User login
CASE Sexually active woman asks about the HPV vaccine
A 26-year-old woman delivered her first child 4 weeks ago. She has had 3 lifetime sexual partners and is now in a mutually faithful monogamous relationship with her partner. She has no known history of sexually transmissible infections. She received only one Pap test 3 years ago, and the cytology showed no abnormal cells. This cervical specimen was not tested for human papillomavirus (HPV) DNA. At the time of her postpartum appointment, she inquires whether she is a candidate for the HPV vaccine.
What should be your response?
Genital HPV infection is the most common sexually transmissible infection in the United States. This virus is the cause of multiple genital malignancies, including cancers of the vagina, vulva, penis, anus, and cervix. The organism is also now the major cause of oropharyngeal cancer.
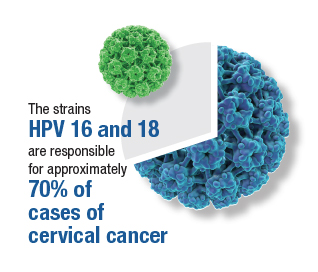
Of the more than 200 different HPV types that have been identified, 12 have been defined as oncogenic (high risk), and 8 to 12 types have been defined as possibly or probably oncogenic. The HPV strain with the highest risk of progression to cancer is HPV 16. The strains HPV 16 and 18 are responsible for approximately 70% of cases of cervical cancer. Each year in the United States, approximately 11,500 new cases of invasive cervical cancer occur. Unfortunately, this malignancy is responsible for about 4,000 deaths annually. Worldwide, HPV causes approximately 690,000 cancers each year.1
To a large extent, most cases of HPV infection would be preventable if patients were to take advantage of the remarkably effective HPV vaccine that is now available. However, acceptance of the vaccine has been disappointing. In 2020, only about half of adolescents, age 13 to 15, had received the appropriate number of vaccine doses.1
As ObGyn physicians, we can take several measures, in concert with our pediatrician colleagues, to improve HPV vaccination rates. In this article, I review the development of the HPV vaccine and describe the components, indications, dosing schedules, contraindications, adverse effects, and cost of the vaccine.
HPV vaccine development and expansion
The first HPV vaccine introduced in the United States was the recombinant quadrivalent vaccine (Gardasil; Merck); it was approved by the US Food and Drug Administration (FDA) in 2006. This vaccine is composed of viral-like particles unique to HPV 16 and 18 (the 2 most common causes of cervical, penile, anal, and oropharyngeal cancer) and HPV 6 and 11 (the 2 most common causes of genital warts). The formulation is prepared in baker’s yeast, and it elicits a robust production of neutralizing antibodies.2
In 2009, the FDA approved the bivalent vaccine (Cervarix; GlaxoSmithKline Biologicals). This vaccine contains viral-like particles unique to HPV 16 and 18, and it also induces a robust immune response. The vaccine is prepared in insect viral vectors.2
Both the quadrivalent and bivalent vaccines are no longer available in the United States. The only HPV vaccine currently marketed is the recombinant 9-valent vaccine (Gardasil 9; Merck), which was approved by the FDA in 2014. This newer vaccine targets the original 4 viral HPV strains in the quadrivalent vaccine (16, 18, 6, 11) plus 5 additional oncogenic strains: 31, 33, 45, 52, 58.2-4 The HPV strains targeted by this vaccine are responsible for approximately 90% of all cancers caused by HPV.
The 9-valent HPV vaccine, like the other 2, is highly effective in preventing cancers of the cervix, vagina, vulva, anus, penis; oropharyngeal cancers; and precancerous lesions such as genital warts.2-5 It will not, however, prevent the progression of preexisting infection or clear an infection that is already present at the time of vaccination.1
Although the original protocol for administration of the vaccine provided for 3 doses, recent studies indicate that 2 doses may be as effective as 3 in eliciting a favorable antibody response.6 There also is evidence that even a single dose of the vaccine can elicit a protective immune response.7 This encouraging finding is particularly important to public health officials responsible for developing HPV vaccination programs in low- and middle-resource countries.
Continue to: Target groups for the HPV vaccine...
Target groups for the HPV vaccine
The primary target group for the HPV vaccine is girls and boys who are aged 11 to 12 years. The key strategy is to immunize these individuals before they become sexually active. The vaccine also should be offered to children who are aged 9 to 10 years of age if they are judged to be at unusual risk, such as because of concern about sexual molestation. Children in these 2 age groups should receive 2 doses of the vaccine, with the second dose administered 6 to 12 months after the first dose.
