indication for surgery, and the patient’s anatomy.
FIGURE 3Guidelines to determine route of hysterectomy.
The authors report no financial relationship with any companies whose products are mentioned in this article.
1. Reich H, DeCaprio J, McGlynn F. Laparoscopic hysterectomy. J Gynecol Surg. 1989;5:213-216.
2. Dicker RC, Greenspan JR, Strauss LT, Cowart MR, Scally MJ, et al. Complications of abdominal and vaginal hysterectomy among women of reproductive age in the United States. Am J Obstet Gynecol. 1982;144:841-848.
3. Johns DA, Carrera B, Jones J, DeLeon F, Vincent R, et al. The medical and economic impact of laparoscopic-assisted vaginal hysterectomy in a large, metropolitan, not-for-profit hospital. Am J Obstet Gynecol. 1995;172:1709-719.
4. Meikle SF, Nugent EW, Orleans M. Complication and recovery for laparoscopic-assisted vaginal hysterectomy compared with abdominal and vaginal hysterectomy. Obstet Gynecol. 1997;89:304-311.
5. Kovac SR, Christie SJ, Bindbeutel GA. Abdominal versus vaginal hysterectomy: a statistical model for determining physician decision making and patient outcomes. Med Decis Making. 1991;11:19-28.
6. Dorsey JH, Steinberg EP, Holtz PM. Clinical indications for hysterectomy route: patient characteristics or physician preference. Am J Obstet Gynecol. 1995;173:1452-1460.
7. Wingo PA, Huezo CM, Rubin GL, Ory HW, Peterson HB. The mortality risk associated with hysterectomy. Am J Obstet Gynecol. 1985;152:803-808.
8. Tollefson DG, Russell KP. The transverse incision in pelvic surgery. Am J Obstet Gynecol. 1954;68:410-422.
9. Helmkamp BF. Abdominal wound dehiscence. Am J Obstet Gynecol. 1977;128:803-807.
10. Hoffman MS, Lynch CM. Minilaparotomy hysterectomy. Am J Obstet Gynecol. 1998;179:316-320.
11. Flynn M, Niloff JM. Minilaparotomy for the ambulatory management of ovarian cysts. Am J Obstet Gynecol. 1995;173:1727-1730.
12. White SC, Wartel LJ, Wade ME. Comparison of abdominal and vaginal hysterectomies. Obstet Gynecol. 1971;37:530-537.
13. Sheth SS, Malpani AN. Vaginal hysterectomy following previous cesarean section. Int J Gynaecol Obstet. 1995;50:65-69.
14. Kovac SR. Guidelines to determine route of hysterectomy. Obstet Gynecol. 1995;85:18-23.
15. Hoffman MS, DeCesare S, Kalter C. Abdominal hysterectomy versus transvaginal morcellation for the removal of enlarged uteri. Am J Obstet Gynecol. 1994;171:309-315.
16. Mazdisnian F, Kurzel RB, Coe S, Bosuk M, Montz F. Vaginal hysterectomy by uterine morcellation: an efficient, non-morbid procedure. Obstet Gynecol. 1995;86:60-64.
17. Kudo R, Yamauchi O, Okazaki T, Sagac S, Ito E, Hashimoto M. Vaginal hysterectomy without ligation of the ligaments of the cervix uteri. Surg Gynecol Obstet. 1990;70:299-305.
18. Kovac SR. Intramyometrial coring as an adjunct to vaginal hysterectomy. Obstet Gynecol. 1986;67:131-136.
19. Stovall TG, Summit RL, Washburn SA, Ling FW. Gonadotropin-releasing hormone agonist use before hysterectomy. Am J Obstet Gynecol. 1994;170:1744-1748.
20. Crosignani PG, Vercellini P, Meschia M, Oldani S, Bramante T. GnRH agonists before surgery for uterine leiomyomas. J Reprod Med. 1996;41:415-421.
21. Hoffman MS. Transvaginal removal of ovaries with endoloop sutures at the time of transvaginal hysterectomy. Am J Obstet Gynecol. 1991;165:407-408.
22. Sheth SS. Ovarian clamp for oophorectomy during vaginal hysterectomy. J Am Assoc Gynecol Laparosc. 1995;2(4):S76-S77.
23. Hefni MA, Davies AE. Vaginal endoscopic oophorectomy with vaginal hysterectomy: a simple minimal access surgery technique. Br J Obstet Gynaecol. 1997;104:621-622.
24. Sheth SS. The place of oophorectomy at vaginal hysterectomy. Br J Obstet Gynaecol. 1991;98:662-666.
25. Cristoforoni PM, Palmieri A, Gerbaldo D, Montz FJ. Frequency and cause of aborted laparoscopic-assisted vaginal hysterectomy. J Am Assoc Gynecol Laparosc. 1995;3:33-37.
26. Summitt RL, Stovall TG, Steege JF, Lipscomb GH. A multicenter randomized comparison of laparoscopic-assisted vaginal hysterectomy and abdominal hysterectomy in abdominal hysterectomy candidates. Obstet Gynecol. 1998;92:321-326.
27. Dorsey JH, Holtz PM, Griffiths RI, McGrath MM, Steinberg EP. Costs and charges associated with three alternative techniques of hysterectomy. N Engl J Med. 1996;335:476-482.
28. Summitt RL, Stovall TG, Lipscomb GH, Ling FW. Randomized comparison of laparoscopic-assisted vaginal hysterectomy with standard vaginal hysterectomy in an outpatient setting. Obstet Gynecol. 1992;80:895-901.
- Choose the abdominal route when extensive intraperitoneal surgery and/or exploration are required in addition to the hysterectomy, i.e., in cases of pelvic organ carcinoma.
- Use a combination of uterine morcellation techniques to accomplish a vaginal hysterectomy, as researchers have found morcellation of an enlarged uterus to be safer than removing it abdominally.
- For laparoscopic-assisted vaginal hysterectomy, use 3 ports and avoid most disposable instruments by using cautery on vascular pedicles to help minimize costs.
- Seek alternatives to abdominal hysterectomy given its less favorable outcome in terms of morbidity and recovery.
