User login
WHAT IS ‘EARLY’ SURGERY?
More than 50% of patients with infective endocarditis undergo cardiac surgery during their initial presentation.1
The 2017 guidelines of the American Association for Thoracic Surgery (AATS) recommend surgery once a surgical indication has been established and effective antimicrobial therapy has been started.2
The American Heart Association/American College of Cardiology (ACC/AHA) guidelines recommend surgery during the initial hospitalization before completion of a full course of antibiotics.3
The European Society of Cardiology guidelines define surgery according to the time since the patient received intravenous antibiotic therapy: emergency surgery is performed within 24 hours of therapy, urgent surgery is performed within a few days, and elective surgery is performed after at least 1 to 2 weeks.4
These slight differences are due to the dearth of large randomized trials addressing this question.
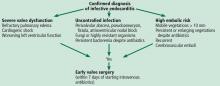
INDICATIONS FOR EARLY SURGERY
Left ventricular dysfunction and heart failure
Of all the complications of infectious endocarditis, concomitant heart failure has the greatest impact on prognosis5 and is one of the most frequent indications for surgery.6
The guidelines recommend emergency surgery during the initial hospitalization for all patients with infective endocarditis who present with refractory pulmonary edema, worsening left ventricular dysfunction, or cardiogenic shock, regardless of whether they have completed a full course of antibiotics. This applies to both native valve endocarditis and prosthetic valve endocarditis.
Uncontrolled persistent infection
Persistent infection is defined as fever and positive cultures persisting after 1 week of appropriate antibiotic treatment.4 However, 1 week is a long time. Persistence of positive blood cultures more than 48 to 72 hours after starting antibiotic therapy is associated with poor outcome and is an independent predictor of in-hospital mortality.7
The ACC/AHA guidelines recommend early surgery in patients with left-sided infective endocarditis caused by fungi or highly resistant organisms such as vancomycin-resistant enterococci or multidrug-resistant gram-negative bacilli.3 Nonetheless, antibiotic resistance is an unusual reason for expediting surgery unless there are additional indications for it.
Extension of the infection beyond the valve annulus, which occurs in about 30% of cases of native valve endocarditis and 50% of cases of prosthetic valve endocarditis,8 is considered a more valid reason to expedite surgery. Similarly, urgent surgery should be considered if there is any evidence of locally uncontrolled infection causing perivalvular abscess, fistula, pseudoaneurysm, or conduction system abnormalities causing atrioventricular nodal block.2–4
Some authors suggest reviewing the surgical pathology and microbial sequencing of excised cardiac valves after surgery to confirm the diagnosis and identify the culprit pathogen.9,10
Right-sided infective endocarditis
Right-sided infective endocarditis has a more favorable prognosis than left-sided infective endocarditis and usually responds well to medical therapy.11
Nevertheless, surgery for right-sided infective endocarditis should be expedited in patients with right heart failure secondary to severe tricuspid regurgitation with poor response to medical therapy or in the case of large tricuspid valve vegetations.12 Likewise, recurrent septic pulmonary emboli can be encountered in the setting of right-sided infective endocarditis and are an indication for early surgery.4,12
Since many patients with right-sided infective endocarditis acquire the infection by intravenous drug use, there is often a reluctance to recommend surgery, given the risk of prosthetic valve infection if they continue to use intravenous drugs.4,12 One study showed that the risk of death or reoperation between 3 and 6 months after surgery for infective endocarditis was 10 times higher in intravenous drug users. Yet their survival after surgery beyond this period was similar to that of patients with endocarditis who did not inject drugs.13 Therefore, the AATS guidelines recommend applying normal indications for surgery to those patients, with emphasis on the need for strict follow-up aimed at addiction treatment.2
Prevention of embolic events
Neurologic embolic events are a frequent complication of infective endocarditis, with the highest risk during the first few days after antibiotics are started. However, this risk decreases significantly after 2 weeks.14
The timing of surgery largely depends on whether the patient has had previous neurologic embolic events and on the size and mobility of the vegetation. The current guidelines recommend early surgery for recurrent emboli and persistent or enlarging vegetations despite appropriate antibiotic therapy, or in case of large vegetations (> 10 mm) on a native valve even in the absence of embolic events.4
A randomized trial by Kang et al15 demonstrated that, compared with conventional care, early surgery (within 48 hours of diagnosis) in patients with native valve endocarditis with large vegetations (> 10 mm) and severe valve dysfunction was associated with a significant reduction in the risk of death and embolic events.
