User login
Postpartum hemorrhage (PPH) is a common complication of birth. In 2019, 4.3% of births in the United States were complicated by at least one episode of PPH.1 Major causes of PPH include uterine atony, retained products of conception, reproductive tract trauma, and coagulopathy.2 Active management of the third stage of labor with the routine administration of postpartum uterotonics reduces the risk of PPH.3,4
PPH treatment requires a systematic approach using appropriate uterotonic medications, tranexamic acid, and procedures performed in a timely sequence to resolve the hemorrhage. Following vaginal birth, procedures that do not require a laparotomy to treat PPH include uterine massage, uterine evacuation to remove retained placental tissue, repair of lacerations, uterine balloon tamponade (UBT), uterine packing, a vacuum-induced hemorrhage control device (VHCD; JADA, Organon), and uterine artery embolization. Following cesarean birth, with an open laparotomy incision, interventions to treat PPH due to atony include vascular ligation, uterine compression sutures, UBT, VHCD, hysterectomy, and pelvic packing.2
Over the past 2 decades, UBT has been widely used for the treatment of PPH with a success rate in observational studies of approximately 86%.5 The uterine balloon creates pressure against the wall of the uterus permitting accumulation of platelets at bleeding sites, enhancing the activity of the clotting system. The uterine balloon provides direct pressure on the bleeding site(s). It is well known in trauma care that the first step to treat a bleeding wound is to apply direct pressure to the bleeding site. During the third stage of labor, a natural process is tetanic uterine contraction, which constricts myometrial vessels and the placenta bed. Placing a balloon in the uterus and inflating the balloon to 200 mL to 500 mL may delay the involution of the uterus that should occur following birth. An observation of great interest is the insight that inducing a vacuum in the uterine cavity may enhance tetanic uterine contraction and constriction of the myometrial vessels. Vacuum-induced hemorrhage control is discussed in detail in this editorial.
Vacuum-induced hemorrhage control device
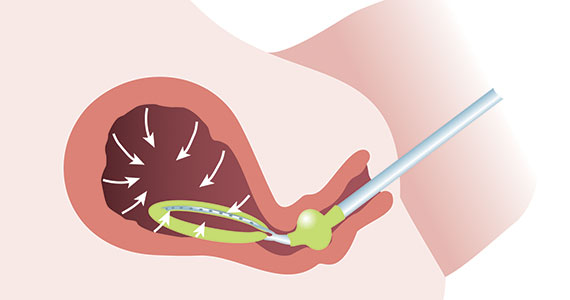
A new device for the treatment of PPH due to uterine atony is the JADA VHCD (FIGURE), which generates negative intrauterine pressure causing the uterus to contract, thereby constricting myometrial vessels and reducing uterine bleeding. The JADA VHCD system is indicated to provide control and treatment of abnormal postpartum uterine bleeding following vaginal or cesarean birth caused by uterine atony when conservative management is indicated.6
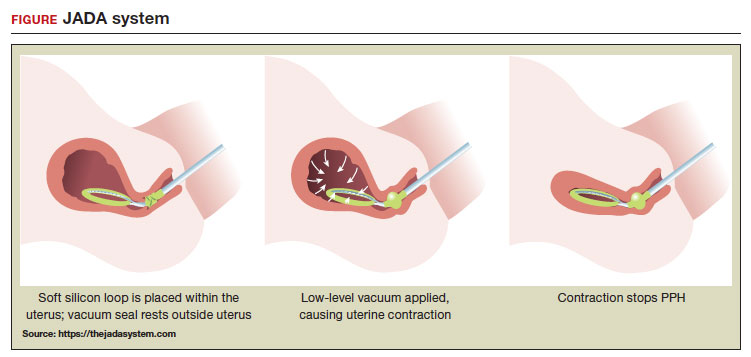 ILLUSTRATION: MARY ELLEN NIATAS FOR OBG MANAGEMENT
ILLUSTRATION: MARY ELLEN NIATAS FOR OBG MANAGEMENT

System components
The JADA VHCD consists of a leading portion intended to be inserted into the uterine cavity, which consists of a silicone elliptical loop with 20 vacuum pores. A soft shield covers the vacuum loop to reduce the risk of the vacuum pores being clogged with biological material, including blood and clots. The elliptical loop is attached to a catheter intended for connection to a vacuum source set to 80 mm Hg ±10 mm Hg (hospital wall suction or portable suction device) with an in-line cannister to collect blood. Approximately 16 cm from the tip of the elliptical loop is a balloon that should be positioned in the upper vagina, not inside the cervix, and inflated with fluid (60 mL to 120 mL) through a dedicated port to occlude the vagina, thereby preserving a stable intrauterine vacuum.
Continue to: Correct usage...
Correct usage
A simple mnemonic to facilitate use of the JADA VHCD is “120/80”—fill the vaginal balloon with 120 mL of sterile fluid and attach the tubing to a source that is set to provide 80 mm Hg of vacuum with an in-line collection cannister. The VHCD may not work correctly if there is a substantial amount of blood in the uterus. Clinical experts advise that an important step prior to placing the elliptical loop in the uterus is to perform a sweep of the uterine cavity with a hand or instrument to remove clots and ensure there is no retained placental tissue. It is preferable to assemble the suction tubing, syringe, sterile fluid, and other instruments (eg, forceps, speculum) needed to insert the device prior to attempting to place the VHCD. When the elliptical loop is compressed for insertion, it is about 2 cm in diameter, necessitating that the cervix be dilated sufficiently to accommodate the device.
