User login
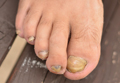
Onychomycosis is a chronic fungal infection of the nails. Dermatophytes are the most common etiologic agents, but yeasts and nondermatophyte molds also constitute a substantial number of cases.1 An accumulation of debris under distorted, deformed, thickened, and discolored nails, particularly with ragged and furrowed edges, strongly suggests tinea unguium.2 Candidal onychomycosis (CO) lacks gross distortion and accumulated detritus and mainly affects fingernails.3 Nondermatophytic molds cause 1.5% to 6% of cases of onychomycosis, mostly seen in toenails of elderly individuals with a history of trauma.4 Onychomycosis affects 5.5% of the world population5 and represents 20% to 40% of all onychopathies and approximately 30% of cutaneous mycotic infections.6
The incidence of onychomycosis ranges from 0.5% to 5% in the general population in India.7 The incidence is particularly high in warm humid climates such as India.8 Researchers have found certain habits of the population in the Indian subcontinent (eg, walking with bare feet, wearing ill-fitting shoes, nail-biting [eg, onychophagia], working with chemicals) to be contributing factors for onychomycosis.9 Several studies have shown that the prevalence of onychomycosis increases with age, possibly due to poor peripheral circulation, diabetes mellitus, repeated nail trauma, prolonged exposure to pathogenic fungi, suboptimal immune function, inactivity, or inability to trim the toenails and care for the feet.10 Nail infection is a cosmetic problem with serious physical and psychological morbidity and also serves as the fungal reservoir for skin infections. Besides destruction and disfigurement of the nail plate, onychomycosis can lead to self-consciousness and impairment of daily functioning.11
Nail dystrophy occurs secondary to various systemic disorders or can be associated with other dermatologic conditions. Nail discoloration and other onychia should be differentiated from onychomycosis by classifying nail lesions as distal lateral subungual onychomycosis, proximal subungual onychomycosis (PSO), CO, white superficial onychomycosis (WSO), and total dystrophic onychomycosis.12 Laboratory investigation is necessary to accurately differentiate between fungal infections and other skin diseases before starting treatment. Our hospital-based study sought to determine the incidence and epidemiology of onychomycosis with an analysis of 134 participants with clinically suspected onychomycosis. We evaluated prevalence based on age, sex, and occupation, as well as the most common pathogens.
Materials and Methods
Study Design and Participants
The study population consisted of 134 patients with clinically suspected onychomycosis who visited the dermatology department at the Veer Chandra Singh Garhwali Government Institute of Medical Sciences and Research Institute in Uttarakhand, India (October 2010 to October 2011). A thorough history was obtained and a detailed examination of the distorted nails was conducted in the microbiology laboratory. Patient history and demographic factors such as age, sex, occupation, and related history of risk factors for onychomycosis were recorded pro forma. Some of the details such as itching, family history of fungal infection, and prior cutaneous infections were recorded. Patients who were undergoing treatment with systemic or topical antifungal agents in the 4 weeks preceding the study period were excluded to rule out false-negative cases and to avoid the influence of antifungal agents on the disease course.
Assessments
Two samples were taken from each patient on different days. Participants were divided into 4 groups based on occupation: farmer, housewife, student, and other (eg, clerk, shopkeeper, painter). Clinical presentation of discoloration, onycholysis, subungual hyperkeratosis, and nail thickening affecting the distal and/or lateral nail plate was defined as distal lateral subungual onychomycosis; discoloration and onycholysis affecting the proximal part of the nail was defined as PSO; association with paronychia and distal and lateral onycholysis was defined as CO; white opaque patches on the nail surface were defined as WSO; and end-stage nail disease was defined as total dystrophic onychomycosis.
Prior to sampling, the nails were cleaned with a 70% alcohol solution. Nail clippings were obtained using presterilized nail clippers and a blunt no. 15 scalpel blade and were placed on sterilized black paper. Each nail sample was divided into 2 parts: one for direct microscopy and one for culture. Nail clippings were subjected to microscopic examination after clearing in 20% potassium hydroxide solution. The slides were examined for fungal hyphae, arthrospores, yeasts, and pseudohyphal forms. Culture was done with Emmons modification of Sabouraud dextrose agar (incubated at 27°C for molds and 37°C for yeasts) as well as with 0.4% chloramphenicol and 5% cycloheximide (incubated at 27°C). Culture tubes were examined daily for the first week and on alternate days thereafter for 4 weeks of incubation.
Dermatophytes were identified based on the colony morphology, growth rate, texture, border, and pigmentation in the obverse and reverse of culture media and microscopic examination using lactophenol cotton blue tease mount. Yeast colonies were identified microscopically with Gram stain, and species were identified by germ tube, carbohydrate assimilation, and fermentation tests.13 Nondermatophyte molds were identified by colony morphology, microscopic examination, and slide culture. Molds were considered as pathogens in the presence of the following criteria: (1) absence of other fungal growth in the same culture tube; (2) presence of mold growth in all 3 samples; and (3) presence of filaments identified on direct examination.
Results
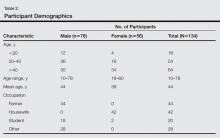
Of 134 clinically suspected cases of onychomycosis, 78 (58.2%) were from fingernails and 56 (41.8%) from toenails. Clinical diagnosis was confirmed in 96 (71.6%) cases by both fungal culture and direct microscopy but was confirmed by direct microscopy alone in only 76 (56.7%) cases. False-negative results were found in 23.9% (32/134) of participants with direct microscopy and 9.0% (12/134) with fungal cultures. The results of direct microscopy and fungal culture are outlined in Table 1. The study included 78 (58.2%) males and 56 (41.8%) females with a mean age of 44 years. Highest prevalence (47.8%) was seen in participants older than 40 years and lowest prevalence (11.9%) in participants younger than 20 years. In total, 32.8% of participants were farmers, 31.3% were housewives, 14.9% were students, and 20.9% performed other occupations. Disease history at the time of first presentation varied from 1 month to more than 2 years; 33.6% of participants had a 1- to 6-month history of disease, while only 3.7% had a disease history of less than 1 month at presentation. The demographic data are further outlined in Table 2.
Distal lateral subungual onychomycosis was the most prevalent clinical pattern found in 66 (49.3%) participants; fungal isolates were found in 60 of these participants. The next most prevalent clinical pattern was PSO, which was found in 34 (25.4%) participants, 12 showing fungal growth. A clinical pattern of CO was noted in 28 (20.9%) participants, 22 showing fungal growth; WSO was noted in 10 (7.5%) participants, 2 showing fungal growth.
