User login
In May 2020, the US Food and Drug Administration (FDA) approved the first 2 targeted treatments for prostate cancer, specifically, the poly-(adenosine diphosphate-ribose) polymerase (PARP) inhibitors rucaparib and olaparib.1,2 For these medications to work, the tumor must have a homologous recombination deficiency (HRD), which is a form of DNA repair deficiency. The PARP pathway is important for DNA repair, and PARP inhibition leads to “synthetic lethality” in cancer cells that already are deficient in DNA repair mechanisms.3 Now, there is evidence that patients with prostate cancer who have HRD tumors and receive PARP inhibitors live longer when compared with those who receive standard of care options.4 These findings offer hope for patients with prostate cancer. They also demonstrate the process and potential benefits of precision oncology efforts; namely, targeted treatments for specific tumor types in cancer patients.
This article discusses the challenges and opportunities of precision oncology for US Department of Veterans Affairs (VA) Veterans Health Administration (VHA). First, the article will discuss working with relatively rare mutations. Second, the article will examine how the trials of olaparib and rucaparib illuminate the VHA contribution to research on new therapies for patients with cancer. Finally, the article will explore the ways in which VHA is becoming a major national contributor in drug discovery and approval of precision medications.
Precision Oncology
Despite advances in screening and treatment, an estimated 600,000 people in the US will die of cancer in 2020.5 Meaningful advances in cancer care depend on both laboratory and clinical research. This combination, known as translational research, takes discoveries in the laboratory and applies them to patients and vice versa. Successful translational research requires many components. These include talented scientists to form hypotheses and perform the work; money for supplies and equipment; platforms for timely dissemination of knowledge; well-trained clinicians to treat patients and lead research teams; and patients to participate in clinical trials. In precision oncology, the ability to find patients for the trials can be daunting, particularly in cases where the frequency of the mutation of interest is low.
During the 20th century, with few exceptions, physicians caring for patients with cancer had blunt instruments at their disposal. Surgery and radiation could lead to survival if the cancer was caught early enough. Systemic therapies, such as chemotherapy, rarely cured but could prolong life in some patients. However, chemotherapy is imprecise and targets any cell growing rapidly, including blood, hair, and gastrointestinal tract cells, which often leads to adverse effects. Sometimes complications from chemotherapy may shorten a person’s life, and certainly the quality of life during and after these treatments could be diminished. The improvements in cancer care occurred more rapidly once scientists had the tools to learn about individual tumors.
In the summer of 2000, researchers announced that the human genome had been sequenced.6 The genome (ie, DNA) consists of introns and exons that form a map for human development. Exons can be converted to proteins that carry out specific actions, such as helping in cell growth, cell death, or DNA repair. Solving the human genome itself did not lead directly to cures, but it did represent a huge advance in medical research. As time passed, sequencing genomes became more affordable, and sequencing just the exome alone was even cheaper.7 Treatments for cancer began to expand with the help of these tools, but questions as to the true benefit of targeted therapy also grew.8
Physicians and scientists have amassed more information about cancer cells and have applied this knowledge to active drug development. In 2001, the FDA approved the first targeted therapy, imatinib, for the treatment of chronic myelogenous leukemia (CML). This rapidly improved patient survival through targeting the mutated protein that leads to CML, rather than just aiming for rapidly dividing cells.9 Those mutations for which there is a drug to target, such as the BCR-ABL translocation in CML, are called actionable mutations.
Precision Oncology Program for Prostate Cancer
In 2016, the VA and the Prostate Cancer Foundation (PCF) established the Precision Oncology Program for Prostate Cancer (POPCaP) Centers of Excellence (COE). This partnership was formed to accelerate treatment and cure for veterans with prostate cancer. The VA Greater Los Angeles Healthcare System in California and VA Puget Sound Health Care System in Washington led this effort, and their principal investigators continue to co-lead POPCaP. Since its inception, 9 additional funded POPCaP COEs have joined, each with a mandate to sequence the tumors of men with metastatic prostate cancer.
The more that is learned about a tumor, the more likely it is that researchers can find mutations that are that tumor’s Achilles heel and defeat it. In fact, many drugs that can target mutations are already available. For example, BRCA2 is an actionable mutation that can be exploited by knocking out another key DNA repair mechanism in the cell, PARP. Today, the effort of sequencing has led to a rich database of mutations present in men with metastatic prostate cancer.
Although there are many targeted therapies, most have not been studied formally in prostate cancer. Occasionally, clinicians treating patients will use these drugs in an unapproved way, hoping that there will be anticancer activity. It is difficult to estimate the likelihood of success with a drug in this situation, and the safety profile may not be well described in that setting. Treatment decisions for incurable cancers must be made knowing the risks and benefits. This helps in shared decision making between the clinician and patient and informs choices concerning which laboratory tests to order and how often to see the patient. However, treatment decisions are sometimes made with the hope of activity when a cancer is known to be incurable. Very little data, which are critical to determine whether this helps or hurts patients, support this approach.
Some data suggest that sequencing and giving a drug for an actionable mutation may lead to better outcomes for patients. Sequencing of pancreatic tumors by Pishvaian and colleagues revealed that 282 of 1,082 (26%) samples harbored actionable mutations.10 Those patients who received a drug that targeted their actionable mutation (n = 46; 24%) lived longer when compared with those who had an actionable mutation but did not receive a drug that targeted it (hazard ratio [HR] 0.42 [95% CI, 0.26-0.68; P = .0004]). Additionally, those who received therapy for an actionable mutation lived longer when compared with those who did not have an actionable mutation (HR 0.34 [95% CI, 0.22-0.53; P < .001]). While this finding is intriguing, it does not mean that treating actionable mutations outside of a clinical trial should be done. To this end, VA established Prostate cancer Analysis for Therapy CHoice (PATCH) as a clinical trials network within POPCaP.
Prostate Cancer Analysis
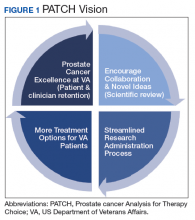
The overall PATCH vision is designed for clinical care and research work to together toward improved care for those with prostate cancer (Figure 1). The resources necessary for successful translational research are substantial, and PATCH aims to streamline those resources. PATCH will support innovative, precision-based clinical research at the POPCaP COEs through its 5 arms.
Arm 1. Dedicated personnel ensure veteran access to trials in PATCH by giving patients and providers accurate information about available trial options; aiding veterans in traveling from home VA to a POPCaP COE for participation on a study; and maintaining the Committee for Veteran Participation in PATCH, where veterans will be represented and asked to provide input into the PATCH process.
