User login
Newly diagnosed major depressive disorder (MDD) in patients age ≥65 often has a vascular component. Concomitant cerebrovascular disease (CVD) does not substantially alter the management of late-life depression, but it may affect presenting symptoms, complicate the diagnosis, and influence treatment outcomes.
The relationship between depression and CVD progression remains to be fully explained, and no disease-specific interventions exist to address vascular depression’s pathophysiology. When planning treatment, however, one can draw inferences from existing studies. This article reviews the evidence on late-life depression accompanied by CVD and vascular risk factors, the “vascular depression” concept, and approaches to primary and secondary prevention and treatment.
CVD etiology of depression
Vascular depression constitutes a subgroup of late-life depression, usually associated with neuroimaging abnormalities in the basal ganglia and white matter on MRI.1 The cause of the structural brain changes is thought to be sclerosis in the small arterioles.2 These end-artery vessels may be particularly susceptible to pulse-wave changes caused by arterial rigidity or hypertension.
Alexopoulos et al1 and Krishnan et al3 proposed the concept of vascular depression on the premise that CVD may be etiologically related to geriatric depressive syndromes. Krishnan et al3 examined clinical and demographic characteristics of depressed elderly patients with vascular lesions on brain MRI. Those with clinically defined vascular depression experienced greater cognitive dysfunction, disability, and psychomotor retardation but less agitation and guilt feelings than patients with nonvascular depression.
Clinically, vascular depression resembles a medial frontal lobe syndrome, with prominent psychomotor retardation, apathy, and pronounced disability.4 Depression with vascular stigmata or cerebrovascular lesions on neuroimaging is characterized by poor outcomes, including persistent depressive symptoms, unstable remission, and increased risk for dementia.5,6 Patients with depression and subcortical vascular lesions have been shown to respond poorly to antidepressants.6
Impaired brain function also may predispose to geriatric depression, described by Alexopoulos as “depression-executive dysfunction syndrome of late life.”7 This common syndrome’s presentation—psychomotor retardation, lack of interest, limited depressive ideation and insight, and prominent disability—is consistent with its underlying abnormalities.5 Executive dysfunction also predicts limited response to antidepressants.8 Thus, the presentation and course of depression-executive dysfunction syndrome are consistent with those of subcortical ischemic depression.
Neuroimaging support
The vascular depression hypothesis is supported by observations related to MRI hyperintensities (HI):
- CT and MRI studies identify HI in persons with late-life depression.
- HI are associated with age and cerebrovascular risk factors.
- Pathophysiologic evidence indicates that HI are associated with widespread diminution in cerebral perfusion.9
Neuropathologic correlates of HI are diverse and represent ischemic changes, together with demyelination, edema, and gliosis.9-11 The putative link between HI and vascular disease is central to the vascular theory of depression.
In a study of 56 patients age ≥50 meeting DSM-III-R criteria for MDD, Fujikawa et al12 reported “silent cerebral infarctions” on MRI in 60% of patients. High rates of abnormalities consistently have been observed on MRIs of older adults with MDD,10,11 and these can be classified into 3 types (Figure):
- Periventricular HI are halos or rims adjacent to ventricles that in severe forms may invade surrounding deep white matter.
- Deep white matter HI are single, patchy, or confluent foci observed in subcortical white matter.
- Deep gray matter HI may be found, particularly in the basal ganglia, thalamus, and pons.9
These leukoaraiosis (or encephalomalacia) occur more frequently in patients with geriatric depression than in normal controls13 or patients with Alzheimer’s disease14 and may be comparable to the rate associated with vascular dementia.15 Observations in older adults11 suggest that diminished brain volume (especially in frontal regions) and HI may provide additive, albeit autonomous, pathways to late-life MDD. Vascular and nonvascular medical comorbidity contribute to HI, which in turn facilitate MDD.
Figure: Subcortical cerebrovascular disease in late-life depression
Structural MRIs of elderly adults with major depressive disorder consistently show high rates of brain abnormalities. Subcortical white matter abnormalities manifest as (1) periventricular hyperintensities [halos or rims adjacent to ventricles] and (2) deep white matter hyperintensities [single, patchy, or confluent foci]. Strategic subcortical gray matter infarctions (3) are observed, particularly in the basal ganglia, thalamus, and pons.
Bidirectional relationship
The relationship between depression and cardiovascular disease appears to be bidirectional:
- Depression may be the first clinical expression of an underlying cardiovascular disease, which is expressed as an increased risk for ischemic events.
- Depression itself, whether or not contributed by a silent cardiovascular disease, increases the risk of vascular damage, which in turn further promotes depression.
- Vascular pathogenesis affecting heart and brain is likely to increase the risk for depression through a variety of mechanisms.
Post-stroke depression (PSD) occurs within 12 to 24 months after a cerebrovascular accident.13 DSM-IV-TR categorizes PSD as a “mood disorder due to a general medical condition with the specifiers of (a) depressive features, (b) major depressive-like episodes, or (c) mixed features.”
Although important in depression’s pathophysiology, the location of stroke lesions is not the exclusive etiologic factor. Personal diathesis for depression, psychosocial factors, and physical and social impairment related to post-stroke neurologic deficits also may contribute to PSD.16
PSD patients with right-sided lesions often have family histories of depressive illness.17 Different serotonergic mechanisms might be responsible for depressive illness associated with right-sided vs left-sided lesions. This notion is supported by observed lateralized changes in serotonin type-2 (5-HT2) receptors18 and the influence of lateralized lesions on prolactin responsivity to d-fenfluramine challenge in PSD.19 Damage closer to the frontal lobes is likely to affect catecholamine-mediated brain activity.
The 8-year Framingham study20 examined the risk of developing cerebrovascular events in persons age ≤65 vs those age >65. Subjects age ≤65 with significant depressive symptoms—Center for Epidemiologic Studies Depression scale score >1621—were 4 times more likely to develop stroke or transient ischemic attack compared with the same age group without depression. Another study found a link between depression and stroke risk across the adult age range.22 Mechanisms by which depressive symptoms may predispose to stroke are not fully known, but depression has been shown to affect autonomic function and platelet activation.23
CHD and depression. In the United States, approximately 20% of coronary heart disease (CHD) patients have clinically significant depressive symptoms.24 A history of depression also has been shown to increase the relative risk of developing CHD by >80%.25
The association between depression and CHD is unclear but likely includes:
- direct biological mechanisms such as autonomic dysfunction and dysregulated inflammation
- behavioral factors such as smoking or poor self-care (Table 1).
A recent analysis of 13 cross-sectional studies26 suggests that reduced heart rate variability (HRV) related to autonomic dysfunction may be the link between depression and CHD risk. The studies’ effect sizes were small, however, and their methodologies varied considerably.
C-reactive protein (CRP), interleukin-6, tumor necrosis factor-α (TNF-α), and fibrinogen are inflammatory markers. In a 2-year follow-up study, Frasure-Smith et al27 investigated the relationship between depression and inflammatory markers in 741 patients (602 male) with acute coronary syndrome. Two months after an acute coronary event, depressive symptoms and elevated CRP levels were overlapping risk factors for future cardiac events in men.
Carney et al28 showed that fibrinogen was most associated with altered heart rate variability in depressed CHD patients and proposed deficits in parasympathetic modulation of immunity and coagulation as the cause. In contrast, Whooley et al29 found no association between major depression and inflammatory markers—including CRP, fibrinogen, and interleukin-6—in 984 outpatients with CHD. Differences in assessment scales and sample heterogeneity may have contributed to these disparate findings.
Diabetes and depression. As with CHD, a bidirectional relationship exists between depression and diabetes mellitus, although depression is only a modest risk factor for diabetes.30 Possible explanations include hypercortisolemia and increased inflammation resulting in increased insulin resistance and metabolic syndrome.
Table 1
Shared risk factors for depression and heart disease
| Decreased heart rate variability |
| Vascular inflammation (increased interleukin-6 and C-reactive protein) |
| Endothelial dysfunction |
| Platelet dysfunction |
| Atherosclerosis |
| Dyslipidemia |
| Smoking |
| Source: References 26-29 |
Diagnosis of vascular depression
Vascular depression is characterized by a clinical diagnosis of DSM-IV-TR-defined MDD, dysthymia, or depression not-otherwise-specified, accompanied by:
- evidence of CVD or
- known vascular risk factors (hypertension, diabetes, hyperlipidemia, stroke, heart failure, etc.).
In performing thorough neurologic, neuropsychiatric, and neuropsychological examinations, look for soft neurologic signs with regional weakness, apathy, and executive dysfunction. Useful bedside scales include the clock-drawing test, word list generation, brief dementia screens, and the Apathy Evaluation Scale.31
CT or MRI can provide supportive evidence by demonstrating signs of subcortical or cortical stroke. Neuroimaging studies may not be necessary, however, when depression onset is temporally associated with strong physical evidence of a stroke (such as falling, peripheral muscle weakness, or incontinence).
Treating depression symptoms
When treating vascular depression, clinical goals are to ameliorate affective symptoms, improve quality of life, and help patients perform activities of daily living (Table 2).
Psychosocial interventions. When depression is less than severe, consider psychosocial interventions as first-line treatment. Investigate environmental factors such as financial and marital problems or loneliness in patients’ depressive symptoms, and develop corresponding interventions—such as education, nutrition, exercise, socialization, or pain and stress management. Cognitive rehabilitation training and cognitive-behavioral therapy can reduce cognitive impairment and associated depression.