Immediately after placing the VHCD, contractions can be monitored by physical examination and the amount of ongoing bleeding can be estimated by observing the amount of blood accumulating in the cannister. Rapid onset of a palpable increase in uterine tone is a prominent feature of successful treatment of PPH with the VHCD. The VHCD should be kept in the uterus with active suction for at least 1 hour. Taping the tubing to the inner thigh may help stabilize the device. Once bleeding is controlled, prior to removing the device, the vacuum should be discontinued, and bleeding activityshould be assessed for at least 30 minutes. If the patient is stable, the vaginal balloon can be deflated, followed by removal of the device. The VHCD should be removed within 24 hours of placement.6
The JADA VHCD system should not be used with ongoing intrauterine pregnancy, untreated uterine rupture, unresolved uterine inversion, current cervical cancer, or serious infection of the uterus.6 The VHCD has not been evaluated for effectiveness in the treatment of placenta accreta or coagulopathy. The VHCD has not been specifically evaluated for safety and effectiveness in patients < 34 weeks’ duration, but clinicians report successful use of the device in cases of PPH that have occurred in the second and early-third trimesters. If the device can be appropriately placed with the elliptical loop in the uterus and the balloon in the vagina, it is theoretically possible to use the device for cases of PPH occurring before 34 weeks’ gestation.
When using the JADA VHCD system, it is important to simultaneously provide cardiovascular support, appropriate transfusion of blood products and timely surgical intervention, if indicated. All obstetricians know that in complicated cases of PPH, where conservative measures have not worked, uterine artery embolization or hysterectomy may be the only interventions that will prevent serious patient morbidity.
Effectiveness data
The VHCD has not been evaluated against an alternative approach, such as UBT, in published randomized clinical trials. However, prospective cohort studies have reported that the JADA is often successful in the treatment of PPH.7-10
In a multicenter cohort study of 107 patients with PPH, including 91 vaginal and 16 cesarean births, 100 patients (93%) were successfully treated with the JADA VHCD.7 Median blood loss before application of the system was 870 mL with vaginal birth and 1,300 mL with cesarean birth. Definitive control of the hemorrhage was observed at a median of 3 minutes after initiation of the intrauterine vacuum. In this study, 32% of patients had reproductive tract lacerations that needed to be repaired, and 2 patients required a hysterectomy. Forty patients required a blood transfusion.
Two patients were treated with a Bakri UBT when the VHCD did not resolve the PPH. In this cohort, the vacuum was applied for a median duration of 144 minutes, and a median total device dwell time was 191 minutes. Compared with UBT, the JADA VHCD intrauterine dwell time was shorter, facilitating patient progression and early transfer to the postpartum unit. The physicians who participated in the study reported that the device was easy to use. The complications reported in this cohort were minor and included endometritis (5 cases), vaginal infection (2 cases), and disruption of a vaginal laceration repair (1 case).7
Novel approaches to generating an intrauterine vacuum to treat PPH
The JADA VHCD is the only vacuum device approved by the US Food and Drug Administration (FDA) for treatment of PPH. However, clinical innovators have reported alternative approaches to generating an intrauterine vacuum using equipment designed for other purposes. In one study, a Bakri balloon was used to generate intrauterine vacuum tamponade to treat PPH.11 In this study, a Bakri balloon was inserted into the uterus, and the balloon was inflated to 50 mL to 100 mL to seal the vacuum. The main Bakri port was attached to a suction aspiration device set to generate a vacuum of 450 mm Hg to 525 mm Hg, a much greater vacuum than used with the JADA VHCD. This study included 44 cases of PPH due to uterine atony and 22 cases due to placental pathology, with successful treatment of PPH in 86% and 73% of the cases, respectively.
Another approach to generate intrauterine vacuum tamponade involves using a Levin stomach tube (FG24 or FG36), which has an open end and 4 side ports near the open tip.12-14 The Levin stomach tube is low cost and has many favorable design features, including a rounded tip, wide-bore, and circumferentially placed side ports. The FG36 Levin stomach tube is 12 mm in diameter and has 10 mm side ports. A vacuum device set to deliver 100 mm Hg to 200 mm Hgwas used in some of the studies evaluating the Levin stomach tube for the treatment of PPH. In 3 cases of severe PPH unresponsive to standard interventions, creation of vacuum tamponade with flexible suction tubing with side ports was successful in controlling the hemorrhage.13
Dr. T.N. Vasudeva Panicker invented an intrauterine cannula 12 mm in diameter and 25 cm in length, with dozens of 4 mm side ports over the distal 12 cm of the cannula.15 The cannula, which is made of stainless steel or plastic, is inserted into the uterus and 700 mm Hgvacuum is applied, a level much greater than the 80 mm Hg vacuum recommended for use with the JADA VHCD. When successful, the high suction clears the uterus of blood and causes uterine contraction. In 4 cases of severe PPH, the device successfully controlled the hemorrhage. In 2 of the 4 cases the device that was initially placed became clogged with blood and needed to be replaced.
