User login
Determining the best route for hysterectomy
- Choose the abdominal route when extensive intraperitoneal surgery and/or exploration are required in addition to the hysterectomy, i.e., in cases of pelvic organ carcinoma.
- Use a combination of uterine morcellation techniques to accomplish a vaginal hysterectomy, as researchers have found morcellation of an enlarged uterus to be safer than removing it abdominally.
- For laparoscopic-assisted vaginal hysterectomy, use 3 ports and avoid most disposable instruments by using cautery on vascular pedicles to help minimize costs.
- Seek alternatives to abdominal hysterectomy given its less favorable outcome in terms of morbidity and recovery.
While hysterectomy is one of the most frequently performed operations in gynecology, how to perform it—abdominally, vaginally, or laparoscopically—is less evident. Numerous studies have been published in an attempt to shed some light on this controversy.
Individualize the approach for each patient rather than rely on a dogmatic assignment of technique.
Prior to the introduction of the laparoscopic-assisted vaginal hysterectomy (LAVH) by Reich et al in 1989,1 several large studies were published that compared the abdominal and vaginal routes for hysterectomy. The largest was the Collaborative Review of Sterilization (CREST) study conducted by the Centers for Disease Control (CDC).2 This report included 1,856 women aged 15 to 44 who underwent non-emergency, non-radical hysterectomies at 9 institutions between 1978 and 1981. Fewer complications were associated with vaginal hysterectomy (VH) than abdominal hysterectomy (AH) (Table 1).
Now, several trials have included LAVH in the comparison of hysterectomy routes. In the most comprehensive study to date, Johns et al reviewed 2,563 hysterectomies performed for nonmalignant indications by 37 private gynecologists from a single institution.3 The researchers found that bowel, bladder, and ureteral injuries were uncommon, and the rates of each were similar among LAVH, abdominal hysterectomy, and vaginal hysterectomy (Table 2). In addition, a review of the literature between 1989 and 1995 revealed that LAVH is associated with a shorter hospital stay, decreased recovery time, and less analgesia compared with AH.4
However, since most of the data on route for hysterectomy are from retrospective and uncontrolled trials, one must interpret the findings carefully. For example, many studies do not control for additional procedures performed at the time of hysterectomy (e.g., enterocele, rectocele, and cystocele repairs). In addition, information on how researchers categorized unsuccessful attempts at VH or LAVH—which then had to be converted to AH—often is excluded. Also, physicians usually select the technique based on personal preference, practice style, and traditional dogma such as uterine size rather than a standard protocol.5,6 Therefore, the increased incidence of postoperative morbidity associated with AH is difficult to decipher. Is it due to the increased number of obese and nulliparous women undergoing AH, the surgeon’s experience, pelvic pathology or operative indication, or is it related to the actual opening of the abdomen and intraperitoneal manipulation? Most likely, it is a combination of these factors.
Overall, hysterectomy is a relatively safe procedure with a mortality rate of 1 to 2 per 1,000.7 Morbidity, however, remains high. Fortunately, most complications are minor and easily remedied with little clinical consequence. Since certain aspects of postoperative morbidity are related to the route for hysterectomy, the surgeon must individualize the approach for each patient and not rely on a dogmatic assignment of technique. Here, we will review the patient selection for and provide pearls on abdominal, vaginal, and laparoscopic-assisted vaginal hysterectomy, as well as look at the advantages and disadvantages of each method.
TABLE 1
CREST*study results
| VH | AH | |
|---|---|---|
| Mean age (yrs) | 34.4 | 35.8 |
| Nulliparous (%) | 1.4 | 13.3 |
| Prior cesarean section (%) | 4.8 | 10.1 |
| Obese** (%) | 38.0 | 44.7 |
| Febrile morbidity (%) | 15.3 | 32.3 |
| Required transfusion (%) | 8.3 | 15.4 |
| Death (%) | 0.2 | 0.1 |
| *The Collaborative Review of Sterilization | ||
| **Greater than 120% ideal body weight | ||
| VH=vaginal hysterectomy | ||
| AH=abdominal hysterectomy | ||
| Source: Dicker RC, Greenspan JR, Strauss LT, Cowart MR, Scally MJ, et al. Complications of abdominal and vaginal hysterectomy among women of reproductive age in the United States. Am J Obstet Gynecol. 1982;144:841-848. | ||
TABLE 2
Complications at hysterectomy
| AH | VH | LAVH | |
|---|---|---|---|
| Operating time (minutes) | 82 | 63 | 102 |
| Uterine weight (grams) | 216 | 113 | 129 |
| Febrile morbidity (%) | 9.1 | 3.2 | 2.0 |
| Required transfusion (%) | 2.5 | 1.0 | 0.06 |
| Bowel, bladder, or ureteral injury (%) | 1.0 | 0.9 | 1.1 |
| Death (%) | 0 | 0.2 | 0 |
| AH=abdominal hysterectomy | |||
| VH=vaginal hysterectomy | |||
| LAVH=laparoscopic-assisted vaginal hysterectomy | |||
| Source: Johns DA, et al. The medical and economic impact of laparoscopically assisted vaginal hysterectomy in a large, metropolitan, not-for-profit hospital. Am J Obstet Gynecol. 1995;172:1709-1719. | |||
Abdominal hysterectomy
Patient selection. Choose the abdominal route when extensive intraperitoneal surgery and/or exploration are required in addition to the hysterectomy, i.e., in cases of pelvic organ carcinoma. Severe pelvic adhesive disease from documented severe endometriosis, salpingitis, or significant adnexal pathology and a considerably enlarged uterus also are best approached abdominally (Figure 1). In addition, use the abdominal route for obstetric emergencies such as postpartum hemorrhage.
Technique. First, determine the type of incision based on the following factors: Which one will allow completion of the procedure in a safe and efficient manner, minimize complications, expedite recovery, and offer a favorable cosmetic result? A vertical midline incision is the most adaptable to unsuspected pathology, especially if found in the upper abdomen. It is the quickest to perform and is associated with the least blood loss, an important advantage when operating emergently on an anemic or hemorrhaging patient. However, bear in mind that vertical incisions that have been extended into the upper abdomen are accompanied by increased postoperative pulmonary morbidity, pain, and risk of hernia formation.8,9
Transverse incisions such as the commonly used Pfannenstiel, Cherney, and Maylard compromise upper abdominal exposure but are more cosmetically appealing than vertical incisions. Each of these involves a lower abdominal transverse skin incision and a transverse fascial incision. With the Pfannenstiel technique, the rectus fascia is separated from the rectus muscles, and the muscles are split vertically in the midline, allowing access to the peritoneal cavity. This is the quickest transverse incision to make but provides the least exposure.
If a Pfannenstiel incision has been made and additional lateral pelvic exposure is needed, convert the incision to a Cherney. Both the Cherney and Maylard incisions provide additional exposure to the pelvis, especially laterally, which is advantageous when complex pathology is limited to the pelvis. The Cherney incision involves dividing the rectus muscles from the pubic symphysis, whereas the Maylard incision entails horizontal transection of the rectus muscles at the level of the fascial incision. With the Maylard technique, the rectus fascia is not separated from the underlying muscles as it is in the Cherney incision. If upper abdominal exposure becomes necessary, all of these incisions can be extended upward from their lateral margin, creating a “J.” Alternatively, a second incision can be made in the upper abdomen.
Another option is mini-laparotomy (an incision less than 6 cm). Hoffman and Lynch reported success with this technique for hysterectomy in select patients.10 Most of the procedures were completed with minimal use of retractors by exteriorizing the uterus via one of the aforementioned incisions. They found this approach to be safe and effective in nonobese women in whom a vaginal approach was precluded due to anatomy.
Use the mini-laparotomy approach when there is a suspicious adnexal mass that can be removed through a small abdominal incision.11 In addition, a large benign-appearing cystic adnexal mass can be drained, and the procedure then completed through this incision. Proceed cautiously, however, since an occasional unanticipated carcinoma will be encountered, and the act of drainage will result in the need for chemotherapy in a woman with otherwise early-stage disease. If cancer is suspected, consider a vertical minilaparotomy, which allows easy extension into the upper abdomen should surgical staging or debulking become necessary.
