User login
- Most women diagnosed with vaginal intraepithelial neoplasia (VAIN) have a history of cervical intraepithelial neoplasia.
- Compelling clinical and laboratory data indicate a causal relationship between human papillomavirus and VAIN.
- Like its cervical counterpart, VAIN 3 is thought to have substantial potential to progress to invasive cancer.
- Diagnosis includes careful gross and colposcopic inspection of the entire vagina (with mapping of involved areas), representative colposcopically directed biopsies, and careful palpation of the vaginal walls, especially the vaginal cuff scar.
- Important factors to consider when selecting appropriate treatment for women with VAIN include prior hysterectomy, prior radiation therapy, age, whether she is sexually active, comorbidities, vaginal anatomy, and prior treatments.
We can easily identify vulvar intraepithelial neoplasia (VIN): The patient complains of itching and has a visible lesion. We find cervical intraepithelial neoplasia (CIN) by investigating an abnormal Pap test. But what about vaginal intraepithelial neoplasia (VAIN)? It does not itch and is invisible to the naked eye. A Pap test sometimes catches it, although this test is used mainly to screen for CIN, not VAIN.
VAIN just does not grab our attention. It is uncommon, and invasive vaginal cancer is rare. But before you slip this article into the “obscure disease” file, consider the following:
- VAIN is difficult to diagnose, but some women are at increased risk.
- It is difficult to manage, but understanding the treatment options is important to success.
- The potential for VAIN to evolve into invasive cancer is probably substantial.
- Treatment of invasive vaginal cancer has a high rate of complications and is often unsuccessful.
This article describes the epidemiology, natural history, diagnosis, and treatment of VAIN, focusing primarily on management.
A range of risk factors
As with many uncommon diseases that are difficult to diagnose, good data on the incidence of VAIN are not available. Women diagnosed with VAIN can be in their late teens or senior citizens; mean age is about 50 years.1-5 Race is not mentioned in most studies.
CIN. Although concomitant or subsequent VAIN is very unusual in the approximately 600,000 women identified with CIN each year in the United States, most patients diagnosed with VAIN have a history of CIN. The small number who still have a cervix and are diagnosed with VAIN have a high incidence of concomitant CIN.1 Of women who have had a hysterectomy for CIN, only 1% to 5% are subsequently diagnosed with VAIN.6,7
Since most women who develop vaginal intraepithelial neoplasia have a prior or current history of cervical neoplasia, the “field effect” also renders them at risk for vulvar neoplasia.
HPV. Compelling data indicate a causal relationship between human papillomavirus (HPV) and CIN; the same is true for VAIN.8
Tobacco use. A history of tobacco use is frequent among women diagnosed with VAIN.1
Pelvic radiotherapy is commonly reported in case series.1,3-5 Generally, malignancy is potentially radiation-related if it develops at least 5 to 10 years after treatment.
We do not know whether radiotherapy induces neoplastic transformation in the vagina, but women with a history of radiation warrant long-term follow-up, as they seem to be at increased risk and diagnosis may be difficult.4,5
Chronically immunocompromised women are at particular risk for multifocal lower genital tract neoplasia.9,10
Natural history
The limited data available do suggest that vaginal intraepithelial neoplasia is a premalignant condition.11,12 Unfortunately, little is known about the relationship between severity of the intraepithelial neoplastic process and degree of risk. Natural history studies of VAIN 3 are even more limited than those of CIN 3.
Like its cervical counterpart, VAIN 3 is thought to have substantial potential for progression to invasive cancer.1-4,10-13
EVALUATIONDiagnosis entails inspection, palpation, and directed biopsies
VAIN is most commonly diagnosed after investigation of an abnormal Pap test taken from the vaginal cuff of a woman who has undergone a prior hysterectomy for cervical neoplasia. Occasionally, the disease is identified during colposcopy as extension of a cervical lesion. In either case, VAIN usually involves the upper third of the vagina.1-5,11 A minority of patients will be found to have diffuse multifocal lesions along the vaginal walls.
Adequate diagnosis mandates:
- careful gross and colposcopic inspection of the entire vagina (with mapping of involved areas),
- representative colposcopically directed biopsies,
- careful palpation of the vaginal walls, especially the vaginal cuff scar, and
- in some cases, excision of the vaginal cuff scar.
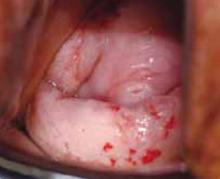
VAIN is often readily visualized with a colposcope, and the appearance may be more prominent than that of a comparable cervical lesion (FIGURE 1). The lesions are sometimes hyperkeratotic and grossly visible. However, colposcopy of the vagina is more difficult than that of the cervix due to vaginal folding, a larger surface area, and vaginal cuff irregularities.
Vaginal atrophy also creates diagnostic difficulties related to colposcopic assessment, and to overreading of vaginal cytology (eg, when a lesion is interpreted as high-grade dysplasia or suspicious). Reassessment after 4 to 6 weeks of estrogen therapy helps resolve these issues.
FIGURE 1 Colposcopic view of VAIN 3
Note the hyperkeratotic, prominent appearance of the VAIN lesions.
TREATMENT
Because women with VAIN are a heterogeneous group, treatment must be individualized. Important factors to consider when selecting appropriate treatment include prior hysterectomy, prior radiation therapy, age, whether the woman is sexually active, comorbidities, vaginal anatomy, and prior treatments.
Prior hysterectomy
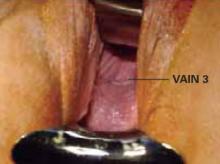
Most women diagnosed with VAIN have previously undergone hysterectomy, usually because of cervical neoplasia.2-5,10 In these women, VAIN is generally confined to the vaginal apex (FIGURE 2).
Watch for occult neoplasia. Buried epithelium within the vaginal cuff scar may harbor occult neoplasia. Patients with VAIN at the vaginal apex5,10,11,13 and a vaginal cuff from a hysterectomy for cervical neoplasia are analogous to women with CIN and an unsatisfactory colposcopy.
Excision is usual management. Most of these women are appropriately managed with excision of the involved vaginal apex, including the cuff scar.1,3-5,11,14 Problematic vaginal shortening is uncommon following this procedure. The status of the resection margin is predictive of the likelihood of recurrence.3
With more extensive vaginal epithelial involvement, consider cuff excision in selected cases to eliminate the potential for occult disease. The remainder of the involved vagina can be treated by other means outlined below.
FIGURE 2 VAIN 3 at vaginal apex
VAIN usually is confined to the apex in women hysterectomized for cervical neoplasia.
When CIN extends onto the vaginal fornix
This is an uncommon scenario, although its underrecognition may explain why some VAIN is diagnosed shortly after hysterectomy for CIN.
Management is simpler than for disease involving the posthysterectomy apex because there is no scarring, distortion, or buried epithelium, and traction on the cervix generally provides good exposure.
Laser vaporization is an option. For many such patients, this is one of the few good indications remaining for laser vaporization.5,15,16
If hysterectomy is indicated, remove the affected portion of the upper vagina along with the cervix.5,17
Multifocal or diffuse pattern: Typical of immunocompromise
Multifocal or diffuse manifestation is likewise uncommon, often involving low-grade neoplasia and condylomatous changes.5,18 This is the pattern typically seen in chronically immunocompromised women.5,10
The natural history of VAIN—especially the malignant potential—is less well understood for women with this disease pattern. When managing such patients, keep in mind the potential for occult neoplasia in the vaginal cuff scar. However, broaden the focus of treatment to encompass the entire vagina. The various management options are described below:
- If the patient has cervical intraepithelial neoplasia 2 or 3, treat with laser, cryotherapy, or large loop excision of the transformation zone at least 2 weeks prior to beginning 5-fluorouracil (5-FU).