Antidepressants. A trial of antidepressant therapy is advisable for moderate-to-severe, chronic vascular depression, even though comorbid CVD may diminish the antidepressant response. In elderly patients, start with one-third to one-half the usual adult antidepressant dosage and increase while balancing efficacy and tolerability.
Match the medication’s side-effect profile with the patient’s target symptoms (such as anxiety vs apathy).32 Selective serotonin reuptake inhibitors are probably first-line, but bupropion, venlafaxine, duloxetine, or mirtazapine may be more appropriate for some patients (Table 3).
In PSD, nortriptyline has shown a significantly greater response rate than fluoxetine or placebo in improving anxiety symptoms and recovery of activities of daily living.33 Tricyclic antidepressants’ anticholinergic properties are a safety concern in patients with heart disease, however. In general, avoid agents with substantial anticholinergic effects in elderly patients to minimize the risk of cognitive impairment and other side effects, such as urinary retention or worsening of glaucoma.
Because of the substantial risk of postural hypotension, nonselective monoamine oxidase inhibitors are probably appropriate only for geriatric patients with highly treatment-refractory depression. Dopaminergic agents such as methylphenidate in a relatively moderate dose (such as 5 to 20 mg/d) may improve apathy and social withdrawal, but research into their use in vascular depression is lacking.
Other options. Clinical experience suggests that electroconvulsive therapy (ECT) is effective for patients who do not respond to antidepressants. ECT appears quite safe in older patients, especially if not used in the first 6 months post-stroke. Strategies to reduce the risk of cognitive side effects include:
- 2 rather than 3 weekly treatments
- unilateral or bifrontal rather than bilateral treatments
- frontal lead placement.34
In the only study of transcranial magnetic stimulation (TMS) for geriatric patients with depression (N=92), those with treatment-resistant vascular depression showed higher remission rates with TMS (27.3%) compared with sham TMS (3.5%). Response rates to TMS were negatively correlated with advancing age and positively correlated with higher frontal gray matter volumes.35
Fish oil or vitamin B complex may be used to manage hyperlipidemia or nutritional deficiencies.36 Herbal preparations such as St. John’s wort (Hypericum perforatum) or S-adenosyl-L-methionine (SAMe) have shown some efficacy in adults with MDD, but further study is needed.
Table 2
Clinical management of late-life vascular depression
| Decision point | Assessment/intervention |
|---|---|
| Diagnosis | Apply DSM-IV-TR diagnostic criteria based on results of comprehensive assessment (neuropsychiatric, neuropsychological, structural neuroimaging, vascular and genetic risk factors) |
| Prevention | Identify and treat modifiable risk factors for the development or worsening of cerebrovascular disease, especially in high-risk populations (Table 4) |
| Treatment goals | Target 1: Achieve remission of depressive symptoms, improved cognition and function Target 2: Maintain remission and prevent relapse |
| Managing psychological and behavioral symptoms | Step 1: Consider psychotherapy addressing existing stressors and environmental management in patients with mild-to-moderate depression Step 2: If depression is severe or Step 1 is ineffective, an antidepressant trial* is highly recommended (Table 3); consider ECT or TMS in severe cases |
| *Avoid medications that could worsen cognition or motor functioning, such as tricyclic antidepressants or neuroleptics | |
| ECT: electroconvulsive therapy; TMS: transcranial magnetic stimulation | |
Table 3
Recommended antidepressant dosing
for elderly patients with vascular depression*
| Drug | Starting daily dosage (usual therapeutic range) | Side effect profile (patient characteristics) |
|---|---|---|
| SSRIs | ||
| Escitalopram | 5 mg (10 to 20 mg) | Nausea, headaches, GI upset, insomnia, anxiety |
| Fluoxetine | 10 mg (10 to 60 mg) | |
| Paroxetine | 10 mg (10 to 30 mg) | |
| Sertraline | 25 mg (50 to 150 mg) | |
| Others | ||
| Bupropion | 75 mg (75 to 300 mg) | GI upset, anxiety (may be useful for patients with high apathy) |
| Mirtazapine | 7.5 mg (15 to 45 mg) | Sedation, weight gain (may be useful for patients with severe insomnia or anorexia) |
| Venlafaxine | 37.5 mg (75 to 300 mg) | Nausea, headaches, anxiety, blood pressure elevation, insomnia (may be useful for patients with chronic pain) |
| Duloxetine | 20 mg (30 to 120 mg) | |
| *Avoid medications that could worsen cognition or motor functioning, such as tricyclic antidepressants or neuroleptics | ||
| GI: gastrointestinal; SSRIs: selective serotonin reuptake inhibitors | ||
Treating vascular factors
In addition to treating your patients’ depressive symptoms, collaborate with their primary care physicians to modify physiologic and behavioral factors that increase the risk for vascular injury—such as hypertension, diabetes mellitus, cigarette smoking, and hyperlipidemia. All can be controlled in presymptomatic or mildly symptomatic stages (Table 4).
Anticoagulation. In appropriate patients, anticoagulation can prevent thromboembolic strokes, although risks such as increased hemorrhagic complications must be considered.37 In elderly adults, base treatment decisions on individual risk factors, goals of treatment, and quality-of-life expectancy. In a study of low-dose aspirin (81 mg/d) and low-intensity oral anticoagulation in men at risk of cardiovascular disease, verbal fluency and mental flexibility were significantly better in men taking antithrombotic medications (especially aspirin) than in those taking placebo.38
Antihypertensives and statins. Patients with vascular depression may benefit from calcium channel blockers or angiotensin-converting enzyme (ACE) inhibitors for hypertension and HMG-CoA reductase inhibitors (statins) for hyperlipidemia. Statins seem to decrease the generation of amyloid precursor protein, the neuronal secretion of β-amyloid, and cholesterol synthesis.39 Some epidemiologic studies suggest an association between statin use for cholesterol reduction and reduced prevalence of Alzheimer’s disease and vascular dementia.40
Potential preventive strategies are not without controversy, however:
- Beta blockers and ACE inhibitors have been linked to depression, although the evidence has been conflicting.
- Lipid-lowering therapies and calcium-channel blockers have been linked to an increased risk of suicide.41
- A more recent population-based study did not support an association between an increased risk of suicide and cardiovascular drugs (except perhaps for angiotensin-receptor antagonists).42
Table 4
Preventing vascular causes of late-life depression
| Decision point | Assessment/intervention | Comment |
|---|---|---|
| Primary, secondary prevention of stroke, vascular depression, and cognitive impairment | Identify and treat modifiable risk factors (hypertension, alcohol use, smoking, hyperlipidemia, diabetes mellitus), especially in high-risk patients | Consider as high-risk patients having ≥1 of these features: age >50; male gender; Asian, Hispanic, or African-American heritage; low educational achievement; concurrent vascular risk factors |
| Tertiary prevention of worsened illness in patients with established vascular disease | Intensively treat vascular risk factors | Collaborate with primary care physician to manage arterial hypertension, myocardial infarction, atrial fibrillation, coronary heart disease, diabetes, atherosclerosis, hyperlipidemia, obesity, and smoking |
| Rapidly identify and treat acute stroke to limit ischemic brain changes and promote recovery | ||
| Prevent stroke recurrence by aggressively treating vascular risk factors | Let CVD etiology guide treatment | |
| CVD: cerebrovascular disease | ||
| Source: Adapted from Lavretsky H. Diagnosis and treatment of vascular dementia. Directions in Psychiatry. 2006;26(1):49-68 | ||
Related resources
- Lavretsky H, Chui H. Vascular dementia. In: Agronin ME, Maletta GJ, eds. Principles and practice of geriatric psychiatry. New York, NY: Lippincott, Williams, and Wilkins; 2005: 301-310.
- Baldwin RC, O’Brien J. Vascular basis of late-onset depressive disorder. Br J Psychiatry. 2002;180:157-160.
- Kendler KS, Gardner CO, Fiske A, et al. Major depression and coronary heart disease in the Swedish twin registry. Arch Gen Psychiatry. 2008;66(8):857-863.
Drug brand names
- Bupropion • Wellbutrin
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Methylphenidate • Ritalin, Concerta, others
- Mirtazapine • Remeron
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosures
Dr. Lavretsky receives grant/research support from Forest Research Institute and is a consultant to Forest Laboratories, Myriad Pharmaceuticals, and Accera, Inc.
Dr. Meeks reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgments
This work was supported by National Institute of Health grants R01 MH077650 and R-21 AT003480 (Dr. Lavretsky), the U.S. Department of Health and Human Services, Health Resources and Services Administration (Geriatric Academic Career Award), and the Sam and Rose Stein Institute for Research on Aging (Dr. Meeks).
1. Alexopoulos GS, Meyers BS, Young RC, et al. Clinically defined vascular depression. Am J Psychiatry. 1997;154:562-565.
2. Newberg AR, Davydow DS, Lee HB. Cerebrovascular disease basis of depression: post-stroke depression and vascular depression. Int Rev Psychiatry. 2006;18:433-441.
3. Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997;154:497-501.
4. Krishnan KR, Taylor WD, McQuoid DR, et al. Clinical characteristics of magnetic resonance imaging-defined subcortical ischemic depression. Biol Psychiatry. 2004;5(4):390-397.
5. Alexopoulos GS, Kiosses DN, Klimstra S, et al. Clinical presentation of the "depression-executive dysfunction syndrome" of late life. Am J Geriatr Psychiatry. 2002;10:98-102.
6. Taylor WD, Steffens DC, Krishnan KR. Psychiatric disease in the twenty-first century: the case for subcortical ischemic depression. Biol Psychiatry. 2006;60(12):1299-1303.
