User login
The Diagnosis: Kaposi Sarcoma
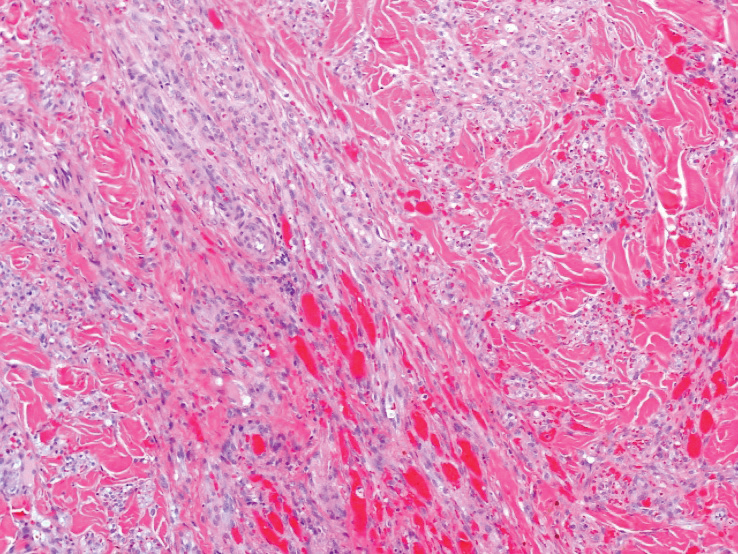
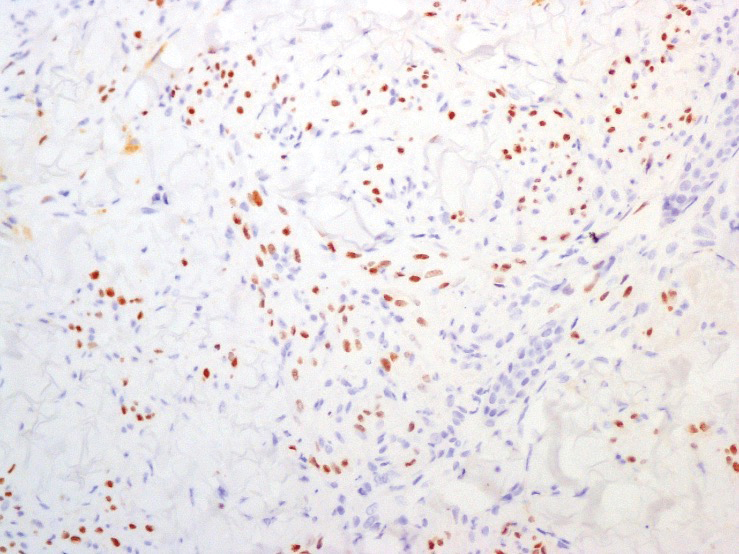
A punch biopsy of a lesion on the right side of the back revealed a diffuse, poorly circumscribed, spindle cell neoplasm of the papillary and reticular dermis with associated vascular and pseudovascular spaces distended by erythrocytes (Figure 1). Immunostaining was positive for human herpesvirus 8 (HHV-8)(Figure 2), ETS-related gene, CD31, and CD34 and negative for pan cytokeratin, confirming the diagnosis of Kaposi sarcoma (KS). Bacterial, fungal, and mycobacterial tissue cultures were negative. The patient was tested for HIV and referred to infectious disease and oncology. He subsequently was found to have HIV with a viral load greater than 1 million copies. He was started on antiretroviral therapy and Pneumocystis jirovecii pneumonia prophylaxis. Computed tomography of the chest, abdomen, and pelvis showed bilateral, multifocal, perihilar, flame-shaped consolidations suggestive of KS. The patient later disclosed having an intermittent dry cough of more than a year’s duration with occasional bright red blood per rectum after bowel movements. After workup, the patient was found to have cytomegalovirus esophagitis/gastritis and candidal esophagitis that were treated with valganciclovir and fluconazole, respectively.