The second target group for vaccination is individuals who are aged 13 to 26 years who have never been vaccinated. They should be offered catch-up vaccination. If older than age 15, they should receive 3 doses of the vaccine, with the second dose administered 1 to 2 months after the first dose and the third dose administered 6 months after the first dose.1
A third target group is individuals who are aged 27 to 45 years and who, in their own opinion or in the opinion of their physician, are at new or increased risk for HPV infection. These individuals should receive the 3-dose vaccine series as outlined above.1
Patients in any age range who are immunocompromised, for example, due to HIV infection, should receive the 3-dose series.1
The approximate retail cost of a single 0.5-mL intramuscular dose of the 9-valent vaccine is $240 (www.goodrx.com).
Vaccine adverse effects
The most common reactions to the HPV vaccine are inflammation at the site of injection, fatigue, headache, fever, gastrointestinal upset, vertigo, cough, and oropharyngeal discomfort. The most serious reaction—which fortunately is very rare—is anaphylaxis.1
Contraindications to the vaccine
The HPV vaccine should not be used in any patient who is hypersensitive to any component of the vaccine, including yeast. It should not be given to a patient who is moderately or severely ill at the time of the scheduled administration. Because of an abundance of caution, the manufacturer also recommends that the vaccine not be given to pregnant women even though the agent does not contain live virus.1
Of note, a study by Scheller and colleagues was very reassuring about the lack of adverse effects of HPV vaccine administration in pregnancy.8 The authors evaluated a large cohort of pregnant women in Demark and found that exposure to the vaccine was not associated with an increase in the frequency of major birth defects, spontaneous abortion, preterm delivery, low birthweight, fetal growth restriction, or stillbirth.8
Barriers to vaccination
One important barrier to HPV vaccination is patient apprehension that the vaccine may cause genital tract or oropharyngeal cancer. The patient should be reassured that the vaccine does not contain infectious viral particles and does not transmit infection. Rather, it builds robust immunity to infection.
Another important barrier is the misconception that the vaccine will promote sexual promiscuity in preteenagers and teenagers. Absolutely no evidence supports this belief. Multiple studies have demonstrated that teenagers do not engage in more high-risk sexual behavior following vaccination.
A specific barrier related to vaccination of young boys is the philosophical viewpoint that, “Why should my young male child be vaccinated to protect against a disease (specifically cervical cancer) that occurs only in girls and women?” The appropriate answer to this question is that the vaccine also protects against penile cancer, anal cancer, oropharyngeal cancer, and genital warts. While penile and anal cancers are rare, the other 2 conditions are not. In fact, oropharyngeal cancer is significantly more common in males than females.
A final important barrier to HPV vaccination is cost. The new evidence that demonstrated the effectiveness of a 2-dose vaccine series, and even single-dose vaccination, is of great importance in minimizing cost of the HPV vaccine series, in the absence of full reimbursement by public and private insurance agencies.
Continue to: Creating an effective vaccination program...
Creating an effective vaccination program
The following commonsense guidelines, which we have implemented at our medical center, should be helpful in organizing an effective HPV vaccination program for your office or department4,9,10:
- One clinician in the department or practice should be designated the “vaccination champion.” This individual should provide colleagues with periodic updates, emphasizing the importance of the HPV vaccine and other vaccines, such as Tdap (tetanus, diphtheria, pertussis), influenza, COVID, pneumococcal, hepatitis B, herpes zoster (shingles), and RSV (respiratory syncytial virus).
- One staff member in the practice or department should be designated as the go-to person for all logistical matters related to vaccines. This individual should be responsible for estimating usage, ordering vaccines, and storing them properly. He or she also should be knowledgeable about the cost of the vaccines and insurance reimbursement for the vaccines.
- Signs and educational materials should be posted in strategic locations in the office, advising patients of the importance of timely vaccination for themselves and their adolescent children.
- At every encounter, patients should be encouraged to receive the HPV vaccine series if they are in the appropriate age range and social situation for vaccination. They should not be required to have HPV testing before vaccine administration.
- Key leaders in the department or practice should lobby effectively with their pediatrician colleagues and with public and private insurance companies to encourage timely administration and proper coverage of this important immunization.
Other measures to reduce the risk of HPV-mediated malignancies
Practitioners should advise their patients to:
- Be circumspect in selection of sexual partners.
- Use male or female condoms when engaging in vaginal, anal, and/or oral sex with multiple partners, particularly those who may have genital or oral condylomas.
