User login
A major placebo-controlled trial has found that a statin can reduce the risk of venous thromboembolism (VTE).1
We do not recommend prescribing this class of drugs for this purpose until much more research has been done, and we certainly do not recommend substituting a statin for anticoagulant therapy in a patient at risk of VTE.
Nevertheless, we are excited by the latest findings, and we find comfort in knowing that if a patient is taking a statin for an approved indication, ie, reducing the risk of cardiovascular disease in a patient with hyperlipidemia or a previous cardiovascular event, the drug will also reduce the risk of VTE.
In the pages that follow, we describe and comment on what is known about the effect of statins on the risk of VTE.
ARTERIAL AND VENOUS THROMBOSIS: HOW ARE THEY LINKED?
The causes of arterial thrombosis may not be entirely distinct from those of deep vein thrombosis and pulmonary embolism, collectively referred to as VTE. Some studies have found that risk factors for arterial thrombosis overlap with those for VTE.2–4 However, other studies have shown no association between venous and arterial events.5–10
Hyperlipidemia, in particular, has been evaluated to see if it is a risk factor for VTE. As with other risk factors for arterial thrombosis, the data have been mixed, with some reports favoring an association with VTE and others not.4,5,11 Even so, preventive strategies targeting arterial risk factors have shown promise in reducing VTE events.12
Although commonly used to treat hyperlipidemia, statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) are believed to reduce the incidence of thrombosis by a number of mechanisms13:
- Decreasing platelet aggregation
- Inhibiting expression of tissue factor and plasminogen activator inhibitor 1
- Increasing expression of tissue plasminogen activator
- Increasing expression of thrombomodulin, which can activate protein C and prevent thrombin-induced platelet and factor V activation and fibrinogen clotting.
STATINS AND VTE IN OBSERVATIONAL AND CASE-CONTROL STUDIES
In view of the multiple effects of statins, several studies have looked at whether these drugs reduce the occurrence of both arterial thrombosis and VTE.14–19
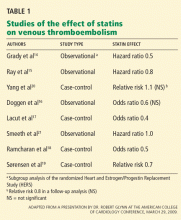
Two prospective observational studies and four case-control studies found that statins reduced the risk of VTE by 20% to 60%.14–19 Interestingly, two of the case-control studies found that antiplatelet therapy did not reduce the risk of VTE.18,19
THE JUPITER STUDY
The Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) study primarily sought to determine if rosuvastatin (Crestor) 20 mg/day, compared with placebo, would reduce the rate of first major cardiovascular events.22 A prespecified secondary end point of the trial was VTE, making JUPITER the first randomized, placebo-controlled trial to specifically test whether statins prevent VTE.1
Inclusion criteria: Normal LDL, high CRP
The study included men age 50 and older and women age 60 and older with no history of cardiovascular disease. In addition, their lowdensity lipoprotein (LDL) cholesterol levels had to be lower than 130 mg/dL (3.4 mmol/L), their triglyceride levels had to be lower than 500 mg/dL (5.6 mmol/L), and their highsensitivity C-reactive protein (hs-CRP) levels had to be 2.0 mg/L or higher.
Since high levels of hs-CRP, a marker of inflammation, predict cardiovascular events and since statins lower hs-CRP levels, the investigators hypothesized that people with elevated hs-CRP but without hyperlipidemia might benefit from statin treatment.21
Patients were excluded if they had received lipid-lowering therapy within 6 weeks of the trial screening, had diabetes mellitus or uncontrolled hypertension, were currently using postmenopausal hormone-replacement therapy, or had had cancer within the previous 5 years, except for certain skin cancers.
Candidates who complied well during a 4-week placebo run-in phase were randomly assigned to receive either rosuvastatin 20 mg daily (an intermediate dose) or a matching placebo. In all, 17,802 people were randomized. The two assigned groups appeared to be well matched.
Patients were to come in for visits twice a year for 60 months after randomization to be assessed for symptomatic deep venous thrombosis and pulmonary embolism. New cases of VTE were confirmed by imaging studies, by the initiation of anticoagulation therapy, or by death ascribed to pulmonary embolism.
Idiopathic VTE was classified as unprovoked if it occurred in the absence of trauma, hospitalization, or surgery within 3 months before the event, and in the absence of any diagnosed cancer within 3 months before and after the event. Provoked VTE events were those that occurred in a participant with cancer or when a precipitating event was associated with trauma, hospitalization, or surgery.
Rosuvastatin prevents heart attack, stroke
On the recommendation of the trial’s independent data and safety monitoring board, JUPITER was stopped early because the trial drug showed evidence of efficacy in preventing the combined primary end point of a first major cardiovascular event—ie, nonfatal myocardial infarction, nonfatal stroke, hospitalization for unstable angina, an arterial revascularization procedure, or confirmed death from a cardiovascular cause.22 (The cardiovascular outcomes of the JUPITER study were reviewed by Shishehbor and Hazen23 in the January 2009 issue of the Cleveland Clinic Journal of Medicine; see doi:10.3949/ccjm.75a.08105).