Kaposi sarcoma is an angioproliferative, AIDSdefining disease associated with HHV-8. There are 4 types of KS as defined by the populations they affect. AIDS-associated KS occurs in individuals with HIV, as seen in our patient. It often is accompanied by extensive mucocutaneous and visceral lesions, as well as systemic symptoms such as fever, weight loss, and diarrhea.1 Classic KS is a variant that presents in older men of Mediterranean, Eastern European, and South American descent. Cutaneous lesions typically are distributed on the lower extremities.2,3 Endemic (African) KS is seen in HIV-negative children and young adults in equatorial Africa. It most commonly affects the lower extremities or lymph nodes and usually follows a more aggressive course.2 Lastly, iatrogenic KS is associated with immunosuppressive medications or conditions, such as organ transplantation, chemotherapy, and rheumatologic disorders.3,4
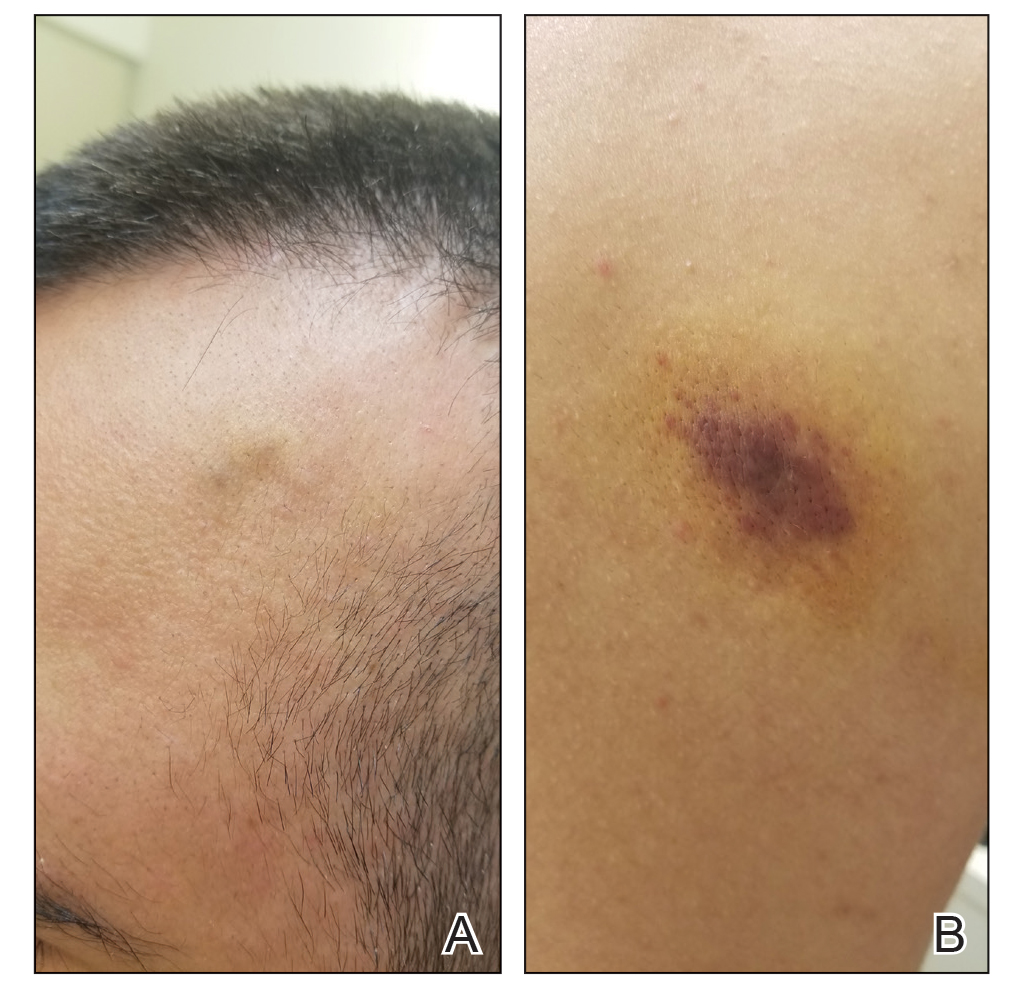
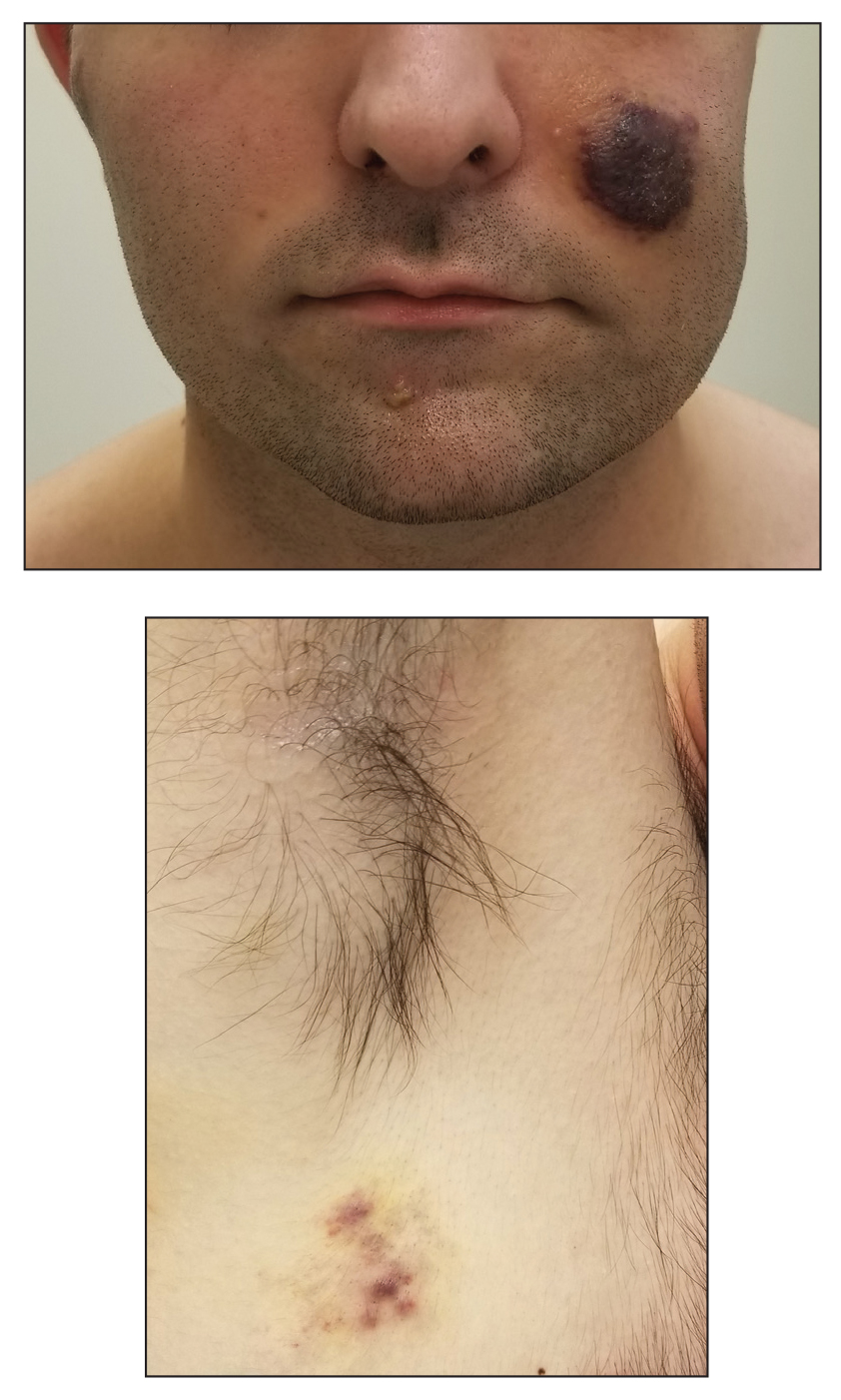
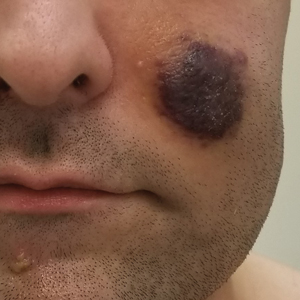
Kaposi sarcoma commonly presents as violaceous or dark red macules, patches, papules, plaques, and nodules on various parts of the body (Figure 3). Lesions typically begin as macules and progress into plaques or nodules. Our patient presented as a deceptively healthy young man with lesions at various stages of development. In addition to the skin and oral mucosa, the lungs, lymph nodes, and gastrointestinal tract commonly are involved in AIDS-associated KS.5 Patients may experience symptoms of internal involvement, including bleeding, hematochezia, odynophagia, or dyspnea.