Arm 2. Coordinators at the coordinating COE in Portland, Orgeon, train investigators and study staff at the local POPCaP COEs to ensure research can be performed in a safe and responsible way.
Arm 3. Personnel experienced in conducting clinical trials liaise with investigators at VA Central Institutional Review Board, monitor trials, build databases for appropriate and efficient data collection, and manage high-risk studies conducted under an Investigational New Drug application. This group works closely with biostatisticians to choose appropriate trial designs, estimate numbers of patients needed, and interpret data once they are collected.
Arm 4. Protocol development and data dissemination is coordinated by a group to assist investigators in drafting protocols and reviewing abstracts and manuscripts.
Arm 5. A core group manages contracts and budgets, as well as relationships between VA and industry, where funding and drugs may be obtained.
Perhaps most importantly, PATCH leverages the genetic data collected by POPCaP COEs to find patients for clinical trials. For example, the trials that examined olaparib and rucaparib assumed that the prevalence of HRD was about 25% in men with advanced prostate cancer.11 As these trials began enrollment, however, researchers discovered that the prevalence was < 20%. In fact, the study of olaparib screened 4,425 patients at 206 sites in 20 countries to identify 778 (18% of screened) patients with HRD.4 With widespread sequencing within VA, it could be possible to identify a substantial number of patients who are already known to have the mutation of interest (Figure 2).
Clinical Trials
There are currently 2 clinical trials in PATCH; 4 additional trials await funding decisions, and more trials are in the concept stage. BRACeD (NCT04038502) is a phase 2 trial examining platinum and taxane chemotherapy in tumors with HRD (specifically, BRCA1, BRCA2, and PALB2). About 15% to 20% of men with advanced prostate cancer will have a DNA repair defect in the tumor that could make them eligible for this study. The primary endpoint is progression-free survival.
A second study, CHOMP (NCT04104893), is a phase 2 trial examining the efficacy of immunotherapy (PD-1 inhibition) in tumors having mismatch repair deficiency or CDK12-/-. Each of those is found in about 7% of men with metastatic prostate cancer, and full accrual of a trial with rare mutations could take 5 to 10 years without a systematic approach of sequencing and identifying potential participants. The primary endpoint is a composite of radiographic response by iRECIST (immune response evaluation criteria in solid tumors), progression-free survival at 6 months and prostate specific antigen reduction by ≥ 50% in ≤ 12 weeks. With 11 POPCaP COEs sequencing the tumors of every man with metastatic prostate cancer, identifying men with the appropriate mutation is possible. PATCH will aid the sites in recruitment through outreach and coordination of travel.
Industry Partnerships
PATCH depends upon pharmaceutical industry partners, as clinical trials of even 40 patients can require significant funding and trial resources to operate. Furthermore, many drugs of interest are not available outside of a clinical trial, and partnerships enable VA researchers to access these medications. PATCH also benefits greatly from foundation partners, such as the PCF, which has made POPCaP possible and will continue to connect talented researchers with VA resources. Finally, access to other publicly available research funds, such as those from VA Office of Research and Development, National Institutes of Health, and US Department of Defense (DoD) Congressionally Directed Research Program are needed for trials.
Funding for these trials remains limited despite public health and broader interests in addressing important questions. Accelerated accrual through PATCH may be an attractive partnership opportunity for companies, foundations and government funding agencies to support the PATCH efforts.
Both POPCaP and PATCH highlight the potential promise of precision oncology within the nation’s largest integrated health care system. The VHA patient population enables prostate cancer researchers to serve an important early target. It also provides a foundational platform for a broader set of activities. These include a tailored approach to identifying tumor profiles and other patient characteristics that may help to elevate standard of care for other common cancers including ones affecting the lungs and/or head and neck.
To this end, VA has been working with the National Cancer Institute (NCI) and DoD to establish a national infrastructure for precision oncology across multiple cancer types.12 In addition to clinical capabilities and the ability to run clinical trials that can accrue sufficient patients to answer key questions, we have developed capabilities for data collection and sharing, and analytical tools to support a learning health care system approach as a core element to precision oncology.
Besides having a research-specific context, such informatics and information technology systems enable clinicians to obtain and apply decision-making data rapidly for a specific patient and cancer type. These systems take particular advantage of the extensive electronic health record that underlies the VHA system, integrating real-world evidence into rigorous trials for precision oncology and other diseases. This is important for facilitating prerequisite activities for quality assessments for incorporation into databases (with appropriate permissions) to enhance treatment options. These activities are a key focus of the APOLLO initiative.13 While a more in-depth discussion of the importance of informatics is beyond the scope of this article, the field represents an important investment that is needed to achieve the goals of precision oncology.
In addition to informatics and data handling capabilities, VA has a longstanding tradition of designing and coordinating multisite clinical trials. This dates to the time of World War II when returning veterans had a high prevalence of tuberculosis. Since then, VA has contributed extensively to landmark findings in cardiovascular disease and surgery, mental health, infectious disease, and cancer. It was a VA study that helped establish colonoscopy as a standard for colorectal cancer screening by detecting colonic neoplasms in asymptomatic patients.14
From such investigations, the VA Cooperative Studies Program (CSP) has developed many strategies to conduct multisite clinical trials. But, CSP also has organized its sites methodically for operational efficiency and the ability to maintain institutional knowledge that crosses different types of studies and diseases. Using its Network of Dedicated Enrollment Sites (NODES) model, VA partnered with NCI to more effectively address administrative and regulatory requirements for initiating trials and recruiting veterans into cancer clinical trials.15 This partnership—the NCI And VA Interagency Group to Accelerate Trials Enrollment (NAVIGATE)—supports 12 sites with a central CSP Coordinating Center (CSPCC).
CSPCC provides support, shares best practices and provides organizational commitment at the senior levels of both agencies to overcome potential barriers. The goals and strategies are described by Schiller and colleagues.16 While still in its early stage as a cancer research network, NAVIGATE may be integrated with POPCaP and other parts of VA clinical research enterprise. This would allow us to specialize in advancing oncology care and to leverage capabilities more specifically to precision oncology. With an emphasis on recruitment, NAVIGATE has established capabilities with VA Informatics and Computing Infrastructure to quickly identify patients who may be eligible for particular clinical trials. We envision further refining these capabilities for precision oncology trials that incorporate genetic and other information for individual patients. VA also hopes to inform trial sponsors about design considerations. This is important since networked investigators will have direct insights into patient-level factors, which may help with more effectively identifying and enrolling them into trials for their particular cancers.