7. Alexopoulos GS. The depression-executive dysfunction syndrome of late life: a specific target for D3 receptor agonists? Am J Geriatr Psychiatry. 2001;9:1-8.
8. Alexopoulos GS. Depression in the elderly. Lancet. 2005;365:1961-1970.
9. Sackeim H. Brain structure and function in late-life depression. In: Morihisa JM, ed. Advances in brain imaging. Arlington, VA: American Psychiatric Publishing, Inc.; 2001:83–122.
10. Kumar A, Bilker W, Jin Z, et al. Atrophy and high intensity lesions: complementary neurobiological mechanisms in late-life major depression. Neuropsychopharmacology. 2000;22:264-274.
11. Kumar A, Mintz J, Bilker W, et al. Autonomous neurobiological pathways to late-life major depressive disorder: clinical and pathophysiological implications. Neuropsychopharmacology. 2002;26:229-236.
12. Fujikawa T, Yamawaki S, Fujita Y, et al. [Clinical study of correlation pre-senile, senile depressive state with silent cerebral infarction—MRI findings and its distribution]. Seishin Shinkeigaku Zasshi. 1992;94(9):851-863.
13. Kumar A, Cummings J. Depression in neurodegenerative disorders and related conditions in Alzheimer’s disease and related conditions. In: Gothier S, Cummings J, eds. Alzheimer’s disease and related disorders. London, UK: Martin Dunitz; 2001:123-141.
14. Erkinjuntti T, Gao F, Lee DH, et al. Lack of difference in brain hyperintensities between patients with early Alzheimer’s disease and control subjects. Arch Neurol. 1994;51:260-268.
15. Zubenko G, Sullivan P, Nelson J, et al. Brain imaging abnormalities in mental disorders of late life. Arch Neurol. 1990;47:1107-1111.
16. Birkett DP. The psychiatry of stroke. Arlington, VA: American Psychiatric Publishing, Inc.; 1996.
17. Robinson PG, Starkstein SE. Current research in affective disorders following stroke. J Neuropsychiatry Clin Neurosci. 1990;2:1-14.
18. Mayberg HS, Parikh RM, Morris PL, et al. Spontaneous remission of post-stroke depression and temporal changes in cortical S2-serotonin receptors. J Neuropsychiatry Clin Neurosci. 1991;3:80-83.
19. Ramasubbu R, Flint A, Brown G, et al. A neuroendocrine study of serotonin function in depressed stroke patients compared to nondepressed stroke patients and healthy controls. J Affect Disord. 1999;52:121-133.
20. Salaycik KJ, Kelly-Hayes M, Beiser A, et al. Depressive symptoms and risk of stroke. The Framingham study. Stroke. 2007;38:16-21.
21. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measurement. 1977;1:385-401.
22. Jonas BS, Mussolino ME. Symptoms of depression as a prospective risk factor for stroke. Psychosom Med. 2000;62:463-471.
23. Whyte EM, Pollock BG, Wagner WR, et al. Influence of serotonin-transporter-linked promoter region polymorphism on platelet activation in geriatric depression. Am J Psychiatry. 2001;158(12):2074-2076.
24. Amin AA, Jones AM, Nugnet K, et al. The prevalence of unrecognized depression in patients with acute coronary syndrome. Am Heart J. 2006;152:928-934.
25. Nicholson A, Kuper H, Hemingway H. Depression as an aetiolgic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27:2763-2774.
26. Rottenberg J. Cardiac vagal control in depression: a critical analysis. Biol Psychol. 2007;74:200-211.
27. Frasure-Smith N, Lesperance F, Irwin MR, et al. Depression, C-reactive protein and two-year major adverse cardiac events in men after acute coronary syndromes. Biol Psychiatry. 2007;62:302-308.
28. Carney RM, Freedland KE, Stein PK, et al. Heart rate variability and markers of inflammation and coagulation in depressed patients with coronary heart disease. J Psychosomatic Res. 2007;62:463-467.
29. Whooley MA, Caska CM, Hendrickson BE, et al. Depression and inflammation in patients with coronary heart disease: findings from the Heart and Soul Study. Biol Psychiatry. 2007;62:314-320.
30. Hill Golden S, Lazo M, Carnethon M, et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008;299(23):2751-2759.
31. Marin RS. Differential diagnosis of apathy and related disorders of diminished motivation. Psychiatric Annals. 1997;27:30-33.
32. Roose S. Treatment of depression in patients with heart disease. Biol Psychiatry. 2003;54:262-268.
33. Robinson RG, Schultz SK, Castillo C, et al. Nortriptyline versus fluoxetine in the treatment of depression and in short-term recovery after stroke: a placebo-controlled, double-blind study. Am J Psychiatry. 2000;157(3):351-359.
34. Katz IR. Diagnosis and treatment of depression in patients with Alzheimer’s disease and other dementias. J Clin Psychiatry. 1998;59(9):38-44.
35. Jorge RE, Moser DJ, Acion L, et al. Treatment of vascular depression using repetitive transcranial magnetic stimulation. Arch Gen Psychiatry. 2008;65(3):268-276.
36. Lavretsky H. The use of complementary and alternative medicine for treatment of late-life neuropsychiatric disorders. J Aging Health. 2009;5(1):61-78.
37. Pantoni L, Inzitari D. New clinical relevance of leukoaraiosis. European force on age-related white-matter changes. Stroke. 1998;29(2):543.-
38. Richards M, Meade TW, Peart S, et al. Is there any evidence for a protective effect of antithrombotic medication on cognitive function in men at risk of cardiovascular disease? Some preliminary findings. J Neurol Neurosurg Psychiatry. 1997;62(3):269-272.
39. Lutjohann D, Papassotiropoulos A, Bjorkhem I, et al. Plasma 24S-hydroxycholesterol (cerebrosterol) is increased in Alzheimer and vascular demented patients. J Lipid Res. 2000;41(2):195-198.
40. Jick H, Zornberg GL, Jick SS, et al. Statins and the risk of dementia. Lancet. 2000;356(9242):1627-1631.
41. Yang CC, Jick SS, Jick H. Lipid-lowering drugs and the risk of depression and suicidal behavior. Arch Intern Med. 2003;163(16):1926-1932.
42. Callréus T, Agerskov Andersen U, Hallas J, et al. Cardiovascular drugs and the risk of suicide: a nested case-control study. Eur J Clin Pharmacol. 2007;63(6):591-596.
Newly diagnosed major depressive disorder (MDD) in patients age ≥65 often has a vascular component. Concomitant cerebrovascular disease (CVD) does not substantially alter the management of late-life depression, but it may affect presenting symptoms, complicate the diagnosis, and influence treatment outcomes.
The relationship between depression and CVD progression remains to be fully explained, and no disease-specific interventions exist to address vascular depression’s pathophysiology. When planning treatment, however, one can draw inferences from existing studies. This article reviews the evidence on late-life depression accompanied by CVD and vascular risk factors, the “vascular depression” concept, and approaches to primary and secondary prevention and treatment.
CVD etiology of depression
Vascular depression constitutes a subgroup of late-life depression, usually associated with neuroimaging abnormalities in the basal ganglia and white matter on MRI.1 The cause of the structural brain changes is thought to be sclerosis in the small arterioles.2 These end-artery vessels may be particularly susceptible to pulse-wave changes caused by arterial rigidity or hypertension.
Alexopoulos et al1 and Krishnan et al3 proposed the concept of vascular depression on the premise that CVD may be etiologically related to geriatric depressive syndromes. Krishnan et al3 examined clinical and demographic characteristics of depressed elderly patients with vascular lesions on brain MRI. Those with clinically defined vascular depression experienced greater cognitive dysfunction, disability, and psychomotor retardation but less agitation and guilt feelings than patients with nonvascular depression.
Clinically, vascular depression resembles a medial frontal lobe syndrome, with prominent psychomotor retardation, apathy, and pronounced disability.4 Depression with vascular stigmata or cerebrovascular lesions on neuroimaging is characterized by poor outcomes, including persistent depressive symptoms, unstable remission, and increased risk for dementia.5,6 Patients with depression and subcortical vascular lesions have been shown to respond poorly to antidepressants.6
Impaired brain function also may predispose to geriatric depression, described by Alexopoulos as “depression-executive dysfunction syndrome of late life.”7 This common syndrome’s presentation—psychomotor retardation, lack of interest, limited depressive ideation and insight, and prominent disability—is consistent with its underlying abnormalities.5 Executive dysfunction also predicts limited response to antidepressants.8 Thus, the presentation and course of depression-executive dysfunction syndrome are consistent with those of subcortical ischemic depression.
Neuroimaging support
The vascular depression hypothesis is supported by observations related to MRI hyperintensities (HI):
- CT and MRI studies identify HI in persons with late-life depression.
- HI are associated with age and cerebrovascular risk factors.
- Pathophysiologic evidence indicates that HI are associated with widespread diminution in cerebral perfusion.9
Neuropathologic correlates of HI are diverse and represent ischemic changes, together with demyelination, edema, and gliosis.9-11 The putative link between HI and vascular disease is central to the vascular theory of depression.
In a study of 56 patients age ≥50 meeting DSM-III-R criteria for MDD, Fujikawa et al12 reported “silent cerebral infarctions” on MRI in 60% of patients. High rates of abnormalities consistently have been observed on MRIs of older adults with MDD,10,11 and these can be classified into 3 types (Figure):
- Periventricular HI are halos or rims adjacent to ventricles that in severe forms may invade surrounding deep white matter.
- Deep white matter HI are single, patchy, or confluent foci observed in subcortical white matter.
