User login
Coronary-subclavian steal syndrome (CSSS) is a rare clinical entity with an incidence of 0.2% to 0.7%.1 Despite its scarcity, CSSS is a condition that can result in devastating clinical consequences, such as myocardial ischemia, ranging from angina to myocardial infarction (MI) and ischemic cardiomyopathy.2
In 1974, Harjola and Valle first reported the angiographic and physiologic descriptions of CSSS in an asymptomatic patient who was found to have flow reversal in the left internal mammary artery (LIMA) graft in a follow-up coronary angiography performed 11 months after coronary artery bypass grafting (CABG).3 Because of the similarity in the pathophysiology of this condition with vertebral-subclavian steal syndrome, this clinical entity was named coronary-subclavian steal syndrome (CSSS).4,5
In steal-syndrome phenomena, there is a significant stenosis in the subclavian artery proximal to the origin of an arterial branch, either LIMA or vertebral artery, resulting in lower pressure in the distal subclavian artery. As a result, the negative pressure gradient might be sufficient to cause retrograde flow; consequently causing arterial branch “flow reversal,” and then “steal” flow from the organ—either heart or brain—supplied by that artery.3,6
Coronary-subclavian steal syndrome is caused by a reversal of flow in a previously constructed internal mammary artery (IMA)-coronary conduit graft. It typically results from hemodynamically significant subclavian artery stenosis proximal to the ipsilateral IMA. The reversal of flow will “steal” the blood from the coronary territory supplied by the IMA conduit.4,5 The absence of proximal subclavian artery stenosis does not preclude the presence of this syndrome; reversal in the IMA conduit can occur in association with upper extremity hemodialysis fistulae or anomalous connection of the left subclavian artery to the pulmonary artery in d-transposition of the great arteries.2 Although the stenosis is most commonly caused by atherosclerotic disease, other clinical entities, including Takayasu vasculitis, radiation, and giant cell arteritis, have been described.6 Patients with CSSS usually present with stable or unstable angina as well as arm claudication and various neurologic symptoms.5 The consequence of CSSS can include ischemic cardiomyopathy, acute MI,7 stroke, and death.5,8
Case Presentation
A 66-year-old man with a previous MI managed with CABG, permanent atrial fibrillation (AF), and moderate aortic stenosis presented to the ambulatory clinic with recurrent symptoms of stable angina despite being on maximal anti-anginal therapy. A coronary angiogram performed 4 years earlier had revealed significant left main artery disease and total occlusion of the right coronary artery.
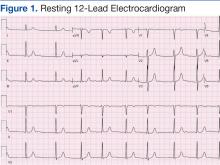
Cardiovascular examination revealed an irregular rhythm with a normal S1, variable S2, and a 3/6 systolic ejection murmur heard best at the right second intercostal space with radiation to the carotids. His peripheral pulses were equal and symmetric in the lower extremities, and no peripheral edema was noted. The remainder of the physical examination was otherwise unremarkable. The resting 12-lead electrocardiogram showed AF at a rate of 60 bpm (Figure 1).
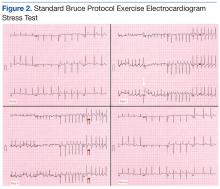
A stress test was performed to elucidate a possible coronary distribution for the cause of the chest pain.
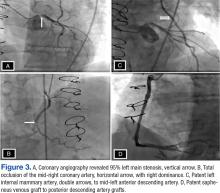
Consequently, coronary angiography was performed and showed 95% left main stenosis and total occlusion of the mid-right coronary artery with right dominance, patent LIMA to mid-LAD and patent saphenous venous graft to posterior descending artery grafts (Figure 3)
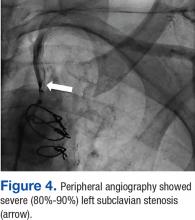
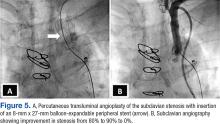
The patient underwent percutaneous transluminal angioplasty (PTA) of the subclavian stenosis with insertion of an 8 mm x 27 mm balloon-expandable peripheral stent (Figure 5) (Supplemental video 6). The patient tolerated the procedure well without complications and with resolution of his symptoms at a 6-month follow-up.