- Have regular Pap tests, every 3 to 5 years, depending upon age. More frequent testing may be indicated if there is a history of previous abnormal testing.
- Seek prompt medical or surgical treatment for genital or oral condylomas.
CASE Resolved with HPV vaccination
This patient is an excellent candidate for catch-up vaccination. She should receive the first dose of the 9-valent HPV vaccine at the time of her postpartum appointment. The second dose should be administered 1 to 2 months later. The third dose should be administered 6 months after the first dose. She also should have a Pap test, either cytology alone or cytology plus HPV screening. If the latter test is chosen and is reassuring, she will not need retesting for 5 years. If the former test is chosen, she should have a repeat test in 3 years. ●

- The overwhelming majority of precancerous lesions and overt malignancies of the genital tract and oropharynx are caused by oncogenic strains of HPV.
- Most of these cancers could be prevented if patients were vaccinated with the 9-valent HPV vaccine.
- The HPV vaccine should be offered to all children beginning at age 11 and to selected high-risk children at age 9. For children aged 14 years and younger, 2 doses of the vaccine are sufficient to induce a robust immune response. The second dose should be administered 6 to 12 months after the first dose.
- Individuals in the age range 13 to 26 years should be offered catch-up vaccination if they have not been previously vaccinated.
- Persons in the age range 27 to 45 years also should be offered vaccination if they have developed a new high-risk profile.
- Persons older than age 15, or those of any age with immunocompromising conditions, should receive 3 doses of the vaccine. The second dose should be administered 1 to 2 months after the first dose, and the third dose should be given 6 months after the first dose.
- The vaccine does not prevent the progression of preexisting infection or clear an infection that is already present at the time of vaccination.
- As a general rule, the vaccine should be deferred during pregnancy, although no adverse effects have been documented when the vaccine has been administered to pregnant women.
- Markowitz LE, Unger ER. Human papilloma virus vaccination. N Engl J Med. 2023;388:1790-1798.
- Schiller JT, Castellsague X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30(suppl 5): F123-F138.
- Lei J, Ploner A, Elfstrom KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383: 1340-1348.
- ACOG Committee Opinion Summary No. 809. Human papillomavirus vaccination. Obstet Gynecol. 2020;136:435-436.
- Barbieri RL. 9vHPV vaccine: prevention of oropharyngeal cancer. OBG Manag. 2020;32:9, 14-15.
- Iversen OE, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316:2411-2421.
- Watson-Jones D, Changalucha J, Whitworth H, et al. Immunogenicity and safety of one-dose human papillomavirus vaccine compared with two or three doses in Tanzanian girls (DoRIS): an open-label, randomised noninferiority trial. Lancet Glob Health. 2022;10:e1473-e1484.
- Scheller NM, Pasternak B, Molgaard-Nielsen D, et al. Quadrivalent HPV vaccination and the risk of adverse pregnancy outcomes. N Engl J Med. 2017;376:1223-1233.
- ACOG Committee Opinion Summary No. 641. Human papillomavirus vaccination. Obstet Gynecol. 2015;126:693.
- Boitano TKL, Ketch PW, Scarinci IC, et al. An update on human papillomavirus vaccination in the United States. Obstet Gynecol. 2023;141:324-330.
CASE Sexually active woman asks about the HPV vaccine
A 26-year-old woman delivered her first child 4 weeks ago. She has had 3 lifetime sexual partners and is now in a mutually faithful monogamous relationship with her partner. She has no known history of sexually transmissible infections. She received only one Pap test 3 years ago, and the cytology showed no abnormal cells. This cervical specimen was not tested for human papillomavirus (HPV) DNA. At the time of her postpartum appointment, she inquires whether she is a candidate for the HPV vaccine.
What should be your response?
Genital HPV infection is the most common sexually transmissible infection in the United States. This virus is the cause of multiple genital malignancies, including cancers of the vagina, vulva, penis, anus, and cervix. The organism is also now the major cause of oropharyngeal cancer.
Of the more than 200 different HPV types that have been identified, 12 have been defined as oncogenic (high risk), and 8 to 12 types have been defined as possibly or probably oncogenic. The HPV strain with the highest risk of progression to cancer is HPV 16. The strains HPV 16 and 18 are responsible for approximately 70% of cases of cervical cancer. Each year in the United States, approximately 11,500 new cases of invasive cervical cancer occur. Unfortunately, this malignancy is responsible for about 4,000 deaths annually. Worldwide, HPV causes approximately 690,000 cancers each year.1
To a large extent, most cases of HPV infection would be preventable if patients were to take advantage of the remarkably effective HPV vaccine that is now available. However, acceptance of the vaccine has been disappointing. In 2020, only about half of adolescents, age 13 to 15, had received the appropriate number of vaccine doses.1
As ObGyn physicians, we can take several measures, in concert with our pediatrician colleagues, to improve HPV vaccination rates. In this article, I review the development of the HPV vaccine and describe the components, indications, dosing schedules, contraindications, adverse effects, and cost of the vaccine.