Formal follow-up for the trial's primary and secondary efficacy end points ended then, but data on VTE continued to be collected until each patient’s closeout visit as part of a safety monitoring protocol. The last closeout visit occurred on August 20, 2008. The primary analysis focused on events occurring up to March 30, 2008, the date the study was stopped.
Secondary end point results: Rosuvastatin prevents VTE
At a median follow-up of 1.9 years, an episode of VTE had occurred in 94 (0.53%) of the 17,802 patients—34 in the rosuvastatin group and 60 in the placebo group.1 This translates to 0.18 and 0.32 events per 100 person-years of follow-up in the rosuvastatin and placebo groups, respectively (hazard ratio for the rosuvastatin group 0.57, 95% confidence interval [CI] 0.37–0.86, P = .007).
Forty-four cases of VTE were classified as provoked and 50 cases were categorized as unprovoked. The risk reduction was statistically significant for provoked cases (hazard ratio 0.52, 95% CI 0.28–0.96, P = .03), but not for unprovoked events (hazard ratio 0.61, 95% CI 0.35–1.09, P = .09).
Subgroup analysis revealed no significant association between patient characteristics and the impact of rosuvastatin on the risk of a VTE event, but, as expected, more benefit was associated with higher baseline lipid levels.
STILL TOO SOON TO ADVISE ROUTINE STATIN USE TO PREVENT VTE
While the JUPITER trial data show an apparent benefit of statin use on the rate of VTE events, advising routine use of statins to prevent VTE is premature, for three main reasons.
Many must be treated to prevent one case of VTE. The number needed to treat (NNT) with rosuvastatin for 5 years to prevent either a case of VTE or a cardiovascular event was 21, and the NNT to prevent one cardiovascular event was 25. In a review of the two most recent case-control studies investigating the effects of statins on VTE,18,19 Cushman24 calculated that the NNT to prevent one VTE event each year was 333 for those age 75 and older. Though the Jupiter data did not provide the specific incidence of VTE at 1 year, except graphically, we can estimate that the NNT to prevent one VTE event at 1 year in the study is also very high.
Practically speaking, the perceived benefits of VTE prevention require large numbers to be treated, and the net clinical gain is still largely in preventing arterial events such as heart attack and stroke rather than VTE.
Statins, though safe, can still have adverse effects. The JUPITER study found a trend (albeit nonsignificant) toward more muscle complaints and elevations on liver function testing in apparently healthy persons taking a statin.22 Although severe complications of statin therapy such as rhabdomyolysis and elevations of creatine phosphokinase are rare, patients taking a statin have a 39% higher risk of an adverse event, most commonly myalgias or abnormalities on liver function testing.25 Were statins to be given routinely to even more people than they are now, more adverse outcomes would be likely.
More study is needed. The JUPITER study did not address a high risk of VTE. In fact, the investigators provided no information as to the VTE history of those enrolled.
Clearly, statins should not be substituted for proven prophylaxis and anticoagulation without further investigation, especially for patients with recurrent deep venous thrombosis, hospitalized patients, postoperative patients, and other patients prone to VTE.
OUR VIEW
The JUPITER study is an important leap forward in adding to our knowledge of how to prevent VTE. For people with another indication for taking a statin (eg, a previous cardiovascular event, hyperlipidemia), it is helpful to know that their risk of VTE may be reduced without exposure to the risks of other kinds of conventional thromboprophylaxis.
We look forward to additional studies to elaborate on the benefits of statins in both the prevention and treatment of VTE for averagerisk and VTE-prone populations.
- Glynn RJ, Danielson E, Fonseca FA, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med 2009; 360:1851–1861.
- Prandoni P, Bilora F, Marchiori A, et al. An association between atherosclerosis and venous thrombosis. N Engl J Med 2003; 348:1435–1441.
- Prandoni P, Ghirarduzzi A, Prins MH, et al. Venous thromboembolism and the risk of subsequent symptomatic atherosclerosis. J Thromb Haemost 2006; 4:1891–1896.
- Braekkan SK, Mathiesen EB, Njolstad I, Wilsgaard T, Stormer J, Hansen JB. Family history of myocardial infarction is an independent risk factor for venous thromboembolism: the Tromso study. J Thromb Haemost 2008; 6:1851–1857.
- Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Polak JF, Folsom AR. Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology. Arch Intern Med 2002; 162:1182–1189.
- van der Hagen PB, Folsom AR, Jenny NS, et al. Subclinical atherosclerosis and the risk of future venous thrombosis in the Cardiovascular Health Study. J Thromb Haemost 2006; 4:1903–1908.
- Reich LM, Folsom AR, Key NS, et al. Prospective study of subclinical atherosclerosis as a risk factor for venous thromboembolism. J Thromb Haemost 2006; 4:1909–1913.
- Huerta C, Johansson S, Wallander MA, Rodriguez LA. Risk of myocardial infarction and overall mortality in survivors of venous thromboembolism. Thromb J 2008; 6:10.
- Linnemann B, Schindewolf M, Zgouras D, Erbe M, Jarosch-Preusche M, Lindhoff-Last E. Are patients with thrombophilia and previous venous thromboembolism at higher risk to arterial thrombosis? Thromb Res 2008; 121:743–750.