UBT vs VHCD
To date there are no published randomized controlled trials comparing Bakri UBT to the JADA VHCD. In one retrospective study, the frequency of massive transfusion of red blood cells (RBCs), defined as the transfusion of 4 units or greater of RBCs, was assessed among 78 patients treated with the Bakri UBT and 36 patients treated with the JADA VHCD.9 In this study, at baseline there was a non ̶ statistically significant trend for JADA VHCD to be used more frequently than the Bakri UBT in cases of PPH occurring during repeat cesarean delivery (33% vs 14%). The Bakri UBT was used more frequently than the JADA VHCD among patients having a PPH following a vaginal delivery (51% vs 31%). Both devices were used at similar rates for operative vaginal delivery (6%) and primary cesarean birth (31% VHCD and 28% UBT).
In this retrospective study, the percentage of patients treated with VHCD or UBT who received 4 or more units of RBCs was 3% and 21%, respectively (P < .01). Among patients treated with VHCD and UBT, the estimated median blood loss was 1,500 mL and 1,850 mL (P=.02), respectively. The median hemoglobin concentration at discharge was similar in the VHCD and UBT groups, 8.8 g/dL and 8.6 g/dL, respectively.9 A randomized controlled trial is necessary to refine our understanding of the comparative effectiveness of UBT and VHCD in controlling PPH following vaginal and cesarean birth.
A welcome addition to treatment options
Every obstetrician knows that, in the next 12 months of their practice, they will encounter multiple cases of PPH. One or two of these cases may require the physician to use every medication and procedure available for the treatment of PPH to save the life of the patient. To prepare to treat the next case of PPH rapidly and effectively, it is important for every obstetrician to develop a standardized cognitive plan for using all available treatmentmodalities in an appropriate and timely sequence, including both the Bakri balloon and the JADA VHCD. The insight that inducing an intrauterine vacuum causes uterine contraction, which may resolve PPH, is an important discovery. The JADA VHCD is a welcome addition to our armamentarium of treatments for PPH. ●
- Corbetta-Rastelli CM, Friedman AM, Sobhani NC, et al. Postpartum hemorrhage trends and outcomes in the United States, 2000-2019. Obstet Gynecol. 2023;141:152-161.
- Bienstock JL, Eke AC, Hueppchen NA. Postpartum hemorrhage. N Engl J Med. 2021;384:16351645.
- Salati JA, Leathersich SJ, Williams MJ, et al. Prophylactic oxytocin for the third stage of labour to prevent postpartum hemorrhage. Cochrane Database Syst Rev. 2019;CD001808.
- Begley CM, Gyte GMI, Devane D, et al. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev. 2019;CD007412.
- Suarez S, Conde-Agudelo A, Borovac-Pinheiro A, et al. Uterine balloon tamponade for the treatment of postpartum hemorrhage: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;222:293.e1-e52.
- US Food and Drug Administration. JADA system approval. Accessed July 25, 2023. https://www .accessdata.fda.gov/cdrh_docs/pdf21/K212757 .pdf
- D’Alton ME, Rood KM, Smid MC, et al. Intrauterine vacuum-induced hemorrhage control device for rapid treatment of postpartum hemorrhage. Obstet Gynecol. 2020;136:882-891.
- D’Alton M, Rood K, Simhan H, et al. Profile of the JADA System: the vacuum-induced hemorrhage control device for treating abnormal postpartum uterine bleeding and postpartum hemorrhage. Expert Rev Med Devices. 2021; 18:849-853.
- Gulersen M, Gerber RP, Rochelson B, et al. Vacuum-induced hemorrhage control versus uterine balloon tamponade for postpartum hemorrhage. J Obstet Gynaecol Can. 2023;45:267-272.
- Purwosunnu Y, Sarkoen W, Arulkumaran S, et al. Control of postpartum hemorrhage using vacuum-induced uterine tamponade. Obstet Gynecol. 2016;128:33-36.
- Haslinger C, Weber K, Zimmerman R. Vacuuminduced tamponade for treatment of postpartum hemorrhage. Obstet Gynecol. 2021;138:361-365.
- Hofmeyr GJ, Middleton K, Singata-Madliki M. Randomized feasibility study of suction-tube uterine tamponade for postpartum hemorrhage. Int J Gynaecol Obstet. 2019;146:339-343.
- Hofmeyr GJ, Singata-Madliki M. Novel suction tube uterine tamponade for treating intractable postpartum hemorrhage: description of technique and report of three cases. BJOG. 2020;127:1280-1283.
- Cebekhulu SN, Abdul H, Batting J, et al. Suction tube uterine tamponade for treatment of refractory postpartum hemorrhage: internal feasibility and acceptability pilot of a randomized clinical trial. Int J Gynaecol Obstet. 2022;158: 79-85.
- Panicker TNV. Panicker’s vacuum suction haemostatic device for treating post-partum hemorrhage. J Obstet Gynaecol India. 2017;67:150-151.