While hysterectomy is one of the most frequently performed operations in gynecology, how to perform it—abdominally, vaginally, or laparoscopically—is less evident. Numerous studies have been published in an attempt to shed some light on this controversy.
Individualize the approach for each patient rather than rely on a dogmatic assignment of technique.
Prior to the introduction of the laparoscopic-assisted vaginal hysterectomy (LAVH) by Reich et al in 1989,1 several large studies were published that compared the abdominal and vaginal routes for hysterectomy. The largest was the Collaborative Review of Sterilization (CREST) study conducted by the Centers for Disease Control (CDC).2 This report included 1,856 women aged 15 to 44 who underwent non-emergency, non-radical hysterectomies at 9 institutions between 1978 and 1981. Fewer complications were associated with vaginal hysterectomy (VH) than abdominal hysterectomy (AH) (Table 1).
Now, several trials have included LAVH in the comparison of hysterectomy routes. In the most comprehensive study to date, Johns et al reviewed 2,563 hysterectomies performed for nonmalignant indications by 37 private gynecologists from a single institution.3 The researchers found that bowel, bladder, and ureteral injuries were uncommon, and the rates of each were similar among LAVH, abdominal hysterectomy, and vaginal hysterectomy (Table 2). In addition, a review of the literature between 1989 and 1995 revealed that LAVH is associated with a shorter hospital stay, decreased recovery time, and less analgesia compared with AH.4
However, since most of the data on route for hysterectomy are from retrospective and uncontrolled trials, one must interpret the findings carefully. For example, many studies do not control for additional procedures performed at the time of hysterectomy (e.g., enterocele, rectocele, and cystocele repairs). In addition, information on how researchers categorized unsuccessful attempts at VH or LAVH—which then had to be converted to AH—often is excluded. Also, physicians usually select the technique based on personal preference, practice style, and traditional dogma such as uterine size rather than a standard protocol.5,6 Therefore, the increased incidence of postoperative morbidity associated with AH is difficult to decipher. Is it due to the increased number of obese and nulliparous women undergoing AH, the surgeon’s experience, pelvic pathology or operative indication, or is it related to the actual opening of the abdomen and intraperitoneal manipulation? Most likely, it is a combination of these factors.
Overall, hysterectomy is a relatively safe procedure with a mortality rate of 1 to 2 per 1,000.7 Morbidity, however, remains high. Fortunately, most complications are minor and easily remedied with little clinical consequence. Since certain aspects of postoperative morbidity are related to the route for hysterectomy, the surgeon must individualize the approach for each patient and not rely on a dogmatic assignment of technique. Here, we will review the patient selection for and provide pearls on abdominal, vaginal, and laparoscopic-assisted vaginal hysterectomy, as well as look at the advantages and disadvantages of each method.
TABLE 1
CREST*study results
| VH | AH | |
|---|---|---|
| Mean age (yrs) | 34.4 | 35.8 |
| Nulliparous (%) | 1.4 | 13.3 |
| Prior cesarean section (%) | 4.8 | 10.1 |
| Obese** (%) | 38.0 | 44.7 |
| Febrile morbidity (%) | 15.3 | 32.3 |
| Required transfusion (%) | 8.3 | 15.4 |
| Death (%) | 0.2 | 0.1 |
| *The Collaborative Review of Sterilization | ||
| **Greater than 120% ideal body weight | ||
| VH=vaginal hysterectomy | ||
| AH=abdominal hysterectomy | ||
| Source: Dicker RC, Greenspan JR, Strauss LT, Cowart MR, Scally MJ, et al. Complications of abdominal and vaginal hysterectomy among women of reproductive age in the United States. Am J Obstet Gynecol. 1982;144:841-848. | ||
TABLE 2
Complications at hysterectomy
| AH | VH | LAVH | |
|---|---|---|---|
| Operating time (minutes) | 82 | 63 | 102 |
| Uterine weight (grams) | 216 | 113 | 129 |
| Febrile morbidity (%) | 9.1 | 3.2 | 2.0 |
| Required transfusion (%) | 2.5 | 1.0 | 0.06 |
| Bowel, bladder, or ureteral injury (%) | 1.0 | 0.9 | 1.1 |
| Death (%) | 0 | 0.2 | 0 |
| AH=abdominal hysterectomy | |||
| VH=vaginal hysterectomy | |||
| LAVH=laparoscopic-assisted vaginal hysterectomy | |||
| Source: Johns DA, et al. The medical and economic impact of laparoscopically assisted vaginal hysterectomy in a large, metropolitan, not-for-profit hospital. Am J Obstet Gynecol. 1995;172:1709-1719. | |||
Abdominal hysterectomy
Patient selection. Choose the abdominal route when extensive intraperitoneal surgery and/or exploration are required in addition to the hysterectomy, i.e., in cases of pelvic organ carcinoma. Severe pelvic adhesive disease from documented severe endometriosis, salpingitis, or significant adnexal pathology and a considerably enlarged uterus also are best approached abdominally (Figure 1). In addition, use the abdominal route for obstetric emergencies such as postpartum hemorrhage.
Technique. First, determine the type of incision based on the following factors: Which one will allow completion of the procedure in a safe and efficient manner, minimize complications, expedite recovery, and offer a favorable cosmetic result? A vertical midline incision is the most adaptable to unsuspected pathology, especially if found in the upper abdomen. It is the quickest to perform and is associated with the least blood loss, an important advantage when operating emergently on an anemic or hemorrhaging patient. However, bear in mind that vertical incisions that have been extended into the upper abdomen are accompanied by increased postoperative pulmonary morbidity, pain, and risk of hernia formation.8,9
Transverse incisions such as the commonly used Pfannenstiel, Cherney, and Maylard compromise upper abdominal exposure but are more cosmetically appealing than vertical incisions. Each of these involves a lower abdominal transverse skin incision and a transverse fascial incision. With the Pfannenstiel technique, the rectus fascia is separated from the rectus muscles, and the muscles are split vertically in the midline, allowing access to the peritoneal cavity. This is the quickest transverse incision to make but provides the least exposure.