- Schwaiger J, Kiechl S, Stockner H, et al. Burden of atherosclerosis and risk of venous thromboembolism in patients with migraine. Neurology 2008; 71:937–943.
- Linnemann B, Zgouras D, Schindewolf M, Schwonberg J, Jarosch-Preusche M, Lindhoff-Last E. Impact of sex and traditional cardiovascular risk factors on the risk of recurrent venous thromboembolism: results from the German MAISTHRO Registry. Blood Coagul Fibrinolysis 2008; 19:159–165.
- Steffen LM, Folsom AR, Cushman M, Jacobs DR, Rosamond WD. Greater fish, fruit, and vegetable intakes are related to lower incidence of venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology. Circulation 2007; 115:188–195.
- Arslan F, Pasterkamp G, de Kleijn DP. Unraveling pleiotropic effects of statins: bit by bit, a slow case with perspective. Circ Res 2008; 103:334–336.
- Grady D, Wenger NK, Herrington D, et al. Postmenopausal hormone therapy increases risk for venous thromboembolic disease. The Heart and Estrogen/progestin Replacement Study. Ann Intern Med 2000; 132:689–696.
- Ray JG, Mamdani M, Tsuyuki RT, Anderson DR, Yeo EL, Laupacis A. Use of statins and the subsequent development of deep vein thrombosis. Arch Intern Med 2001; 161:1405–1410.
- Doggen CJ, Lemaitre RN, Smith NL, Heckbert SR, Psaty BM. HMG CoA reductase inhibitors and the risk of venous thrombosis among postmenopausal women. J Thromb Haemost 2004; 2:700–701.
- Lacut K, Oger E, Le Gal G, et al. Statins but not fibrates are associated with a reduced risk of venous thromboembolism: a hospitalbased case-control study. Fundam Clin Pharmacol 2004; 18:477–482.
- Ramcharan AS, Van Stralen KJ, Snoep JD, Mantel-Teeuwisse AK, Rosendaal FR, Doggen CJ. HMG-CoA reductase inhibitors, other lipid-lowering medication, antiplatelet therapy, and the risk of venous thrombosis. J Thromb Haemost 2009; 7:514–520.
- Sørensen HT, Horvath-Puho E, Sogaard KK, et al. Arterial cardiovascular events, statins, low-dose aspirin and subsequent risk of venous thromboembolism: a population-based case-control study. J Thromb Haemost 2009; 7:521–528.
- Yang CC, Jick SS, Jick H. Statins and the risk of idiopathic venous thromboembolism. Br J Clin Pharmacol 2002; 53:101–105.
- Smeeth L, Douglas I, Hall AJ, Hubbard R, Evans S. Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials. Br J Clin Pharmacol 2009; 67:99–109.
- Ridker PM, Danielson E, Fonseca FA, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Shishehbor MH, Hazen SL. JUPITER to Earth: A statin helps peole with normal LDL-C and high hs-CRP, but what does it mean? Cleve Clin J Med 2009; 76:37–44.
- Cushman M. A new indication for statins to prevent venous thromboembolism? Not yet. J Thromb Haemost 2009; 7:511–513.
- Silva MA, Swanson AC, Gandhi PJ, Tataronis GR. Statin-related adverse events: a meta-analysis. Clin Ther 2006; 28:26–35.
A major placebo-controlled trial has found that a statin can reduce the risk of venous thromboembolism (VTE).1
We do not recommend prescribing this class of drugs for this purpose until much more research has been done, and we certainly do not recommend substituting a statin for anticoagulant therapy in a patient at risk of VTE.
Nevertheless, we are excited by the latest findings, and we find comfort in knowing that if a patient is taking a statin for an approved indication, ie, reducing the risk of cardiovascular disease in a patient with hyperlipidemia or a previous cardiovascular event, the drug will also reduce the risk of VTE.
In the pages that follow, we describe and comment on what is known about the effect of statins on the risk of VTE.
ARTERIAL AND VENOUS THROMBOSIS: HOW ARE THEY LINKED?
The causes of arterial thrombosis may not be entirely distinct from those of deep vein thrombosis and pulmonary embolism, collectively referred to as VTE. Some studies have found that risk factors for arterial thrombosis overlap with those for VTE.2–4 However, other studies have shown no association between venous and arterial events.5–10
Hyperlipidemia, in particular, has been evaluated to see if it is a risk factor for VTE. As with other risk factors for arterial thrombosis, the data have been mixed, with some reports favoring an association with VTE and others not.4,5,11 Even so, preventive strategies targeting arterial risk factors have shown promise in reducing VTE events.12
Although commonly used to treat hyperlipidemia, statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) are believed to reduce the incidence of thrombosis by a number of mechanisms13:
- Decreasing platelet aggregation
- Inhibiting expression of tissue factor and plasminogen activator inhibitor 1
- Increasing expression of tissue plasminogen activator
- Increasing expression of thrombomodulin, which can activate protein C and prevent thrombin-induced platelet and factor V activation and fibrinogen clotting.