Conclusions
VA may have an opportunity to reach out to veterans who may not have immediate access to facilities running clinical trials. As it develops capabilities to bring the trial to the veteran, VA could have more virtual and/or centralized recruitment strategies. This would broaden opportunities for considering novel approaches that may not rely on a more traditional facility-based recruitment approach.
Ultimately, VA can be a critical part of a national effort to fight and, perhaps even, defeat cancers. With its extensive resources and capabilities, VA has the ability to advance a precision oncology agenda that provides veterans with the highest standard of care. It has built upon many key elements in clinical, technological and scientific fields of study that would challenge most health care systems given the extensive costs involved. In addition, creating strong partnerships with organizations such as PCF, NCI, and DoD that are complementary in resources and expertise will help VA to build a national network for cancer care. Putting this all together will support and facilitate a vision for more precise care for any veteran with cancer by more rapidly enabling the testing and approval of medications developed for this purpose.
Acknowledgments
The authors would like to thank Daphne Swancutt for comments and edits on drafts of this article.
1. Lynparza (Olaparib) [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP Inc, 2019.
2. Rubraca (rucaparib) [package insert]: Clovis Oncology, Inc., Boulder, CO: 2018.
3. McLornan DP, List A, Mufti GJ. Applying synthetic lethality for the selective targeting of cancer. N Engl J Med. 2014;371(18):1725-1735. doi:10.1056/NEJMra1407390
4. de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091-2102. doi:10.1056/NEJMoa1911440
5. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. doi:10.3322/caac.21590
6. Bentley DR. Decoding the human genome sequence. Hum Mol Genet. 2000;9(16):2353-2358. doi:10.1093/hmg/9.16.2353
7. National Human Genome Research institute. The cost of sequencing a human genome. https://www.genome.gov/about-genomics/fact-sheets/Sequencing-Human-Genome-cost. Updated October 30, 2019. Accessed July 31, 2020. 8. Paggio JCD, Sullivan R, Booth CM. Targeting the value of targeted therapy. Oncotarget. 2017;8(53):90612-90613. Published 2017 Oct 7. doi:10.18632/oncotarget.21596
9. Druker BJ, Guilhot F, O’Brien SG, et al; IRIS Investigators. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408-2417. doi:10.1056/NEJMoa062867
10. Pishvaian MJ, Blais EM, Brody JR, et al. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the Know Your Tumor registry trial [published correction appears in Lancet Oncol. 2020 Apr;21(4):e182]. Lancet Oncol. 2020;21(4):508-518. doi:10.1016/S1470-2045(20)30074-7
11. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer [published correction appears in Cell. 2015 Jul 16;162(2):454]. Cell. 2015;161(5):1215-1228. doi:10.1016/j.cell.2015.05.001
12. Fiore LD, Brophy MT, Ferguson RE, et al. Data sharing, clinical trials, and biomarkers in precision oncology: challenges, opportunities, and programs at the Department of Veterans Affairs. Clin Pharmacol Ther. 2017;101(5):586-589. doi:10.1002/cpt.660
13. Lee JSH, Darcy KM, Hu H, et al. From discovery to practice and survivorship: building a national real-world data learning healthcare framework for military and veteran cancer patients. Clin Pharmacol Ther. 2019;106(1):52-57. doi:10.1002/cpt.1425
14. Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380 [published correction appears in N Engl J Med 2000 Oct 19;343(16):1204]. N Engl J Med. 2000;343(3):162-168. doi:10.1056/NEJM200007203430301
15. Condon DL, Beck D, Kenworthy-Heinige T, et al. A cross-cutting approach to enhancing clinical trial site success: The Department of Veterans Affairs’ Network of Dedicated Enrollment Sites (NODES) model. Contemp Clin Trials Commun. 2017;6:78-84. Published 2017 Mar 29. doi:10.1016/j.conctc.2017.03.006
16. Schiller SJ, Shannon C, Brophy MT, et al. The National Cancer Institute and Department of Veterans Affairs Interagency Group to Accelerate Trials Enrollment (NAVIGATE): A federal collaboration to improve cancer care. Semin Oncol. 2019;46(4-5):308-313. doi:10.1053/j.seminoncol.2019.09.005
In May 2020, the US Food and Drug Administration (FDA) approved the first 2 targeted treatments for prostate cancer, specifically, the poly-(adenosine diphosphate-ribose) polymerase (PARP) inhibitors rucaparib and olaparib.1,2 For these medications to work, the tumor must have a homologous recombination deficiency (HRD), which is a form of DNA repair deficiency. The PARP pathway is important for DNA repair, and PARP inhibition leads to “synthetic lethality” in cancer cells that already are deficient in DNA repair mechanisms.3 Now, there is evidence that patients with prostate cancer who have HRD tumors and receive PARP inhibitors live longer when compared with those who receive standard of care options.4 These findings offer hope for patients with prostate cancer. They also demonstrate the process and potential benefits of precision oncology efforts; namely, targeted treatments for specific tumor types in cancer patients.
This article discusses the challenges and opportunities of precision oncology for US Department of Veterans Affairs (VA) Veterans Health Administration (VHA). First, the article will discuss working with relatively rare mutations. Second, the article will examine how the trials of olaparib and rucaparib illuminate the VHA contribution to research on new therapies for patients with cancer. Finally, the article will explore the ways in which VHA is becoming a major national contributor in drug discovery and approval of precision medications.
Precision Oncology
Despite advances in screening and treatment, an estimated 600,000 people in the US will die of cancer in 2020.5 Meaningful advances in cancer care depend on both laboratory and clinical research. This combination, known as translational research, takes discoveries in the laboratory and applies them to patients and vice versa. Successful translational research requires many components. These include talented scientists to form hypotheses and perform the work; money for supplies and equipment; platforms for timely dissemination of knowledge; well-trained clinicians to treat patients and lead research teams; and patients to participate in clinical trials. In precision oncology, the ability to find patients for the trials can be daunting, particularly in cases where the frequency of the mutation of interest is low.