Advantages and disadvantages. The abdominal approach offers the best exposure of the pelvic and upper abdominal cavity but is associated with a high rate of complications, including fever and excessive blood loss. We surmise that postoperative recovery with a mini-laparotomy is likely to be improved compared with the traditional abdominal technique.
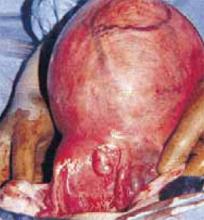
Figure 1
Opt for abdominal hysterectomy when a considerably enlarged uterus due to numerous fibroids is encountered.
Vaginal hysterectomy
Patient selection. Choose the vaginal route when pelvic support defects are present (Figure 2), but bear in mind that the course of the ureter changes with worsening degrees of uterine prolapse. We have found the ureter to be palpable in the bladder pillar in most women undergoing VH for prolapse, which helps avoid intraoperative ureteral injury.
The ovaries can be safely removed through the vagina in a large percentage of patients.
Obese, elderly, and otherwise medically debilitated patients also will benefit from the vaginal approach. Avoiding an abdominal wound in these women has obvious advantages. Also, vaginal surgery is associated with a reduction in postoperative pulmonary complications when compared with AH.6,12
Vaginal accessibility inevitably influences the decision to proceed vaginally with a hysterectomy. An inadequate bony pelvis is reason to forgo a VH. Orthopedic conditions and muscular contractures of the lower extremities, which prevent safe positioning, also inhibit this approach. Some surgeons also consider prior abdominal or pelvic surgery a reason to avoid vaginal hysterectomy, while others find this route a means of eluding potential adhesions.
We are strong advocates of vaginal surgery and do not consider nulliparity, lack of uterine descent, or prior abdominopelvic surgery to be strict contraindications for VH. A Schuchardt incision may overcome a small introitus, and a mobile uterus often will descend as the uterosacral and cardinal ligaments are divided.
In women with a prior cesarean delivery, it may be easier to enter the vesicouterine space by approaching it from the less-scarred vaginal side. In a study of more than 200 patients with a previous cesarean who underwent VH, Sheth and Malpani found no increase in complications and concluded that VH is the route of choice in this patient population.13 When unsuspected pelvic adhesions are encountered, carefully dissect them transvaginally and complete the procedure.
Besides accessibility, uterine size must be considered. However, size alone should present a dilemma in only about 15% of patients since most hysterectomy specimens are 12 weeks’ gestational size or smaller.14
Technique. Large uteri can be removed vaginally, depending on the surgeon’s technical ability and experience. A familiarity with the various methods of morcellation—hemisection, posterior fundal morcellation, intramyometrial coring, and myomectomy—is mandatory. It often is beneficial to use a combination of these techniques to accomplish the procedure vaginally, as opposed to a single means of morcellation. With any morcellation procedure, maintain a midline orientation to avoid dissection into the broad ligament. Continued caudal traction also is helpful in eliminating excessive blood loss during this prolonged procedure. Do not morcellate a uterus when endometrial carcinoma is suspected.
In a prospective observational study comparing vaginal morcellation to AH, Hoffman et al15 found the former to be safer. Other researchers also have demonstrated the procedure’s safety in retrospective reports16-18 and as subgroups in other series.14 Alternatively, preoperative treatment with a gonadotropin-releasing hor-Surgical Techniques mone (GnRH) agonist can reduce uterine size and result in a technically less challenging VH in some women.19,20
Also, whether or not the ovaries will be resected may influence the decision to perform a VH. While some surgeons believe that oophorectomy precludes the vaginal approach, the ovaries can be safely removed through the vagina in a large percentage of patients.21-23 In fact, Sheth24 and Kovac14 have published 95% and 97% success rates in 2 separate series in which vaginal oophorectomy was attempted in 740 and 142 women, respectively. However, do not attempt adnexal removal without adequate transvaginal access and visibility, as it increases the risk of hemorrhage and ureteral injury. In these cases, LAVH or mini-laparotomy AH may be preferable if oophorectomy is mandatory, i.e., in women who are predisposed to ovarian malignancies because of genetic mutations. However, when patients are appropriately selected, there are essentially no disadvantages to VH.
Advantages and disadvantages.VH has many advantages over AH. Minimal intraperitoneal manipulation and the avoidance of an abdominal wound lead to a shorter hospital stay and decreased recovery time, which is coupled with less cost. Patients also find the lack of an abdominal scar to be an attractive feature of VH. A disadvantage is reduced operative exposure, making it difficult to manipulate pelvic pathology and resect the adnexa.
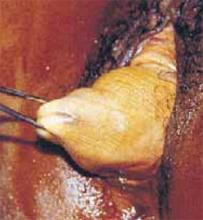
Figure 2
Choose the vaginal route when pelvic support defects are present, but bear in mind that the course of the ureter changes due to prolapse.
Laparoscopic-assisted vaginal hysterectomy
Patient selection. When in doubt about which approach to use, turn to LAVH. The laparoscope allows the surgeon to thoroughly assess intra-abdominal pathology, which aids in the appropriate selection of a hysterectomy route. For example, if cancer is suspected based on the visual inspection, opt for an AH. On the other hand, if only extensive adhesions are noted, laparoscopic adhesiolysis can be performed to allow the hysterectomy to be completed vaginally. As previously noted, LAVH is appropriate in patients undergoing hysterectomy with prophylactic oophorectomy due to genetic or familial risk factors.
Technique. Bear in mind that the laparoscope does not permit all patients to undergo a VH. In fact, about 10% of attempted LAVHs will be unsuccessful.25,26 Some authors have reported failure during the laparoscopic portion,25,26 while others have attributed the lack of success to the vaginal portion of the procedure.27 Thus, it is important to be skilled at both laparoscopic and vaginal surgery in order to be successful with an LAVH.
LAVH requires three to five 5-to 10-mm abdominal incisions for port sites. An umbilical port is commonly used for the camera, and the remaining ports are used for operating. We typically use 3 ports and avoid most disposable instruments by using cautery on vascular pedicles, which helps minimize costs.3 Other alternatives include the use of endoscopic staplers, the Harmonic Scalpel (Ethicon Endo-Surgery, a Johnson & Johnson company, Cincinnati, Ohio), and suture ligatures with extra-or intracorporeal knot tying. Whichever instrument is used, familiarize yourself with all available equipment given the occasional malfunction encountered during laparoscopy.
Advantages and disadvantages. LAVH requires special equipment and expertise beyond that needed for an abdominal or vaginal hysterectomy. Additionally, this procedure increases operative time, cost, and morbidity (depending on the surgeon’s experience),4,27,28 while the postoperative recovery is similar to that of VH.
Although we use the laparoscope infrequently and believe there is little need to perform excessive laparoscopy at the time of routine hysterectomy, we do not wish to understate the role of the laparoscope. Its use in specific instances can certainly avoid a laparotomy or help determine when an AH is more appropriate.
Conclusion
Emergent situations and patients with excessively enlarged uteri, significant pelvic pathology, or cancer are obvious candidates for AH. On the other hand, VH is frequently chosen for the small uterus in a multiparous woman with a large pelvis and no prior pelvic inflammatory disease or surgery. The dilemma arises when determining the approach for those patients with moderately enlarged uteri or presumptive risk factors for serious pelvic disease.
Kovac reported a standard protocol for selecting the route for hysterectomy. Uterine size, other pelvic pathology (endometriosis, adnexal disease, chronic pain, etc.), and uterine and adnexal accessibility (bony architecture, uterine support, and vaginal diameter) were each considered in the decision-making. A simplification of Kovac’s guidelines applicable to women undergoing hysterectomy for benign indications is summarized in Figure 3. Using these guidelines, Kovac reported a 99% (608/611) success rate for women assigned to VH or LAVH. The laparoscope was deemed necessary in only 19% of those assigned to the LAVH group, and ultimately only 9 patients required AH, yielding a 1:68 ratio for AH to VH.14
Gynecologists should seek alternatives to AH given its less favorable outcome in terms of morbidity and recovery. However, the surgeon who is competent with AH better serves the patient by performing the procedure via this route than by attempting an alternative procedure without the necessary proficiency. In other words, pelvic surgeons must be cognizant of their abilities and practice within that realm. Ultimately, the final selection of hysterectomy route should be based on the surgeon’s experience, the indication for surgery, and the patient’s anatomy.
