User login
To the Editor:
A 41-year-old man presented to our clinic with concerns of ulceration, skin thickening, and loss of eyebrows. The ulceration developed on the knees approximately 1 year prior to presentation; however, he reported loss of sensation in the knees after an accident involving his back 7 years prior. Loss of the eyebrows and skin thickening on the abdomen occurred within the same time frame of the knee ulceration. He also experienced generalized loss of skin sensation. The patient had a history of hunting and fishing but denied any contact with an armadillo.
On clinical examination thin yellow plaques were present on both cheeks, as well as typical leonine facies, a saddle nose deformity, loss of bilateral eyebrows, slate gray color on the face, and infiltrated plaques with telangiectases on the nose. Subcutaneous nodules developed on the arms, chest, and abdomen. He also had angulated ulcers with granulation tissue on the knees.
Laboratory data collected included a complete metabolic panel and complete blood cell count with differential. The complete metabolic panel was within reference range excluding a moderately high glucose level of 114 mg/dL (reference range, 70–100 mg/dL) and a low creatinine level of 0.7 mg/dL (reference range, 0.5–1.2 mg/dL). The complete blood cell count showed mild anemia with a hemoglobin to hematocrit ratio of 11.0 g/dL (reference range, 12.0–16.0 g/dL) to 32.6% (reference range, 37.0%–48.5%) and a nonspecific elevated mean corpuscular volume of 94.4 fL (reference range, 82–95 fL). The differential revealed a low percentage of lymphocytes (11.4% [reference range, 21%–44%]) and a high percentage of granulocytes (85.6% [reference range, 38%–73%]).
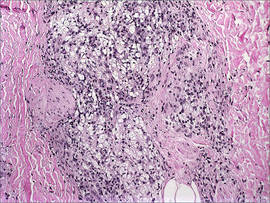
A total of 3 biopsies were taken from the abdomen, upper chest, and right wrist. Histology showed a diffuse infiltrate of epithelioid histiocytes forming ill-defined nodules in the dermis, extending into the represented subcutis (Figure 1). Many of the histiocytes had prominent foamy cytoplasms (Figure 2). Scattered lymphocytes and plasma cells also were present. Fite-modified acid-fast staining was performed on all of the biopsies and yielded large numbers of acid-fast bacilli dispersed throughout the specimens (Figure 3), which confirmed the diagnosis of lepromatous leprosy. The patient was referred to the National Hansen’s Disease (Leprosy) Program in Baton Rouge, Louisiana. The physician team started him on high-dose clofazimine, dapsone, and rifampin. He also was diagnosed with erythema nodosum leprosum for which he was given thalidomide.
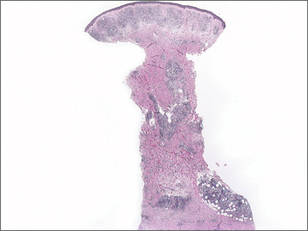
Hansen disease, also known as leprosy, is a chronic inflammatory disease caused by Mycobacterium leprae and Mycobacterium lepromatosis. The mode of transmission is postulated to be through respiratory droplets and nasal secretions. Armadillo exposure, poor sanitary conditions, endemic location, and infected family members are considered risk factors for the development of leprosy. The incubation period is approximately 5 years, with symptoms arising up to 20 years after acquisition of the bacillus. Replication tends to begin in cooler regions of the body. The disease spectrum ranges from lepromatous to tuberculoid.1-3 Lepromatous leprosy consists of an uncontrolled high-titer replication of the mycobacteria, resulting mostly in cutaneous changes and late nerve damage. Histologically, foamy or undifferentiated macrophages predominate the field; they often include many bacilli that can be demonstrated by the modified Fite-Faraco method, a clinically relevant carbol-fuchsin stain.3-5 Acid-fast organisms stain red. A Ziehl-Neelsen stain and Harada modified Allochrome method also may be used.
The damage to peripheral nerves and the cutis can cause substantial loss of function and lead to painless ulceration of the skin. Changes in the skin may include nodules, hypopigmented macules, sores, and skin thickening. Leonine facies and disfigurement also may be observed. A type III hypersensitivity reaction may occur, resulting in erythema nodosum leprosum, a type II lepra reaction characterized by elevated tumor necrosis factor α.6
Treatment requires multidrug therapy involving dapsone, rifampin, and clofazimine.4 The World Health Organization is targeted at eliminating the disease and providing free multidrug therapy to patients.7

