User login
Kaposi sarcoma (KS) is caused by infection with human herpesvirus 8, a DNA virus and member of the Gammaherpesvirinae subfamily.1,2 Cutaneous KS lesions generally appear as red to purple macules, plaques, and/or nodules that can become ulcerated, causing pain and bleeding. Lesions often are localized on the feet, which may impede walking and daily activities. Early diagnosis and management are important to avoid or minimize severe symptoms (eg, enlargement of lesions, ulceration with bleeding and pain). Injection therapy is recommended for the management of localized disease, while systemic therapy is appropriate for disseminated malignancy.3 There currently is no curative therapy available for KS; however, palliative therapy can avoid or reduce cosmetically unacceptable lesions and painful edema.
Vinblastine (VNB) is a natural vinca alkaloid whose primary cytotoxic effect is prevention of mitotic activity.4 However, antiangiogenetic activity has been demonstrated with low concentrations of VNB.5 The use of intralesional VNB injections for the management of oral KS in open-label studies4 using different doses and multiple courses has proven to be therapeutic.6-8 There are limited studies regarding the use of intralesional VNB injections in patients with cutaneous KS, particularly in those with associated type 2 diabetes mellitus.6-8 Thus, we evaluated the clinical efficacy of intralesional VNB injections in the treatment of KS lesions in diabetic patients with no visceral involvement.
Methods
This study was conducted in the department of dermatology from January 1, 2009, to December 31, 2011, and was approved by the institutional review board of the Fondazione IRCCS Policlinico San Matteo (Pavia, Italy). Six patients with type 2 diabetes mellitus and histologically diagnosed cutaneous KS were enrolled in the study. Inclusion criteria included histological diagnosis of KS, negative human immunodeficiency virus testing, history of type 2 diabetes mellitus, more than 5 cutaneous KS lesions, and no visceral involvement (confirmed by colonoscopy, gastroscopy, lymph node and abdominal echography, and chest radiograph). Informed written consent was obtained from all participants.
Treated plaques and nodules measured more than 1 to 2 cm in diameter. Intralesional vinblastine injections were administered, not perilesional, with a standard concentration of 0.5 mg/mL per 1-cm2 lesion (maximum dose, 2 mg daily). All participants received 1 injection per nodule and a maximum of 2 injections per plaque. Monitoring of the target lesions was done via photography and mapping. Clinical evaluation of the treated lesions and assessment of side effects was conducted at 1-week and 1-, 3-, and 6-month follow-up. Complete response was defined as 95% to 100% remission of the target lesions, partial response was defined as 50% to 95% remission, and minimal response was defined as less than 50% remission, all at 3-month follow-up. No response was defined as no change or increase in size of the target lesions. Side effects including pain, hyperpigmentation, and ulcer formation were evaluated.
Results
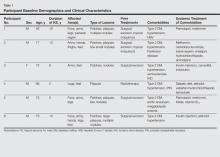
A total of 6 participants (5 men, 1 woman; age range, 66–82 years) completed the study. Baseline demographics and clinical characteristics of the participants are reported in Table 1. Three participants were currently undergoing diabetic therapy with metformin, 2 were receiving insulin injections, and 1 was following a diabetic diet. None of the participants had internal KS. All 5 participants with nodular KS lesions previously underwent surgical excision. One participant also underwent radiotherapy with a good initial outcome, but lesions recurred at the same site within 1 year.
Complete response of nodular KS lesions and partial response of plaques to intralesional VNB injections was observed at 3-month follow-up in all treated lesions (Table 2). Five participants achieved a greater than 50% reduction (overall decrease in size and flattening of the plaque) of the KS plaque lesions. No participants showed minimal or no response.
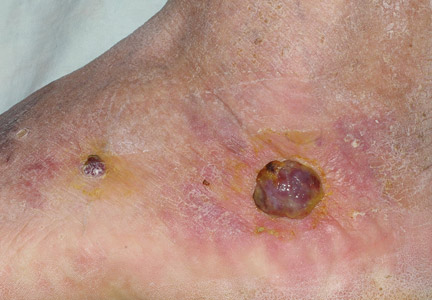
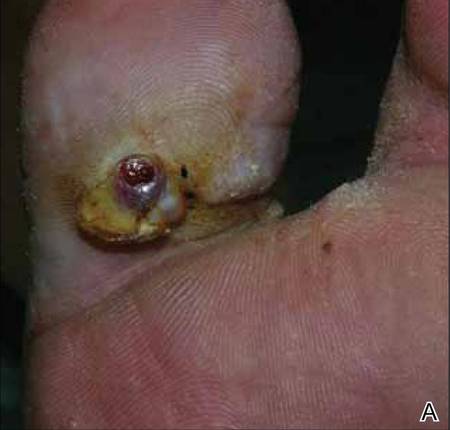
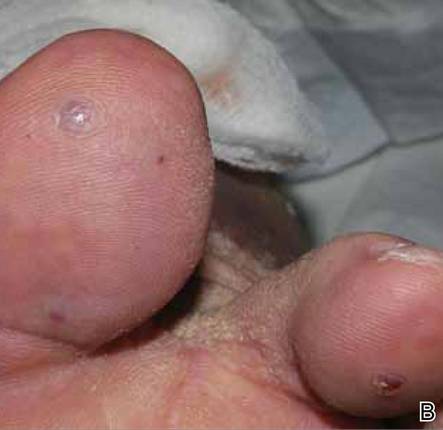
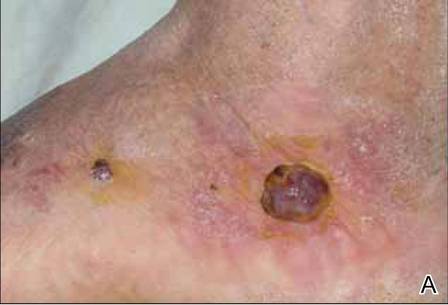
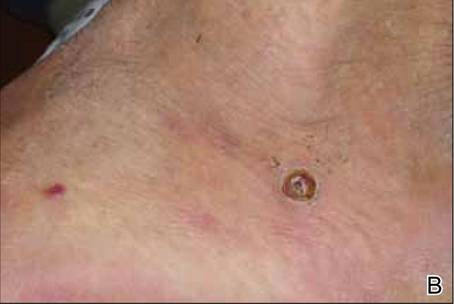
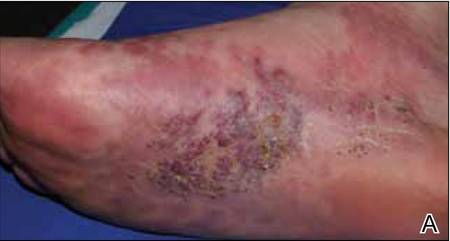
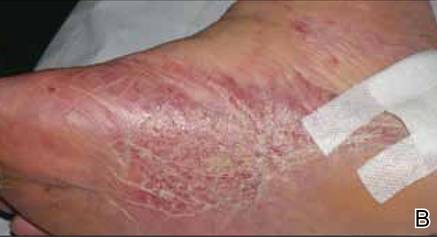
At 1-week follow-up, shrinkage of nodular lesions was evident with necrotic crusts. Participant 4 who presented with plaques under the feet developed lymphatic serum exudation in the first 3 days following treatment on the legs. All the treated nodular lesions resolved completely within 3 months after treatment (Figures 1 and 2), though some new lesions appeared in new locations. All the treated plaques showed partial response to treatment after 1- to 3-month follow-up (Figure 3).