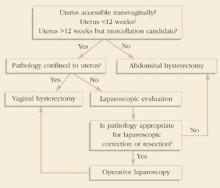
FIGURE 3Guidelines to determine route of hysterectomy.
The authors report no financial relationship with any companies whose products are mentioned in this article.
1. Reich H, DeCaprio J, McGlynn F. Laparoscopic hysterectomy. J Gynecol Surg. 1989;5:213-216.
2. Dicker RC, Greenspan JR, Strauss LT, Cowart MR, Scally MJ, et al. Complications of abdominal and vaginal hysterectomy among women of reproductive age in the United States. Am J Obstet Gynecol. 1982;144:841-848.
3. Johns DA, Carrera B, Jones J, DeLeon F, Vincent R, et al. The medical and economic impact of laparoscopic-assisted vaginal hysterectomy in a large, metropolitan, not-for-profit hospital. Am J Obstet Gynecol. 1995;172:1709-719.
4. Meikle SF, Nugent EW, Orleans M. Complication and recovery for laparoscopic-assisted vaginal hysterectomy compared with abdominal and vaginal hysterectomy. Obstet Gynecol. 1997;89:304-311.
5. Kovac SR, Christie SJ, Bindbeutel GA. Abdominal versus vaginal hysterectomy: a statistical model for determining physician decision making and patient outcomes. Med Decis Making. 1991;11:19-28.
6. Dorsey JH, Steinberg EP, Holtz PM. Clinical indications for hysterectomy route: patient characteristics or physician preference. Am J Obstet Gynecol. 1995;173:1452-1460.
7. Wingo PA, Huezo CM, Rubin GL, Ory HW, Peterson HB. The mortality risk associated with hysterectomy. Am J Obstet Gynecol. 1985;152:803-808.
8. Tollefson DG, Russell KP. The transverse incision in pelvic surgery. Am J Obstet Gynecol. 1954;68:410-422.
9. Helmkamp BF. Abdominal wound dehiscence. Am J Obstet Gynecol. 1977;128:803-807.
10. Hoffman MS, Lynch CM. Minilaparotomy hysterectomy. Am J Obstet Gynecol. 1998;179:316-320.
11. Flynn M, Niloff JM. Minilaparotomy for the ambulatory management of ovarian cysts. Am J Obstet Gynecol. 1995;173:1727-1730.
12. White SC, Wartel LJ, Wade ME. Comparison of abdominal and vaginal hysterectomies. Obstet Gynecol. 1971;37:530-537.
13. Sheth SS, Malpani AN. Vaginal hysterectomy following previous cesarean section. Int J Gynaecol Obstet. 1995;50:65-69.
14. Kovac SR. Guidelines to determine route of hysterectomy. Obstet Gynecol. 1995;85:18-23.
15. Hoffman MS, DeCesare S, Kalter C. Abdominal hysterectomy versus transvaginal morcellation for the removal of enlarged uteri. Am J Obstet Gynecol. 1994;171:309-315.
16. Mazdisnian F, Kurzel RB, Coe S, Bosuk M, Montz F. Vaginal hysterectomy by uterine morcellation: an efficient, non-morbid procedure. Obstet Gynecol. 1995;86:60-64.
17. Kudo R, Yamauchi O, Okazaki T, Sagac S, Ito E, Hashimoto M. Vaginal hysterectomy without ligation of the ligaments of the cervix uteri. Surg Gynecol Obstet. 1990;70:299-305.
18. Kovac SR. Intramyometrial coring as an adjunct to vaginal hysterectomy. Obstet Gynecol. 1986;67:131-136.
19. Stovall TG, Summit RL, Washburn SA, Ling FW. Gonadotropin-releasing hormone agonist use before hysterectomy. Am J Obstet Gynecol. 1994;170:1744-1748.
20. Crosignani PG, Vercellini P, Meschia M, Oldani S, Bramante T. GnRH agonists before surgery for uterine leiomyomas. J Reprod Med. 1996;41:415-421.
21. Hoffman MS. Transvaginal removal of ovaries with endoloop sutures at the time of transvaginal hysterectomy. Am J Obstet Gynecol. 1991;165:407-408.
22. Sheth SS. Ovarian clamp for oophorectomy during vaginal hysterectomy. J Am Assoc Gynecol Laparosc. 1995;2(4):S76-S77.
23. Hefni MA, Davies AE. Vaginal endoscopic oophorectomy with vaginal hysterectomy: a simple minimal access surgery technique. Br J Obstet Gynaecol. 1997;104:621-622.
24. Sheth SS. The place of oophorectomy at vaginal hysterectomy. Br J Obstet Gynaecol. 1991;98:662-666.
25. Cristoforoni PM, Palmieri A, Gerbaldo D, Montz FJ. Frequency and cause of aborted laparoscopic-assisted vaginal hysterectomy. J Am Assoc Gynecol Laparosc. 1995;3:33-37.
26. Summitt RL, Stovall TG, Steege JF, Lipscomb GH. A multicenter randomized comparison of laparoscopic-assisted vaginal hysterectomy and abdominal hysterectomy in abdominal hysterectomy candidates. Obstet Gynecol. 1998;92:321-326.
27. Dorsey JH, Holtz PM, Griffiths RI, McGrath MM, Steinberg EP. Costs and charges associated with three alternative techniques of hysterectomy. N Engl J Med. 1996;335:476-482.
28. Summitt RL, Stovall TG, Lipscomb GH, Ling FW. Randomized comparison of laparoscopic-assisted vaginal hysterectomy with standard vaginal hysterectomy in an outpatient setting. Obstet Gynecol. 1992;80:895-901.
- Choose the abdominal route when extensive intraperitoneal surgery and/or exploration are required in addition to the hysterectomy, i.e., in cases of pelvic organ carcinoma.
- Use a combination of uterine morcellation techniques to accomplish a vaginal hysterectomy, as researchers have found morcellation of an enlarged uterus to be safer than removing it abdominally.
- For laparoscopic-assisted vaginal hysterectomy, use 3 ports and avoid most disposable instruments by using cautery on vascular pedicles to help minimize costs.
- Seek alternatives to abdominal hysterectomy given its less favorable outcome in terms of morbidity and recovery.
While hysterectomy is one of the most frequently performed operations in gynecology, how to perform it—abdominally, vaginally, or laparoscopically—is less evident. Numerous studies have been published in an attempt to shed some light on this controversy.
Individualize the approach for each patient rather than rely on a dogmatic assignment of technique.
Prior to the introduction of the laparoscopic-assisted vaginal hysterectomy (LAVH) by Reich et al in 1989,1 several large studies were published that compared the abdominal and vaginal routes for hysterectomy. The largest was the Collaborative Review of Sterilization (CREST) study conducted by the Centers for Disease Control (CDC).2 This report included 1,856 women aged 15 to 44 who underwent non-emergency, non-radical hysterectomies at 9 institutions between 1978 and 1981. Fewer complications were associated with vaginal hysterectomy (VH) than abdominal hysterectomy (AH) (Table 1).
Now, several trials have included LAVH in the comparison of hysterectomy routes. In the most comprehensive study to date, Johns et al reviewed 2,563 hysterectomies performed for nonmalignant indications by 37 private gynecologists from a single institution.3 The researchers found that bowel, bladder, and ureteral injuries were uncommon, and the rates of each were similar among LAVH, abdominal hysterectomy, and vaginal hysterectomy (Table 2). In addition, a review of the literature between 1989 and 1995 revealed that LAVH is associated with a shorter hospital stay, decreased recovery time, and less analgesia compared with AH.4
However, since most of the data on route for hysterectomy are from retrospective and uncontrolled trials, one must interpret the findings carefully. For example, many studies do not control for additional procedures performed at the time of hysterectomy (e.g., enterocele, rectocele, and cystocele repairs). In addition, information on how researchers categorized unsuccessful attempts at VH or LAVH—which then had to be converted to AH—often is excluded. Also, physicians usually select the technique based on personal preference, practice style, and traditional dogma such as uterine size rather than a standard protocol.5,6 Therefore, the increased incidence of postoperative morbidity associated with AH is difficult to decipher. Is it due to the increased number of obese and nulliparous women undergoing AH, the surgeon’s experience, pelvic pathology or operative indication, or is it related to the actual opening of the abdomen and intraperitoneal manipulation? Most likely, it is a combination of these factors.