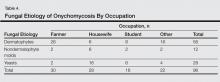
Of 96 culture-positive cases, dermatophytes were the most common pathogens isolated in 56 (58.3%) participants, followed by Candida species in 28 (29.2%) participants. Nondermatophyte molds were isolated in 12 (12.5%) participants. The various dermatophytes, Candida species, and nondermatophyte molds that were isolated on fungal culture are outlined in Table 3. Of the 96 participants with positive fungal cultures, 30 (31.2%) were farmers working with soil, 28 (29.2%) were housewives associated with wet work, 16 (16.7%) were students associated with increased physical exercise from extracurricular activity, and 22 (22.9%) were in other occupations (Table 4).
Comment
The term onychomycosis is derived from onyx, the Greek word for nail, and mykes, the Greek word for fungus. Onychomycosis is a chronic mycotic infection of the fingernails and toenails that can have a serious impact on patients’ quality of life. The fungi known to cause onychomycosis vary among geographic areas, primarily due to differences in climate.14 The isolation rate of onychomycosis in our hospital-based study was 71.6%, which is in accordance with various studies in India and abroad, including 60% in Karnataka, India5; 82.3% in Sikkim, India6; and 86.9% in Turkey.1 However, other studies have shown lower isolation rates of 39.5% in Central Delhi, India,15 and 37.6% in Himachal Pradesh, India.16 Some patients with onychomycosis may not seek medical attention, which may explain the difference in the prevalence of onychomycosis observed worldwide.17 The prevalence of onychomycosis by age also varies. In our study, participants older than 40 years showed the highest prevalence (47.8%), which is in accordance with other studies from India18 and abroad.19,20 In contrast, some Indian studies15,21,22 have reported a higher prevalence in younger adults (ie, 21–30 years), which may be attributed to greater self-consciousness about nail discoloration and disfigurement as well as increased physical activity and different shoe-wearing habits. A higher prevalence in older adults, as observed in our study as well some other studies,19,21 may be due to poor peripheral circulation, diabetes mellitus, repeated nail trauma, longer exposure to pathogenic fungi, suboptimal immune function, inactivity, and poor hygiene.10
In our study, suspected onychomycosis was more common in males (58.2%) than in females (41.8%). These results are in accordance with many of the studies in the worldwide literature.1,10,11,15,16,23-25 A higher isolation rate in males worldwide may be due to common use of occlusive footwear, more exposure to outdoor conditions, and increased physical activity, leading to an increased likelihood of trauma. The importance of trauma to the nails as a predisposing factor for onychomycosis is well established.24 In our study, the majority of males wore shoes regardless of occupation. Perspiration of the feet when wearing socks and/or shoes can generate a warm moist environment that promotes the growth of fungi and predisposes patients to onychomycosis. Similar observations have been reported by other investigators.21,22,25,26
The incidence of onychomycosis was almost evenly distributed among farmers, housewives, and the miscellaneous group, whereas a high isolation rate was noted among students. Of 20 students included in our study, onychomycosis was confirmed in 16, which may be related to an increased use of synthetic sports shoes and socks that retain sweat as well as vigorous physical activity frequently resulting in nail injuries among this patient population.11 Younger patients may be more conscious of their appearance and therefore may be more likely to seek treatment. Similar observations have been reported by other researchers.15,21,22
In our study, dermatophytes were the most commonly found pathogens (58.3%), which is comparable to other studies.15,18,22Trichophyton mentagrophytes was the most frequently isolated dermatophyte from cultures, which was in concordance with a study from Delhi.15 In some studies,18,20,22Trichophyton rubrum has been reported as the most prevalent dermatophyte, but we identified Trichophyton rubrum in only 18 participants, which can be attributed to variations in epidemiology based on geographic region. Nondermatophyte molds were isolated in 12.5% of participants, with Aspergillus niger being the most common isolate found in 8 cases. Other isolated species were Alternaria alternata and Fusarium solani found in 2 cases each. Aspergillus niger has been reported in worldwide studies as an important cause of onychomycosis.15,18,19,21,22
In 28 cases (29.2%) involving Candida species, Candida albicans, Candida parapsilosis, and Candida tropicalis were the most common pathogens, respectively, which is in accordance with many studies.15,20-22,25 In 28 cases of CO, females (n=16) were affected more than males (n=12). All of the females were housewives and C albicans was predominantly isolated from the fingernails. Household responsibilities involving kitchen work (eg, cutting and peeling vegetables, washing utensils, cleaning the house/laundry) may chronically expose housewives to moist environments and make them more prone to injury, thus facilitating easy entry of fungal agents.
Distal lateral subungual onychomycosis was the most prevalent clinical type found (n=66), which is comparable to other reports.20,22,25 Proximal subungual onychomycosis was the second most common type; however, a greater incidence has been reported by some researchers,23,24 while others have reported a lower incidence.20,21 Candidial onychomycosis and WSO were not common in our study, and PSO was not associated with any immunodeficiency disease, as reported by other researchers.15,20
Of 134 suspected cases of onychomycosis, 71.6% were confirmed by both direct microscopy and fungal culture, but only 56.7% were confirmed by direct microscopy alone. If we had relied on microscopy with potassium hydroxide only, we would have missed 23.9% of cases. Therefore, nail scrapings should always be subjected to fungal culture as well as direct microscopy, as both are necessary for accurate diagnosis and treatment of onychomycosis. If onychomycosis is not successfully treated, it can act as a reservoir of fungal infection affecting other parts of the body with the potential to pass infection on to others.
Conclusion
Clinical examination alone is not sufficient for diagnosing onychomycosis14,18,20; in many cases of suspected onychomycosis with nail changes, mycologic examination does not confirm fungal infection. In our study, only 71.6% of participants with nail changes proved to be of fungal etiology. Other researchers from different geographic locations have reported similar results with lower incidence (eg, 39.5%,15 37.6%,16 51.7%,18 45.3%21) of fungal etiology in such cases. Therefore, both clinical and mycologic examinations are important for establishing the diagnosis and selecting the most suitable antifungal agent, which is possible only if the underlying pathogen is correctly identified.
1. Yenişehirli G, Bulut Y, Sezer E, et al. Onychomycosis infections in the Middle Black Sea Region, Turkey. Int J Dermatol. 2009;48:956-959.
2. Kouskoukis CE, Scher RK, Ackerman AB. What histologic finding distinguishes onychomycosis and psoriasis? Am J Dermatopathol. 1983;5:501-503.