- Treat vulvar, anal, or urethral lesions with a laser or local excision prior to 5-FU.
- Treat vulvovaginitis prior to 5-FU.
- Have the patient administer 1/2 vaginal applicator of 5% 5-FU (Efudex; 2.5 g) deep in the vagina weekly at bedtime for 10 weeks. Instruct her to place a tampon or cotton balls into the outer vagina, unless the distal vagina is involved. Also have her apply petroleum jelly to all vulvar tissues, around the urethra, and to the anus. In the morning, she should remove the tampon, wash the vulva with soap and water, and dry carefully. This should be followed by another application of petroleum jelly (sparingly) to vulvar tissues. A mini napkin may help prevent staining of garments.
- When 5-FU treatment ends, have the patient administer 1/3 applicator of vaginal estrogen cream nightly for 2 weeks beginning 3 weeks prior to reevaluation.
- Evaluate with colposcopy and cytology 8 weeks after completing 5-FU therapy.
Observation only is particularly suited for women who have low-grade disease or who are severely debilitated and chronically immunocompromised.
5-fluorouracil (5-FU) cream is a very good treatment when VAIN involves the upper half to two thirds of the vagina.2,9 However, the cream does not reach buried epithelium in the vaginal cuff scar and probably does not consistently treat the lower vagina when applied with the standard vaginal applicator. Applying the cream directly to the lower vagina may be more effective when that region is involved (see “6-step vaginal fluorouracil therapy for intraepithelial neoplasia,” above).
Laser vaporization is well accepted and commonly used.2,9,15,19,20 Advantages are versatility in treatment of multifocal disease without sacrificing vaginal epithelium, and low likelihood of complications. Disadvantages include inability to treat buried vaginal cuff epithelium; technical difficulties in applying the laser to a folding and often distorted (cuff) surface within a confined space; and the expensive equipment, technical support, and surgical expertise required.1,4,5,11,15,16,20
Planned combined treatment using laser vaporization followed by 5-FU cream has been reported efficacious in the treatment of diffuse vaginal condylomata.21 Selected patients with VAIN also may benefit,22 such as those with plaque-like disease (where a thick layer of keratin can reduce penetration of the 5-FU cream), diffuse/multifocal disease where laser vaporization is likely to be incomplete, or vaginal anatomy that makes it difficult to accomplish complete laser vaporization.
Vaginectomy is definitive management for selected patients with extensive VAIN. The operation is done transperineally, although hysterovaginectomy may require a combined approach.23 Leaving the distal third of the vagina intact (when disease distribution allows) makes the operation easier and may help avert iatrogenic urinary incontinence. Follow-up examination—and treatment, if necessary—of the remaining short vaginal stump is fairly easy.
Removal of the vagina is technically demanding in some women. A Schuchardt incision is useful in such instances.
The other obvious disadvantage of vaginectomy is loss of coital function, although placement of a skin graft is an option.
Brachytherapy is another option for treating extensive VAIN in highly selected patients.24,25
A cylindrical apparatus placed in the vagina delivers radiotherapy to the vaginal epithelium; the likelihood of significant morbidity is low. This method is most applicable to poor surgical candidates with extensive VAIN. Disadvantages include fibrosis of the vagina, limited data on efficacy (and particular concern about inadequate dosing to buried or distorted vaginal cuff epithelium), and potential difficulties with follow-up and treatment of recurrence.3,5
“Chemosurgery,” specifically 5-FU cream followed by surgical removal of the then-partially-detached VAIN, followed by additional 5-FU cream, has been used effectively at 1 center.10 Other reported-but-less-investigated methods include cryotherapy, electrocautery, loop electrosurgical excision, and cavitational ultrasonic surgical aspiration (CUSA).3-5
VAIN in a radiated vagina
Most women who develop VAIN in this scenario received radiation therapy many years earlier for carcinoma of the cervix.5,11
The most common sites are the upper third to upper half of the remaining vagina, where radiation changes are prominent.
Diagnosis often is problematic due to:
- difficulties with interpreting cytologic preparations in such patients;
- radiation changes in the vagina (pale and fibrotic with telangiectasis), which largely obscure colposcopic findings;
- the difficulty and potential hazards of biopsy of a thin, fibrotic upper vagina; and
- obliterative coaptation of the upper third to half of the vagina.
Significantly abnormal cytology in the absence of a colposcopically identified lesion (or palpable abnormality) is of particular concern. In such patients, view the abnormal cytologic interpretation with caution and consider further initial evaluation, including outside review of the cytology slides, treatment of the vagina with estrogen, and repeat cytologic and colposcopic evaluation.
When there is clear cytologic evidence of a severe abnormality, consider the possibility of occult neoplasia within the coapted upper vagina and/or cervix.
Management of VAIN in a radiated vagina.
Take into account the anatomic distortion of the upper vagina, the thin and fibrotic nature of the epithelium, and the potential for fistula formation with excisional procedures or other treatments that produce injury beneath the surface of the epithelium. Among the options:
- 5-FU cream or laser vaporization. VAIN that is completely visualized within the remaining vagina is probably best treated with one of these modalities, provided the physician is experienced in managing such patients.5,9
- Excision of the upper vagina,5 including the coapted portion (and sometimes the residual cervix with or without the uterus) is appropriate for highly selected cases, but only after careful consideration of:
- the likelihood of finding significant neoplasia,
- the anatomic feasibility (it is desirable to perform the procedure transvaginally), and
- the overall risk versus benefit. Such procedures should be performed only by an experienced physician.
The chronically immunocompromised
These patients often have undergone organ transplants or are human immunodeficiency virus (HIV)-positive. Diffuse HPV infection of the lower genital tract is pervasive in these women,5,10,26 and diffuse/multifocal lower genital tract intraepithelial neoplasia is often present as well.
These women may be at increased risk for progression to invasive cancer.
Eradication may not be possible. Attempts to eliminate intraepithelial disease are usually unsuccessful.
Management. Many of these women are severely debilitated, with other, more significant medical problems and a short life expectancy. Vigilance is required, as other lower genital tract (and anal) sites are frequently involved. Since eradication of diffuse/multifocal intraepithelial disease is not a realistic goal, treatment followed by chronic suppressive therapy (such as a low intermittent dose of 5-FU) is reasonable.9,10,22
Another approach is close observation, including frequent examinations, with prompt intervention when invasive disease is suspected.
FOLLOW-UP
After treatment for VAIN, follow-up is similar to that for a comparable cervical lesion. Once the vagina has healed, see the patient every 3 to 6 months for 2 years and annually thereafter. In addition to obtaining vaginal cytology, carefully inspect and palpate the vagina (including the vaginal cuff scar).
Effects of 5-FU. Following a course of 5-FU cream, vaginal mucosal ulcerations may persist for several weeks. Occasionally, these may lead to partial coaptations.27 In addition, subsequent islands of columnar epithelium have been described.28 Keep these factors in mind during the follow-up of women who have been treated with transvaginal 5-FU cream.
Long-term annual follow-up is reasonable, since these women are probably at increased risk for developing a second primary vaginal lesion and/or lower anogenital tract neoplasia at other sites.
When extra vigilance is warranted. Some patients merit closer follow-up, such as the chronically immunosuppressed women described; also, women who have been treated for vaginal apical disease without resection of the vaginal cuff scar, previously radiated patients, and women whose VAIN was treated with brachytherapy.
Dr. Hoffman reports no financial relationships relevant to this article.
1. Dodge JA, Eltabbakh GH, Mount SL, Walker RP, Morgan A. Clinical features and risk of recurrence among patients with vaginal intraepithelial neoplasia. Gynecol Oncol. 2001;83:363-369.