Postpartum hemorrhage (PPH) is a common complication of birth. In 2019, 4.3% of births in the United States were complicated by at least one episode of PPH.1 Major causes of PPH include uterine atony, retained products of conception, reproductive tract trauma, and coagulopathy.2 Active management of the third stage of labor with the routine administration of postpartum uterotonics reduces the risk of PPH.3,4
PPH treatment requires a systematic approach using appropriate uterotonic medications, tranexamic acid, and procedures performed in a timely sequence to resolve the hemorrhage. Following vaginal birth, procedures that do not require a laparotomy to treat PPH include uterine massage, uterine evacuation to remove retained placental tissue, repair of lacerations, uterine balloon tamponade (UBT), uterine packing, a vacuum-induced hemorrhage control device (VHCD; JADA, Organon), and uterine artery embolization. Following cesarean birth, with an open laparotomy incision, interventions to treat PPH due to atony include vascular ligation, uterine compression sutures, UBT, VHCD, hysterectomy, and pelvic packing.2
Over the past 2 decades, UBT has been widely used for the treatment of PPH with a success rate in observational studies of approximately 86%.5 The uterine balloon creates pressure against the wall of the uterus permitting accumulation of platelets at bleeding sites, enhancing the activity of the clotting system. The uterine balloon provides direct pressure on the bleeding site(s). It is well known in trauma care that the first step to treat a bleeding wound is to apply direct pressure to the bleeding site. During the third stage of labor, a natural process is tetanic uterine contraction, which constricts myometrial vessels and the placenta bed. Placing a balloon in the uterus and inflating the balloon to 200 mL to 500 mL may delay the involution of the uterus that should occur following birth. An observation of great interest is the insight that inducing a vacuum in the uterine cavity may enhance tetanic uterine contraction and constriction of the myometrial vessels. Vacuum-induced hemorrhage control is discussed in detail in this editorial.
Vacuum-induced hemorrhage control device
A new device for the treatment of PPH due to uterine atony is the JADA VHCD (FIGURE), which generates negative intrauterine pressure causing the uterus to contract, thereby constricting myometrial vessels and reducing uterine bleeding. The JADA VHCD system is indicated to provide control and treatment of abnormal postpartum uterine bleeding following vaginal or cesarean birth caused by uterine atony when conservative management is indicated.6
 ILLUSTRATION: MARY ELLEN NIATAS FOR OBG MANAGEMENT
ILLUSTRATION: MARY ELLEN NIATAS FOR OBG MANAGEMENT

System components
The JADA VHCD consists of a leading portion intended to be inserted into the uterine cavity, which consists of a silicone elliptical loop with 20 vacuum pores. A soft shield covers the vacuum loop to reduce the risk of the vacuum pores being clogged with biological material, including blood and clots. The elliptical loop is attached to a catheter intended for connection to a vacuum source set to 80 mm Hg ±10 mm Hg (hospital wall suction or portable suction device) with an in-line cannister to collect blood. Approximately 16 cm from the tip of the elliptical loop is a balloon that should be positioned in the upper vagina, not inside the cervix, and inflated with fluid (60 mL to 120 mL) through a dedicated port to occlude the vagina, thereby preserving a stable intrauterine vacuum.
Continue to: Correct usage...
Correct usage
A simple mnemonic to facilitate use of the JADA VHCD is “120/80”—fill the vaginal balloon with 120 mL of sterile fluid and attach the tubing to a source that is set to provide 80 mm Hg of vacuum with an in-line collection cannister. The VHCD may not work correctly if there is a substantial amount of blood in the uterus. Clinical experts advise that an important step prior to placing the elliptical loop in the uterus is to perform a sweep of the uterine cavity with a hand or instrument to remove clots and ensure there is no retained placental tissue. It is preferable to assemble the suction tubing, syringe, sterile fluid, and other instruments (eg, forceps, speculum) needed to insert the device prior to attempting to place the VHCD. When the elliptical loop is compressed for insertion, it is about 2 cm in diameter, necessitating that the cervix be dilated sufficiently to accommodate the device.
Immediately after placing the VHCD, contractions can be monitored by physical examination and the amount of ongoing bleeding can be estimated by observing the amount of blood accumulating in the cannister. Rapid onset of a palpable increase in uterine tone is a prominent feature of successful treatment of PPH with the VHCD. The VHCD should be kept in the uterus with active suction for at least 1 hour. Taping the tubing to the inner thigh may help stabilize the device. Once bleeding is controlled, prior to removing the device, the vacuum should be discontinued, and bleeding activityshould be assessed for at least 30 minutes. If the patient is stable, the vaginal balloon can be deflated, followed by removal of the device. The VHCD should be removed within 24 hours of placement.6
The JADA VHCD system should not be used with ongoing intrauterine pregnancy, untreated uterine rupture, unresolved uterine inversion, current cervical cancer, or serious infection of the uterus.6 The VHCD has not been evaluated for effectiveness in the treatment of placenta accreta or coagulopathy. The VHCD has not been specifically evaluated for safety and effectiveness in patients < 34 weeks’ duration, but clinicians report successful use of the device in cases of PPH that have occurred in the second and early-third trimesters. If the device can be appropriately placed with the elliptical loop in the uterus and the balloon in the vagina, it is theoretically possible to use the device for cases of PPH occurring before 34 weeks’ gestation.
When using the JADA VHCD system, it is important to simultaneously provide cardiovascular support, appropriate transfusion of blood products and timely surgical intervention, if indicated. All obstetricians know that in complicated cases of PPH, where conservative measures have not worked, uterine artery embolization or hysterectomy may be the only interventions that will prevent serious patient morbidity.
Effectiveness data
The VHCD has not been evaluated against an alternative approach, such as UBT, in published randomized clinical trials. However, prospective cohort studies have reported that the JADA is often successful in the treatment of PPH.7-10
In a multicenter cohort study of 107 patients with PPH, including 91 vaginal and 16 cesarean births, 100 patients (93%) were successfully treated with the JADA VHCD.7 Median blood loss before application of the system was 870 mL with vaginal birth and 1,300 mL with cesarean birth. Definitive control of the hemorrhage was observed at a median of 3 minutes after initiation of the intrauterine vacuum. In this study, 32% of patients had reproductive tract lacerations that needed to be repaired, and 2 patients required a hysterectomy. Forty patients required a blood transfusion.