Overall, hysterectomy is a relatively safe procedure with a mortality rate of 1 to 2 per 1,000.7 Morbidity, however, remains high. Fortunately, most complications are minor and easily remedied with little clinical consequence. Since certain aspects of postoperative morbidity are related to the route for hysterectomy, the surgeon must individualize the approach for each patient and not rely on a dogmatic assignment of technique. Here, we will review the patient selection for and provide pearls on abdominal, vaginal, and laparoscopic-assisted vaginal hysterectomy, as well as look at the advantages and disadvantages of each method.
TABLE 1
CREST*study results
| VH | AH | |
|---|---|---|
| Mean age (yrs) | 34.4 | 35.8 |
| Nulliparous (%) | 1.4 | 13.3 |
| Prior cesarean section (%) | 4.8 | 10.1 |
| Obese** (%) | 38.0 | 44.7 |
| Febrile morbidity (%) | 15.3 | 32.3 |
| Required transfusion (%) | 8.3 | 15.4 |
| Death (%) | 0.2 | 0.1 |
| *The Collaborative Review of Sterilization | ||
| **Greater than 120% ideal body weight | ||
| VH=vaginal hysterectomy | ||
| AH=abdominal hysterectomy | ||
| Source: Dicker RC, Greenspan JR, Strauss LT, Cowart MR, Scally MJ, et al. Complications of abdominal and vaginal hysterectomy among women of reproductive age in the United States. Am J Obstet Gynecol. 1982;144:841-848. | ||
TABLE 2
Complications at hysterectomy
| AH | VH | LAVH | |
|---|---|---|---|
| Operating time (minutes) | 82 | 63 | 102 |
| Uterine weight (grams) | 216 | 113 | 129 |
| Febrile morbidity (%) | 9.1 | 3.2 | 2.0 |
| Required transfusion (%) | 2.5 | 1.0 | 0.06 |
| Bowel, bladder, or ureteral injury (%) | 1.0 | 0.9 | 1.1 |
| Death (%) | 0 | 0.2 | 0 |
| AH=abdominal hysterectomy | |||
| VH=vaginal hysterectomy | |||
| LAVH=laparoscopic-assisted vaginal hysterectomy | |||
| Source: Johns DA, et al. The medical and economic impact of laparoscopically assisted vaginal hysterectomy in a large, metropolitan, not-for-profit hospital. Am J Obstet Gynecol. 1995;172:1709-1719. | |||
Abdominal hysterectomy
Patient selection. Choose the abdominal route when extensive intraperitoneal surgery and/or exploration are required in addition to the hysterectomy, i.e., in cases of pelvic organ carcinoma. Severe pelvic adhesive disease from documented severe endometriosis, salpingitis, or significant adnexal pathology and a considerably enlarged uterus also are best approached abdominally (Figure 1). In addition, use the abdominal route for obstetric emergencies such as postpartum hemorrhage.
Technique. First, determine the type of incision based on the following factors: Which one will allow completion of the procedure in a safe and efficient manner, minimize complications, expedite recovery, and offer a favorable cosmetic result? A vertical midline incision is the most adaptable to unsuspected pathology, especially if found in the upper abdomen. It is the quickest to perform and is associated with the least blood loss, an important advantage when operating emergently on an anemic or hemorrhaging patient. However, bear in mind that vertical incisions that have been extended into the upper abdomen are accompanied by increased postoperative pulmonary morbidity, pain, and risk of hernia formation.8,9
Transverse incisions such as the commonly used Pfannenstiel, Cherney, and Maylard compromise upper abdominal exposure but are more cosmetically appealing than vertical incisions. Each of these involves a lower abdominal transverse skin incision and a transverse fascial incision. With the Pfannenstiel technique, the rectus fascia is separated from the rectus muscles, and the muscles are split vertically in the midline, allowing access to the peritoneal cavity. This is the quickest transverse incision to make but provides the least exposure.
If a Pfannenstiel incision has been made and additional lateral pelvic exposure is needed, convert the incision to a Cherney. Both the Cherney and Maylard incisions provide additional exposure to the pelvis, especially laterally, which is advantageous when complex pathology is limited to the pelvis. The Cherney incision involves dividing the rectus muscles from the pubic symphysis, whereas the Maylard incision entails horizontal transection of the rectus muscles at the level of the fascial incision. With the Maylard technique, the rectus fascia is not separated from the underlying muscles as it is in the Cherney incision. If upper abdominal exposure becomes necessary, all of these incisions can be extended upward from their lateral margin, creating a “J.” Alternatively, a second incision can be made in the upper abdomen.
Another option is mini-laparotomy (an incision less than 6 cm). Hoffman and Lynch reported success with this technique for hysterectomy in select patients.10 Most of the procedures were completed with minimal use of retractors by exteriorizing the uterus via one of the aforementioned incisions. They found this approach to be safe and effective in nonobese women in whom a vaginal approach was precluded due to anatomy.
Use the mini-laparotomy approach when there is a suspicious adnexal mass that can be removed through a small abdominal incision.11 In addition, a large benign-appearing cystic adnexal mass can be drained, and the procedure then completed through this incision. Proceed cautiously, however, since an occasional unanticipated carcinoma will be encountered, and the act of drainage will result in the need for chemotherapy in a woman with otherwise early-stage disease. If cancer is suspected, consider a vertical minilaparotomy, which allows easy extension into the upper abdomen should surgical staging or debulking become necessary.
Advantages and disadvantages. The abdominal approach offers the best exposure of the pelvic and upper abdominal cavity but is associated with a high rate of complications, including fever and excessive blood loss. We surmise that postoperative recovery with a mini-laparotomy is likely to be improved compared with the traditional abdominal technique.
Figure 1
Opt for abdominal hysterectomy when a considerably enlarged uterus due to numerous fibroids is encountered.
Vaginal hysterectomy
Patient selection. Choose the vaginal route when pelvic support defects are present (Figure 2), but bear in mind that the course of the ureter changes with worsening degrees of uterine prolapse. We have found the ureter to be palpable in the bladder pillar in most women undergoing VH for prolapse, which helps avoid intraoperative ureteral injury.
The ovaries can be safely removed through the vagina in a large percentage of patients.
Obese, elderly, and otherwise medically debilitated patients also will benefit from the vaginal approach. Avoiding an abdominal wound in these women has obvious advantages. Also, vaginal surgery is associated with a reduction in postoperative pulmonary complications when compared with AH.6,12
Vaginal accessibility inevitably influences the decision to proceed vaginally with a hysterectomy. An inadequate bony pelvis is reason to forgo a VH. Orthopedic conditions and muscular contractures of the lower extremities, which prevent safe positioning, also inhibit this approach. Some surgeons also consider prior abdominal or pelvic surgery a reason to avoid vaginal hysterectomy, while others find this route a means of eluding potential adhesions.
We are strong advocates of vaginal surgery and do not consider nulliparity, lack of uterine descent, or prior abdominopelvic surgery to be strict contraindications for VH. A Schuchardt incision may overcome a small introitus, and a mobile uterus often will descend as the uterosacral and cardinal ligaments are divided.
In women with a prior cesarean delivery, it may be easier to enter the vesicouterine space by approaching it from the less-scarred vaginal side. In a study of more than 200 patients with a previous cesarean who underwent VH, Sheth and Malpani found no increase in complications and concluded that VH is the route of choice in this patient population.13 When unsuspected pelvic adhesions are encountered, carefully dissect them transvaginally and complete the procedure.