  |
  |
  |
All participants reported pain on the second and third days posttreatment, but analgesics were only prescribed to participants 2 and 4. Hyperpigmentation developed after 3 to 6 months in 3 participants. Two participants developed small ulcers within 2 weeks following treatment that healed within 3 months.
Comment
Classic KS is characterized by a localized onset with slow progression of systemic involvement. Cutaneous lesions progress from mild to severe.9 Systemic therapeutic strategies currently employed for the management of KS include single-agent and multiagent cytotoxic chemotherapy.10 Because these drugs have been associated with remarkable systemic side effects and patients often are elderly individuals, systemic therapy may be unsuitable for long-term use. In an epidemiologic study of 37 patients with KS, 12 patients (32%) were found to have concurrent diabetes mellitus.11 Some authors suggest that KS in diabetic patients is a subtype of KS because of the immunosuppressive state caused by diabetes.12-15
Some of the reported therapies for lesions of classic KS include cryotherapy, surgical excision, radiation therapy, alitretinoin gel 0.1%, and infrared photocoagulation.3-16 Cryotherapy of a small papular lesion requires a 30- to 60-second freeze time that usually needs to be followed by further cryotherapy applications and is not known to be effective in lesions larger than 1 cm.17 Surgical procedures in the management of cutaneous KS may cause discomfort and bleeding and also are expensive.18 Radiotherapy is an alternative therapy, especially in plaque lesions.19 Alitretinoin gel 0.1% has been shown to be effective and well tolerated for the treatment of cutaneous lesions in patients with AIDS-related KS. Although Morganroth20 reported alitretinoin gel 0.1% as a promising therapy for control of classic KS cutaneous lesions, Rongioletti et al21 described failure of this topical treatment for classic KS. The efficacy of topical alitretinoin in classic KS needs to be confirmed by further studies. Short pulses of the infrared coagulator have been reported for the treatment of 10 cutaneous AIDS-related KS lesions in 7 patients. The infrared coagulator may be a useful addition in the palliative cosmetic treatment of KS, producing acceptable results in small (ie, <2 cm in diameter) lesions of the arms and trunk but not in those situated on the legs, as in our patients.16
The use of VNB in classic KS was pioneered in 1980 by Klein et al22 who described 14 patients with KS who were systemically treated with VNB sulfate at a low dose with excellent therapeutic results leading to regression of cutaneous lesions. In this study, intravenous VNB therapy was supplemented with intralesional VNB.22 In other reports, intralesional VNB injections have proved to be effective in reducing lesion size and symptoms in oral KS with mild adverse effects.19-29 For this reason, we decided to employ intralesional VNB injections in nodules and plaques of KS patients with diabetes. After treatment, lesion size was reduced in all 6 participants, with 4 participants achieving more than 50% reduction of the lesion size. In other studies reporting oral lesions,3 the highest proportion of complete responses was achieved with injections done periodically until evidence of clinical resolution was obtained, with a mean number of 2.4 injections per patient. In contrast, another study reported complete response of 48% in patients who received a single dose of VNB,7 as in our study.
Minimal complications following intralesional VNB injections have been reported. In our study, moderate pain following VNB injection was common during the first week posttreatment but typically resolved partially or completely during the following weeks. Additionally, pain described as needlelike during the procedure was minimal except for 1 participant who reported severe pain a day after the treatment. Unlike the study by Smith et al,30 lidocaine with epinephrine was not used in our study as a pretreatment.
At 1-month follow-up, clinical evaluation of the injection site revealed ulceration in 2 participants, but no ulcers were noted at 3-month follow-up. To avoid this potentially dangerous complication, which can involve the foot in diabetic patients, further studies of a larger sample of patients using a lower dose of VNB are needed.
In our small group of diabetic patients, intralesional injections of VNB proved to be effective in the management of cutaneous lesions of classic KS with minimal adverse side effects. Even in cases where only one intralesional injection was administered, improvement was observed in all treated lesions. Imiquimod also has been proposed for the treatment of KS. Célestin Schartz et al31 conducted a study of 17 patients who were treated with imiquimod cream applied under occlusion 3 times weekly for 24 weeks. Eight patients had an objective clinical response (2 complete and 6 partial responses), and tumor progression was noted in 6 patients.31 This treatment appears to be suitable for new small skin lesions in immunocompetent patients; however, no known comparative studies have been conducted, possibly because of the relatively high cost of treatment due to the chronic nature of the disease. Intralesional bleomycin also has been reported as useful in the treatment of cutaneous KS. The best results were obtained with macular lesions.32 However, the risk for necrosis is high. Therefore, bleomycin is not the first choice for diabetics, particularly those who have lesions localized on legs.