HPV vaccine development and expansion
The first HPV vaccine introduced in the United States was the recombinant quadrivalent vaccine (Gardasil; Merck); it was approved by the US Food and Drug Administration (FDA) in 2006. This vaccine is composed of viral-like particles unique to HPV 16 and 18 (the 2 most common causes of cervical, penile, anal, and oropharyngeal cancer) and HPV 6 and 11 (the 2 most common causes of genital warts). The formulation is prepared in baker’s yeast, and it elicits a robust production of neutralizing antibodies.2
In 2009, the FDA approved the bivalent vaccine (Cervarix; GlaxoSmithKline Biologicals). This vaccine contains viral-like particles unique to HPV 16 and 18, and it also induces a robust immune response. The vaccine is prepared in insect viral vectors.2
Both the quadrivalent and bivalent vaccines are no longer available in the United States. The only HPV vaccine currently marketed is the recombinant 9-valent vaccine (Gardasil 9; Merck), which was approved by the FDA in 2014. This newer vaccine targets the original 4 viral HPV strains in the quadrivalent vaccine (16, 18, 6, 11) plus 5 additional oncogenic strains: 31, 33, 45, 52, 58.2-4 The HPV strains targeted by this vaccine are responsible for approximately 90% of all cancers caused by HPV.
The 9-valent HPV vaccine, like the other 2, is highly effective in preventing cancers of the cervix, vagina, vulva, anus, penis; oropharyngeal cancers; and precancerous lesions such as genital warts.2-5 It will not, however, prevent the progression of preexisting infection or clear an infection that is already present at the time of vaccination.1
Although the original protocol for administration of the vaccine provided for 3 doses, recent studies indicate that 2 doses may be as effective as 3 in eliciting a favorable antibody response.6 There also is evidence that even a single dose of the vaccine can elicit a protective immune response.7 This encouraging finding is particularly important to public health officials responsible for developing HPV vaccination programs in low- and middle-resource countries.

Continue to: Target groups for the HPV vaccine...
Target groups for the HPV vaccine
The primary target group for the HPV vaccine is girls and boys who are aged 11 to 12 years. The key strategy is to immunize these individuals before they become sexually active. The vaccine also should be offered to children who are aged 9 to 10 years of age if they are judged to be at unusual risk, such as because of concern about sexual molestation. Children in these 2 age groups should receive 2 doses of the vaccine, with the second dose administered 6 to 12 months after the first dose.
The second target group for vaccination is individuals who are aged 13 to 26 years who have never been vaccinated. They should be offered catch-up vaccination. If older than age 15, they should receive 3 doses of the vaccine, with the second dose administered 1 to 2 months after the first dose and the third dose administered 6 months after the first dose.1
A third target group is individuals who are aged 27 to 45 years and who, in their own opinion or in the opinion of their physician, are at new or increased risk for HPV infection. These individuals should receive the 3-dose vaccine series as outlined above.1
Patients in any age range who are immunocompromised, for example, due to HIV infection, should receive the 3-dose series.1
The approximate retail cost of a single 0.5-mL intramuscular dose of the 9-valent vaccine is $240 (www.goodrx.com).
Vaccine adverse effects
The most common reactions to the HPV vaccine are inflammation at the site of injection, fatigue, headache, fever, gastrointestinal upset, vertigo, cough, and oropharyngeal discomfort. The most serious reaction—which fortunately is very rare—is anaphylaxis.1
Contraindications to the vaccine
The HPV vaccine should not be used in any patient who is hypersensitive to any component of the vaccine, including yeast. It should not be given to a patient who is moderately or severely ill at the time of the scheduled administration. Because of an abundance of caution, the manufacturer also recommends that the vaccine not be given to pregnant women even though the agent does not contain live virus.1
Of note, a study by Scheller and colleagues was very reassuring about the lack of adverse effects of HPV vaccine administration in pregnancy.8 The authors evaluated a large cohort of pregnant women in Demark and found that exposure to the vaccine was not associated with an increase in the frequency of major birth defects, spontaneous abortion, preterm delivery, low birthweight, fetal growth restriction, or stillbirth.8
Barriers to vaccination
One important barrier to HPV vaccination is patient apprehension that the vaccine may cause genital tract or oropharyngeal cancer. The patient should be reassured that the vaccine does not contain infectious viral particles and does not transmit infection. Rather, it builds robust immunity to infection.