STATINS AND VTE IN OBSERVATIONAL AND CASE-CONTROL STUDIES
In view of the multiple effects of statins, several studies have looked at whether these drugs reduce the occurrence of both arterial thrombosis and VTE.14–19
Two prospective observational studies and four case-control studies found that statins reduced the risk of VTE by 20% to 60%.14–19 Interestingly, two of the case-control studies found that antiplatelet therapy did not reduce the risk of VTE.18,19
THE JUPITER STUDY
The Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) study primarily sought to determine if rosuvastatin (Crestor) 20 mg/day, compared with placebo, would reduce the rate of first major cardiovascular events.22 A prespecified secondary end point of the trial was VTE, making JUPITER the first randomized, placebo-controlled trial to specifically test whether statins prevent VTE.1
Inclusion criteria: Normal LDL, high CRP
The study included men age 50 and older and women age 60 and older with no history of cardiovascular disease. In addition, their lowdensity lipoprotein (LDL) cholesterol levels had to be lower than 130 mg/dL (3.4 mmol/L), their triglyceride levels had to be lower than 500 mg/dL (5.6 mmol/L), and their highsensitivity C-reactive protein (hs-CRP) levels had to be 2.0 mg/L or higher.
Since high levels of hs-CRP, a marker of inflammation, predict cardiovascular events and since statins lower hs-CRP levels, the investigators hypothesized that people with elevated hs-CRP but without hyperlipidemia might benefit from statin treatment.21
Patients were excluded if they had received lipid-lowering therapy within 6 weeks of the trial screening, had diabetes mellitus or uncontrolled hypertension, were currently using postmenopausal hormone-replacement therapy, or had had cancer within the previous 5 years, except for certain skin cancers.
Candidates who complied well during a 4-week placebo run-in phase were randomly assigned to receive either rosuvastatin 20 mg daily (an intermediate dose) or a matching placebo. In all, 17,802 people were randomized. The two assigned groups appeared to be well matched.
Patients were to come in for visits twice a year for 60 months after randomization to be assessed for symptomatic deep venous thrombosis and pulmonary embolism. New cases of VTE were confirmed by imaging studies, by the initiation of anticoagulation therapy, or by death ascribed to pulmonary embolism.
Idiopathic VTE was classified as unprovoked if it occurred in the absence of trauma, hospitalization, or surgery within 3 months before the event, and in the absence of any diagnosed cancer within 3 months before and after the event. Provoked VTE events were those that occurred in a participant with cancer or when a precipitating event was associated with trauma, hospitalization, or surgery.
Rosuvastatin prevents heart attack, stroke
On the recommendation of the trial’s independent data and safety monitoring board, JUPITER was stopped early because the trial drug showed evidence of efficacy in preventing the combined primary end point of a first major cardiovascular event—ie, nonfatal myocardial infarction, nonfatal stroke, hospitalization for unstable angina, an arterial revascularization procedure, or confirmed death from a cardiovascular cause.22 (The cardiovascular outcomes of the JUPITER study were reviewed by Shishehbor and Hazen23 in the January 2009 issue of the Cleveland Clinic Journal of Medicine; see doi:10.3949/ccjm.75a.08105).
Formal follow-up for the trial's primary and secondary efficacy end points ended then, but data on VTE continued to be collected until each patient’s closeout visit as part of a safety monitoring protocol. The last closeout visit occurred on August 20, 2008. The primary analysis focused on events occurring up to March 30, 2008, the date the study was stopped.
Secondary end point results: Rosuvastatin prevents VTE
At a median follow-up of 1.9 years, an episode of VTE had occurred in 94 (0.53%) of the 17,802 patients—34 in the rosuvastatin group and 60 in the placebo group.1 This translates to 0.18 and 0.32 events per 100 person-years of follow-up in the rosuvastatin and placebo groups, respectively (hazard ratio for the rosuvastatin group 0.57, 95% confidence interval [CI] 0.37–0.86, P = .007).
Forty-four cases of VTE were classified as provoked and 50 cases were categorized as unprovoked. The risk reduction was statistically significant for provoked cases (hazard ratio 0.52, 95% CI 0.28–0.96, P = .03), but not for unprovoked events (hazard ratio 0.61, 95% CI 0.35–1.09, P = .09).
Subgroup analysis revealed no significant association between patient characteristics and the impact of rosuvastatin on the risk of a VTE event, but, as expected, more benefit was associated with higher baseline lipid levels.
STILL TOO SOON TO ADVISE ROUTINE STATIN USE TO PREVENT VTE
While the JUPITER trial data show an apparent benefit of statin use on the rate of VTE events, advising routine use of statins to prevent VTE is premature, for three main reasons.
Many must be treated to prevent one case of VTE. The number needed to treat (NNT) with rosuvastatin for 5 years to prevent either a case of VTE or a cardiovascular event was 21, and the NNT to prevent one cardiovascular event was 25. In a review of the two most recent case-control studies investigating the effects of statins on VTE,18,19 Cushman24 calculated that the NNT to prevent one VTE event each year was 333 for those age 75 and older. Though the Jupiter data did not provide the specific incidence of VTE at 1 year, except graphically, we can estimate that the NNT to prevent one VTE event at 1 year in the study is also very high.
