User login
Lichen planus (LP) is a chronic inflammatory dermatosis of unknown origin that involves the skin and mucous membranes, and lichenoid drug eruption (LDE) is an uncommon cutaneous adverse reaction to a medication.1 The manifestations resemble each other clinically, and sometimes it is difficult to differentiate between them on histology. The pathogenesis still is not well characterized, especially the key initiating event that leads to the development of LP or LDE postimmunization. There have been reports of LP or LDEs after certain vaccines, especially the hepatitis B and influenza vaccines.2-4 Both vaccines are routinely administered in the United States; more than 100 million individuals have received the hepatitis B vaccine in the United States since it became available in 1982,5 and the Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention (CDC) recommends that all individuals 6 months or older receive an influenza vaccine every year.6 Currently, influenza vaccine coverage among adults 18 years or older reaches approximately 40% annually in the United States.6
Although certain viral infections (eg, hepatitis C virus) seem to play a role in the development of LP,7,8 the link between LP and hepatitis B vaccination is less well recognized. Reports of LP and LDE after vaccination have been largely limited to case reports and case series.2-4,9,10 Therefore, we aimed to characterize and review cases of LP and LDE following vaccination by analyzing the Vaccine Adverse Event Reporting System (VAERS) database.
Methods
The VAERS is a national vaccine safety surveillance database maintained jointly by the CDC and the US Food and Drug Administration to analyze adverse events (AEs) following immunizations. Serious AEs and deaths recorded in the VAERS were followed up periodically by VAERS staff. Information on vaccine-associated LP or LDE was retrieved from the VAERS database using the CDC WONDER online interface (http://wonder.cdc.gov/vaers.html). To examine if LP or LDE after vaccination occurred more frequently in patients with certain demographic risk factors, all reported cases of LP and LDE associated with vaccines administered from July 1990 to November 2014 were identified in the symptoms section of the VAERS system using the search terms lichen planus, oral lichen planus, and lichenoid drug eruption. Characteristics such as age, gender, time to onset, type of vaccine, method of diagnosis, and clinical outcome were collected.
The statistical package for social sciences (SPSS version 22) was utilized for the descriptive analysis. Fisher exact and χ2 tests were used to evaluate statistical significance. A 2-sided P value of <.05 was considered statistically significant.
Results
There were 434,943 reported AEs following vaccination in the VAERS database from July 1990 to November 2014; among them, 33 cases involved LP or LDE. Of these vaccine-associated AEs, LP was diagnosed in 23 (69.7%) cases, while LDE and oral LP were diagnosed in 6 (18.2%) and 4 (12.1%) cases, respectively. Females represented slightly more than half (57.6% [19/33]) of the total cases. The median age of onset was 47 years. Approximately two-thirds of the identified cases were confirmed on skin biopsy and histology, while the rest were diagnosed either by a dermatologist or a primary care physician. The time to onset of symptoms ranged from 1 to 297 days after vaccination, with a median time of 14 days.