Conclusion
Standard treatments of KS lesions such as radiotherapy, surgery, and chemotherapy are associated with more severe adverse events, especially in diabetic patients, as well as higher costs due to the use of more sophisticated techniques and equipment. Because KS is a multicentric disease, local therapy should not be expected to prevent the development of new lesions on other sites of the skin or internal disease. The best candidates for localized therapy are patients affected by nodular classic KS without visceral involvement, with fewer than 10 lesions on acral sites.
1. Cathomas G. Human herpes virus 8: a new virus discloses its face. Virchows Arch. 2000;436:195-206.
2. Chang Y, Cesarman CE, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865-1869.
3. Tur E, Brenner S. Treatment of Kaposi’s sarcoma. Arch Dermatol. 1996;132:327-331.
4. Gorsky M, Epstein JB. A case series of acquired immunodeficiency syndrome patients with initial neoplastic diagnoses of intraoral Kaposi’s sarcoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:612-617.
5. Chaplin DJ. The effect of therapy on tumor vascular function. Int J Radiat Biol. 1991;60:311-325.
6. Donehower RC, Rowinsky EK. Anticancer drugs derived from plants. In: De Vita VT, Hellman S, Rosenberg SA, eds. Cancer: Principles and Practice of Oncology. 4th ed. Philadelphia, PA: Lippincott; 1993:409-417.
7. Epstein JB. Treatment of oral Kaposi’s sarcoma with intralesional vinblastine. Cancer. 1993;71:1722-1725.
8. McCormick SU. Intralesional vinblastine injections for the treatment of oral Kaposi’s sarcoma: report of 10 patients with 2-year follow-up. J Oral Maxillofac Surg. 1996;54:583-587.
9. Schwartz RA, Micali G, Nasca MR, et al. Kaposi sarcoma: a continuing conundrum. J Am Acad Dermatol. 2008;59:179-206.
10. Schwartz RA. Kaposi’s sarcoma: an update. J Surg Oncol. 2004;87:146-151.
11. Laor Y, Schwartz RA. Epidemiologic aspects of American Kaposi’s sarcoma. J Surg Oncol. 1979;12:299-303.
12. Gill K, Shah J. Kaposi sarcoma in patients with diabetes and wounds. Adv Skin Wound Care. 2006;19:196-198, 201.
13. Hurlbut W, Lincoln CS Jr. Multiple hemorrhagic sarcoma and diabetes mellitus; review of a series, with report of two cases. Arch Intern Med (Chic). 1949;84:738-750.
14. Fischer JW, Cohen DM. Simultaneous occurrence of Kaposi’s sarcoma, leukemia and diabetes mellitus; report of a case. Am J Clin Pathol. 1951;21:586-589.
15. Tankel R. Kaposi’s sarcoma and diabetes mellitus. Arch Dermatol. 1971;104:442-444.
16. Langtry JA, Bottomley DM, Phillips RH, et al. The infra-red coagulator in the treatment of AIDS-related Kaposi’s sarcoma and a comparison with radiotherapy. Clin Exp Dermatol. 1994;19:23-25.
17. Von Roenn JH, Cianfrocca M. Treatment of Kaposi’s sarcoma. Cancer Treat Res. 2001;104:127-148.
18. Dezube BJ. AIDS-related Kaposi sarcoma. the role of local therapy for a systemic disease. Arch Dermatol. 2000;136:1554-1556.
19. Oysul K, Beyzadeoglu M, Surenkok S, et al. A dose-response analysis for classical Kaposi’s sarcoma management by radiotherapy. Saudi Med J. 2008;29:837-840.
20. Morganroth GS. Topical 0.1% alitretinoin gel for classic Kaposi sarcoma. Arch Dermatol. 2002;138:542-543.
21. Rongioletti F, Zaccaria E, Viglizzo G. Failure of topical 0.1% alitretinoin gel for classic Kaposi sarcoma: first European experience. Br J Dermatol. 2006;155:856-857.
22. Klein E, Schwartz RA, Laor Y, et al. Treatment of Kaposi’s sarcoma with vinblastine. Cancer. 1980;45:427-431.
23. Flaitz CM, Nichols CM, Hicks MJ. Role of intralesional vinblastine administration in treatment of intraoral Kaposi’s sarcoma in AIDS. Eur J Cancer B Oral Oncol. 1995;31B:280-285.
24. Epstein JB, Lozada-Nur F, McLeod WA, et al. Oral Kaposi’s sarcoma in acquired immunodeficiency syndrome. review of management and report of the efficacy of intralesional vinblastine. Cancer. 1989;64:2424-2430.
25. Antman K, Chang Y. Kaposi’s sarcoma. N Engl J Med. 2000;342:1027-1038.
26. Ramírez-Amador V, Esquivel-Pedraza L, Lozada-Nur F, et al. Intralesional vinblastine vs. 3% sodium tetradecyl sulfate for the treatment of oral Kaposi’s sarcoma. a double blind, randomized clinical trial. Oral Oncol. 2002;38:460-467.
27. Boudreaux AA, Smith LL, Cosby CD, et al. Intralesional vinblastine for cutaneous Kaposi’s sarcoma associated with acquired immunodeficiency syndrome. a clinical trial to evaluate efficacy and discomfort associated with infection. J Am Acad Dermatol. 1993;28:61-65.
28. Odom RB, Goette DK. Treatment of cutaneous Kaposi’s sarcoma with intralesional vincristine. Arch Dermatol. 1978;114:1693-1694.
29. Tappero JW, Conant MA, Wolfe SF, et al. Kaposi’s sarcoma. epidemiology, pathogenesis, histology, clinical spectrum, staging criteria and therapy. J Am Acad Dermatol. 1993;28:371-395.
30. Smith KJ, Skelton HG, Turiansky G, et al. Hyaluronidase enhances the therapeutic effect of vinblastine in intralesional treatment of Kaposi’s sarcoma. Military Medical Consortium for the Advancement of Retroviral Research (MMCARR). J Am Acad Dermatol. 1997;36(2, pt 1):239-242.
31. Célestin Schartz NE, Chevret S, Paz C, et al. Imiquimod 5% cream for treatment of HIV-negative Kaposi’s sarcoma skin lesions: a phase I to II, open-label trial in 17 patients [published online ahead of print February 20, 2008]. J Am Acad Dermatol. 2008;58:585-591.