Practically speaking, the perceived benefits of VTE prevention require large numbers to be treated, and the net clinical gain is still largely in preventing arterial events such as heart attack and stroke rather than VTE.
Statins, though safe, can still have adverse effects. The JUPITER study found a trend (albeit nonsignificant) toward more muscle complaints and elevations on liver function testing in apparently healthy persons taking a statin.22 Although severe complications of statin therapy such as rhabdomyolysis and elevations of creatine phosphokinase are rare, patients taking a statin have a 39% higher risk of an adverse event, most commonly myalgias or abnormalities on liver function testing.25 Were statins to be given routinely to even more people than they are now, more adverse outcomes would be likely.
More study is needed. The JUPITER study did not address a high risk of VTE. In fact, the investigators provided no information as to the VTE history of those enrolled.
Clearly, statins should not be substituted for proven prophylaxis and anticoagulation without further investigation, especially for patients with recurrent deep venous thrombosis, hospitalized patients, postoperative patients, and other patients prone to VTE.
OUR VIEW
The JUPITER study is an important leap forward in adding to our knowledge of how to prevent VTE. For people with another indication for taking a statin (eg, a previous cardiovascular event, hyperlipidemia), it is helpful to know that their risk of VTE may be reduced without exposure to the risks of other kinds of conventional thromboprophylaxis.
We look forward to additional studies to elaborate on the benefits of statins in both the prevention and treatment of VTE for averagerisk and VTE-prone populations.
A major placebo-controlled trial has found that a statin can reduce the risk of venous thromboembolism (VTE).1
We do not recommend prescribing this class of drugs for this purpose until much more research has been done, and we certainly do not recommend substituting a statin for anticoagulant therapy in a patient at risk of VTE.
Nevertheless, we are excited by the latest findings, and we find comfort in knowing that if a patient is taking a statin for an approved indication, ie, reducing the risk of cardiovascular disease in a patient with hyperlipidemia or a previous cardiovascular event, the drug will also reduce the risk of VTE.
In the pages that follow, we describe and comment on what is known about the effect of statins on the risk of VTE.
ARTERIAL AND VENOUS THROMBOSIS: HOW ARE THEY LINKED?
The causes of arterial thrombosis may not be entirely distinct from those of deep vein thrombosis and pulmonary embolism, collectively referred to as VTE. Some studies have found that risk factors for arterial thrombosis overlap with those for VTE.2–4 However, other studies have shown no association between venous and arterial events.5–10
Hyperlipidemia, in particular, has been evaluated to see if it is a risk factor for VTE. As with other risk factors for arterial thrombosis, the data have been mixed, with some reports favoring an association with VTE and others not.4,5,11 Even so, preventive strategies targeting arterial risk factors have shown promise in reducing VTE events.12
Although commonly used to treat hyperlipidemia, statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) are believed to reduce the incidence of thrombosis by a number of mechanisms13:
- Decreasing platelet aggregation
- Inhibiting expression of tissue factor and plasminogen activator inhibitor 1
- Increasing expression of tissue plasminogen activator
- Increasing expression of thrombomodulin, which can activate protein C and prevent thrombin-induced platelet and factor V activation and fibrinogen clotting.
STATINS AND VTE IN OBSERVATIONAL AND CASE-CONTROL STUDIES
In view of the multiple effects of statins, several studies have looked at whether these drugs reduce the occurrence of both arterial thrombosis and VTE.14–19
Two prospective observational studies and four case-control studies found that statins reduced the risk of VTE by 20% to 60%.14–19 Interestingly, two of the case-control studies found that antiplatelet therapy did not reduce the risk of VTE.18,19
THE JUPITER STUDY
The Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) study primarily sought to determine if rosuvastatin (Crestor) 20 mg/day, compared with placebo, would reduce the rate of first major cardiovascular events.22 A prespecified secondary end point of the trial was VTE, making JUPITER the first randomized, placebo-controlled trial to specifically test whether statins prevent VTE.1
Inclusion criteria: Normal LDL, high CRP
The study included men age 50 and older and women age 60 and older with no history of cardiovascular disease. In addition, their lowdensity lipoprotein (LDL) cholesterol levels had to be lower than 130 mg/dL (3.4 mmol/L), their triglyceride levels had to be lower than 500 mg/dL (5.6 mmol/L), and their highsensitivity C-reactive protein (hs-CRP) levels had to be 2.0 mg/L or higher.
Since high levels of hs-CRP, a marker of inflammation, predict cardiovascular events and since statins lower hs-CRP levels, the investigators hypothesized that people with elevated hs-CRP but without hyperlipidemia might benefit from statin treatment.21
Patients were excluded if they had received lipid-lowering therapy within 6 weeks of the trial screening, had diabetes mellitus or uncontrolled hypertension, were currently using postmenopausal hormone-replacement therapy, or had had cancer within the previous 5 years, except for certain skin cancers.