If a Pfannenstiel incision has been made and additional lateral pelvic exposure is needed, convert the incision to a Cherney. Both the Cherney and Maylard incisions provide additional exposure to the pelvis, especially laterally, which is advantageous when complex pathology is limited to the pelvis. The Cherney incision involves dividing the rectus muscles from the pubic symphysis, whereas the Maylard incision entails horizontal transection of the rectus muscles at the level of the fascial incision. With the Maylard technique, the rectus fascia is not separated from the underlying muscles as it is in the Cherney incision. If upper abdominal exposure becomes necessary, all of these incisions can be extended upward from their lateral margin, creating a “J.” Alternatively, a second incision can be made in the upper abdomen.
Another option is mini-laparotomy (an incision less than 6 cm). Hoffman and Lynch reported success with this technique for hysterectomy in select patients.10 Most of the procedures were completed with minimal use of retractors by exteriorizing the uterus via one of the aforementioned incisions. They found this approach to be safe and effective in nonobese women in whom a vaginal approach was precluded due to anatomy.
Use the mini-laparotomy approach when there is a suspicious adnexal mass that can be removed through a small abdominal incision.11 In addition, a large benign-appearing cystic adnexal mass can be drained, and the procedure then completed through this incision. Proceed cautiously, however, since an occasional unanticipated carcinoma will be encountered, and the act of drainage will result in the need for chemotherapy in a woman with otherwise early-stage disease. If cancer is suspected, consider a vertical minilaparotomy, which allows easy extension into the upper abdomen should surgical staging or debulking become necessary.
Advantages and disadvantages. The abdominal approach offers the best exposure of the pelvic and upper abdominal cavity but is associated with a high rate of complications, including fever and excessive blood loss. We surmise that postoperative recovery with a mini-laparotomy is likely to be improved compared with the traditional abdominal technique.
Figure 1
Opt for abdominal hysterectomy when a considerably enlarged uterus due to numerous fibroids is encountered.
Vaginal hysterectomy
Patient selection. Choose the vaginal route when pelvic support defects are present (Figure 2), but bear in mind that the course of the ureter changes with worsening degrees of uterine prolapse. We have found the ureter to be palpable in the bladder pillar in most women undergoing VH for prolapse, which helps avoid intraoperative ureteral injury.
The ovaries can be safely removed through the vagina in a large percentage of patients.
Obese, elderly, and otherwise medically debilitated patients also will benefit from the vaginal approach. Avoiding an abdominal wound in these women has obvious advantages. Also, vaginal surgery is associated with a reduction in postoperative pulmonary complications when compared with AH.6,12
Vaginal accessibility inevitably influences the decision to proceed vaginally with a hysterectomy. An inadequate bony pelvis is reason to forgo a VH. Orthopedic conditions and muscular contractures of the lower extremities, which prevent safe positioning, also inhibit this approach. Some surgeons also consider prior abdominal or pelvic surgery a reason to avoid vaginal hysterectomy, while others find this route a means of eluding potential adhesions.
We are strong advocates of vaginal surgery and do not consider nulliparity, lack of uterine descent, or prior abdominopelvic surgery to be strict contraindications for VH. A Schuchardt incision may overcome a small introitus, and a mobile uterus often will descend as the uterosacral and cardinal ligaments are divided.
In women with a prior cesarean delivery, it may be easier to enter the vesicouterine space by approaching it from the less-scarred vaginal side. In a study of more than 200 patients with a previous cesarean who underwent VH, Sheth and Malpani found no increase in complications and concluded that VH is the route of choice in this patient population.13 When unsuspected pelvic adhesions are encountered, carefully dissect them transvaginally and complete the procedure.
Besides accessibility, uterine size must be considered. However, size alone should present a dilemma in only about 15% of patients since most hysterectomy specimens are 12 weeks’ gestational size or smaller.14
Technique. Large uteri can be removed vaginally, depending on the surgeon’s technical ability and experience. A familiarity with the various methods of morcellation—hemisection, posterior fundal morcellation, intramyometrial coring, and myomectomy—is mandatory. It often is beneficial to use a combination of these techniques to accomplish the procedure vaginally, as opposed to a single means of morcellation. With any morcellation procedure, maintain a midline orientation to avoid dissection into the broad ligament. Continued caudal traction also is helpful in eliminating excessive blood loss during this prolonged procedure. Do not morcellate a uterus when endometrial carcinoma is suspected.
In a prospective observational study comparing vaginal morcellation to AH, Hoffman et al15 found the former to be safer. Other researchers also have demonstrated the procedure’s safety in retrospective reports16-18 and as subgroups in other series.14 Alternatively, preoperative treatment with a gonadotropin-releasing hor-Surgical Techniques mone (GnRH) agonist can reduce uterine size and result in a technically less challenging VH in some women.19,20
Also, whether or not the ovaries will be resected may influence the decision to perform a VH. While some surgeons believe that oophorectomy precludes the vaginal approach, the ovaries can be safely removed through the vagina in a large percentage of patients.21-23 In fact, Sheth24 and Kovac14 have published 95% and 97% success rates in 2 separate series in which vaginal oophorectomy was attempted in 740 and 142 women, respectively. However, do not attempt adnexal removal without adequate transvaginal access and visibility, as it increases the risk of hemorrhage and ureteral injury. In these cases, LAVH or mini-laparotomy AH may be preferable if oophorectomy is mandatory, i.e., in women who are predisposed to ovarian malignancies because of genetic mutations. However, when patients are appropriately selected, there are essentially no disadvantages to VH.
Advantages and disadvantages.VH has many advantages over AH. Minimal intraperitoneal manipulation and the avoidance of an abdominal wound lead to a shorter hospital stay and decreased recovery time, which is coupled with less cost. Patients also find the lack of an abdominal scar to be an attractive feature of VH. A disadvantage is reduced operative exposure, making it difficult to manipulate pelvic pathology and resect the adnexa.
Figure 2
Choose the vaginal route when pelvic support defects are present, but bear in mind that the course of the ureter changes due to prolapse.