During the 20th century, with few exceptions, physicians caring for patients with cancer had blunt instruments at their disposal. Surgery and radiation could lead to survival if the cancer was caught early enough. Systemic therapies, such as chemotherapy, rarely cured but could prolong life in some patients. However, chemotherapy is imprecise and targets any cell growing rapidly, including blood, hair, and gastrointestinal tract cells, which often leads to adverse effects. Sometimes complications from chemotherapy may shorten a person’s life, and certainly the quality of life during and after these treatments could be diminished. The improvements in cancer care occurred more rapidly once scientists had the tools to learn about individual tumors.
In the summer of 2000, researchers announced that the human genome had been sequenced.6 The genome (ie, DNA) consists of introns and exons that form a map for human development. Exons can be converted to proteins that carry out specific actions, such as helping in cell growth, cell death, or DNA repair. Solving the human genome itself did not lead directly to cures, but it did represent a huge advance in medical research. As time passed, sequencing genomes became more affordable, and sequencing just the exome alone was even cheaper.7 Treatments for cancer began to expand with the help of these tools, but questions as to the true benefit of targeted therapy also grew.8
Physicians and scientists have amassed more information about cancer cells and have applied this knowledge to active drug development. In 2001, the FDA approved the first targeted therapy, imatinib, for the treatment of chronic myelogenous leukemia (CML). This rapidly improved patient survival through targeting the mutated protein that leads to CML, rather than just aiming for rapidly dividing cells.9 Those mutations for which there is a drug to target, such as the BCR-ABL translocation in CML, are called actionable mutations.
Precision Oncology Program for Prostate Cancer
In 2016, the VA and the Prostate Cancer Foundation (PCF) established the Precision Oncology Program for Prostate Cancer (POPCaP) Centers of Excellence (COE). This partnership was formed to accelerate treatment and cure for veterans with prostate cancer. The VA Greater Los Angeles Healthcare System in California and VA Puget Sound Health Care System in Washington led this effort, and their principal investigators continue to co-lead POPCaP. Since its inception, 9 additional funded POPCaP COEs have joined, each with a mandate to sequence the tumors of men with metastatic prostate cancer.
The more that is learned about a tumor, the more likely it is that researchers can find mutations that are that tumor’s Achilles heel and defeat it. In fact, many drugs that can target mutations are already available. For example, BRCA2 is an actionable mutation that can be exploited by knocking out another key DNA repair mechanism in the cell, PARP. Today, the effort of sequencing has led to a rich database of mutations present in men with metastatic prostate cancer.
Although there are many targeted therapies, most have not been studied formally in prostate cancer. Occasionally, clinicians treating patients will use these drugs in an unapproved way, hoping that there will be anticancer activity. It is difficult to estimate the likelihood of success with a drug in this situation, and the safety profile may not be well described in that setting. Treatment decisions for incurable cancers must be made knowing the risks and benefits. This helps in shared decision making between the clinician and patient and informs choices concerning which laboratory tests to order and how often to see the patient. However, treatment decisions are sometimes made with the hope of activity when a cancer is known to be incurable. Very little data, which are critical to determine whether this helps or hurts patients, support this approach.
Some data suggest that sequencing and giving a drug for an actionable mutation may lead to better outcomes for patients. Sequencing of pancreatic tumors by Pishvaian and colleagues revealed that 282 of 1,082 (26%) samples harbored actionable mutations.10 Those patients who received a drug that targeted their actionable mutation (n = 46; 24%) lived longer when compared with those who had an actionable mutation but did not receive a drug that targeted it (hazard ratio [HR] 0.42 [95% CI, 0.26-0.68; P = .0004]). Additionally, those who received therapy for an actionable mutation lived longer when compared with those who did not have an actionable mutation (HR 0.34 [95% CI, 0.22-0.53; P < .001]). While this finding is intriguing, it does not mean that treating actionable mutations outside of a clinical trial should be done. To this end, VA established Prostate cancer Analysis for Therapy CHoice (PATCH) as a clinical trials network within POPCaP.
Prostate Cancer Analysis
The overall PATCH vision is designed for clinical care and research work to together toward improved care for those with prostate cancer (Figure 1). The resources necessary for successful translational research are substantial, and PATCH aims to streamline those resources. PATCH will support innovative, precision-based clinical research at the POPCaP COEs through its 5 arms.
Arm 1. Dedicated personnel ensure veteran access to trials in PATCH by giving patients and providers accurate information about available trial options; aiding veterans in traveling from home VA to a POPCaP COE for participation on a study; and maintaining the Committee for Veteran Participation in PATCH, where veterans will be represented and asked to provide input into the PATCH process.
Arm 2. Coordinators at the coordinating COE in Portland, Orgeon, train investigators and study staff at the local POPCaP COEs to ensure research can be performed in a safe and responsible way.
Arm 3. Personnel experienced in conducting clinical trials liaise with investigators at VA Central Institutional Review Board, monitor trials, build databases for appropriate and efficient data collection, and manage high-risk studies conducted under an Investigational New Drug application. This group works closely with biostatisticians to choose appropriate trial designs, estimate numbers of patients needed, and interpret data once they are collected.
Arm 4. Protocol development and data dissemination is coordinated by a group to assist investigators in drafting protocols and reviewing abstracts and manuscripts.
Arm 5. A core group manages contracts and budgets, as well as relationships between VA and industry, where funding and drugs may be obtained.
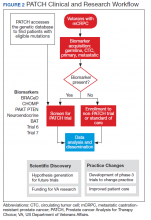
Perhaps most importantly, PATCH leverages the genetic data collected by POPCaP COEs to find patients for clinical trials. For example, the trials that examined olaparib and rucaparib assumed that the prevalence of HRD was about 25% in men with advanced prostate cancer.11 As these trials began enrollment, however, researchers discovered that the prevalence was < 20%. In fact, the study of olaparib screened 4,425 patients at 206 sites in 20 countries to identify 778 (18% of screened) patients with HRD.4 With widespread sequencing within VA, it could be possible to identify a substantial number of patients who are already known to have the mutation of interest (Figure 2).
Clinical Trials
There are currently 2 clinical trials in PATCH; 4 additional trials await funding decisions, and more trials are in the concept stage. BRACeD (NCT04038502) is a phase 2 trial examining platinum and taxane chemotherapy in tumors with HRD (specifically, BRCA1, BRCA2, and PALB2). About 15% to 20% of men with advanced prostate cancer will have a DNA repair defect in the tumor that could make them eligible for this study. The primary endpoint is progression-free survival.