2. Petrilli ES, Townsend DE, Morrow CP, Nakao CY. Vaginal intraepithelial neoplasia: biologic aspects and treatment with topical 5-fluorouracil and the carbon dioxide laser. Am J Obstet Gynecol. 1980;138:321-328.
3. Benedet JL, Sanders BH. Carcinoma in situ of the vagina. Am J Obstet Gynecol. 1984;148:695-700.
4. Lenehan PM, Meffe F, Lickrish GM. Vaginal intraepithelial neoplasia: biologic aspects and management. Obstet Gynecol. 1986;68:333-337.
5. Audet-Lapointe P, Body G, Vauclair R, Drouin P, Ayoub J. Vaginal intraepithelial neoplasia. Gynecol Oncol. 1990;36:232-239.
6. Gallup DG, Morley GW. Carcinoma in situ of the vagina—a study and review. Obstet Gynecol. 1975;46:334-440.
7. Woodruff JD. Treatment of recurrent carcinoma in situ in the lower genital canal. Clin Obstet Gynecol. 1965;8:757-770.
8. McCance DJ, Clarkson PK, Dyson JL, Walker PG, Singer A. Human papillomavirus types 6 and 16 in multifocal intraepithelial neoplasias of the female lower genital tract. Br J Obstet Gynaecol. 1985;92:1093-1100.
9. Krebs HB. Treatment of vaginal intraepithelial neoplasia with laser and topical 5-fluorouracil. Obstet Gynecol. 1989;73:657-660.
10. Sillman FH, Fruchter RG, Chen YS, Camilien L, Sedlis A, McTigue E. Vaginal intraepithelial neoplasia: risk factors for persistence, recurrence, and invasion and its management. Am J Obstet Gynecol. 1997;176:93-99.
11. Woodman CBJ, Jordan JA, Wade-Evans T. The management of vaginal intraepithelial neoplasia after hysterectomy. Br J Obstet Gynaecol. 1984;91:103-105.
12. Aho M, Vesterinen E, Meyer B, Purola E, Paavonen J. Natural history of vaginal intraepithelial neoplasia. Cancer. 1991;68:195-197.
13. Hoffman MS, Roberts WS, LaPolla JP, Sterghos S, Jr, Cavanagh D. Neoplasia in vaginal cuff epithelial inclusion cysts after hysterectomy. J Reprod Med. 1989;34:412-416.
14. Hoffman MS, DeCesare SL, Roberts WS, Fiorica JV, Finan MA, Cavanagh D. Upper vaginectomy for in situ and occult, superficially invasive carcinoma of the vagina. Am J Obstet Gynecol. 1992;166:30-33.
15. Curtin JP, Twiggs LB, Julian TM. Treatment of vaginal intraepithelial neoplasia with the CO2laser. J Reprod Med. 1985;30:942-944.
16. Hoffman MS, Roberts WS, LaPolla JP, Fiorica JV, Cavanagh D. Laser vaporization of grade 3 vaginal intraepithelial neoplasia. Am J Obstet Gynecol. 1991;165:1342-1344.
17. Hoffman MS, Spellacy WN. The Difficult Vaginal Hysterectomy. New York, NY: Springer-Verlag; 1995;117-119.
18. Reid R. Human papillomaviral infection. The key to rational triage of cervical neoplasia. Obstet Gynecol Clin North Am. 1987;14:407-429.
19. Townsend DE, Levine RU, Crum CP, Richart RM. Treatment of vaginal carcinoma in situ with the carbon dioxide laser. Am J Obstet Gynecol. 1982;143:565-568.
20. Sherman AI. Laser therapy for vaginal intraepithelial neoplasia after hysterectomy. J Reprod Med. 1990;35—941-944.
21. Krebs HB. Combination of laser plus 5-fluorouracil for the treatment of extensive genital condylomata acuminata. Lasers Surg Med. 1988;8:135-138.
22. Krebs HB. Prophylactic topical 5-fluorouracil following treatment of human papillomavirus-associated lesions of the vulva and vagina. Obstet Gynecol. 1986;68:837-841.
23. Hoffman MS, Bomalaski JJ, Lockhart JL, Greenwald DP. Total removal of the normal non-prolapsed vagina. Technique, results and anatomic observations. J Pelvic Surg. 2002;8:89-94.
24. MacLeod C, Fowler A, Dalrymple C, Atkinson K, Elliott P, Carter J. High-dose-rate brachytherapy in the management of high-grade intraepithelial neoplasia of the vagina. Gynecol Oncol. 1997;65:74-77.
25. Teruya Y, Sakumoto K, Moromizato H, et al. High dose-rate intracavitary brachytherapy for carcinoma in situ of the vagina occurring after hysterectomy: a rational prescription of radiation dose. Am J Obstet Gynecol. 2002;187:360-364.
26. Sillman FH, Sedlis A. Anogenital HPV/neoplasia in immunosuppressed women: an update. Dermatol Clin North Am. 1991;9:353-369.
27. Krebs HB, Helmkamp BF. Chronic ulcerations following topical therapy with 5-fluorouracil for vaginal human papillomavirus-associated lesions. Obstet Gynecol. 1991;78:205-212.
28. Dungar CF, Wilkinson EJ. Vaginal columnar cell metaplasia: an acquired adenosis associated with topical 5-FU therapy. J Reprod Med. 1995;40:361-366.
- Most women diagnosed with vaginal intraepithelial neoplasia (VAIN) have a history of cervical intraepithelial neoplasia.
- Compelling clinical and laboratory data indicate a causal relationship between human papillomavirus and VAIN.
- Like its cervical counterpart, VAIN 3 is thought to have substantial potential to progress to invasive cancer.
- Diagnosis includes careful gross and colposcopic inspection of the entire vagina (with mapping of involved areas), representative colposcopically directed biopsies, and careful palpation of the vaginal walls, especially the vaginal cuff scar.
- Important factors to consider when selecting appropriate treatment for women with VAIN include prior hysterectomy, prior radiation therapy, age, whether she is sexually active, comorbidities, vaginal anatomy, and prior treatments.
We can easily identify vulvar intraepithelial neoplasia (VIN): The patient complains of itching and has a visible lesion. We find cervical intraepithelial neoplasia (CIN) by investigating an abnormal Pap test. But what about vaginal intraepithelial neoplasia (VAIN)? It does not itch and is invisible to the naked eye. A Pap test sometimes catches it, although this test is used mainly to screen for CIN, not VAIN.
VAIN just does not grab our attention. It is uncommon, and invasive vaginal cancer is rare. But before you slip this article into the “obscure disease” file, consider the following:
- VAIN is difficult to diagnose, but some women are at increased risk.
- It is difficult to manage, but understanding the treatment options is important to success.
- The potential for VAIN to evolve into invasive cancer is probably substantial.
- Treatment of invasive vaginal cancer has a high rate of complications and is often unsuccessful.
This article describes the epidemiology, natural history, diagnosis, and treatment of VAIN, focusing primarily on management.
A range of risk factors
As with many uncommon diseases that are difficult to diagnose, good data on the incidence of VAIN are not available. Women diagnosed with VAIN can be in their late teens or senior citizens; mean age is about 50 years.1-5 Race is not mentioned in most studies.
CIN. Although concomitant or subsequent VAIN is very unusual in the approximately 600,000 women identified with CIN each year in the United States, most patients diagnosed with VAIN have a history of CIN. The small number who still have a cervix and are diagnosed with VAIN have a high incidence of concomitant CIN.1 Of women who have had a hysterectomy for CIN, only 1% to 5% are subsequently diagnosed with VAIN.6,7
Since most women who develop vaginal intraepithelial neoplasia have a prior or current history of cervical neoplasia, the “field effect” also renders them at risk for vulvar neoplasia.