Timing of surgery after a neurologic complication
Determining the right time for surgery is challenging in patients with infective endocarditis who have had neurologic complications, given the risk of hemorrhagic conversion of existing stroke with anticoagulation or exacerbation of cerebral ischemia in case of intraoperative hypotension. The decision should take into account the severity of cardiac decompensation, weighed against the severity of neurologic symptoms.
In general, surgery should be postponed for at least 4 weeks after intracerebral hemorrhage. However, it should be expedited in the event of silent cerebral embolism or transient ischemic attack, or in patients with infective endocarditis with stroke who have other indications for early surgery, as long as cerebral hemorrhage has been excluded by appropriate imaging.4
Early surgery for prosthetic valve endocarditis
The timing of surgery for prosthetic valve endocarditis follows the same general principles as for native valve endocarditis.2–4,12
One study showed that early surgery for prosthetic valve endocarditis was not associated with lower in-hospital and 1-year mortality rates compared with medical therapy.16 On the other hand, a subgroup analysis demonstrated surgery to be significantly beneficial in those with the strongest indications for surgery, including severe valve regurgitation, heart failure, paravalvular abscess, fistula, or prosthetic valve dehiscence.
The decision to proceed with surgery in prosthetic valve endocarditis should be weighed carefully, taking into consideration the patient’s overall clinical condition and estimated surgical risk.16
COLLABORATION IS HELPFUL
Early surgery is indicated for infective endocarditis patients presenting with:
- Refractory heart failure symptoms
- Persistent infection
- Large vegetations with a high risk of embolism.
Expeditious and successful treatment entails multidisciplinary collaboration among experts in cardiology and infectious diseases with access to cardiac surgery input early in the evaluation.
- Lalani T, Cabell CH, Benjamin DK, et al; International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators. Analysis of the impact of early surgery on in-hospital mortality of native valve endocarditis: use of propensity score and instrumental variable methods to adjust for treatment-selection bias. Circulation 2010; 121(8):1005–1013. doi:10.1161/CIRCULATIONAHA.109.864488
- AATS Surgical Treatment of Infective Endocarditis Consensus Guidelines Writing Committee Chairs; Pettersson GB, Coselli JS; Writing Committee, et al. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: surgical treatment of infective endocarditis: executive summary. J Thorac Cardiovasc Surg 2017; 153(6):1241–1258.e29. doi:10.1016/j.jtcvs.2016.09.093
- Nishimura RA, Otto CM, Bonow RO, et al; ACC/AHA Task Force Members. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(23):2440–2492. doi:10.1161/CIR.0000000000000029
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis. Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Prendergast BD, Tornos P. Surgery for infective endocarditis. Who and when? Circulation 2010; 121(9):1141–1152. doi:10.1161/CIRCULATIONAHA.108.773598
- Tornos P, Iung B, Permanyer-Miralda G, et al. Infective endocarditis in Europe: lessons from the Euro heart survey. Heart 2005; 91(5):571–575. doi:10.1136/hrt.2003.032128
- López J, Sevilla T, Vilacosta I, et al. Prognostic role of persistent positive blood cultures after initiation of antibiotic therapy in left-sided infective endocarditis. Eur Heart J 2013; 34(23):1749–1754. doi:10.1093/eurheartj/ehs379
- Graupner C, Vilacosta I, SanRoman J, et al. Periannular extension of infective endocarditis. J Am Coll Cardiol 2002; 39(7):1204–1211. doi:10.1016/S0735-1097(02)01747-3
