User login
2018-2019 season retrospective
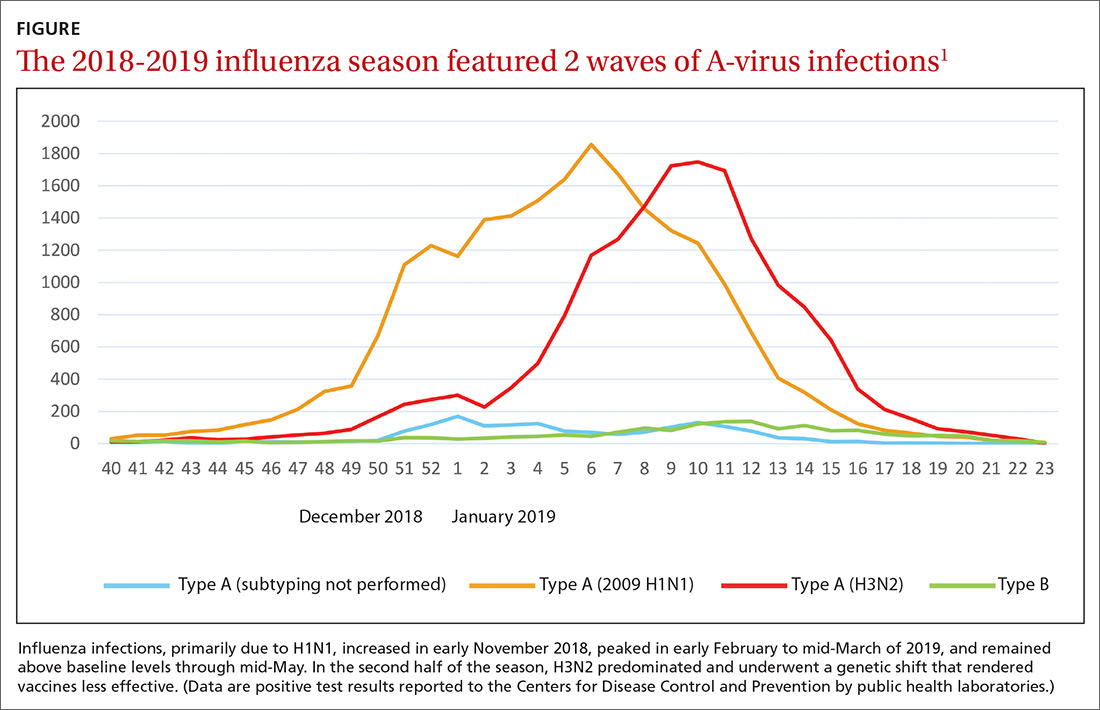
Last year’s influenza season was longer than usual. Infections, as measured by the percentage of outpatient visits due to influenza-like illness, increased in early November 2018, peaked in early February to mid-March of 2019, and remained above baseline levels through mid-May.1,2 Ninety six percent of influenza-positive samples were influenza A,1 and 57% of those were H1N1.2 In the second half of the season, H3N2 became the predominant circulating virus and there was a genetic shift in this strain that caused a decrease in the effectiveness of influenza vaccines (FIGURE).1 The influenza-confirmed hospitalization rate was 65.3/100,000, with the highest rate (221.7/100,000) occurring among those 65 years of age and older.2 Of those hospitalized with influenza, 93% of adults and 55% of children had an underlying medical condition and 29% of women of childbearing age were pregnant.2
Morbidity and mortality from influenza during the 2018-2019 influenza season were moderate compared with previous years. Pneumonia and influenza mortality reached close to 8% of all deaths during the peak of the season (considered a modest peak), but stayed above the epidemic threshold for 10 weeks.2 There were 119 pediatric deaths.1 Overall, in the United States, there were an estimated 37 to 43 million influenza-related illnesses, 17 to 20 million flu-related medical visits, 531,000 to 647,000 flu-related hospitalizations, and 36,400 to 61,200 deaths.1
Influenza viral resistance to oseltamivir remained very low throughout the season for both A and B viruses.2
Vaccine effectiveness was subpar
The effectiveness of influenza vaccine last season was disappointing. When assessed using laboratory-confirmed medically attended influenza, the vaccine was 29% effective; when assessed by age group, the confidence intervals included 0 in ages 9 to 17 years and 50 years and older.3 In the age group 6 months to 8 years, the vaccine was 49% effective.3 The vaccine was not effective against the predominant H3N2 strain circulating. It was 25% effective in preventing hospitalization, with a lack of benefit seen in individuals ages 18 to 49 years and those 65 and older.3
Vaccination was associated with increased rates of hospitalizations from infections cause by H3N2. It is not known if this finding was due to chance, unstable results from small numbers, an unknown bias, or some biological cause not yet understood. This is a topic of ongoing research.