Laparoscopic-assisted vaginal hysterectomy
Patient selection. When in doubt about which approach to use, turn to LAVH. The laparoscope allows the surgeon to thoroughly assess intra-abdominal pathology, which aids in the appropriate selection of a hysterectomy route. For example, if cancer is suspected based on the visual inspection, opt for an AH. On the other hand, if only extensive adhesions are noted, laparoscopic adhesiolysis can be performed to allow the hysterectomy to be completed vaginally. As previously noted, LAVH is appropriate in patients undergoing hysterectomy with prophylactic oophorectomy due to genetic or familial risk factors.
Technique. Bear in mind that the laparoscope does not permit all patients to undergo a VH. In fact, about 10% of attempted LAVHs will be unsuccessful.25,26 Some authors have reported failure during the laparoscopic portion,25,26 while others have attributed the lack of success to the vaginal portion of the procedure.27 Thus, it is important to be skilled at both laparoscopic and vaginal surgery in order to be successful with an LAVH.
LAVH requires three to five 5-to 10-mm abdominal incisions for port sites. An umbilical port is commonly used for the camera, and the remaining ports are used for operating. We typically use 3 ports and avoid most disposable instruments by using cautery on vascular pedicles, which helps minimize costs.3 Other alternatives include the use of endoscopic staplers, the Harmonic Scalpel (Ethicon Endo-Surgery, a Johnson & Johnson company, Cincinnati, Ohio), and suture ligatures with extra-or intracorporeal knot tying. Whichever instrument is used, familiarize yourself with all available equipment given the occasional malfunction encountered during laparoscopy.
Advantages and disadvantages. LAVH requires special equipment and expertise beyond that needed for an abdominal or vaginal hysterectomy. Additionally, this procedure increases operative time, cost, and morbidity (depending on the surgeon’s experience),4,27,28 while the postoperative recovery is similar to that of VH.
Although we use the laparoscope infrequently and believe there is little need to perform excessive laparoscopy at the time of routine hysterectomy, we do not wish to understate the role of the laparoscope. Its use in specific instances can certainly avoid a laparotomy or help determine when an AH is more appropriate.
Conclusion
Emergent situations and patients with excessively enlarged uteri, significant pelvic pathology, or cancer are obvious candidates for AH. On the other hand, VH is frequently chosen for the small uterus in a multiparous woman with a large pelvis and no prior pelvic inflammatory disease or surgery. The dilemma arises when determining the approach for those patients with moderately enlarged uteri or presumptive risk factors for serious pelvic disease.
Kovac reported a standard protocol for selecting the route for hysterectomy. Uterine size, other pelvic pathology (endometriosis, adnexal disease, chronic pain, etc.), and uterine and adnexal accessibility (bony architecture, uterine support, and vaginal diameter) were each considered in the decision-making. A simplification of Kovac’s guidelines applicable to women undergoing hysterectomy for benign indications is summarized in Figure 3. Using these guidelines, Kovac reported a 99% (608/611) success rate for women assigned to VH or LAVH. The laparoscope was deemed necessary in only 19% of those assigned to the LAVH group, and ultimately only 9 patients required AH, yielding a 1:68 ratio for AH to VH.14
Gynecologists should seek alternatives to AH given its less favorable outcome in terms of morbidity and recovery. However, the surgeon who is competent with AH better serves the patient by performing the procedure via this route than by attempting an alternative procedure without the necessary proficiency. In other words, pelvic surgeons must be cognizant of their abilities and practice within that realm. Ultimately, the final selection of hysterectomy route should be based on the surgeon’s experience, the indication for surgery, and the patient’s anatomy.
FIGURE 3Guidelines to determine route of hysterectomy.
The authors report no financial relationship with any companies whose products are mentioned in this article.
- Choose the abdominal route when extensive intraperitoneal surgery and/or exploration are required in addition to the hysterectomy, i.e., in cases of pelvic organ carcinoma.
- Use a combination of uterine morcellation techniques to accomplish a vaginal hysterectomy, as researchers have found morcellation of an enlarged uterus to be safer than removing it abdominally.
- For laparoscopic-assisted vaginal hysterectomy, use 3 ports and avoid most disposable instruments by using cautery on vascular pedicles to help minimize costs.
- Seek alternatives to abdominal hysterectomy given its less favorable outcome in terms of morbidity and recovery.
While hysterectomy is one of the most frequently performed operations in gynecology, how to perform it—abdominally, vaginally, or laparoscopically—is less evident. Numerous studies have been published in an attempt to shed some light on this controversy.
Individualize the approach for each patient rather than rely on a dogmatic assignment of technique.
Prior to the introduction of the laparoscopic-assisted vaginal hysterectomy (LAVH) by Reich et al in 1989,1 several large studies were published that compared the abdominal and vaginal routes for hysterectomy. The largest was the Collaborative Review of Sterilization (CREST) study conducted by the Centers for Disease Control (CDC).2 This report included 1,856 women aged 15 to 44 who underwent non-emergency, non-radical hysterectomies at 9 institutions between 1978 and 1981. Fewer complications were associated with vaginal hysterectomy (VH) than abdominal hysterectomy (AH) (Table 1).
Now, several trials have included LAVH in the comparison of hysterectomy routes. In the most comprehensive study to date, Johns et al reviewed 2,563 hysterectomies performed for nonmalignant indications by 37 private gynecologists from a single institution.3 The researchers found that bowel, bladder, and ureteral injuries were uncommon, and the rates of each were similar among LAVH, abdominal hysterectomy, and vaginal hysterectomy (Table 2). In addition, a review of the literature between 1989 and 1995 revealed that LAVH is associated with a shorter hospital stay, decreased recovery time, and less analgesia compared with AH.4
However, since most of the data on route for hysterectomy are from retrospective and uncontrolled trials, one must interpret the findings carefully. For example, many studies do not control for additional procedures performed at the time of hysterectomy (e.g., enterocele, rectocele, and cystocele repairs). In addition, information on how researchers categorized unsuccessful attempts at VH or LAVH—which then had to be converted to AH—often is excluded. Also, physicians usually select the technique based on personal preference, practice style, and traditional dogma such as uterine size rather than a standard protocol.5,6 Therefore, the increased incidence of postoperative morbidity associated with AH is difficult to decipher. Is it due to the increased number of obese and nulliparous women undergoing AH, the surgeon’s experience, pelvic pathology or operative indication, or is it related to the actual opening of the abdomen and intraperitoneal manipulation? Most likely, it is a combination of these factors.