Two patients were treated with a Bakri UBT when the VHCD did not resolve the PPH. In this cohort, the vacuum was applied for a median duration of 144 minutes, and a median total device dwell time was 191 minutes. Compared with UBT, the JADA VHCD intrauterine dwell time was shorter, facilitating patient progression and early transfer to the postpartum unit. The physicians who participated in the study reported that the device was easy to use. The complications reported in this cohort were minor and included endometritis (5 cases), vaginal infection (2 cases), and disruption of a vaginal laceration repair (1 case).7
Novel approaches to generating an intrauterine vacuum to treat PPH
The JADA VHCD is the only vacuum device approved by the US Food and Drug Administration (FDA) for treatment of PPH. However, clinical innovators have reported alternative approaches to generating an intrauterine vacuum using equipment designed for other purposes. In one study, a Bakri balloon was used to generate intrauterine vacuum tamponade to treat PPH.11 In this study, a Bakri balloon was inserted into the uterus, and the balloon was inflated to 50 mL to 100 mL to seal the vacuum. The main Bakri port was attached to a suction aspiration device set to generate a vacuum of 450 mm Hg to 525 mm Hg, a much greater vacuum than used with the JADA VHCD. This study included 44 cases of PPH due to uterine atony and 22 cases due to placental pathology, with successful treatment of PPH in 86% and 73% of the cases, respectively.
Another approach to generate intrauterine vacuum tamponade involves using a Levin stomach tube (FG24 or FG36), which has an open end and 4 side ports near the open tip.12-14 The Levin stomach tube is low cost and has many favorable design features, including a rounded tip, wide-bore, and circumferentially placed side ports. The FG36 Levin stomach tube is 12 mm in diameter and has 10 mm side ports. A vacuum device set to deliver 100 mm Hg to 200 mm Hgwas used in some of the studies evaluating the Levin stomach tube for the treatment of PPH. In 3 cases of severe PPH unresponsive to standard interventions, creation of vacuum tamponade with flexible suction tubing with side ports was successful in controlling the hemorrhage.13
Dr. T.N. Vasudeva Panicker invented an intrauterine cannula 12 mm in diameter and 25 cm in length, with dozens of 4 mm side ports over the distal 12 cm of the cannula.15 The cannula, which is made of stainless steel or plastic, is inserted into the uterus and 700 mm Hgvacuum is applied, a level much greater than the 80 mm Hg vacuum recommended for use with the JADA VHCD. When successful, the high suction clears the uterus of blood and causes uterine contraction. In 4 cases of severe PPH, the device successfully controlled the hemorrhage. In 2 of the 4 cases the device that was initially placed became clogged with blood and needed to be replaced.
UBT vs VHCD
To date there are no published randomized controlled trials comparing Bakri UBT to the JADA VHCD. In one retrospective study, the frequency of massive transfusion of red blood cells (RBCs), defined as the transfusion of 4 units or greater of RBCs, was assessed among 78 patients treated with the Bakri UBT and 36 patients treated with the JADA VHCD.9 In this study, at baseline there was a non ̶ statistically significant trend for JADA VHCD to be used more frequently than the Bakri UBT in cases of PPH occurring during repeat cesarean delivery (33% vs 14%). The Bakri UBT was used more frequently than the JADA VHCD among patients having a PPH following a vaginal delivery (51% vs 31%). Both devices were used at similar rates for operative vaginal delivery (6%) and primary cesarean birth (31% VHCD and 28% UBT).
In this retrospective study, the percentage of patients treated with VHCD or UBT who received 4 or more units of RBCs was 3% and 21%, respectively (P < .01). Among patients treated with VHCD and UBT, the estimated median blood loss was 1,500 mL and 1,850 mL (P=.02), respectively. The median hemoglobin concentration at discharge was similar in the VHCD and UBT groups, 8.8 g/dL and 8.6 g/dL, respectively.9 A randomized controlled trial is necessary to refine our understanding of the comparative effectiveness of UBT and VHCD in controlling PPH following vaginal and cesarean birth.
A welcome addition to treatment options
Every obstetrician knows that, in the next 12 months of their practice, they will encounter multiple cases of PPH. One or two of these cases may require the physician to use every medication and procedure available for the treatment of PPH to save the life of the patient. To prepare to treat the next case of PPH rapidly and effectively, it is important for every obstetrician to develop a standardized cognitive plan for using all available treatmentmodalities in an appropriate and timely sequence, including both the Bakri balloon and the JADA VHCD. The insight that inducing an intrauterine vacuum causes uterine contraction, which may resolve PPH, is an important discovery. The JADA VHCD is a welcome addition to our armamentarium of treatments for PPH. ●
Postpartum hemorrhage (PPH) is a common complication of birth. In 2019, 4.3% of births in the United States were complicated by at least one episode of PPH.1 Major causes of PPH include uterine atony, retained products of conception, reproductive tract trauma, and coagulopathy.2 Active management of the third stage of labor with the routine administration of postpartum uterotonics reduces the risk of PPH.3,4
PPH treatment requires a systematic approach using appropriate uterotonic medications, tranexamic acid, and procedures performed in a timely sequence to resolve the hemorrhage. Following vaginal birth, procedures that do not require a laparotomy to treat PPH include uterine massage, uterine evacuation to remove retained placental tissue, repair of lacerations, uterine balloon tamponade (UBT), uterine packing, a vacuum-induced hemorrhage control device (VHCD; JADA, Organon), and uterine artery embolization. Following cesarean birth, with an open laparotomy incision, interventions to treat PPH due to atony include vascular ligation, uterine compression sutures, UBT, VHCD, hysterectomy, and pelvic packing.2
Over the past 2 decades, UBT has been widely used for the treatment of PPH with a success rate in observational studies of approximately 86%.5 The uterine balloon creates pressure against the wall of the uterus permitting accumulation of platelets at bleeding sites, enhancing the activity of the clotting system. The uterine balloon provides direct pressure on the bleeding site(s). It is well known in trauma care that the first step to treat a bleeding wound is to apply direct pressure to the bleeding site. During the third stage of labor, a natural process is tetanic uterine contraction, which constricts myometrial vessels and the placenta bed. Placing a balloon in the uterus and inflating the balloon to 200 mL to 500 mL may delay the involution of the uterus that should occur following birth. An observation of great interest is the insight that inducing a vacuum in the uterine cavity may enhance tetanic uterine contraction and constriction of the myometrial vessels. Vacuum-induced hemorrhage control is discussed in detail in this editorial.