The differential diagnosis includes conditions that can mimic KS, including bacillary angiomatosis, angioinvasive fungal disease, sarcoid, and other malignancies. A skin biopsy is the gold standard for definitive diagnosis of KS. Histopathology shows a vascular proliferation in the dermis and spindle cell proliferation.6 Kaposi sarcoma stains positively for factor VIII–related antigen, CD31, and CD34.2 Additionally, staining for HHV-8 gene products, such as latency-associated nuclear antigen 1, is helpful in differentiating KS from other conditions.7
In HIV-associated KS, the mainstay of treatment is initiation of highly active antiretroviral therapy. Typically, as the CD4 count rises with treatment, the tumor burden classic KS, effective treatment options include recurrent cryotherapy or intralesional chemotherapeutics, such as vincristine, for localized lesions; for widespread disease, pegylated liposomal doxorubicin or radiation have been found to be effective options. Lastly, for patients with iatrogenic KS, reducing immunosuppressive medications is a reasonable first step in management. If this does not yield adequate improvement, transitioning from calcineurin inhibitors (eg, cyclosporine) to proliferation signal inhibitors (eg, sirolimus) may lead to resolution.7
- Friedman-Kien AE, Saltzman BR. Clinical manifestations of classical, endemic African, and epidemic AIDS-associated Kaposi’s sarcoma. J Am Acad Dermatol. 1990;22:1237-1250.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol. 2019;58:538-542.
- Klepp O, Dahl O, Stenwig JT. Association of Kaposi’s sarcoma and prior immunosuppressive therapy. a 5‐year material of Kaposi’s sarcoma in Norway. Cancer. 1978;42:2626-2630.
- Lemlich G, Schwam L, Lebwohl M. Kaposi’s sarcoma and acquired immunodeficiency syndrome: postmortem findings in twenty-four cases. J Am Acad Dermatol. 1987;16:319-325.
- Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:10.
- Curtiss P, Strazzulla LC, Friedman-Kien AE. An update on Kaposi’s sarcoma: epidemiology, pathogenesis and treatment. Dermatol Ther. 2016;6:465-470.
The Diagnosis: Kaposi Sarcoma
A punch biopsy of a lesion on the right side of the back revealed a diffuse, poorly circumscribed, spindle cell neoplasm of the papillary and reticular dermis with associated vascular and pseudovascular spaces distended by erythrocytes (Figure 1). Immunostaining was positive for human herpesvirus 8 (HHV-8)(Figure 2), ETS-related gene, CD31, and CD34 and negative for pan cytokeratin, confirming the diagnosis of Kaposi sarcoma (KS). Bacterial, fungal, and mycobacterial tissue cultures were negative. The patient was tested for HIV and referred to infectious disease and oncology. He subsequently was found to have HIV with a viral load greater than 1 million copies. He was started on antiretroviral therapy and Pneumocystis jirovecii pneumonia prophylaxis. Computed tomography of the chest, abdomen, and pelvis showed bilateral, multifocal, perihilar, flame-shaped consolidations suggestive of KS. The patient later disclosed having an intermittent dry cough of more than a year’s duration with occasional bright red blood per rectum after bowel movements. After workup, the patient was found to have cytomegalovirus esophagitis/gastritis and candidal esophagitis that were treated with valganciclovir and fluconazole, respectively.