Overall, hysterectomy is a relatively safe procedure with a mortality rate of 1 to 2 per 1,000.7 Morbidity, however, remains high. Fortunately, most complications are minor and easily remedied with little clinical consequence. Since certain aspects of postoperative morbidity are related to the route for hysterectomy, the surgeon must individualize the approach for each patient and not rely on a dogmatic assignment of technique. Here, we will review the patient selection for and provide pearls on abdominal, vaginal, and laparoscopic-assisted vaginal hysterectomy, as well as look at the advantages and disadvantages of each method.
TABLE 1
CREST*study results
| VH | AH | |
|---|---|---|
| Mean age (yrs) | 34.4 | 35.8 |
| Nulliparous (%) | 1.4 | 13.3 |
| Prior cesarean section (%) | 4.8 | 10.1 |
| Obese** (%) | 38.0 | 44.7 |
| Febrile morbidity (%) | 15.3 | 32.3 |
| Required transfusion (%) | 8.3 | 15.4 |
| Death (%) | 0.2 | 0.1 |
| *The Collaborative Review of Sterilization | ||
| **Greater than 120% ideal body weight | ||
| VH=vaginal hysterectomy | ||
| AH=abdominal hysterectomy | ||
| Source: Dicker RC, Greenspan JR, Strauss LT, Cowart MR, Scally MJ, et al. Complications of abdominal and vaginal hysterectomy among women of reproductive age in the United States. Am J Obstet Gynecol. 1982;144:841-848. | ||
TABLE 2
Complications at hysterectomy
| AH | VH | LAVH | |
|---|---|---|---|
| Operating time (minutes) | 82 | 63 | 102 |
| Uterine weight (grams) | 216 | 113 | 129 |
| Febrile morbidity (%) | 9.1 | 3.2 | 2.0 |
| Required transfusion (%) | 2.5 | 1.0 | 0.06 |
| Bowel, bladder, or ureteral injury (%) | 1.0 | 0.9 | 1.1 |
| Death (%) | 0 | 0.2 | 0 |
| AH=abdominal hysterectomy | |||
| VH=vaginal hysterectomy | |||
| LAVH=laparoscopic-assisted vaginal hysterectomy | |||
| Source: Johns DA, et al. The medical and economic impact of laparoscopically assisted vaginal hysterectomy in a large, metropolitan, not-for-profit hospital. Am J Obstet Gynecol. 1995;172:1709-1719. | |||
Abdominal hysterectomy
Patient selection. Choose the abdominal route when extensive intraperitoneal surgery and/or exploration are required in addition to the hysterectomy, i.e., in cases of pelvic organ carcinoma. Severe pelvic adhesive disease from documented severe endometriosis, salpingitis, or significant adnexal pathology and a considerably enlarged uterus also are best approached abdominally (Figure 1). In addition, use the abdominal route for obstetric emergencies such as postpartum hemorrhage.
Technique. First, determine the type of incision based on the following factors: Which one will allow completion of the procedure in a safe and efficient manner, minimize complications, expedite recovery, and offer a favorable cosmetic result? A vertical midline incision is the most adaptable to unsuspected pathology, especially if found in the upper abdomen. It is the quickest to perform and is associated with the least blood loss, an important advantage when operating emergently on an anemic or hemorrhaging patient. However, bear in mind that vertical incisions that have been extended into the upper abdomen are accompanied by increased postoperative pulmonary morbidity, pain, and risk of hernia formation.8,9
Transverse incisions such as the commonly used Pfannenstiel, Cherney, and Maylard compromise upper abdominal exposure but are more cosmetically appealing than vertical incisions. Each of these involves a lower abdominal transverse skin incision and a transverse fascial incision. With the Pfannenstiel technique, the rectus fascia is separated from the rectus muscles, and the muscles are split vertically in the midline, allowing access to the peritoneal cavity. This is the quickest transverse incision to make but provides the least exposure.
If a Pfannenstiel incision has been made and additional lateral pelvic exposure is needed, convert the incision to a Cherney. Both the Cherney and Maylard incisions provide additional exposure to the pelvis, especially laterally, which is advantageous when complex pathology is limited to the pelvis. The Cherney incision involves dividing the rectus muscles from the pubic symphysis, whereas the Maylard incision entails horizontal transection of the rectus muscles at the level of the fascial incision. With the Maylard technique, the rectus fascia is not separated from the underlying muscles as it is in the Cherney incision. If upper abdominal exposure becomes necessary, all of these incisions can be extended upward from their lateral margin, creating a “J.” Alternatively, a second incision can be made in the upper abdomen.
Another option is mini-laparotomy (an incision less than 6 cm). Hoffman and Lynch reported success with this technique for hysterectomy in select patients.10 Most of the procedures were completed with minimal use of retractors by exteriorizing the uterus via one of the aforementioned incisions. They found this approach to be safe and effective in nonobese women in whom a vaginal approach was precluded due to anatomy.
Use the mini-laparotomy approach when there is a suspicious adnexal mass that can be removed through a small abdominal incision.11 In addition, a large benign-appearing cystic adnexal mass can be drained, and the procedure then completed through this incision. Proceed cautiously, however, since an occasional unanticipated carcinoma will be encountered, and the act of drainage will result in the need for chemotherapy in a woman with otherwise early-stage disease. If cancer is suspected, consider a vertical minilaparotomy, which allows easy extension into the upper abdomen should surgical staging or debulking become necessary.
Advantages and disadvantages. The abdominal approach offers the best exposure of the pelvic and upper abdominal cavity but is associated with a high rate of complications, including fever and excessive blood loss. We surmise that postoperative recovery with a mini-laparotomy is likely to be improved compared with the traditional abdominal technique.
Figure 1
Opt for abdominal hysterectomy when a considerably enlarged uterus due to numerous fibroids is encountered.