3. Rippon JW. Medical mycology. In: Wonsiewicz M, ed. The Pathogenic Fungi and the Pathogenic Actinomycetes. 3rd ed. Philadelphia, PA: WB Saunders; 1988:169-275.
4. Greer DL. Evolving role of nondermatophytes in onychomycosis. Int J Dermatol. 1995;34:521-524.
5. Murray SC, Dawber RP. Onychomycosis of toenails: orthopaedic and podiatric considerations. Australas J Dermatol. 2002;43:105-112.
6. Achten G, Wanet-Rouard J. Onychomycoses in the laboratory. Mykosen Suppl. 1978;1:125-127.
7. Sobhanadri C, Rao DT, Babu KS. Clinical and mycological study of superficial fungal infections at Government General Hospital: guntur and their response to treatment with hamycin, dermostatin and dermamycin. Indian J Dermatol Venereol. 1970;36:209-214.
8. Jain S, Sehgal VN. Commentary: onychomycosis: an epidemio-etiologic perspective. Int J Dermatol. 2000;39:100-103.
9. Sehgal VN, Aggarwal AK, Srivastava G, et al. Onychomycosis: a 3 year clinicomycologic hospital-based study. Skinmed. 2007;6:11-17.
10. Elewski BE, Charif MA. Prevalence of onychomycosis in patients attending a dermatology clinic in northeastern Ohio for the other conditions. Arch Dermatol. 1997;133:1172-1173.
11. Scher RK. Onychomycosis is more than a cosmetic problem. Br J Dermatol. 1994;130(suppl 43):S15.
12. Godoy-Martinez PG, Nunes FG, Tomimori-Yamashita J, et al. Onychomycosis in São Paulo, Brazil [published online ahead of print May 8, 2009]. Mycopathologia. 2009;168:111-116.
13. Larone DH. Medically Important Fungi: A Guide to Identification. 4th ed. Washington, DC: American Society for Microbiology Press; 2002.
14. Sehgal VN, Srivastava G, Dogra S, et al. Onychomycosis: an Asian perspective. Skinmed. 2010;8:37-45.
15. Sanjiv A, Shalini M, Charoo H. Etiological agents of onychomycosis from a tertiary care hospital in Central Delhi, India. Indian J Fund Appl Life Science. 2011;1:11-14.
16. Gupta M, Sharma NL, Kanga AK, et al. Onychomycosis: clinic-mycologic study of 130 patients from Himachal Pradesh, India. Indian J Dermatol Venereol Leprol. 2007;73:389-392.
17. Eleweski BE. Diagnostic techniques for confirming onychomycosis. J Am Acad Dermatol. 1996;35(3, pt 2):S6-S9.
18. Das NK, Ghosh P, Das S, et al. A study on the etiological agent and clinico-mycological correlation of fingernail onychomycosis in eastern India. Indian J Dermatol. 2008;53:75-79.
19. Bassiri-Jahromi S, Khaksar AA. Nondermatophytic moulds as a causative agent of onychomycosis in Tehran. Indian J Dermatol. 2010;55:140-143.
20. Bokhari MA, Hussain I, Jahangir M, et al. Onychomycosis in Lahore, Pakistan. Int J Dermatol. 1999;38:591-595.
21. Jesudanam TM, Rao GR, Lakshmi DJ, et al. Onychomycosis: a significant medical problem. Indian J Dermatol Venereol Leprol. 2002;68:326-329.
22. Ahmad M, Gupta S, Gupte S. A clinico-mycological study of onychomycosis. EDOJ. 2010;6:1-9.
23. Vinod S, Grover S, Dash K, et al. A clinico-mycological evaluation of onychomycosis. Indian J Dermatol Venereol Leprol. 2000;66:238-240.
24. Veer P, Patwardhan NS, Damle AS. Study of onychomycosis: prevailing fungi and pattern of infection. Indian J Med Microbiol. 2007;25:53-56.
25. Garg A, Venkatesh V, Singh M, et al. Onychomycosis in central India: a clinicoetiologic correlation. Int J Dermatol. 2004;43:498-502.
26. Adhikari L, Das Gupta A, Pal R, et al. Clinico-etiologic correlates of onychomycosis in Sikkim. Indian J Pathol Microbiol. 2009;52:194-197.
Onychomycosis is a chronic fungal infection of the nails. Dermatophytes are the most common etiologic agents, but yeasts and nondermatophyte molds also constitute a substantial number of cases.1 An accumulation of debris under distorted, deformed, thickened, and discolored nails, particularly with ragged and furrowed edges, strongly suggests tinea unguium.2 Candidal onychomycosis (CO) lacks gross distortion and accumulated detritus and mainly affects fingernails.3 Nondermatophytic molds cause 1.5% to 6% of cases of onychomycosis, mostly seen in toenails of elderly individuals with a history of trauma.4 Onychomycosis affects 5.5% of the world population5 and represents 20% to 40% of all onychopathies and approximately 30% of cutaneous mycotic infections.6
The incidence of onychomycosis ranges from 0.5% to 5% in the general population in India.7 The incidence is particularly high in warm humid climates such as India.8 Researchers have found certain habits of the population in the Indian subcontinent (eg, walking with bare feet, wearing ill-fitting shoes, nail-biting [eg, onychophagia], working with chemicals) to be contributing factors for onychomycosis.9 Several studies have shown that the prevalence of onychomycosis increases with age, possibly due to poor peripheral circulation, diabetes mellitus, repeated nail trauma, prolonged exposure to pathogenic fungi, suboptimal immune function, inactivity, or inability to trim the toenails and care for the feet.10 Nail infection is a cosmetic problem with serious physical and psychological morbidity and also serves as the fungal reservoir for skin infections. Besides destruction and disfigurement of the nail plate, onychomycosis can lead to self-consciousness and impairment of daily functioning.11
Nail dystrophy occurs secondary to various systemic disorders or can be associated with other dermatologic conditions. Nail discoloration and other onychia should be differentiated from onychomycosis by classifying nail lesions as distal lateral subungual onychomycosis, proximal subungual onychomycosis (PSO), CO, white superficial onychomycosis (WSO), and total dystrophic onychomycosis.12 Laboratory investigation is necessary to accurately differentiate between fungal infections and other skin diseases before starting treatment. Our hospital-based study sought to determine the incidence and epidemiology of onychomycosis with an analysis of 134 participants with clinically suspected onychomycosis. We evaluated prevalence based on age, sex, and occupation, as well as the most common pathogens.