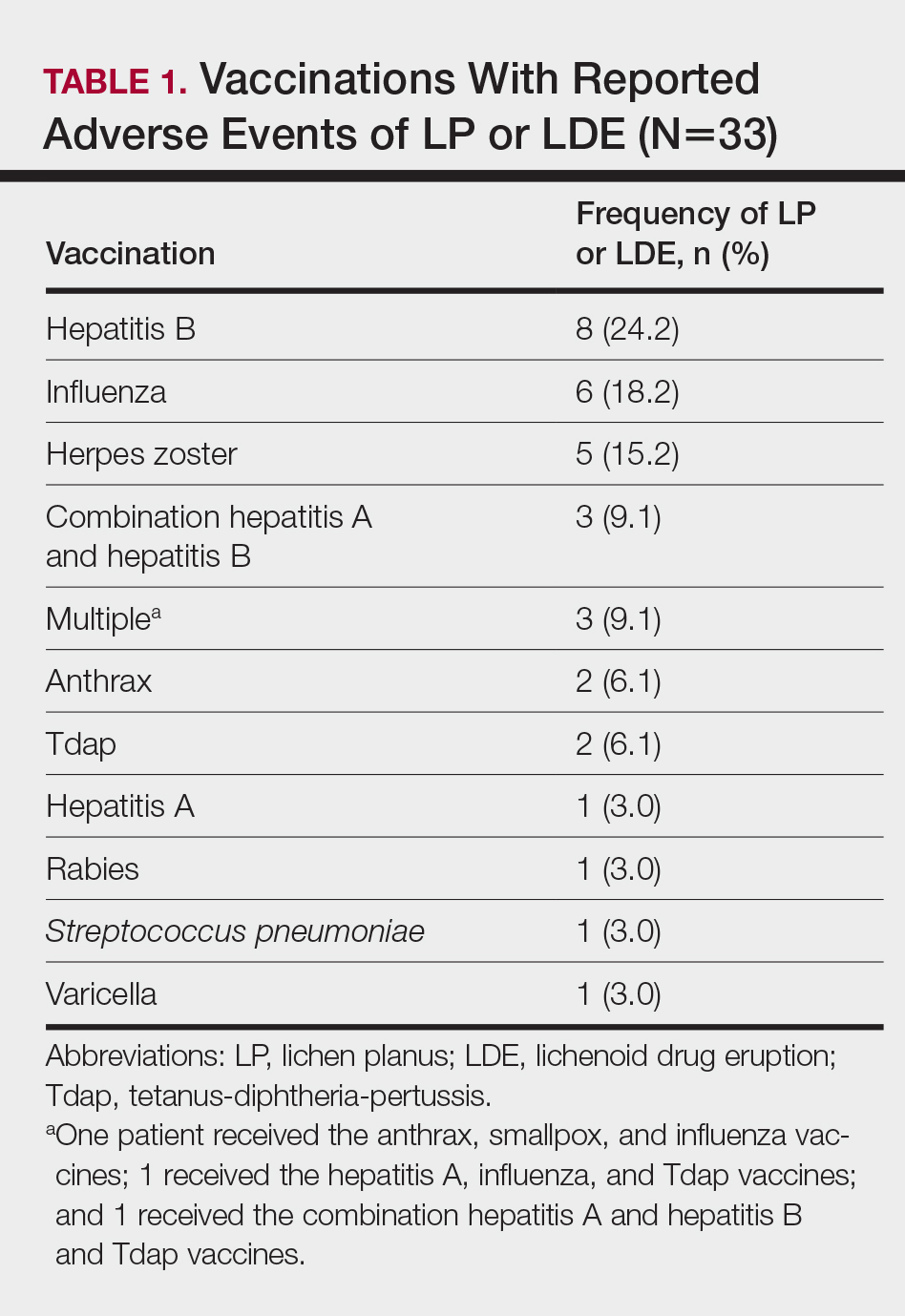
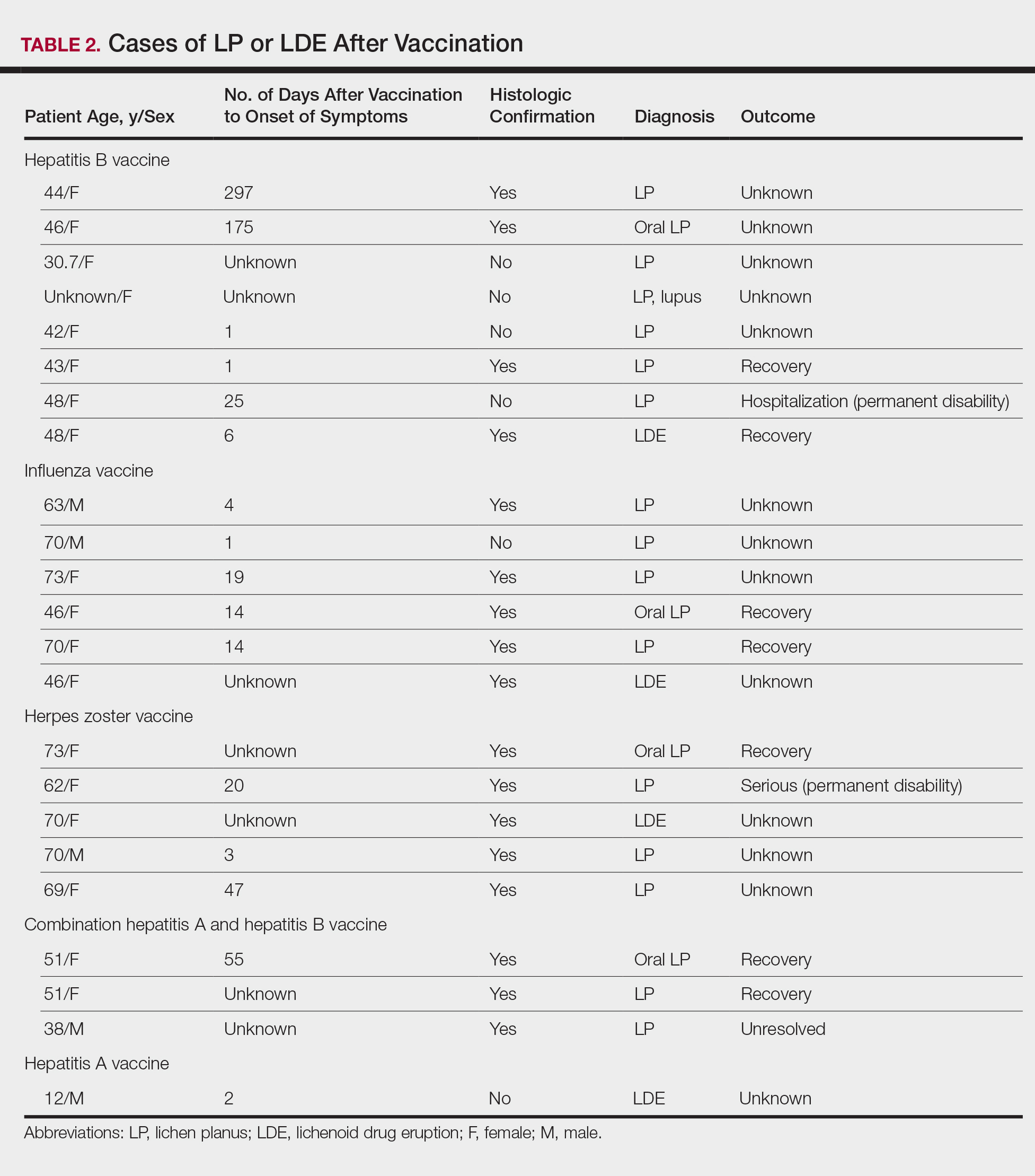
Patients with LP or LDE were significantly older compared to the reported AEs overall (P<.001); the median age of onset was 47 years for LP or LDE compared to 24 years for all reported AEs. Table 1 shows the various vaccines associated with LP or LDE. The hepatitis B, influenza, and herpes zoster vaccines were the 3 most common types of vaccines associated with these conditions. The hepatitis B vaccine accounted for 24.2% (8/33) of the reported events, followed by influenza (18.2% [6/33]) and herpes zoster (15.2% [5/33]) vaccines. In addition, there were 3 cases of cutaneous reaction after receiving the combination hepatitis A and hepatitis B vaccine. Table 2 presents details of the reported events associated with hepatitis B, influenza, herpes zoster, combination hepatitis A and hepatitis B, and hepatitis A vaccination.