Discussion
Long-term follow-up of LIMA as a conduit to LAD has shown a 10-year patency of 95% compared with 76% for saphenous vein and an associated 10-year survival of 93.4% for LIMA compared with 88% for saphenous vein graft.9,10 Because of the superiority of LIMA outcomes, it has become the preferred graft in CABG. However, this approach is associated with 0.1% to 0.2% risk of ischemia related to flow reversal in the LIMA b
Greater awareness and improvement in diagnostic imaging have contributed to the increased incidence of CSSS and its consequences.2 Although symptoms related to myocardial ischemia, as in this case, are the most dominant in CSSS, other brachiocephalic symptoms, including vertebral-subclavian steal, transient ischemic attacks, and strokes, have been reported.11 Additionally, the same disease might compromise distal flow, resulting in extremity claudication or even distal microembolization.12
It is important to recognize that significant brachiocephalic stenosis has been reported in about 0.2% to 2.5% of patients undergoing elective CABG.6,8 Therefore, it is essential to screen for brachiocephalic artery disease before undergoing CABG. Different strategies have been suggested, including assessing pressure gradient between the upper extremities as the initial step; CSSS should be considered when the pressure gradient is > 20 mm Hg.
Other strategies include ultrasonic duplex scanning with provocation test using arm exercise or reactive hyperemia.13 Many high-volume centers are performing screening by proximal subclavian angiography in all patients undergoing coronary angiography. When significant disease is detected, arch aortography and 4-vessel cerebral angiography is performed.6 In addition, other centers have adopted the routine use of computerized tomographic angiography before CABG.14
Surgical correction of CSSS is considered to be the gold standard and can be accomplished by performing aorta-subclavian bypass, carotid-subclavian bypass, axillo-axillary bypass, or relocation of the IMA graft.2 Although this approach is invasive and carries many disadvantages related to patient comfort,surgical revascularization can be performed safely at the time of CABG and may not carry additional risk of morbidity or mortality.15 Moreover, surgical correction is the preferred modality for treatment of CSSS when the anatomy is not favorable for percutaneous intervention, such as chronic total occlusion of the subclavian artery.15Alternatively, CSSS can effectively be managed less invasively by percutaneous intervention, including PTA with stent placement,16,17 thrombectomy18 or atherectomy of the stenotic subclavian artery.19
In this patient, PTA was performed with primary stent placement. The lesion was crossed with a sheath, using combined femoral and radial access. After proper positioning, a balloon-expandable stent was deployed that resulted in complete angiographic resolution of the lesion and improvement of symptoms at 6-month follow-up. In line with previous reports, this case demonstrated that percutaneous intervention is a feasible and less invasive approach for management of CSSS.16,17 The effectiveness of the percutaneous approach has effectiveness equivalent to surgical bypass with minimal complications and good long-term success. Therefore, it has been suggested as first-line therapy in CSSS.8,16
Although preoperative screening for brachiocephalic disease before undergoing ipsilateral IMA coronary artery bypass can prevent the development of CSSS, there is controversy about the best approach for managing these concomitant conditions. Many institutions use all-vein coronary conduits, but that forgoes the benefit of a LIMA graft. Therefore, others still perform an IMA conduit after brachiocephalic reconstruction. An alternative method is to use free IMA or radial artery conduit. Currently, there are limited data about the use of endovascular treatment for brachiocephalic disease with a CABG.2
Conclusion
Coronary-subclavian steal syndrome is an important clinical condition that is associated with significant morbidity and mortality. In the Sullivan and colleagues report of 27 patients with CSSS, 59.3% had stable angina and 40.7% had acute coronary syndrome, among which 14.8% presented with acute MI.7 Therefore, early recognition is essential to prevent catastrophic consequences.
Patients with CSSS usually present with cardiac symptoms, but symptoms related to vertebral-subclavian steal and posterior cerebral insufficiency can coexist. The authors suggest routine preoperative screening for the presence of brachiocephalic disease, using ultrasonic duplex or angiography. This practice is cost-effective and essential to prevent the development of CSSS. Optimal management of brachiocephalic disease prior to CABG is debatable; however, IMA grafting and reconstruction of the brachiocephalic system seems to be a promising approach.
When CSSS develops after CABG, the condition can be successfully treated with percutaneous intervention and outcomes comparable with those of surgical bypass.
Acknowledgments
Special thanks to the division of cardiology at New Jersey VA Health Care System, in particular Steve Tsai, MD; Ronald L. Vaillancourt, RN, and Preciosa Yap, RN.
1. Marques KM, Ernst SM, Mast EG, Bal ET, Suttorp MJ, Plokker HW. Percutaneous transluminal angioplasty of the left subclavian artery to prevent or treat the coronary-subclavian steal syndrome. Am J Cardiol. 1996;78(6):687-690.