- Shrestha NK, Ledtke CS, Wang H, et al. Heart valve culture and sequencing to identify the infective endocarditis pathogen in surgically treated patients. Ann Thorac Surg 2015; 99(1):33–37. doi:10.1016/j.athoracsur.2014.07.028
- Shapira N, Merin O, Rosenmann E, et al. Latent infective endocarditis: epidemiology and clinical characteristics of patients with unsuspected endocarditis detected after elective valve replacement. Ann Thorac Surg 2004; 78(5):1623–1629. doi:10.1016/j.athoracsur.2004.05.052
- Hecht SR, Berger M. Right-sided endocarditis in intravenous drug users. Prognostic features in 102 episodes. Ann Intern Med 1992; 117(7):560–566. doi:10.7326/0003-4819-117-7-560
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Shrestha NK, Jue J, Hussain ST, et al. Injection drug use and outcomes after surgical intervention for infective endocarditis. Ann Thorac Surg 2015; 100(3):875–882. doi:10.1016/j.athoracsur.2015.03.019
- Garcia-Cabrera E, Fernandez-Hidalgo N, Almirante B, et al. Neurological complications of infective endocarditis: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation 2013; 127(23):2272–2284. doi:10.1161/CIRCULATIONAHA.112.000813
- Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012; 366(26):2466–2473. doi:10.1056/NEJMoa1112843
- Lalani T, Chu VH, Park LP, et al; International Collaboration on Endocarditis–Prospective Cohort Study Investigators. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern Med 2013; 173(16):1495–1504. doi:10.1001/jamainternmed.2013.8203
WHAT IS ‘EARLY’ SURGERY?
More than 50% of patients with infective endocarditis undergo cardiac surgery during their initial presentation.1
The 2017 guidelines of the American Association for Thoracic Surgery (AATS) recommend surgery once a surgical indication has been established and effective antimicrobial therapy has been started.2
The American Heart Association/American College of Cardiology (ACC/AHA) guidelines recommend surgery during the initial hospitalization before completion of a full course of antibiotics.3
The European Society of Cardiology guidelines define surgery according to the time since the patient received intravenous antibiotic therapy: emergency surgery is performed within 24 hours of therapy, urgent surgery is performed within a few days, and elective surgery is performed after at least 1 to 2 weeks.4
These slight differences are due to the dearth of large randomized trials addressing this question.
INDICATIONS FOR EARLY SURGERY
Left ventricular dysfunction and heart failure
Of all the complications of infectious endocarditis, concomitant heart failure has the greatest impact on prognosis5 and is one of the most frequent indications for surgery.6
The guidelines recommend emergency surgery during the initial hospitalization for all patients with infective endocarditis who present with refractory pulmonary edema, worsening left ventricular dysfunction, or cardiogenic shock, regardless of whether they have completed a full course of antibiotics. This applies to both native valve endocarditis and prosthetic valve endocarditis.
Uncontrolled persistent infection
Persistent infection is defined as fever and positive cultures persisting after 1 week of appropriate antibiotic treatment.4 However, 1 week is a long time. Persistence of positive blood cultures more than 48 to 72 hours after starting antibiotic therapy is associated with poor outcome and is an independent predictor of in-hospital mortality.7
The ACC/AHA guidelines recommend early surgery in patients with left-sided infective endocarditis caused by fungi or highly resistant organisms such as vancomycin-resistant enterococci or multidrug-resistant gram-negative bacilli.3 Nonetheless, antibiotic resistance is an unusual reason for expediting surgery unless there are additional indications for it.