Effectiveness in preventing pediatric hospitalizations was estimated at 31%, again with no effectiveness against H3N2.3 The estimate of vaccine effectiveness in the United States was similar to that in Canada.2
While these results are much lower than desired, influenza vaccine did prevent an estimated 40,000 to 90,000 hospitalizations and decreased influenza-like illnesses by 44%.3
Continue to: A look at vaccine safety
A look at vaccine safety
Numerous studies of influenza vaccine safety were presented at the June 2019 meeting of the Advisory Committee on Immunization Practices (ACIP).4 These studies included assessments using the Vaccine Adverse Events Reporting System; the Vaccine Safety Datalink (VSD), which conducts ongoing rapid analysis of adverse events throughout the influenza season; and Food and Drug Administration (FDA)-sponsored studies of Medicare patients. These vaccine safety monitoring systems have been described in a prior Practice Alert.5
Possible vaccine reactions studied included Guillain-Barre Syndrome (GBS), anaphylaxis, encephalitis, Bell’s palsy, febrile seizures, and pregnancy-related adverse events such as miscarriage and congenital anomalies. While preliminary safety signals were detected for anaphylaxis, Bell’s palsy, febrile seizures, and GBS, a more in-depth investigation found no association of any adverse events with vaccination except for febrile seizures, with an attributable risk of 4.24/100,000 doses in children ages 6 to 23 months and 1.8/100,000 in those ages 24 to 59 months.4 The incidence of febrile seizures was similar to that of previous seasons and primarily occurred when the vaccine was administered in conjunction with another vaccine. A preliminary FDA analysis found a small elevated risk of GBS with high-dose trivalent inactivated vaccine, with an attributable risk of 0.98 per million doses, but this was not confirmed by the VSD analysis.4
What you need to know about the upcoming season
ACIP recommendations on influenza vaccines for 2019 to 2020 are essentially unchanged from last year.6 All individuals ages 6 months and older, who do not have a contraindication, should receive a flu vaccine in the fall of 2019. The composition of this season’s vaccine contains new H1N1 and H3N2 variants to more closely match the circulating strains. ACIP has updated or clarified 4 logistical issues in this year’s recommendations:
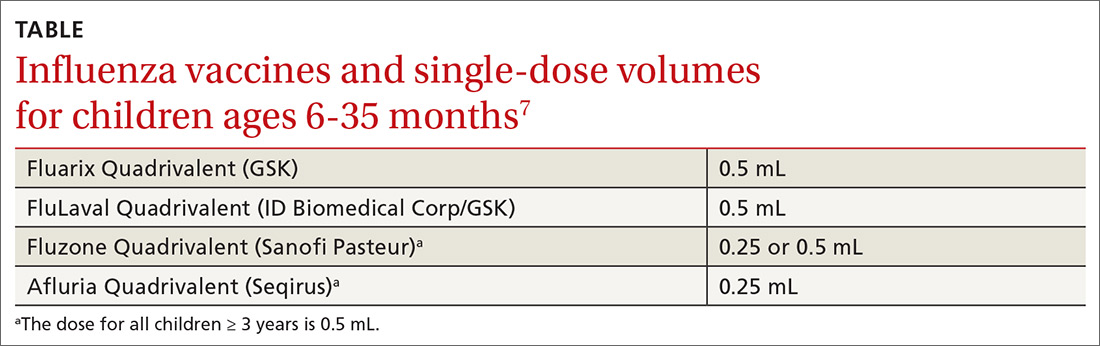
- Four inactivated-influenza vaccines are now available for children ages 6 to 35 months. Dose volumes are not the same for all 4 (TABLE).7
- Vaccination is now encouraged for September or later for those requiring only 1 dose of vaccine. Earlier administration can result in waning immunity by the end of the flu season, especially in older adults.7
- Children ages 6 months to 8 years may require 2 doses if they haven’t received any previous influenza vaccine, and the second dose should be given even if the child turns 9 between doses 1 and 2.7
- One adjuvanted influenza vaccine containing MF59—the trivalent inactivated influenza vaccine, Fluad—is approved for those ages 65 years and older. One note of caution is that licensed vaccines for other conditions also contain new nonaluminum adjuvants and there are few data on the safety and effectiveness of simultaneous or sequential administration of Fluad with the 2 novel nonaluminum adjuvant-containing vaccines. These vaccines are the recombinant zoster subunit vaccine (Shingrix), which contains the liposome-based adjuvant ASO1, and the recombinant hepatitis B surface antigen vaccine (Heplisav-B), which contains cytosine phosphoguanine oligodeoxynucleotide. Given the lack of data and the availability of other influenza vaccine options, ACIP advises that selecting a nonadjuvanted influenza vaccine may be the best option when an older adult needs both an influenza vaccine and either Shingrix or Heplisav-B. However, do not delay giving any vaccine if a specific alternate product is unavailable.7
All recommendations concerning the use of influenza vaccine for the 2019-2020 influenza season and a listing of all available influenza vaccine products can be found on the ACIP Web site (cdc.gov/vaccines/acip/index.html) or in the Morbidity and Mortality Weekly Report.8
1. Brammer L. Influenza Surveillance Update. Presented to the ACIP June 27, 2019. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/flu-2-Brammer-508.pdf. Accessed August 21, 2019.