HPV. Compelling data indicate a causal relationship between human papillomavirus (HPV) and CIN; the same is true for VAIN.8
Tobacco use. A history of tobacco use is frequent among women diagnosed with VAIN.1
Pelvic radiotherapy is commonly reported in case series.1,3-5 Generally, malignancy is potentially radiation-related if it develops at least 5 to 10 years after treatment.
We do not know whether radiotherapy induces neoplastic transformation in the vagina, but women with a history of radiation warrant long-term follow-up, as they seem to be at increased risk and diagnosis may be difficult.4,5
Chronically immunocompromised women are at particular risk for multifocal lower genital tract neoplasia.9,10
Natural history
The limited data available do suggest that vaginal intraepithelial neoplasia is a premalignant condition.11,12 Unfortunately, little is known about the relationship between severity of the intraepithelial neoplastic process and degree of risk. Natural history studies of VAIN 3 are even more limited than those of CIN 3.
Like its cervical counterpart, VAIN 3 is thought to have substantial potential for progression to invasive cancer.1-4,10-13
EVALUATIONDiagnosis entails inspection, palpation, and directed biopsies
VAIN is most commonly diagnosed after investigation of an abnormal Pap test taken from the vaginal cuff of a woman who has undergone a prior hysterectomy for cervical neoplasia. Occasionally, the disease is identified during colposcopy as extension of a cervical lesion. In either case, VAIN usually involves the upper third of the vagina.1-5,11 A minority of patients will be found to have diffuse multifocal lesions along the vaginal walls.
Adequate diagnosis mandates:
- careful gross and colposcopic inspection of the entire vagina (with mapping of involved areas),
- representative colposcopically directed biopsies,
- careful palpation of the vaginal walls, especially the vaginal cuff scar, and
- in some cases, excision of the vaginal cuff scar.
VAIN is often readily visualized with a colposcope, and the appearance may be more prominent than that of a comparable cervical lesion (FIGURE 1). The lesions are sometimes hyperkeratotic and grossly visible. However, colposcopy of the vagina is more difficult than that of the cervix due to vaginal folding, a larger surface area, and vaginal cuff irregularities.
Vaginal atrophy also creates diagnostic difficulties related to colposcopic assessment, and to overreading of vaginal cytology (eg, when a lesion is interpreted as high-grade dysplasia or suspicious). Reassessment after 4 to 6 weeks of estrogen therapy helps resolve these issues.
FIGURE 1 Colposcopic view of VAIN 3
Note the hyperkeratotic, prominent appearance of the VAIN lesions.
TREATMENT
Because women with VAIN are a heterogeneous group, treatment must be individualized. Important factors to consider when selecting appropriate treatment include prior hysterectomy, prior radiation therapy, age, whether the woman is sexually active, comorbidities, vaginal anatomy, and prior treatments.
Prior hysterectomy
Most women diagnosed with VAIN have previously undergone hysterectomy, usually because of cervical neoplasia.2-5,10 In these women, VAIN is generally confined to the vaginal apex (FIGURE 2).
Watch for occult neoplasia. Buried epithelium within the vaginal cuff scar may harbor occult neoplasia. Patients with VAIN at the vaginal apex5,10,11,13 and a vaginal cuff from a hysterectomy for cervical neoplasia are analogous to women with CIN and an unsatisfactory colposcopy.
Excision is usual management. Most of these women are appropriately managed with excision of the involved vaginal apex, including the cuff scar.1,3-5,11,14 Problematic vaginal shortening is uncommon following this procedure. The status of the resection margin is predictive of the likelihood of recurrence.3
With more extensive vaginal epithelial involvement, consider cuff excision in selected cases to eliminate the potential for occult disease. The remainder of the involved vagina can be treated by other means outlined below.
FIGURE 2 VAIN 3 at vaginal apex
VAIN usually is confined to the apex in women hysterectomized for cervical neoplasia.
When CIN extends onto the vaginal fornix
This is an uncommon scenario, although its underrecognition may explain why some VAIN is diagnosed shortly after hysterectomy for CIN.
Management is simpler than for disease involving the posthysterectomy apex because there is no scarring, distortion, or buried epithelium, and traction on the cervix generally provides good exposure.
Laser vaporization is an option. For many such patients, this is one of the few good indications remaining for laser vaporization.5,15,16
If hysterectomy is indicated, remove the affected portion of the upper vagina along with the cervix.5,17
Multifocal or diffuse pattern: Typical of immunocompromise
Multifocal or diffuse manifestation is likewise uncommon, often involving low-grade neoplasia and condylomatous changes.5,18 This is the pattern typically seen in chronically immunocompromised women.5,10
The natural history of VAIN—especially the malignant potential—is less well understood for women with this disease pattern. When managing such patients, keep in mind the potential for occult neoplasia in the vaginal cuff scar. However, broaden the focus of treatment to encompass the entire vagina. The various management options are described below:
- If the patient has cervical intraepithelial neoplasia 2 or 3, treat with laser, cryotherapy, or large loop excision of the transformation zone at least 2 weeks prior to beginning 5-fluorouracil (5-FU).
- Treat vulvar, anal, or urethral lesions with a laser or local excision prior to 5-FU.
- Treat vulvovaginitis prior to 5-FU.
- Have the patient administer 1/2 vaginal applicator of 5% 5-FU (Efudex; 2.5 g) deep in the vagina weekly at bedtime for 10 weeks. Instruct her to place a tampon or cotton balls into the outer vagina, unless the distal vagina is involved. Also have her apply petroleum jelly to all vulvar tissues, around the urethra, and to the anus. In the morning, she should remove the tampon, wash the vulva with soap and water, and dry carefully. This should be followed by another application of petroleum jelly (sparingly) to vulvar tissues. A mini napkin may help prevent staining of garments.
- When 5-FU treatment ends, have the patient administer 1/3 applicator of vaginal estrogen cream nightly for 2 weeks beginning 3 weeks prior to reevaluation.
- Evaluate with colposcopy and cytology 8 weeks after completing 5-FU therapy.
Observation only is particularly suited for women who have low-grade disease or who are severely debilitated and chronically immunocompromised.
5-fluorouracil (5-FU) cream is a very good treatment when VAIN involves the upper half to two thirds of the vagina.2,9 However, the cream does not reach buried epithelium in the vaginal cuff scar and probably does not consistently treat the lower vagina when applied with the standard vaginal applicator. Applying the cream directly to the lower vagina may be more effective when that region is involved (see “6-step vaginal fluorouracil therapy for intraepithelial neoplasia,” above).
Laser vaporization is well accepted and commonly used.2,9,15,19,20 Advantages are versatility in treatment of multifocal disease without sacrificing vaginal epithelium, and low likelihood of complications. Disadvantages include inability to treat buried vaginal cuff epithelium; technical difficulties in applying the laser to a folding and often distorted (cuff) surface within a confined space; and the expensive equipment, technical support, and surgical expertise required.1,4,5,11,15,16,20
Planned combined treatment using laser vaporization followed by 5-FU cream has been reported efficacious in the treatment of diffuse vaginal condylomata.21 Selected patients with VAIN also may benefit,22 such as those with plaque-like disease (where a thick layer of keratin can reduce penetration of the 5-FU cream), diffuse/multifocal disease where laser vaporization is likely to be incomplete, or vaginal anatomy that makes it difficult to accomplish complete laser vaporization.
Vaginectomy is definitive management for selected patients with extensive VAIN. The operation is done transperineally, although hysterovaginectomy may require a combined approach.23 Leaving the distal third of the vagina intact (when disease distribution allows) makes the operation easier and may help avert iatrogenic urinary incontinence. Follow-up examination—and treatment, if necessary—of the remaining short vaginal stump is fairly easy.