Extension of the infection beyond the valve annulus, which occurs in about 30% of cases of native valve endocarditis and 50% of cases of prosthetic valve endocarditis,8 is considered a more valid reason to expedite surgery. Similarly, urgent surgery should be considered if there is any evidence of locally uncontrolled infection causing perivalvular abscess, fistula, pseudoaneurysm, or conduction system abnormalities causing atrioventricular nodal block.2–4
Some authors suggest reviewing the surgical pathology and microbial sequencing of excised cardiac valves after surgery to confirm the diagnosis and identify the culprit pathogen.9,10
Right-sided infective endocarditis
Right-sided infective endocarditis has a more favorable prognosis than left-sided infective endocarditis and usually responds well to medical therapy.11
Nevertheless, surgery for right-sided infective endocarditis should be expedited in patients with right heart failure secondary to severe tricuspid regurgitation with poor response to medical therapy or in the case of large tricuspid valve vegetations.12 Likewise, recurrent septic pulmonary emboli can be encountered in the setting of right-sided infective endocarditis and are an indication for early surgery.4,12
Since many patients with right-sided infective endocarditis acquire the infection by intravenous drug use, there is often a reluctance to recommend surgery, given the risk of prosthetic valve infection if they continue to use intravenous drugs.4,12 One study showed that the risk of death or reoperation between 3 and 6 months after surgery for infective endocarditis was 10 times higher in intravenous drug users. Yet their survival after surgery beyond this period was similar to that of patients with endocarditis who did not inject drugs.13 Therefore, the AATS guidelines recommend applying normal indications for surgery to those patients, with emphasis on the need for strict follow-up aimed at addiction treatment.2
Prevention of embolic events
Neurologic embolic events are a frequent complication of infective endocarditis, with the highest risk during the first few days after antibiotics are started. However, this risk decreases significantly after 2 weeks.14
The timing of surgery largely depends on whether the patient has had previous neurologic embolic events and on the size and mobility of the vegetation. The current guidelines recommend early surgery for recurrent emboli and persistent or enlarging vegetations despite appropriate antibiotic therapy, or in case of large vegetations (> 10 mm) on a native valve even in the absence of embolic events.4
A randomized trial by Kang et al15 demonstrated that, compared with conventional care, early surgery (within 48 hours of diagnosis) in patients with native valve endocarditis with large vegetations (> 10 mm) and severe valve dysfunction was associated with a significant reduction in the risk of death and embolic events.
Timing of surgery after a neurologic complication
Determining the right time for surgery is challenging in patients with infective endocarditis who have had neurologic complications, given the risk of hemorrhagic conversion of existing stroke with anticoagulation or exacerbation of cerebral ischemia in case of intraoperative hypotension. The decision should take into account the severity of cardiac decompensation, weighed against the severity of neurologic symptoms.
In general, surgery should be postponed for at least 4 weeks after intracerebral hemorrhage. However, it should be expedited in the event of silent cerebral embolism or transient ischemic attack, or in patients with infective endocarditis with stroke who have other indications for early surgery, as long as cerebral hemorrhage has been excluded by appropriate imaging.4
Early surgery for prosthetic valve endocarditis
The timing of surgery for prosthetic valve endocarditis follows the same general principles as for native valve endocarditis.2–4,12
One study showed that early surgery for prosthetic valve endocarditis was not associated with lower in-hospital and 1-year mortality rates compared with medical therapy.16 On the other hand, a subgroup analysis demonstrated surgery to be significantly beneficial in those with the strongest indications for surgery, including severe valve regurgitation, heart failure, paravalvular abscess, fistula, or prosthetic valve dehiscence.
The decision to proceed with surgery in prosthetic valve endocarditis should be weighed carefully, taking into consideration the patient’s overall clinical condition and estimated surgical risk.16
COLLABORATION IS HELPFUL
Early surgery is indicated for infective endocarditis patients presenting with:
- Refractory heart failure symptoms
- Persistent infection
- Large vegetations with a high risk of embolism.
Expeditious and successful treatment entails multidisciplinary collaboration among experts in cardiology and infectious diseases with access to cardiac surgery input early in the evaluation.
WHAT IS ‘EARLY’ SURGERY?
More than 50% of patients with infective endocarditis undergo cardiac surgery during their initial presentation.1
The 2017 guidelines of the American Association for Thoracic Surgery (AATS) recommend surgery once a surgical indication has been established and effective antimicrobial therapy has been started.2
The American Heart Association/American College of Cardiology (ACC/AHA) guidelines recommend surgery during the initial hospitalization before completion of a full course of antibiotics.3
The European Society of Cardiology guidelines define surgery according to the time since the patient received intravenous antibiotic therapy: emergency surgery is performed within 24 hours of therapy, urgent surgery is performed within a few days, and elective surgery is performed after at least 1 to 2 weeks.4
These slight differences are due to the dearth of large randomized trials addressing this question.