Besides accessibility, uterine size must be considered. However, size alone should present a dilemma in only about 15% of patients since most hysterectomy specimens are 12 weeks’ gestational size or smaller.14
Technique. Large uteri can be removed vaginally, depending on the surgeon’s technical ability and experience. A familiarity with the various methods of morcellation—hemisection, posterior fundal morcellation, intramyometrial coring, and myomectomy—is mandatory. It often is beneficial to use a combination of these techniques to accomplish the procedure vaginally, as opposed to a single means of morcellation. With any morcellation procedure, maintain a midline orientation to avoid dissection into the broad ligament. Continued caudal traction also is helpful in eliminating excessive blood loss during this prolonged procedure. Do not morcellate a uterus when endometrial carcinoma is suspected.
In a prospective observational study comparing vaginal morcellation to AH, Hoffman et al15 found the former to be safer. Other researchers also have demonstrated the procedure’s safety in retrospective reports16-18 and as subgroups in other series.14 Alternatively, preoperative treatment with a gonadotropin-releasing hor-Surgical Techniques mone (GnRH) agonist can reduce uterine size and result in a technically less challenging VH in some women.19,20
Also, whether or not the ovaries will be resected may influence the decision to perform a VH. While some surgeons believe that oophorectomy precludes the vaginal approach, the ovaries can be safely removed through the vagina in a large percentage of patients.21-23 In fact, Sheth24 and Kovac14 have published 95% and 97% success rates in 2 separate series in which vaginal oophorectomy was attempted in 740 and 142 women, respectively. However, do not attempt adnexal removal without adequate transvaginal access and visibility, as it increases the risk of hemorrhage and ureteral injury. In these cases, LAVH or mini-laparotomy AH may be preferable if oophorectomy is mandatory, i.e., in women who are predisposed to ovarian malignancies because of genetic mutations. However, when patients are appropriately selected, there are essentially no disadvantages to VH.
Advantages and disadvantages.VH has many advantages over AH. Minimal intraperitoneal manipulation and the avoidance of an abdominal wound lead to a shorter hospital stay and decreased recovery time, which is coupled with less cost. Patients also find the lack of an abdominal scar to be an attractive feature of VH. A disadvantage is reduced operative exposure, making it difficult to manipulate pelvic pathology and resect the adnexa.
Figure 2
Choose the vaginal route when pelvic support defects are present, but bear in mind that the course of the ureter changes due to prolapse.
Laparoscopic-assisted vaginal hysterectomy
Patient selection. When in doubt about which approach to use, turn to LAVH. The laparoscope allows the surgeon to thoroughly assess intra-abdominal pathology, which aids in the appropriate selection of a hysterectomy route. For example, if cancer is suspected based on the visual inspection, opt for an AH. On the other hand, if only extensive adhesions are noted, laparoscopic adhesiolysis can be performed to allow the hysterectomy to be completed vaginally. As previously noted, LAVH is appropriate in patients undergoing hysterectomy with prophylactic oophorectomy due to genetic or familial risk factors.
Technique. Bear in mind that the laparoscope does not permit all patients to undergo a VH. In fact, about 10% of attempted LAVHs will be unsuccessful.25,26 Some authors have reported failure during the laparoscopic portion,25,26 while others have attributed the lack of success to the vaginal portion of the procedure.27 Thus, it is important to be skilled at both laparoscopic and vaginal surgery in order to be successful with an LAVH.
LAVH requires three to five 5-to 10-mm abdominal incisions for port sites. An umbilical port is commonly used for the camera, and the remaining ports are used for operating. We typically use 3 ports and avoid most disposable instruments by using cautery on vascular pedicles, which helps minimize costs.3 Other alternatives include the use of endoscopic staplers, the Harmonic Scalpel (Ethicon Endo-Surgery, a Johnson & Johnson company, Cincinnati, Ohio), and suture ligatures with extra-or intracorporeal knot tying. Whichever instrument is used, familiarize yourself with all available equipment given the occasional malfunction encountered during laparoscopy.
Advantages and disadvantages. LAVH requires special equipment and expertise beyond that needed for an abdominal or vaginal hysterectomy. Additionally, this procedure increases operative time, cost, and morbidity (depending on the surgeon’s experience),4,27,28 while the postoperative recovery is similar to that of VH.
Although we use the laparoscope infrequently and believe there is little need to perform excessive laparoscopy at the time of routine hysterectomy, we do not wish to understate the role of the laparoscope. Its use in specific instances can certainly avoid a laparotomy or help determine when an AH is more appropriate.
Conclusion
Emergent situations and patients with excessively enlarged uteri, significant pelvic pathology, or cancer are obvious candidates for AH. On the other hand, VH is frequently chosen for the small uterus in a multiparous woman with a large pelvis and no prior pelvic inflammatory disease or surgery. The dilemma arises when determining the approach for those patients with moderately enlarged uteri or presumptive risk factors for serious pelvic disease.
Kovac reported a standard protocol for selecting the route for hysterectomy. Uterine size, other pelvic pathology (endometriosis, adnexal disease, chronic pain, etc.), and uterine and adnexal accessibility (bony architecture, uterine support, and vaginal diameter) were each considered in the decision-making. A simplification of Kovac’s guidelines applicable to women undergoing hysterectomy for benign indications is summarized in Figure 3. Using these guidelines, Kovac reported a 99% (608/611) success rate for women assigned to VH or LAVH. The laparoscope was deemed necessary in only 19% of those assigned to the LAVH group, and ultimately only 9 patients required AH, yielding a 1:68 ratio for AH to VH.14
Gynecologists should seek alternatives to AH given its less favorable outcome in terms of morbidity and recovery. However, the surgeon who is competent with AH better serves the patient by performing the procedure via this route than by attempting an alternative procedure without the necessary proficiency. In other words, pelvic surgeons must be cognizant of their abilities and practice within that realm. Ultimately, the final selection of hysterectomy route should be based on the surgeon’s experience, the indication for surgery, and the patient’s anatomy.
FIGURE 3Guidelines to determine route of hysterectomy.
The authors report no financial relationship with any companies whose products are mentioned in this article.
- Choose the abdominal route when extensive intraperitoneal surgery and/or exploration are required in addition to the hysterectomy, i.e., in cases of pelvic organ carcinoma.
- Use a combination of uterine morcellation techniques to accomplish a vaginal hysterectomy, as researchers have found morcellation of an enlarged uterus to be safer than removing it abdominally.
- For laparoscopic-assisted vaginal hysterectomy, use 3 ports and avoid most disposable instruments by using cautery on vascular pedicles to help minimize costs.
- Seek alternatives to abdominal hysterectomy given its less favorable outcome in terms of morbidity and recovery.
While hysterectomy is one of the most frequently performed operations in gynecology, how to perform it—abdominally, vaginally, or laparoscopically—is less evident. Numerous studies have been published in an attempt to shed some light on this controversy.
Individualize the approach for each patient rather than rely on a dogmatic assignment of technique.
Prior to the introduction of the laparoscopic-assisted vaginal hysterectomy (LAVH) by Reich et al in 1989,1 several large studies were published that compared the abdominal and vaginal routes for hysterectomy. The largest was the Collaborative Review of Sterilization (CREST) study conducted by the Centers for Disease Control (CDC).2 This report included 1,856 women aged 15 to 44 who underwent non-emergency, non-radical hysterectomies at 9 institutions between 1978 and 1981. Fewer complications were associated with vaginal hysterectomy (VH) than abdominal hysterectomy (AH) (Table 1).
Now, several trials have included LAVH in the comparison of hysterectomy routes. In the most comprehensive study to date, Johns et al reviewed 2,563 hysterectomies performed for nonmalignant indications by 37 private gynecologists from a single institution.3 The researchers found that bowel, bladder, and ureteral injuries were uncommon, and the rates of each were similar among LAVH, abdominal hysterectomy, and vaginal hysterectomy (Table 2). In addition, a review of the literature between 1989 and 1995 revealed that LAVH is associated with a shorter hospital stay, decreased recovery time, and less analgesia compared with AH.4
However, since most of the data on route for hysterectomy are from retrospective and uncontrolled trials, one must interpret the findings carefully. For example, many studies do not control for additional procedures performed at the time of hysterectomy (e.g., enterocele, rectocele, and cystocele repairs). In addition, information on how researchers categorized unsuccessful attempts at VH or LAVH—which then had to be converted to AH—often is excluded. Also, physicians usually select the technique based on personal preference, practice style, and traditional dogma such as uterine size rather than a standard protocol.5,6 Therefore, the increased incidence of postoperative morbidity associated with AH is difficult to decipher. Is it due to the increased number of obese and nulliparous women undergoing AH, the surgeon’s experience, pelvic pathology or operative indication, or is it related to the actual opening of the abdomen and intraperitoneal manipulation? Most likely, it is a combination of these factors.