Kaposi sarcoma is an angioproliferative, AIDSdefining disease associated with HHV-8. There are 4 types of KS as defined by the populations they affect. AIDS-associated KS occurs in individuals with HIV, as seen in our patient. It often is accompanied by extensive mucocutaneous and visceral lesions, as well as systemic symptoms such as fever, weight loss, and diarrhea.1 Classic KS is a variant that presents in older men of Mediterranean, Eastern European, and South American descent. Cutaneous lesions typically are distributed on the lower extremities.2,3 Endemic (African) KS is seen in HIV-negative children and young adults in equatorial Africa. It most commonly affects the lower extremities or lymph nodes and usually follows a more aggressive course.2 Lastly, iatrogenic KS is associated with immunosuppressive medications or conditions, such as organ transplantation, chemotherapy, and rheumatologic disorders.3,4
Kaposi sarcoma commonly presents as violaceous or dark red macules, patches, papules, plaques, and nodules on various parts of the body (Figure 3). Lesions typically begin as macules and progress into plaques or nodules. Our patient presented as a deceptively healthy young man with lesions at various stages of development. In addition to the skin and oral mucosa, the lungs, lymph nodes, and gastrointestinal tract commonly are involved in AIDS-associated KS.5 Patients may experience symptoms of internal involvement, including bleeding, hematochezia, odynophagia, or dyspnea.
The differential diagnosis includes conditions that can mimic KS, including bacillary angiomatosis, angioinvasive fungal disease, sarcoid, and other malignancies. A skin biopsy is the gold standard for definitive diagnosis of KS. Histopathology shows a vascular proliferation in the dermis and spindle cell proliferation.6 Kaposi sarcoma stains positively for factor VIII–related antigen, CD31, and CD34.2 Additionally, staining for HHV-8 gene products, such as latency-associated nuclear antigen 1, is helpful in differentiating KS from other conditions.7
In HIV-associated KS, the mainstay of treatment is initiation of highly active antiretroviral therapy. Typically, as the CD4 count rises with treatment, the tumor burden classic KS, effective treatment options include recurrent cryotherapy or intralesional chemotherapeutics, such as vincristine, for localized lesions; for widespread disease, pegylated liposomal doxorubicin or radiation have been found to be effective options. Lastly, for patients with iatrogenic KS, reducing immunosuppressive medications is a reasonable first step in management. If this does not yield adequate improvement, transitioning from calcineurin inhibitors (eg, cyclosporine) to proliferation signal inhibitors (eg, sirolimus) may lead to resolution.7
The Diagnosis: Kaposi Sarcoma
A punch biopsy of a lesion on the right side of the back revealed a diffuse, poorly circumscribed, spindle cell neoplasm of the papillary and reticular dermis with associated vascular and pseudovascular spaces distended by erythrocytes (Figure 1). Immunostaining was positive for human herpesvirus 8 (HHV-8)(Figure 2), ETS-related gene, CD31, and CD34 and negative for pan cytokeratin, confirming the diagnosis of Kaposi sarcoma (KS). Bacterial, fungal, and mycobacterial tissue cultures were negative. The patient was tested for HIV and referred to infectious disease and oncology. He subsequently was found to have HIV with a viral load greater than 1 million copies. He was started on antiretroviral therapy and Pneumocystis jirovecii pneumonia prophylaxis. Computed tomography of the chest, abdomen, and pelvis showed bilateral, multifocal, perihilar, flame-shaped consolidations suggestive of KS. The patient later disclosed having an intermittent dry cough of more than a year’s duration with occasional bright red blood per rectum after bowel movements. After workup, the patient was found to have cytomegalovirus esophagitis/gastritis and candidal esophagitis that were treated with valganciclovir and fluconazole, respectively.
Kaposi sarcoma is an angioproliferative, AIDSdefining disease associated with HHV-8. There are 4 types of KS as defined by the populations they affect. AIDS-associated KS occurs in individuals with HIV, as seen in our patient. It often is accompanied by extensive mucocutaneous and visceral lesions, as well as systemic symptoms such as fever, weight loss, and diarrhea.1 Classic KS is a variant that presents in older men of Mediterranean, Eastern European, and South American descent. Cutaneous lesions typically are distributed on the lower extremities.2,3 Endemic (African) KS is seen in HIV-negative children and young adults in equatorial Africa. It most commonly affects the lower extremities or lymph nodes and usually follows a more aggressive course.2 Lastly, iatrogenic KS is associated with immunosuppressive medications or conditions, such as organ transplantation, chemotherapy, and rheumatologic disorders.3,4
Kaposi sarcoma commonly presents as violaceous or dark red macules, patches, papules, plaques, and nodules on various parts of the body (Figure 3). Lesions typically begin as macules and progress into plaques or nodules. Our patient presented as a deceptively healthy young man with lesions at various stages of development. In addition to the skin and oral mucosa, the lungs, lymph nodes, and gastrointestinal tract commonly are involved in AIDS-associated KS.5 Patients may experience symptoms of internal involvement, including bleeding, hematochezia, odynophagia, or dyspnea.