INDICATIONS FOR EARLY SURGERY
Left ventricular dysfunction and heart failure
Of all the complications of infectious endocarditis, concomitant heart failure has the greatest impact on prognosis5 and is one of the most frequent indications for surgery.6
The guidelines recommend emergency surgery during the initial hospitalization for all patients with infective endocarditis who present with refractory pulmonary edema, worsening left ventricular dysfunction, or cardiogenic shock, regardless of whether they have completed a full course of antibiotics. This applies to both native valve endocarditis and prosthetic valve endocarditis.
Uncontrolled persistent infection
Persistent infection is defined as fever and positive cultures persisting after 1 week of appropriate antibiotic treatment.4 However, 1 week is a long time. Persistence of positive blood cultures more than 48 to 72 hours after starting antibiotic therapy is associated with poor outcome and is an independent predictor of in-hospital mortality.7
The ACC/AHA guidelines recommend early surgery in patients with left-sided infective endocarditis caused by fungi or highly resistant organisms such as vancomycin-resistant enterococci or multidrug-resistant gram-negative bacilli.3 Nonetheless, antibiotic resistance is an unusual reason for expediting surgery unless there are additional indications for it.
Extension of the infection beyond the valve annulus, which occurs in about 30% of cases of native valve endocarditis and 50% of cases of prosthetic valve endocarditis,8 is considered a more valid reason to expedite surgery. Similarly, urgent surgery should be considered if there is any evidence of locally uncontrolled infection causing perivalvular abscess, fistula, pseudoaneurysm, or conduction system abnormalities causing atrioventricular nodal block.2–4
Some authors suggest reviewing the surgical pathology and microbial sequencing of excised cardiac valves after surgery to confirm the diagnosis and identify the culprit pathogen.9,10
Right-sided infective endocarditis
Right-sided infective endocarditis has a more favorable prognosis than left-sided infective endocarditis and usually responds well to medical therapy.11
Nevertheless, surgery for right-sided infective endocarditis should be expedited in patients with right heart failure secondary to severe tricuspid regurgitation with poor response to medical therapy or in the case of large tricuspid valve vegetations.12 Likewise, recurrent septic pulmonary emboli can be encountered in the setting of right-sided infective endocarditis and are an indication for early surgery.4,12
Since many patients with right-sided infective endocarditis acquire the infection by intravenous drug use, there is often a reluctance to recommend surgery, given the risk of prosthetic valve infection if they continue to use intravenous drugs.4,12 One study showed that the risk of death or reoperation between 3 and 6 months after surgery for infective endocarditis was 10 times higher in intravenous drug users. Yet their survival after surgery beyond this period was similar to that of patients with endocarditis who did not inject drugs.13 Therefore, the AATS guidelines recommend applying normal indications for surgery to those patients, with emphasis on the need for strict follow-up aimed at addiction treatment.2
Prevention of embolic events
Neurologic embolic events are a frequent complication of infective endocarditis, with the highest risk during the first few days after antibiotics are started. However, this risk decreases significantly after 2 weeks.14
The timing of surgery largely depends on whether the patient has had previous neurologic embolic events and on the size and mobility of the vegetation. The current guidelines recommend early surgery for recurrent emboli and persistent or enlarging vegetations despite appropriate antibiotic therapy, or in case of large vegetations (> 10 mm) on a native valve even in the absence of embolic events.4
A randomized trial by Kang et al15 demonstrated that, compared with conventional care, early surgery (within 48 hours of diagnosis) in patients with native valve endocarditis with large vegetations (> 10 mm) and severe valve dysfunction was associated with a significant reduction in the risk of death and embolic events.
Timing of surgery after a neurologic complication
Determining the right time for surgery is challenging in patients with infective endocarditis who have had neurologic complications, given the risk of hemorrhagic conversion of existing stroke with anticoagulation or exacerbation of cerebral ischemia in case of intraoperative hypotension. The decision should take into account the severity of cardiac decompensation, weighed against the severity of neurologic symptoms.