- Deep gray matter HI may be found, particularly in the basal ganglia, thalamus, and pons.9
These leukoaraiosis (or encephalomalacia) occur more frequently in patients with geriatric depression than in normal controls13 or patients with Alzheimer’s disease14 and may be comparable to the rate associated with vascular dementia.15 Observations in older adults11 suggest that diminished brain volume (especially in frontal regions) and HI may provide additive, albeit autonomous, pathways to late-life MDD. Vascular and nonvascular medical comorbidity contribute to HI, which in turn facilitate MDD.
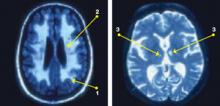
Figure: Subcortical cerebrovascular disease in late-life depression
Structural MRIs of elderly adults with major depressive disorder consistently show high rates of brain abnormalities. Subcortical white matter abnormalities manifest as (1) periventricular hyperintensities [halos or rims adjacent to ventricles] and (2) deep white matter hyperintensities [single, patchy, or confluent foci]. Strategic subcortical gray matter infarctions (3) are observed, particularly in the basal ganglia, thalamus, and pons.
Bidirectional relationship
The relationship between depression and cardiovascular disease appears to be bidirectional:
- Depression may be the first clinical expression of an underlying cardiovascular disease, which is expressed as an increased risk for ischemic events.
- Depression itself, whether or not contributed by a silent cardiovascular disease, increases the risk of vascular damage, which in turn further promotes depression.
- Vascular pathogenesis affecting heart and brain is likely to increase the risk for depression through a variety of mechanisms.
Post-stroke depression (PSD) occurs within 12 to 24 months after a cerebrovascular accident.13 DSM-IV-TR categorizes PSD as a “mood disorder due to a general medical condition with the specifiers of (a) depressive features, (b) major depressive-like episodes, or (c) mixed features.”
Although important in depression’s pathophysiology, the location of stroke lesions is not the exclusive etiologic factor. Personal diathesis for depression, psychosocial factors, and physical and social impairment related to post-stroke neurologic deficits also may contribute to PSD.16
PSD patients with right-sided lesions often have family histories of depressive illness.17 Different serotonergic mechanisms might be responsible for depressive illness associated with right-sided vs left-sided lesions. This notion is supported by observed lateralized changes in serotonin type-2 (5-HT2) receptors18 and the influence of lateralized lesions on prolactin responsivity to d-fenfluramine challenge in PSD.19 Damage closer to the frontal lobes is likely to affect catecholamine-mediated brain activity.
The 8-year Framingham study20 examined the risk of developing cerebrovascular events in persons age ≤65 vs those age >65. Subjects age ≤65 with significant depressive symptoms—Center for Epidemiologic Studies Depression scale score >1621—were 4 times more likely to develop stroke or transient ischemic attack compared with the same age group without depression. Another study found a link between depression and stroke risk across the adult age range.22 Mechanisms by which depressive symptoms may predispose to stroke are not fully known, but depression has been shown to affect autonomic function and platelet activation.23
CHD and depression. In the United States, approximately 20% of coronary heart disease (CHD) patients have clinically significant depressive symptoms.24 A history of depression also has been shown to increase the relative risk of developing CHD by >80%.25
The association between depression and CHD is unclear but likely includes:
- direct biological mechanisms such as autonomic dysfunction and dysregulated inflammation
- behavioral factors such as smoking or poor self-care (Table 1).
A recent analysis of 13 cross-sectional studies26 suggests that reduced heart rate variability (HRV) related to autonomic dysfunction may be the link between depression and CHD risk. The studies’ effect sizes were small, however, and their methodologies varied considerably.
C-reactive protein (CRP), interleukin-6, tumor necrosis factor-α (TNF-α), and fibrinogen are inflammatory markers. In a 2-year follow-up study, Frasure-Smith et al27 investigated the relationship between depression and inflammatory markers in 741 patients (602 male) with acute coronary syndrome. Two months after an acute coronary event, depressive symptoms and elevated CRP levels were overlapping risk factors for future cardiac events in men.
Carney et al28 showed that fibrinogen was most associated with altered heart rate variability in depressed CHD patients and proposed deficits in parasympathetic modulation of immunity and coagulation as the cause. In contrast, Whooley et al29 found no association between major depression and inflammatory markers—including CRP, fibrinogen, and interleukin-6—in 984 outpatients with CHD. Differences in assessment scales and sample heterogeneity may have contributed to these disparate findings.
Diabetes and depression. As with CHD, a bidirectional relationship exists between depression and diabetes mellitus, although depression is only a modest risk factor for diabetes.30 Possible explanations include hypercortisolemia and increased inflammation resulting in increased insulin resistance and metabolic syndrome.
Table 1
Shared risk factors for depression and heart disease
| Decreased heart rate variability |
| Vascular inflammation (increased interleukin-6 and C-reactive protein) |
| Endothelial dysfunction |
| Platelet dysfunction |
| Atherosclerosis |
| Dyslipidemia |
| Smoking |
| Source: References 26-29 |
Diagnosis of vascular depression
Vascular depression is characterized by a clinical diagnosis of DSM-IV-TR-defined MDD, dysthymia, or depression not-otherwise-specified, accompanied by:
- evidence of CVD or
- known vascular risk factors (hypertension, diabetes, hyperlipidemia, stroke, heart failure, etc.).
In performing thorough neurologic, neuropsychiatric, and neuropsychological examinations, look for soft neurologic signs with regional weakness, apathy, and executive dysfunction. Useful bedside scales include the clock-drawing test, word list generation, brief dementia screens, and the Apathy Evaluation Scale.31
CT or MRI can provide supportive evidence by demonstrating signs of subcortical or cortical stroke. Neuroimaging studies may not be necessary, however, when depression onset is temporally associated with strong physical evidence of a stroke (such as falling, peripheral muscle weakness, or incontinence).
Treating depression symptoms
When treating vascular depression, clinical goals are to ameliorate affective symptoms, improve quality of life, and help patients perform activities of daily living (Table 2).
Psychosocial interventions. When depression is less than severe, consider psychosocial interventions as first-line treatment. Investigate environmental factors such as financial and marital problems or loneliness in patients’ depressive symptoms, and develop corresponding interventions—such as education, nutrition, exercise, socialization, or pain and stress management. Cognitive rehabilitation training and cognitive-behavioral therapy can reduce cognitive impairment and associated depression.
Antidepressants. A trial of antidepressant therapy is advisable for moderate-to-severe, chronic vascular depression, even though comorbid CVD may diminish the antidepressant response. In elderly patients, start with one-third to one-half the usual adult antidepressant dosage and increase while balancing efficacy and tolerability.
Match the medication’s side-effect profile with the patient’s target symptoms (such as anxiety vs apathy).32 Selective serotonin reuptake inhibitors are probably first-line, but bupropion, venlafaxine, duloxetine, or mirtazapine may be more appropriate for some patients (Table 3).
In PSD, nortriptyline has shown a significantly greater response rate than fluoxetine or placebo in improving anxiety symptoms and recovery of activities of daily living.33 Tricyclic antidepressants’ anticholinergic properties are a safety concern in patients with heart disease, however. In general, avoid agents with substantial anticholinergic effects in elderly patients to minimize the risk of cognitive impairment and other side effects, such as urinary retention or worsening of glaucoma.
Because of the substantial risk of postural hypotension, nonselective monoamine oxidase inhibitors are probably appropriate only for geriatric patients with highly treatment-refractory depression. Dopaminergic agents such as methylphenidate in a relatively moderate dose (such as 5 to 20 mg/d) may improve apathy and social withdrawal, but research into their use in vascular depression is lacking.
Other options. Clinical experience suggests that electroconvulsive therapy (ECT) is effective for patients who do not respond to antidepressants. ECT appears quite safe in older patients, especially if not used in the first 6 months post-stroke. Strategies to reduce the risk of cognitive side effects include:
- 2 rather than 3 weekly treatments
- unilateral or bifrontal rather than bilateral treatments
- frontal lead placement.34
In the only study of transcranial magnetic stimulation (TMS) for geriatric patients with depression (N=92), those with treatment-resistant vascular depression showed higher remission rates with TMS (27.3%) compared with sham TMS (3.5%). Response rates to TMS were negatively correlated with advancing age and positively correlated with higher frontal gray matter volumes.35
Fish oil or vitamin B complex may be used to manage hyperlipidemia or nutritional deficiencies.36 Herbal preparations such as St. John’s wort (Hypericum perforatum) or S-adenosyl-L-methionine (SAMe) have shown some efficacy in adults with MDD, but further study is needed.