Another important barrier is the misconception that the vaccine will promote sexual promiscuity in preteenagers and teenagers. Absolutely no evidence supports this belief. Multiple studies have demonstrated that teenagers do not engage in more high-risk sexual behavior following vaccination.
A specific barrier related to vaccination of young boys is the philosophical viewpoint that, “Why should my young male child be vaccinated to protect against a disease (specifically cervical cancer) that occurs only in girls and women?” The appropriate answer to this question is that the vaccine also protects against penile cancer, anal cancer, oropharyngeal cancer, and genital warts. While penile and anal cancers are rare, the other 2 conditions are not. In fact, oropharyngeal cancer is significantly more common in males than females.
A final important barrier to HPV vaccination is cost. The new evidence that demonstrated the effectiveness of a 2-dose vaccine series, and even single-dose vaccination, is of great importance in minimizing cost of the HPV vaccine series, in the absence of full reimbursement by public and private insurance agencies.
Continue to: Creating an effective vaccination program...
Creating an effective vaccination program
The following commonsense guidelines, which we have implemented at our medical center, should be helpful in organizing an effective HPV vaccination program for your office or department4,9,10:
- One clinician in the department or practice should be designated the “vaccination champion.” This individual should provide colleagues with periodic updates, emphasizing the importance of the HPV vaccine and other vaccines, such as Tdap (tetanus, diphtheria, pertussis), influenza, COVID, pneumococcal, hepatitis B, herpes zoster (shingles), and RSV (respiratory syncytial virus).
- One staff member in the practice or department should be designated as the go-to person for all logistical matters related to vaccines. This individual should be responsible for estimating usage, ordering vaccines, and storing them properly. He or she also should be knowledgeable about the cost of the vaccines and insurance reimbursement for the vaccines.
- Signs and educational materials should be posted in strategic locations in the office, advising patients of the importance of timely vaccination for themselves and their adolescent children.
- At every encounter, patients should be encouraged to receive the HPV vaccine series if they are in the appropriate age range and social situation for vaccination. They should not be required to have HPV testing before vaccine administration.
- Key leaders in the department or practice should lobby effectively with their pediatrician colleagues and with public and private insurance companies to encourage timely administration and proper coverage of this important immunization.
Other measures to reduce the risk of HPV-mediated malignancies
Practitioners should advise their patients to:
- Be circumspect in selection of sexual partners.
- Use male or female condoms when engaging in vaginal, anal, and/or oral sex with multiple partners, particularly those who may have genital or oral condylomas.
- Have regular Pap tests, every 3 to 5 years, depending upon age. More frequent testing may be indicated if there is a history of previous abnormal testing.
- Seek prompt medical or surgical treatment for genital or oral condylomas.
CASE Resolved with HPV vaccination
This patient is an excellent candidate for catch-up vaccination. She should receive the first dose of the 9-valent HPV vaccine at the time of her postpartum appointment. The second dose should be administered 1 to 2 months later. The third dose should be administered 6 months after the first dose. She also should have a Pap test, either cytology alone or cytology plus HPV screening. If the latter test is chosen and is reassuring, she will not need retesting for 5 years. If the former test is chosen, she should have a repeat test in 3 years. ●

- The overwhelming majority of precancerous lesions and overt malignancies of the genital tract and oropharynx are caused by oncogenic strains of HPV.
- Most of these cancers could be prevented if patients were vaccinated with the 9-valent HPV vaccine.
- The HPV vaccine should be offered to all children beginning at age 11 and to selected high-risk children at age 9. For children aged 14 years and younger, 2 doses of the vaccine are sufficient to induce a robust immune response. The second dose should be administered 6 to 12 months after the first dose.
- Individuals in the age range 13 to 26 years should be offered catch-up vaccination if they have not been previously vaccinated.
- Persons in the age range 27 to 45 years also should be offered vaccination if they have developed a new high-risk profile.
- Persons older than age 15, or those of any age with immunocompromising conditions, should receive 3 doses of the vaccine. The second dose should be administered 1 to 2 months after the first dose, and the third dose should be given 6 months after the first dose.