- Leprosy (Hansen’s disease): Technical Information. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/nczved/divisions/dfbmd/diseases/hansens_disease/technical.html/. Updated May 17, 2010. Accessed July 2, 2014.
- Esfandbod M. Images in clinical medicine. tuberculoid leprosy. N Engl J Med. 2011;364:1657.
- Robati RM, Rahimi H, Asadi-Kani Z, et al. Photoclinic. lepromatous leprosy. Arch Iran Med. 2010;13:443-444.
- Moschella SL. An update on the diagnosis and treatment of leprosy. J Am Acad Dermatol. 2004;51:417-426.
- Wolff K, Johnson RA, Saavedra AP, eds. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 7th ed. New York, NY: McGraw-Hill; 2013.
- Walker SL, Lockwood DN. Leprosy. Clin Dermatol. 2007;25:165-172.
- World Health Organization Committee on Leprosy. WHO Expert Committee on Leprosy: Seventh Report. Geneva, Switzerland: World Health Organization Committee on Leprosy; 1997.
To the Editor:
A 41-year-old man presented to our clinic with concerns of ulceration, skin thickening, and loss of eyebrows. The ulceration developed on the knees approximately 1 year prior to presentation; however, he reported loss of sensation in the knees after an accident involving his back 7 years prior. Loss of the eyebrows and skin thickening on the abdomen occurred within the same time frame of the knee ulceration. He also experienced generalized loss of skin sensation. The patient had a history of hunting and fishing but denied any contact with an armadillo.
On clinical examination thin yellow plaques were present on both cheeks, as well as typical leonine facies, a saddle nose deformity, loss of bilateral eyebrows, slate gray color on the face, and infiltrated plaques with telangiectases on the nose. Subcutaneous nodules developed on the arms, chest, and abdomen. He also had angulated ulcers with granulation tissue on the knees.
Laboratory data collected included a complete metabolic panel and complete blood cell count with differential. The complete metabolic panel was within reference range excluding a moderately high glucose level of 114 mg/dL (reference range, 70–100 mg/dL) and a low creatinine level of 0.7 mg/dL (reference range, 0.5–1.2 mg/dL). The complete blood cell count showed mild anemia with a hemoglobin to hematocrit ratio of 11.0 g/dL (reference range, 12.0–16.0 g/dL) to 32.6% (reference range, 37.0%–48.5%) and a nonspecific elevated mean corpuscular volume of 94.4 fL (reference range, 82–95 fL). The differential revealed a low percentage of lymphocytes (11.4% [reference range, 21%–44%]) and a high percentage of granulocytes (85.6% [reference range, 38%–73%]).
A total of 3 biopsies were taken from the abdomen, upper chest, and right wrist. Histology showed a diffuse infiltrate of epithelioid histiocytes forming ill-defined nodules in the dermis, extending into the represented subcutis (Figure 1). Many of the histiocytes had prominent foamy cytoplasms (Figure 2). Scattered lymphocytes and plasma cells also were present. Fite-modified acid-fast staining was performed on all of the biopsies and yielded large numbers of acid-fast bacilli dispersed throughout the specimens (Figure 3), which confirmed the diagnosis of lepromatous leprosy. The patient was referred to the National Hansen’s Disease (Leprosy) Program in Baton Rouge, Louisiana. The physician team started him on high-dose clofazimine, dapsone, and rifampin. He also was diagnosed with erythema nodosum leprosum for which he was given thalidomide.

Hansen disease, also known as leprosy, is a chronic inflammatory disease caused by Mycobacterium leprae and Mycobacterium lepromatosis. The mode of transmission is postulated to be through respiratory droplets and nasal secretions. Armadillo exposure, poor sanitary conditions, endemic location, and infected family members are considered risk factors for the development of leprosy. The incubation period is approximately 5 years, with symptoms arising up to 20 years after acquisition of the bacillus. Replication tends to begin in cooler regions of the body. The disease spectrum ranges from lepromatous to tuberculoid.1-3 Lepromatous leprosy consists of an uncontrolled high-titer replication of the mycobacteria, resulting mostly in cutaneous changes and late nerve damage. Histologically, foamy or undifferentiated macrophages predominate the field; they often include many bacilli that can be demonstrated by the modified Fite-Faraco method, a clinically relevant carbol-fuchsin stain.3-5 Acid-fast organisms stain red. A Ziehl-Neelsen stain and Harada modified Allochrome method also may be used.

The damage to peripheral nerves and the cutis can cause substantial loss of function and lead to painless ulceration of the skin. Changes in the skin may include nodules, hypopigmented macules, sores, and skin thickening. Leonine facies and disfigurement also may be observed. A type III hypersensitivity reaction may occur, resulting in erythema nodosum leprosum, a type II lepra reaction characterized by elevated tumor necrosis factor α.6
Treatment requires multidrug therapy involving dapsone, rifampin, and clofazimine.4 The World Health Organization is targeted at eliminating the disease and providing free multidrug therapy to patients.7