Candidates who complied well during a 4-week placebo run-in phase were randomly assigned to receive either rosuvastatin 20 mg daily (an intermediate dose) or a matching placebo. In all, 17,802 people were randomized. The two assigned groups appeared to be well matched.
Patients were to come in for visits twice a year for 60 months after randomization to be assessed for symptomatic deep venous thrombosis and pulmonary embolism. New cases of VTE were confirmed by imaging studies, by the initiation of anticoagulation therapy, or by death ascribed to pulmonary embolism.
Idiopathic VTE was classified as unprovoked if it occurred in the absence of trauma, hospitalization, or surgery within 3 months before the event, and in the absence of any diagnosed cancer within 3 months before and after the event. Provoked VTE events were those that occurred in a participant with cancer or when a precipitating event was associated with trauma, hospitalization, or surgery.
Rosuvastatin prevents heart attack, stroke
On the recommendation of the trial’s independent data and safety monitoring board, JUPITER was stopped early because the trial drug showed evidence of efficacy in preventing the combined primary end point of a first major cardiovascular event—ie, nonfatal myocardial infarction, nonfatal stroke, hospitalization for unstable angina, an arterial revascularization procedure, or confirmed death from a cardiovascular cause.22 (The cardiovascular outcomes of the JUPITER study were reviewed by Shishehbor and Hazen23 in the January 2009 issue of the Cleveland Clinic Journal of Medicine; see doi:10.3949/ccjm.75a.08105).
Formal follow-up for the trial's primary and secondary efficacy end points ended then, but data on VTE continued to be collected until each patient’s closeout visit as part of a safety monitoring protocol. The last closeout visit occurred on August 20, 2008. The primary analysis focused on events occurring up to March 30, 2008, the date the study was stopped.
Secondary end point results: Rosuvastatin prevents VTE
At a median follow-up of 1.9 years, an episode of VTE had occurred in 94 (0.53%) of the 17,802 patients—34 in the rosuvastatin group and 60 in the placebo group.1 This translates to 0.18 and 0.32 events per 100 person-years of follow-up in the rosuvastatin and placebo groups, respectively (hazard ratio for the rosuvastatin group 0.57, 95% confidence interval [CI] 0.37–0.86, P = .007).
Forty-four cases of VTE were classified as provoked and 50 cases were categorized as unprovoked. The risk reduction was statistically significant for provoked cases (hazard ratio 0.52, 95% CI 0.28–0.96, P = .03), but not for unprovoked events (hazard ratio 0.61, 95% CI 0.35–1.09, P = .09).
Subgroup analysis revealed no significant association between patient characteristics and the impact of rosuvastatin on the risk of a VTE event, but, as expected, more benefit was associated with higher baseline lipid levels.
STILL TOO SOON TO ADVISE ROUTINE STATIN USE TO PREVENT VTE
While the JUPITER trial data show an apparent benefit of statin use on the rate of VTE events, advising routine use of statins to prevent VTE is premature, for three main reasons.
Many must be treated to prevent one case of VTE. The number needed to treat (NNT) with rosuvastatin for 5 years to prevent either a case of VTE or a cardiovascular event was 21, and the NNT to prevent one cardiovascular event was 25. In a review of the two most recent case-control studies investigating the effects of statins on VTE,18,19 Cushman24 calculated that the NNT to prevent one VTE event each year was 333 for those age 75 and older. Though the Jupiter data did not provide the specific incidence of VTE at 1 year, except graphically, we can estimate that the NNT to prevent one VTE event at 1 year in the study is also very high.
Practically speaking, the perceived benefits of VTE prevention require large numbers to be treated, and the net clinical gain is still largely in preventing arterial events such as heart attack and stroke rather than VTE.
Statins, though safe, can still have adverse effects. The JUPITER study found a trend (albeit nonsignificant) toward more muscle complaints and elevations on liver function testing in apparently healthy persons taking a statin.22 Although severe complications of statin therapy such as rhabdomyolysis and elevations of creatine phosphokinase are rare, patients taking a statin have a 39% higher risk of an adverse event, most commonly myalgias or abnormalities on liver function testing.25 Were statins to be given routinely to even more people than they are now, more adverse outcomes would be likely.
More study is needed. The JUPITER study did not address a high risk of VTE. In fact, the investigators provided no information as to the VTE history of those enrolled.
Clearly, statins should not be substituted for proven prophylaxis and anticoagulation without further investigation, especially for patients with recurrent deep venous thrombosis, hospitalized patients, postoperative patients, and other patients prone to VTE.
OUR VIEW
The JUPITER study is an important leap forward in adding to our knowledge of how to prevent VTE. For people with another indication for taking a statin (eg, a previous cardiovascular event, hyperlipidemia), it is helpful to know that their risk of VTE may be reduced without exposure to the risks of other kinds of conventional thromboprophylaxis.
We look forward to additional studies to elaborate on the benefits of statins in both the prevention and treatment of VTE for averagerisk and VTE-prone populations.
- Glynn RJ, Danielson E, Fonseca FA, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med 2009; 360:1851–1861.
- Prandoni P, Bilora F, Marchiori A, et al. An association between atherosclerosis and venous thrombosis. N Engl J Med 2003; 348:1435–1441.