Table 2
Clinical management of late-life vascular depression
| Decision point | Assessment/intervention |
|---|---|
| Diagnosis | Apply DSM-IV-TR diagnostic criteria based on results of comprehensive assessment (neuropsychiatric, neuropsychological, structural neuroimaging, vascular and genetic risk factors) |
| Prevention | Identify and treat modifiable risk factors for the development or worsening of cerebrovascular disease, especially in high-risk populations (Table 4) |
| Treatment goals | Target 1: Achieve remission of depressive symptoms, improved cognition and function Target 2: Maintain remission and prevent relapse |
| Managing psychological and behavioral symptoms | Step 1: Consider psychotherapy addressing existing stressors and environmental management in patients with mild-to-moderate depression Step 2: If depression is severe or Step 1 is ineffective, an antidepressant trial* is highly recommended (Table 3); consider ECT or TMS in severe cases |
| *Avoid medications that could worsen cognition or motor functioning, such as tricyclic antidepressants or neuroleptics | |
| ECT: electroconvulsive therapy; TMS: transcranial magnetic stimulation | |
Table 3
Recommended antidepressant dosing
for elderly patients with vascular depression*
| Drug | Starting daily dosage (usual therapeutic range) | Side effect profile (patient characteristics) |
|---|---|---|
| SSRIs | ||
| Escitalopram | 5 mg (10 to 20 mg) | Nausea, headaches, GI upset, insomnia, anxiety |
| Fluoxetine | 10 mg (10 to 60 mg) | |
| Paroxetine | 10 mg (10 to 30 mg) | |
| Sertraline | 25 mg (50 to 150 mg) | |
| Others | ||
| Bupropion | 75 mg (75 to 300 mg) | GI upset, anxiety (may be useful for patients with high apathy) |
| Mirtazapine | 7.5 mg (15 to 45 mg) | Sedation, weight gain (may be useful for patients with severe insomnia or anorexia) |
| Venlafaxine | 37.5 mg (75 to 300 mg) | Nausea, headaches, anxiety, blood pressure elevation, insomnia (may be useful for patients with chronic pain) |
| Duloxetine | 20 mg (30 to 120 mg) | |
| *Avoid medications that could worsen cognition or motor functioning, such as tricyclic antidepressants or neuroleptics | ||
| GI: gastrointestinal; SSRIs: selective serotonin reuptake inhibitors | ||
Treating vascular factors
In addition to treating your patients’ depressive symptoms, collaborate with their primary care physicians to modify physiologic and behavioral factors that increase the risk for vascular injury—such as hypertension, diabetes mellitus, cigarette smoking, and hyperlipidemia. All can be controlled in presymptomatic or mildly symptomatic stages (Table 4).
Anticoagulation. In appropriate patients, anticoagulation can prevent thromboembolic strokes, although risks such as increased hemorrhagic complications must be considered.37 In elderly adults, base treatment decisions on individual risk factors, goals of treatment, and quality-of-life expectancy. In a study of low-dose aspirin (81 mg/d) and low-intensity oral anticoagulation in men at risk of cardiovascular disease, verbal fluency and mental flexibility were significantly better in men taking antithrombotic medications (especially aspirin) than in those taking placebo.38
Antihypertensives and statins. Patients with vascular depression may benefit from calcium channel blockers or angiotensin-converting enzyme (ACE) inhibitors for hypertension and HMG-CoA reductase inhibitors (statins) for hyperlipidemia. Statins seem to decrease the generation of amyloid precursor protein, the neuronal secretion of β-amyloid, and cholesterol synthesis.39 Some epidemiologic studies suggest an association between statin use for cholesterol reduction and reduced prevalence of Alzheimer’s disease and vascular dementia.40
Potential preventive strategies are not without controversy, however:
- Beta blockers and ACE inhibitors have been linked to depression, although the evidence has been conflicting.
- Lipid-lowering therapies and calcium-channel blockers have been linked to an increased risk of suicide.41
- A more recent population-based study did not support an association between an increased risk of suicide and cardiovascular drugs (except perhaps for angiotensin-receptor antagonists).42
Table 4
Preventing vascular causes of late-life depression
| Decision point | Assessment/intervention | Comment |
|---|---|---|
| Primary, secondary prevention of stroke, vascular depression, and cognitive impairment | Identify and treat modifiable risk factors (hypertension, alcohol use, smoking, hyperlipidemia, diabetes mellitus), especially in high-risk patients | Consider as high-risk patients having ≥1 of these features: age >50; male gender; Asian, Hispanic, or African-American heritage; low educational achievement; concurrent vascular risk factors |
| Tertiary prevention of worsened illness in patients with established vascular disease | Intensively treat vascular risk factors | Collaborate with primary care physician to manage arterial hypertension, myocardial infarction, atrial fibrillation, coronary heart disease, diabetes, atherosclerosis, hyperlipidemia, obesity, and smoking |
| Rapidly identify and treat acute stroke to limit ischemic brain changes and promote recovery | ||
| Prevent stroke recurrence by aggressively treating vascular risk factors | Let CVD etiology guide treatment | |
| CVD: cerebrovascular disease | ||
| Source: Adapted from Lavretsky H. Diagnosis and treatment of vascular dementia. Directions in Psychiatry. 2006;26(1):49-68 | ||
Related resources
- Lavretsky H, Chui H. Vascular dementia. In: Agronin ME, Maletta GJ, eds. Principles and practice of geriatric psychiatry. New York, NY: Lippincott, Williams, and Wilkins; 2005: 301-310.
- Baldwin RC, O’Brien J. Vascular basis of late-onset depressive disorder. Br J Psychiatry. 2002;180:157-160.
- Kendler KS, Gardner CO, Fiske A, et al. Major depression and coronary heart disease in the Swedish twin registry. Arch Gen Psychiatry. 2008;66(8):857-863.
Drug brand names
- Bupropion • Wellbutrin
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Methylphenidate • Ritalin, Concerta, others
- Mirtazapine • Remeron
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosures
Dr. Lavretsky receives grant/research support from Forest Research Institute and is a consultant to Forest Laboratories, Myriad Pharmaceuticals, and Accera, Inc.
Dr. Meeks reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgments
This work was supported by National Institute of Health grants R01 MH077650 and R-21 AT003480 (Dr. Lavretsky), the U.S. Department of Health and Human Services, Health Resources and Services Administration (Geriatric Academic Career Award), and the Sam and Rose Stein Institute for Research on Aging (Dr. Meeks).
Newly diagnosed major depressive disorder (MDD) in patients age ≥65 often has a vascular component. Concomitant cerebrovascular disease (CVD) does not substantially alter the management of late-life depression, but it may affect presenting symptoms, complicate the diagnosis, and influence treatment outcomes.
The relationship between depression and CVD progression remains to be fully explained, and no disease-specific interventions exist to address vascular depression’s pathophysiology. When planning treatment, however, one can draw inferences from existing studies. This article reviews the evidence on late-life depression accompanied by CVD and vascular risk factors, the “vascular depression” concept, and approaches to primary and secondary prevention and treatment.
CVD etiology of depression
Vascular depression constitutes a subgroup of late-life depression, usually associated with neuroimaging abnormalities in the basal ganglia and white matter on MRI.1 The cause of the structural brain changes is thought to be sclerosis in the small arterioles.2 These end-artery vessels may be particularly susceptible to pulse-wave changes caused by arterial rigidity or hypertension.
Alexopoulos et al1 and Krishnan et al3 proposed the concept of vascular depression on the premise that CVD may be etiologically related to geriatric depressive syndromes. Krishnan et al3 examined clinical and demographic characteristics of depressed elderly patients with vascular lesions on brain MRI. Those with clinically defined vascular depression experienced greater cognitive dysfunction, disability, and psychomotor retardation but less agitation and guilt feelings than patients with nonvascular depression.
Clinically, vascular depression resembles a medial frontal lobe syndrome, with prominent psychomotor retardation, apathy, and pronounced disability.4 Depression with vascular stigmata or cerebrovascular lesions on neuroimaging is characterized by poor outcomes, including persistent depressive symptoms, unstable remission, and increased risk for dementia.5,6 Patients with depression and subcortical vascular lesions have been shown to respond poorly to antidepressants.6
Impaired brain function also may predispose to geriatric depression, described by Alexopoulos as “depression-executive dysfunction syndrome of late life.”7 This common syndrome’s presentation—psychomotor retardation, lack of interest, limited depressive ideation and insight, and prominent disability—is consistent with its underlying abnormalities.5 Executive dysfunction also predicts limited response to antidepressants.8 Thus, the presentation and course of depression-executive dysfunction syndrome are consistent with those of subcortical ischemic depression.
Neuroimaging support
The vascular depression hypothesis is supported by observations related to MRI hyperintensities (HI):
- CT and MRI studies identify HI in persons with late-life depression.
- HI are associated with age and cerebrovascular risk factors.
- Pathophysiologic evidence indicates that HI are associated with widespread diminution in cerebral perfusion.9
Neuropathologic correlates of HI are diverse and represent ischemic changes, together with demyelination, edema, and gliosis.9-11 The putative link between HI and vascular disease is central to the vascular theory of depression.
In a study of 56 patients age ≥50 meeting DSM-III-R criteria for MDD, Fujikawa et al12 reported “silent cerebral infarctions” on MRI in 60% of patients. High rates of abnormalities consistently have been observed on MRIs of older adults with MDD,10,11 and these can be classified into 3 types (Figure):
- Periventricular HI are halos or rims adjacent to ventricles that in severe forms may invade surrounding deep white matter.
- Deep white matter HI are single, patchy, or confluent foci observed in subcortical white matter.
- Deep gray matter HI may be found, particularly in the basal ganglia, thalamus, and pons.9
These leukoaraiosis (or encephalomalacia) occur more frequently in patients with geriatric depression than in normal controls13 or patients with Alzheimer’s disease14 and may be comparable to the rate associated with vascular dementia.15 Observations in older adults11 suggest that diminished brain volume (especially in frontal regions) and HI may provide additive, albeit autonomous, pathways to late-life MDD. Vascular and nonvascular medical comorbidity contribute to HI, which in turn facilitate MDD.
Figure: Subcortical cerebrovascular disease in late-life depression
Structural MRIs of elderly adults with major depressive disorder consistently show high rates of brain abnormalities. Subcortical white matter abnormalities manifest as (1) periventricular hyperintensities [halos or rims adjacent to ventricles] and (2) deep white matter hyperintensities [single, patchy, or confluent foci]. Strategic subcortical gray matter infarctions (3) are observed, particularly in the basal ganglia, thalamus, and pons.