32. Poignonec S, Lachiver LD, Lamas G, et al. Intralesional bleomycin for acquired immunodeficiency syndrome-associated cutaneous Kaposi’s sarcoma. Arch Dermatol. 1995;131:228.
Kaposi sarcoma (KS) is caused by infection with human herpesvirus 8, a DNA virus and member of the Gammaherpesvirinae subfamily.1,2 Cutaneous KS lesions generally appear as red to purple macules, plaques, and/or nodules that can become ulcerated, causing pain and bleeding. Lesions often are localized on the feet, which may impede walking and daily activities. Early diagnosis and management are important to avoid or minimize severe symptoms (eg, enlargement of lesions, ulceration with bleeding and pain). Injection therapy is recommended for the management of localized disease, while systemic therapy is appropriate for disseminated malignancy.3 There currently is no curative therapy available for KS; however, palliative therapy can avoid or reduce cosmetically unacceptable lesions and painful edema.
Vinblastine (VNB) is a natural vinca alkaloid whose primary cytotoxic effect is prevention of mitotic activity.4 However, antiangiogenetic activity has been demonstrated with low concentrations of VNB.5 The use of intralesional VNB injections for the management of oral KS in open-label studies4 using different doses and multiple courses has proven to be therapeutic.6-8 There are limited studies regarding the use of intralesional VNB injections in patients with cutaneous KS, particularly in those with associated type 2 diabetes mellitus.6-8 Thus, we evaluated the clinical efficacy of intralesional VNB injections in the treatment of KS lesions in diabetic patients with no visceral involvement.
Methods
This study was conducted in the department of dermatology from January 1, 2009, to December 31, 2011, and was approved by the institutional review board of the Fondazione IRCCS Policlinico San Matteo (Pavia, Italy). Six patients with type 2 diabetes mellitus and histologically diagnosed cutaneous KS were enrolled in the study. Inclusion criteria included histological diagnosis of KS, negative human immunodeficiency virus testing, history of type 2 diabetes mellitus, more than 5 cutaneous KS lesions, and no visceral involvement (confirmed by colonoscopy, gastroscopy, lymph node and abdominal echography, and chest radiograph). Informed written consent was obtained from all participants.
Treated plaques and nodules measured more than 1 to 2 cm in diameter. Intralesional vinblastine injections were administered, not perilesional, with a standard concentration of 0.5 mg/mL per 1-cm2 lesion (maximum dose, 2 mg daily). All participants received 1 injection per nodule and a maximum of 2 injections per plaque. Monitoring of the target lesions was done via photography and mapping. Clinical evaluation of the treated lesions and assessment of side effects was conducted at 1-week and 1-, 3-, and 6-month follow-up. Complete response was defined as 95% to 100% remission of the target lesions, partial response was defined as 50% to 95% remission, and minimal response was defined as less than 50% remission, all at 3-month follow-up. No response was defined as no change or increase in size of the target lesions. Side effects including pain, hyperpigmentation, and ulcer formation were evaluated.
Results
A total of 6 participants (5 men, 1 woman; age range, 66–82 years) completed the study. Baseline demographics and clinical characteristics of the participants are reported in Table 1. Three participants were currently undergoing diabetic therapy with metformin, 2 were receiving insulin injections, and 1 was following a diabetic diet. None of the participants had internal KS. All 5 participants with nodular KS lesions previously underwent surgical excision. One participant also underwent radiotherapy with a good initial outcome, but lesions recurred at the same site within 1 year.
Complete response of nodular KS lesions and partial response of plaques to intralesional VNB injections was observed at 3-month follow-up in all treated lesions (Table 2). Five participants achieved a greater than 50% reduction (overall decrease in size and flattening of the plaque) of the KS plaque lesions. No participants showed minimal or no response.
At 1-week follow-up, shrinkage of nodular lesions was evident with necrotic crusts. Participant 4 who presented with plaques under the feet developed lymphatic serum exudation in the first 3 days following treatment on the legs. All the treated nodular lesions resolved completely within 3 months after treatment (Figures 1 and 2), though some new lesions appeared in new locations. All the treated plaques showed partial response to treatment after 1- to 3-month follow-up (Figure 3).
  |
  |
  |
All participants reported pain on the second and third days posttreatment, but analgesics were only prescribed to participants 2 and 4. Hyperpigmentation developed after 3 to 6 months in 3 participants. Two participants developed small ulcers within 2 weeks following treatment that healed within 3 months.
Comment
Classic KS is characterized by a localized onset with slow progression of systemic involvement. Cutaneous lesions progress from mild to severe.9 Systemic therapeutic strategies currently employed for the management of KS include single-agent and multiagent cytotoxic chemotherapy.10 Because these drugs have been associated with remarkable systemic side effects and patients often are elderly individuals, systemic therapy may be unsuitable for long-term use. In an epidemiologic study of 37 patients with KS, 12 patients (32%) were found to have concurrent diabetes mellitus.11 Some authors suggest that KS in diabetic patients is a subtype of KS because of the immunosuppressive state caused by diabetes.12-15
Some of the reported therapies for lesions of classic KS include cryotherapy, surgical excision, radiation therapy, alitretinoin gel 0.1%, and infrared photocoagulation.3-16 Cryotherapy of a small papular lesion requires a 30- to 60-second freeze time that usually needs to be followed by further cryotherapy applications and is not known to be effective in lesions larger than 1 cm.17 Surgical procedures in the management of cutaneous KS may cause discomfort and bleeding and also are expensive.18 Radiotherapy is an alternative therapy, especially in plaque lesions.19 Alitretinoin gel 0.1% has been shown to be effective and well tolerated for the treatment of cutaneous lesions in patients with AIDS-related KS. Although Morganroth20 reported alitretinoin gel 0.1% as a promising therapy for control of classic KS cutaneous lesions, Rongioletti et al21 described failure of this topical treatment for classic KS. The efficacy of topical alitretinoin in classic KS needs to be confirmed by further studies. Short pulses of the infrared coagulator have been reported for the treatment of 10 cutaneous AIDS-related KS lesions in 7 patients. The infrared coagulator may be a useful addition in the palliative cosmetic treatment of KS, producing acceptable results in small (ie, <2 cm in diameter) lesions of the arms and trunk but not in those situated on the legs, as in our patients.16
The use of VNB in classic KS was pioneered in 1980 by Klein et al22 who described 14 patients with KS who were systemically treated with VNB sulfate at a low dose with excellent therapeutic results leading to regression of cutaneous lesions. In this study, intravenous VNB therapy was supplemented with intralesional VNB.22 In other reports, intralesional VNB injections have proved to be effective in reducing lesion size and symptoms in oral KS with mild adverse effects.19-29 For this reason, we decided to employ intralesional VNB injections in nodules and plaques of KS patients with diabetes. After treatment, lesion size was reduced in all 6 participants, with 4 participants achieving more than 50% reduction of the lesion size. In other studies reporting oral lesions,3 the highest proportion of complete responses was achieved with injections done periodically until evidence of clinical resolution was obtained, with a mean number of 2.4 injections per patient. In contrast, another study reported complete response of 48% in patients who received a single dose of VNB,7 as in our study.