Removal of the vagina is technically demanding in some women. A Schuchardt incision is useful in such instances.
The other obvious disadvantage of vaginectomy is loss of coital function, although placement of a skin graft is an option.
Brachytherapy is another option for treating extensive VAIN in highly selected patients.24,25
A cylindrical apparatus placed in the vagina delivers radiotherapy to the vaginal epithelium; the likelihood of significant morbidity is low. This method is most applicable to poor surgical candidates with extensive VAIN. Disadvantages include fibrosis of the vagina, limited data on efficacy (and particular concern about inadequate dosing to buried or distorted vaginal cuff epithelium), and potential difficulties with follow-up and treatment of recurrence.3,5
“Chemosurgery,” specifically 5-FU cream followed by surgical removal of the then-partially-detached VAIN, followed by additional 5-FU cream, has been used effectively at 1 center.10 Other reported-but-less-investigated methods include cryotherapy, electrocautery, loop electrosurgical excision, and cavitational ultrasonic surgical aspiration (CUSA).3-5
VAIN in a radiated vagina
Most women who develop VAIN in this scenario received radiation therapy many years earlier for carcinoma of the cervix.5,11
The most common sites are the upper third to upper half of the remaining vagina, where radiation changes are prominent.
Diagnosis often is problematic due to:
- difficulties with interpreting cytologic preparations in such patients;
- radiation changes in the vagina (pale and fibrotic with telangiectasis), which largely obscure colposcopic findings;
- the difficulty and potential hazards of biopsy of a thin, fibrotic upper vagina; and
- obliterative coaptation of the upper third to half of the vagina.
Significantly abnormal cytology in the absence of a colposcopically identified lesion (or palpable abnormality) is of particular concern. In such patients, view the abnormal cytologic interpretation with caution and consider further initial evaluation, including outside review of the cytology slides, treatment of the vagina with estrogen, and repeat cytologic and colposcopic evaluation.
When there is clear cytologic evidence of a severe abnormality, consider the possibility of occult neoplasia within the coapted upper vagina and/or cervix.
Management of VAIN in a radiated vagina.
Take into account the anatomic distortion of the upper vagina, the thin and fibrotic nature of the epithelium, and the potential for fistula formation with excisional procedures or other treatments that produce injury beneath the surface of the epithelium. Among the options:
- 5-FU cream or laser vaporization. VAIN that is completely visualized within the remaining vagina is probably best treated with one of these modalities, provided the physician is experienced in managing such patients.5,9
- Excision of the upper vagina,5 including the coapted portion (and sometimes the residual cervix with or without the uterus) is appropriate for highly selected cases, but only after careful consideration of:
- the likelihood of finding significant neoplasia,
- the anatomic feasibility (it is desirable to perform the procedure transvaginally), and
- the overall risk versus benefit. Such procedures should be performed only by an experienced physician.
The chronically immunocompromised
These patients often have undergone organ transplants or are human immunodeficiency virus (HIV)-positive. Diffuse HPV infection of the lower genital tract is pervasive in these women,5,10,26 and diffuse/multifocal lower genital tract intraepithelial neoplasia is often present as well.
These women may be at increased risk for progression to invasive cancer.
Eradication may not be possible. Attempts to eliminate intraepithelial disease are usually unsuccessful.
Management. Many of these women are severely debilitated, with other, more significant medical problems and a short life expectancy. Vigilance is required, as other lower genital tract (and anal) sites are frequently involved. Since eradication of diffuse/multifocal intraepithelial disease is not a realistic goal, treatment followed by chronic suppressive therapy (such as a low intermittent dose of 5-FU) is reasonable.9,10,22
Another approach is close observation, including frequent examinations, with prompt intervention when invasive disease is suspected.
FOLLOW-UP
After treatment for VAIN, follow-up is similar to that for a comparable cervical lesion. Once the vagina has healed, see the patient every 3 to 6 months for 2 years and annually thereafter. In addition to obtaining vaginal cytology, carefully inspect and palpate the vagina (including the vaginal cuff scar).
Effects of 5-FU. Following a course of 5-FU cream, vaginal mucosal ulcerations may persist for several weeks. Occasionally, these may lead to partial coaptations.27 In addition, subsequent islands of columnar epithelium have been described.28 Keep these factors in mind during the follow-up of women who have been treated with transvaginal 5-FU cream.
Long-term annual follow-up is reasonable, since these women are probably at increased risk for developing a second primary vaginal lesion and/or lower anogenital tract neoplasia at other sites.
When extra vigilance is warranted. Some patients merit closer follow-up, such as the chronically immunosuppressed women described; also, women who have been treated for vaginal apical disease without resection of the vaginal cuff scar, previously radiated patients, and women whose VAIN was treated with brachytherapy.
Dr. Hoffman reports no financial relationships relevant to this article.
- Most women diagnosed with vaginal intraepithelial neoplasia (VAIN) have a history of cervical intraepithelial neoplasia.
- Compelling clinical and laboratory data indicate a causal relationship between human papillomavirus and VAIN.
- Like its cervical counterpart, VAIN 3 is thought to have substantial potential to progress to invasive cancer.
- Diagnosis includes careful gross and colposcopic inspection of the entire vagina (with mapping of involved areas), representative colposcopically directed biopsies, and careful palpation of the vaginal walls, especially the vaginal cuff scar.
- Important factors to consider when selecting appropriate treatment for women with VAIN include prior hysterectomy, prior radiation therapy, age, whether she is sexually active, comorbidities, vaginal anatomy, and prior treatments.
We can easily identify vulvar intraepithelial neoplasia (VIN): The patient complains of itching and has a visible lesion. We find cervical intraepithelial neoplasia (CIN) by investigating an abnormal Pap test. But what about vaginal intraepithelial neoplasia (VAIN)? It does not itch and is invisible to the naked eye. A Pap test sometimes catches it, although this test is used mainly to screen for CIN, not VAIN.
VAIN just does not grab our attention. It is uncommon, and invasive vaginal cancer is rare. But before you slip this article into the “obscure disease” file, consider the following:
- VAIN is difficult to diagnose, but some women are at increased risk.
- It is difficult to manage, but understanding the treatment options is important to success.
- The potential for VAIN to evolve into invasive cancer is probably substantial.
- Treatment of invasive vaginal cancer has a high rate of complications and is often unsuccessful.
This article describes the epidemiology, natural history, diagnosis, and treatment of VAIN, focusing primarily on management.
A range of risk factors
As with many uncommon diseases that are difficult to diagnose, good data on the incidence of VAIN are not available. Women diagnosed with VAIN can be in their late teens or senior citizens; mean age is about 50 years.1-5 Race is not mentioned in most studies.
CIN. Although concomitant or subsequent VAIN is very unusual in the approximately 600,000 women identified with CIN each year in the United States, most patients diagnosed with VAIN have a history of CIN. The small number who still have a cervix and are diagnosed with VAIN have a high incidence of concomitant CIN.1 Of women who have had a hysterectomy for CIN, only 1% to 5% are subsequently diagnosed with VAIN.6,7
Since most women who develop vaginal intraepithelial neoplasia have a prior or current history of cervical neoplasia, the “field effect” also renders them at risk for vulvar neoplasia.
HPV. Compelling data indicate a causal relationship between human papillomavirus (HPV) and CIN; the same is true for VAIN.8
Tobacco use. A history of tobacco use is frequent among women diagnosed with VAIN.1
Pelvic radiotherapy is commonly reported in case series.1,3-5 Generally, malignancy is potentially radiation-related if it develops at least 5 to 10 years after treatment.
