User login
Mycosis fungoides (MF), the most common cutaneous T-cell lymphoma (CTCL), is characterized by clonal proliferation of predominantly CD4+ T cells with localization to the skin.1 Mycosis fungoides typically affects older adults with a male to female ratio of 2:1 but also can occur in children and younger adults.2,3 Known as the great imitator, the manifestations of MF can be variable with considerable clinical and pathologic overlap with benign inflammatory skin diseases, rendering definitive diagnosis challenging.4-7 The early stages of classic MF manifest as pruritic erythematous patches and plaques with variable scaling that can progress in later stages to ulceration and tumors.8 Histopathologically, classic MF is characterized by epidermotropic proliferation of small- to intermediate-sized pleomorphic lymphocytes with cerebriform nuclei and a haloed appearance; intraepidermal nests of atypical lymphocytes known as Pautrier microabscesses occasionally are observed.5 Mycosis fungoides typically follows an indolent clinical course, with advanced-stage MF portending a poor prognosis.9,10 Current treatment is focused on halting disease progression, with topical therapies, phototherapy, and radiation therapy as the standard therapies for early-stage MF.11-13 For advanced-stage MF, treatment may include systemic therapies such as interferon alfa and oral retinoids along with chemotherapies for more refractive cases.14 Allogenic hematopoietic cell transplantation is the only curative treatment.11
Current staging guidelines for MF do not address anatomic location as there is little known about its impact on patient outcomes.11,15 Due to the indolent nature of MF leading to diagnostic challenges, the exact frequency of each primary disease site for MF also remains unclear, though the suggested incidence of MF of the head and neck ranges from 30% to 70%.16,17 Involvement of the head and neck16,18 or external ear and external auditory canal19 is associated with worse prognosis. The purpose of this study was to examine the impact of anatomic location of primary disease site on survival in MF.
Methods
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database includes patient records from 18 registries and encompasses approximately 48% of the US population.20 Using SEER*STAT software (version 8.4.0.1), we conducted a search of patients diagnosed with MF (International Classification of Diseases for Oncology, Third Edition [ICD-O-3] histologic code 9700/3 [mycosis fungoides]) between 2000 and 2019. For inclusion in the study, patients were required to have a known age, specified primary site, and a known cause of death (if applicable). Patients with known Sézary syndrome (SS)—an aggressive form of CTCL that is characterized by the presence of clonally related neoplastic T cells in the skin, lymph nodes, and peripheral blood—were not included because the World Health Organization/European Organisation for Research and Treatment of Cancer considers SS and MF to be separate entities1,15; SS does not necessarily arise from preexisting MF and is associated with markedly poorer survival. This study was exempt from institutional review board approval because the data were publicly available and anonymized.
Data Collection—For age at diagnosis, patients were divided into the following categories: younger than 40 years, 40 to 59 years, 60 to 79 years, and 80 years and older. Demographics, tumor characteristics, and surgical management (if applicable) were obtained for each patient. The designations of chemotherapy and radiation treatment in the SEER database are not reliable and prone to false negatives. As such, these were excluded from analysis.
The primary outcomes of interest were overall survival (OS) and disease-specific survival (DSS), which were calculated as time from MF diagnosis to death. Although OS included all patients who died of any cause, DSS only included patients who died of MF.
Statistical Analysis—Demographics (age, sex, race, ethnicity), tumor characteristics (tumor size, primary site, T stage, lymph node involvement, metastasis), and surgical management (if applicable) were summarized. Overall survival and DSS were calculated using Kaplan-Meier analysis. Univariate and multivariable Cox proportional hazards regression models were generated to determine which prognostic factors for MF were associated with poorer OS and DSS. Only statistically significant variables in the univariate analysis were used to construct the multivariable analysis. Hazard ratios (HRs) and their associated 95% CIs were reported. Incidence rates were calculated and age adjusted to the 2000 US standard population. The SEER JoinPoint Regression program was used to determine the annual percent change (APC)—change in incidence rate over time. P<.05 was considered statistically significant. All statistical analyses were conducted with R version 4.0.2.
Results
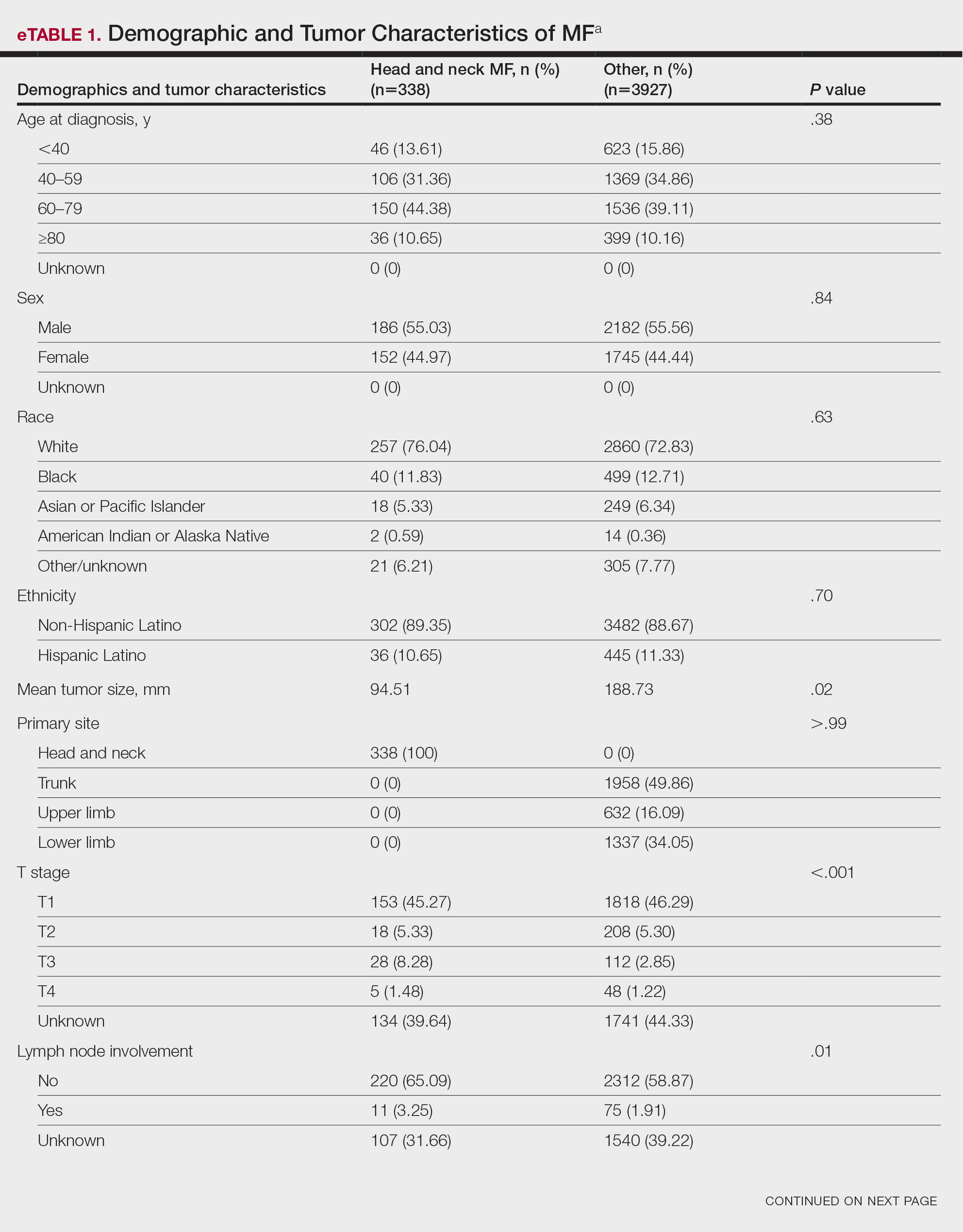
Patient Demographics and Tumor Characteristics—There were 4265 patients diagnosed with MF from 2000 to 2019. The overall incidence of MF was 2.55 per million (95% CI, 2.48-2.63) when age adjusted to the 2000 US standard population, which increased with time (mean APC, 0.97% per year; P=.01). The mean age at diagnosis was 56.4 years with a male to female ratio of 1.2:1. Males (3.07 per million; 95% CI, 2.94-3.20) had a higher incidence of MF than females (2.16 per million; 95% CI, 2.06-2.26), with incidence in females increasing over time (mean APC, 1.52% per year; P=.02) while incidence in males remained stable (mean APC, 1.09%; P=.37). Patients predominantly self-identified as White (73.08%). Patients with MF of the head and neck were more likely to have smaller tumors (P=.02), a more advanced T stage (P<.001), and lymph node involvement (P=.01) at the time of diagnosis. Additional demographics and tumor characteristics are summarized in eTable 1.