2. Hammond A, Hundal K, Laurenson-Shafer H, et al. Review of the 2018–2019 influenza season in the northern hemisphere. WHO Wkly Epidemiol Record. 2019;94:345-364.
3. Flannery B, Chung J, Ferdinands J, et al. Preliminary estimates of the 2018-2019 seasonal influenza vaccine effectiveness against medically attended influenza from three U.S. networks. Presented to ACIP June 27, 2019. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/flu-3-flannery-508.pdf. Accessed August 21, 2019.
4. Shimabukuro T. End-of-season update: 2018-2019 influenza vaccine safety monitoring. Presented to the ACIP meeting June 27, 2019. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/flu-4-Shimabukuro-508.pdf. Accessed August 21, 2019.
5. Campos-Outcalt D. Facts to help you keep pace with the vaccine conversation. J Fam Pract. 2019;68:341-346.
6. Campos-Outcalt D. CDC recommendations for the 2018-2019 influenza season. J Fam Pract. 2018;67:550-553.
7. Grohskopf L. Influenza work group considerations and proposed 2019-2020 season recommendations. Presented to the ACIP June 27, 2019. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/flu-5-grohskopf-508.pdf. Accessed August 21, 2019.
8. Grohskopf LA, Alyanak E, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices —United States, 2019-20 influenza season. MMWR Recomm Rep. 2019;68:1-21.
2018-2019 season retrospective
Last year’s influenza season was longer than usual. Infections, as measured by the percentage of outpatient visits due to influenza-like illness, increased in early November 2018, peaked in early February to mid-March of 2019, and remained above baseline levels through mid-May.1,2 Ninety six percent of influenza-positive samples were influenza A,1 and 57% of those were H1N1.2 In the second half of the season, H3N2 became the predominant circulating virus and there was a genetic shift in this strain that caused a decrease in the effectiveness of influenza vaccines (FIGURE).1 The influenza-confirmed hospitalization rate was 65.3/100,000, with the highest rate (221.7/100,000) occurring among those 65 years of age and older.2 Of those hospitalized with influenza, 93% of adults and 55% of children had an underlying medical condition and 29% of women of childbearing age were pregnant.2
Morbidity and mortality from influenza during the 2018-2019 influenza season were moderate compared with previous years. Pneumonia and influenza mortality reached close to 8% of all deaths during the peak of the season (considered a modest peak), but stayed above the epidemic threshold for 10 weeks.2 There were 119 pediatric deaths.1 Overall, in the United States, there were an estimated 37 to 43 million influenza-related illnesses, 17 to 20 million flu-related medical visits, 531,000 to 647,000 flu-related hospitalizations, and 36,400 to 61,200 deaths.1
Influenza viral resistance to oseltamivir remained very low throughout the season for both A and B viruses.2
Vaccine effectiveness was subpar
The effectiveness of influenza vaccine last season was disappointing. When assessed using laboratory-confirmed medically attended influenza, the vaccine was 29% effective; when assessed by age group, the confidence intervals included 0 in ages 9 to 17 years and 50 years and older.3 In the age group 6 months to 8 years, the vaccine was 49% effective.3 The vaccine was not effective against the predominant H3N2 strain circulating. It was 25% effective in preventing hospitalization, with a lack of benefit seen in individuals ages 18 to 49 years and those 65 and older.3
Vaccination was associated with increased rates of hospitalizations from infections cause by H3N2. It is not known if this finding was due to chance, unstable results from small numbers, an unknown bias, or some biological cause not yet understood. This is a topic of ongoing research.