Vaginal hysterectomy
Patient selection. Choose the vaginal route when pelvic support defects are present (Figure 2), but bear in mind that the course of the ureter changes with worsening degrees of uterine prolapse. We have found the ureter to be palpable in the bladder pillar in most women undergoing VH for prolapse, which helps avoid intraoperative ureteral injury.
The ovaries can be safely removed through the vagina in a large percentage of patients.
Obese, elderly, and otherwise medically debilitated patients also will benefit from the vaginal approach. Avoiding an abdominal wound in these women has obvious advantages. Also, vaginal surgery is associated with a reduction in postoperative pulmonary complications when compared with AH.6,12
Vaginal accessibility inevitably influences the decision to proceed vaginally with a hysterectomy. An inadequate bony pelvis is reason to forgo a VH. Orthopedic conditions and muscular contractures of the lower extremities, which prevent safe positioning, also inhibit this approach. Some surgeons also consider prior abdominal or pelvic surgery a reason to avoid vaginal hysterectomy, while others find this route a means of eluding potential adhesions.
We are strong advocates of vaginal surgery and do not consider nulliparity, lack of uterine descent, or prior abdominopelvic surgery to be strict contraindications for VH. A Schuchardt incision may overcome a small introitus, and a mobile uterus often will descend as the uterosacral and cardinal ligaments are divided.
In women with a prior cesarean delivery, it may be easier to enter the vesicouterine space by approaching it from the less-scarred vaginal side. In a study of more than 200 patients with a previous cesarean who underwent VH, Sheth and Malpani found no increase in complications and concluded that VH is the route of choice in this patient population.13 When unsuspected pelvic adhesions are encountered, carefully dissect them transvaginally and complete the procedure.
Besides accessibility, uterine size must be considered. However, size alone should present a dilemma in only about 15% of patients since most hysterectomy specimens are 12 weeks’ gestational size or smaller.14
Technique. Large uteri can be removed vaginally, depending on the surgeon’s technical ability and experience. A familiarity with the various methods of morcellation—hemisection, posterior fundal morcellation, intramyometrial coring, and myomectomy—is mandatory. It often is beneficial to use a combination of these techniques to accomplish the procedure vaginally, as opposed to a single means of morcellation. With any morcellation procedure, maintain a midline orientation to avoid dissection into the broad ligament. Continued caudal traction also is helpful in eliminating excessive blood loss during this prolonged procedure. Do not morcellate a uterus when endometrial carcinoma is suspected.
In a prospective observational study comparing vaginal morcellation to AH, Hoffman et al15 found the former to be safer. Other researchers also have demonstrated the procedure’s safety in retrospective reports16-18 and as subgroups in other series.14 Alternatively, preoperative treatment with a gonadotropin-releasing hor-Surgical Techniques mone (GnRH) agonist can reduce uterine size and result in a technically less challenging VH in some women.19,20
Also, whether or not the ovaries will be resected may influence the decision to perform a VH. While some surgeons believe that oophorectomy precludes the vaginal approach, the ovaries can be safely removed through the vagina in a large percentage of patients.21-23 In fact, Sheth24 and Kovac14 have published 95% and 97% success rates in 2 separate series in which vaginal oophorectomy was attempted in 740 and 142 women, respectively. However, do not attempt adnexal removal without adequate transvaginal access and visibility, as it increases the risk of hemorrhage and ureteral injury. In these cases, LAVH or mini-laparotomy AH may be preferable if oophorectomy is mandatory, i.e., in women who are predisposed to ovarian malignancies because of genetic mutations. However, when patients are appropriately selected, there are essentially no disadvantages to VH.
Advantages and disadvantages.VH has many advantages over AH. Minimal intraperitoneal manipulation and the avoidance of an abdominal wound lead to a shorter hospital stay and decreased recovery time, which is coupled with less cost. Patients also find the lack of an abdominal scar to be an attractive feature of VH. A disadvantage is reduced operative exposure, making it difficult to manipulate pelvic pathology and resect the adnexa.
Figure 2
Choose the vaginal route when pelvic support defects are present, but bear in mind that the course of the ureter changes due to prolapse.
Laparoscopic-assisted vaginal hysterectomy
Patient selection. When in doubt about which approach to use, turn to LAVH. The laparoscope allows the surgeon to thoroughly assess intra-abdominal pathology, which aids in the appropriate selection of a hysterectomy route. For example, if cancer is suspected based on the visual inspection, opt for an AH. On the other hand, if only extensive adhesions are noted, laparoscopic adhesiolysis can be performed to allow the hysterectomy to be completed vaginally. As previously noted, LAVH is appropriate in patients undergoing hysterectomy with prophylactic oophorectomy due to genetic or familial risk factors.
Technique. Bear in mind that the laparoscope does not permit all patients to undergo a VH. In fact, about 10% of attempted LAVHs will be unsuccessful.25,26 Some authors have reported failure during the laparoscopic portion,25,26 while others have attributed the lack of success to the vaginal portion of the procedure.27 Thus, it is important to be skilled at both laparoscopic and vaginal surgery in order to be successful with an LAVH.
LAVH requires three to five 5-to 10-mm abdominal incisions for port sites. An umbilical port is commonly used for the camera, and the remaining ports are used for operating. We typically use 3 ports and avoid most disposable instruments by using cautery on vascular pedicles, which helps minimize costs.3 Other alternatives include the use of endoscopic staplers, the Harmonic Scalpel (Ethicon Endo-Surgery, a Johnson & Johnson company, Cincinnati, Ohio), and suture ligatures with extra-or intracorporeal knot tying. Whichever instrument is used, familiarize yourself with all available equipment given the occasional malfunction encountered during laparoscopy.
Advantages and disadvantages. LAVH requires special equipment and expertise beyond that needed for an abdominal or vaginal hysterectomy. Additionally, this procedure increases operative time, cost, and morbidity (depending on the surgeon’s experience),4,27,28 while the postoperative recovery is similar to that of VH.
Although we use the laparoscope infrequently and believe there is little need to perform excessive laparoscopy at the time of routine hysterectomy, we do not wish to understate the role of the laparoscope. Its use in specific instances can certainly avoid a laparotomy or help determine when an AH is more appropriate.