2. Takach TJ, Reul GJ, Cooley DA, et al. Myocardial thievery: the coronary-subclavian steal syndrome. Ann Thorac Surg. 2006;81(1):386-392.
3. Harjola PT, Valle M. The importance of aortic arch or subclavian angiography before coronary reconstruction. Chest. 1974;66(4):436-438.
4. Tyras DH, Barner HB. Coronary-subclavian steal. Arch Surg. 1977;112(9):1125-1127.
5. Brown AH. Coronary steal by internal mammary graft with subclavian stenosis. J Thorac Cardiovasc Surg. 1977;73(5):690-693.
6. Takach TJ, Reul GJ, Duncan JM, et al. Concomitant brachiocephalic and coronary artery disease: outcome and decision analysis. Ann Thorac Surg. 2005;80(2):564-569.
7. Sullivan TM, Gray BH, Bacharach JM, et al. Angioplasty and primary stenting of the subclavian, innominate, and common carotid arteries in 83 patients. J Vasc Surg. 1998;28(6):1059-1065.
8. Hwang HY, Kim JH, Lee W, Park JH, Kim KB. Left subclavian artery stenosis in coronary artery bypass: prevalence and revascularization strategies. Ann Thorac Surg. 2010;89(4):1146-11 50.
9. Zeff RH, Kongtahworn C, Iannone LA, et al. Internal mammary artery versus saphenous vein graft to the left anterior descending coronary artery: prospective randomized study with 10-year follow-up. Ann Thorac Surg.1988;45(5):533-536.
10. Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314(1):1-6.
11. Lee SR, Jeong MH, Rhew JY, et al. Simultaneous coronary-subclavian and vertebral-subclavian steal syndrome. Circ J. 2003;67(5):464-466.
12. Takach TJ, Beggs ML, Nykamp VJ, Reul GJ Jr. Concomitant cerebral and coronary subclavian steal. Ann Thorac Surg. 1997;63(3):853-854.
13. Branchereau A, Magnan PE, Espinoza H, Bartoli JM. Subclavian artery stenosis: hemodynamic aspects and surgical outcome. J Cardiovasc Surg (Torino). 1991;32(5):604-661.
14. Park KH, Lee HY, Lim C, et al. Clinical impact of computerised tomographic angiography performed for preoperative evaluation before coronary artery bypass grafting. Eur J Cardiothorac Surg. 2010;37(6):1346-1352.
15. Sintek M, Coverstone E, Singh J. Coronary subclavian steal syndrome. Curr Opin Cardiol. 2014;29(6):506-513.
16. Eisenhauer AC. Subclavian and innominate revascularization: surgical therapy versus catheter-based intervention. Curr Interv Cardiol Rep. 2000;2(2):101-110.
17. Bates MC, Broce M, Lavigne PS, Stone P. Subclavian artery stenting: factors influencing long-term outcome. Catheter Cardiovasc Interv. 2004;61(1):5-11.
18. Zeller T, Frank U, Burgelin K, Sinn L, Horn B, Roskamm H. Acute thrombotic subclavian artery occlusion treated with a new rotational thrombectomy device. J Endovasc Ther. 2002;9(6):917-921.
19. Breall JA, Grossman W, Stillman IE, Gianturco LE, Kim D. Atherectomy of the subclavian artery for patients with symptomatic coronary-subclavian steal syndrome. J Am Coll Cardiol. 1993;21(7):1564-1567.