Bidirectional relationship
The relationship between depression and cardiovascular disease appears to be bidirectional:
- Depression may be the first clinical expression of an underlying cardiovascular disease, which is expressed as an increased risk for ischemic events.
- Depression itself, whether or not contributed by a silent cardiovascular disease, increases the risk of vascular damage, which in turn further promotes depression.
- Vascular pathogenesis affecting heart and brain is likely to increase the risk for depression through a variety of mechanisms.
Post-stroke depression (PSD) occurs within 12 to 24 months after a cerebrovascular accident.13 DSM-IV-TR categorizes PSD as a “mood disorder due to a general medical condition with the specifiers of (a) depressive features, (b) major depressive-like episodes, or (c) mixed features.”
Although important in depression’s pathophysiology, the location of stroke lesions is not the exclusive etiologic factor. Personal diathesis for depression, psychosocial factors, and physical and social impairment related to post-stroke neurologic deficits also may contribute to PSD.16
PSD patients with right-sided lesions often have family histories of depressive illness.17 Different serotonergic mechanisms might be responsible for depressive illness associated with right-sided vs left-sided lesions. This notion is supported by observed lateralized changes in serotonin type-2 (5-HT2) receptors18 and the influence of lateralized lesions on prolactin responsivity to d-fenfluramine challenge in PSD.19 Damage closer to the frontal lobes is likely to affect catecholamine-mediated brain activity.
The 8-year Framingham study20 examined the risk of developing cerebrovascular events in persons age ≤65 vs those age >65. Subjects age ≤65 with significant depressive symptoms—Center for Epidemiologic Studies Depression scale score >1621—were 4 times more likely to develop stroke or transient ischemic attack compared with the same age group without depression. Another study found a link between depression and stroke risk across the adult age range.22 Mechanisms by which depressive symptoms may predispose to stroke are not fully known, but depression has been shown to affect autonomic function and platelet activation.23
CHD and depression. In the United States, approximately 20% of coronary heart disease (CHD) patients have clinically significant depressive symptoms.24 A history of depression also has been shown to increase the relative risk of developing CHD by >80%.25
The association between depression and CHD is unclear but likely includes:
- direct biological mechanisms such as autonomic dysfunction and dysregulated inflammation
- behavioral factors such as smoking or poor self-care (Table 1).
A recent analysis of 13 cross-sectional studies26 suggests that reduced heart rate variability (HRV) related to autonomic dysfunction may be the link between depression and CHD risk. The studies’ effect sizes were small, however, and their methodologies varied considerably.
C-reactive protein (CRP), interleukin-6, tumor necrosis factor-α (TNF-α), and fibrinogen are inflammatory markers. In a 2-year follow-up study, Frasure-Smith et al27 investigated the relationship between depression and inflammatory markers in 741 patients (602 male) with acute coronary syndrome. Two months after an acute coronary event, depressive symptoms and elevated CRP levels were overlapping risk factors for future cardiac events in men.
Carney et al28 showed that fibrinogen was most associated with altered heart rate variability in depressed CHD patients and proposed deficits in parasympathetic modulation of immunity and coagulation as the cause. In contrast, Whooley et al29 found no association between major depression and inflammatory markers—including CRP, fibrinogen, and interleukin-6—in 984 outpatients with CHD. Differences in assessment scales and sample heterogeneity may have contributed to these disparate findings.
Diabetes and depression. As with CHD, a bidirectional relationship exists between depression and diabetes mellitus, although depression is only a modest risk factor for diabetes.30 Possible explanations include hypercortisolemia and increased inflammation resulting in increased insulin resistance and metabolic syndrome.
Table 1
Shared risk factors for depression and heart disease
| Decreased heart rate variability |
| Vascular inflammation (increased interleukin-6 and C-reactive protein) |
| Endothelial dysfunction |
| Platelet dysfunction |
| Atherosclerosis |
| Dyslipidemia |
| Smoking |
| Source: References 26-29 |
Diagnosis of vascular depression
Vascular depression is characterized by a clinical diagnosis of DSM-IV-TR-defined MDD, dysthymia, or depression not-otherwise-specified, accompanied by:
- evidence of CVD or
- known vascular risk factors (hypertension, diabetes, hyperlipidemia, stroke, heart failure, etc.).
In performing thorough neurologic, neuropsychiatric, and neuropsychological examinations, look for soft neurologic signs with regional weakness, apathy, and executive dysfunction. Useful bedside scales include the clock-drawing test, word list generation, brief dementia screens, and the Apathy Evaluation Scale.31
CT or MRI can provide supportive evidence by demonstrating signs of subcortical or cortical stroke. Neuroimaging studies may not be necessary, however, when depression onset is temporally associated with strong physical evidence of a stroke (such as falling, peripheral muscle weakness, or incontinence).
Treating depression symptoms
When treating vascular depression, clinical goals are to ameliorate affective symptoms, improve quality of life, and help patients perform activities of daily living (Table 2).
Psychosocial interventions. When depression is less than severe, consider psychosocial interventions as first-line treatment. Investigate environmental factors such as financial and marital problems or loneliness in patients’ depressive symptoms, and develop corresponding interventions—such as education, nutrition, exercise, socialization, or pain and stress management. Cognitive rehabilitation training and cognitive-behavioral therapy can reduce cognitive impairment and associated depression.
Antidepressants. A trial of antidepressant therapy is advisable for moderate-to-severe, chronic vascular depression, even though comorbid CVD may diminish the antidepressant response. In elderly patients, start with one-third to one-half the usual adult antidepressant dosage and increase while balancing efficacy and tolerability.
Match the medication’s side-effect profile with the patient’s target symptoms (such as anxiety vs apathy).32 Selective serotonin reuptake inhibitors are probably first-line, but bupropion, venlafaxine, duloxetine, or mirtazapine may be more appropriate for some patients (Table 3).
In PSD, nortriptyline has shown a significantly greater response rate than fluoxetine or placebo in improving anxiety symptoms and recovery of activities of daily living.33 Tricyclic antidepressants’ anticholinergic properties are a safety concern in patients with heart disease, however. In general, avoid agents with substantial anticholinergic effects in elderly patients to minimize the risk of cognitive impairment and other side effects, such as urinary retention or worsening of glaucoma.
Because of the substantial risk of postural hypotension, nonselective monoamine oxidase inhibitors are probably appropriate only for geriatric patients with highly treatment-refractory depression. Dopaminergic agents such as methylphenidate in a relatively moderate dose (such as 5 to 20 mg/d) may improve apathy and social withdrawal, but research into their use in vascular depression is lacking.
Other options. Clinical experience suggests that electroconvulsive therapy (ECT) is effective for patients who do not respond to antidepressants. ECT appears quite safe in older patients, especially if not used in the first 6 months post-stroke. Strategies to reduce the risk of cognitive side effects include:
- 2 rather than 3 weekly treatments
- unilateral or bifrontal rather than bilateral treatments
- frontal lead placement.34
In the only study of transcranial magnetic stimulation (TMS) for geriatric patients with depression (N=92), those with treatment-resistant vascular depression showed higher remission rates with TMS (27.3%) compared with sham TMS (3.5%). Response rates to TMS were negatively correlated with advancing age and positively correlated with higher frontal gray matter volumes.35
Fish oil or vitamin B complex may be used to manage hyperlipidemia or nutritional deficiencies.36 Herbal preparations such as St. John’s wort (Hypericum perforatum) or S-adenosyl-L-methionine (SAMe) have shown some efficacy in adults with MDD, but further study is needed.
Table 2
Clinical management of late-life vascular depression
| Decision point | Assessment/intervention |
|---|---|
| Diagnosis | Apply DSM-IV-TR diagnostic criteria based on results of comprehensive assessment (neuropsychiatric, neuropsychological, structural neuroimaging, vascular and genetic risk factors) |
| Prevention | Identify and treat modifiable risk factors for the development or worsening of cerebrovascular disease, especially in high-risk populations (Table 4) |
| Treatment goals | Target 1: Achieve remission of depressive symptoms, improved cognition and function Target 2: Maintain remission and prevent relapse |
| Managing psychological and behavioral symptoms | Step 1: Consider psychotherapy addressing existing stressors and environmental management in patients with mild-to-moderate depression Step 2: If depression is severe or Step 1 is ineffective, an antidepressant trial* is highly recommended (Table 3); consider ECT or TMS in severe cases |
| *Avoid medications that could worsen cognition or motor functioning, such as tricyclic antidepressants or neuroleptics | |
| ECT: electroconvulsive therapy; TMS: transcranial magnetic stimulation | |
Table 3
Recommended antidepressant dosing
for elderly patients with vascular depression*
| Drug | Starting daily dosage (usual therapeutic range) | Side effect profile (patient characteristics) |
|---|---|---|
| SSRIs | ||
| Escitalopram | 5 mg (10 to 20 mg) | Nausea, headaches, GI upset, insomnia, anxiety |
| Fluoxetine | 10 mg (10 to 60 mg) | |
| Paroxetine | 10 mg (10 to 30 mg) | |
| Sertraline | 25 mg (50 to 150 mg) | |
| Others | ||
| Bupropion | 75 mg (75 to 300 mg) | GI upset, anxiety (may be useful for patients with high apathy) |
| Mirtazapine | 7.5 mg (15 to 45 mg) | Sedation, weight gain (may be useful for patients with severe insomnia or anorexia) |
| Venlafaxine | 37.5 mg (75 to 300 mg) | Nausea, headaches, anxiety, blood pressure elevation, insomnia (may be useful for patients with chronic pain) |
| Duloxetine | 20 mg (30 to 120 mg) | |
| *Avoid medications that could worsen cognition or motor functioning, such as tricyclic antidepressants or neuroleptics | ||
| GI: gastrointestinal; SSRIs: selective serotonin reuptake inhibitors | ||
Treating vascular factors
In addition to treating your patients’ depressive symptoms, collaborate with their primary care physicians to modify physiologic and behavioral factors that increase the risk for vascular injury—such as hypertension, diabetes mellitus, cigarette smoking, and hyperlipidemia. All can be controlled in presymptomatic or mildly symptomatic stages (Table 4).