- The vaccine does not prevent the progression of preexisting infection or clear an infection that is already present at the time of vaccination.
- As a general rule, the vaccine should be deferred during pregnancy, although no adverse effects have been documented when the vaccine has been administered to pregnant women.
CASE Sexually active woman asks about the HPV vaccine
A 26-year-old woman delivered her first child 4 weeks ago. She has had 3 lifetime sexual partners and is now in a mutually faithful monogamous relationship with her partner. She has no known history of sexually transmissible infections. She received only one Pap test 3 years ago, and the cytology showed no abnormal cells. This cervical specimen was not tested for human papillomavirus (HPV) DNA. At the time of her postpartum appointment, she inquires whether she is a candidate for the HPV vaccine.
What should be your response?
Genital HPV infection is the most common sexually transmissible infection in the United States. This virus is the cause of multiple genital malignancies, including cancers of the vagina, vulva, penis, anus, and cervix. The organism is also now the major cause of oropharyngeal cancer.
Of the more than 200 different HPV types that have been identified, 12 have been defined as oncogenic (high risk), and 8 to 12 types have been defined as possibly or probably oncogenic. The HPV strain with the highest risk of progression to cancer is HPV 16. The strains HPV 16 and 18 are responsible for approximately 70% of cases of cervical cancer. Each year in the United States, approximately 11,500 new cases of invasive cervical cancer occur. Unfortunately, this malignancy is responsible for about 4,000 deaths annually. Worldwide, HPV causes approximately 690,000 cancers each year.1
To a large extent, most cases of HPV infection would be preventable if patients were to take advantage of the remarkably effective HPV vaccine that is now available. However, acceptance of the vaccine has been disappointing. In 2020, only about half of adolescents, age 13 to 15, had received the appropriate number of vaccine doses.1
As ObGyn physicians, we can take several measures, in concert with our pediatrician colleagues, to improve HPV vaccination rates. In this article, I review the development of the HPV vaccine and describe the components, indications, dosing schedules, contraindications, adverse effects, and cost of the vaccine.
HPV vaccine development and expansion
The first HPV vaccine introduced in the United States was the recombinant quadrivalent vaccine (Gardasil; Merck); it was approved by the US Food and Drug Administration (FDA) in 2006. This vaccine is composed of viral-like particles unique to HPV 16 and 18 (the 2 most common causes of cervical, penile, anal, and oropharyngeal cancer) and HPV 6 and 11 (the 2 most common causes of genital warts). The formulation is prepared in baker’s yeast, and it elicits a robust production of neutralizing antibodies.2
In 2009, the FDA approved the bivalent vaccine (Cervarix; GlaxoSmithKline Biologicals). This vaccine contains viral-like particles unique to HPV 16 and 18, and it also induces a robust immune response. The vaccine is prepared in insect viral vectors.2
Both the quadrivalent and bivalent vaccines are no longer available in the United States. The only HPV vaccine currently marketed is the recombinant 9-valent vaccine (Gardasil 9; Merck), which was approved by the FDA in 2014. This newer vaccine targets the original 4 viral HPV strains in the quadrivalent vaccine (16, 18, 6, 11) plus 5 additional oncogenic strains: 31, 33, 45, 52, 58.2-4 The HPV strains targeted by this vaccine are responsible for approximately 90% of all cancers caused by HPV.
The 9-valent HPV vaccine, like the other 2, is highly effective in preventing cancers of the cervix, vagina, vulva, anus, penis; oropharyngeal cancers; and precancerous lesions such as genital warts.2-5 It will not, however, prevent the progression of preexisting infection or clear an infection that is already present at the time of vaccination.1
Although the original protocol for administration of the vaccine provided for 3 doses, recent studies indicate that 2 doses may be as effective as 3 in eliciting a favorable antibody response.6 There also is evidence that even a single dose of the vaccine can elicit a protective immune response.7 This encouraging finding is particularly important to public health officials responsible for developing HPV vaccination programs in low- and middle-resource countries.

Continue to: Target groups for the HPV vaccine...
Target groups for the HPV vaccine
The primary target group for the HPV vaccine is girls and boys who are aged 11 to 12 years. The key strategy is to immunize these individuals before they become sexually active. The vaccine also should be offered to children who are aged 9 to 10 years of age if they are judged to be at unusual risk, such as because of concern about sexual molestation. Children in these 2 age groups should receive 2 doses of the vaccine, with the second dose administered 6 to 12 months after the first dose.