Vacuum-induced hemorrhage control device
A new device for the treatment of PPH due to uterine atony is the JADA VHCD (FIGURE), which generates negative intrauterine pressure causing the uterus to contract, thereby constricting myometrial vessels and reducing uterine bleeding. The JADA VHCD system is indicated to provide control and treatment of abnormal postpartum uterine bleeding following vaginal or cesarean birth caused by uterine atony when conservative management is indicated.6
 ILLUSTRATION: MARY ELLEN NIATAS FOR OBG MANAGEMENT
ILLUSTRATION: MARY ELLEN NIATAS FOR OBG MANAGEMENT

System components
The JADA VHCD consists of a leading portion intended to be inserted into the uterine cavity, which consists of a silicone elliptical loop with 20 vacuum pores. A soft shield covers the vacuum loop to reduce the risk of the vacuum pores being clogged with biological material, including blood and clots. The elliptical loop is attached to a catheter intended for connection to a vacuum source set to 80 mm Hg ±10 mm Hg (hospital wall suction or portable suction device) with an in-line cannister to collect blood. Approximately 16 cm from the tip of the elliptical loop is a balloon that should be positioned in the upper vagina, not inside the cervix, and inflated with fluid (60 mL to 120 mL) through a dedicated port to occlude the vagina, thereby preserving a stable intrauterine vacuum.
Continue to: Correct usage...
Correct usage
A simple mnemonic to facilitate use of the JADA VHCD is “120/80”—fill the vaginal balloon with 120 mL of sterile fluid and attach the tubing to a source that is set to provide 80 mm Hg of vacuum with an in-line collection cannister. The VHCD may not work correctly if there is a substantial amount of blood in the uterus. Clinical experts advise that an important step prior to placing the elliptical loop in the uterus is to perform a sweep of the uterine cavity with a hand or instrument to remove clots and ensure there is no retained placental tissue. It is preferable to assemble the suction tubing, syringe, sterile fluid, and other instruments (eg, forceps, speculum) needed to insert the device prior to attempting to place the VHCD. When the elliptical loop is compressed for insertion, it is about 2 cm in diameter, necessitating that the cervix be dilated sufficiently to accommodate the device.
Immediately after placing the VHCD, contractions can be monitored by physical examination and the amount of ongoing bleeding can be estimated by observing the amount of blood accumulating in the cannister. Rapid onset of a palpable increase in uterine tone is a prominent feature of successful treatment of PPH with the VHCD. The VHCD should be kept in the uterus with active suction for at least 1 hour. Taping the tubing to the inner thigh may help stabilize the device. Once bleeding is controlled, prior to removing the device, the vacuum should be discontinued, and bleeding activityshould be assessed for at least 30 minutes. If the patient is stable, the vaginal balloon can be deflated, followed by removal of the device. The VHCD should be removed within 24 hours of placement.6
The JADA VHCD system should not be used with ongoing intrauterine pregnancy, untreated uterine rupture, unresolved uterine inversion, current cervical cancer, or serious infection of the uterus.6 The VHCD has not been evaluated for effectiveness in the treatment of placenta accreta or coagulopathy. The VHCD has not been specifically evaluated for safety and effectiveness in patients < 34 weeks’ duration, but clinicians report successful use of the device in cases of PPH that have occurred in the second and early-third trimesters. If the device can be appropriately placed with the elliptical loop in the uterus and the balloon in the vagina, it is theoretically possible to use the device for cases of PPH occurring before 34 weeks’ gestation.
When using the JADA VHCD system, it is important to simultaneously provide cardiovascular support, appropriate transfusion of blood products and timely surgical intervention, if indicated. All obstetricians know that in complicated cases of PPH, where conservative measures have not worked, uterine artery embolization or hysterectomy may be the only interventions that will prevent serious patient morbidity.
Effectiveness data
The VHCD has not been evaluated against an alternative approach, such as UBT, in published randomized clinical trials. However, prospective cohort studies have reported that the JADA is often successful in the treatment of PPH.7-10
In a multicenter cohort study of 107 patients with PPH, including 91 vaginal and 16 cesarean births, 100 patients (93%) were successfully treated with the JADA VHCD.7 Median blood loss before application of the system was 870 mL with vaginal birth and 1,300 mL with cesarean birth. Definitive control of the hemorrhage was observed at a median of 3 minutes after initiation of the intrauterine vacuum. In this study, 32% of patients had reproductive tract lacerations that needed to be repaired, and 2 patients required a hysterectomy. Forty patients required a blood transfusion.