Minimal complications following intralesional VNB injections have been reported. In our study, moderate pain following VNB injection was common during the first week posttreatment but typically resolved partially or completely during the following weeks. Additionally, pain described as needlelike during the procedure was minimal except for 1 participant who reported severe pain a day after the treatment. Unlike the study by Smith et al,30 lidocaine with epinephrine was not used in our study as a pretreatment.
At 1-month follow-up, clinical evaluation of the injection site revealed ulceration in 2 participants, but no ulcers were noted at 3-month follow-up. To avoid this potentially dangerous complication, which can involve the foot in diabetic patients, further studies of a larger sample of patients using a lower dose of VNB are needed.
In our small group of diabetic patients, intralesional injections of VNB proved to be effective in the management of cutaneous lesions of classic KS with minimal adverse side effects. Even in cases where only one intralesional injection was administered, improvement was observed in all treated lesions. Imiquimod also has been proposed for the treatment of KS. Célestin Schartz et al31 conducted a study of 17 patients who were treated with imiquimod cream applied under occlusion 3 times weekly for 24 weeks. Eight patients had an objective clinical response (2 complete and 6 partial responses), and tumor progression was noted in 6 patients.31 This treatment appears to be suitable for new small skin lesions in immunocompetent patients; however, no known comparative studies have been conducted, possibly because of the relatively high cost of treatment due to the chronic nature of the disease. Intralesional bleomycin also has been reported as useful in the treatment of cutaneous KS. The best results were obtained with macular lesions.32 However, the risk for necrosis is high. Therefore, bleomycin is not the first choice for diabetics, particularly those who have lesions localized on legs.
Conclusion
Standard treatments of KS lesions such as radiotherapy, surgery, and chemotherapy are associated with more severe adverse events, especially in diabetic patients, as well as higher costs due to the use of more sophisticated techniques and equipment. Because KS is a multicentric disease, local therapy should not be expected to prevent the development of new lesions on other sites of the skin or internal disease. The best candidates for localized therapy are patients affected by nodular classic KS without visceral involvement, with fewer than 10 lesions on acral sites.
Kaposi sarcoma (KS) is caused by infection with human herpesvirus 8, a DNA virus and member of the Gammaherpesvirinae subfamily.1,2 Cutaneous KS lesions generally appear as red to purple macules, plaques, and/or nodules that can become ulcerated, causing pain and bleeding. Lesions often are localized on the feet, which may impede walking and daily activities. Early diagnosis and management are important to avoid or minimize severe symptoms (eg, enlargement of lesions, ulceration with bleeding and pain). Injection therapy is recommended for the management of localized disease, while systemic therapy is appropriate for disseminated malignancy.3 There currently is no curative therapy available for KS; however, palliative therapy can avoid or reduce cosmetically unacceptable lesions and painful edema.
Vinblastine (VNB) is a natural vinca alkaloid whose primary cytotoxic effect is prevention of mitotic activity.4 However, antiangiogenetic activity has been demonstrated with low concentrations of VNB.5 The use of intralesional VNB injections for the management of oral KS in open-label studies4 using different doses and multiple courses has proven to be therapeutic.6-8 There are limited studies regarding the use of intralesional VNB injections in patients with cutaneous KS, particularly in those with associated type 2 diabetes mellitus.6-8 Thus, we evaluated the clinical efficacy of intralesional VNB injections in the treatment of KS lesions in diabetic patients with no visceral involvement.
Methods
This study was conducted in the department of dermatology from January 1, 2009, to December 31, 2011, and was approved by the institutional review board of the Fondazione IRCCS Policlinico San Matteo (Pavia, Italy). Six patients with type 2 diabetes mellitus and histologically diagnosed cutaneous KS were enrolled in the study. Inclusion criteria included histological diagnosis of KS, negative human immunodeficiency virus testing, history of type 2 diabetes mellitus, more than 5 cutaneous KS lesions, and no visceral involvement (confirmed by colonoscopy, gastroscopy, lymph node and abdominal echography, and chest radiograph). Informed written consent was obtained from all participants.
Treated plaques and nodules measured more than 1 to 2 cm in diameter. Intralesional vinblastine injections were administered, not perilesional, with a standard concentration of 0.5 mg/mL per 1-cm2 lesion (maximum dose, 2 mg daily). All participants received 1 injection per nodule and a maximum of 2 injections per plaque. Monitoring of the target lesions was done via photography and mapping. Clinical evaluation of the treated lesions and assessment of side effects was conducted at 1-week and 1-, 3-, and 6-month follow-up. Complete response was defined as 95% to 100% remission of the target lesions, partial response was defined as 50% to 95% remission, and minimal response was defined as less than 50% remission, all at 3-month follow-up. No response was defined as no change or increase in size of the target lesions. Side effects including pain, hyperpigmentation, and ulcer formation were evaluated.
Results
A total of 6 participants (5 men, 1 woman; age range, 66–82 years) completed the study. Baseline demographics and clinical characteristics of the participants are reported in Table 1. Three participants were currently undergoing diabetic therapy with metformin, 2 were receiving insulin injections, and 1 was following a diabetic diet. None of the participants had internal KS. All 5 participants with nodular KS lesions previously underwent surgical excision. One participant also underwent radiotherapy with a good initial outcome, but lesions recurred at the same site within 1 year.