The second target group for vaccination is individuals who are aged 13 to 26 years who have never been vaccinated. They should be offered catch-up vaccination. If older than age 15, they should receive 3 doses of the vaccine, with the second dose administered 1 to 2 months after the first dose and the third dose administered 6 months after the first dose.1
A third target group is individuals who are aged 27 to 45 years and who, in their own opinion or in the opinion of their physician, are at new or increased risk for HPV infection. These individuals should receive the 3-dose vaccine series as outlined above.1
Patients in any age range who are immunocompromised, for example, due to HIV infection, should receive the 3-dose series.1
The approximate retail cost of a single 0.5-mL intramuscular dose of the 9-valent vaccine is $240 (www.goodrx.com).
Vaccine adverse effects
The most common reactions to the HPV vaccine are inflammation at the site of injection, fatigue, headache, fever, gastrointestinal upset, vertigo, cough, and oropharyngeal discomfort. The most serious reaction—which fortunately is very rare—is anaphylaxis.1
Contraindications to the vaccine
The HPV vaccine should not be used in any patient who is hypersensitive to any component of the vaccine, including yeast. It should not be given to a patient who is moderately or severely ill at the time of the scheduled administration. Because of an abundance of caution, the manufacturer also recommends that the vaccine not be given to pregnant women even though the agent does not contain live virus.1
Of note, a study by Scheller and colleagues was very reassuring about the lack of adverse effects of HPV vaccine administration in pregnancy.8 The authors evaluated a large cohort of pregnant women in Demark and found that exposure to the vaccine was not associated with an increase in the frequency of major birth defects, spontaneous abortion, preterm delivery, low birthweight, fetal growth restriction, or stillbirth.8
Barriers to vaccination
One important barrier to HPV vaccination is patient apprehension that the vaccine may cause genital tract or oropharyngeal cancer. The patient should be reassured that the vaccine does not contain infectious viral particles and does not transmit infection. Rather, it builds robust immunity to infection.
Another important barrier is the misconception that the vaccine will promote sexual promiscuity in preteenagers and teenagers. Absolutely no evidence supports this belief. Multiple studies have demonstrated that teenagers do not engage in more high-risk sexual behavior following vaccination.
A specific barrier related to vaccination of young boys is the philosophical viewpoint that, “Why should my young male child be vaccinated to protect against a disease (specifically cervical cancer) that occurs only in girls and women?” The appropriate answer to this question is that the vaccine also protects against penile cancer, anal cancer, oropharyngeal cancer, and genital warts. While penile and anal cancers are rare, the other 2 conditions are not. In fact, oropharyngeal cancer is significantly more common in males than females.
A final important barrier to HPV vaccination is cost. The new evidence that demonstrated the effectiveness of a 2-dose vaccine series, and even single-dose vaccination, is of great importance in minimizing cost of the HPV vaccine series, in the absence of full reimbursement by public and private insurance agencies.
Continue to: Creating an effective vaccination program...
Creating an effective vaccination program
The following commonsense guidelines, which we have implemented at our medical center, should be helpful in organizing an effective HPV vaccination program for your office or department4,9,10:
- One clinician in the department or practice should be designated the “vaccination champion.” This individual should provide colleagues with periodic updates, emphasizing the importance of the HPV vaccine and other vaccines, such as Tdap (tetanus, diphtheria, pertussis), influenza, COVID, pneumococcal, hepatitis B, herpes zoster (shingles), and RSV (respiratory syncytial virus).
- One staff member in the practice or department should be designated as the go-to person for all logistical matters related to vaccines. This individual should be responsible for estimating usage, ordering vaccines, and storing them properly. He or she also should be knowledgeable about the cost of the vaccines and insurance reimbursement for the vaccines.
- Signs and educational materials should be posted in strategic locations in the office, advising patients of the importance of timely vaccination for themselves and their adolescent children.
- At every encounter, patients should be encouraged to receive the HPV vaccine series if they are in the appropriate age range and social situation for vaccination. They should not be required to have HPV testing before vaccine administration.
- Key leaders in the department or practice should lobby effectively with their pediatrician colleagues and with public and private insurance companies to encourage timely administration and proper coverage of this important immunization.
Other measures to reduce the risk of HPV-mediated malignancies
Practitioners should advise their patients to:
- Be circumspect in selection of sexual partners.
- Use male or female condoms when engaging in vaginal, anal, and/or oral sex with multiple partners, particularly those who may have genital or oral condylomas.
- Have regular Pap tests, every 3 to 5 years, depending upon age. More frequent testing may be indicated if there is a history of previous abnormal testing.