A second study, CHOMP (NCT04104893), is a phase 2 trial examining the efficacy of immunotherapy (PD-1 inhibition) in tumors having mismatch repair deficiency or CDK12-/-. Each of those is found in about 7% of men with metastatic prostate cancer, and full accrual of a trial with rare mutations could take 5 to 10 years without a systematic approach of sequencing and identifying potential participants. The primary endpoint is a composite of radiographic response by iRECIST (immune response evaluation criteria in solid tumors), progression-free survival at 6 months and prostate specific antigen reduction by ≥ 50% in ≤ 12 weeks. With 11 POPCaP COEs sequencing the tumors of every man with metastatic prostate cancer, identifying men with the appropriate mutation is possible. PATCH will aid the sites in recruitment through outreach and coordination of travel.
Industry Partnerships
PATCH depends upon pharmaceutical industry partners, as clinical trials of even 40 patients can require significant funding and trial resources to operate. Furthermore, many drugs of interest are not available outside of a clinical trial, and partnerships enable VA researchers to access these medications. PATCH also benefits greatly from foundation partners, such as the PCF, which has made POPCaP possible and will continue to connect talented researchers with VA resources. Finally, access to other publicly available research funds, such as those from VA Office of Research and Development, National Institutes of Health, and US Department of Defense (DoD) Congressionally Directed Research Program are needed for trials.
Funding for these trials remains limited despite public health and broader interests in addressing important questions. Accelerated accrual through PATCH may be an attractive partnership opportunity for companies, foundations and government funding agencies to support the PATCH efforts.
Both POPCaP and PATCH highlight the potential promise of precision oncology within the nation’s largest integrated health care system. The VHA patient population enables prostate cancer researchers to serve an important early target. It also provides a foundational platform for a broader set of activities. These include a tailored approach to identifying tumor profiles and other patient characteristics that may help to elevate standard of care for other common cancers including ones affecting the lungs and/or head and neck.
To this end, VA has been working with the National Cancer Institute (NCI) and DoD to establish a national infrastructure for precision oncology across multiple cancer types.12 In addition to clinical capabilities and the ability to run clinical trials that can accrue sufficient patients to answer key questions, we have developed capabilities for data collection and sharing, and analytical tools to support a learning health care system approach as a core element to precision oncology.
Besides having a research-specific context, such informatics and information technology systems enable clinicians to obtain and apply decision-making data rapidly for a specific patient and cancer type. These systems take particular advantage of the extensive electronic health record that underlies the VHA system, integrating real-world evidence into rigorous trials for precision oncology and other diseases. This is important for facilitating prerequisite activities for quality assessments for incorporation into databases (with appropriate permissions) to enhance treatment options. These activities are a key focus of the APOLLO initiative.13 While a more in-depth discussion of the importance of informatics is beyond the scope of this article, the field represents an important investment that is needed to achieve the goals of precision oncology.
In addition to informatics and data handling capabilities, VA has a longstanding tradition of designing and coordinating multisite clinical trials. This dates to the time of World War II when returning veterans had a high prevalence of tuberculosis. Since then, VA has contributed extensively to landmark findings in cardiovascular disease and surgery, mental health, infectious disease, and cancer. It was a VA study that helped establish colonoscopy as a standard for colorectal cancer screening by detecting colonic neoplasms in asymptomatic patients.14
From such investigations, the VA Cooperative Studies Program (CSP) has developed many strategies to conduct multisite clinical trials. But, CSP also has organized its sites methodically for operational efficiency and the ability to maintain institutional knowledge that crosses different types of studies and diseases. Using its Network of Dedicated Enrollment Sites (NODES) model, VA partnered with NCI to more effectively address administrative and regulatory requirements for initiating trials and recruiting veterans into cancer clinical trials.15 This partnership—the NCI And VA Interagency Group to Accelerate Trials Enrollment (NAVIGATE)—supports 12 sites with a central CSP Coordinating Center (CSPCC).
CSPCC provides support, shares best practices and provides organizational commitment at the senior levels of both agencies to overcome potential barriers. The goals and strategies are described by Schiller and colleagues.16 While still in its early stage as a cancer research network, NAVIGATE may be integrated with POPCaP and other parts of VA clinical research enterprise. This would allow us to specialize in advancing oncology care and to leverage capabilities more specifically to precision oncology. With an emphasis on recruitment, NAVIGATE has established capabilities with VA Informatics and Computing Infrastructure to quickly identify patients who may be eligible for particular clinical trials. We envision further refining these capabilities for precision oncology trials that incorporate genetic and other information for individual patients. VA also hopes to inform trial sponsors about design considerations. This is important since networked investigators will have direct insights into patient-level factors, which may help with more effectively identifying and enrolling them into trials for their particular cancers.
Conclusions
VA may have an opportunity to reach out to veterans who may not have immediate access to facilities running clinical trials. As it develops capabilities to bring the trial to the veteran, VA could have more virtual and/or centralized recruitment strategies. This would broaden opportunities for considering novel approaches that may not rely on a more traditional facility-based recruitment approach.
Ultimately, VA can be a critical part of a national effort to fight and, perhaps even, defeat cancers. With its extensive resources and capabilities, VA has the ability to advance a precision oncology agenda that provides veterans with the highest standard of care. It has built upon many key elements in clinical, technological and scientific fields of study that would challenge most health care systems given the extensive costs involved. In addition, creating strong partnerships with organizations such as PCF, NCI, and DoD that are complementary in resources and expertise will help VA to build a national network for cancer care. Putting this all together will support and facilitate a vision for more precise care for any veteran with cancer by more rapidly enabling the testing and approval of medications developed for this purpose.
Acknowledgments
The authors would like to thank Daphne Swancutt for comments and edits on drafts of this article.
In May 2020, the US Food and Drug Administration (FDA) approved the first 2 targeted treatments for prostate cancer, specifically, the poly-(adenosine diphosphate-ribose) polymerase (PARP) inhibitors rucaparib and olaparib.1,2 For these medications to work, the tumor must have a homologous recombination deficiency (HRD), which is a form of DNA repair deficiency. The PARP pathway is important for DNA repair, and PARP inhibition leads to “synthetic lethality” in cancer cells that already are deficient in DNA repair mechanisms.3 Now, there is evidence that patients with prostate cancer who have HRD tumors and receive PARP inhibitors live longer when compared with those who receive standard of care options.4 These findings offer hope for patients with prostate cancer. They also demonstrate the process and potential benefits of precision oncology efforts; namely, targeted treatments for specific tumor types in cancer patients.