Coronary-subclavian steal syndrome (CSSS) is a rare clinical entity with an incidence of 0.2% to 0.7%.1 Despite its scarcity, CSSS is a condition that can result in devastating clinical consequences, such as myocardial ischemia, ranging from angina to myocardial infarction (MI) and ischemic cardiomyopathy.2
In 1974, Harjola and Valle first reported the angiographic and physiologic descriptions of CSSS in an asymptomatic patient who was found to have flow reversal in the left internal mammary artery (LIMA) graft in a follow-up coronary angiography performed 11 months after coronary artery bypass grafting (CABG).3 Because of the similarity in the pathophysiology of this condition with vertebral-subclavian steal syndrome, this clinical entity was named coronary-subclavian steal syndrome (CSSS).4,5
In steal-syndrome phenomena, there is a significant stenosis in the subclavian artery proximal to the origin of an arterial branch, either LIMA or vertebral artery, resulting in lower pressure in the distal subclavian artery. As a result, the negative pressure gradient might be sufficient to cause retrograde flow; consequently causing arterial branch “flow reversal,” and then “steal” flow from the organ—either heart or brain—supplied by that artery.3,6
Coronary-subclavian steal syndrome is caused by a reversal of flow in a previously constructed internal mammary artery (IMA)-coronary conduit graft. It typically results from hemodynamically significant subclavian artery stenosis proximal to the ipsilateral IMA. The reversal of flow will “steal” the blood from the coronary territory supplied by the IMA conduit.4,5 The absence of proximal subclavian artery stenosis does not preclude the presence of this syndrome; reversal in the IMA conduit can occur in association with upper extremity hemodialysis fistulae or anomalous connection of the left subclavian artery to the pulmonary artery in d-transposition of the great arteries.2 Although the stenosis is most commonly caused by atherosclerotic disease, other clinical entities, including Takayasu vasculitis, radiation, and giant cell arteritis, have been described.6 Patients with CSSS usually present with stable or unstable angina as well as arm claudication and various neurologic symptoms.5 The consequence of CSSS can include ischemic cardiomyopathy, acute MI,7 stroke, and death.5,8
Case Presentation
A 66-year-old man with a previous MI managed with CABG, permanent atrial fibrillation (AF), and moderate aortic stenosis presented to the ambulatory clinic with recurrent symptoms of stable angina despite being on maximal anti-anginal therapy. A coronary angiogram performed 4 years earlier had revealed significant left main artery disease and total occlusion of the right coronary artery.
Cardiovascular examination revealed an irregular rhythm with a normal S1, variable S2, and a 3/6 systolic ejection murmur heard best at the right second intercostal space with radiation to the carotids. His peripheral pulses were equal and symmetric in the lower extremities, and no peripheral edema was noted. The remainder of the physical examination was otherwise unremarkable. The resting 12-lead electrocardiogram showed AF at a rate of 60 bpm (Figure 1).
A stress test was performed to elucidate a possible coronary distribution for the cause of the chest pain.
Consequently, coronary angiography was performed and showed 95% left main stenosis and total occlusion of the mid-right coronary artery with right dominance, patent LIMA to mid-LAD and patent saphenous venous graft to posterior descending artery grafts (Figure 3)
The patient underwent percutaneous transluminal angioplasty (PTA) of the subclavian stenosis with insertion of an 8 mm x 27 mm balloon-expandable peripheral stent (Figure 5) (Supplemental video 6). The patient tolerated the procedure well without complications and with resolution of his symptoms at a 6-month follow-up.
Discussion
Long-term follow-up of LIMA as a conduit to LAD has shown a 10-year patency of 95% compared with 76% for saphenous vein and an associated 10-year survival of 93.4% for LIMA compared with 88% for saphenous vein graft.9,10 Because of the superiority of LIMA outcomes, it has become the preferred graft in CABG. However, this approach is associated with 0.1% to 0.2% risk of ischemia related to flow reversal in the LIMA b
Greater awareness and improvement in diagnostic imaging have contributed to the increased incidence of CSSS and its consequences.2 Although symptoms related to myocardial ischemia, as in this case, are the most dominant in CSSS, other brachiocephalic symptoms, including vertebral-subclavian steal, transient ischemic attacks, and strokes, have been reported.11 Additionally, the same disease might compromise distal flow, resulting in extremity claudication or even distal microembolization.12
It is important to recognize that significant brachiocephalic stenosis has been reported in about 0.2% to 2.5% of patients undergoing elective CABG.6,8 Therefore, it is essential to screen for brachiocephalic artery disease before undergoing CABG. Different strategies have been suggested, including assessing pressure gradient between the upper extremities as the initial step; CSSS should be considered when the pressure gradient is > 20 mm Hg.