We do not know whether radiotherapy induces neoplastic transformation in the vagina, but women with a history of radiation warrant long-term follow-up, as they seem to be at increased risk and diagnosis may be difficult.4,5
Chronically immunocompromised women are at particular risk for multifocal lower genital tract neoplasia.9,10
Natural history
The limited data available do suggest that vaginal intraepithelial neoplasia is a premalignant condition.11,12 Unfortunately, little is known about the relationship between severity of the intraepithelial neoplastic process and degree of risk. Natural history studies of VAIN 3 are even more limited than those of CIN 3.
Like its cervical counterpart, VAIN 3 is thought to have substantial potential for progression to invasive cancer.1-4,10-13
EVALUATIONDiagnosis entails inspection, palpation, and directed biopsies
VAIN is most commonly diagnosed after investigation of an abnormal Pap test taken from the vaginal cuff of a woman who has undergone a prior hysterectomy for cervical neoplasia. Occasionally, the disease is identified during colposcopy as extension of a cervical lesion. In either case, VAIN usually involves the upper third of the vagina.1-5,11 A minority of patients will be found to have diffuse multifocal lesions along the vaginal walls.
Adequate diagnosis mandates:
- careful gross and colposcopic inspection of the entire vagina (with mapping of involved areas),
- representative colposcopically directed biopsies,
- careful palpation of the vaginal walls, especially the vaginal cuff scar, and
- in some cases, excision of the vaginal cuff scar.
VAIN is often readily visualized with a colposcope, and the appearance may be more prominent than that of a comparable cervical lesion (FIGURE 1). The lesions are sometimes hyperkeratotic and grossly visible. However, colposcopy of the vagina is more difficult than that of the cervix due to vaginal folding, a larger surface area, and vaginal cuff irregularities.
Vaginal atrophy also creates diagnostic difficulties related to colposcopic assessment, and to overreading of vaginal cytology (eg, when a lesion is interpreted as high-grade dysplasia or suspicious). Reassessment after 4 to 6 weeks of estrogen therapy helps resolve these issues.
FIGURE 1 Colposcopic view of VAIN 3
Note the hyperkeratotic, prominent appearance of the VAIN lesions.
TREATMENT
Because women with VAIN are a heterogeneous group, treatment must be individualized. Important factors to consider when selecting appropriate treatment include prior hysterectomy, prior radiation therapy, age, whether the woman is sexually active, comorbidities, vaginal anatomy, and prior treatments.
Prior hysterectomy
Most women diagnosed with VAIN have previously undergone hysterectomy, usually because of cervical neoplasia.2-5,10 In these women, VAIN is generally confined to the vaginal apex (FIGURE 2).
Watch for occult neoplasia. Buried epithelium within the vaginal cuff scar may harbor occult neoplasia. Patients with VAIN at the vaginal apex5,10,11,13 and a vaginal cuff from a hysterectomy for cervical neoplasia are analogous to women with CIN and an unsatisfactory colposcopy.
Excision is usual management. Most of these women are appropriately managed with excision of the involved vaginal apex, including the cuff scar.1,3-5,11,14 Problematic vaginal shortening is uncommon following this procedure. The status of the resection margin is predictive of the likelihood of recurrence.3
With more extensive vaginal epithelial involvement, consider cuff excision in selected cases to eliminate the potential for occult disease. The remainder of the involved vagina can be treated by other means outlined below.
FIGURE 2 VAIN 3 at vaginal apex
VAIN usually is confined to the apex in women hysterectomized for cervical neoplasia.
When CIN extends onto the vaginal fornix
This is an uncommon scenario, although its underrecognition may explain why some VAIN is diagnosed shortly after hysterectomy for CIN.
Management is simpler than for disease involving the posthysterectomy apex because there is no scarring, distortion, or buried epithelium, and traction on the cervix generally provides good exposure.
Laser vaporization is an option. For many such patients, this is one of the few good indications remaining for laser vaporization.5,15,16
If hysterectomy is indicated, remove the affected portion of the upper vagina along with the cervix.5,17
Multifocal or diffuse pattern: Typical of immunocompromise
Multifocal or diffuse manifestation is likewise uncommon, often involving low-grade neoplasia and condylomatous changes.5,18 This is the pattern typically seen in chronically immunocompromised women.5,10
The natural history of VAIN—especially the malignant potential—is less well understood for women with this disease pattern. When managing such patients, keep in mind the potential for occult neoplasia in the vaginal cuff scar. However, broaden the focus of treatment to encompass the entire vagina. The various management options are described below:
- If the patient has cervical intraepithelial neoplasia 2 or 3, treat with laser, cryotherapy, or large loop excision of the transformation zone at least 2 weeks prior to beginning 5-fluorouracil (5-FU).
- Treat vulvar, anal, or urethral lesions with a laser or local excision prior to 5-FU.
- Treat vulvovaginitis prior to 5-FU.
- Have the patient administer 1/2 vaginal applicator of 5% 5-FU (Efudex; 2.5 g) deep in the vagina weekly at bedtime for 10 weeks. Instruct her to place a tampon or cotton balls into the outer vagina, unless the distal vagina is involved. Also have her apply petroleum jelly to all vulvar tissues, around the urethra, and to the anus. In the morning, she should remove the tampon, wash the vulva with soap and water, and dry carefully. This should be followed by another application of petroleum jelly (sparingly) to vulvar tissues. A mini napkin may help prevent staining of garments.
- When 5-FU treatment ends, have the patient administer 1/3 applicator of vaginal estrogen cream nightly for 2 weeks beginning 3 weeks prior to reevaluation.
- Evaluate with colposcopy and cytology 8 weeks after completing 5-FU therapy.
Observation only is particularly suited for women who have low-grade disease or who are severely debilitated and chronically immunocompromised.
5-fluorouracil (5-FU) cream is a very good treatment when VAIN involves the upper half to two thirds of the vagina.2,9 However, the cream does not reach buried epithelium in the vaginal cuff scar and probably does not consistently treat the lower vagina when applied with the standard vaginal applicator. Applying the cream directly to the lower vagina may be more effective when that region is involved (see “6-step vaginal fluorouracil therapy for intraepithelial neoplasia,” above).
Laser vaporization is well accepted and commonly used.2,9,15,19,20 Advantages are versatility in treatment of multifocal disease without sacrificing vaginal epithelium, and low likelihood of complications. Disadvantages include inability to treat buried vaginal cuff epithelium; technical difficulties in applying the laser to a folding and often distorted (cuff) surface within a confined space; and the expensive equipment, technical support, and surgical expertise required.1,4,5,11,15,16,20
Planned combined treatment using laser vaporization followed by 5-FU cream has been reported efficacious in the treatment of diffuse vaginal condylomata.21 Selected patients with VAIN also may benefit,22 such as those with plaque-like disease (where a thick layer of keratin can reduce penetration of the 5-FU cream), diffuse/multifocal disease where laser vaporization is likely to be incomplete, or vaginal anatomy that makes it difficult to accomplish complete laser vaporization.
Vaginectomy is definitive management for selected patients with extensive VAIN. The operation is done transperineally, although hysterovaginectomy may require a combined approach.23 Leaving the distal third of the vagina intact (when disease distribution allows) makes the operation easier and may help avert iatrogenic urinary incontinence. Follow-up examination—and treatment, if necessary—of the remaining short vaginal stump is fairly easy.
Removal of the vagina is technically demanding in some women. A Schuchardt incision is useful in such instances.
The other obvious disadvantage of vaginectomy is loss of coital function, although placement of a skin graft is an option.