Materials and Methods
Study Design and Participants
The study population consisted of 134 patients with clinically suspected onychomycosis who visited the dermatology department at the Veer Chandra Singh Garhwali Government Institute of Medical Sciences and Research Institute in Uttarakhand, India (October 2010 to October 2011). A thorough history was obtained and a detailed examination of the distorted nails was conducted in the microbiology laboratory. Patient history and demographic factors such as age, sex, occupation, and related history of risk factors for onychomycosis were recorded pro forma. Some of the details such as itching, family history of fungal infection, and prior cutaneous infections were recorded. Patients who were undergoing treatment with systemic or topical antifungal agents in the 4 weeks preceding the study period were excluded to rule out false-negative cases and to avoid the influence of antifungal agents on the disease course.
Assessments
Two samples were taken from each patient on different days. Participants were divided into 4 groups based on occupation: farmer, housewife, student, and other (eg, clerk, shopkeeper, painter). Clinical presentation of discoloration, onycholysis, subungual hyperkeratosis, and nail thickening affecting the distal and/or lateral nail plate was defined as distal lateral subungual onychomycosis; discoloration and onycholysis affecting the proximal part of the nail was defined as PSO; association with paronychia and distal and lateral onycholysis was defined as CO; white opaque patches on the nail surface were defined as WSO; and end-stage nail disease was defined as total dystrophic onychomycosis.
Prior to sampling, the nails were cleaned with a 70% alcohol solution. Nail clippings were obtained using presterilized nail clippers and a blunt no. 15 scalpel blade and were placed on sterilized black paper. Each nail sample was divided into 2 parts: one for direct microscopy and one for culture. Nail clippings were subjected to microscopic examination after clearing in 20% potassium hydroxide solution. The slides were examined for fungal hyphae, arthrospores, yeasts, and pseudohyphal forms. Culture was done with Emmons modification of Sabouraud dextrose agar (incubated at 27°C for molds and 37°C for yeasts) as well as with 0.4% chloramphenicol and 5% cycloheximide (incubated at 27°C). Culture tubes were examined daily for the first week and on alternate days thereafter for 4 weeks of incubation.
Dermatophytes were identified based on the colony morphology, growth rate, texture, border, and pigmentation in the obverse and reverse of culture media and microscopic examination using lactophenol cotton blue tease mount. Yeast colonies were identified microscopically with Gram stain, and species were identified by germ tube, carbohydrate assimilation, and fermentation tests.13 Nondermatophyte molds were identified by colony morphology, microscopic examination, and slide culture. Molds were considered as pathogens in the presence of the following criteria: (1) absence of other fungal growth in the same culture tube; (2) presence of mold growth in all 3 samples; and (3) presence of filaments identified on direct examination.
Results
Of 134 clinically suspected cases of onychomycosis, 78 (58.2%) were from fingernails and 56 (41.8%) from toenails. Clinical diagnosis was confirmed in 96 (71.6%) cases by both fungal culture and direct microscopy but was confirmed by direct microscopy alone in only 76 (56.7%) cases. False-negative results were found in 23.9% (32/134) of participants with direct microscopy and 9.0% (12/134) with fungal cultures. The results of direct microscopy and fungal culture are outlined in Table 1. The study included 78 (58.2%) males and 56 (41.8%) females with a mean age of 44 years. Highest prevalence (47.8%) was seen in participants older than 40 years and lowest prevalence (11.9%) in participants younger than 20 years. In total, 32.8% of participants were farmers, 31.3% were housewives, 14.9% were students, and 20.9% performed other occupations. Disease history at the time of first presentation varied from 1 month to more than 2 years; 33.6% of participants had a 1- to 6-month history of disease, while only 3.7% had a disease history of less than 1 month at presentation. The demographic data are further outlined in Table 2.
Distal lateral subungual onychomycosis was the most prevalent clinical pattern found in 66 (49.3%) participants; fungal isolates were found in 60 of these participants. The next most prevalent clinical pattern was PSO, which was found in 34 (25.4%) participants, 12 showing fungal growth. A clinical pattern of CO was noted in 28 (20.9%) participants, 22 showing fungal growth; WSO was noted in 10 (7.5%) participants, 2 showing fungal growth.
Of 96 culture-positive cases, dermatophytes were the most common pathogens isolated in 56 (58.3%) participants, followed by Candida species in 28 (29.2%) participants. Nondermatophyte molds were isolated in 12 (12.5%) participants. The various dermatophytes, Candida species, and nondermatophyte molds that were isolated on fungal culture are outlined in Table 3. Of the 96 participants with positive fungal cultures, 30 (31.2%) were farmers working with soil, 28 (29.2%) were housewives associated with wet work, 16 (16.7%) were students associated with increased physical exercise from extracurricular activity, and 22 (22.9%) were in other occupations (Table 4).