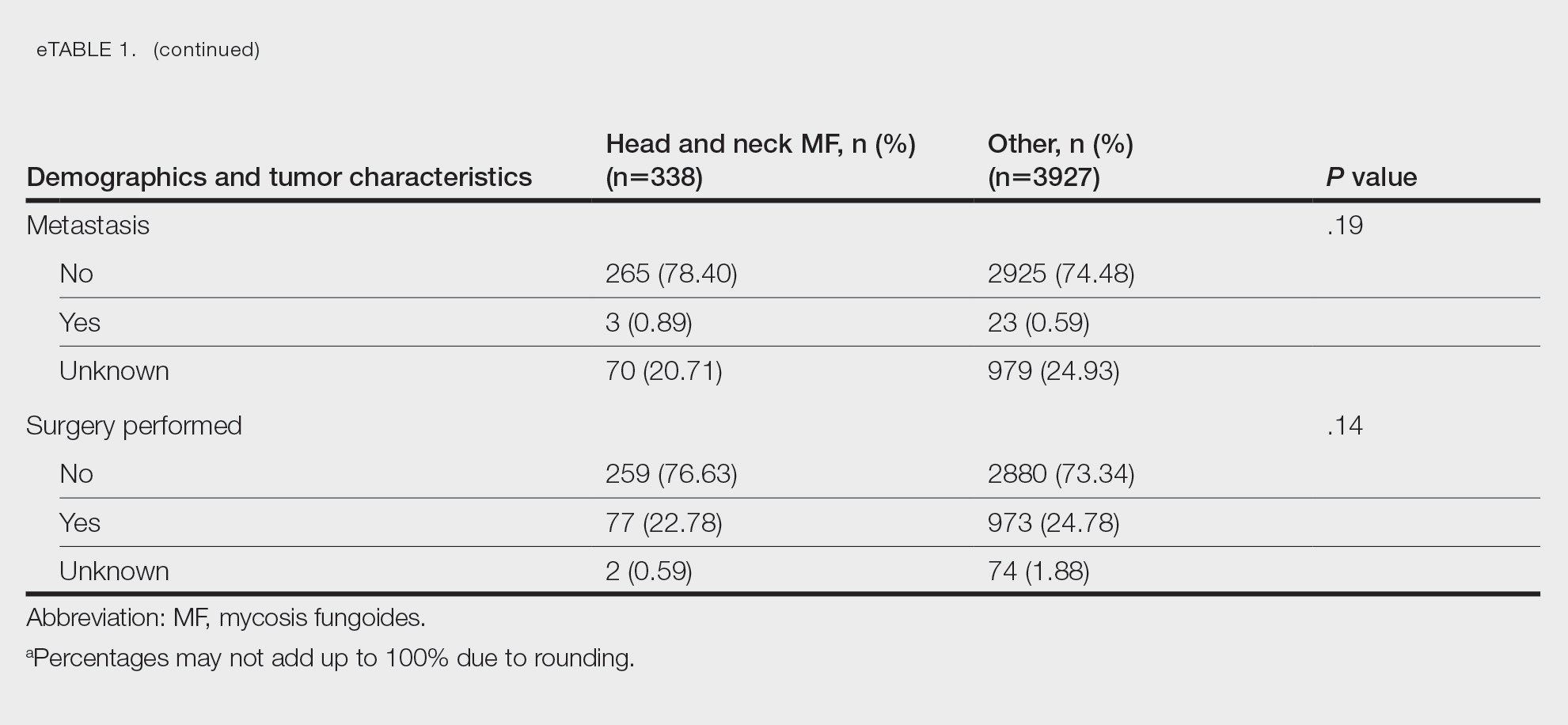
Survival Outcomes—The mean follow-up time was 86.9 months. The 5- and 10-year OS rates were 85.4% (95% CI, 84.2%-86.6%) and 75.0% (95% CI, 73.4%-76.7%), respectively (Figure 1)(Table). The 5- and 10-year DSS rates were 93.3% (95% CI, 92.4%-94.1%) and 89.5% (95% CI, 88.3%-90.6%), respectively. For OS, univariate analysis indicated that significant prognostic factors included increasing age (P<.001), female sex (P<.001), self-identifying as Asian or Pacific Islander (P<.001), self-identifying as Hispanic Latino (P<.001), primary tumor sites of either the head and neck or upper limb (P<.001), T3 or T4 staging (P=.001), lymph node involvement at the time of diagnosis (P<.001), and metastasis (P<.001).
For DSS, univariate analysis had similar risk factors with self-identifying as Black being an additional risk factor (P=.02), though self-identifying as Asian/Pacific Islander or Hispanic Latino were not significant nor was location on the lower limb. For recorded tumor size, the HR increased by 1.001 per each 1-mm increase in size (eTable 2).
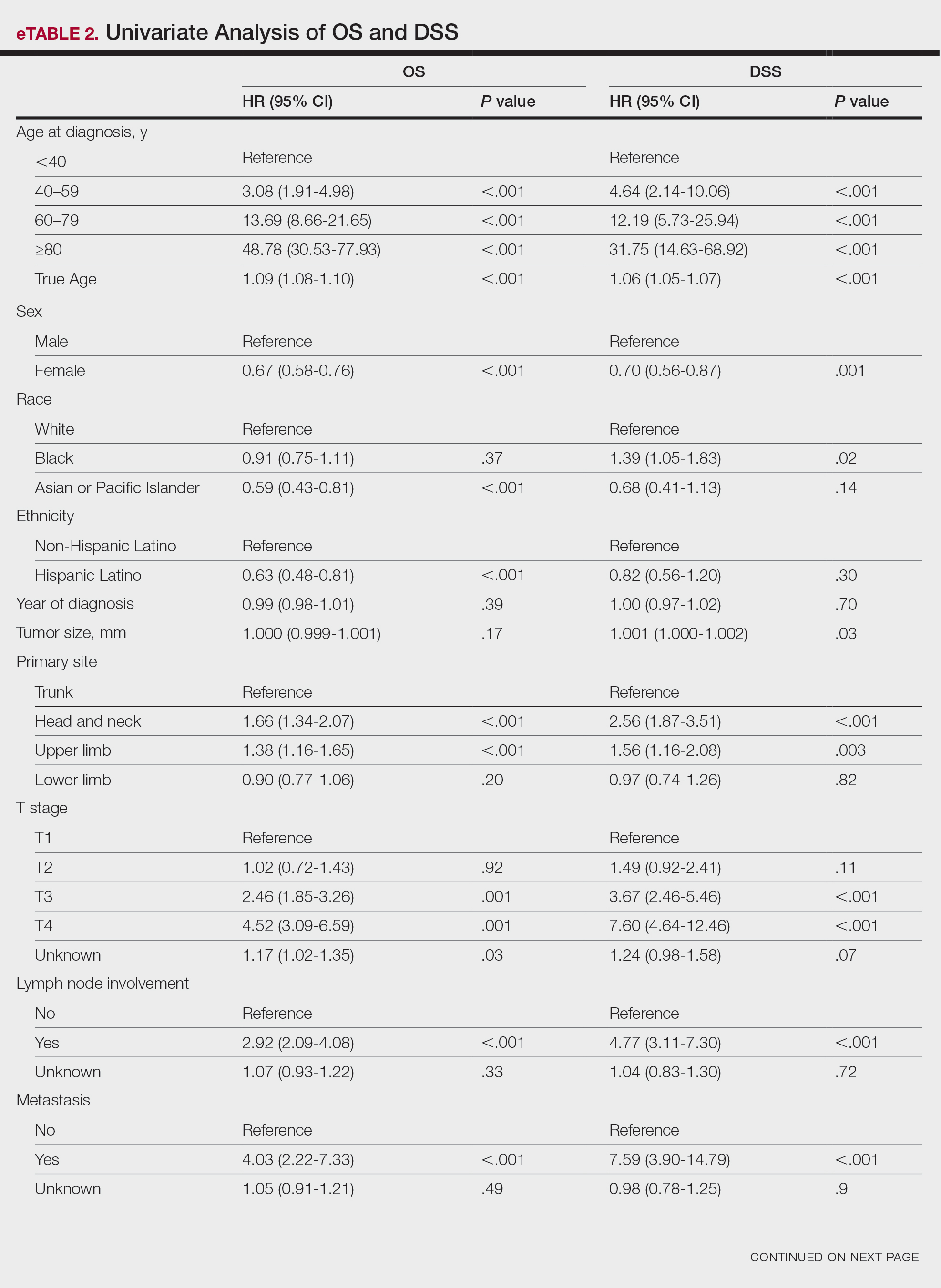
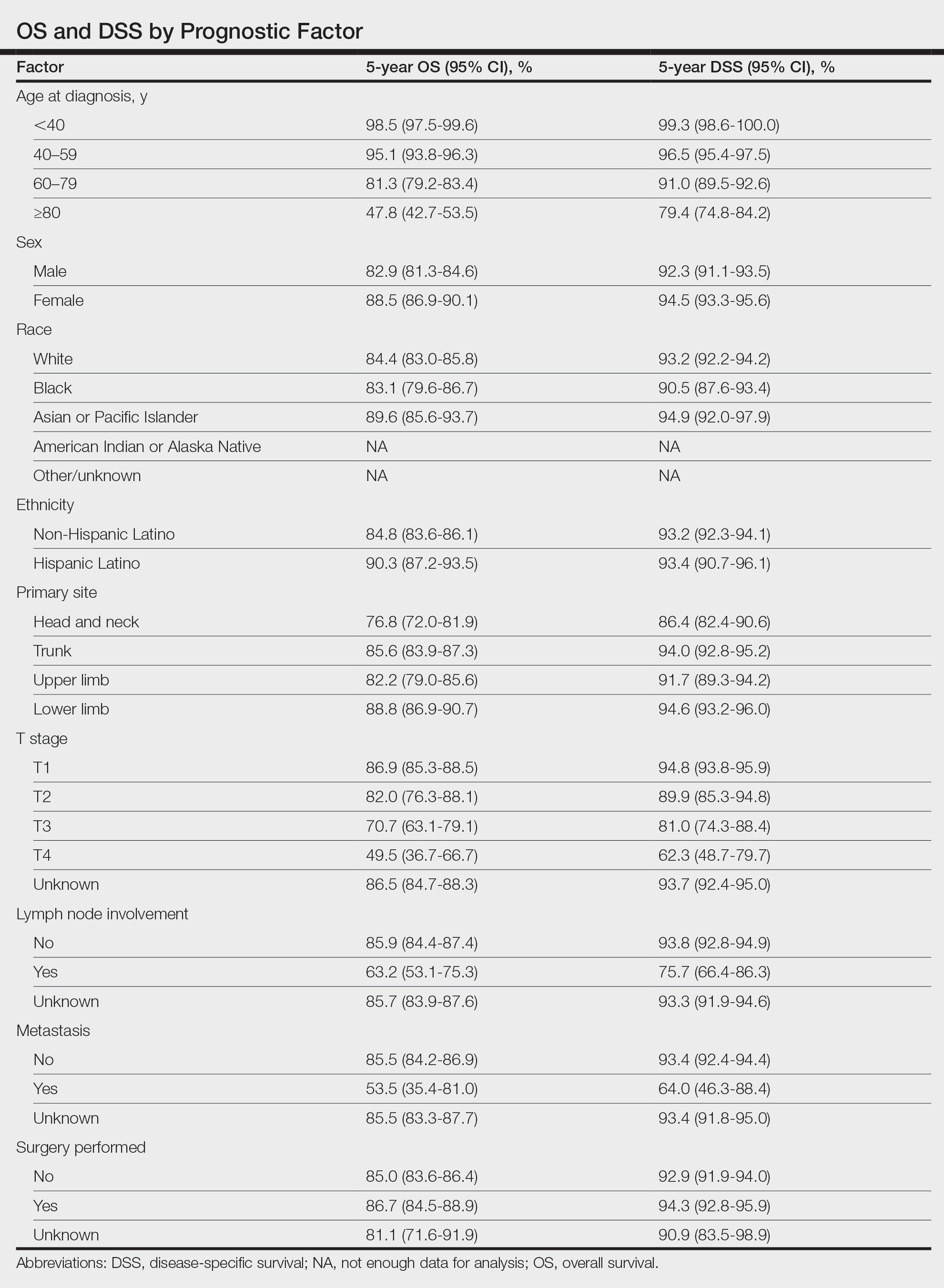
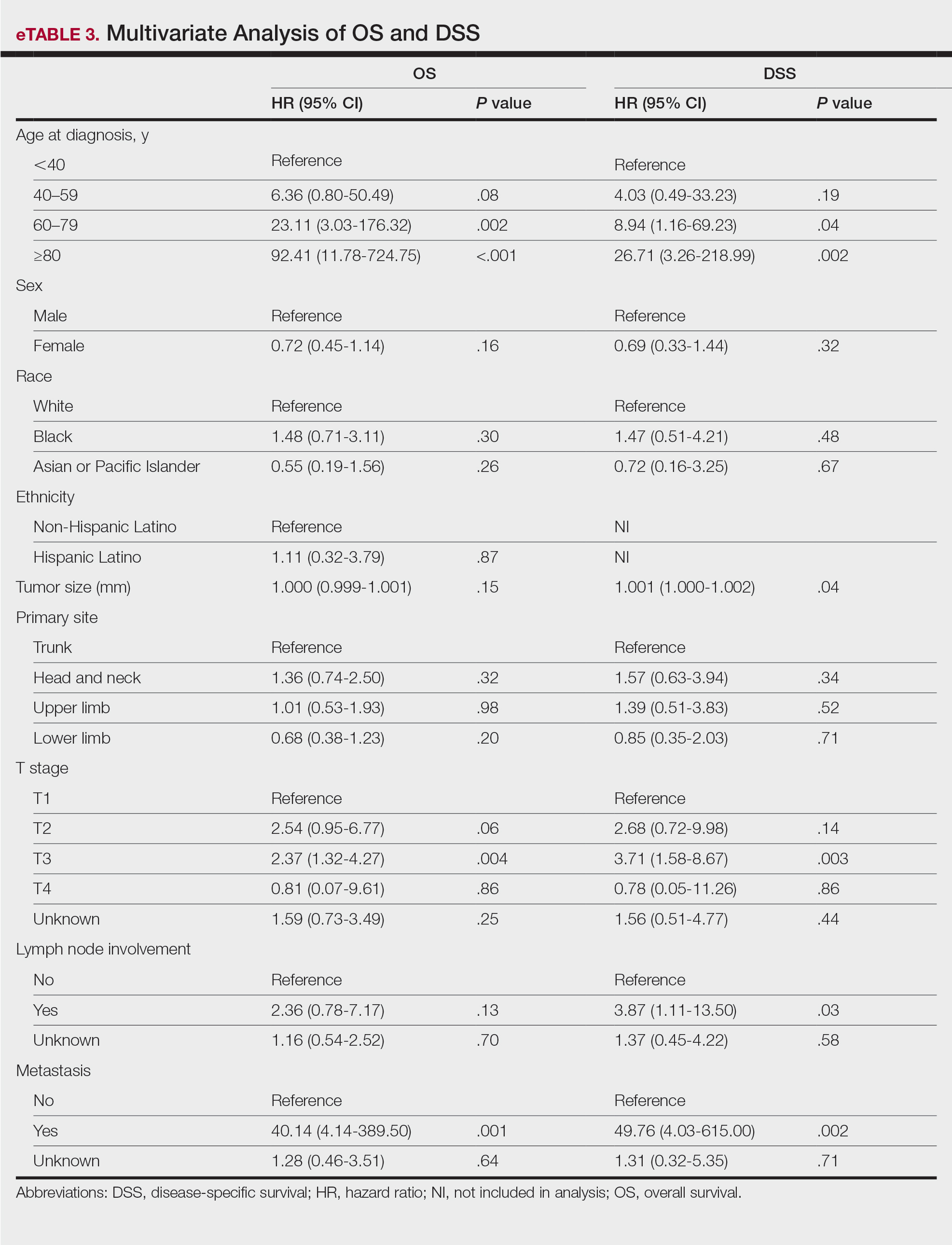