- Seek prompt medical or surgical treatment for genital or oral condylomas.
CASE Resolved with HPV vaccination
This patient is an excellent candidate for catch-up vaccination. She should receive the first dose of the 9-valent HPV vaccine at the time of her postpartum appointment. The second dose should be administered 1 to 2 months later. The third dose should be administered 6 months after the first dose. She also should have a Pap test, either cytology alone or cytology plus HPV screening. If the latter test is chosen and is reassuring, she will not need retesting for 5 years. If the former test is chosen, she should have a repeat test in 3 years. ●

- The overwhelming majority of precancerous lesions and overt malignancies of the genital tract and oropharynx are caused by oncogenic strains of HPV.
- Most of these cancers could be prevented if patients were vaccinated with the 9-valent HPV vaccine.
- The HPV vaccine should be offered to all children beginning at age 11 and to selected high-risk children at age 9. For children aged 14 years and younger, 2 doses of the vaccine are sufficient to induce a robust immune response. The second dose should be administered 6 to 12 months after the first dose.
- Individuals in the age range 13 to 26 years should be offered catch-up vaccination if they have not been previously vaccinated.
- Persons in the age range 27 to 45 years also should be offered vaccination if they have developed a new high-risk profile.
- Persons older than age 15, or those of any age with immunocompromising conditions, should receive 3 doses of the vaccine. The second dose should be administered 1 to 2 months after the first dose, and the third dose should be given 6 months after the first dose.
- The vaccine does not prevent the progression of preexisting infection or clear an infection that is already present at the time of vaccination.
- As a general rule, the vaccine should be deferred during pregnancy, although no adverse effects have been documented when the vaccine has been administered to pregnant women.
- Markowitz LE, Unger ER. Human papilloma virus vaccination. N Engl J Med. 2023;388:1790-1798.
- Schiller JT, Castellsague X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30(suppl 5): F123-F138.
- Lei J, Ploner A, Elfstrom KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383: 1340-1348.
- ACOG Committee Opinion Summary No. 809. Human papillomavirus vaccination. Obstet Gynecol. 2020;136:435-436.
- Barbieri RL. 9vHPV vaccine: prevention of oropharyngeal cancer. OBG Manag. 2020;32:9, 14-15.
- Iversen OE, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316:2411-2421.
- Watson-Jones D, Changalucha J, Whitworth H, et al. Immunogenicity and safety of one-dose human papillomavirus vaccine compared with two or three doses in Tanzanian girls (DoRIS): an open-label, randomised noninferiority trial. Lancet Glob Health. 2022;10:e1473-e1484.
- Scheller NM, Pasternak B, Molgaard-Nielsen D, et al. Quadrivalent HPV vaccination and the risk of adverse pregnancy outcomes. N Engl J Med. 2017;376:1223-1233.
- ACOG Committee Opinion Summary No. 641. Human papillomavirus vaccination. Obstet Gynecol. 2015;126:693.
- Boitano TKL, Ketch PW, Scarinci IC, et al. An update on human papillomavirus vaccination in the United States. Obstet Gynecol. 2023;141:324-330.
- Markowitz LE, Unger ER. Human papilloma virus vaccination. N Engl J Med. 2023;388:1790-1798.
- Schiller JT, Castellsague X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30(suppl 5): F123-F138.
- Lei J, Ploner A, Elfstrom KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383: 1340-1348.
- ACOG Committee Opinion Summary No. 809. Human papillomavirus vaccination. Obstet Gynecol. 2020;136:435-436.
- Barbieri RL. 9vHPV vaccine: prevention of oropharyngeal cancer. OBG Manag. 2020;32:9, 14-15.
- Iversen OE, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316:2411-2421.
- Watson-Jones D, Changalucha J, Whitworth H, et al. Immunogenicity and safety of one-dose human papillomavirus vaccine compared with two or three doses in Tanzanian girls (DoRIS): an open-label, randomised noninferiority trial. Lancet Glob Health. 2022;10:e1473-e1484.
- Scheller NM, Pasternak B, Molgaard-Nielsen D, et al. Quadrivalent HPV vaccination and the risk of adverse pregnancy outcomes. N Engl J Med. 2017;376:1223-1233.
- ACOG Committee Opinion Summary No. 641. Human papillomavirus vaccination. Obstet Gynecol. 2015;126:693.
- Boitano TKL, Ketch PW, Scarinci IC, et al. An update on human papillomavirus vaccination in the United States. Obstet Gynecol. 2023;141:324-330.