Brachytherapy is another option for treating extensive VAIN in highly selected patients.24,25
A cylindrical apparatus placed in the vagina delivers radiotherapy to the vaginal epithelium; the likelihood of significant morbidity is low. This method is most applicable to poor surgical candidates with extensive VAIN. Disadvantages include fibrosis of the vagina, limited data on efficacy (and particular concern about inadequate dosing to buried or distorted vaginal cuff epithelium), and potential difficulties with follow-up and treatment of recurrence.3,5
“Chemosurgery,” specifically 5-FU cream followed by surgical removal of the then-partially-detached VAIN, followed by additional 5-FU cream, has been used effectively at 1 center.10 Other reported-but-less-investigated methods include cryotherapy, electrocautery, loop electrosurgical excision, and cavitational ultrasonic surgical aspiration (CUSA).3-5
VAIN in a radiated vagina
Most women who develop VAIN in this scenario received radiation therapy many years earlier for carcinoma of the cervix.5,11
The most common sites are the upper third to upper half of the remaining vagina, where radiation changes are prominent.
Diagnosis often is problematic due to:
- difficulties with interpreting cytologic preparations in such patients;
- radiation changes in the vagina (pale and fibrotic with telangiectasis), which largely obscure colposcopic findings;
- the difficulty and potential hazards of biopsy of a thin, fibrotic upper vagina; and
- obliterative coaptation of the upper third to half of the vagina.
Significantly abnormal cytology in the absence of a colposcopically identified lesion (or palpable abnormality) is of particular concern. In such patients, view the abnormal cytologic interpretation with caution and consider further initial evaluation, including outside review of the cytology slides, treatment of the vagina with estrogen, and repeat cytologic and colposcopic evaluation.
When there is clear cytologic evidence of a severe abnormality, consider the possibility of occult neoplasia within the coapted upper vagina and/or cervix.
Management of VAIN in a radiated vagina.
Take into account the anatomic distortion of the upper vagina, the thin and fibrotic nature of the epithelium, and the potential for fistula formation with excisional procedures or other treatments that produce injury beneath the surface of the epithelium. Among the options:
- 5-FU cream or laser vaporization. VAIN that is completely visualized within the remaining vagina is probably best treated with one of these modalities, provided the physician is experienced in managing such patients.5,9
- Excision of the upper vagina,5 including the coapted portion (and sometimes the residual cervix with or without the uterus) is appropriate for highly selected cases, but only after careful consideration of:
- the likelihood of finding significant neoplasia,
- the anatomic feasibility (it is desirable to perform the procedure transvaginally), and
- the overall risk versus benefit. Such procedures should be performed only by an experienced physician.
The chronically immunocompromised
These patients often have undergone organ transplants or are human immunodeficiency virus (HIV)-positive. Diffuse HPV infection of the lower genital tract is pervasive in these women,5,10,26 and diffuse/multifocal lower genital tract intraepithelial neoplasia is often present as well.
These women may be at increased risk for progression to invasive cancer.
Eradication may not be possible. Attempts to eliminate intraepithelial disease are usually unsuccessful.
Management. Many of these women are severely debilitated, with other, more significant medical problems and a short life expectancy. Vigilance is required, as other lower genital tract (and anal) sites are frequently involved. Since eradication of diffuse/multifocal intraepithelial disease is not a realistic goal, treatment followed by chronic suppressive therapy (such as a low intermittent dose of 5-FU) is reasonable.9,10,22
Another approach is close observation, including frequent examinations, with prompt intervention when invasive disease is suspected.
FOLLOW-UP
After treatment for VAIN, follow-up is similar to that for a comparable cervical lesion. Once the vagina has healed, see the patient every 3 to 6 months for 2 years and annually thereafter. In addition to obtaining vaginal cytology, carefully inspect and palpate the vagina (including the vaginal cuff scar).
Effects of 5-FU. Following a course of 5-FU cream, vaginal mucosal ulcerations may persist for several weeks. Occasionally, these may lead to partial coaptations.27 In addition, subsequent islands of columnar epithelium have been described.28 Keep these factors in mind during the follow-up of women who have been treated with transvaginal 5-FU cream.
Long-term annual follow-up is reasonable, since these women are probably at increased risk for developing a second primary vaginal lesion and/or lower anogenital tract neoplasia at other sites.
When extra vigilance is warranted. Some patients merit closer follow-up, such as the chronically immunosuppressed women described; also, women who have been treated for vaginal apical disease without resection of the vaginal cuff scar, previously radiated patients, and women whose VAIN was treated with brachytherapy.
Dr. Hoffman reports no financial relationships relevant to this article.
1. Dodge JA, Eltabbakh GH, Mount SL, Walker RP, Morgan A. Clinical features and risk of recurrence among patients with vaginal intraepithelial neoplasia. Gynecol Oncol. 2001;83:363-369.
2. Petrilli ES, Townsend DE, Morrow CP, Nakao CY. Vaginal intraepithelial neoplasia: biologic aspects and treatment with topical 5-fluorouracil and the carbon dioxide laser. Am J Obstet Gynecol. 1980;138:321-328.
3. Benedet JL, Sanders BH. Carcinoma in situ of the vagina. Am J Obstet Gynecol. 1984;148:695-700.
4. Lenehan PM, Meffe F, Lickrish GM. Vaginal intraepithelial neoplasia: biologic aspects and management. Obstet Gynecol. 1986;68:333-337.
5. Audet-Lapointe P, Body G, Vauclair R, Drouin P, Ayoub J. Vaginal intraepithelial neoplasia. Gynecol Oncol. 1990;36:232-239.
6. Gallup DG, Morley GW. Carcinoma in situ of the vagina—a study and review. Obstet Gynecol. 1975;46:334-440.
7. Woodruff JD. Treatment of recurrent carcinoma in situ in the lower genital canal. Clin Obstet Gynecol. 1965;8:757-770.
8. McCance DJ, Clarkson PK, Dyson JL, Walker PG, Singer A. Human papillomavirus types 6 and 16 in multifocal intraepithelial neoplasias of the female lower genital tract. Br J Obstet Gynaecol. 1985;92:1093-1100.
9. Krebs HB. Treatment of vaginal intraepithelial neoplasia with laser and topical 5-fluorouracil. Obstet Gynecol. 1989;73:657-660.
10. Sillman FH, Fruchter RG, Chen YS, Camilien L, Sedlis A, McTigue E. Vaginal intraepithelial neoplasia: risk factors for persistence, recurrence, and invasion and its management. Am J Obstet Gynecol. 1997;176:93-99.
11. Woodman CBJ, Jordan JA, Wade-Evans T. The management of vaginal intraepithelial neoplasia after hysterectomy. Br J Obstet Gynaecol. 1984;91:103-105.
12. Aho M, Vesterinen E, Meyer B, Purola E, Paavonen J. Natural history of vaginal intraepithelial neoplasia. Cancer. 1991;68:195-197.
13. Hoffman MS, Roberts WS, LaPolla JP, Sterghos S, Jr, Cavanagh D. Neoplasia in vaginal cuff epithelial inclusion cysts after hysterectomy. J Reprod Med. 1989;34:412-416.
14. Hoffman MS, DeCesare SL, Roberts WS, Fiorica JV, Finan MA, Cavanagh D. Upper vaginectomy for in situ and occult, superficially invasive carcinoma of the vagina. Am J Obstet Gynecol. 1992;166:30-33.
15. Curtin JP, Twiggs LB, Julian TM. Treatment of vaginal intraepithelial neoplasia with the CO2laser. J Reprod Med. 1985;30:942-944.
16. Hoffman MS, Roberts WS, LaPolla JP, Fiorica JV, Cavanagh D. Laser vaporization of grade 3 vaginal intraepithelial neoplasia. Am J Obstet Gynecol. 1991;165:1342-1344.