Multivariate analysis showed age at diagnosis (60–79 years: HR, 23.11 [95% CI, 3.03-176.32]; P=.002; ≥80 years: HR, 92.41 [95% CI, 11.78-724.75]; P<.001), T3 staging (HR, 2.37 [95% CI, 1.32-4.27]; P=.004), and metastasis (HR, 40.14 [95% CI, 4.14-389.50]; P=.001) significantly influenced OS. For DSS, multivariate analysis indicated the significant prognostic factors were age at diagnosis (60–79 years: HR, 8.94 [95% CI, 1.16-69.23]; P=.04];≥80 years: HR, 26.71; [95% CI, 3.26-218.99]; P=.002), tumor size (HR, 1.001 [95% CI, 1.000-1.002]; P=.04), T3 staging (HR, 3.71 [95% CI, 1.58-8.67]; P=.003), lymph node involvement (HR, 3.87 [95% CI, 1.11-13.50]; P=.03) and metastasis (HR, 49.76 [95% CI, 4.03-615.00]; P=.002)(Figure 2). When controlling for the aforementioned factors, the primary disease site was not significant (eTable 3).
Comment
Although the prognostic significance of primary disease sites on various types of CTCLs has been examined, limited research exists on MF due to its rarity. For the 4265 patients with MF included in our study, statistically significant prognostic factors on multivariate analysis for DSS included age at diagnosis, tumor size, T staging, lymph node involvement, and presence of metastasis. For OS, only age at diagnosis, T staging, and presence of metastasis were statistically significant predictors. Although initially statistically significant on univariate analysis for both OS and DSS, tumor location was not significant when controlling for confounders.