Effectiveness in preventing pediatric hospitalizations was estimated at 31%, again with no effectiveness against H3N2.3 The estimate of vaccine effectiveness in the United States was similar to that in Canada.2
While these results are much lower than desired, influenza vaccine did prevent an estimated 40,000 to 90,000 hospitalizations and decreased influenza-like illnesses by 44%.3
Continue to: A look at vaccine safety
A look at vaccine safety
Numerous studies of influenza vaccine safety were presented at the June 2019 meeting of the Advisory Committee on Immunization Practices (ACIP).4 These studies included assessments using the Vaccine Adverse Events Reporting System; the Vaccine Safety Datalink (VSD), which conducts ongoing rapid analysis of adverse events throughout the influenza season; and Food and Drug Administration (FDA)-sponsored studies of Medicare patients. These vaccine safety monitoring systems have been described in a prior Practice Alert.5
Possible vaccine reactions studied included Guillain-Barre Syndrome (GBS), anaphylaxis, encephalitis, Bell’s palsy, febrile seizures, and pregnancy-related adverse events such as miscarriage and congenital anomalies. While preliminary safety signals were detected for anaphylaxis, Bell’s palsy, febrile seizures, and GBS, a more in-depth investigation found no association of any adverse events with vaccination except for febrile seizures, with an attributable risk of 4.24/100,000 doses in children ages 6 to 23 months and 1.8/100,000 in those ages 24 to 59 months.4 The incidence of febrile seizures was similar to that of previous seasons and primarily occurred when the vaccine was administered in conjunction with another vaccine. A preliminary FDA analysis found a small elevated risk of GBS with high-dose trivalent inactivated vaccine, with an attributable risk of 0.98 per million doses, but this was not confirmed by the VSD analysis.4
What you need to know about the upcoming season
ACIP recommendations on influenza vaccines for 2019 to 2020 are essentially unchanged from last year.6 All individuals ages 6 months and older, who do not have a contraindication, should receive a flu vaccine in the fall of 2019. The composition of this season’s vaccine contains new H1N1 and H3N2 variants to more closely match the circulating strains. ACIP has updated or clarified 4 logistical issues in this year’s recommendations:
- Four inactivated-influenza vaccines are now available for children ages 6 to 35 months. Dose volumes are not the same for all 4 (TABLE).7
- Vaccination is now encouraged for September or later for those requiring only 1 dose of vaccine. Earlier administration can result in waning immunity by the end of the flu season, especially in older adults.7
- Children ages 6 months to 8 years may require 2 doses if they haven’t received any previous influenza vaccine, and the second dose should be given even if the child turns 9 between doses 1 and 2.7
- One adjuvanted influenza vaccine containing MF59—the trivalent inactivated influenza vaccine, Fluad—is approved for those ages 65 years and older. One note of caution is that licensed vaccines for other conditions also contain new nonaluminum adjuvants and there are few data on the safety and effectiveness of simultaneous or sequential administration of Fluad with the 2 novel nonaluminum adjuvant-containing vaccines. These vaccines are the recombinant zoster subunit vaccine (Shingrix), which contains the liposome-based adjuvant ASO1, and the recombinant hepatitis B surface antigen vaccine (Heplisav-B), which contains cytosine phosphoguanine oligodeoxynucleotide. Given the lack of data and the availability of other influenza vaccine options, ACIP advises that selecting a nonadjuvanted influenza vaccine may be the best option when an older adult needs both an influenza vaccine and either Shingrix or Heplisav-B. However, do not delay giving any vaccine if a specific alternate product is unavailable.7
All recommendations concerning the use of influenza vaccine for the 2019-2020 influenza season and a listing of all available influenza vaccine products can be found on the ACIP Web site (cdc.gov/vaccines/acip/index.html) or in the Morbidity and Mortality Weekly Report.8
2018-2019 season retrospective