17. Hoffman MS, Spellacy WN. The Difficult Vaginal Hysterectomy. New York, NY: Springer-Verlag; 1995;117-119.
18. Reid R. Human papillomaviral infection. The key to rational triage of cervical neoplasia. Obstet Gynecol Clin North Am. 1987;14:407-429.
19. Townsend DE, Levine RU, Crum CP, Richart RM. Treatment of vaginal carcinoma in situ with the carbon dioxide laser. Am J Obstet Gynecol. 1982;143:565-568.
20. Sherman AI. Laser therapy for vaginal intraepithelial neoplasia after hysterectomy. J Reprod Med. 1990;35—941-944.
21. Krebs HB. Combination of laser plus 5-fluorouracil for the treatment of extensive genital condylomata acuminata. Lasers Surg Med. 1988;8:135-138.
22. Krebs HB. Prophylactic topical 5-fluorouracil following treatment of human papillomavirus-associated lesions of the vulva and vagina. Obstet Gynecol. 1986;68:837-841.
23. Hoffman MS, Bomalaski JJ, Lockhart JL, Greenwald DP. Total removal of the normal non-prolapsed vagina. Technique, results and anatomic observations. J Pelvic Surg. 2002;8:89-94.
24. MacLeod C, Fowler A, Dalrymple C, Atkinson K, Elliott P, Carter J. High-dose-rate brachytherapy in the management of high-grade intraepithelial neoplasia of the vagina. Gynecol Oncol. 1997;65:74-77.
25. Teruya Y, Sakumoto K, Moromizato H, et al. High dose-rate intracavitary brachytherapy for carcinoma in situ of the vagina occurring after hysterectomy: a rational prescription of radiation dose. Am J Obstet Gynecol. 2002;187:360-364.
26. Sillman FH, Sedlis A. Anogenital HPV/neoplasia in immunosuppressed women: an update. Dermatol Clin North Am. 1991;9:353-369.
27. Krebs HB, Helmkamp BF. Chronic ulcerations following topical therapy with 5-fluorouracil for vaginal human papillomavirus-associated lesions. Obstet Gynecol. 1991;78:205-212.
28. Dungar CF, Wilkinson EJ. Vaginal columnar cell metaplasia: an acquired adenosis associated with topical 5-FU therapy. J Reprod Med. 1995;40:361-366.
1. Dodge JA, Eltabbakh GH, Mount SL, Walker RP, Morgan A. Clinical features and risk of recurrence among patients with vaginal intraepithelial neoplasia. Gynecol Oncol. 2001;83:363-369.
2. Petrilli ES, Townsend DE, Morrow CP, Nakao CY. Vaginal intraepithelial neoplasia: biologic aspects and treatment with topical 5-fluorouracil and the carbon dioxide laser. Am J Obstet Gynecol. 1980;138:321-328.
3. Benedet JL, Sanders BH. Carcinoma in situ of the vagina. Am J Obstet Gynecol. 1984;148:695-700.
4. Lenehan PM, Meffe F, Lickrish GM. Vaginal intraepithelial neoplasia: biologic aspects and management. Obstet Gynecol. 1986;68:333-337.
5. Audet-Lapointe P, Body G, Vauclair R, Drouin P, Ayoub J. Vaginal intraepithelial neoplasia. Gynecol Oncol. 1990;36:232-239.
6. Gallup DG, Morley GW. Carcinoma in situ of the vagina—a study and review. Obstet Gynecol. 1975;46:334-440.
7. Woodruff JD. Treatment of recurrent carcinoma in situ in the lower genital canal. Clin Obstet Gynecol. 1965;8:757-770.
8. McCance DJ, Clarkson PK, Dyson JL, Walker PG, Singer A. Human papillomavirus types 6 and 16 in multifocal intraepithelial neoplasias of the female lower genital tract. Br J Obstet Gynaecol. 1985;92:1093-1100.
9. Krebs HB. Treatment of vaginal intraepithelial neoplasia with laser and topical 5-fluorouracil. Obstet Gynecol. 1989;73:657-660.
10. Sillman FH, Fruchter RG, Chen YS, Camilien L, Sedlis A, McTigue E. Vaginal intraepithelial neoplasia: risk factors for persistence, recurrence, and invasion and its management. Am J Obstet Gynecol. 1997;176:93-99.
11. Woodman CBJ, Jordan JA, Wade-Evans T. The management of vaginal intraepithelial neoplasia after hysterectomy. Br J Obstet Gynaecol. 1984;91:103-105.
12. Aho M, Vesterinen E, Meyer B, Purola E, Paavonen J. Natural history of vaginal intraepithelial neoplasia. Cancer. 1991;68:195-197.
13. Hoffman MS, Roberts WS, LaPolla JP, Sterghos S, Jr, Cavanagh D. Neoplasia in vaginal cuff epithelial inclusion cysts after hysterectomy. J Reprod Med. 1989;34:412-416.
14. Hoffman MS, DeCesare SL, Roberts WS, Fiorica JV, Finan MA, Cavanagh D. Upper vaginectomy for in situ and occult, superficially invasive carcinoma of the vagina. Am J Obstet Gynecol. 1992;166:30-33.
15. Curtin JP, Twiggs LB, Julian TM. Treatment of vaginal intraepithelial neoplasia with the CO2laser. J Reprod Med. 1985;30:942-944.
16. Hoffman MS, Roberts WS, LaPolla JP, Fiorica JV, Cavanagh D. Laser vaporization of grade 3 vaginal intraepithelial neoplasia. Am J Obstet Gynecol. 1991;165:1342-1344.
17. Hoffman MS, Spellacy WN. The Difficult Vaginal Hysterectomy. New York, NY: Springer-Verlag; 1995;117-119.
18. Reid R. Human papillomaviral infection. The key to rational triage of cervical neoplasia. Obstet Gynecol Clin North Am. 1987;14:407-429.
19. Townsend DE, Levine RU, Crum CP, Richart RM. Treatment of vaginal carcinoma in situ with the carbon dioxide laser. Am J Obstet Gynecol. 1982;143:565-568.
20. Sherman AI. Laser therapy for vaginal intraepithelial neoplasia after hysterectomy. J Reprod Med. 1990;35—941-944.
21. Krebs HB. Combination of laser plus 5-fluorouracil for the treatment of extensive genital condylomata acuminata. Lasers Surg Med. 1988;8:135-138.
22. Krebs HB. Prophylactic topical 5-fluorouracil following treatment of human papillomavirus-associated lesions of the vulva and vagina. Obstet Gynecol. 1986;68:837-841.
23. Hoffman MS, Bomalaski JJ, Lockhart JL, Greenwald DP. Total removal of the normal non-prolapsed vagina. Technique, results and anatomic observations. J Pelvic Surg. 2002;8:89-94.
24. MacLeod C, Fowler A, Dalrymple C, Atkinson K, Elliott P, Carter J. High-dose-rate brachytherapy in the management of high-grade intraepithelial neoplasia of the vagina. Gynecol Oncol. 1997;65:74-77.
25. Teruya Y, Sakumoto K, Moromizato H, et al. High dose-rate intracavitary brachytherapy for carcinoma in situ of the vagina occurring after hysterectomy: a rational prescription of radiation dose. Am J Obstet Gynecol. 2002;187:360-364.
26. Sillman FH, Sedlis A. Anogenital HPV/neoplasia in immunosuppressed women: an update. Dermatol Clin North Am. 1991;9:353-369.
27. Krebs HB, Helmkamp BF. Chronic ulcerations following topical therapy with 5-fluorouracil for vaginal human papillomavirus-associated lesions. Obstet Gynecol. 1991;78:205-212.
28. Dungar CF, Wilkinson EJ. Vaginal columnar cell metaplasia: an acquired adenosis associated with topical 5-FU therapy. J Reprod Med. 1995;40:361-366.