Complete response of nodular KS lesions and partial response of plaques to intralesional VNB injections was observed at 3-month follow-up in all treated lesions (Table 2). Five participants achieved a greater than 50% reduction (overall decrease in size and flattening of the plaque) of the KS plaque lesions. No participants showed minimal or no response.
At 1-week follow-up, shrinkage of nodular lesions was evident with necrotic crusts. Participant 4 who presented with plaques under the feet developed lymphatic serum exudation in the first 3 days following treatment on the legs. All the treated nodular lesions resolved completely within 3 months after treatment (Figures 1 and 2), though some new lesions appeared in new locations. All the treated plaques showed partial response to treatment after 1- to 3-month follow-up (Figure 3).
  |
  |
  |
All participants reported pain on the second and third days posttreatment, but analgesics were only prescribed to participants 2 and 4. Hyperpigmentation developed after 3 to 6 months in 3 participants. Two participants developed small ulcers within 2 weeks following treatment that healed within 3 months.
Comment
Classic KS is characterized by a localized onset with slow progression of systemic involvement. Cutaneous lesions progress from mild to severe.9 Systemic therapeutic strategies currently employed for the management of KS include single-agent and multiagent cytotoxic chemotherapy.10 Because these drugs have been associated with remarkable systemic side effects and patients often are elderly individuals, systemic therapy may be unsuitable for long-term use. In an epidemiologic study of 37 patients with KS, 12 patients (32%) were found to have concurrent diabetes mellitus.11 Some authors suggest that KS in diabetic patients is a subtype of KS because of the immunosuppressive state caused by diabetes.12-15
Some of the reported therapies for lesions of classic KS include cryotherapy, surgical excision, radiation therapy, alitretinoin gel 0.1%, and infrared photocoagulation.3-16 Cryotherapy of a small papular lesion requires a 30- to 60-second freeze time that usually needs to be followed by further cryotherapy applications and is not known to be effective in lesions larger than 1 cm.17 Surgical procedures in the management of cutaneous KS may cause discomfort and bleeding and also are expensive.18 Radiotherapy is an alternative therapy, especially in plaque lesions.19 Alitretinoin gel 0.1% has been shown to be effective and well tolerated for the treatment of cutaneous lesions in patients with AIDS-related KS. Although Morganroth20 reported alitretinoin gel 0.1% as a promising therapy for control of classic KS cutaneous lesions, Rongioletti et al21 described failure of this topical treatment for classic KS. The efficacy of topical alitretinoin in classic KS needs to be confirmed by further studies. Short pulses of the infrared coagulator have been reported for the treatment of 10 cutaneous AIDS-related KS lesions in 7 patients. The infrared coagulator may be a useful addition in the palliative cosmetic treatment of KS, producing acceptable results in small (ie, <2 cm in diameter) lesions of the arms and trunk but not in those situated on the legs, as in our patients.16
The use of VNB in classic KS was pioneered in 1980 by Klein et al22 who described 14 patients with KS who were systemically treated with VNB sulfate at a low dose with excellent therapeutic results leading to regression of cutaneous lesions. In this study, intravenous VNB therapy was supplemented with intralesional VNB.22 In other reports, intralesional VNB injections have proved to be effective in reducing lesion size and symptoms in oral KS with mild adverse effects.19-29 For this reason, we decided to employ intralesional VNB injections in nodules and plaques of KS patients with diabetes. After treatment, lesion size was reduced in all 6 participants, with 4 participants achieving more than 50% reduction of the lesion size. In other studies reporting oral lesions,3 the highest proportion of complete responses was achieved with injections done periodically until evidence of clinical resolution was obtained, with a mean number of 2.4 injections per patient. In contrast, another study reported complete response of 48% in patients who received a single dose of VNB,7 as in our study.
Minimal complications following intralesional VNB injections have been reported. In our study, moderate pain following VNB injection was common during the first week posttreatment but typically resolved partially or completely during the following weeks. Additionally, pain described as needlelike during the procedure was minimal except for 1 participant who reported severe pain a day after the treatment. Unlike the study by Smith et al,30 lidocaine with epinephrine was not used in our study as a pretreatment.
At 1-month follow-up, clinical evaluation of the injection site revealed ulceration in 2 participants, but no ulcers were noted at 3-month follow-up. To avoid this potentially dangerous complication, which can involve the foot in diabetic patients, further studies of a larger sample of patients using a lower dose of VNB are needed.
In our small group of diabetic patients, intralesional injections of VNB proved to be effective in the management of cutaneous lesions of classic KS with minimal adverse side effects. Even in cases where only one intralesional injection was administered, improvement was observed in all treated lesions. Imiquimod also has been proposed for the treatment of KS. Célestin Schartz et al31 conducted a study of 17 patients who were treated with imiquimod cream applied under occlusion 3 times weekly for 24 weeks. Eight patients had an objective clinical response (2 complete and 6 partial responses), and tumor progression was noted in 6 patients.31 This treatment appears to be suitable for new small skin lesions in immunocompetent patients; however, no known comparative studies have been conducted, possibly because of the relatively high cost of treatment due to the chronic nature of the disease. Intralesional bleomycin also has been reported as useful in the treatment of cutaneous KS. The best results were obtained with macular lesions.32 However, the risk for necrosis is high. Therefore, bleomycin is not the first choice for diabetics, particularly those who have lesions localized on legs.
Conclusion
Standard treatments of KS lesions such as radiotherapy, surgery, and chemotherapy are associated with more severe adverse events, especially in diabetic patients, as well as higher costs due to the use of more sophisticated techniques and equipment. Because KS is a multicentric disease, local therapy should not be expected to prevent the development of new lesions on other sites of the skin or internal disease. The best candidates for localized therapy are patients affected by nodular classic KS without visceral involvement, with fewer than 10 lesions on acral sites.