To the Editor:
A 41-year-old man presented to our clinic with concerns of ulceration, skin thickening, and loss of eyebrows. The ulceration developed on the knees approximately 1 year prior to presentation; however, he reported loss of sensation in the knees after an accident involving his back 7 years prior. Loss of the eyebrows and skin thickening on the abdomen occurred within the same time frame of the knee ulceration. He also experienced generalized loss of skin sensation. The patient had a history of hunting and fishing but denied any contact with an armadillo.
On clinical examination thin yellow plaques were present on both cheeks, as well as typical leonine facies, a saddle nose deformity, loss of bilateral eyebrows, slate gray color on the face, and infiltrated plaques with telangiectases on the nose. Subcutaneous nodules developed on the arms, chest, and abdomen. He also had angulated ulcers with granulation tissue on the knees.
Laboratory data collected included a complete metabolic panel and complete blood cell count with differential. The complete metabolic panel was within reference range excluding a moderately high glucose level of 114 mg/dL (reference range, 70–100 mg/dL) and a low creatinine level of 0.7 mg/dL (reference range, 0.5–1.2 mg/dL). The complete blood cell count showed mild anemia with a hemoglobin to hematocrit ratio of 11.0 g/dL (reference range, 12.0–16.0 g/dL) to 32.6% (reference range, 37.0%–48.5%) and a nonspecific elevated mean corpuscular volume of 94.4 fL (reference range, 82–95 fL). The differential revealed a low percentage of lymphocytes (11.4% [reference range, 21%–44%]) and a high percentage of granulocytes (85.6% [reference range, 38%–73%]).
A total of 3 biopsies were taken from the abdomen, upper chest, and right wrist. Histology showed a diffuse infiltrate of epithelioid histiocytes forming ill-defined nodules in the dermis, extending into the represented subcutis (Figure 1). Many of the histiocytes had prominent foamy cytoplasms (Figure 2). Scattered lymphocytes and plasma cells also were present. Fite-modified acid-fast staining was performed on all of the biopsies and yielded large numbers of acid-fast bacilli dispersed throughout the specimens (Figure 3), which confirmed the diagnosis of lepromatous leprosy. The patient was referred to the National Hansen’s Disease (Leprosy) Program in Baton Rouge, Louisiana. The physician team started him on high-dose clofazimine, dapsone, and rifampin. He also was diagnosed with erythema nodosum leprosum for which he was given thalidomide.

Hansen disease, also known as leprosy, is a chronic inflammatory disease caused by Mycobacterium leprae and Mycobacterium lepromatosis. The mode of transmission is postulated to be through respiratory droplets and nasal secretions. Armadillo exposure, poor sanitary conditions, endemic location, and infected family members are considered risk factors for the development of leprosy. The incubation period is approximately 5 years, with symptoms arising up to 20 years after acquisition of the bacillus. Replication tends to begin in cooler regions of the body. The disease spectrum ranges from lepromatous to tuberculoid.1-3 Lepromatous leprosy consists of an uncontrolled high-titer replication of the mycobacteria, resulting mostly in cutaneous changes and late nerve damage. Histologically, foamy or undifferentiated macrophages predominate the field; they often include many bacilli that can be demonstrated by the modified Fite-Faraco method, a clinically relevant carbol-fuchsin stain.3-5 Acid-fast organisms stain red. A Ziehl-Neelsen stain and Harada modified Allochrome method also may be used.

The damage to peripheral nerves and the cutis can cause substantial loss of function and lead to painless ulceration of the skin. Changes in the skin may include nodules, hypopigmented macules, sores, and skin thickening. Leonine facies and disfigurement also may be observed. A type III hypersensitivity reaction may occur, resulting in erythema nodosum leprosum, a type II lepra reaction characterized by elevated tumor necrosis factor α.6
Treatment requires multidrug therapy involving dapsone, rifampin, and clofazimine.4 The World Health Organization is targeted at eliminating the disease and providing free multidrug therapy to patients.7

- Leprosy (Hansen’s disease): Technical Information. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/nczved/divisions/dfbmd/diseases/hansens_disease/technical.html/. Updated May 17, 2010. Accessed July 2, 2014.
- Esfandbod M. Images in clinical medicine. tuberculoid leprosy. N Engl J Med. 2011;364:1657.
- Robati RM, Rahimi H, Asadi-Kani Z, et al. Photoclinic. lepromatous leprosy. Arch Iran Med. 2010;13:443-444.
- Moschella SL. An update on the diagnosis and treatment of leprosy. J Am Acad Dermatol. 2004;51:417-426.
- Wolff K, Johnson RA, Saavedra AP, eds. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 7th ed. New York, NY: McGraw-Hill; 2013.
- Walker SL, Lockwood DN. Leprosy. Clin Dermatol. 2007;25:165-172.
- World Health Organization Committee on Leprosy. WHO Expert Committee on Leprosy: Seventh Report. Geneva, Switzerland: World Health Organization Committee on Leprosy; 1997.
- Leprosy (Hansen’s disease): Technical Information. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/nczved/divisions/dfbmd/diseases/hansens_disease/technical.html/. Updated May 17, 2010. Accessed July 2, 2014.
- Esfandbod M. Images in clinical medicine. tuberculoid leprosy. N Engl J Med. 2011;364:1657.
- Robati RM, Rahimi H, Asadi-Kani Z, et al. Photoclinic. lepromatous leprosy. Arch Iran Med. 2010;13:443-444.
- Moschella SL. An update on the diagnosis and treatment of leprosy. J Am Acad Dermatol. 2004;51:417-426.
- Wolff K, Johnson RA, Saavedra AP, eds. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 7th ed. New York, NY: McGraw-Hill; 2013.
- Walker SL, Lockwood DN. Leprosy. Clin Dermatol. 2007;25:165-172.
- World Health Organization Committee on Leprosy. WHO Expert Committee on Leprosy: Seventh Report. Geneva, Switzerland: World Health Organization Committee on Leprosy; 1997.