Other strategies include ultrasonic duplex scanning with provocation test using arm exercise or reactive hyperemia.13 Many high-volume centers are performing screening by proximal subclavian angiography in all patients undergoing coronary angiography. When significant disease is detected, arch aortography and 4-vessel cerebral angiography is performed.6 In addition, other centers have adopted the routine use of computerized tomographic angiography before CABG.14
Surgical correction of CSSS is considered to be the gold standard and can be accomplished by performing aorta-subclavian bypass, carotid-subclavian bypass, axillo-axillary bypass, or relocation of the IMA graft.2 Although this approach is invasive and carries many disadvantages related to patient comfort,surgical revascularization can be performed safely at the time of CABG and may not carry additional risk of morbidity or mortality.15 Moreover, surgical correction is the preferred modality for treatment of CSSS when the anatomy is not favorable for percutaneous intervention, such as chronic total occlusion of the subclavian artery.15Alternatively, CSSS can effectively be managed less invasively by percutaneous intervention, including PTA with stent placement,16,17 thrombectomy18 or atherectomy of the stenotic subclavian artery.19
In this patient, PTA was performed with primary stent placement. The lesion was crossed with a sheath, using combined femoral and radial access. After proper positioning, a balloon-expandable stent was deployed that resulted in complete angiographic resolution of the lesion and improvement of symptoms at 6-month follow-up. In line with previous reports, this case demonstrated that percutaneous intervention is a feasible and less invasive approach for management of CSSS.16,17 The effectiveness of the percutaneous approach has effectiveness equivalent to surgical bypass with minimal complications and good long-term success. Therefore, it has been suggested as first-line therapy in CSSS.8,16
Although preoperative screening for brachiocephalic disease before undergoing ipsilateral IMA coronary artery bypass can prevent the development of CSSS, there is controversy about the best approach for managing these concomitant conditions. Many institutions use all-vein coronary conduits, but that forgoes the benefit of a LIMA graft. Therefore, others still perform an IMA conduit after brachiocephalic reconstruction. An alternative method is to use free IMA or radial artery conduit. Currently, there are limited data about the use of endovascular treatment for brachiocephalic disease with a CABG.2
Conclusion
Coronary-subclavian steal syndrome is an important clinical condition that is associated with significant morbidity and mortality. In the Sullivan and colleagues report of 27 patients with CSSS, 59.3% had stable angina and 40.7% had acute coronary syndrome, among which 14.8% presented with acute MI.7 Therefore, early recognition is essential to prevent catastrophic consequences.
Patients with CSSS usually present with cardiac symptoms, but symptoms related to vertebral-subclavian steal and posterior cerebral insufficiency can coexist. The authors suggest routine preoperative screening for the presence of brachiocephalic disease, using ultrasonic duplex or angiography. This practice is cost-effective and essential to prevent the development of CSSS. Optimal management of brachiocephalic disease prior to CABG is debatable; however, IMA grafting and reconstruction of the brachiocephalic system seems to be a promising approach.
When CSSS develops after CABG, the condition can be successfully treated with percutaneous intervention and outcomes comparable with those of surgical bypass.
Acknowledgments
Special thanks to the division of cardiology at New Jersey VA Health Care System, in particular Steve Tsai, MD; Ronald L. Vaillancourt, RN, and Preciosa Yap, RN.
Coronary-subclavian steal syndrome (CSSS) is a rare clinical entity with an incidence of 0.2% to 0.7%.1 Despite its scarcity, CSSS is a condition that can result in devastating clinical consequences, such as myocardial ischemia, ranging from angina to myocardial infarction (MI) and ischemic cardiomyopathy.2
In 1974, Harjola and Valle first reported the angiographic and physiologic descriptions of CSSS in an asymptomatic patient who was found to have flow reversal in the left internal mammary artery (LIMA) graft in a follow-up coronary angiography performed 11 months after coronary artery bypass grafting (CABG).3 Because of the similarity in the pathophysiology of this condition with vertebral-subclavian steal syndrome, this clinical entity was named coronary-subclavian steal syndrome (CSSS).4,5
In steal-syndrome phenomena, there is a significant stenosis in the subclavian artery proximal to the origin of an arterial branch, either LIMA or vertebral artery, resulting in lower pressure in the distal subclavian artery. As a result, the negative pressure gradient might be sufficient to cause retrograde flow; consequently causing arterial branch “flow reversal,” and then “steal” flow from the organ—either heart or brain—supplied by that artery.3,6
Coronary-subclavian steal syndrome is caused by a reversal of flow in a previously constructed internal mammary artery (IMA)-coronary conduit graft. It typically results from hemodynamically significant subclavian artery stenosis proximal to the ipsilateral IMA. The reversal of flow will “steal” the blood from the coronary territory supplied by the IMA conduit.4,5 The absence of proximal subclavian artery stenosis does not preclude the presence of this syndrome; reversal in the IMA conduit can occur in association with upper extremity hemodialysis fistulae or anomalous connection of the left subclavian artery to the pulmonary artery in d-transposition of the great arteries.2 Although the stenosis is most commonly caused by atherosclerotic disease, other clinical entities, including Takayasu vasculitis, radiation, and giant cell arteritis, have been described.6 Patients with CSSS usually present with stable or unstable angina as well as arm claudication and various neurologic symptoms.5 The consequence of CSSS can include ischemic cardiomyopathy, acute MI,7 stroke, and death.5,8
Case Presentation
A 66-year-old man with a previous MI managed with CABG, permanent atrial fibrillation (AF), and moderate aortic stenosis presented to the ambulatory clinic with recurrent symptoms of stable angina despite being on maximal anti-anginal therapy. A coronary angiogram performed 4 years earlier had revealed significant left main artery disease and total occlusion of the right coronary artery.