Two patients were treated with a Bakri UBT when the VHCD did not resolve the PPH. In this cohort, the vacuum was applied for a median duration of 144 minutes, and a median total device dwell time was 191 minutes. Compared with UBT, the JADA VHCD intrauterine dwell time was shorter, facilitating patient progression and early transfer to the postpartum unit. The physicians who participated in the study reported that the device was easy to use. The complications reported in this cohort were minor and included endometritis (5 cases), vaginal infection (2 cases), and disruption of a vaginal laceration repair (1 case).7
Novel approaches to generating an intrauterine vacuum to treat PPH
The JADA VHCD is the only vacuum device approved by the US Food and Drug Administration (FDA) for treatment of PPH. However, clinical innovators have reported alternative approaches to generating an intrauterine vacuum using equipment designed for other purposes. In one study, a Bakri balloon was used to generate intrauterine vacuum tamponade to treat PPH.11 In this study, a Bakri balloon was inserted into the uterus, and the balloon was inflated to 50 mL to 100 mL to seal the vacuum. The main Bakri port was attached to a suction aspiration device set to generate a vacuum of 450 mm Hg to 525 mm Hg, a much greater vacuum than used with the JADA VHCD. This study included 44 cases of PPH due to uterine atony and 22 cases due to placental pathology, with successful treatment of PPH in 86% and 73% of the cases, respectively.
Another approach to generate intrauterine vacuum tamponade involves using a Levin stomach tube (FG24 or FG36), which has an open end and 4 side ports near the open tip.12-14 The Levin stomach tube is low cost and has many favorable design features, including a rounded tip, wide-bore, and circumferentially placed side ports. The FG36 Levin stomach tube is 12 mm in diameter and has 10 mm side ports. A vacuum device set to deliver 100 mm Hg to 200 mm Hgwas used in some of the studies evaluating the Levin stomach tube for the treatment of PPH. In 3 cases of severe PPH unresponsive to standard interventions, creation of vacuum tamponade with flexible suction tubing with side ports was successful in controlling the hemorrhage.13
Dr. T.N. Vasudeva Panicker invented an intrauterine cannula 12 mm in diameter and 25 cm in length, with dozens of 4 mm side ports over the distal 12 cm of the cannula.15 The cannula, which is made of stainless steel or plastic, is inserted into the uterus and 700 mm Hgvacuum is applied, a level much greater than the 80 mm Hg vacuum recommended for use with the JADA VHCD. When successful, the high suction clears the uterus of blood and causes uterine contraction. In 4 cases of severe PPH, the device successfully controlled the hemorrhage. In 2 of the 4 cases the device that was initially placed became clogged with blood and needed to be replaced.
UBT vs VHCD
To date there are no published randomized controlled trials comparing Bakri UBT to the JADA VHCD. In one retrospective study, the frequency of massive transfusion of red blood cells (RBCs), defined as the transfusion of 4 units or greater of RBCs, was assessed among 78 patients treated with the Bakri UBT and 36 patients treated with the JADA VHCD.9 In this study, at baseline there was a non ̶ statistically significant trend for JADA VHCD to be used more frequently than the Bakri UBT in cases of PPH occurring during repeat cesarean delivery (33% vs 14%). The Bakri UBT was used more frequently than the JADA VHCD among patients having a PPH following a vaginal delivery (51% vs 31%). Both devices were used at similar rates for operative vaginal delivery (6%) and primary cesarean birth (31% VHCD and 28% UBT).
In this retrospective study, the percentage of patients treated with VHCD or UBT who received 4 or more units of RBCs was 3% and 21%, respectively (P < .01). Among patients treated with VHCD and UBT, the estimated median blood loss was 1,500 mL and 1,850 mL (P=.02), respectively. The median hemoglobin concentration at discharge was similar in the VHCD and UBT groups, 8.8 g/dL and 8.6 g/dL, respectively.9 A randomized controlled trial is necessary to refine our understanding of the comparative effectiveness of UBT and VHCD in controlling PPH following vaginal and cesarean birth.
A welcome addition to treatment options
Every obstetrician knows that, in the next 12 months of their practice, they will encounter multiple cases of PPH. One or two of these cases may require the physician to use every medication and procedure available for the treatment of PPH to save the life of the patient. To prepare to treat the next case of PPH rapidly and effectively, it is important for every obstetrician to develop a standardized cognitive plan for using all available treatmentmodalities in an appropriate and timely sequence, including both the Bakri balloon and the JADA VHCD. The insight that inducing an intrauterine vacuum causes uterine contraction, which may resolve PPH, is an important discovery. The JADA VHCD is a welcome addition to our armamentarium of treatments for PPH. ●
- Corbetta-Rastelli CM, Friedman AM, Sobhani NC, et al. Postpartum hemorrhage trends and outcomes in the United States, 2000-2019. Obstet Gynecol. 2023;141:152-161.
- Bienstock JL, Eke AC, Hueppchen NA. Postpartum hemorrhage. N Engl J Med. 2021;384:16351645.