Conclusion
Emergent situations and patients with excessively enlarged uteri, significant pelvic pathology, or cancer are obvious candidates for AH. On the other hand, VH is frequently chosen for the small uterus in a multiparous woman with a large pelvis and no prior pelvic inflammatory disease or surgery. The dilemma arises when determining the approach for those patients with moderately enlarged uteri or presumptive risk factors for serious pelvic disease.
Kovac reported a standard protocol for selecting the route for hysterectomy. Uterine size, other pelvic pathology (endometriosis, adnexal disease, chronic pain, etc.), and uterine and adnexal accessibility (bony architecture, uterine support, and vaginal diameter) were each considered in the decision-making. A simplification of Kovac’s guidelines applicable to women undergoing hysterectomy for benign indications is summarized in Figure 3. Using these guidelines, Kovac reported a 99% (608/611) success rate for women assigned to VH or LAVH. The laparoscope was deemed necessary in only 19% of those assigned to the LAVH group, and ultimately only 9 patients required AH, yielding a 1:68 ratio for AH to VH.14
Gynecologists should seek alternatives to AH given its less favorable outcome in terms of morbidity and recovery. However, the surgeon who is competent with AH better serves the patient by performing the procedure via this route than by attempting an alternative procedure without the necessary proficiency. In other words, pelvic surgeons must be cognizant of their abilities and practice within that realm. Ultimately, the final selection of hysterectomy route should be based on the surgeon’s experience, the indication for surgery, and the patient’s anatomy.
FIGURE 3Guidelines to determine route of hysterectomy.
The authors report no financial relationship with any companies whose products are mentioned in this article.
1. Reich H, DeCaprio J, McGlynn F. Laparoscopic hysterectomy. J Gynecol Surg. 1989;5:213-216.
2. Dicker RC, Greenspan JR, Strauss LT, Cowart MR, Scally MJ, et al. Complications of abdominal and vaginal hysterectomy among women of reproductive age in the United States. Am J Obstet Gynecol. 1982;144:841-848.
3. Johns DA, Carrera B, Jones J, DeLeon F, Vincent R, et al. The medical and economic impact of laparoscopic-assisted vaginal hysterectomy in a large, metropolitan, not-for-profit hospital. Am J Obstet Gynecol. 1995;172:1709-719.
4. Meikle SF, Nugent EW, Orleans M. Complication and recovery for laparoscopic-assisted vaginal hysterectomy compared with abdominal and vaginal hysterectomy. Obstet Gynecol. 1997;89:304-311.
5. Kovac SR, Christie SJ, Bindbeutel GA. Abdominal versus vaginal hysterectomy: a statistical model for determining physician decision making and patient outcomes. Med Decis Making. 1991;11:19-28.
6. Dorsey JH, Steinberg EP, Holtz PM. Clinical indications for hysterectomy route: patient characteristics or physician preference. Am J Obstet Gynecol. 1995;173:1452-1460.
7. Wingo PA, Huezo CM, Rubin GL, Ory HW, Peterson HB. The mortality risk associated with hysterectomy. Am J Obstet Gynecol. 1985;152:803-808.
8. Tollefson DG, Russell KP. The transverse incision in pelvic surgery. Am J Obstet Gynecol. 1954;68:410-422.
9. Helmkamp BF. Abdominal wound dehiscence. Am J Obstet Gynecol. 1977;128:803-807.
10. Hoffman MS, Lynch CM. Minilaparotomy hysterectomy. Am J Obstet Gynecol. 1998;179:316-320.
11. Flynn M, Niloff JM. Minilaparotomy for the ambulatory management of ovarian cysts. Am J Obstet Gynecol. 1995;173:1727-1730.
12. White SC, Wartel LJ, Wade ME. Comparison of abdominal and vaginal hysterectomies. Obstet Gynecol. 1971;37:530-537.
13. Sheth SS, Malpani AN. Vaginal hysterectomy following previous cesarean section. Int J Gynaecol Obstet. 1995;50:65-69.
14. Kovac SR. Guidelines to determine route of hysterectomy. Obstet Gynecol. 1995;85:18-23.
15. Hoffman MS, DeCesare S, Kalter C. Abdominal hysterectomy versus transvaginal morcellation for the removal of enlarged uteri. Am J Obstet Gynecol. 1994;171:309-315.
16. Mazdisnian F, Kurzel RB, Coe S, Bosuk M, Montz F. Vaginal hysterectomy by uterine morcellation: an efficient, non-morbid procedure. Obstet Gynecol. 1995;86:60-64.
17. Kudo R, Yamauchi O, Okazaki T, Sagac S, Ito E, Hashimoto M. Vaginal hysterectomy without ligation of the ligaments of the cervix uteri. Surg Gynecol Obstet. 1990;70:299-305.
18. Kovac SR. Intramyometrial coring as an adjunct to vaginal hysterectomy. Obstet Gynecol. 1986;67:131-136.
19. Stovall TG, Summit RL, Washburn SA, Ling FW. Gonadotropin-releasing hormone agonist use before hysterectomy. Am J Obstet Gynecol. 1994;170:1744-1748.
20. Crosignani PG, Vercellini P, Meschia M, Oldani S, Bramante T. GnRH agonists before surgery for uterine leiomyomas. J Reprod Med. 1996;41:415-421.
21. Hoffman MS. Transvaginal removal of ovaries with endoloop sutures at the time of transvaginal hysterectomy. Am J Obstet Gynecol. 1991;165:407-408.
22. Sheth SS. Ovarian clamp for oophorectomy during vaginal hysterectomy. J Am Assoc Gynecol Laparosc. 1995;2(4):S76-S77.
23. Hefni MA, Davies AE. Vaginal endoscopic oophorectomy with vaginal hysterectomy: a simple minimal access surgery technique. Br J Obstet Gynaecol. 1997;104:621-622.
24. Sheth SS. The place of oophorectomy at vaginal hysterectomy. Br J Obstet Gynaecol. 1991;98:662-666.
25. Cristoforoni PM, Palmieri A, Gerbaldo D, Montz FJ. Frequency and cause of aborted laparoscopic-assisted vaginal hysterectomy. J Am Assoc Gynecol Laparosc. 1995;3:33-37.