1. Cathomas G. Human herpes virus 8: a new virus discloses its face. Virchows Arch. 2000;436:195-206.
2. Chang Y, Cesarman CE, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865-1869.
3. Tur E, Brenner S. Treatment of Kaposi’s sarcoma. Arch Dermatol. 1996;132:327-331.
4. Gorsky M, Epstein JB. A case series of acquired immunodeficiency syndrome patients with initial neoplastic diagnoses of intraoral Kaposi’s sarcoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:612-617.
5. Chaplin DJ. The effect of therapy on tumor vascular function. Int J Radiat Biol. 1991;60:311-325.
6. Donehower RC, Rowinsky EK. Anticancer drugs derived from plants. In: De Vita VT, Hellman S, Rosenberg SA, eds. Cancer: Principles and Practice of Oncology. 4th ed. Philadelphia, PA: Lippincott; 1993:409-417.
7. Epstein JB. Treatment of oral Kaposi’s sarcoma with intralesional vinblastine. Cancer. 1993;71:1722-1725.
8. McCormick SU. Intralesional vinblastine injections for the treatment of oral Kaposi’s sarcoma: report of 10 patients with 2-year follow-up. J Oral Maxillofac Surg. 1996;54:583-587.
9. Schwartz RA, Micali G, Nasca MR, et al. Kaposi sarcoma: a continuing conundrum. J Am Acad Dermatol. 2008;59:179-206.
10. Schwartz RA. Kaposi’s sarcoma: an update. J Surg Oncol. 2004;87:146-151.
11. Laor Y, Schwartz RA. Epidemiologic aspects of American Kaposi’s sarcoma. J Surg Oncol. 1979;12:299-303.
12. Gill K, Shah J. Kaposi sarcoma in patients with diabetes and wounds. Adv Skin Wound Care. 2006;19:196-198, 201.
13. Hurlbut W, Lincoln CS Jr. Multiple hemorrhagic sarcoma and diabetes mellitus; review of a series, with report of two cases. Arch Intern Med (Chic). 1949;84:738-750.
14. Fischer JW, Cohen DM. Simultaneous occurrence of Kaposi’s sarcoma, leukemia and diabetes mellitus; report of a case. Am J Clin Pathol. 1951;21:586-589.
15. Tankel R. Kaposi’s sarcoma and diabetes mellitus. Arch Dermatol. 1971;104:442-444.
16. Langtry JA, Bottomley DM, Phillips RH, et al. The infra-red coagulator in the treatment of AIDS-related Kaposi’s sarcoma and a comparison with radiotherapy. Clin Exp Dermatol. 1994;19:23-25.
17. Von Roenn JH, Cianfrocca M. Treatment of Kaposi’s sarcoma. Cancer Treat Res. 2001;104:127-148.
18. Dezube BJ. AIDS-related Kaposi sarcoma. the role of local therapy for a systemic disease. Arch Dermatol. 2000;136:1554-1556.
19. Oysul K, Beyzadeoglu M, Surenkok S, et al. A dose-response analysis for classical Kaposi’s sarcoma management by radiotherapy. Saudi Med J. 2008;29:837-840.
20. Morganroth GS. Topical 0.1% alitretinoin gel for classic Kaposi sarcoma. Arch Dermatol. 2002;138:542-543.
21. Rongioletti F, Zaccaria E, Viglizzo G. Failure of topical 0.1% alitretinoin gel for classic Kaposi sarcoma: first European experience. Br J Dermatol. 2006;155:856-857.
22. Klein E, Schwartz RA, Laor Y, et al. Treatment of Kaposi’s sarcoma with vinblastine. Cancer. 1980;45:427-431.
23. Flaitz CM, Nichols CM, Hicks MJ. Role of intralesional vinblastine administration in treatment of intraoral Kaposi’s sarcoma in AIDS. Eur J Cancer B Oral Oncol. 1995;31B:280-285.
24. Epstein JB, Lozada-Nur F, McLeod WA, et al. Oral Kaposi’s sarcoma in acquired immunodeficiency syndrome. review of management and report of the efficacy of intralesional vinblastine. Cancer. 1989;64:2424-2430.
25. Antman K, Chang Y. Kaposi’s sarcoma. N Engl J Med. 2000;342:1027-1038.
26. Ramírez-Amador V, Esquivel-Pedraza L, Lozada-Nur F, et al. Intralesional vinblastine vs. 3% sodium tetradecyl sulfate for the treatment of oral Kaposi’s sarcoma. a double blind, randomized clinical trial. Oral Oncol. 2002;38:460-467.
27. Boudreaux AA, Smith LL, Cosby CD, et al. Intralesional vinblastine for cutaneous Kaposi’s sarcoma associated with acquired immunodeficiency syndrome. a clinical trial to evaluate efficacy and discomfort associated with infection. J Am Acad Dermatol. 1993;28:61-65.
28. Odom RB, Goette DK. Treatment of cutaneous Kaposi’s sarcoma with intralesional vincristine. Arch Dermatol. 1978;114:1693-1694.
29. Tappero JW, Conant MA, Wolfe SF, et al. Kaposi’s sarcoma. epidemiology, pathogenesis, histology, clinical spectrum, staging criteria and therapy. J Am Acad Dermatol. 1993;28:371-395.
30. Smith KJ, Skelton HG, Turiansky G, et al. Hyaluronidase enhances the therapeutic effect of vinblastine in intralesional treatment of Kaposi’s sarcoma. Military Medical Consortium for the Advancement of Retroviral Research (MMCARR). J Am Acad Dermatol. 1997;36(2, pt 1):239-242.
31. Célestin Schartz NE, Chevret S, Paz C, et al. Imiquimod 5% cream for treatment of HIV-negative Kaposi’s sarcoma skin lesions: a phase I to II, open-label trial in 17 patients [published online ahead of print February 20, 2008]. J Am Acad Dermatol. 2008;58:585-591.
32. Poignonec S, Lachiver LD, Lamas G, et al. Intralesional bleomycin for acquired immunodeficiency syndrome-associated cutaneous Kaposi’s sarcoma. Arch Dermatol. 1995;131:228.