Overall, hysterectomy is a relatively safe procedure with a mortality rate of 1 to 2 per 1,000.7 Morbidity, however, remains high. Fortunately, most complications are minor and easily remedied with little clinical consequence. Since certain aspects of postoperative morbidity are related to the route for hysterectomy, the surgeon must individualize the approach for each patient and not rely on a dogmatic assignment of technique. Here, we will review the patient selection for and provide pearls on abdominal, vaginal, and laparoscopic-assisted vaginal hysterectomy, as well as look at the advantages and disadvantages of each method.
TABLE 1
CREST*study results
| VH | AH | |
|---|---|---|
| Mean age (yrs) | 34.4 | 35.8 |
| Nulliparous (%) | 1.4 | 13.3 |
| Prior cesarean section (%) | 4.8 | 10.1 |
| Obese** (%) | 38.0 | 44.7 |
| Febrile morbidity (%) | 15.3 | 32.3 |
| Required transfusion (%) | 8.3 | 15.4 |
| Death (%) | 0.2 | 0.1 |
| *The Collaborative Review of Sterilization | ||
| **Greater than 120% ideal body weight | ||
| VH=vaginal hysterectomy | ||
| AH=abdominal hysterectomy | ||
| Source: Dicker RC, Greenspan JR, Strauss LT, Cowart MR, Scally MJ, et al. Complications of abdominal and vaginal hysterectomy among women of reproductive age in the United States. Am J Obstet Gynecol. 1982;144:841-848. | ||
TABLE 2
Complications at hysterectomy
| AH | VH | LAVH | |
|---|---|---|---|
| Operating time (minutes) | 82 | 63 | 102 |
| Uterine weight (grams) | 216 | 113 | 129 |
| Febrile morbidity (%) | 9.1 | 3.2 | 2.0 |
| Required transfusion (%) | 2.5 | 1.0 | 0.06 |
| Bowel, bladder, or ureteral injury (%) | 1.0 | 0.9 | 1.1 |
| Death (%) | 0 | 0.2 | 0 |
| AH=abdominal hysterectomy | |||
| VH=vaginal hysterectomy | |||
| LAVH=laparoscopic-assisted vaginal hysterectomy | |||
| Source: Johns DA, et al. The medical and economic impact of laparoscopically assisted vaginal hysterectomy in a large, metropolitan, not-for-profit hospital. Am J Obstet Gynecol. 1995;172:1709-1719. | |||
Abdominal hysterectomy
Patient selection. Choose the abdominal route when extensive intraperitoneal surgery and/or exploration are required in addition to the hysterectomy, i.e., in cases of pelvic organ carcinoma. Severe pelvic adhesive disease from documented severe endometriosis, salpingitis, or significant adnexal pathology and a considerably enlarged uterus also are best approached abdominally (Figure 1). In addition, use the abdominal route for obstetric emergencies such as postpartum hemorrhage.
Technique. First, determine the type of incision based on the following factors: Which one will allow completion of the procedure in a safe and efficient manner, minimize complications, expedite recovery, and offer a favorable cosmetic result? A vertical midline incision is the most adaptable to unsuspected pathology, especially if found in the upper abdomen. It is the quickest to perform and is associated with the least blood loss, an important advantage when operating emergently on an anemic or hemorrhaging patient. However, bear in mind that vertical incisions that have been extended into the upper abdomen are accompanied by increased postoperative pulmonary morbidity, pain, and risk of hernia formation.8,9
Transverse incisions such as the commonly used Pfannenstiel, Cherney, and Maylard compromise upper abdominal exposure but are more cosmetically appealing than vertical incisions. Each of these involves a lower abdominal transverse skin incision and a transverse fascial incision. With the Pfannenstiel technique, the rectus fascia is separated from the rectus muscles, and the muscles are split vertically in the midline, allowing access to the peritoneal cavity. This is the quickest transverse incision to make but provides the least exposure.
If a Pfannenstiel incision has been made and additional lateral pelvic exposure is needed, convert the incision to a Cherney. Both the Cherney and Maylard incisions provide additional exposure to the pelvis, especially laterally, which is advantageous when complex pathology is limited to the pelvis. The Cherney incision involves dividing the rectus muscles from the pubic symphysis, whereas the Maylard incision entails horizontal transection of the rectus muscles at the level of the fascial incision. With the Maylard technique, the rectus fascia is not separated from the underlying muscles as it is in the Cherney incision. If upper abdominal exposure becomes necessary, all of these incisions can be extended upward from their lateral margin, creating a “J.” Alternatively, a second incision can be made in the upper abdomen.
Another option is mini-laparotomy (an incision less than 6 cm). Hoffman and Lynch reported success with this technique for hysterectomy in select patients.10 Most of the procedures were completed with minimal use of retractors by exteriorizing the uterus via one of the aforementioned incisions. They found this approach to be safe and effective in nonobese women in whom a vaginal approach was precluded due to anatomy.
Use the mini-laparotomy approach when there is a suspicious adnexal mass that can be removed through a small abdominal incision.11 In addition, a large benign-appearing cystic adnexal mass can be drained, and the procedure then completed through this incision. Proceed cautiously, however, since an occasional unanticipated carcinoma will be encountered, and the act of drainage will result in the need for chemotherapy in a woman with otherwise early-stage disease. If cancer is suspected, consider a vertical minilaparotomy, which allows easy extension into the upper abdomen should surgical staging or debulking become necessary.
Advantages and disadvantages. The abdominal approach offers the best exposure of the pelvic and upper abdominal cavity but is associated with a high rate of complications, including fever and excessive blood loss. We surmise that postoperative recovery with a mini-laparotomy is likely to be improved compared with the traditional abdominal technique.
Figure 1
Opt for abdominal hysterectomy when a considerably enlarged uterus due to numerous fibroids is encountered.
Vaginal hysterectomy
Patient selection. Choose the vaginal route when pelvic support defects are present (Figure 2), but bear in mind that the course of the ureter changes with worsening degrees of uterine prolapse. We have found the ureter to be palpable in the bladder pillar in most women undergoing VH for prolapse, which helps avoid intraoperative ureteral injury.
The ovaries can be safely removed through the vagina in a large percentage of patients.
Obese, elderly, and otherwise medically debilitated patients also will benefit from the vaginal approach. Avoiding an abdominal wound in these women has obvious advantages. Also, vaginal surgery is associated with a reduction in postoperative pulmonary complications when compared with AH.6,12
Vaginal accessibility inevitably influences the decision to proceed vaginally with a hysterectomy. An inadequate bony pelvis is reason to forgo a VH. Orthopedic conditions and muscular contractures of the lower extremities, which prevent safe positioning, also inhibit this approach. Some surgeons also consider prior abdominal or pelvic surgery a reason to avoid vaginal hysterectomy, while others find this route a means of eluding potential adhesions.
We are strong advocates of vaginal surgery and do not consider nulliparity, lack of uterine descent, or prior abdominopelvic surgery to be strict contraindications for VH. A Schuchardt incision may overcome a small introitus, and a mobile uterus often will descend as the uterosacral and cardinal ligaments are divided.
In women with a prior cesarean delivery, it may be easier to enter the vesicouterine space by approaching it from the less-scarred vaginal side. In a study of more than 200 patients with a previous cesarean who underwent VH, Sheth and Malpani found no increase in complications and concluded that VH is the route of choice in this patient population.13 When unsuspected pelvic adhesions are encountered, carefully dissect them transvaginally and complete the procedure.