Anticoagulation. In appropriate patients, anticoagulation can prevent thromboembolic strokes, although risks such as increased hemorrhagic complications must be considered.37 In elderly adults, base treatment decisions on individual risk factors, goals of treatment, and quality-of-life expectancy. In a study of low-dose aspirin (81 mg/d) and low-intensity oral anticoagulation in men at risk of cardiovascular disease, verbal fluency and mental flexibility were significantly better in men taking antithrombotic medications (especially aspirin) than in those taking placebo.38
Antihypertensives and statins. Patients with vascular depression may benefit from calcium channel blockers or angiotensin-converting enzyme (ACE) inhibitors for hypertension and HMG-CoA reductase inhibitors (statins) for hyperlipidemia. Statins seem to decrease the generation of amyloid precursor protein, the neuronal secretion of β-amyloid, and cholesterol synthesis.39 Some epidemiologic studies suggest an association between statin use for cholesterol reduction and reduced prevalence of Alzheimer’s disease and vascular dementia.40
Potential preventive strategies are not without controversy, however:
- Beta blockers and ACE inhibitors have been linked to depression, although the evidence has been conflicting.
- Lipid-lowering therapies and calcium-channel blockers have been linked to an increased risk of suicide.41
- A more recent population-based study did not support an association between an increased risk of suicide and cardiovascular drugs (except perhaps for angiotensin-receptor antagonists).42
Table 4
Preventing vascular causes of late-life depression
| Decision point | Assessment/intervention | Comment |
|---|---|---|
| Primary, secondary prevention of stroke, vascular depression, and cognitive impairment | Identify and treat modifiable risk factors (hypertension, alcohol use, smoking, hyperlipidemia, diabetes mellitus), especially in high-risk patients | Consider as high-risk patients having ≥1 of these features: age >50; male gender; Asian, Hispanic, or African-American heritage; low educational achievement; concurrent vascular risk factors |
| Tertiary prevention of worsened illness in patients with established vascular disease | Intensively treat vascular risk factors | Collaborate with primary care physician to manage arterial hypertension, myocardial infarction, atrial fibrillation, coronary heart disease, diabetes, atherosclerosis, hyperlipidemia, obesity, and smoking |
| Rapidly identify and treat acute stroke to limit ischemic brain changes and promote recovery | ||
| Prevent stroke recurrence by aggressively treating vascular risk factors | Let CVD etiology guide treatment | |
| CVD: cerebrovascular disease | ||
| Source: Adapted from Lavretsky H. Diagnosis and treatment of vascular dementia. Directions in Psychiatry. 2006;26(1):49-68 | ||
Related resources
- Lavretsky H, Chui H. Vascular dementia. In: Agronin ME, Maletta GJ, eds. Principles and practice of geriatric psychiatry. New York, NY: Lippincott, Williams, and Wilkins; 2005: 301-310.
- Baldwin RC, O’Brien J. Vascular basis of late-onset depressive disorder. Br J Psychiatry. 2002;180:157-160.
- Kendler KS, Gardner CO, Fiske A, et al. Major depression and coronary heart disease in the Swedish twin registry. Arch Gen Psychiatry. 2008;66(8):857-863.
Drug brand names
- Bupropion • Wellbutrin
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Methylphenidate • Ritalin, Concerta, others
- Mirtazapine • Remeron
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosures
Dr. Lavretsky receives grant/research support from Forest Research Institute and is a consultant to Forest Laboratories, Myriad Pharmaceuticals, and Accera, Inc.
Dr. Meeks reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgments
This work was supported by National Institute of Health grants R01 MH077650 and R-21 AT003480 (Dr. Lavretsky), the U.S. Department of Health and Human Services, Health Resources and Services Administration (Geriatric Academic Career Award), and the Sam and Rose Stein Institute for Research on Aging (Dr. Meeks).
1. Alexopoulos GS, Meyers BS, Young RC, et al. Clinically defined vascular depression. Am J Psychiatry. 1997;154:562-565.
2. Newberg AR, Davydow DS, Lee HB. Cerebrovascular disease basis of depression: post-stroke depression and vascular depression. Int Rev Psychiatry. 2006;18:433-441.
3. Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997;154:497-501.
4. Krishnan KR, Taylor WD, McQuoid DR, et al. Clinical characteristics of magnetic resonance imaging-defined subcortical ischemic depression. Biol Psychiatry. 2004;5(4):390-397.
5. Alexopoulos GS, Kiosses DN, Klimstra S, et al. Clinical presentation of the "depression-executive dysfunction syndrome" of late life. Am J Geriatr Psychiatry. 2002;10:98-102.
6. Taylor WD, Steffens DC, Krishnan KR. Psychiatric disease in the twenty-first century: the case for subcortical ischemic depression. Biol Psychiatry. 2006;60(12):1299-1303.
7. Alexopoulos GS. The depression-executive dysfunction syndrome of late life: a specific target for D3 receptor agonists? Am J Geriatr Psychiatry. 2001;9:1-8.
8. Alexopoulos GS. Depression in the elderly. Lancet. 2005;365:1961-1970.
9. Sackeim H. Brain structure and function in late-life depression. In: Morihisa JM, ed. Advances in brain imaging. Arlington, VA: American Psychiatric Publishing, Inc.; 2001:83–122.
10. Kumar A, Bilker W, Jin Z, et al. Atrophy and high intensity lesions: complementary neurobiological mechanisms in late-life major depression. Neuropsychopharmacology. 2000;22:264-274.
11. Kumar A, Mintz J, Bilker W, et al. Autonomous neurobiological pathways to late-life major depressive disorder: clinical and pathophysiological implications. Neuropsychopharmacology. 2002;26:229-236.
12. Fujikawa T, Yamawaki S, Fujita Y, et al. [Clinical study of correlation pre-senile, senile depressive state with silent cerebral infarction—MRI findings and its distribution]. Seishin Shinkeigaku Zasshi. 1992;94(9):851-863.
13. Kumar A, Cummings J. Depression in neurodegenerative disorders and related conditions in Alzheimer’s disease and related conditions. In: Gothier S, Cummings J, eds. Alzheimer’s disease and related disorders. London, UK: Martin Dunitz; 2001:123-141.
14. Erkinjuntti T, Gao F, Lee DH, et al. Lack of difference in brain hyperintensities between patients with early Alzheimer’s disease and control subjects. Arch Neurol. 1994;51:260-268.
15. Zubenko G, Sullivan P, Nelson J, et al. Brain imaging abnormalities in mental disorders of late life. Arch Neurol. 1990;47:1107-1111.
16. Birkett DP. The psychiatry of stroke. Arlington, VA: American Psychiatric Publishing, Inc.; 1996.
17. Robinson PG, Starkstein SE. Current research in affective disorders following stroke. J Neuropsychiatry Clin Neurosci. 1990;2:1-14.
18. Mayberg HS, Parikh RM, Morris PL, et al. Spontaneous remission of post-stroke depression and temporal changes in cortical S2-serotonin receptors. J Neuropsychiatry Clin Neurosci. 1991;3:80-83.
19. Ramasubbu R, Flint A, Brown G, et al. A neuroendocrine study of serotonin function in depressed stroke patients compared to nondepressed stroke patients and healthy controls. J Affect Disord. 1999;52:121-133.
20. Salaycik KJ, Kelly-Hayes M, Beiser A, et al. Depressive symptoms and risk of stroke. The Framingham study. Stroke. 2007;38:16-21.
21. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measurement. 1977;1:385-401.
22. Jonas BS, Mussolino ME. Symptoms of depression as a prospective risk factor for stroke. Psychosom Med. 2000;62:463-471.
23. Whyte EM, Pollock BG, Wagner WR, et al. Influence of serotonin-transporter-linked promoter region polymorphism on platelet activation in geriatric depression. Am J Psychiatry. 2001;158(12):2074-2076.
24. Amin AA, Jones AM, Nugnet K, et al. The prevalence of unrecognized depression in patients with acute coronary syndrome. Am Heart J. 2006;152:928-934.
25. Nicholson A, Kuper H, Hemingway H. Depression as an aetiolgic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27:2763-2774.
26. Rottenberg J. Cardiac vagal control in depression: a critical analysis. Biol Psychol. 2007;74:200-211.
27. Frasure-Smith N, Lesperance F, Irwin MR, et al. Depression, C-reactive protein and two-year major adverse cardiac events in men after acute coronary syndromes. Biol Psychiatry. 2007;62:302-308.
28. Carney RM, Freedland KE, Stein PK, et al. Heart rate variability and markers of inflammation and coagulation in depressed patients with coronary heart disease. J Psychosomatic Res. 2007;62:463-467.
29. Whooley MA, Caska CM, Hendrickson BE, et al. Depression and inflammation in patients with coronary heart disease: findings from the Heart and Soul Study. Biol Psychiatry. 2007;62:314-320.