Comment
The term onychomycosis is derived from onyx, the Greek word for nail, and mykes, the Greek word for fungus. Onychomycosis is a chronic mycotic infection of the fingernails and toenails that can have a serious impact on patients’ quality of life. The fungi known to cause onychomycosis vary among geographic areas, primarily due to differences in climate.14 The isolation rate of onychomycosis in our hospital-based study was 71.6%, which is in accordance with various studies in India and abroad, including 60% in Karnataka, India5; 82.3% in Sikkim, India6; and 86.9% in Turkey.1 However, other studies have shown lower isolation rates of 39.5% in Central Delhi, India,15 and 37.6% in Himachal Pradesh, India.16 Some patients with onychomycosis may not seek medical attention, which may explain the difference in the prevalence of onychomycosis observed worldwide.17 The prevalence of onychomycosis by age also varies. In our study, participants older than 40 years showed the highest prevalence (47.8%), which is in accordance with other studies from India18 and abroad.19,20 In contrast, some Indian studies15,21,22 have reported a higher prevalence in younger adults (ie, 21–30 years), which may be attributed to greater self-consciousness about nail discoloration and disfigurement as well as increased physical activity and different shoe-wearing habits. A higher prevalence in older adults, as observed in our study as well some other studies,19,21 may be due to poor peripheral circulation, diabetes mellitus, repeated nail trauma, longer exposure to pathogenic fungi, suboptimal immune function, inactivity, and poor hygiene.10
In our study, suspected onychomycosis was more common in males (58.2%) than in females (41.8%). These results are in accordance with many of the studies in the worldwide literature.1,10,11,15,16,23-25 A higher isolation rate in males worldwide may be due to common use of occlusive footwear, more exposure to outdoor conditions, and increased physical activity, leading to an increased likelihood of trauma. The importance of trauma to the nails as a predisposing factor for onychomycosis is well established.24 In our study, the majority of males wore shoes regardless of occupation. Perspiration of the feet when wearing socks and/or shoes can generate a warm moist environment that promotes the growth of fungi and predisposes patients to onychomycosis. Similar observations have been reported by other investigators.21,22,25,26
The incidence of onychomycosis was almost evenly distributed among farmers, housewives, and the miscellaneous group, whereas a high isolation rate was noted among students. Of 20 students included in our study, onychomycosis was confirmed in 16, which may be related to an increased use of synthetic sports shoes and socks that retain sweat as well as vigorous physical activity frequently resulting in nail injuries among this patient population.11 Younger patients may be more conscious of their appearance and therefore may be more likely to seek treatment. Similar observations have been reported by other researchers.15,21,22
In our study, dermatophytes were the most commonly found pathogens (58.3%), which is comparable to other studies.15,18,22Trichophyton mentagrophytes was the most frequently isolated dermatophyte from cultures, which was in concordance with a study from Delhi.15 In some studies,18,20,22Trichophyton rubrum has been reported as the most prevalent dermatophyte, but we identified Trichophyton rubrum in only 18 participants, which can be attributed to variations in epidemiology based on geographic region. Nondermatophyte molds were isolated in 12.5% of participants, with Aspergillus niger being the most common isolate found in 8 cases. Other isolated species were Alternaria alternata and Fusarium solani found in 2 cases each. Aspergillus niger has been reported in worldwide studies as an important cause of onychomycosis.15,18,19,21,22
In 28 cases (29.2%) involving Candida species, Candida albicans, Candida parapsilosis, and Candida tropicalis were the most common pathogens, respectively, which is in accordance with many studies.15,20-22,25 In 28 cases of CO, females (n=16) were affected more than males (n=12). All of the females were housewives and C albicans was predominantly isolated from the fingernails. Household responsibilities involving kitchen work (eg, cutting and peeling vegetables, washing utensils, cleaning the house/laundry) may chronically expose housewives to moist environments and make them more prone to injury, thus facilitating easy entry of fungal agents.
Distal lateral subungual onychomycosis was the most prevalent clinical type found (n=66), which is comparable to other reports.20,22,25 Proximal subungual onychomycosis was the second most common type; however, a greater incidence has been reported by some researchers,23,24 while others have reported a lower incidence.20,21 Candidial onychomycosis and WSO were not common in our study, and PSO was not associated with any immunodeficiency disease, as reported by other researchers.15,20
Of 134 suspected cases of onychomycosis, 71.6% were confirmed by both direct microscopy and fungal culture, but only 56.7% were confirmed by direct microscopy alone. If we had relied on microscopy with potassium hydroxide only, we would have missed 23.9% of cases. Therefore, nail scrapings should always be subjected to fungal culture as well as direct microscopy, as both are necessary for accurate diagnosis and treatment of onychomycosis. If onychomycosis is not successfully treated, it can act as a reservoir of fungal infection affecting other parts of the body with the potential to pass infection on to others.
Conclusion
Clinical examination alone is not sufficient for diagnosing onychomycosis14,18,20; in many cases of suspected onychomycosis with nail changes, mycologic examination does not confirm fungal infection. In our study, only 71.6% of participants with nail changes proved to be of fungal etiology. Other researchers from different geographic locations have reported similar results with lower incidence (eg, 39.5%,15 37.6%,16 51.7%,18 45.3%21) of fungal etiology in such cases. Therefore, both clinical and mycologic examinations are important for establishing the diagnosis and selecting the most suitable antifungal agent, which is possible only if the underlying pathogen is correctly identified.
Onychomycosis is a chronic fungal infection of the nails. Dermatophytes are the most common etiologic agents, but yeasts and nondermatophyte molds also constitute a substantial number of cases.1 An accumulation of debris under distorted, deformed, thickened, and discolored nails, particularly with ragged and furrowed edges, strongly suggests tinea unguium.2 Candidal onychomycosis (CO) lacks gross distortion and accumulated detritus and mainly affects fingernails.3 Nondermatophytic molds cause 1.5% to 6% of cases of onychomycosis, mostly seen in toenails of elderly individuals with a history of trauma.4 Onychomycosis affects 5.5% of the world population5 and represents 20% to 40% of all onychopathies and approximately 30% of cutaneous mycotic infections.6
The incidence of onychomycosis ranges from 0.5% to 5% in the general population in India.7 The incidence is particularly high in warm humid climates such as India.8 Researchers have found certain habits of the population in the Indian subcontinent (eg, walking with bare feet, wearing ill-fitting shoes, nail-biting [eg, onychophagia], working with chemicals) to be contributing factors for onychomycosis.9 Several studies have shown that the prevalence of onychomycosis increases with age, possibly due to poor peripheral circulation, diabetes mellitus, repeated nail trauma, prolonged exposure to pathogenic fungi, suboptimal immune function, inactivity, or inability to trim the toenails and care for the feet.10 Nail infection is a cosmetic problem with serious physical and psychological morbidity and also serves as the fungal reservoir for skin infections. Besides destruction and disfigurement of the nail plate, onychomycosis can lead to self-consciousness and impairment of daily functioning.11
Nail dystrophy occurs secondary to various systemic disorders or can be associated with other dermatologic conditions. Nail discoloration and other onychia should be differentiated from onychomycosis by classifying nail lesions as distal lateral subungual onychomycosis, proximal subungual onychomycosis (PSO), CO, white superficial onychomycosis (WSO), and total dystrophic onychomycosis.12 Laboratory investigation is necessary to accurately differentiate between fungal infections and other skin diseases before starting treatment. Our hospital-based study sought to determine the incidence and epidemiology of onychomycosis with an analysis of 134 participants with clinically suspected onychomycosis. We evaluated prevalence based on age, sex, and occupation, as well as the most common pathogens.
Materials and Methods
Study Design and Participants
The study population consisted of 134 patients with clinically suspected onychomycosis who visited the dermatology department at the Veer Chandra Singh Garhwali Government Institute of Medical Sciences and Research Institute in Uttarakhand, India (October 2010 to October 2011). A thorough history was obtained and a detailed examination of the distorted nails was conducted in the microbiology laboratory. Patient history and demographic factors such as age, sex, occupation, and related history of risk factors for onychomycosis were recorded pro forma. Some of the details such as itching, family history of fungal infection, and prior cutaneous infections were recorded. Patients who were undergoing treatment with systemic or topical antifungal agents in the 4 weeks preceding the study period were excluded to rule out false-negative cases and to avoid the influence of antifungal agents on the disease course.