- Prandoni P, Ghirarduzzi A, Prins MH, et al. Venous thromboembolism and the risk of subsequent symptomatic atherosclerosis. J Thromb Haemost 2006; 4:1891–1896.
- Braekkan SK, Mathiesen EB, Njolstad I, Wilsgaard T, Stormer J, Hansen JB. Family history of myocardial infarction is an independent risk factor for venous thromboembolism: the Tromso study. J Thromb Haemost 2008; 6:1851–1857.
- Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Polak JF, Folsom AR. Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology. Arch Intern Med 2002; 162:1182–1189.
- van der Hagen PB, Folsom AR, Jenny NS, et al. Subclinical atherosclerosis and the risk of future venous thrombosis in the Cardiovascular Health Study. J Thromb Haemost 2006; 4:1903–1908.
- Reich LM, Folsom AR, Key NS, et al. Prospective study of subclinical atherosclerosis as a risk factor for venous thromboembolism. J Thromb Haemost 2006; 4:1909–1913.
- Huerta C, Johansson S, Wallander MA, Rodriguez LA. Risk of myocardial infarction and overall mortality in survivors of venous thromboembolism. Thromb J 2008; 6:10.
- Linnemann B, Schindewolf M, Zgouras D, Erbe M, Jarosch-Preusche M, Lindhoff-Last E. Are patients with thrombophilia and previous venous thromboembolism at higher risk to arterial thrombosis? Thromb Res 2008; 121:743–750.
- Schwaiger J, Kiechl S, Stockner H, et al. Burden of atherosclerosis and risk of venous thromboembolism in patients with migraine. Neurology 2008; 71:937–943.
- Linnemann B, Zgouras D, Schindewolf M, Schwonberg J, Jarosch-Preusche M, Lindhoff-Last E. Impact of sex and traditional cardiovascular risk factors on the risk of recurrent venous thromboembolism: results from the German MAISTHRO Registry. Blood Coagul Fibrinolysis 2008; 19:159–165.
- Steffen LM, Folsom AR, Cushman M, Jacobs DR, Rosamond WD. Greater fish, fruit, and vegetable intakes are related to lower incidence of venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology. Circulation 2007; 115:188–195.
- Arslan F, Pasterkamp G, de Kleijn DP. Unraveling pleiotropic effects of statins: bit by bit, a slow case with perspective. Circ Res 2008; 103:334–336.
- Grady D, Wenger NK, Herrington D, et al. Postmenopausal hormone therapy increases risk for venous thromboembolic disease. The Heart and Estrogen/progestin Replacement Study. Ann Intern Med 2000; 132:689–696.
- Ray JG, Mamdani M, Tsuyuki RT, Anderson DR, Yeo EL, Laupacis A. Use of statins and the subsequent development of deep vein thrombosis. Arch Intern Med 2001; 161:1405–1410.
- Doggen CJ, Lemaitre RN, Smith NL, Heckbert SR, Psaty BM. HMG CoA reductase inhibitors and the risk of venous thrombosis among postmenopausal women. J Thromb Haemost 2004; 2:700–701.
- Lacut K, Oger E, Le Gal G, et al. Statins but not fibrates are associated with a reduced risk of venous thromboembolism: a hospitalbased case-control study. Fundam Clin Pharmacol 2004; 18:477–482.
- Ramcharan AS, Van Stralen KJ, Snoep JD, Mantel-Teeuwisse AK, Rosendaal FR, Doggen CJ. HMG-CoA reductase inhibitors, other lipid-lowering medication, antiplatelet therapy, and the risk of venous thrombosis. J Thromb Haemost 2009; 7:514–520.
- Sørensen HT, Horvath-Puho E, Sogaard KK, et al. Arterial cardiovascular events, statins, low-dose aspirin and subsequent risk of venous thromboembolism: a population-based case-control study. J Thromb Haemost 2009; 7:521–528.
- Yang CC, Jick SS, Jick H. Statins and the risk of idiopathic venous thromboembolism. Br J Clin Pharmacol 2002; 53:101–105.
- Smeeth L, Douglas I, Hall AJ, Hubbard R, Evans S. Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials. Br J Clin Pharmacol 2009; 67:99–109.
- Ridker PM, Danielson E, Fonseca FA, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Shishehbor MH, Hazen SL. JUPITER to Earth: A statin helps peole with normal LDL-C and high hs-CRP, but what does it mean? Cleve Clin J Med 2009; 76:37–44.
- Cushman M. A new indication for statins to prevent venous thromboembolism? Not yet. J Thromb Haemost 2009; 7:511–513.
- Silva MA, Swanson AC, Gandhi PJ, Tataronis GR. Statin-related adverse events: a meta-analysis. Clin Ther 2006; 28:26–35.
- Glynn RJ, Danielson E, Fonseca FA, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med 2009; 360:1851–1861.
- Prandoni P, Bilora F, Marchiori A, et al. An association between atherosclerosis and venous thrombosis. N Engl J Med 2003; 348:1435–1441.