Our population-based analysis found that 5- and 10-year OS for patients with head and neck MF were 85.4% and 75.0%, respectively, and 5- and 10-year DSS were 93.3% and 89.5%, respectively. Our 10-year OS survival rate of 75.0% was slightly worse than the 81.6% reported by Jung et al16 in a study of 39 cases of MF of the head and neck from the Asan Medical Center database. The difference in survival rate may not only be due to differences in sample size but also because the Asan Medical Center database had a higher proportion of Asian patients as a Korean registry. In our univariate analysis, Asian/Pacific Islander race was shown to be a statistically significant predictor of worse prognosis for OS (P<.001). When comparing survival in patients with head and neck MF vs all primary tumor sites, our OS rate for head and neck MF was more favorable than the 5-year OS of 75% reported by Agar et al21 in their analysis of 1502 patients with MF of all locations, though their cohort also included patients with SS, which is known to have a poorer prognosis. Additionally, our 10-year OS rate of 75.0% for patients with MF with a primary tumor site of the head and neck was slightly less favorable than the 81.0% reported by a prior analysis of the SEER database for MF of all locations,22 which initially may be suggestive of worse outcomes associated with MF originating from the head and neck.
Although MF originating in the head and neck region was found to be a statistically significant prognostic factor under univariate analysis (P<.001), tumor location was not significant upon adjusting for confounders in the multivariate analysis. These results are consistent with those reported in a multivariable analysis conducted by Jung et al,16 which compared 39 cases of head and neck MF to 85 cases without head and neck involvement. The investigators found that the head and neck as the primary site was a significant prognostic factor associated with worsened rates of OS when patients had stages IA to IIA (P=.009) and T2 stage tumors (P=.012) but not in either T1 stage or advanced stage IIB to IVB tumors.16 In contrast, a study by Su et al18 evaluating patients with MF from the National Cancer Database found that patients with MF originating in the head and neck region had similar survival compared with MF originating in the lower limbs after pairwise propensity matching. It previously has been postulated that primary MF lesions originating in the head and neck region have relatively higher frequencies of biological markers believed to be associated with more aggressive tumor behavior and poorer prognosis, such as histopathologic folliculotropism, T-cell receptor gene rearrangements, and large-cell transformations.16 However, MF typically is an indolent disease with advanced-stage MF following an aggressive disease course that often is refractory to treatment. A review from a single academic center noted that 5-year DSS was 97.3% for T1a but only 37.5% for T4.23 Similarly, a meta-analysis evaluating survival in patients with MF noted the 5-year OS for stage IB was 85.8% while for stage IVB it was only 23.3%.24 As such, having advanced-stage MF influences survival to a far greater extent than the presence of head and neck involvement alone. Accordingly, the significantly higher prevalence of advanced T stage disease and increased likelihood of lymph node involvement in MF lesions originating in the head and neck region (both P<.001) may explain why previous studies noted a poorer survival rate with head and neck involvement, as they did not have the sample size to adjust for these factors. Controlling for the above factors likely explains the nonsignificance of this region as a prognostic indicator in our multivariate analysis of OS and DSS.
Similar to MF originating in the head and neck region, the upper limb as a primary tumor site initially was found to be a significant predictor of both OS and DSS on univariate analysis but not on multivariate analysis. By contrast, Su et al18 found survival outcomes were worse for patients diagnosed with MF with the upper limb as the primary tumor site compared with the lower limb on multivariate Cox proportional hazards analysis but not on pairwise propensity score matching. The difference in our results compared with Su et al18 may be because the National Cancer Database only reports OS, while DSS may be more useful in determining prognostic factors associated with poorer survival, especially in an older patient population with greater comorbidities. Furthermore, the nonsignificance of the upper limb as a primary tumor site on further multivariate analysis may be due to similar reasonings as for the head and neck, including more advanced T staging and an anatomic location close to lymph nodes.
Study Limitations—The SEER database is a national registry, which lends itself to potential data heterogeneity in recording and miscoding. Additionally, there may be higher rates of unconfirmed or missing information given the retrospective nature of the SEER database; the database also does not delineate facility type, insurance status, or Charlson/Deyo comorbidity index as demographic factors, which could influence the multivariable analysis. Finally, the SEER database does not further demarcate subtypes of MF, such as the aggressive folliculotropic variant commonly seen in head and neck MF lesions, which precludes independent analysis of disease course by subtype.
Conclusion
Our study evaluated primary disease site as a prognostic factor for OS and DSS in patients with MF. Although head and neck and upper limb as primary disease sites were found to be significant on univariate analysis, they were found to be an insignificant prognostic variable for OS or DSS in our multivariable analysis, potentially due to the aggressive nature of advanced-stage MF and localization close to lymph nodes. Further research including a deeper dive into MF of all stages and subtypes is needed to fully investigate primary disease site as a prognostic indicator. Older age, larger tumor size, a higher T stage, lymph node involvement, and presence of metastasis were associated with worse DSS, and patients with these attributes should be counseled regarding expected disease course and prognosis.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785. doi:10.1182/blood-2004-09-3502
- Hwang ST, Janik JE, Jaffe ES, et al. Mycosis fungoides and Sézary syndrome. Lancet. 2008;371:945-957. doi:10.1016/S0140-6736(08)60420-1
- Jung JM, Lim DJ, Won CH, et al. Mycosis fungoides in children and adolescents: a systematic review. JAMA Dermatol. 2021;157:431-438. doi:10.1001/jamadermatol.2021.0083
- Hodak E, Amitay-Laish I. Mycosis fungoides: a great imitator. Clin Dermatol. 2019;37:255-267. doi:10.1016/j.clindermatol.2019.01.004
- Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063. doi:10.1016/j.jaad.2005.08.057
- Spieth K, Grundmann-Kollmann M, Runne U, et al. Mycosis-fungoides-type Cutaneous T cell lymphoma of the hands and soles: a variant causing delay in diagnosis and adequate treatment of patients with palmoplantar eczema. Dermatology. 2002;205:239-244. doi:10.1159/000065862
- Scarisbrick JJ, Quaglino P, Prince HM, et al. The PROCLIPI international registry of early-stage mycosis fungoides identifies substantial diagnostic delay in most patients. Br J Dermatol. 2019;181:350-357. doi:10.1111/bjd.17258
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part i. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-205.e16. doi:10.1016/j.jaad.2013.07.049
- Suh KS, Jang MS, Jung JH, et al. Clinical characteristics and long-term outcome of 223 patients with mycosis fungoides at a single tertiary center in Korea: a 29-year review. J Am Acad Dermatol. 2022;86:1275-1284. doi:10.1016/j.jaad.2021.06.860
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sézary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866. doi:10.1001/archderm.139.7.857
- Trautinger F, Eder J, Assaf C, et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome—update 2017. Eur J Cancer. 2017;77:57-74. doi:10.1016/j.ejca.2017.02.027
- Quaglino P, Prince HM, Cowan R, et al. Treatment of early-stage mycosis fungoides: results from the PROspective Cutaneous Lymphoma International Prognostic Index (PROCLIPI) study. Br J Dermatol. 2021;184:722-730. doi:10.1111/bjd.19252
- Specht L, Dabaja B, Illidge T, et al. Modern radiation therapy for primary cutaneous lymphomas: field and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2015;92:32-39. doi:10.1016/j.ijrobp.2015.01.008
- Alberti-Violetti S, Talpur R, Schlichte M, et al. Advanced-stagemycosis fungoides and Sézary syndrome: survival and response to treatment. Clin Lymphoma Myeloma Leuk. 2015;15:E105-E112. doi:10.1016/j.clml.2015.02.027
- Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:1713-1722. doi:10.1182/blood-2007-03-055749
- Jung JM, Yoo H, Lim DJ, et al. Clinicoprognostic implications of head and neck involvement by mycosis fungoides: a retrospective cohort study. J Am Acad Dermatol. 2022;86:1258-1265. doi:10.1016/j.jaad.2021.03.056
- Brennan JA. The head and neck manifestations of mycosis fungoides. Laryngoscope. 1995;105(5, pt 1):478-480. doi:10.1288/00005537-199505000-00005
- Su C, Tang R, Bai HX, et al. Disease site as a prognostic factor for mycosis fungoides: an analysis of 2428 cases from the US National Cancer Database. Br J Haematol. 2019;185:592-595. doi:10.1111/bjh.15570
- Wilkinson AJ, Nader ME, Roberts D, et al. Survival outcomes of patients with mycosis fungoides involving the external ear and ear canal. Laryngoscope. 2023;133:1486-1491. doi:10.1002/lary.30377
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) surveillance research program. Published July 2021. Accessed March 14, 2024. https://seer.cancer.gov/about/factsheets/SEER_Overview.pdf
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer Staging proposal. J Clin Oncol. 2010;28:4730-4739. doi:10.1200/JCO.2009.27.7665
- Vollmer RT. A review of survival in mycosis fungoides. Am J Clin Pathol. 2014;141:706-711. doi:10.1309/AJCPH2PHXFCX3BOX
- Desai M, Liu S, Parker S. Clinical characteristics, prognostic factors, and survival of 393 patients with mycosis fungoides and Sézary syndrome in the southeastern United States: a single-institution cohort. J Am Acad Dermatol. 2015;72:276-285. doi:10.1016/j.jaad.2014.10.019
- Mourad A, Gniadecki R. Overall survival in mycosis fungoides: a systematic review and meta-analysis. J Invest Dermatol. 2020;140:495-497.e5. doi:10.1016/j.jid.2019.07.712
Mycosis fungoides (MF), the most common cutaneous T-cell lymphoma (CTCL), is characterized by clonal proliferation of predominantly CD4+ T cells with localization to the skin.1 Mycosis fungoides typically affects older adults with a male to female ratio of 2:1 but also can occur in children and younger adults.2,3 Known as the great imitator, the manifestations of MF can be variable with considerable clinical and pathologic overlap with benign inflammatory skin diseases, rendering definitive diagnosis challenging.4-7 The early stages of classic MF manifest as pruritic erythematous patches and plaques with variable scaling that can progress in later stages to ulceration and tumors.8 Histopathologically, classic MF is characterized by epidermotropic proliferation of small- to intermediate-sized pleomorphic lymphocytes with cerebriform nuclei and a haloed appearance; intraepidermal nests of atypical lymphocytes known as Pautrier microabscesses occasionally are observed.5 Mycosis fungoides typically follows an indolent clinical course, with advanced-stage MF portending a poor prognosis.9,10 Current treatment is focused on halting disease progression, with topical therapies, phototherapy, and radiation therapy as the standard therapies for early-stage MF.11-13 For advanced-stage MF, treatment may include systemic therapies such as interferon alfa and oral retinoids along with chemotherapies for more refractive cases.14 Allogenic hematopoietic cell transplantation is the only curative treatment.11
Current staging guidelines for MF do not address anatomic location as there is little known about its impact on patient outcomes.11,15 Due to the indolent nature of MF leading to diagnostic challenges, the exact frequency of each primary disease site for MF also remains unclear, though the suggested incidence of MF of the head and neck ranges from 30% to 70%.16,17 Involvement of the head and neck16,18 or external ear and external auditory canal19 is associated with worse prognosis. The purpose of this study was to examine the impact of anatomic location of primary disease site on survival in MF.