26. Summitt RL, Stovall TG, Steege JF, Lipscomb GH. A multicenter randomized comparison of laparoscopic-assisted vaginal hysterectomy and abdominal hysterectomy in abdominal hysterectomy candidates. Obstet Gynecol. 1998;92:321-326.
27. Dorsey JH, Holtz PM, Griffiths RI, McGrath MM, Steinberg EP. Costs and charges associated with three alternative techniques of hysterectomy. N Engl J Med. 1996;335:476-482.
28. Summitt RL, Stovall TG, Lipscomb GH, Ling FW. Randomized comparison of laparoscopic-assisted vaginal hysterectomy with standard vaginal hysterectomy in an outpatient setting. Obstet Gynecol. 1992;80:895-901.
1. Reich H, DeCaprio J, McGlynn F. Laparoscopic hysterectomy. J Gynecol Surg. 1989;5:213-216.
2. Dicker RC, Greenspan JR, Strauss LT, Cowart MR, Scally MJ, et al. Complications of abdominal and vaginal hysterectomy among women of reproductive age in the United States. Am J Obstet Gynecol. 1982;144:841-848.
3. Johns DA, Carrera B, Jones J, DeLeon F, Vincent R, et al. The medical and economic impact of laparoscopic-assisted vaginal hysterectomy in a large, metropolitan, not-for-profit hospital. Am J Obstet Gynecol. 1995;172:1709-719.
4. Meikle SF, Nugent EW, Orleans M. Complication and recovery for laparoscopic-assisted vaginal hysterectomy compared with abdominal and vaginal hysterectomy. Obstet Gynecol. 1997;89:304-311.
5. Kovac SR, Christie SJ, Bindbeutel GA. Abdominal versus vaginal hysterectomy: a statistical model for determining physician decision making and patient outcomes. Med Decis Making. 1991;11:19-28.
6. Dorsey JH, Steinberg EP, Holtz PM. Clinical indications for hysterectomy route: patient characteristics or physician preference. Am J Obstet Gynecol. 1995;173:1452-1460.
7. Wingo PA, Huezo CM, Rubin GL, Ory HW, Peterson HB. The mortality risk associated with hysterectomy. Am J Obstet Gynecol. 1985;152:803-808.
8. Tollefson DG, Russell KP. The transverse incision in pelvic surgery. Am J Obstet Gynecol. 1954;68:410-422.
9. Helmkamp BF. Abdominal wound dehiscence. Am J Obstet Gynecol. 1977;128:803-807.
10. Hoffman MS, Lynch CM. Minilaparotomy hysterectomy. Am J Obstet Gynecol. 1998;179:316-320.
11. Flynn M, Niloff JM. Minilaparotomy for the ambulatory management of ovarian cysts. Am J Obstet Gynecol. 1995;173:1727-1730.
12. White SC, Wartel LJ, Wade ME. Comparison of abdominal and vaginal hysterectomies. Obstet Gynecol. 1971;37:530-537.
13. Sheth SS, Malpani AN. Vaginal hysterectomy following previous cesarean section. Int J Gynaecol Obstet. 1995;50:65-69.
14. Kovac SR. Guidelines to determine route of hysterectomy. Obstet Gynecol. 1995;85:18-23.
15. Hoffman MS, DeCesare S, Kalter C. Abdominal hysterectomy versus transvaginal morcellation for the removal of enlarged uteri. Am J Obstet Gynecol. 1994;171:309-315.
16. Mazdisnian F, Kurzel RB, Coe S, Bosuk M, Montz F. Vaginal hysterectomy by uterine morcellation: an efficient, non-morbid procedure. Obstet Gynecol. 1995;86:60-64.
17. Kudo R, Yamauchi O, Okazaki T, Sagac S, Ito E, Hashimoto M. Vaginal hysterectomy without ligation of the ligaments of the cervix uteri. Surg Gynecol Obstet. 1990;70:299-305.
18. Kovac SR. Intramyometrial coring as an adjunct to vaginal hysterectomy. Obstet Gynecol. 1986;67:131-136.
19. Stovall TG, Summit RL, Washburn SA, Ling FW. Gonadotropin-releasing hormone agonist use before hysterectomy. Am J Obstet Gynecol. 1994;170:1744-1748.
20. Crosignani PG, Vercellini P, Meschia M, Oldani S, Bramante T. GnRH agonists before surgery for uterine leiomyomas. J Reprod Med. 1996;41:415-421.
21. Hoffman MS. Transvaginal removal of ovaries with endoloop sutures at the time of transvaginal hysterectomy. Am J Obstet Gynecol. 1991;165:407-408.
22. Sheth SS. Ovarian clamp for oophorectomy during vaginal hysterectomy. J Am Assoc Gynecol Laparosc. 1995;2(4):S76-S77.
23. Hefni MA, Davies AE. Vaginal endoscopic oophorectomy with vaginal hysterectomy: a simple minimal access surgery technique. Br J Obstet Gynaecol. 1997;104:621-622.
24. Sheth SS. The place of oophorectomy at vaginal hysterectomy. Br J Obstet Gynaecol. 1991;98:662-666.
25. Cristoforoni PM, Palmieri A, Gerbaldo D, Montz FJ. Frequency and cause of aborted laparoscopic-assisted vaginal hysterectomy. J Am Assoc Gynecol Laparosc. 1995;3:33-37.
26. Summitt RL, Stovall TG, Steege JF, Lipscomb GH. A multicenter randomized comparison of laparoscopic-assisted vaginal hysterectomy and abdominal hysterectomy in abdominal hysterectomy candidates. Obstet Gynecol. 1998;92:321-326.
27. Dorsey JH, Holtz PM, Griffiths RI, McGrath MM, Steinberg EP. Costs and charges associated with three alternative techniques of hysterectomy. N Engl J Med. 1996;335:476-482.
28. Summitt RL, Stovall TG, Lipscomb GH, Ling FW. Randomized comparison of laparoscopic-assisted vaginal hysterectomy with standard vaginal hysterectomy in an outpatient setting. Obstet Gynecol. 1992;80:895-901.