- Prandoni P, Ghirarduzzi A, Prins MH, et al. Venous thromboembolism and the risk of subsequent symptomatic atherosclerosis. J Thromb Haemost 2006; 4:1891–1896.
- Braekkan SK, Mathiesen EB, Njolstad I, Wilsgaard T, Stormer J, Hansen JB. Family history of myocardial infarction is an independent risk factor for venous thromboembolism: the Tromso study. J Thromb Haemost 2008; 6:1851–1857.
- Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Polak JF, Folsom AR. Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology. Arch Intern Med 2002; 162:1182–1189.
- van der Hagen PB, Folsom AR, Jenny NS, et al. Subclinical atherosclerosis and the risk of future venous thrombosis in the Cardiovascular Health Study. J Thromb Haemost 2006; 4:1903–1908.
- Reich LM, Folsom AR, Key NS, et al. Prospective study of subclinical atherosclerosis as a risk factor for venous thromboembolism. J Thromb Haemost 2006; 4:1909–1913.
- Huerta C, Johansson S, Wallander MA, Rodriguez LA. Risk of myocardial infarction and overall mortality in survivors of venous thromboembolism. Thromb J 2008; 6:10.
- Linnemann B, Schindewolf M, Zgouras D, Erbe M, Jarosch-Preusche M, Lindhoff-Last E. Are patients with thrombophilia and previous venous thromboembolism at higher risk to arterial thrombosis? Thromb Res 2008; 121:743–750.
- Schwaiger J, Kiechl S, Stockner H, et al. Burden of atherosclerosis and risk of venous thromboembolism in patients with migraine. Neurology 2008; 71:937–943.
- Linnemann B, Zgouras D, Schindewolf M, Schwonberg J, Jarosch-Preusche M, Lindhoff-Last E. Impact of sex and traditional cardiovascular risk factors on the risk of recurrent venous thromboembolism: results from the German MAISTHRO Registry. Blood Coagul Fibrinolysis 2008; 19:159–165.
- Steffen LM, Folsom AR, Cushman M, Jacobs DR, Rosamond WD. Greater fish, fruit, and vegetable intakes are related to lower incidence of venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology. Circulation 2007; 115:188–195.
- Arslan F, Pasterkamp G, de Kleijn DP. Unraveling pleiotropic effects of statins: bit by bit, a slow case with perspective. Circ Res 2008; 103:334–336.
- Grady D, Wenger NK, Herrington D, et al. Postmenopausal hormone therapy increases risk for venous thromboembolic disease. The Heart and Estrogen/progestin Replacement Study. Ann Intern Med 2000; 132:689–696.
- Ray JG, Mamdani M, Tsuyuki RT, Anderson DR, Yeo EL, Laupacis A. Use of statins and the subsequent development of deep vein thrombosis. Arch Intern Med 2001; 161:1405–1410.
- Doggen CJ, Lemaitre RN, Smith NL, Heckbert SR, Psaty BM. HMG CoA reductase inhibitors and the risk of venous thrombosis among postmenopausal women. J Thromb Haemost 2004; 2:700–701.
- Lacut K, Oger E, Le Gal G, et al. Statins but not fibrates are associated with a reduced risk of venous thromboembolism: a hospitalbased case-control study. Fundam Clin Pharmacol 2004; 18:477–482.
- Ramcharan AS, Van Stralen KJ, Snoep JD, Mantel-Teeuwisse AK, Rosendaal FR, Doggen CJ. HMG-CoA reductase inhibitors, other lipid-lowering medication, antiplatelet therapy, and the risk of venous thrombosis. J Thromb Haemost 2009; 7:514–520.
- Sørensen HT, Horvath-Puho E, Sogaard KK, et al. Arterial cardiovascular events, statins, low-dose aspirin and subsequent risk of venous thromboembolism: a population-based case-control study. J Thromb Haemost 2009; 7:521–528.
- Yang CC, Jick SS, Jick H. Statins and the risk of idiopathic venous thromboembolism. Br J Clin Pharmacol 2002; 53:101–105.
- Smeeth L, Douglas I, Hall AJ, Hubbard R, Evans S. Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials. Br J Clin Pharmacol 2009; 67:99–109.
- Ridker PM, Danielson E, Fonseca FA, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Shishehbor MH, Hazen SL. JUPITER to Earth: A statin helps peole with normal LDL-C and high hs-CRP, but what does it mean? Cleve Clin J Med 2009; 76:37–44.
- Cushman M. A new indication for statins to prevent venous thromboembolism? Not yet. J Thromb Haemost 2009; 7:511–513.
- Silva MA, Swanson AC, Gandhi PJ, Tataronis GR. Statin-related adverse events: a meta-analysis. Clin Ther 2006; 28:26–35.
KEY POINTS
- Risk factors for VTE overlap with those for arterial thrombosis, although the data are mixed.
- The statin drugs have a number of effects on factors other than lipid levels, notably on markers of inflammation and on clotting factors.
- In the JUPITER trial, the incidence of VTE in people taking rosuvastatin (Crestor) 20 mg/day was about half that in people taking placebo. This was a relatively healthy population, and the incidence in both groups was low.
- Further study is needed in patients at risk of VTE.