Of 8 AEs associated with hepatitis B vaccination, 1 AE resulted in permanent disability and required hospitalization. O
Comment
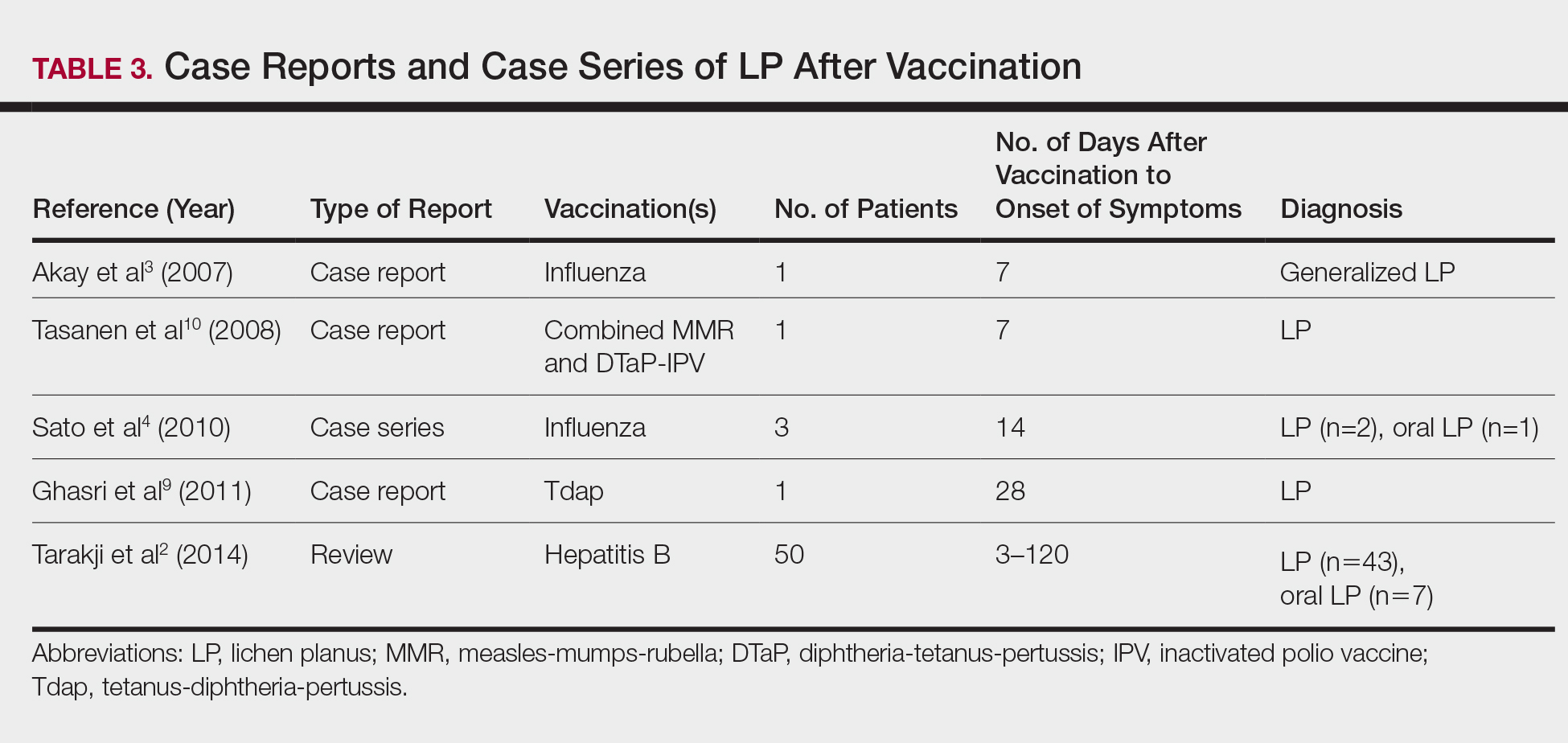
The estimated prevalence of LP ranges from 0.22% to 5% worldwide,11-15 with an incidence of 0.032% to 0.037%.16 Although rare, LP and LDE can occur from certain medications or vaccines. Cases of LP have been reported after hepatitis B and influenza vaccinations. The first case of LP following hepatitis B vaccination was described by Ciaccio and Rebora17 in 1990. Since then, a total of 50 similar cases have been reported worldwide.2 There also have been reports of LP following influenza, tetanus-diphtheria-pertussis, measles-mumps-rubella, and inactivated polio vaccines.3,4,9,10 Table 3 summarizes cases of LP following various vaccinations.
The key initiating event of the pathogenesis for both LP and LDE is not completely understood. Both conditions share similar immunologic mechanisms of persistently activated CD8 autocytotoxic T lymphocytes against epidermal cells.18 These cells can induce apoptosis of basal epidermal keratinocytes and generate various cytokines (eg, IFN-γ, IL-5) to enhance expression of class II MHC molecules and antigen presentation to CD4 T cells.19-22 It is conceivable that one of the initiating factors may be related to components in vaccines.