Methods
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database includes patient records from 18 registries and encompasses approximately 48% of the US population.20 Using SEER*STAT software (version 8.4.0.1), we conducted a search of patients diagnosed with MF (International Classification of Diseases for Oncology, Third Edition [ICD-O-3] histologic code 9700/3 [mycosis fungoides]) between 2000 and 2019. For inclusion in the study, patients were required to have a known age, specified primary site, and a known cause of death (if applicable). Patients with known Sézary syndrome (SS)—an aggressive form of CTCL that is characterized by the presence of clonally related neoplastic T cells in the skin, lymph nodes, and peripheral blood—were not included because the World Health Organization/European Organisation for Research and Treatment of Cancer considers SS and MF to be separate entities1,15; SS does not necessarily arise from preexisting MF and is associated with markedly poorer survival. This study was exempt from institutional review board approval because the data were publicly available and anonymized.
Data Collection—For age at diagnosis, patients were divided into the following categories: younger than 40 years, 40 to 59 years, 60 to 79 years, and 80 years and older. Demographics, tumor characteristics, and surgical management (if applicable) were obtained for each patient. The designations of chemotherapy and radiation treatment in the SEER database are not reliable and prone to false negatives. As such, these were excluded from analysis.
The primary outcomes of interest were overall survival (OS) and disease-specific survival (DSS), which were calculated as time from MF diagnosis to death. Although OS included all patients who died of any cause, DSS only included patients who died of MF.
Statistical Analysis—Demographics (age, sex, race, ethnicity), tumor characteristics (tumor size, primary site, T stage, lymph node involvement, metastasis), and surgical management (if applicable) were summarized. Overall survival and DSS were calculated using Kaplan-Meier analysis. Univariate and multivariable Cox proportional hazards regression models were generated to determine which prognostic factors for MF were associated with poorer OS and DSS. Only statistically significant variables in the univariate analysis were used to construct the multivariable analysis. Hazard ratios (HRs) and their associated 95% CIs were reported. Incidence rates were calculated and age adjusted to the 2000 US standard population. The SEER JoinPoint Regression program was used to determine the annual percent change (APC)—change in incidence rate over time. P<.05 was considered statistically significant. All statistical analyses were conducted with R version 4.0.2.
Results
Patient Demographics and Tumor Characteristics—There were 4265 patients diagnosed with MF from 2000 to 2019. The overall incidence of MF was 2.55 per million (95% CI, 2.48-2.63) when age adjusted to the 2000 US standard population, which increased with time (mean APC, 0.97% per year; P=.01). The mean age at diagnosis was 56.4 years with a male to female ratio of 1.2:1. Males (3.07 per million; 95% CI, 2.94-3.20) had a higher incidence of MF than females (2.16 per million; 95% CI, 2.06-2.26), with incidence in females increasing over time (mean APC, 1.52% per year; P=.02) while incidence in males remained stable (mean APC, 1.09%; P=.37). Patients predominantly self-identified as White (73.08%). Patients with MF of the head and neck were more likely to have smaller tumors (P=.02), a more advanced T stage (P<.001), and lymph node involvement (P=.01) at the time of diagnosis. Additional demographics and tumor characteristics are summarized in eTable 1.


Survival Outcomes—The mean follow-up time was 86.9 months. The 5- and 10-year OS rates were 85.4% (95% CI, 84.2%-86.6%) and 75.0% (95% CI, 73.4%-76.7%), respectively (Figure 1)(Table). The 5- and 10-year DSS rates were 93.3% (95% CI, 92.4%-94.1%) and 89.5% (95% CI, 88.3%-90.6%), respectively. For OS, univariate analysis indicated that significant prognostic factors included increasing age (P<.001), female sex (P<.001), self-identifying as Asian or Pacific Islander (P<.001), self-identifying as Hispanic Latino (P<.001), primary tumor sites of either the head and neck or upper limb (P<.001), T3 or T4 staging (P=.001), lymph node involvement at the time of diagnosis (P<.001), and metastasis (P<.001).


For DSS, univariate analysis had similar risk factors with self-identifying as Black being an additional risk factor (P=.02), though self-identifying as Asian/Pacific Islander or Hispanic Latino were not significant nor was location on the lower limb. For recorded tumor size, the HR increased by 1.001 per each 1-mm increase in size (eTable 2).


Multivariate analysis showed age at diagnosis (60–79 years: HR, 23.11 [95% CI, 3.03-176.32]; P=.002; ≥80 years: HR, 92.41 [95% CI, 11.78-724.75]; P<.001), T3 staging (HR, 2.37 [95% CI, 1.32-4.27]; P=.004), and metastasis (HR, 40.14 [95% CI, 4.14-389.50]; P=.001) significantly influenced OS. For DSS, multivariate analysis indicated the significant prognostic factors were age at diagnosis (60–79 years: HR, 8.94 [95% CI, 1.16-69.23]; P=.04];≥80 years: HR, 26.71; [95% CI, 3.26-218.99]; P=.002), tumor size (HR, 1.001 [95% CI, 1.000-1.002]; P=.04), T3 staging (HR, 3.71 [95% CI, 1.58-8.67]; P=.003), lymph node involvement (HR, 3.87 [95% CI, 1.11-13.50]; P=.03) and metastasis (HR, 49.76 [95% CI, 4.03-615.00]; P=.002)(Figure 2). When controlling for the aforementioned factors, the primary disease site was not significant (eTable 3).


Comment
Although the prognostic significance of primary disease sites on various types of CTCLs has been examined, limited research exists on MF due to its rarity. For the 4265 patients with MF included in our study, statistically significant prognostic factors on multivariate analysis for DSS included age at diagnosis, tumor size, T staging, lymph node involvement, and presence of metastasis. For OS, only age at diagnosis, T staging, and presence of metastasis were statistically significant predictors. Although initially statistically significant on univariate analysis for both OS and DSS, tumor location was not significant when controlling for confounders.