This article discusses the challenges and opportunities of precision oncology for US Department of Veterans Affairs (VA) Veterans Health Administration (VHA). First, the article will discuss working with relatively rare mutations. Second, the article will examine how the trials of olaparib and rucaparib illuminate the VHA contribution to research on new therapies for patients with cancer. Finally, the article will explore the ways in which VHA is becoming a major national contributor in drug discovery and approval of precision medications.
Precision Oncology
Despite advances in screening and treatment, an estimated 600,000 people in the US will die of cancer in 2020.5 Meaningful advances in cancer care depend on both laboratory and clinical research. This combination, known as translational research, takes discoveries in the laboratory and applies them to patients and vice versa. Successful translational research requires many components. These include talented scientists to form hypotheses and perform the work; money for supplies and equipment; platforms for timely dissemination of knowledge; well-trained clinicians to treat patients and lead research teams; and patients to participate in clinical trials. In precision oncology, the ability to find patients for the trials can be daunting, particularly in cases where the frequency of the mutation of interest is low.
During the 20th century, with few exceptions, physicians caring for patients with cancer had blunt instruments at their disposal. Surgery and radiation could lead to survival if the cancer was caught early enough. Systemic therapies, such as chemotherapy, rarely cured but could prolong life in some patients. However, chemotherapy is imprecise and targets any cell growing rapidly, including blood, hair, and gastrointestinal tract cells, which often leads to adverse effects. Sometimes complications from chemotherapy may shorten a person’s life, and certainly the quality of life during and after these treatments could be diminished. The improvements in cancer care occurred more rapidly once scientists had the tools to learn about individual tumors.
In the summer of 2000, researchers announced that the human genome had been sequenced.6 The genome (ie, DNA) consists of introns and exons that form a map for human development. Exons can be converted to proteins that carry out specific actions, such as helping in cell growth, cell death, or DNA repair. Solving the human genome itself did not lead directly to cures, but it did represent a huge advance in medical research. As time passed, sequencing genomes became more affordable, and sequencing just the exome alone was even cheaper.7 Treatments for cancer began to expand with the help of these tools, but questions as to the true benefit of targeted therapy also grew.8
Physicians and scientists have amassed more information about cancer cells and have applied this knowledge to active drug development. In 2001, the FDA approved the first targeted therapy, imatinib, for the treatment of chronic myelogenous leukemia (CML). This rapidly improved patient survival through targeting the mutated protein that leads to CML, rather than just aiming for rapidly dividing cells.9 Those mutations for which there is a drug to target, such as the BCR-ABL translocation in CML, are called actionable mutations.
Precision Oncology Program for Prostate Cancer
In 2016, the VA and the Prostate Cancer Foundation (PCF) established the Precision Oncology Program for Prostate Cancer (POPCaP) Centers of Excellence (COE). This partnership was formed to accelerate treatment and cure for veterans with prostate cancer. The VA Greater Los Angeles Healthcare System in California and VA Puget Sound Health Care System in Washington led this effort, and their principal investigators continue to co-lead POPCaP. Since its inception, 9 additional funded POPCaP COEs have joined, each with a mandate to sequence the tumors of men with metastatic prostate cancer.
The more that is learned about a tumor, the more likely it is that researchers can find mutations that are that tumor’s Achilles heel and defeat it. In fact, many drugs that can target mutations are already available. For example, BRCA2 is an actionable mutation that can be exploited by knocking out another key DNA repair mechanism in the cell, PARP. Today, the effort of sequencing has led to a rich database of mutations present in men with metastatic prostate cancer.
Although there are many targeted therapies, most have not been studied formally in prostate cancer. Occasionally, clinicians treating patients will use these drugs in an unapproved way, hoping that there will be anticancer activity. It is difficult to estimate the likelihood of success with a drug in this situation, and the safety profile may not be well described in that setting. Treatment decisions for incurable cancers must be made knowing the risks and benefits. This helps in shared decision making between the clinician and patient and informs choices concerning which laboratory tests to order and how often to see the patient. However, treatment decisions are sometimes made with the hope of activity when a cancer is known to be incurable. Very little data, which are critical to determine whether this helps or hurts patients, support this approach.
Some data suggest that sequencing and giving a drug for an actionable mutation may lead to better outcomes for patients. Sequencing of pancreatic tumors by Pishvaian and colleagues revealed that 282 of 1,082 (26%) samples harbored actionable mutations.10 Those patients who received a drug that targeted their actionable mutation (n = 46; 24%) lived longer when compared with those who had an actionable mutation but did not receive a drug that targeted it (hazard ratio [HR] 0.42 [95% CI, 0.26-0.68; P = .0004]). Additionally, those who received therapy for an actionable mutation lived longer when compared with those who did not have an actionable mutation (HR 0.34 [95% CI, 0.22-0.53; P < .001]). While this finding is intriguing, it does not mean that treating actionable mutations outside of a clinical trial should be done. To this end, VA established Prostate cancer Analysis for Therapy CHoice (PATCH) as a clinical trials network within POPCaP.
Prostate Cancer Analysis
The overall PATCH vision is designed for clinical care and research work to together toward improved care for those with prostate cancer (Figure 1). The resources necessary for successful translational research are substantial, and PATCH aims to streamline those resources. PATCH will support innovative, precision-based clinical research at the POPCaP COEs through its 5 arms.
Arm 1. Dedicated personnel ensure veteran access to trials in PATCH by giving patients and providers accurate information about available trial options; aiding veterans in traveling from home VA to a POPCaP COE for participation on a study; and maintaining the Committee for Veteran Participation in PATCH, where veterans will be represented and asked to provide input into the PATCH process.
Arm 2. Coordinators at the coordinating COE in Portland, Orgeon, train investigators and study staff at the local POPCaP COEs to ensure research can be performed in a safe and responsible way.
Arm 3. Personnel experienced in conducting clinical trials liaise with investigators at VA Central Institutional Review Board, monitor trials, build databases for appropriate and efficient data collection, and manage high-risk studies conducted under an Investigational New Drug application. This group works closely with biostatisticians to choose appropriate trial designs, estimate numbers of patients needed, and interpret data once they are collected.
Arm 4. Protocol development and data dissemination is coordinated by a group to assist investigators in drafting protocols and reviewing abstracts and manuscripts.
Arm 5. A core group manages contracts and budgets, as well as relationships between VA and industry, where funding and drugs may be obtained.