Last year’s influenza season was longer than usual. Infections, as measured by the percentage of outpatient visits due to influenza-like illness, increased in early November 2018, peaked in early February to mid-March of 2019, and remained above baseline levels through mid-May.1,2 Ninety six percent of influenza-positive samples were influenza A,1 and 57% of those were H1N1.2 In the second half of the season, H3N2 became the predominant circulating virus and there was a genetic shift in this strain that caused a decrease in the effectiveness of influenza vaccines (FIGURE).1 The influenza-confirmed hospitalization rate was 65.3/100,000, with the highest rate (221.7/100,000) occurring among those 65 years of age and older.2 Of those hospitalized with influenza, 93% of adults and 55% of children had an underlying medical condition and 29% of women of childbearing age were pregnant.2
Morbidity and mortality from influenza during the 2018-2019 influenza season were moderate compared with previous years. Pneumonia and influenza mortality reached close to 8% of all deaths during the peak of the season (considered a modest peak), but stayed above the epidemic threshold for 10 weeks.2 There were 119 pediatric deaths.1 Overall, in the United States, there were an estimated 37 to 43 million influenza-related illnesses, 17 to 20 million flu-related medical visits, 531,000 to 647,000 flu-related hospitalizations, and 36,400 to 61,200 deaths.1
Influenza viral resistance to oseltamivir remained very low throughout the season for both A and B viruses.2
Vaccine effectiveness was subpar
The effectiveness of influenza vaccine last season was disappointing. When assessed using laboratory-confirmed medically attended influenza, the vaccine was 29% effective; when assessed by age group, the confidence intervals included 0 in ages 9 to 17 years and 50 years and older.3 In the age group 6 months to 8 years, the vaccine was 49% effective.3 The vaccine was not effective against the predominant H3N2 strain circulating. It was 25% effective in preventing hospitalization, with a lack of benefit seen in individuals ages 18 to 49 years and those 65 and older.3
Vaccination was associated with increased rates of hospitalizations from infections cause by H3N2. It is not known if this finding was due to chance, unstable results from small numbers, an unknown bias, or some biological cause not yet understood. This is a topic of ongoing research.
Effectiveness in preventing pediatric hospitalizations was estimated at 31%, again with no effectiveness against H3N2.3 The estimate of vaccine effectiveness in the United States was similar to that in Canada.2
While these results are much lower than desired, influenza vaccine did prevent an estimated 40,000 to 90,000 hospitalizations and decreased influenza-like illnesses by 44%.3
Continue to: A look at vaccine safety
A look at vaccine safety
Numerous studies of influenza vaccine safety were presented at the June 2019 meeting of the Advisory Committee on Immunization Practices (ACIP).4 These studies included assessments using the Vaccine Adverse Events Reporting System; the Vaccine Safety Datalink (VSD), which conducts ongoing rapid analysis of adverse events throughout the influenza season; and Food and Drug Administration (FDA)-sponsored studies of Medicare patients. These vaccine safety monitoring systems have been described in a prior Practice Alert.5
Possible vaccine reactions studied included Guillain-Barre Syndrome (GBS), anaphylaxis, encephalitis, Bell’s palsy, febrile seizures, and pregnancy-related adverse events such as miscarriage and congenital anomalies. While preliminary safety signals were detected for anaphylaxis, Bell’s palsy, febrile seizures, and GBS, a more in-depth investigation found no association of any adverse events with vaccination except for febrile seizures, with an attributable risk of 4.24/100,000 doses in children ages 6 to 23 months and 1.8/100,000 in those ages 24 to 59 months.4 The incidence of febrile seizures was similar to that of previous seasons and primarily occurred when the vaccine was administered in conjunction with another vaccine. A preliminary FDA analysis found a small elevated risk of GBS with high-dose trivalent inactivated vaccine, with an attributable risk of 0.98 per million doses, but this was not confirmed by the VSD analysis.4
What you need to know about the upcoming season
ACIP recommendations on influenza vaccines for 2019 to 2020 are essentially unchanged from last year.6 All individuals ages 6 months and older, who do not have a contraindication, should receive a flu vaccine in the fall of 2019. The composition of this season’s vaccine contains new H1N1 and H3N2 variants to more closely match the circulating strains. ACIP has updated or clarified 4 logistical issues in this year’s recommendations:
- Four inactivated-influenza vaccines are now available for children ages 6 to 35 months. Dose volumes are not the same for all 4 (TABLE).7