Cardiovascular examination revealed an irregular rhythm with a normal S1, variable S2, and a 3/6 systolic ejection murmur heard best at the right second intercostal space with radiation to the carotids. His peripheral pulses were equal and symmetric in the lower extremities, and no peripheral edema was noted. The remainder of the physical examination was otherwise unremarkable. The resting 12-lead electrocardiogram showed AF at a rate of 60 bpm (Figure 1).
A stress test was performed to elucidate a possible coronary distribution for the cause of the chest pain.
Consequently, coronary angiography was performed and showed 95% left main stenosis and total occlusion of the mid-right coronary artery with right dominance, patent LIMA to mid-LAD and patent saphenous venous graft to posterior descending artery grafts (Figure 3)
The patient underwent percutaneous transluminal angioplasty (PTA) of the subclavian stenosis with insertion of an 8 mm x 27 mm balloon-expandable peripheral stent (Figure 5) (Supplemental video 6). The patient tolerated the procedure well without complications and with resolution of his symptoms at a 6-month follow-up.
Discussion
Long-term follow-up of LIMA as a conduit to LAD has shown a 10-year patency of 95% compared with 76% for saphenous vein and an associated 10-year survival of 93.4% for LIMA compared with 88% for saphenous vein graft.9,10 Because of the superiority of LIMA outcomes, it has become the preferred graft in CABG. However, this approach is associated with 0.1% to 0.2% risk of ischemia related to flow reversal in the LIMA b
Greater awareness and improvement in diagnostic imaging have contributed to the increased incidence of CSSS and its consequences.2 Although symptoms related to myocardial ischemia, as in this case, are the most dominant in CSSS, other brachiocephalic symptoms, including vertebral-subclavian steal, transient ischemic attacks, and strokes, have been reported.11 Additionally, the same disease might compromise distal flow, resulting in extremity claudication or even distal microembolization.12
It is important to recognize that significant brachiocephalic stenosis has been reported in about 0.2% to 2.5% of patients undergoing elective CABG.6,8 Therefore, it is essential to screen for brachiocephalic artery disease before undergoing CABG. Different strategies have been suggested, including assessing pressure gradient between the upper extremities as the initial step; CSSS should be considered when the pressure gradient is > 20 mm Hg.
Other strategies include ultrasonic duplex scanning with provocation test using arm exercise or reactive hyperemia.13 Many high-volume centers are performing screening by proximal subclavian angiography in all patients undergoing coronary angiography. When significant disease is detected, arch aortography and 4-vessel cerebral angiography is performed.6 In addition, other centers have adopted the routine use of computerized tomographic angiography before CABG.14
Surgical correction of CSSS is considered to be the gold standard and can be accomplished by performing aorta-subclavian bypass, carotid-subclavian bypass, axillo-axillary bypass, or relocation of the IMA graft.2 Although this approach is invasive and carries many disadvantages related to patient comfort,surgical revascularization can be performed safely at the time of CABG and may not carry additional risk of morbidity or mortality.15 Moreover, surgical correction is the preferred modality for treatment of CSSS when the anatomy is not favorable for percutaneous intervention, such as chronic total occlusion of the subclavian artery.15Alternatively, CSSS can effectively be managed less invasively by percutaneous intervention, including PTA with stent placement,16,17 thrombectomy18 or atherectomy of the stenotic subclavian artery.19
In this patient, PTA was performed with primary stent placement. The lesion was crossed with a sheath, using combined femoral and radial access. After proper positioning, a balloon-expandable stent was deployed that resulted in complete angiographic resolution of the lesion and improvement of symptoms at 6-month follow-up. In line with previous reports, this case demonstrated that percutaneous intervention is a feasible and less invasive approach for management of CSSS.16,17 The effectiveness of the percutaneous approach has effectiveness equivalent to surgical bypass with minimal complications and good long-term success. Therefore, it has been suggested as first-line therapy in CSSS.8,16
Although preoperative screening for brachiocephalic disease before undergoing ipsilateral IMA coronary artery bypass can prevent the development of CSSS, there is controversy about the best approach for managing these concomitant conditions. Many institutions use all-vein coronary conduits, but that forgoes the benefit of a LIMA graft. Therefore, others still perform an IMA conduit after brachiocephalic reconstruction. An alternative method is to use free IMA or radial artery conduit. Currently, there are limited data about the use of endovascular treatment for brachiocephalic disease with a CABG.2
Conclusion
Coronary-subclavian steal syndrome is an important clinical condition that is associated with significant morbidity and mortality. In the Sullivan and colleagues report of 27 patients with CSSS, 59.3% had stable angina and 40.7% had acute coronary syndrome, among which 14.8% presented with acute MI.7 Therefore, early recognition is essential to prevent catastrophic consequences.
