User login
An implantable device that provides measurements of pulmonary arterial pressure in patients with class III heart failure has been approved by the Food and Drug Administration, based on a study that showed the use of the device to remotely monitor patients reduced heart failure hospitalization rates.
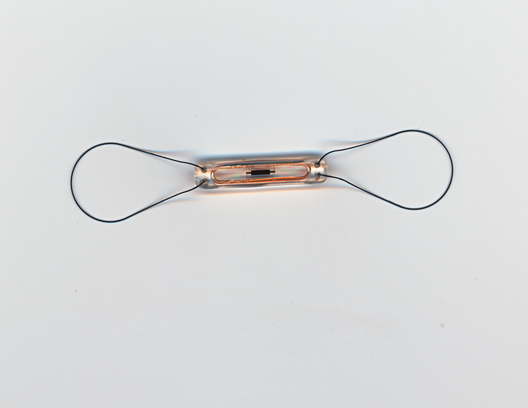
The CardioMEMS HF System "is the first permanently implantable wireless system intended to provide PA pressure measurements, including systolic, diastolic, and mean PA pressures," according to the FDA statement announcing the approval on May 28. This information is remotely reviewed by the patient’s physician, who "can make decisions regarding the status of the patient and, if necessary, initiate changes in medical therapy, with the goal of reducing hospitalization due to heart failure," the statement said.
It is specifically approved for patients with New York Heart Association (NYHA) class III heart failure (HF) who have been hospitalized for heart failure in the previous year.
The three components of the system are the battery-free sensor/monitor that is permanently implanted in the pulmonary artery, a transvenous catheter that deploys the sensor in the distal PA, and an electronic system that receives and processes the signals from the sensor/monitor and transfers the PA pressure measurements to a secure database, the statement said. Patients can be monitored from their home or another remote location.
Approval was based on a study of 550 patients with NYHA class III HF and a recent hospitalization for HF, who had the device implanted. Physicians had access to daily PA measurements only for the patients randomized to the treatment group, and adjusted HF medications based on the values provided. At 6 months, the HF hospitalization rate was significantly lower among those in the treatment group. The FDA statement noted that at 6 months, almost 99% of the patients who had the device implanted or in whom implantation was attempted had no complications related to the device or system, and all of the devices that were implanted were operating normally.
However, concerns about the study held up approval of the device for several years, and at a meeting in December 2011, the majority of the FDA’s Circulatory System Devices Panel agreed that the risks of the device outweighed the benefits. The manufacturer, CardioMEMS, provided follow-up data and further analyses of the data that were provided at another meeting of the panel, in October 2013. At that meeting, the majority of the panel agreed that the benefits of the device outweighed its risks for monitoring patients who met the criteria specified in the indication that has been approved, patients with NYHA class III heart failure who have been hospitalized for HF in the previous year.
In the May 28 statement, the FDA said that the company is required to conduct a postmarketing study to evaluate the performance of the device when used outside of a clinical trial. One concern of the panelists who supported approval at the 2013 meeting was that the benefit in terms of HF hospitalizations was not evident in women in the study, which they said could have been due to the low number of women enrolled in the trial, and they recommended that the device be studied in more women.
An implantable device that provides measurements of pulmonary arterial pressure in patients with class III heart failure has been approved by the Food and Drug Administration, based on a study that showed the use of the device to remotely monitor patients reduced heart failure hospitalization rates.
The CardioMEMS HF System "is the first permanently implantable wireless system intended to provide PA pressure measurements, including systolic, diastolic, and mean PA pressures," according to the FDA statement announcing the approval on May 28. This information is remotely reviewed by the patient’s physician, who "can make decisions regarding the status of the patient and, if necessary, initiate changes in medical therapy, with the goal of reducing hospitalization due to heart failure," the statement said.
It is specifically approved for patients with New York Heart Association (NYHA) class III heart failure (HF) who have been hospitalized for heart failure in the previous year.