- Vaccination is now encouraged for September or later for those requiring only 1 dose of vaccine. Earlier administration can result in waning immunity by the end of the flu season, especially in older adults.7
- Children ages 6 months to 8 years may require 2 doses if they haven’t received any previous influenza vaccine, and the second dose should be given even if the child turns 9 between doses 1 and 2.7
- One adjuvanted influenza vaccine containing MF59—the trivalent inactivated influenza vaccine, Fluad—is approved for those ages 65 years and older. One note of caution is that licensed vaccines for other conditions also contain new nonaluminum adjuvants and there are few data on the safety and effectiveness of simultaneous or sequential administration of Fluad with the 2 novel nonaluminum adjuvant-containing vaccines. These vaccines are the recombinant zoster subunit vaccine (Shingrix), which contains the liposome-based adjuvant ASO1, and the recombinant hepatitis B surface antigen vaccine (Heplisav-B), which contains cytosine phosphoguanine oligodeoxynucleotide. Given the lack of data and the availability of other influenza vaccine options, ACIP advises that selecting a nonadjuvanted influenza vaccine may be the best option when an older adult needs both an influenza vaccine and either Shingrix or Heplisav-B. However, do not delay giving any vaccine if a specific alternate product is unavailable.7
All recommendations concerning the use of influenza vaccine for the 2019-2020 influenza season and a listing of all available influenza vaccine products can be found on the ACIP Web site (cdc.gov/vaccines/acip/index.html) or in the Morbidity and Mortality Weekly Report.8
1. Brammer L. Influenza Surveillance Update. Presented to the ACIP June 27, 2019. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/flu-2-Brammer-508.pdf. Accessed August 21, 2019.
2. Hammond A, Hundal K, Laurenson-Shafer H, et al. Review of the 2018–2019 influenza season in the northern hemisphere. WHO Wkly Epidemiol Record. 2019;94:345-364.
3. Flannery B, Chung J, Ferdinands J, et al. Preliminary estimates of the 2018-2019 seasonal influenza vaccine effectiveness against medically attended influenza from three U.S. networks. Presented to ACIP June 27, 2019. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/flu-3-flannery-508.pdf. Accessed August 21, 2019.
4. Shimabukuro T. End-of-season update: 2018-2019 influenza vaccine safety monitoring. Presented to the ACIP meeting June 27, 2019. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/flu-4-Shimabukuro-508.pdf. Accessed August 21, 2019.
5. Campos-Outcalt D. Facts to help you keep pace with the vaccine conversation. J Fam Pract. 2019;68:341-346.
6. Campos-Outcalt D. CDC recommendations for the 2018-2019 influenza season. J Fam Pract. 2018;67:550-553.
7. Grohskopf L. Influenza work group considerations and proposed 2019-2020 season recommendations. Presented to the ACIP June 27, 2019. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/flu-5-grohskopf-508.pdf. Accessed August 21, 2019.
8. Grohskopf LA, Alyanak E, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices —United States, 2019-20 influenza season. MMWR Recomm Rep. 2019;68:1-21.
1. Brammer L. Influenza Surveillance Update. Presented to the ACIP June 27, 2019. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/flu-2-Brammer-508.pdf. Accessed August 21, 2019.
2. Hammond A, Hundal K, Laurenson-Shafer H, et al. Review of the 2018–2019 influenza season in the northern hemisphere. WHO Wkly Epidemiol Record. 2019;94:345-364.
3. Flannery B, Chung J, Ferdinands J, et al. Preliminary estimates of the 2018-2019 seasonal influenza vaccine effectiveness against medically attended influenza from three U.S. networks. Presented to ACIP June 27, 2019. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/flu-3-flannery-508.pdf. Accessed August 21, 2019.
4. Shimabukuro T. End-of-season update: 2018-2019 influenza vaccine safety monitoring. Presented to the ACIP meeting June 27, 2019. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/flu-4-Shimabukuro-508.pdf. Accessed August 21, 2019.
5. Campos-Outcalt D. Facts to help you keep pace with the vaccine conversation. J Fam Pract. 2019;68:341-346.
6. Campos-Outcalt D. CDC recommendations for the 2018-2019 influenza season. J Fam Pract. 2018;67:550-553.
7. Grohskopf L. Influenza work group considerations and proposed 2019-2020 season recommendations. Presented to the ACIP June 27, 2019. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/flu-5-grohskopf-508.pdf. Accessed August 21, 2019.
8. Grohskopf LA, Alyanak E, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices —United States, 2019-20 influenza season. MMWR Recomm Rep. 2019;68:1-21.