Our population-based analysis found that 5- and 10-year OS for patients with head and neck MF were 85.4% and 75.0%, respectively, and 5- and 10-year DSS were 93.3% and 89.5%, respectively. Our 10-year OS survival rate of 75.0% was slightly worse than the 81.6% reported by Jung et al16 in a study of 39 cases of MF of the head and neck from the Asan Medical Center database. The difference in survival rate may not only be due to differences in sample size but also because the Asan Medical Center database had a higher proportion of Asian patients as a Korean registry. In our univariate analysis, Asian/Pacific Islander race was shown to be a statistically significant predictor of worse prognosis for OS (P<.001). When comparing survival in patients with head and neck MF vs all primary tumor sites, our OS rate for head and neck MF was more favorable than the 5-year OS of 75% reported by Agar et al21 in their analysis of 1502 patients with MF of all locations, though their cohort also included patients with SS, which is known to have a poorer prognosis. Additionally, our 10-year OS rate of 75.0% for patients with MF with a primary tumor site of the head and neck was slightly less favorable than the 81.0% reported by a prior analysis of the SEER database for MF of all locations,22 which initially may be suggestive of worse outcomes associated with MF originating from the head and neck.
Although MF originating in the head and neck region was found to be a statistically significant prognostic factor under univariate analysis (P<.001), tumor location was not significant upon adjusting for confounders in the multivariate analysis. These results are consistent with those reported in a multivariable analysis conducted by Jung et al,16 which compared 39 cases of head and neck MF to 85 cases without head and neck involvement. The investigators found that the head and neck as the primary site was a significant prognostic factor associated with worsened rates of OS when patients had stages IA to IIA (P=.009) and T2 stage tumors (P=.012) but not in either T1 stage or advanced stage IIB to IVB tumors.16 In contrast, a study by Su et al18 evaluating patients with MF from the National Cancer Database found that patients with MF originating in the head and neck region had similar survival compared with MF originating in the lower limbs after pairwise propensity matching. It previously has been postulated that primary MF lesions originating in the head and neck region have relatively higher frequencies of biological markers believed to be associated with more aggressive tumor behavior and poorer prognosis, such as histopathologic folliculotropism, T-cell receptor gene rearrangements, and large-cell transformations.16 However, MF typically is an indolent disease with advanced-stage MF following an aggressive disease course that often is refractory to treatment. A review from a single academic center noted that 5-year DSS was 97.3% for T1a but only 37.5% for T4.23 Similarly, a meta-analysis evaluating survival in patients with MF noted the 5-year OS for stage IB was 85.8% while for stage IVB it was only 23.3%.24 As such, having advanced-stage MF influences survival to a far greater extent than the presence of head and neck involvement alone. Accordingly, the significantly higher prevalence of advanced T stage disease and increased likelihood of lymph node involvement in MF lesions originating in the head and neck region (both P<.001) may explain why previous studies noted a poorer survival rate with head and neck involvement, as they did not have the sample size to adjust for these factors. Controlling for the above factors likely explains the nonsignificance of this region as a prognostic indicator in our multivariate analysis of OS and DSS.
Similar to MF originating in the head and neck region, the upper limb as a primary tumor site initially was found to be a significant predictor of both OS and DSS on univariate analysis but not on multivariate analysis. By contrast, Su et al18 found survival outcomes were worse for patients diagnosed with MF with the upper limb as the primary tumor site compared with the lower limb on multivariate Cox proportional hazards analysis but not on pairwise propensity score matching. The difference in our results compared with Su et al18 may be because the National Cancer Database only reports OS, while DSS may be more useful in determining prognostic factors associated with poorer survival, especially in an older patient population with greater comorbidities. Furthermore, the nonsignificance of the upper limb as a primary tumor site on further multivariate analysis may be due to similar reasonings as for the head and neck, including more advanced T staging and an anatomic location close to lymph nodes.
Study Limitations—The SEER database is a national registry, which lends itself to potential data heterogeneity in recording and miscoding. Additionally, there may be higher rates of unconfirmed or missing information given the retrospective nature of the SEER database; the database also does not delineate facility type, insurance status, or Charlson/Deyo comorbidity index as demographic factors, which could influence the multivariable analysis. Finally, the SEER database does not further demarcate subtypes of MF, such as the aggressive folliculotropic variant commonly seen in head and neck MF lesions, which precludes independent analysis of disease course by subtype.
Conclusion
Our study evaluated primary disease site as a prognostic factor for OS and DSS in patients with MF. Although head and neck and upper limb as primary disease sites were found to be significant on univariate analysis, they were found to be an insignificant prognostic variable for OS or DSS in our multivariable analysis, potentially due to the aggressive nature of advanced-stage MF and localization close to lymph nodes. Further research including a deeper dive into MF of all stages and subtypes is needed to fully investigate primary disease site as a prognostic indicator. Older age, larger tumor size, a higher T stage, lymph node involvement, and presence of metastasis were associated with worse DSS, and patients with these attributes should be counseled regarding expected disease course and prognosis.
Mycosis fungoides (MF), the most common cutaneous T-cell lymphoma (CTCL), is characterized by clonal proliferation of predominantly CD4+ T cells with localization to the skin.1 Mycosis fungoides typically affects older adults with a male to female ratio of 2:1 but also can occur in children and younger adults.2,3 Known as the great imitator, the manifestations of MF can be variable with considerable clinical and pathologic overlap with benign inflammatory skin diseases, rendering definitive diagnosis challenging.4-7 The early stages of classic MF manifest as pruritic erythematous patches and plaques with variable scaling that can progress in later stages to ulceration and tumors.8 Histopathologically, classic MF is characterized by epidermotropic proliferation of small- to intermediate-sized pleomorphic lymphocytes with cerebriform nuclei and a haloed appearance; intraepidermal nests of atypical lymphocytes known as Pautrier microabscesses occasionally are observed.5 Mycosis fungoides typically follows an indolent clinical course, with advanced-stage MF portending a poor prognosis.9,10 Current treatment is focused on halting disease progression, with topical therapies, phototherapy, and radiation therapy as the standard therapies for early-stage MF.11-13 For advanced-stage MF, treatment may include systemic therapies such as interferon alfa and oral retinoids along with chemotherapies for more refractive cases.14 Allogenic hematopoietic cell transplantation is the only curative treatment.11
Current staging guidelines for MF do not address anatomic location as there is little known about its impact on patient outcomes.11,15 Due to the indolent nature of MF leading to diagnostic challenges, the exact frequency of each primary disease site for MF also remains unclear, though the suggested incidence of MF of the head and neck ranges from 30% to 70%.16,17 Involvement of the head and neck16,18 or external ear and external auditory canal19 is associated with worse prognosis. The purpose of this study was to examine the impact of anatomic location of primary disease site on survival in MF.
Methods
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database includes patient records from 18 registries and encompasses approximately 48% of the US population.20 Using SEER*STAT software (version 8.4.0.1), we conducted a search of patients diagnosed with MF (International Classification of Diseases for Oncology, Third Edition [ICD-O-3] histologic code 9700/3 [mycosis fungoides]) between 2000 and 2019. For inclusion in the study, patients were required to have a known age, specified primary site, and a known cause of death (if applicable). Patients with known Sézary syndrome (SS)—an aggressive form of CTCL that is characterized by the presence of clonally related neoplastic T cells in the skin, lymph nodes, and peripheral blood—were not included because the World Health Organization/European Organisation for Research and Treatment of Cancer considers SS and MF to be separate entities1,15; SS does not necessarily arise from preexisting MF and is associated with markedly poorer survival. This study was exempt from institutional review board approval because the data were publicly available and anonymized.
Data Collection—For age at diagnosis, patients were divided into the following categories: younger than 40 years, 40 to 59 years, 60 to 79 years, and 80 years and older. Demographics, tumor characteristics, and surgical management (if applicable) were obtained for each patient. The designations of chemotherapy and radiation treatment in the SEER database are not reliable and prone to false negatives. As such, these were excluded from analysis.
The primary outcomes of interest were overall survival (OS) and disease-specific survival (DSS), which were calculated as time from MF diagnosis to death. Although OS included all patients who died of any cause, DSS only included patients who died of MF.
Statistical Analysis—Demographics (age, sex, race, ethnicity), tumor characteristics (tumor size, primary site, T stage, lymph node involvement, metastasis), and surgical management (if applicable) were summarized. Overall survival and DSS were calculated using Kaplan-Meier analysis. Univariate and multivariable Cox proportional hazards regression models were generated to determine which prognostic factors for MF were associated with poorer OS and DSS. Only statistically significant variables in the univariate analysis were used to construct the multivariable analysis. Hazard ratios (HRs) and their associated 95% CIs were reported. Incidence rates were calculated and age adjusted to the 2000 US standard population. The SEER JoinPoint Regression program was used to determine the annual percent change (APC)—change in incidence rate over time. P<.05 was considered statistically significant. All statistical analyses were conducted with R version 4.0.2.
Results
Patient Demographics and Tumor Characteristics—There were 4265 patients diagnosed with MF from 2000 to 2019. The overall incidence of MF was 2.55 per million (95% CI, 2.48-2.63) when age adjusted to the 2000 US standard population, which increased with time (mean APC, 0.97% per year; P=.01). The mean age at diagnosis was 56.4 years with a male to female ratio of 1.2:1. Males (3.07 per million; 95% CI, 2.94-3.20) had a higher incidence of MF than females (2.16 per million; 95% CI, 2.06-2.26), with incidence in females increasing over time (mean APC, 1.52% per year; P=.02) while incidence in males remained stable (mean APC, 1.09%; P=.37). Patients predominantly self-identified as White (73.08%). Patients with MF of the head and neck were more likely to have smaller tumors (P=.02), a more advanced T stage (P<.001), and lymph node involvement (P=.01) at the time of diagnosis. Additional demographics and tumor characteristics are summarized in eTable 1.