Assessments
Two samples were taken from each patient on different days. Participants were divided into 4 groups based on occupation: farmer, housewife, student, and other (eg, clerk, shopkeeper, painter). Clinical presentation of discoloration, onycholysis, subungual hyperkeratosis, and nail thickening affecting the distal and/or lateral nail plate was defined as distal lateral subungual onychomycosis; discoloration and onycholysis affecting the proximal part of the nail was defined as PSO; association with paronychia and distal and lateral onycholysis was defined as CO; white opaque patches on the nail surface were defined as WSO; and end-stage nail disease was defined as total dystrophic onychomycosis.
Prior to sampling, the nails were cleaned with a 70% alcohol solution. Nail clippings were obtained using presterilized nail clippers and a blunt no. 15 scalpel blade and were placed on sterilized black paper. Each nail sample was divided into 2 parts: one for direct microscopy and one for culture. Nail clippings were subjected to microscopic examination after clearing in 20% potassium hydroxide solution. The slides were examined for fungal hyphae, arthrospores, yeasts, and pseudohyphal forms. Culture was done with Emmons modification of Sabouraud dextrose agar (incubated at 27°C for molds and 37°C for yeasts) as well as with 0.4% chloramphenicol and 5% cycloheximide (incubated at 27°C). Culture tubes were examined daily for the first week and on alternate days thereafter for 4 weeks of incubation.
Dermatophytes were identified based on the colony morphology, growth rate, texture, border, and pigmentation in the obverse and reverse of culture media and microscopic examination using lactophenol cotton blue tease mount. Yeast colonies were identified microscopically with Gram stain, and species were identified by germ tube, carbohydrate assimilation, and fermentation tests.13 Nondermatophyte molds were identified by colony morphology, microscopic examination, and slide culture. Molds were considered as pathogens in the presence of the following criteria: (1) absence of other fungal growth in the same culture tube; (2) presence of mold growth in all 3 samples; and (3) presence of filaments identified on direct examination.
Results
Of 134 clinically suspected cases of onychomycosis, 78 (58.2%) were from fingernails and 56 (41.8%) from toenails. Clinical diagnosis was confirmed in 96 (71.6%) cases by both fungal culture and direct microscopy but was confirmed by direct microscopy alone in only 76 (56.7%) cases. False-negative results were found in 23.9% (32/134) of participants with direct microscopy and 9.0% (12/134) with fungal cultures. The results of direct microscopy and fungal culture are outlined in Table 1. The study included 78 (58.2%) males and 56 (41.8%) females with a mean age of 44 years. Highest prevalence (47.8%) was seen in participants older than 40 years and lowest prevalence (11.9%) in participants younger than 20 years. In total, 32.8% of participants were farmers, 31.3% were housewives, 14.9% were students, and 20.9% performed other occupations. Disease history at the time of first presentation varied from 1 month to more than 2 years; 33.6% of participants had a 1- to 6-month history of disease, while only 3.7% had a disease history of less than 1 month at presentation. The demographic data are further outlined in Table 2.
Distal lateral subungual onychomycosis was the most prevalent clinical pattern found in 66 (49.3%) participants; fungal isolates were found in 60 of these participants. The next most prevalent clinical pattern was PSO, which was found in 34 (25.4%) participants, 12 showing fungal growth. A clinical pattern of CO was noted in 28 (20.9%) participants, 22 showing fungal growth; WSO was noted in 10 (7.5%) participants, 2 showing fungal growth.
Of 96 culture-positive cases, dermatophytes were the most common pathogens isolated in 56 (58.3%) participants, followed by Candida species in 28 (29.2%) participants. Nondermatophyte molds were isolated in 12 (12.5%) participants. The various dermatophytes, Candida species, and nondermatophyte molds that were isolated on fungal culture are outlined in Table 3. Of the 96 participants with positive fungal cultures, 30 (31.2%) were farmers working with soil, 28 (29.2%) were housewives associated with wet work, 16 (16.7%) were students associated with increased physical exercise from extracurricular activity, and 22 (22.9%) were in other occupations (Table 4).
Comment
The term onychomycosis is derived from onyx, the Greek word for nail, and mykes, the Greek word for fungus. Onychomycosis is a chronic mycotic infection of the fingernails and toenails that can have a serious impact on patients’ quality of life. The fungi known to cause onychomycosis vary among geographic areas, primarily due to differences in climate.14 The isolation rate of onychomycosis in our hospital-based study was 71.6%, which is in accordance with various studies in India and abroad, including 60% in Karnataka, India5; 82.3% in Sikkim, India6; and 86.9% in Turkey.1 However, other studies have shown lower isolation rates of 39.5% in Central Delhi, India,15 and 37.6% in Himachal Pradesh, India.16 Some patients with onychomycosis may not seek medical attention, which may explain the difference in the prevalence of onychomycosis observed worldwide.17 The prevalence of onychomycosis by age also varies. In our study, participants older than 40 years showed the highest prevalence (47.8%), which is in accordance with other studies from India18 and abroad.19,20 In contrast, some Indian studies15,21,22 have reported a higher prevalence in younger adults (ie, 21–30 years), which may be attributed to greater self-consciousness about nail discoloration and disfigurement as well as increased physical activity and different shoe-wearing habits. A higher prevalence in older adults, as observed in our study as well some other studies,19,21 may be due to poor peripheral circulation, diabetes mellitus, repeated nail trauma, longer exposure to pathogenic fungi, suboptimal immune function, inactivity, and poor hygiene.10
In our study, suspected onychomycosis was more common in males (58.2%) than in females (41.8%). These results are in accordance with many of the studies in the worldwide literature.1,10,11,15,16,23-25 A higher isolation rate in males worldwide may be due to common use of occlusive footwear, more exposure to outdoor conditions, and increased physical activity, leading to an increased likelihood of trauma. The importance of trauma to the nails as a predisposing factor for onychomycosis is well established.24 In our study, the majority of males wore shoes regardless of occupation. Perspiration of the feet when wearing socks and/or shoes can generate a warm moist environment that promotes the growth of fungi and predisposes patients to onychomycosis. Similar observations have been reported by other investigators.21,22,25,26
The incidence of onychomycosis was almost evenly distributed among farmers, housewives, and the miscellaneous group, whereas a high isolation rate was noted among students. Of 20 students included in our study, onychomycosis was confirmed in 16, which may be related to an increased use of synthetic sports shoes and socks that retain sweat as well as vigorous physical activity frequently resulting in nail injuries among this patient population.11 Younger patients may be more conscious of their appearance and therefore may be more likely to seek treatment. Similar observations have been reported by other researchers.15,21,22
In our study, dermatophytes were the most commonly found pathogens (58.3%), which is comparable to other studies.15,18,22Trichophyton mentagrophytes was the most frequently isolated dermatophyte from cultures, which was in concordance with a study from Delhi.15 In some studies,18,20,22Trichophyton rubrum has been reported as the most prevalent dermatophyte, but we identified Trichophyton rubrum in only 18 participants, which can be attributed to variations in epidemiology based on geographic region. Nondermatophyte molds were isolated in 12.5% of participants, with Aspergillus niger being the most common isolate found in 8 cases. Other isolated species were Alternaria alternata and Fusarium solani found in 2 cases each. Aspergillus niger has been reported in worldwide studies as an important cause of onychomycosis.15,18,19,21,22
In 28 cases (29.2%) involving Candida species, Candida albicans, Candida parapsilosis, and Candida tropicalis were the most common pathogens, respectively, which is in accordance with many studies.15,20-22,25 In 28 cases of CO, females (n=16) were affected more than males (n=12). All of the females were housewives and C albicans was predominantly isolated from the fingernails. Household responsibilities involving kitchen work (eg, cutting and peeling vegetables, washing utensils, cleaning the house/laundry) may chronically expose housewives to moist environments and make them more prone to injury, thus facilitating easy entry of fungal agents.