Perhaps most importantly, PATCH leverages the genetic data collected by POPCaP COEs to find patients for clinical trials. For example, the trials that examined olaparib and rucaparib assumed that the prevalence of HRD was about 25% in men with advanced prostate cancer.11 As these trials began enrollment, however, researchers discovered that the prevalence was < 20%. In fact, the study of olaparib screened 4,425 patients at 206 sites in 20 countries to identify 778 (18% of screened) patients with HRD.4 With widespread sequencing within VA, it could be possible to identify a substantial number of patients who are already known to have the mutation of interest (Figure 2).
Clinical Trials
There are currently 2 clinical trials in PATCH; 4 additional trials await funding decisions, and more trials are in the concept stage. BRACeD (NCT04038502) is a phase 2 trial examining platinum and taxane chemotherapy in tumors with HRD (specifically, BRCA1, BRCA2, and PALB2). About 15% to 20% of men with advanced prostate cancer will have a DNA repair defect in the tumor that could make them eligible for this study. The primary endpoint is progression-free survival.
A second study, CHOMP (NCT04104893), is a phase 2 trial examining the efficacy of immunotherapy (PD-1 inhibition) in tumors having mismatch repair deficiency or CDK12-/-. Each of those is found in about 7% of men with metastatic prostate cancer, and full accrual of a trial with rare mutations could take 5 to 10 years without a systematic approach of sequencing and identifying potential participants. The primary endpoint is a composite of radiographic response by iRECIST (immune response evaluation criteria in solid tumors), progression-free survival at 6 months and prostate specific antigen reduction by ≥ 50% in ≤ 12 weeks. With 11 POPCaP COEs sequencing the tumors of every man with metastatic prostate cancer, identifying men with the appropriate mutation is possible. PATCH will aid the sites in recruitment through outreach and coordination of travel.
Industry Partnerships
PATCH depends upon pharmaceutical industry partners, as clinical trials of even 40 patients can require significant funding and trial resources to operate. Furthermore, many drugs of interest are not available outside of a clinical trial, and partnerships enable VA researchers to access these medications. PATCH also benefits greatly from foundation partners, such as the PCF, which has made POPCaP possible and will continue to connect talented researchers with VA resources. Finally, access to other publicly available research funds, such as those from VA Office of Research and Development, National Institutes of Health, and US Department of Defense (DoD) Congressionally Directed Research Program are needed for trials.
Funding for these trials remains limited despite public health and broader interests in addressing important questions. Accelerated accrual through PATCH may be an attractive partnership opportunity for companies, foundations and government funding agencies to support the PATCH efforts.
Both POPCaP and PATCH highlight the potential promise of precision oncology within the nation’s largest integrated health care system. The VHA patient population enables prostate cancer researchers to serve an important early target. It also provides a foundational platform for a broader set of activities. These include a tailored approach to identifying tumor profiles and other patient characteristics that may help to elevate standard of care for other common cancers including ones affecting the lungs and/or head and neck.
To this end, VA has been working with the National Cancer Institute (NCI) and DoD to establish a national infrastructure for precision oncology across multiple cancer types.12 In addition to clinical capabilities and the ability to run clinical trials that can accrue sufficient patients to answer key questions, we have developed capabilities for data collection and sharing, and analytical tools to support a learning health care system approach as a core element to precision oncology.
Besides having a research-specific context, such informatics and information technology systems enable clinicians to obtain and apply decision-making data rapidly for a specific patient and cancer type. These systems take particular advantage of the extensive electronic health record that underlies the VHA system, integrating real-world evidence into rigorous trials for precision oncology and other diseases. This is important for facilitating prerequisite activities for quality assessments for incorporation into databases (with appropriate permissions) to enhance treatment options. These activities are a key focus of the APOLLO initiative.13 While a more in-depth discussion of the importance of informatics is beyond the scope of this article, the field represents an important investment that is needed to achieve the goals of precision oncology.
In addition to informatics and data handling capabilities, VA has a longstanding tradition of designing and coordinating multisite clinical trials. This dates to the time of World War II when returning veterans had a high prevalence of tuberculosis. Since then, VA has contributed extensively to landmark findings in cardiovascular disease and surgery, mental health, infectious disease, and cancer. It was a VA study that helped establish colonoscopy as a standard for colorectal cancer screening by detecting colonic neoplasms in asymptomatic patients.14
From such investigations, the VA Cooperative Studies Program (CSP) has developed many strategies to conduct multisite clinical trials. But, CSP also has organized its sites methodically for operational efficiency and the ability to maintain institutional knowledge that crosses different types of studies and diseases. Using its Network of Dedicated Enrollment Sites (NODES) model, VA partnered with NCI to more effectively address administrative and regulatory requirements for initiating trials and recruiting veterans into cancer clinical trials.15 This partnership—the NCI And VA Interagency Group to Accelerate Trials Enrollment (NAVIGATE)—supports 12 sites with a central CSP Coordinating Center (CSPCC).
CSPCC provides support, shares best practices and provides organizational commitment at the senior levels of both agencies to overcome potential barriers. The goals and strategies are described by Schiller and colleagues.16 While still in its early stage as a cancer research network, NAVIGATE may be integrated with POPCaP and other parts of VA clinical research enterprise. This would allow us to specialize in advancing oncology care and to leverage capabilities more specifically to precision oncology. With an emphasis on recruitment, NAVIGATE has established capabilities with VA Informatics and Computing Infrastructure to quickly identify patients who may be eligible for particular clinical trials. We envision further refining these capabilities for precision oncology trials that incorporate genetic and other information for individual patients. VA also hopes to inform trial sponsors about design considerations. This is important since networked investigators will have direct insights into patient-level factors, which may help with more effectively identifying and enrolling them into trials for their particular cancers.
Conclusions
VA may have an opportunity to reach out to veterans who may not have immediate access to facilities running clinical trials. As it develops capabilities to bring the trial to the veteran, VA could have more virtual and/or centralized recruitment strategies. This would broaden opportunities for considering novel approaches that may not rely on a more traditional facility-based recruitment approach.
Ultimately, VA can be a critical part of a national effort to fight and, perhaps even, defeat cancers. With its extensive resources and capabilities, VA has the ability to advance a precision oncology agenda that provides veterans with the highest standard of care. It has built upon many key elements in clinical, technological and scientific fields of study that would challenge most health care systems given the extensive costs involved. In addition, creating strong partnerships with organizations such as PCF, NCI, and DoD that are complementary in resources and expertise will help VA to build a national network for cancer care. Putting this all together will support and facilitate a vision for more precise care for any veteran with cancer by more rapidly enabling the testing and approval of medications developed for this purpose.