Patients with CSSS usually present with cardiac symptoms, but symptoms related to vertebral-subclavian steal and posterior cerebral insufficiency can coexist. The authors suggest routine preoperative screening for the presence of brachiocephalic disease, using ultrasonic duplex or angiography. This practice is cost-effective and essential to prevent the development of CSSS. Optimal management of brachiocephalic disease prior to CABG is debatable; however, IMA grafting and reconstruction of the brachiocephalic system seems to be a promising approach.
When CSSS develops after CABG, the condition can be successfully treated with percutaneous intervention and outcomes comparable with those of surgical bypass.
Acknowledgments
Special thanks to the division of cardiology at New Jersey VA Health Care System, in particular Steve Tsai, MD; Ronald L. Vaillancourt, RN, and Preciosa Yap, RN.
1. Marques KM, Ernst SM, Mast EG, Bal ET, Suttorp MJ, Plokker HW. Percutaneous transluminal angioplasty of the left subclavian artery to prevent or treat the coronary-subclavian steal syndrome. Am J Cardiol. 1996;78(6):687-690.
2. Takach TJ, Reul GJ, Cooley DA, et al. Myocardial thievery: the coronary-subclavian steal syndrome. Ann Thorac Surg. 2006;81(1):386-392.
3. Harjola PT, Valle M. The importance of aortic arch or subclavian angiography before coronary reconstruction. Chest. 1974;66(4):436-438.
4. Tyras DH, Barner HB. Coronary-subclavian steal. Arch Surg. 1977;112(9):1125-1127.
5. Brown AH. Coronary steal by internal mammary graft with subclavian stenosis. J Thorac Cardiovasc Surg. 1977;73(5):690-693.
6. Takach TJ, Reul GJ, Duncan JM, et al. Concomitant brachiocephalic and coronary artery disease: outcome and decision analysis. Ann Thorac Surg. 2005;80(2):564-569.
7. Sullivan TM, Gray BH, Bacharach JM, et al. Angioplasty and primary stenting of the subclavian, innominate, and common carotid arteries in 83 patients. J Vasc Surg. 1998;28(6):1059-1065.
8. Hwang HY, Kim JH, Lee W, Park JH, Kim KB. Left subclavian artery stenosis in coronary artery bypass: prevalence and revascularization strategies. Ann Thorac Surg. 2010;89(4):1146-11 50.
9. Zeff RH, Kongtahworn C, Iannone LA, et al. Internal mammary artery versus saphenous vein graft to the left anterior descending coronary artery: prospective randomized study with 10-year follow-up. Ann Thorac Surg.1988;45(5):533-536.
10. Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314(1):1-6.
11. Lee SR, Jeong MH, Rhew JY, et al. Simultaneous coronary-subclavian and vertebral-subclavian steal syndrome. Circ J. 2003;67(5):464-466.
12. Takach TJ, Beggs ML, Nykamp VJ, Reul GJ Jr. Concomitant cerebral and coronary subclavian steal. Ann Thorac Surg. 1997;63(3):853-854.
13. Branchereau A, Magnan PE, Espinoza H, Bartoli JM. Subclavian artery stenosis: hemodynamic aspects and surgical outcome. J Cardiovasc Surg (Torino). 1991;32(5):604-661.
14. Park KH, Lee HY, Lim C, et al. Clinical impact of computerised tomographic angiography performed for preoperative evaluation before coronary artery bypass grafting. Eur J Cardiothorac Surg. 2010;37(6):1346-1352.
15. Sintek M, Coverstone E, Singh J. Coronary subclavian steal syndrome. Curr Opin Cardiol. 2014;29(6):506-513.
16. Eisenhauer AC. Subclavian and innominate revascularization: surgical therapy versus catheter-based intervention. Curr Interv Cardiol Rep. 2000;2(2):101-110.