The three components of the system are the battery-free sensor/monitor that is permanently implanted in the pulmonary artery, a transvenous catheter that deploys the sensor in the distal PA, and an electronic system that receives and processes the signals from the sensor/monitor and transfers the PA pressure measurements to a secure database, the statement said. Patients can be monitored from their home or another remote location.
Approval was based on a study of 550 patients with NYHA class III HF and a recent hospitalization for HF, who had the device implanted. Physicians had access to daily PA measurements only for the patients randomized to the treatment group, and adjusted HF medications based on the values provided. At 6 months, the HF hospitalization rate was significantly lower among those in the treatment group. The FDA statement noted that at 6 months, almost 99% of the patients who had the device implanted or in whom implantation was attempted had no complications related to the device or system, and all of the devices that were implanted were operating normally.
However, concerns about the study held up approval of the device for several years, and at a meeting in December 2011, the majority of the FDA’s Circulatory System Devices Panel agreed that the risks of the device outweighed the benefits. The manufacturer, CardioMEMS, provided follow-up data and further analyses of the data that were provided at another meeting of the panel, in October 2013. At that meeting, the majority of the panel agreed that the benefits of the device outweighed its risks for monitoring patients who met the criteria specified in the indication that has been approved, patients with NYHA class III heart failure who have been hospitalized for HF in the previous year.
In the May 28 statement, the FDA said that the company is required to conduct a postmarketing study to evaluate the performance of the device when used outside of a clinical trial. One concern of the panelists who supported approval at the 2013 meeting was that the benefit in terms of HF hospitalizations was not evident in women in the study, which they said could have been due to the low number of women enrolled in the trial, and they recommended that the device be studied in more women.
An implantable device that provides measurements of pulmonary arterial pressure in patients with class III heart failure has been approved by the Food and Drug Administration, based on a study that showed the use of the device to remotely monitor patients reduced heart failure hospitalization rates.
The CardioMEMS HF System "is the first permanently implantable wireless system intended to provide PA pressure measurements, including systolic, diastolic, and mean PA pressures," according to the FDA statement announcing the approval on May 28. This information is remotely reviewed by the patient’s physician, who "can make decisions regarding the status of the patient and, if necessary, initiate changes in medical therapy, with the goal of reducing hospitalization due to heart failure," the statement said.
It is specifically approved for patients with New York Heart Association (NYHA) class III heart failure (HF) who have been hospitalized for heart failure in the previous year.
The three components of the system are the battery-free sensor/monitor that is permanently implanted in the pulmonary artery, a transvenous catheter that deploys the sensor in the distal PA, and an electronic system that receives and processes the signals from the sensor/monitor and transfers the PA pressure measurements to a secure database, the statement said. Patients can be monitored from their home or another remote location.
Approval was based on a study of 550 patients with NYHA class III HF and a recent hospitalization for HF, who had the device implanted. Physicians had access to daily PA measurements only for the patients randomized to the treatment group, and adjusted HF medications based on the values provided. At 6 months, the HF hospitalization rate was significantly lower among those in the treatment group. The FDA statement noted that at 6 months, almost 99% of the patients who had the device implanted or in whom implantation was attempted had no complications related to the device or system, and all of the devices that were implanted were operating normally.
However, concerns about the study held up approval of the device for several years, and at a meeting in December 2011, the majority of the FDA’s Circulatory System Devices Panel agreed that the risks of the device outweighed the benefits. The manufacturer, CardioMEMS, provided follow-up data and further analyses of the data that were provided at another meeting of the panel, in October 2013. At that meeting, the majority of the panel agreed that the benefits of the device outweighed its risks for monitoring patients who met the criteria specified in the indication that has been approved, patients with NYHA class III heart failure who have been hospitalized for HF in the previous year.
In the May 28 statement, the FDA said that the company is required to conduct a postmarketing study to evaluate the performance of the device when used outside of a clinical trial. One concern of the panelists who supported approval at the 2013 meeting was that the benefit in terms of HF hospitalizations was not evident in women in the study, which they said could have been due to the low number of women enrolled in the trial, and they recommended that the device be studied in more women.