Survival Outcomes—The mean follow-up time was 86.9 months. The 5- and 10-year OS rates were 85.4% (95% CI, 84.2%-86.6%) and 75.0% (95% CI, 73.4%-76.7%), respectively (Figure 1)(Table). The 5- and 10-year DSS rates were 93.3% (95% CI, 92.4%-94.1%) and 89.5% (95% CI, 88.3%-90.6%), respectively. For OS, univariate analysis indicated that significant prognostic factors included increasing age (P<.001), female sex (P<.001), self-identifying as Asian or Pacific Islander (P<.001), self-identifying as Hispanic Latino (P<.001), primary tumor sites of either the head and neck or upper limb (P<.001), T3 or T4 staging (P=.001), lymph node involvement at the time of diagnosis (P<.001), and metastasis (P<.001).


For DSS, univariate analysis had similar risk factors with self-identifying as Black being an additional risk factor (P=.02), though self-identifying as Asian/Pacific Islander or Hispanic Latino were not significant nor was location on the lower limb. For recorded tumor size, the HR increased by 1.001 per each 1-mm increase in size (eTable 2).


Multivariate analysis showed age at diagnosis (60–79 years: HR, 23.11 [95% CI, 3.03-176.32]; P=.002; ≥80 years: HR, 92.41 [95% CI, 11.78-724.75]; P<.001), T3 staging (HR, 2.37 [95% CI, 1.32-4.27]; P=.004), and metastasis (HR, 40.14 [95% CI, 4.14-389.50]; P=.001) significantly influenced OS. For DSS, multivariate analysis indicated the significant prognostic factors were age at diagnosis (60–79 years: HR, 8.94 [95% CI, 1.16-69.23]; P=.04];≥80 years: HR, 26.71; [95% CI, 3.26-218.99]; P=.002), tumor size (HR, 1.001 [95% CI, 1.000-1.002]; P=.04), T3 staging (HR, 3.71 [95% CI, 1.58-8.67]; P=.003), lymph node involvement (HR, 3.87 [95% CI, 1.11-13.50]; P=.03) and metastasis (HR, 49.76 [95% CI, 4.03-615.00]; P=.002)(Figure 2). When controlling for the aforementioned factors, the primary disease site was not significant (eTable 3).


Comment
Although the prognostic significance of primary disease sites on various types of CTCLs has been examined, limited research exists on MF due to its rarity. For the 4265 patients with MF included in our study, statistically significant prognostic factors on multivariate analysis for DSS included age at diagnosis, tumor size, T staging, lymph node involvement, and presence of metastasis. For OS, only age at diagnosis, T staging, and presence of metastasis were statistically significant predictors. Although initially statistically significant on univariate analysis for both OS and DSS, tumor location was not significant when controlling for confounders.
Our population-based analysis found that 5- and 10-year OS for patients with head and neck MF were 85.4% and 75.0%, respectively, and 5- and 10-year DSS were 93.3% and 89.5%, respectively. Our 10-year OS survival rate of 75.0% was slightly worse than the 81.6% reported by Jung et al16 in a study of 39 cases of MF of the head and neck from the Asan Medical Center database. The difference in survival rate may not only be due to differences in sample size but also because the Asan Medical Center database had a higher proportion of Asian patients as a Korean registry. In our univariate analysis, Asian/Pacific Islander race was shown to be a statistically significant predictor of worse prognosis for OS (P<.001). When comparing survival in patients with head and neck MF vs all primary tumor sites, our OS rate for head and neck MF was more favorable than the 5-year OS of 75% reported by Agar et al21 in their analysis of 1502 patients with MF of all locations, though their cohort also included patients with SS, which is known to have a poorer prognosis. Additionally, our 10-year OS rate of 75.0% for patients with MF with a primary tumor site of the head and neck was slightly less favorable than the 81.0% reported by a prior analysis of the SEER database for MF of all locations,22 which initially may be suggestive of worse outcomes associated with MF originating from the head and neck.
Although MF originating in the head and neck region was found to be a statistically significant prognostic factor under univariate analysis (P<.001), tumor location was not significant upon adjusting for confounders in the multivariate analysis. These results are consistent with those reported in a multivariable analysis conducted by Jung et al,16 which compared 39 cases of head and neck MF to 85 cases without head and neck involvement. The investigators found that the head and neck as the primary site was a significant prognostic factor associated with worsened rates of OS when patients had stages IA to IIA (P=.009) and T2 stage tumors (P=.012) but not in either T1 stage or advanced stage IIB to IVB tumors.16 In contrast, a study by Su et al18 evaluating patients with MF from the National Cancer Database found that patients with MF originating in the head and neck region had similar survival compared with MF originating in the lower limbs after pairwise propensity matching. It previously has been postulated that primary MF lesions originating in the head and neck region have relatively higher frequencies of biological markers believed to be associated with more aggressive tumor behavior and poorer prognosis, such as histopathologic folliculotropism, T-cell receptor gene rearrangements, and large-cell transformations.16 However, MF typically is an indolent disease with advanced-stage MF following an aggressive disease course that often is refractory to treatment. A review from a single academic center noted that 5-year DSS was 97.3% for T1a but only 37.5% for T4.23 Similarly, a meta-analysis evaluating survival in patients with MF noted the 5-year OS for stage IB was 85.8% while for stage IVB it was only 23.3%.24 As such, having advanced-stage MF influences survival to a far greater extent than the presence of head and neck involvement alone. Accordingly, the significantly higher prevalence of advanced T stage disease and increased likelihood of lymph node involvement in MF lesions originating in the head and neck region (both P<.001) may explain why previous studies noted a poorer survival rate with head and neck involvement, as they did not have the sample size to adjust for these factors. Controlling for the above factors likely explains the nonsignificance of this region as a prognostic indicator in our multivariate analysis of OS and DSS.
Similar to MF originating in the head and neck region, the upper limb as a primary tumor site initially was found to be a significant predictor of both OS and DSS on univariate analysis but not on multivariate analysis. By contrast, Su et al18 found survival outcomes were worse for patients diagnosed with MF with the upper limb as the primary tumor site compared with the lower limb on multivariate Cox proportional hazards analysis but not on pairwise propensity score matching. The difference in our results compared with Su et al18 may be because the National Cancer Database only reports OS, while DSS may be more useful in determining prognostic factors associated with poorer survival, especially in an older patient population with greater comorbidities. Furthermore, the nonsignificance of the upper limb as a primary tumor site on further multivariate analysis may be due to similar reasonings as for the head and neck, including more advanced T staging and an anatomic location close to lymph nodes.
Study Limitations—The SEER database is a national registry, which lends itself to potential data heterogeneity in recording and miscoding. Additionally, there may be higher rates of unconfirmed or missing information given the retrospective nature of the SEER database; the database also does not delineate facility type, insurance status, or Charlson/Deyo comorbidity index as demographic factors, which could influence the multivariable analysis. Finally, the SEER database does not further demarcate subtypes of MF, such as the aggressive folliculotropic variant commonly seen in head and neck MF lesions, which precludes independent analysis of disease course by subtype.