- Salati JA, Leathersich SJ, Williams MJ, et al. Prophylactic oxytocin for the third stage of labour to prevent postpartum hemorrhage. Cochrane Database Syst Rev. 2019;CD001808.
- Begley CM, Gyte GMI, Devane D, et al. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev. 2019;CD007412.
- Suarez S, Conde-Agudelo A, Borovac-Pinheiro A, et al. Uterine balloon tamponade for the treatment of postpartum hemorrhage: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;222:293.e1-e52.
- US Food and Drug Administration. JADA system approval. Accessed July 25, 2023. https://www .accessdata.fda.gov/cdrh_docs/pdf21/K212757 .pdf
- D’Alton ME, Rood KM, Smid MC, et al. Intrauterine vacuum-induced hemorrhage control device for rapid treatment of postpartum hemorrhage. Obstet Gynecol. 2020;136:882-891.
- D’Alton M, Rood K, Simhan H, et al. Profile of the JADA System: the vacuum-induced hemorrhage control device for treating abnormal postpartum uterine bleeding and postpartum hemorrhage. Expert Rev Med Devices. 2021; 18:849-853.
- Gulersen M, Gerber RP, Rochelson B, et al. Vacuum-induced hemorrhage control versus uterine balloon tamponade for postpartum hemorrhage. J Obstet Gynaecol Can. 2023;45:267-272.
- Purwosunnu Y, Sarkoen W, Arulkumaran S, et al. Control of postpartum hemorrhage using vacuum-induced uterine tamponade. Obstet Gynecol. 2016;128:33-36.
- Haslinger C, Weber K, Zimmerman R. Vacuuminduced tamponade for treatment of postpartum hemorrhage. Obstet Gynecol. 2021;138:361-365.
- Hofmeyr GJ, Middleton K, Singata-Madliki M. Randomized feasibility study of suction-tube uterine tamponade for postpartum hemorrhage. Int J Gynaecol Obstet. 2019;146:339-343.
- Hofmeyr GJ, Singata-Madliki M. Novel suction tube uterine tamponade for treating intractable postpartum hemorrhage: description of technique and report of three cases. BJOG. 2020;127:1280-1283.
- Cebekhulu SN, Abdul H, Batting J, et al. Suction tube uterine tamponade for treatment of refractory postpartum hemorrhage: internal feasibility and acceptability pilot of a randomized clinical trial. Int J Gynaecol Obstet. 2022;158: 79-85.
- Panicker TNV. Panicker’s vacuum suction haemostatic device for treating post-partum hemorrhage. J Obstet Gynaecol India. 2017;67:150-151.
- Corbetta-Rastelli CM, Friedman AM, Sobhani NC, et al. Postpartum hemorrhage trends and outcomes in the United States, 2000-2019. Obstet Gynecol. 2023;141:152-161.
- Bienstock JL, Eke AC, Hueppchen NA. Postpartum hemorrhage. N Engl J Med. 2021;384:16351645.
- Salati JA, Leathersich SJ, Williams MJ, et al. Prophylactic oxytocin for the third stage of labour to prevent postpartum hemorrhage. Cochrane Database Syst Rev. 2019;CD001808.
- Begley CM, Gyte GMI, Devane D, et al. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev. 2019;CD007412.
- Suarez S, Conde-Agudelo A, Borovac-Pinheiro A, et al. Uterine balloon tamponade for the treatment of postpartum hemorrhage: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;222:293.e1-e52.
- US Food and Drug Administration. JADA system approval. Accessed July 25, 2023. https://www .accessdata.fda.gov/cdrh_docs/pdf21/K212757 .pdf
- D’Alton ME, Rood KM, Smid MC, et al. Intrauterine vacuum-induced hemorrhage control device for rapid treatment of postpartum hemorrhage. Obstet Gynecol. 2020;136:882-891.
- D’Alton M, Rood K, Simhan H, et al. Profile of the JADA System: the vacuum-induced hemorrhage control device for treating abnormal postpartum uterine bleeding and postpartum hemorrhage. Expert Rev Med Devices. 2021; 18:849-853.
- Gulersen M, Gerber RP, Rochelson B, et al. Vacuum-induced hemorrhage control versus uterine balloon tamponade for postpartum hemorrhage. J Obstet Gynaecol Can. 2023;45:267-272.
- Purwosunnu Y, Sarkoen W, Arulkumaran S, et al. Control of postpartum hemorrhage using vacuum-induced uterine tamponade. Obstet Gynecol. 2016;128:33-36.
- Haslinger C, Weber K, Zimmerman R. Vacuuminduced tamponade for treatment of postpartum hemorrhage. Obstet Gynecol. 2021;138:361-365.
- Hofmeyr GJ, Middleton K, Singata-Madliki M. Randomized feasibility study of suction-tube uterine tamponade for postpartum hemorrhage. Int J Gynaecol Obstet. 2019;146:339-343.
- Hofmeyr GJ, Singata-Madliki M. Novel suction tube uterine tamponade for treating intractable postpartum hemorrhage: description of technique and report of three cases. BJOG. 2020;127:1280-1283.
- Cebekhulu SN, Abdul H, Batting J, et al. Suction tube uterine tamponade for treatment of refractory postpartum hemorrhage: internal feasibility and acceptability pilot of a randomized clinical trial. Int J Gynaecol Obstet. 2022;158: 79-85.
- Panicker TNV. Panicker’s vacuum suction haemostatic device for treating post-partum hemorrhage. J Obstet Gynaecol India. 2017;67:150-151.