Acknowledgments
The authors would like to thank Daphne Swancutt for comments and edits on drafts of this article.
1. Lynparza (Olaparib) [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP Inc, 2019.
2. Rubraca (rucaparib) [package insert]: Clovis Oncology, Inc., Boulder, CO: 2018.
3. McLornan DP, List A, Mufti GJ. Applying synthetic lethality for the selective targeting of cancer. N Engl J Med. 2014;371(18):1725-1735. doi:10.1056/NEJMra1407390
4. de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091-2102. doi:10.1056/NEJMoa1911440
5. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. doi:10.3322/caac.21590
6. Bentley DR. Decoding the human genome sequence. Hum Mol Genet. 2000;9(16):2353-2358. doi:10.1093/hmg/9.16.2353
7. National Human Genome Research institute. The cost of sequencing a human genome. https://www.genome.gov/about-genomics/fact-sheets/Sequencing-Human-Genome-cost. Updated October 30, 2019. Accessed July 31, 2020. 8. Paggio JCD, Sullivan R, Booth CM. Targeting the value of targeted therapy. Oncotarget. 2017;8(53):90612-90613. Published 2017 Oct 7. doi:10.18632/oncotarget.21596
9. Druker BJ, Guilhot F, O’Brien SG, et al; IRIS Investigators. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408-2417. doi:10.1056/NEJMoa062867
10. Pishvaian MJ, Blais EM, Brody JR, et al. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the Know Your Tumor registry trial [published correction appears in Lancet Oncol. 2020 Apr;21(4):e182]. Lancet Oncol. 2020;21(4):508-518. doi:10.1016/S1470-2045(20)30074-7
11. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer [published correction appears in Cell. 2015 Jul 16;162(2):454]. Cell. 2015;161(5):1215-1228. doi:10.1016/j.cell.2015.05.001
12. Fiore LD, Brophy MT, Ferguson RE, et al. Data sharing, clinical trials, and biomarkers in precision oncology: challenges, opportunities, and programs at the Department of Veterans Affairs. Clin Pharmacol Ther. 2017;101(5):586-589. doi:10.1002/cpt.660
13. Lee JSH, Darcy KM, Hu H, et al. From discovery to practice and survivorship: building a national real-world data learning healthcare framework for military and veteran cancer patients. Clin Pharmacol Ther. 2019;106(1):52-57. doi:10.1002/cpt.1425
14. Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380 [published correction appears in N Engl J Med 2000 Oct 19;343(16):1204]. N Engl J Med. 2000;343(3):162-168. doi:10.1056/NEJM200007203430301
15. Condon DL, Beck D, Kenworthy-Heinige T, et al. A cross-cutting approach to enhancing clinical trial site success: The Department of Veterans Affairs’ Network of Dedicated Enrollment Sites (NODES) model. Contemp Clin Trials Commun. 2017;6:78-84. Published 2017 Mar 29. doi:10.1016/j.conctc.2017.03.006
16. Schiller SJ, Shannon C, Brophy MT, et al. The National Cancer Institute and Department of Veterans Affairs Interagency Group to Accelerate Trials Enrollment (NAVIGATE): A federal collaboration to improve cancer care. Semin Oncol. 2019;46(4-5):308-313. doi:10.1053/j.seminoncol.2019.09.005
1. Lynparza (Olaparib) [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP Inc, 2019.
2. Rubraca (rucaparib) [package insert]: Clovis Oncology, Inc., Boulder, CO: 2018.
3. McLornan DP, List A, Mufti GJ. Applying synthetic lethality for the selective targeting of cancer. N Engl J Med. 2014;371(18):1725-1735. doi:10.1056/NEJMra1407390
4. de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091-2102. doi:10.1056/NEJMoa1911440
5. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. doi:10.3322/caac.21590
6. Bentley DR. Decoding the human genome sequence. Hum Mol Genet. 2000;9(16):2353-2358. doi:10.1093/hmg/9.16.2353
7. National Human Genome Research institute. The cost of sequencing a human genome. https://www.genome.gov/about-genomics/fact-sheets/Sequencing-Human-Genome-cost. Updated October 30, 2019. Accessed July 31, 2020. 8. Paggio JCD, Sullivan R, Booth CM. Targeting the value of targeted therapy. Oncotarget. 2017;8(53):90612-90613. Published 2017 Oct 7. doi:10.18632/oncotarget.21596
9. Druker BJ, Guilhot F, O’Brien SG, et al; IRIS Investigators. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408-2417. doi:10.1056/NEJMoa062867
10. Pishvaian MJ, Blais EM, Brody JR, et al. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the Know Your Tumor registry trial [published correction appears in Lancet Oncol. 2020 Apr;21(4):e182]. Lancet Oncol. 2020;21(4):508-518. doi:10.1016/S1470-2045(20)30074-7
11. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer [published correction appears in Cell. 2015 Jul 16;162(2):454]. Cell. 2015;161(5):1215-1228. doi:10.1016/j.cell.2015.05.001
12. Fiore LD, Brophy MT, Ferguson RE, et al. Data sharing, clinical trials, and biomarkers in precision oncology: challenges, opportunities, and programs at the Department of Veterans Affairs. Clin Pharmacol Ther. 2017;101(5):586-589. doi:10.1002/cpt.660
13. Lee JSH, Darcy KM, Hu H, et al. From discovery to practice and survivorship: building a national real-world data learning healthcare framework for military and veteran cancer patients. Clin Pharmacol Ther. 2019;106(1):52-57. doi:10.1002/cpt.1425
14. Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380 [published correction appears in N Engl J Med 2000 Oct 19;343(16):1204]. N Engl J Med. 2000;343(3):162-168. doi:10.1056/NEJM200007203430301
15. Condon DL, Beck D, Kenworthy-Heinige T, et al. A cross-cutting approach to enhancing clinical trial site success: The Department of Veterans Affairs’ Network of Dedicated Enrollment Sites (NODES) model. Contemp Clin Trials Commun. 2017;6:78-84. Published 2017 Mar 29. doi:10.1016/j.conctc.2017.03.006
16. Schiller SJ, Shannon C, Brophy MT, et al. The National Cancer Institute and Department of Veterans Affairs Interagency Group to Accelerate Trials Enrollment (NAVIGATE): A federal collaboration to improve cancer care. Semin Oncol. 2019;46(4-5):308-313. doi:10.1053/j.seminoncol.2019.09.005