Distal lateral subungual onychomycosis was the most prevalent clinical type found (n=66), which is comparable to other reports.20,22,25 Proximal subungual onychomycosis was the second most common type; however, a greater incidence has been reported by some researchers,23,24 while others have reported a lower incidence.20,21 Candidial onychomycosis and WSO were not common in our study, and PSO was not associated with any immunodeficiency disease, as reported by other researchers.15,20
Of 134 suspected cases of onychomycosis, 71.6% were confirmed by both direct microscopy and fungal culture, but only 56.7% were confirmed by direct microscopy alone. If we had relied on microscopy with potassium hydroxide only, we would have missed 23.9% of cases. Therefore, nail scrapings should always be subjected to fungal culture as well as direct microscopy, as both are necessary for accurate diagnosis and treatment of onychomycosis. If onychomycosis is not successfully treated, it can act as a reservoir of fungal infection affecting other parts of the body with the potential to pass infection on to others.
Conclusion
Clinical examination alone is not sufficient for diagnosing onychomycosis14,18,20; in many cases of suspected onychomycosis with nail changes, mycologic examination does not confirm fungal infection. In our study, only 71.6% of participants with nail changes proved to be of fungal etiology. Other researchers from different geographic locations have reported similar results with lower incidence (eg, 39.5%,15 37.6%,16 51.7%,18 45.3%21) of fungal etiology in such cases. Therefore, both clinical and mycologic examinations are important for establishing the diagnosis and selecting the most suitable antifungal agent, which is possible only if the underlying pathogen is correctly identified.
1. Yenişehirli G, Bulut Y, Sezer E, et al. Onychomycosis infections in the Middle Black Sea Region, Turkey. Int J Dermatol. 2009;48:956-959.
2. Kouskoukis CE, Scher RK, Ackerman AB. What histologic finding distinguishes onychomycosis and psoriasis? Am J Dermatopathol. 1983;5:501-503.
3. Rippon JW. Medical mycology. In: Wonsiewicz M, ed. The Pathogenic Fungi and the Pathogenic Actinomycetes. 3rd ed. Philadelphia, PA: WB Saunders; 1988:169-275.
4. Greer DL. Evolving role of nondermatophytes in onychomycosis. Int J Dermatol. 1995;34:521-524.
5. Murray SC, Dawber RP. Onychomycosis of toenails: orthopaedic and podiatric considerations. Australas J Dermatol. 2002;43:105-112.
6. Achten G, Wanet-Rouard J. Onychomycoses in the laboratory. Mykosen Suppl. 1978;1:125-127.
7. Sobhanadri C, Rao DT, Babu KS. Clinical and mycological study of superficial fungal infections at Government General Hospital: guntur and their response to treatment with hamycin, dermostatin and dermamycin. Indian J Dermatol Venereol. 1970;36:209-214.
8. Jain S, Sehgal VN. Commentary: onychomycosis: an epidemio-etiologic perspective. Int J Dermatol. 2000;39:100-103.
9. Sehgal VN, Aggarwal AK, Srivastava G, et al. Onychomycosis: a 3 year clinicomycologic hospital-based study. Skinmed. 2007;6:11-17.
10. Elewski BE, Charif MA. Prevalence of onychomycosis in patients attending a dermatology clinic in northeastern Ohio for the other conditions. Arch Dermatol. 1997;133:1172-1173.
11. Scher RK. Onychomycosis is more than a cosmetic problem. Br J Dermatol. 1994;130(suppl 43):S15.
12. Godoy-Martinez PG, Nunes FG, Tomimori-Yamashita J, et al. Onychomycosis in São Paulo, Brazil [published online ahead of print May 8, 2009]. Mycopathologia. 2009;168:111-116.
13. Larone DH. Medically Important Fungi: A Guide to Identification. 4th ed. Washington, DC: American Society for Microbiology Press; 2002.
14. Sehgal VN, Srivastava G, Dogra S, et al. Onychomycosis: an Asian perspective. Skinmed. 2010;8:37-45.
15. Sanjiv A, Shalini M, Charoo H. Etiological agents of onychomycosis from a tertiary care hospital in Central Delhi, India. Indian J Fund Appl Life Science. 2011;1:11-14.
16. Gupta M, Sharma NL, Kanga AK, et al. Onychomycosis: clinic-mycologic study of 130 patients from Himachal Pradesh, India. Indian J Dermatol Venereol Leprol. 2007;73:389-392.
17. Eleweski BE. Diagnostic techniques for confirming onychomycosis. J Am Acad Dermatol. 1996;35(3, pt 2):S6-S9.
18. Das NK, Ghosh P, Das S, et al. A study on the etiological agent and clinico-mycological correlation of fingernail onychomycosis in eastern India. Indian J Dermatol. 2008;53:75-79.