17. Bates MC, Broce M, Lavigne PS, Stone P. Subclavian artery stenting: factors influencing long-term outcome. Catheter Cardiovasc Interv. 2004;61(1):5-11.
18. Zeller T, Frank U, Burgelin K, Sinn L, Horn B, Roskamm H. Acute thrombotic subclavian artery occlusion treated with a new rotational thrombectomy device. J Endovasc Ther. 2002;9(6):917-921.
19. Breall JA, Grossman W, Stillman IE, Gianturco LE, Kim D. Atherectomy of the subclavian artery for patients with symptomatic coronary-subclavian steal syndrome. J Am Coll Cardiol. 1993;21(7):1564-1567.
1. Marques KM, Ernst SM, Mast EG, Bal ET, Suttorp MJ, Plokker HW. Percutaneous transluminal angioplasty of the left subclavian artery to prevent or treat the coronary-subclavian steal syndrome. Am J Cardiol. 1996;78(6):687-690.
2. Takach TJ, Reul GJ, Cooley DA, et al. Myocardial thievery: the coronary-subclavian steal syndrome. Ann Thorac Surg. 2006;81(1):386-392.
3. Harjola PT, Valle M. The importance of aortic arch or subclavian angiography before coronary reconstruction. Chest. 1974;66(4):436-438.
4. Tyras DH, Barner HB. Coronary-subclavian steal. Arch Surg. 1977;112(9):1125-1127.
5. Brown AH. Coronary steal by internal mammary graft with subclavian stenosis. J Thorac Cardiovasc Surg. 1977;73(5):690-693.
6. Takach TJ, Reul GJ, Duncan JM, et al. Concomitant brachiocephalic and coronary artery disease: outcome and decision analysis. Ann Thorac Surg. 2005;80(2):564-569.
7. Sullivan TM, Gray BH, Bacharach JM, et al. Angioplasty and primary stenting of the subclavian, innominate, and common carotid arteries in 83 patients. J Vasc Surg. 1998;28(6):1059-1065.
8. Hwang HY, Kim JH, Lee W, Park JH, Kim KB. Left subclavian artery stenosis in coronary artery bypass: prevalence and revascularization strategies. Ann Thorac Surg. 2010;89(4):1146-11 50.
9. Zeff RH, Kongtahworn C, Iannone LA, et al. Internal mammary artery versus saphenous vein graft to the left anterior descending coronary artery: prospective randomized study with 10-year follow-up. Ann Thorac Surg.1988;45(5):533-536.
10. Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314(1):1-6.
11. Lee SR, Jeong MH, Rhew JY, et al. Simultaneous coronary-subclavian and vertebral-subclavian steal syndrome. Circ J. 2003;67(5):464-466.
12. Takach TJ, Beggs ML, Nykamp VJ, Reul GJ Jr. Concomitant cerebral and coronary subclavian steal. Ann Thorac Surg. 1997;63(3):853-854.
13. Branchereau A, Magnan PE, Espinoza H, Bartoli JM. Subclavian artery stenosis: hemodynamic aspects and surgical outcome. J Cardiovasc Surg (Torino). 1991;32(5):604-661.
14. Park KH, Lee HY, Lim C, et al. Clinical impact of computerised tomographic angiography performed for preoperative evaluation before coronary artery bypass grafting. Eur J Cardiothorac Surg. 2010;37(6):1346-1352.
15. Sintek M, Coverstone E, Singh J. Coronary subclavian steal syndrome. Curr Opin Cardiol. 2014;29(6):506-513.
16. Eisenhauer AC. Subclavian and innominate revascularization: surgical therapy versus catheter-based intervention. Curr Interv Cardiol Rep. 2000;2(2):101-110.
17. Bates MC, Broce M, Lavigne PS, Stone P. Subclavian artery stenting: factors influencing long-term outcome. Catheter Cardiovasc Interv. 2004;61(1):5-11.
18. Zeller T, Frank U, Burgelin K, Sinn L, Horn B, Roskamm H. Acute thrombotic subclavian artery occlusion treated with a new rotational thrombectomy device. J Endovasc Ther. 2002;9(6):917-921.
19. Breall JA, Grossman W, Stillman IE, Gianturco LE, Kim D. Atherectomy of the subclavian artery for patients with symptomatic coronary-subclavian steal syndrome. J Am Coll Cardiol. 1993;21(7):1564-1567.