In general, surgery should be postponed for at least 4 weeks after intracerebral hemorrhage. However, it should be expedited in the event of silent cerebral embolism or transient ischemic attack, or in patients with infective endocarditis with stroke who have other indications for early surgery, as long as cerebral hemorrhage has been excluded by appropriate imaging.4
Early surgery for prosthetic valve endocarditis
The timing of surgery for prosthetic valve endocarditis follows the same general principles as for native valve endocarditis.2–4,12
One study showed that early surgery for prosthetic valve endocarditis was not associated with lower in-hospital and 1-year mortality rates compared with medical therapy.16 On the other hand, a subgroup analysis demonstrated surgery to be significantly beneficial in those with the strongest indications for surgery, including severe valve regurgitation, heart failure, paravalvular abscess, fistula, or prosthetic valve dehiscence.
The decision to proceed with surgery in prosthetic valve endocarditis should be weighed carefully, taking into consideration the patient’s overall clinical condition and estimated surgical risk.16
COLLABORATION IS HELPFUL
Early surgery is indicated for infective endocarditis patients presenting with:
- Refractory heart failure symptoms
- Persistent infection
- Large vegetations with a high risk of embolism.
Expeditious and successful treatment entails multidisciplinary collaboration among experts in cardiology and infectious diseases with access to cardiac surgery input early in the evaluation.
- Lalani T, Cabell CH, Benjamin DK, et al; International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators. Analysis of the impact of early surgery on in-hospital mortality of native valve endocarditis: use of propensity score and instrumental variable methods to adjust for treatment-selection bias. Circulation 2010; 121(8):1005–1013. doi:10.1161/CIRCULATIONAHA.109.864488
- AATS Surgical Treatment of Infective Endocarditis Consensus Guidelines Writing Committee Chairs; Pettersson GB, Coselli JS; Writing Committee, et al. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: surgical treatment of infective endocarditis: executive summary. J Thorac Cardiovasc Surg 2017; 153(6):1241–1258.e29. doi:10.1016/j.jtcvs.2016.09.093
- Nishimura RA, Otto CM, Bonow RO, et al; ACC/AHA Task Force Members. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(23):2440–2492. doi:10.1161/CIR.0000000000000029
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis. Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Prendergast BD, Tornos P. Surgery for infective endocarditis. Who and when? Circulation 2010; 121(9):1141–1152. doi:10.1161/CIRCULATIONAHA.108.773598
- Tornos P, Iung B, Permanyer-Miralda G, et al. Infective endocarditis in Europe: lessons from the Euro heart survey. Heart 2005; 91(5):571–575. doi:10.1136/hrt.2003.032128
- López J, Sevilla T, Vilacosta I, et al. Prognostic role of persistent positive blood cultures after initiation of antibiotic therapy in left-sided infective endocarditis. Eur Heart J 2013; 34(23):1749–1754. doi:10.1093/eurheartj/ehs379
- Graupner C, Vilacosta I, SanRoman J, et al. Periannular extension of infective endocarditis. J Am Coll Cardiol 2002; 39(7):1204–1211. doi:10.1016/S0735-1097(02)01747-3
- Shrestha NK, Ledtke CS, Wang H, et al. Heart valve culture and sequencing to identify the infective endocarditis pathogen in surgically treated patients. Ann Thorac Surg 2015; 99(1):33–37. doi:10.1016/j.athoracsur.2014.07.028
- Shapira N, Merin O, Rosenmann E, et al. Latent infective endocarditis: epidemiology and clinical characteristics of patients with unsuspected endocarditis detected after elective valve replacement. Ann Thorac Surg 2004; 78(5):1623–1629. doi:10.1016/j.athoracsur.2004.05.052
- Hecht SR, Berger M. Right-sided endocarditis in intravenous drug users. Prognostic features in 102 episodes. Ann Intern Med 1992; 117(7):560–566. doi:10.7326/0003-4819-117-7-560