19. Bassiri-Jahromi S, Khaksar AA. Nondermatophytic moulds as a causative agent of onychomycosis in Tehran. Indian J Dermatol. 2010;55:140-143.
20. Bokhari MA, Hussain I, Jahangir M, et al. Onychomycosis in Lahore, Pakistan. Int J Dermatol. 1999;38:591-595.
21. Jesudanam TM, Rao GR, Lakshmi DJ, et al. Onychomycosis: a significant medical problem. Indian J Dermatol Venereol Leprol. 2002;68:326-329.
22. Ahmad M, Gupta S, Gupte S. A clinico-mycological study of onychomycosis. EDOJ. 2010;6:1-9.
23. Vinod S, Grover S, Dash K, et al. A clinico-mycological evaluation of onychomycosis. Indian J Dermatol Venereol Leprol. 2000;66:238-240.
24. Veer P, Patwardhan NS, Damle AS. Study of onychomycosis: prevailing fungi and pattern of infection. Indian J Med Microbiol. 2007;25:53-56.
25. Garg A, Venkatesh V, Singh M, et al. Onychomycosis in central India: a clinicoetiologic correlation. Int J Dermatol. 2004;43:498-502.
26. Adhikari L, Das Gupta A, Pal R, et al. Clinico-etiologic correlates of onychomycosis in Sikkim. Indian J Pathol Microbiol. 2009;52:194-197.
1. Yenişehirli G, Bulut Y, Sezer E, et al. Onychomycosis infections in the Middle Black Sea Region, Turkey. Int J Dermatol. 2009;48:956-959.
2. Kouskoukis CE, Scher RK, Ackerman AB. What histologic finding distinguishes onychomycosis and psoriasis? Am J Dermatopathol. 1983;5:501-503.
3. Rippon JW. Medical mycology. In: Wonsiewicz M, ed. The Pathogenic Fungi and the Pathogenic Actinomycetes. 3rd ed. Philadelphia, PA: WB Saunders; 1988:169-275.
4. Greer DL. Evolving role of nondermatophytes in onychomycosis. Int J Dermatol. 1995;34:521-524.
5. Murray SC, Dawber RP. Onychomycosis of toenails: orthopaedic and podiatric considerations. Australas J Dermatol. 2002;43:105-112.
6. Achten G, Wanet-Rouard J. Onychomycoses in the laboratory. Mykosen Suppl. 1978;1:125-127.
7. Sobhanadri C, Rao DT, Babu KS. Clinical and mycological study of superficial fungal infections at Government General Hospital: guntur and their response to treatment with hamycin, dermostatin and dermamycin. Indian J Dermatol Venereol. 1970;36:209-214.
8. Jain S, Sehgal VN. Commentary: onychomycosis: an epidemio-etiologic perspective. Int J Dermatol. 2000;39:100-103.
9. Sehgal VN, Aggarwal AK, Srivastava G, et al. Onychomycosis: a 3 year clinicomycologic hospital-based study. Skinmed. 2007;6:11-17.
10. Elewski BE, Charif MA. Prevalence of onychomycosis in patients attending a dermatology clinic in northeastern Ohio for the other conditions. Arch Dermatol. 1997;133:1172-1173.
11. Scher RK. Onychomycosis is more than a cosmetic problem. Br J Dermatol. 1994;130(suppl 43):S15.
12. Godoy-Martinez PG, Nunes FG, Tomimori-Yamashita J, et al. Onychomycosis in São Paulo, Brazil [published online ahead of print May 8, 2009]. Mycopathologia. 2009;168:111-116.
13. Larone DH. Medically Important Fungi: A Guide to Identification. 4th ed. Washington, DC: American Society for Microbiology Press; 2002.
14. Sehgal VN, Srivastava G, Dogra S, et al. Onychomycosis: an Asian perspective. Skinmed. 2010;8:37-45.
15. Sanjiv A, Shalini M, Charoo H. Etiological agents of onychomycosis from a tertiary care hospital in Central Delhi, India. Indian J Fund Appl Life Science. 2011;1:11-14.
16. Gupta M, Sharma NL, Kanga AK, et al. Onychomycosis: clinic-mycologic study of 130 patients from Himachal Pradesh, India. Indian J Dermatol Venereol Leprol. 2007;73:389-392.
17. Eleweski BE. Diagnostic techniques for confirming onychomycosis. J Am Acad Dermatol. 1996;35(3, pt 2):S6-S9.
18. Das NK, Ghosh P, Das S, et al. A study on the etiological agent and clinico-mycological correlation of fingernail onychomycosis in eastern India. Indian J Dermatol. 2008;53:75-79.
19. Bassiri-Jahromi S, Khaksar AA. Nondermatophytic moulds as a causative agent of onychomycosis in Tehran. Indian J Dermatol. 2010;55:140-143.
20. Bokhari MA, Hussain I, Jahangir M, et al. Onychomycosis in Lahore, Pakistan. Int J Dermatol. 1999;38:591-595.
21. Jesudanam TM, Rao GR, Lakshmi DJ, et al. Onychomycosis: a significant medical problem. Indian J Dermatol Venereol Leprol. 2002;68:326-329.
22. Ahmad M, Gupta S, Gupte S. A clinico-mycological study of onychomycosis. EDOJ. 2010;6:1-9.
23. Vinod S, Grover S, Dash K, et al. A clinico-mycological evaluation of onychomycosis. Indian J Dermatol Venereol Leprol. 2000;66:238-240.
24. Veer P, Patwardhan NS, Damle AS. Study of onychomycosis: prevailing fungi and pattern of infection. Indian J Med Microbiol. 2007;25:53-56.
25. Garg A, Venkatesh V, Singh M, et al. Onychomycosis in central India: a clinicoetiologic correlation. Int J Dermatol. 2004;43:498-502.
26. Adhikari L, Das Gupta A, Pal R, et al. Clinico-etiologic correlates of onychomycosis in Sikkim. Indian J Pathol Microbiol. 2009;52:194-197.
Practice Points
- Onychomycosis is a chronic fungal infection of the nails and represents 20% to 40% of all onycho-pathies worldwide.
- Apart from dermatophytes as etiologic agents, nondermatophyte molds and yeasts also can contribute to the disease.
- Categorization of onychomycosis clinically as well as mycologically will surely ensure better patient care.
- Avoiding certain habits (eg, walking with bare feet, wearing ill-fitting shoes, onychophagia) can decrease disease incidence.