Besides accessibility, uterine size must be considered. However, size alone should present a dilemma in only about 15% of patients since most hysterectomy specimens are 12 weeks’ gestational size or smaller.14
Technique. Large uteri can be removed vaginally, depending on the surgeon’s technical ability and experience. A familiarity with the various methods of morcellation—hemisection, posterior fundal morcellation, intramyometrial coring, and myomectomy—is mandatory. It often is beneficial to use a combination of these techniques to accomplish the procedure vaginally, as opposed to a single means of morcellation. With any morcellation procedure, maintain a midline orientation to avoid dissection into the broad ligament. Continued caudal traction also is helpful in eliminating excessive blood loss during this prolonged procedure. Do not morcellate a uterus when endometrial carcinoma is suspected.
In a prospective observational study comparing vaginal morcellation to AH, Hoffman et al15 found the former to be safer. Other researchers also have demonstrated the procedure’s safety in retrospective reports16-18 and as subgroups in other series.14 Alternatively, preoperative treatment with a gonadotropin-releasing hor-Surgical Techniques mone (GnRH) agonist can reduce uterine size and result in a technically less challenging VH in some women.19,20
Also, whether or not the ovaries will be resected may influence the decision to perform a VH. While some surgeons believe that oophorectomy precludes the vaginal approach, the ovaries can be safely removed through the vagina in a large percentage of patients.21-23 In fact, Sheth24 and Kovac14 have published 95% and 97% success rates in 2 separate series in which vaginal oophorectomy was attempted in 740 and 142 women, respectively. However, do not attempt adnexal removal without adequate transvaginal access and visibility, as it increases the risk of hemorrhage and ureteral injury. In these cases, LAVH or mini-laparotomy AH may be preferable if oophorectomy is mandatory, i.e., in women who are predisposed to ovarian malignancies because of genetic mutations. However, when patients are appropriately selected, there are essentially no disadvantages to VH.
Advantages and disadvantages.VH has many advantages over AH. Minimal intraperitoneal manipulation and the avoidance of an abdominal wound lead to a shorter hospital stay and decreased recovery time, which is coupled with less cost. Patients also find the lack of an abdominal scar to be an attractive feature of VH. A disadvantage is reduced operative exposure, making it difficult to manipulate pelvic pathology and resect the adnexa.
Figure 2
Choose the vaginal route when pelvic support defects are present, but bear in mind that the course of the ureter changes due to prolapse.
Laparoscopic-assisted vaginal hysterectomy
Patient selection. When in doubt about which approach to use, turn to LAVH. The laparoscope allows the surgeon to thoroughly assess intra-abdominal pathology, which aids in the appropriate selection of a hysterectomy route. For example, if cancer is suspected based on the visual inspection, opt for an AH. On the other hand, if only extensive adhesions are noted, laparoscopic adhesiolysis can be performed to allow the hysterectomy to be completed vaginally. As previously noted, LAVH is appropriate in patients undergoing hysterectomy with prophylactic oophorectomy due to genetic or familial risk factors.
Technique. Bear in mind that the laparoscope does not permit all patients to undergo a VH. In fact, about 10% of attempted LAVHs will be unsuccessful.25,26 Some authors have reported failure during the laparoscopic portion,25,26 while others have attributed the lack of success to the vaginal portion of the procedure.27 Thus, it is important to be skilled at both laparoscopic and vaginal surgery in order to be successful with an LAVH.
LAVH requires three to five 5-to 10-mm abdominal incisions for port sites. An umbilical port is commonly used for the camera, and the remaining ports are used for operating. We typically use 3 ports and avoid most disposable instruments by using cautery on vascular pedicles, which helps minimize costs.3 Other alternatives include the use of endoscopic staplers, the Harmonic Scalpel (Ethicon Endo-Surgery, a Johnson & Johnson company, Cincinnati, Ohio), and suture ligatures with extra-or intracorporeal knot tying. Whichever instrument is used, familiarize yourself with all available equipment given the occasional malfunction encountered during laparoscopy.
Advantages and disadvantages. LAVH requires special equipment and expertise beyond that needed for an abdominal or vaginal hysterectomy. Additionally, this procedure increases operative time, cost, and morbidity (depending on the surgeon’s experience),4,27,28 while the postoperative recovery is similar to that of VH.
Although we use the laparoscope infrequently and believe there is little need to perform excessive laparoscopy at the time of routine hysterectomy, we do not wish to understate the role of the laparoscope. Its use in specific instances can certainly avoid a laparotomy or help determine when an AH is more appropriate.
Conclusion
Emergent situations and patients with excessively enlarged uteri, significant pelvic pathology, or cancer are obvious candidates for AH. On the other hand, VH is frequently chosen for the small uterus in a multiparous woman with a large pelvis and no prior pelvic inflammatory disease or surgery. The dilemma arises when determining the approach for those patients with moderately enlarged uteri or presumptive risk factors for serious pelvic disease.
Kovac reported a standard protocol for selecting the route for hysterectomy. Uterine size, other pelvic pathology (endometriosis, adnexal disease, chronic pain, etc.), and uterine and adnexal accessibility (bony architecture, uterine support, and vaginal diameter) were each considered in the decision-making. A simplification of Kovac’s guidelines applicable to women undergoing hysterectomy for benign indications is summarized in Figure 3. Using these guidelines, Kovac reported a 99% (608/611) success rate for women assigned to VH or LAVH. The laparoscope was deemed necessary in only 19% of those assigned to the LAVH group, and ultimately only 9 patients required AH, yielding a 1:68 ratio for AH to VH.14
Gynecologists should seek alternatives to AH given its less favorable outcome in terms of morbidity and recovery. However, the surgeon who is competent with AH better serves the patient by performing the procedure via this route than by attempting an alternative procedure without the necessary proficiency. In other words, pelvic surgeons must be cognizant of their abilities and practice within that realm. Ultimately, the final selection of hysterectomy route should be based on the surgeon’s experience, the indication for surgery, and the patient’s anatomy.
FIGURE 3Guidelines to determine route of hysterectomy.
The authors report no financial relationship with any companies whose products are mentioned in this article.
1. Reich H, DeCaprio J, McGlynn F. Laparoscopic hysterectomy. J Gynecol Surg. 1989;5:213-216.
2. Dicker RC, Greenspan JR, Strauss LT, Cowart MR, Scally MJ, et al. Complications of abdominal and vaginal hysterectomy among women of reproductive age in the United States. Am J Obstet Gynecol. 1982;144:841-848.
3. Johns DA, Carrera B, Jones J, DeLeon F, Vincent R, et al. The medical and economic impact of laparoscopic-assisted vaginal hysterectomy in a large, metropolitan, not-for-profit hospital. Am J Obstet Gynecol. 1995;172:1709-719.
4. Meikle SF, Nugent EW, Orleans M. Complication and recovery for laparoscopic-assisted vaginal hysterectomy compared with abdominal and vaginal hysterectomy. Obstet Gynecol. 1997;89:304-311.
5. Kovac SR, Christie SJ, Bindbeutel GA. Abdominal versus vaginal hysterectomy: a statistical model for determining physician decision making and patient outcomes. Med Decis Making. 1991;11:19-28.
6. Dorsey JH, Steinberg EP, Holtz PM. Clinical indications for hysterectomy route: patient characteristics or physician preference. Am J Obstet Gynecol. 1995;173:1452-1460.
7. Wingo PA, Huezo CM, Rubin GL, Ory HW, Peterson HB. The mortality risk associated with hysterectomy. Am J Obstet Gynecol. 1985;152:803-808.
8. Tollefson DG, Russell KP. The transverse incision in pelvic surgery. Am J Obstet Gynecol. 1954;68:410-422.
9. Helmkamp BF. Abdominal wound dehiscence. Am J Obstet Gynecol. 1977;128:803-807.
10. Hoffman MS, Lynch CM. Minilaparotomy hysterectomy. Am J Obstet Gynecol. 1998;179:316-320.
11. Flynn M, Niloff JM. Minilaparotomy for the ambulatory management of ovarian cysts. Am J Obstet Gynecol. 1995;173:1727-1730.
12. White SC, Wartel LJ, Wade ME. Comparison of abdominal and vaginal hysterectomies. Obstet Gynecol. 1971;37:530-537.
13. Sheth SS, Malpani AN. Vaginal hysterectomy following previous cesarean section. Int J Gynaecol Obstet. 1995;50:65-69.