Conclusion
Our study evaluated primary disease site as a prognostic factor for OS and DSS in patients with MF. Although head and neck and upper limb as primary disease sites were found to be significant on univariate analysis, they were found to be an insignificant prognostic variable for OS or DSS in our multivariable analysis, potentially due to the aggressive nature of advanced-stage MF and localization close to lymph nodes. Further research including a deeper dive into MF of all stages and subtypes is needed to fully investigate primary disease site as a prognostic indicator. Older age, larger tumor size, a higher T stage, lymph node involvement, and presence of metastasis were associated with worse DSS, and patients with these attributes should be counseled regarding expected disease course and prognosis.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785. doi:10.1182/blood-2004-09-3502
- Hwang ST, Janik JE, Jaffe ES, et al. Mycosis fungoides and Sézary syndrome. Lancet. 2008;371:945-957. doi:10.1016/S0140-6736(08)60420-1
- Jung JM, Lim DJ, Won CH, et al. Mycosis fungoides in children and adolescents: a systematic review. JAMA Dermatol. 2021;157:431-438. doi:10.1001/jamadermatol.2021.0083
- Hodak E, Amitay-Laish I. Mycosis fungoides: a great imitator. Clin Dermatol. 2019;37:255-267. doi:10.1016/j.clindermatol.2019.01.004
- Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063. doi:10.1016/j.jaad.2005.08.057
- Spieth K, Grundmann-Kollmann M, Runne U, et al. Mycosis-fungoides-type Cutaneous T cell lymphoma of the hands and soles: a variant causing delay in diagnosis and adequate treatment of patients with palmoplantar eczema. Dermatology. 2002;205:239-244. doi:10.1159/000065862
- Scarisbrick JJ, Quaglino P, Prince HM, et al. The PROCLIPI international registry of early-stage mycosis fungoides identifies substantial diagnostic delay in most patients. Br J Dermatol. 2019;181:350-357. doi:10.1111/bjd.17258
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part i. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-205.e16. doi:10.1016/j.jaad.2013.07.049
- Suh KS, Jang MS, Jung JH, et al. Clinical characteristics and long-term outcome of 223 patients with mycosis fungoides at a single tertiary center in Korea: a 29-year review. J Am Acad Dermatol. 2022;86:1275-1284. doi:10.1016/j.jaad.2021.06.860
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sézary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866. doi:10.1001/archderm.139.7.857
- Trautinger F, Eder J, Assaf C, et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome—update 2017. Eur J Cancer. 2017;77:57-74. doi:10.1016/j.ejca.2017.02.027
- Quaglino P, Prince HM, Cowan R, et al. Treatment of early-stage mycosis fungoides: results from the PROspective Cutaneous Lymphoma International Prognostic Index (PROCLIPI) study. Br J Dermatol. 2021;184:722-730. doi:10.1111/bjd.19252
- Specht L, Dabaja B, Illidge T, et al. Modern radiation therapy for primary cutaneous lymphomas: field and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2015;92:32-39. doi:10.1016/j.ijrobp.2015.01.008
- Alberti-Violetti S, Talpur R, Schlichte M, et al. Advanced-stagemycosis fungoides and Sézary syndrome: survival and response to treatment. Clin Lymphoma Myeloma Leuk. 2015;15:E105-E112. doi:10.1016/j.clml.2015.02.027
- Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:1713-1722. doi:10.1182/blood-2007-03-055749
- Jung JM, Yoo H, Lim DJ, et al. Clinicoprognostic implications of head and neck involvement by mycosis fungoides: a retrospective cohort study. J Am Acad Dermatol. 2022;86:1258-1265. doi:10.1016/j.jaad.2021.03.056
- Brennan JA. The head and neck manifestations of mycosis fungoides. Laryngoscope. 1995;105(5, pt 1):478-480. doi:10.1288/00005537-199505000-00005
- Su C, Tang R, Bai HX, et al. Disease site as a prognostic factor for mycosis fungoides: an analysis of 2428 cases from the US National Cancer Database. Br J Haematol. 2019;185:592-595. doi:10.1111/bjh.15570
- Wilkinson AJ, Nader ME, Roberts D, et al. Survival outcomes of patients with mycosis fungoides involving the external ear and ear canal. Laryngoscope. 2023;133:1486-1491. doi:10.1002/lary.30377
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) surveillance research program. Published July 2021. Accessed March 14, 2024. https://seer.cancer.gov/about/factsheets/SEER_Overview.pdf
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer Staging proposal. J Clin Oncol. 2010;28:4730-4739. doi:10.1200/JCO.2009.27.7665
- Vollmer RT. A review of survival in mycosis fungoides. Am J Clin Pathol. 2014;141:706-711. doi:10.1309/AJCPH2PHXFCX3BOX
- Desai M, Liu S, Parker S. Clinical characteristics, prognostic factors, and survival of 393 patients with mycosis fungoides and Sézary syndrome in the southeastern United States: a single-institution cohort. J Am Acad Dermatol. 2015;72:276-285. doi:10.1016/j.jaad.2014.10.019
- Mourad A, Gniadecki R. Overall survival in mycosis fungoides: a systematic review and meta-analysis. J Invest Dermatol. 2020;140:495-497.e5. doi:10.1016/j.jid.2019.07.712
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785. doi:10.1182/blood-2004-09-3502
- Hwang ST, Janik JE, Jaffe ES, et al. Mycosis fungoides and Sézary syndrome. Lancet. 2008;371:945-957. doi:10.1016/S0140-6736(08)60420-1
- Jung JM, Lim DJ, Won CH, et al. Mycosis fungoides in children and adolescents: a systematic review. JAMA Dermatol. 2021;157:431-438. doi:10.1001/jamadermatol.2021.0083
- Hodak E, Amitay-Laish I. Mycosis fungoides: a great imitator. Clin Dermatol. 2019;37:255-267. doi:10.1016/j.clindermatol.2019.01.004
- Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063. doi:10.1016/j.jaad.2005.08.057
- Spieth K, Grundmann-Kollmann M, Runne U, et al. Mycosis-fungoides-type Cutaneous T cell lymphoma of the hands and soles: a variant causing delay in diagnosis and adequate treatment of patients with palmoplantar eczema. Dermatology. 2002;205:239-244. doi:10.1159/000065862
- Scarisbrick JJ, Quaglino P, Prince HM, et al. The PROCLIPI international registry of early-stage mycosis fungoides identifies substantial diagnostic delay in most patients. Br J Dermatol. 2019;181:350-357. doi:10.1111/bjd.17258
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part i. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-205.e16. doi:10.1016/j.jaad.2013.07.049
- Suh KS, Jang MS, Jung JH, et al. Clinical characteristics and long-term outcome of 223 patients with mycosis fungoides at a single tertiary center in Korea: a 29-year review. J Am Acad Dermatol. 2022;86:1275-1284. doi:10.1016/j.jaad.2021.06.860
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sézary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866. doi:10.1001/archderm.139.7.857
- Trautinger F, Eder J, Assaf C, et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome—update 2017. Eur J Cancer. 2017;77:57-74. doi:10.1016/j.ejca.2017.02.027
- Quaglino P, Prince HM, Cowan R, et al. Treatment of early-stage mycosis fungoides: results from the PROspective Cutaneous Lymphoma International Prognostic Index (PROCLIPI) study. Br J Dermatol. 2021;184:722-730. doi:10.1111/bjd.19252
- Specht L, Dabaja B, Illidge T, et al. Modern radiation therapy for primary cutaneous lymphomas: field and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2015;92:32-39. doi:10.1016/j.ijrobp.2015.01.008
- Alberti-Violetti S, Talpur R, Schlichte M, et al. Advanced-stagemycosis fungoides and Sézary syndrome: survival and response to treatment. Clin Lymphoma Myeloma Leuk. 2015;15:E105-E112. doi:10.1016/j.clml.2015.02.027
- Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:1713-1722. doi:10.1182/blood-2007-03-055749
- Jung JM, Yoo H, Lim DJ, et al. Clinicoprognostic implications of head and neck involvement by mycosis fungoides: a retrospective cohort study. J Am Acad Dermatol. 2022;86:1258-1265. doi:10.1016/j.jaad.2021.03.056
- Brennan JA. The head and neck manifestations of mycosis fungoides. Laryngoscope. 1995;105(5, pt 1):478-480. doi:10.1288/00005537-199505000-00005
- Su C, Tang R, Bai HX, et al. Disease site as a prognostic factor for mycosis fungoides: an analysis of 2428 cases from the US National Cancer Database. Br J Haematol. 2019;185:592-595. doi:10.1111/bjh.15570
- Wilkinson AJ, Nader ME, Roberts D, et al. Survival outcomes of patients with mycosis fungoides involving the external ear and ear canal. Laryngoscope. 2023;133:1486-1491. doi:10.1002/lary.30377
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) surveillance research program. Published July 2021. Accessed March 14, 2024. https://seer.cancer.gov/about/factsheets/SEER_Overview.pdf
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer Staging proposal. J Clin Oncol. 2010;28:4730-4739. doi:10.1200/JCO.2009.27.7665
- Vollmer RT. A review of survival in mycosis fungoides. Am J Clin Pathol. 2014;141:706-711. doi:10.1309/AJCPH2PHXFCX3BOX
- Desai M, Liu S, Parker S. Clinical characteristics, prognostic factors, and survival of 393 patients with mycosis fungoides and Sézary syndrome in the southeastern United States: a single-institution cohort. J Am Acad Dermatol. 2015;72:276-285. doi:10.1016/j.jaad.2014.10.019
- Mourad A, Gniadecki R. Overall survival in mycosis fungoides: a systematic review and meta-analysis. J Invest Dermatol. 2020;140:495-497.e5. doi:10.1016/j.jid.2019.07.712
Practice Points
- Mycosis fungoides (MF) is the most common cutaneous T-cell lymphoma.
- Because MF is associated with diagnostic challenges due to its indolent course, data regarding primary tumor site as a prognostic factor are limited.
- Although MF originating from the head and neck region did not appear to influence survival, it was found that patients who were older or who had a larger tumor size at diagnosis, a higher T stage, lymph node involvement, or presence of metastasis had poorer survival overall and may benefit from additional counseling regarding their prognosis.