The differential diagnosis includes conditions that can mimic KS, including bacillary angiomatosis, angioinvasive fungal disease, sarcoid, and other malignancies. A skin biopsy is the gold standard for definitive diagnosis of KS. Histopathology shows a vascular proliferation in the dermis and spindle cell proliferation.6 Kaposi sarcoma stains positively for factor VIII–related antigen, CD31, and CD34.2 Additionally, staining for HHV-8 gene products, such as latency-associated nuclear antigen 1, is helpful in differentiating KS from other conditions.7
In HIV-associated KS, the mainstay of treatment is initiation of highly active antiretroviral therapy. Typically, as the CD4 count rises with treatment, the tumor burden classic KS, effective treatment options include recurrent cryotherapy or intralesional chemotherapeutics, such as vincristine, for localized lesions; for widespread disease, pegylated liposomal doxorubicin or radiation have been found to be effective options. Lastly, for patients with iatrogenic KS, reducing immunosuppressive medications is a reasonable first step in management. If this does not yield adequate improvement, transitioning from calcineurin inhibitors (eg, cyclosporine) to proliferation signal inhibitors (eg, sirolimus) may lead to resolution.7
- Friedman-Kien AE, Saltzman BR. Clinical manifestations of classical, endemic African, and epidemic AIDS-associated Kaposi’s sarcoma. J Am Acad Dermatol. 1990;22:1237-1250.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol. 2019;58:538-542.
- Klepp O, Dahl O, Stenwig JT. Association of Kaposi’s sarcoma and prior immunosuppressive therapy. a 5‐year material of Kaposi’s sarcoma in Norway. Cancer. 1978;42:2626-2630.
- Lemlich G, Schwam L, Lebwohl M. Kaposi’s sarcoma and acquired immunodeficiency syndrome: postmortem findings in twenty-four cases. J Am Acad Dermatol. 1987;16:319-325.
- Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:10.
- Curtiss P, Strazzulla LC, Friedman-Kien AE. An update on Kaposi’s sarcoma: epidemiology, pathogenesis and treatment. Dermatol Ther. 2016;6:465-470.
- Friedman-Kien AE, Saltzman BR. Clinical manifestations of classical, endemic African, and epidemic AIDS-associated Kaposi’s sarcoma. J Am Acad Dermatol. 1990;22:1237-1250.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol. 2019;58:538-542.
- Klepp O, Dahl O, Stenwig JT. Association of Kaposi’s sarcoma and prior immunosuppressive therapy. a 5‐year material of Kaposi’s sarcoma in Norway. Cancer. 1978;42:2626-2630.
- Lemlich G, Schwam L, Lebwohl M. Kaposi’s sarcoma and acquired immunodeficiency syndrome: postmortem findings in twenty-four cases. J Am Acad Dermatol. 1987;16:319-325.
- Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:10.
- Curtiss P, Strazzulla LC, Friedman-Kien AE. An update on Kaposi’s sarcoma: epidemiology, pathogenesis and treatment. Dermatol Ther. 2016;6:465-470.
A 25-year-old man with no notable medical history presented to the dermatology clinic with growing selfdescribed cysts on the face, trunk, and legs of 6 months’ duration. The lesions started as bruiselike discolorations and progressed to become firm nodules and inflamed masses. Some were minimally itchy and sensitive to touch, but there was no history of bleeding or drainage. The patient denied any new or recent environmental or animal exposures, use of illicit drugs, or travel correlating with the rash onset. He denied any prior treatments. He reported being in his normal state of health and was not taking any medications. Physical examination revealed indurated, violaceous, purpuric subcutaneous nodules, plaques, and masses on the forehead, cheek (top), jaw, flank, axillae (bottom), and back.