14. Kovac SR. Guidelines to determine route of hysterectomy. Obstet Gynecol. 1995;85:18-23.
15. Hoffman MS, DeCesare S, Kalter C. Abdominal hysterectomy versus transvaginal morcellation for the removal of enlarged uteri. Am J Obstet Gynecol. 1994;171:309-315.
16. Mazdisnian F, Kurzel RB, Coe S, Bosuk M, Montz F. Vaginal hysterectomy by uterine morcellation: an efficient, non-morbid procedure. Obstet Gynecol. 1995;86:60-64.
17. Kudo R, Yamauchi O, Okazaki T, Sagac S, Ito E, Hashimoto M. Vaginal hysterectomy without ligation of the ligaments of the cervix uteri. Surg Gynecol Obstet. 1990;70:299-305.
18. Kovac SR. Intramyometrial coring as an adjunct to vaginal hysterectomy. Obstet Gynecol. 1986;67:131-136.
19. Stovall TG, Summit RL, Washburn SA, Ling FW. Gonadotropin-releasing hormone agonist use before hysterectomy. Am J Obstet Gynecol. 1994;170:1744-1748.
20. Crosignani PG, Vercellini P, Meschia M, Oldani S, Bramante T. GnRH agonists before surgery for uterine leiomyomas. J Reprod Med. 1996;41:415-421.
21. Hoffman MS. Transvaginal removal of ovaries with endoloop sutures at the time of transvaginal hysterectomy. Am J Obstet Gynecol. 1991;165:407-408.
22. Sheth SS. Ovarian clamp for oophorectomy during vaginal hysterectomy. J Am Assoc Gynecol Laparosc. 1995;2(4):S76-S77.
23. Hefni MA, Davies AE. Vaginal endoscopic oophorectomy with vaginal hysterectomy: a simple minimal access surgery technique. Br J Obstet Gynaecol. 1997;104:621-622.
24. Sheth SS. The place of oophorectomy at vaginal hysterectomy. Br J Obstet Gynaecol. 1991;98:662-666.
25. Cristoforoni PM, Palmieri A, Gerbaldo D, Montz FJ. Frequency and cause of aborted laparoscopic-assisted vaginal hysterectomy. J Am Assoc Gynecol Laparosc. 1995;3:33-37.
26. Summitt RL, Stovall TG, Steege JF, Lipscomb GH. A multicenter randomized comparison of laparoscopic-assisted vaginal hysterectomy and abdominal hysterectomy in abdominal hysterectomy candidates. Obstet Gynecol. 1998;92:321-326.
27. Dorsey JH, Holtz PM, Griffiths RI, McGrath MM, Steinberg EP. Costs and charges associated with three alternative techniques of hysterectomy. N Engl J Med. 1996;335:476-482.
28. Summitt RL, Stovall TG, Lipscomb GH, Ling FW. Randomized comparison of laparoscopic-assisted vaginal hysterectomy with standard vaginal hysterectomy in an outpatient setting. Obstet Gynecol. 1992;80:895-901.
1. Reich H, DeCaprio J, McGlynn F. Laparoscopic hysterectomy. J Gynecol Surg. 1989;5:213-216.
2. Dicker RC, Greenspan JR, Strauss LT, Cowart MR, Scally MJ, et al. Complications of abdominal and vaginal hysterectomy among women of reproductive age in the United States. Am J Obstet Gynecol. 1982;144:841-848.
3. Johns DA, Carrera B, Jones J, DeLeon F, Vincent R, et al. The medical and economic impact of laparoscopic-assisted vaginal hysterectomy in a large, metropolitan, not-for-profit hospital. Am J Obstet Gynecol. 1995;172:1709-719.
4. Meikle SF, Nugent EW, Orleans M. Complication and recovery for laparoscopic-assisted vaginal hysterectomy compared with abdominal and vaginal hysterectomy. Obstet Gynecol. 1997;89:304-311.
5. Kovac SR, Christie SJ, Bindbeutel GA. Abdominal versus vaginal hysterectomy: a statistical model for determining physician decision making and patient outcomes. Med Decis Making. 1991;11:19-28.
6. Dorsey JH, Steinberg EP, Holtz PM. Clinical indications for hysterectomy route: patient characteristics or physician preference. Am J Obstet Gynecol. 1995;173:1452-1460.
7. Wingo PA, Huezo CM, Rubin GL, Ory HW, Peterson HB. The mortality risk associated with hysterectomy. Am J Obstet Gynecol. 1985;152:803-808.
8. Tollefson DG, Russell KP. The transverse incision in pelvic surgery. Am J Obstet Gynecol. 1954;68:410-422.
9. Helmkamp BF. Abdominal wound dehiscence. Am J Obstet Gynecol. 1977;128:803-807.
10. Hoffman MS, Lynch CM. Minilaparotomy hysterectomy. Am J Obstet Gynecol. 1998;179:316-320.
11. Flynn M, Niloff JM. Minilaparotomy for the ambulatory management of ovarian cysts. Am J Obstet Gynecol. 1995;173:1727-1730.
12. White SC, Wartel LJ, Wade ME. Comparison of abdominal and vaginal hysterectomies. Obstet Gynecol. 1971;37:530-537.
13. Sheth SS, Malpani AN. Vaginal hysterectomy following previous cesarean section. Int J Gynaecol Obstet. 1995;50:65-69.
14. Kovac SR. Guidelines to determine route of hysterectomy. Obstet Gynecol. 1995;85:18-23.
15. Hoffman MS, DeCesare S, Kalter C. Abdominal hysterectomy versus transvaginal morcellation for the removal of enlarged uteri. Am J Obstet Gynecol. 1994;171:309-315.
16. Mazdisnian F, Kurzel RB, Coe S, Bosuk M, Montz F. Vaginal hysterectomy by uterine morcellation: an efficient, non-morbid procedure. Obstet Gynecol. 1995;86:60-64.
17. Kudo R, Yamauchi O, Okazaki T, Sagac S, Ito E, Hashimoto M. Vaginal hysterectomy without ligation of the ligaments of the cervix uteri. Surg Gynecol Obstet. 1990;70:299-305.
18. Kovac SR. Intramyometrial coring as an adjunct to vaginal hysterectomy. Obstet Gynecol. 1986;67:131-136.
19. Stovall TG, Summit RL, Washburn SA, Ling FW. Gonadotropin-releasing hormone agonist use before hysterectomy. Am J Obstet Gynecol. 1994;170:1744-1748.
20. Crosignani PG, Vercellini P, Meschia M, Oldani S, Bramante T. GnRH agonists before surgery for uterine leiomyomas. J Reprod Med. 1996;41:415-421.
21. Hoffman MS. Transvaginal removal of ovaries with endoloop sutures at the time of transvaginal hysterectomy. Am J Obstet Gynecol. 1991;165:407-408.
22. Sheth SS. Ovarian clamp for oophorectomy during vaginal hysterectomy. J Am Assoc Gynecol Laparosc. 1995;2(4):S76-S77.
23. Hefni MA, Davies AE. Vaginal endoscopic oophorectomy with vaginal hysterectomy: a simple minimal access surgery technique. Br J Obstet Gynaecol. 1997;104:621-622.
24. Sheth SS. The place of oophorectomy at vaginal hysterectomy. Br J Obstet Gynaecol. 1991;98:662-666.
25. Cristoforoni PM, Palmieri A, Gerbaldo D, Montz FJ. Frequency and cause of aborted laparoscopic-assisted vaginal hysterectomy. J Am Assoc Gynecol Laparosc. 1995;3:33-37.
26. Summitt RL, Stovall TG, Steege JF, Lipscomb GH. A multicenter randomized comparison of laparoscopic-assisted vaginal hysterectomy and abdominal hysterectomy in abdominal hysterectomy candidates. Obstet Gynecol. 1998;92:321-326.
27. Dorsey JH, Holtz PM, Griffiths RI, McGrath MM, Steinberg EP. Costs and charges associated with three alternative techniques of hysterectomy. N Engl J Med. 1996;335:476-482.
28. Summitt RL, Stovall TG, Lipscomb GH, Ling FW. Randomized comparison of laparoscopic-assisted vaginal hysterectomy with standard vaginal hysterectomy in an outpatient setting. Obstet Gynecol. 1992;80:895-901.