1. Cathomas G. Human herpes virus 8: a new virus discloses its face. Virchows Arch. 2000;436:195-206.
2. Chang Y, Cesarman CE, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865-1869.
3. Tur E, Brenner S. Treatment of Kaposi’s sarcoma. Arch Dermatol. 1996;132:327-331.
4. Gorsky M, Epstein JB. A case series of acquired immunodeficiency syndrome patients with initial neoplastic diagnoses of intraoral Kaposi’s sarcoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:612-617.
5. Chaplin DJ. The effect of therapy on tumor vascular function. Int J Radiat Biol. 1991;60:311-325.
6. Donehower RC, Rowinsky EK. Anticancer drugs derived from plants. In: De Vita VT, Hellman S, Rosenberg SA, eds. Cancer: Principles and Practice of Oncology. 4th ed. Philadelphia, PA: Lippincott; 1993:409-417.
7. Epstein JB. Treatment of oral Kaposi’s sarcoma with intralesional vinblastine. Cancer. 1993;71:1722-1725.
8. McCormick SU. Intralesional vinblastine injections for the treatment of oral Kaposi’s sarcoma: report of 10 patients with 2-year follow-up. J Oral Maxillofac Surg. 1996;54:583-587.
9. Schwartz RA, Micali G, Nasca MR, et al. Kaposi sarcoma: a continuing conundrum. J Am Acad Dermatol. 2008;59:179-206.
10. Schwartz RA. Kaposi’s sarcoma: an update. J Surg Oncol. 2004;87:146-151.
11. Laor Y, Schwartz RA. Epidemiologic aspects of American Kaposi’s sarcoma. J Surg Oncol. 1979;12:299-303.
12. Gill K, Shah J. Kaposi sarcoma in patients with diabetes and wounds. Adv Skin Wound Care. 2006;19:196-198, 201.
13. Hurlbut W, Lincoln CS Jr. Multiple hemorrhagic sarcoma and diabetes mellitus; review of a series, with report of two cases. Arch Intern Med (Chic). 1949;84:738-750.
14. Fischer JW, Cohen DM. Simultaneous occurrence of Kaposi’s sarcoma, leukemia and diabetes mellitus; report of a case. Am J Clin Pathol. 1951;21:586-589.
15. Tankel R. Kaposi’s sarcoma and diabetes mellitus. Arch Dermatol. 1971;104:442-444.
16. Langtry JA, Bottomley DM, Phillips RH, et al. The infra-red coagulator in the treatment of AIDS-related Kaposi’s sarcoma and a comparison with radiotherapy. Clin Exp Dermatol. 1994;19:23-25.
17. Von Roenn JH, Cianfrocca M. Treatment of Kaposi’s sarcoma. Cancer Treat Res. 2001;104:127-148.
18. Dezube BJ. AIDS-related Kaposi sarcoma. the role of local therapy for a systemic disease. Arch Dermatol. 2000;136:1554-1556.
19. Oysul K, Beyzadeoglu M, Surenkok S, et al. A dose-response analysis for classical Kaposi’s sarcoma management by radiotherapy. Saudi Med J. 2008;29:837-840.
20. Morganroth GS. Topical 0.1% alitretinoin gel for classic Kaposi sarcoma. Arch Dermatol. 2002;138:542-543.
21. Rongioletti F, Zaccaria E, Viglizzo G. Failure of topical 0.1% alitretinoin gel for classic Kaposi sarcoma: first European experience. Br J Dermatol. 2006;155:856-857.
22. Klein E, Schwartz RA, Laor Y, et al. Treatment of Kaposi’s sarcoma with vinblastine. Cancer. 1980;45:427-431.
23. Flaitz CM, Nichols CM, Hicks MJ. Role of intralesional vinblastine administration in treatment of intraoral Kaposi’s sarcoma in AIDS. Eur J Cancer B Oral Oncol. 1995;31B:280-285.
24. Epstein JB, Lozada-Nur F, McLeod WA, et al. Oral Kaposi’s sarcoma in acquired immunodeficiency syndrome. review of management and report of the efficacy of intralesional vinblastine. Cancer. 1989;64:2424-2430.
25. Antman K, Chang Y. Kaposi’s sarcoma. N Engl J Med. 2000;342:1027-1038.
26. Ramírez-Amador V, Esquivel-Pedraza L, Lozada-Nur F, et al. Intralesional vinblastine vs. 3% sodium tetradecyl sulfate for the treatment of oral Kaposi’s sarcoma. a double blind, randomized clinical trial. Oral Oncol. 2002;38:460-467.
27. Boudreaux AA, Smith LL, Cosby CD, et al. Intralesional vinblastine for cutaneous Kaposi’s sarcoma associated with acquired immunodeficiency syndrome. a clinical trial to evaluate efficacy and discomfort associated with infection. J Am Acad Dermatol. 1993;28:61-65.
28. Odom RB, Goette DK. Treatment of cutaneous Kaposi’s sarcoma with intralesional vincristine. Arch Dermatol. 1978;114:1693-1694.
29. Tappero JW, Conant MA, Wolfe SF, et al. Kaposi’s sarcoma. epidemiology, pathogenesis, histology, clinical spectrum, staging criteria and therapy. J Am Acad Dermatol. 1993;28:371-395.
30. Smith KJ, Skelton HG, Turiansky G, et al. Hyaluronidase enhances the therapeutic effect of vinblastine in intralesional treatment of Kaposi’s sarcoma. Military Medical Consortium for the Advancement of Retroviral Research (MMCARR). J Am Acad Dermatol. 1997;36(2, pt 1):239-242.
31. Célestin Schartz NE, Chevret S, Paz C, et al. Imiquimod 5% cream for treatment of HIV-negative Kaposi’s sarcoma skin lesions: a phase I to II, open-label trial in 17 patients [published online ahead of print February 20, 2008]. J Am Acad Dermatol. 2008;58:585-591.
32. Poignonec S, Lachiver LD, Lamas G, et al. Intralesional bleomycin for acquired immunodeficiency syndrome-associated cutaneous Kaposi’s sarcoma. Arch Dermatol. 1995;131:228.
Practice Points
- Intralesional injection of low-dose vinblastine is a safe therapy for localized nodular Kaposi sarcoma (KS)(classic type).
- The best candidates for localized therapy are patients affected by nodular classic KS without visceral involvement, with fewer than 10 lesions on acral sites.