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Shrestha NK, Jue J, Hussain ST, et al. Injection drug use and outcomes after surgical intervention for infective endocarditis. Ann Thorac Surg 2015; 100(3):875–882. doi:10.1016/j.athoracsur.2015.03.019
- Garcia-Cabrera E, Fernandez-Hidalgo N, Almirante B, et al. Neurological complications of infective endocarditis: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation 2013; 127(23):2272–2284. doi:10.1161/CIRCULATIONAHA.112.000813
- Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012; 366(26):2466–2473. doi:10.1056/NEJMoa1112843
- Lalani T, Chu VH, Park LP, et al; International Collaboration on Endocarditis–Prospective Cohort Study Investigators. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern Med 2013; 173(16):1495–1504. doi:10.1001/jamainternmed.2013.8203
- Lalani T, Cabell CH, Benjamin DK, et al; International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators. Analysis of the impact of early surgery on in-hospital mortality of native valve endocarditis: use of propensity score and instrumental variable methods to adjust for treatment-selection bias. Circulation 2010; 121(8):1005–1013. doi:10.1161/CIRCULATIONAHA.109.864488
- AATS Surgical Treatment of Infective Endocarditis Consensus Guidelines Writing Committee Chairs; Pettersson GB, Coselli JS; Writing Committee, et al. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: surgical treatment of infective endocarditis: executive summary. J Thorac Cardiovasc Surg 2017; 153(6):1241–1258.e29. doi:10.1016/j.jtcvs.2016.09.093
- Nishimura RA, Otto CM, Bonow RO, et al; ACC/AHA Task Force Members. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(23):2440–2492. doi:10.1161/CIR.0000000000000029
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis. Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Prendergast BD, Tornos P. Surgery for infective endocarditis. Who and when? Circulation 2010; 121(9):1141–1152. doi:10.1161/CIRCULATIONAHA.108.773598
- Tornos P, Iung B, Permanyer-Miralda G, et al. Infective endocarditis in Europe: lessons from the Euro heart survey. Heart 2005; 91(5):571–575. doi:10.1136/hrt.2003.032128
- López J, Sevilla T, Vilacosta I, et al. Prognostic role of persistent positive blood cultures after initiation of antibiotic therapy in left-sided infective endocarditis. Eur Heart J 2013; 34(23):1749–1754. doi:10.1093/eurheartj/ehs379
- Graupner C, Vilacosta I, SanRoman J, et al. Periannular extension of infective endocarditis. J Am Coll Cardiol 2002; 39(7):1204–1211. doi:10.1016/S0735-1097(02)01747-3
- Shrestha NK, Ledtke CS, Wang H, et al. Heart valve culture and sequencing to identify the infective endocarditis pathogen in surgically treated patients. Ann Thorac Surg 2015; 99(1):33–37. doi:10.1016/j.athoracsur.2014.07.028
- Shapira N, Merin O, Rosenmann E, et al. Latent infective endocarditis: epidemiology and clinical characteristics of patients with unsuspected endocarditis detected after elective valve replacement. Ann Thorac Surg 2004; 78(5):1623–1629. doi:10.1016/j.athoracsur.2004.05.052
- Hecht SR, Berger M. Right-sided endocarditis in intravenous drug users. Prognostic features in 102 episodes. Ann Intern Med 1992; 117(7):560–566. doi:10.7326/0003-4819-117-7-560
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Shrestha NK, Jue J, Hussain ST, et al. Injection drug use and outcomes after surgical intervention for infective endocarditis. Ann Thorac Surg 2015; 100(3):875–882. doi:10.1016/j.athoracsur.2015.03.019
- Garcia-Cabrera E, Fernandez-Hidalgo N, Almirante B, et al. Neurological complications of infective endocarditis: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation 2013; 127(23):2272–2284. doi:10.1161/CIRCULATIONAHA.112.000813
- Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012; 366(26):2466–2473. doi:10.1056/NEJMoa1112843
- Lalani T, Chu VH, Park LP, et al; International Collaboration on Endocarditis–Prospective Cohort Study Investigators. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern Med 2013; 173(16):1495–1504. doi:10.1001/jamainternmed.2013.8203