30. Hill Golden S, Lazo M, Carnethon M, et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008;299(23):2751-2759.
31. Marin RS. Differential diagnosis of apathy and related disorders of diminished motivation. Psychiatric Annals. 1997;27:30-33.
32. Roose S. Treatment of depression in patients with heart disease. Biol Psychiatry. 2003;54:262-268.
33. Robinson RG, Schultz SK, Castillo C, et al. Nortriptyline versus fluoxetine in the treatment of depression and in short-term recovery after stroke: a placebo-controlled, double-blind study. Am J Psychiatry. 2000;157(3):351-359.
34. Katz IR. Diagnosis and treatment of depression in patients with Alzheimer’s disease and other dementias. J Clin Psychiatry. 1998;59(9):38-44.
35. Jorge RE, Moser DJ, Acion L, et al. Treatment of vascular depression using repetitive transcranial magnetic stimulation. Arch Gen Psychiatry. 2008;65(3):268-276.
36. Lavretsky H. The use of complementary and alternative medicine for treatment of late-life neuropsychiatric disorders. J Aging Health. 2009;5(1):61-78.
37. Pantoni L, Inzitari D. New clinical relevance of leukoaraiosis. European force on age-related white-matter changes. Stroke. 1998;29(2):543.-
38. Richards M, Meade TW, Peart S, et al. Is there any evidence for a protective effect of antithrombotic medication on cognitive function in men at risk of cardiovascular disease? Some preliminary findings. J Neurol Neurosurg Psychiatry. 1997;62(3):269-272.
39. Lutjohann D, Papassotiropoulos A, Bjorkhem I, et al. Plasma 24S-hydroxycholesterol (cerebrosterol) is increased in Alzheimer and vascular demented patients. J Lipid Res. 2000;41(2):195-198.
40. Jick H, Zornberg GL, Jick SS, et al. Statins and the risk of dementia. Lancet. 2000;356(9242):1627-1631.
41. Yang CC, Jick SS, Jick H. Lipid-lowering drugs and the risk of depression and suicidal behavior. Arch Intern Med. 2003;163(16):1926-1932.
42. Callréus T, Agerskov Andersen U, Hallas J, et al. Cardiovascular drugs and the risk of suicide: a nested case-control study. Eur J Clin Pharmacol. 2007;63(6):591-596.
1. Alexopoulos GS, Meyers BS, Young RC, et al. Clinically defined vascular depression. Am J Psychiatry. 1997;154:562-565.
2. Newberg AR, Davydow DS, Lee HB. Cerebrovascular disease basis of depression: post-stroke depression and vascular depression. Int Rev Psychiatry. 2006;18:433-441.
3. Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997;154:497-501.
4. Krishnan KR, Taylor WD, McQuoid DR, et al. Clinical characteristics of magnetic resonance imaging-defined subcortical ischemic depression. Biol Psychiatry. 2004;5(4):390-397.
5. Alexopoulos GS, Kiosses DN, Klimstra S, et al. Clinical presentation of the "depression-executive dysfunction syndrome" of late life. Am J Geriatr Psychiatry. 2002;10:98-102.
6. Taylor WD, Steffens DC, Krishnan KR. Psychiatric disease in the twenty-first century: the case for subcortical ischemic depression. Biol Psychiatry. 2006;60(12):1299-1303.
7. Alexopoulos GS. The depression-executive dysfunction syndrome of late life: a specific target for D3 receptor agonists? Am J Geriatr Psychiatry. 2001;9:1-8.
8. Alexopoulos GS. Depression in the elderly. Lancet. 2005;365:1961-1970.
9. Sackeim H. Brain structure and function in late-life depression. In: Morihisa JM, ed. Advances in brain imaging. Arlington, VA: American Psychiatric Publishing, Inc.; 2001:83–122.
10. Kumar A, Bilker W, Jin Z, et al. Atrophy and high intensity lesions: complementary neurobiological mechanisms in late-life major depression. Neuropsychopharmacology. 2000;22:264-274.
11. Kumar A, Mintz J, Bilker W, et al. Autonomous neurobiological pathways to late-life major depressive disorder: clinical and pathophysiological implications. Neuropsychopharmacology. 2002;26:229-236.
12. Fujikawa T, Yamawaki S, Fujita Y, et al. [Clinical study of correlation pre-senile, senile depressive state with silent cerebral infarction—MRI findings and its distribution]. Seishin Shinkeigaku Zasshi. 1992;94(9):851-863.
13. Kumar A, Cummings J. Depression in neurodegenerative disorders and related conditions in Alzheimer’s disease and related conditions. In: Gothier S, Cummings J, eds. Alzheimer’s disease and related disorders. London, UK: Martin Dunitz; 2001:123-141.
14. Erkinjuntti T, Gao F, Lee DH, et al. Lack of difference in brain hyperintensities between patients with early Alzheimer’s disease and control subjects. Arch Neurol. 1994;51:260-268.
15. Zubenko G, Sullivan P, Nelson J, et al. Brain imaging abnormalities in mental disorders of late life. Arch Neurol. 1990;47:1107-1111.
16. Birkett DP. The psychiatry of stroke. Arlington, VA: American Psychiatric Publishing, Inc.; 1996.
17. Robinson PG, Starkstein SE. Current research in affective disorders following stroke. J Neuropsychiatry Clin Neurosci. 1990;2:1-14.
18. Mayberg HS, Parikh RM, Morris PL, et al. Spontaneous remission of post-stroke depression and temporal changes in cortical S2-serotonin receptors. J Neuropsychiatry Clin Neurosci. 1991;3:80-83.
19. Ramasubbu R, Flint A, Brown G, et al. A neuroendocrine study of serotonin function in depressed stroke patients compared to nondepressed stroke patients and healthy controls. J Affect Disord. 1999;52:121-133.
20. Salaycik KJ, Kelly-Hayes M, Beiser A, et al. Depressive symptoms and risk of stroke. The Framingham study. Stroke. 2007;38:16-21.
21. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measurement. 1977;1:385-401.
22. Jonas BS, Mussolino ME. Symptoms of depression as a prospective risk factor for stroke. Psychosom Med. 2000;62:463-471.
23. Whyte EM, Pollock BG, Wagner WR, et al. Influence of serotonin-transporter-linked promoter region polymorphism on platelet activation in geriatric depression. Am J Psychiatry. 2001;158(12):2074-2076.
24. Amin AA, Jones AM, Nugnet K, et al. The prevalence of unrecognized depression in patients with acute coronary syndrome. Am Heart J. 2006;152:928-934.
25. Nicholson A, Kuper H, Hemingway H. Depression as an aetiolgic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27:2763-2774.
26. Rottenberg J. Cardiac vagal control in depression: a critical analysis. Biol Psychol. 2007;74:200-211.
27. Frasure-Smith N, Lesperance F, Irwin MR, et al. Depression, C-reactive protein and two-year major adverse cardiac events in men after acute coronary syndromes. Biol Psychiatry. 2007;62:302-308.
28. Carney RM, Freedland KE, Stein PK, et al. Heart rate variability and markers of inflammation and coagulation in depressed patients with coronary heart disease. J Psychosomatic Res. 2007;62:463-467.
29. Whooley MA, Caska CM, Hendrickson BE, et al. Depression and inflammation in patients with coronary heart disease: findings from the Heart and Soul Study. Biol Psychiatry. 2007;62:314-320.
30. Hill Golden S, Lazo M, Carnethon M, et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008;299(23):2751-2759.
31. Marin RS. Differential diagnosis of apathy and related disorders of diminished motivation. Psychiatric Annals. 1997;27:30-33.
32. Roose S. Treatment of depression in patients with heart disease. Biol Psychiatry. 2003;54:262-268.
33. Robinson RG, Schultz SK, Castillo C, et al. Nortriptyline versus fluoxetine in the treatment of depression and in short-term recovery after stroke: a placebo-controlled, double-blind study. Am J Psychiatry. 2000;157(3):351-359.
34. Katz IR. Diagnosis and treatment of depression in patients with Alzheimer’s disease and other dementias. J Clin Psychiatry. 1998;59(9):38-44.
35. Jorge RE, Moser DJ, Acion L, et al. Treatment of vascular depression using repetitive transcranial magnetic stimulation. Arch Gen Psychiatry. 2008;65(3):268-276.
36. Lavretsky H. The use of complementary and alternative medicine for treatment of late-life neuropsychiatric disorders. J Aging Health. 2009;5(1):61-78.
37. Pantoni L, Inzitari D. New clinical relevance of leukoaraiosis. European force on age-related white-matter changes. Stroke. 1998;29(2):543.-
38. Richards M, Meade TW, Peart S, et al. Is there any evidence for a protective effect of antithrombotic medication on cognitive function in men at risk of cardiovascular disease? Some preliminary findings. J Neurol Neurosurg Psychiatry. 1997;62(3):269-272.
39. Lutjohann D, Papassotiropoulos A, Bjorkhem I, et al. Plasma 24S-hydroxycholesterol (cerebrosterol) is increased in Alzheimer and vascular demented patients. J Lipid Res. 2000;41(2):195-198.
40. Jick H, Zornberg GL, Jick SS, et al. Statins and the risk of dementia. Lancet. 2000;356(9242):1627-1631.
41. Yang CC, Jick SS, Jick H. Lipid-lowering drugs and the risk of depression and suicidal behavior. Arch Intern Med. 2003;163(16):1926-1932.
42. Callréus T, Agerskov Andersen U, Hallas J, et al. Cardiovascular drugs and the risk of suicide: a nested case-control study. Eur J Clin Pharmacol. 2007;63(6):591-596.