Hepatitis B, influenza, and herpes zoster vaccines were the 3 most common vaccines implicated in postimmunization LP or LDEs in our study. The excipients of these vaccines were compared based on the product inserts to identify any common components. It was found that all 3 vaccines contain either yeast protein or egg protein with various forms of phosphate buffers, while the hepatitis A and herpes zoster vaccines share Medical Research Council cell strain 5 (human diploid) cells as well as other cellular components.23 Sato et al4 suggested that specific vaccine components, such as the vaccine itself or egg proteins, could have contributed to the development of LP following vaccination. It has been postulated that the protein S fraction of hepatitis B surface antigen plays a crucial role in the pathogenesis of both LP and LDE after hepatitis B vaccination.2,24 It is likely that protein S shares common epitopes on keratinocytes that are recognized by the immune system, thus activating cytotoxic T lymphocytes and inducing apoptosis.2,24
In this study, the median time to onset of vaccine-related LP was 14 days, which is consistent with a case series by Sato et al,4 suggesting that adverse reactions mainly occurred within 2 weeks after influenza vaccination. Onset of symptoms within 2 weeks of vaccination would therefore be a crucial clue for diagnosing possible vaccine-related LP or LDE. On the other hand, at least 4 patients in our study had onset of LP and LDE more than 1 month after vaccination; 2 of 4 cases even reported symptom onset at 175 and 297 days after hepatitis B vaccination, which were much longer than the 120 days reported by Tarakji et al.2 It is not known if these cases constitute true vaccine-associated LP or LDE or if unmeasured confounding factors such as concurrent medications or comorbidities may have contributed to the development of these AEs.
It also is interesting to note that LP and LDE affected mainly middle-aged women. An increased risk of autoimmunity in female adults partly explains this observation.25 Some vaccines, such as herpes zoster and influenza vaccines, generally are recommended for older adults who also are more likely to have multiple comorbidities or take multiple medications/supplements, which can potentially skew the prevalence of AEs toward an older age group. It should be noted, however, that LP and LDE were relatively uncommon AEs following vaccination in the current study. In this study, LP and LDE consisted of only 0.01% (N=42,230) of all AEs after hepatitis B vaccination, while the more common AEs such as pyrexia, nonspecific rashes, nonspecific gastrointestinal symptoms, and headache contributed to approximately 66.5% of all reported events.
One of the strengths of our study is that up to two-thirds of cases were confirmed histologically and all patients were seen and followed up by dermatologists or physicians. The VAERS is an easily accessible, up-to-date, and live reporting system that collects all AEs associated with vaccines in the United States. Important clinical and laboratory information usually is available in the database; however, the main limitation is that this study can only demonstrate a possible association but not a causal relationship between vaccination and LP or LDE. There can be various sources of biases such as underreporting, overreporting, or inaccurate reporting.26,27 Pertinent clinical information (eg, new medications, new dental fillings/implants) that could potentially misrepresent the actual relationship between vaccination and development of AEs also was not available in the VAERS database. A cohort study with long-term follow-up or a large-scale case-control study would be useful in evaluating such associations.
Conclusion
Lichen planus and LDE can occur, albeit rarely, after vaccination, especially following hepatitis B vaccination. When middle-aged adults present to the clinic with LP or LDE, it is important to inquire about recent vaccination history in addition to a detailed medication history.
- Asarch A, Gottlieb AB, Lee J, et al. Lichen planus-like eruptions: an emerging side effect of tumor necrosis factor-alpha antagonists. J Am Acad Dermatol. 2009;61:104-111.
- Tarakji B, Ashok N, Alakeel R, et al. Hepatitis B vaccination and associated oral manifestations: a non-systemic review of literature and case reports. Ann Med Health Sci Res. 2014;4:829-836.
- Akay BN, Arslan A, Cekirge S, et al. The first reported case of lichen planus following inactivated influenza vaccination. J Drugs Dermatol. 2007;6:536-538.
- Sato NA, Kano Y, Shiohara T. Lichen planus occurring after influenza vaccination: report of three cases and review of the literature. Dermatology. 2010;221:296-299.
- Centers for Disease Control and Prevention. Hepatitis B FAQs for the public. https://www.cdc.gov/hepatitis/hbv/bfaq.htm. Updated May 23, 2016. Accessed April 4, 2017.
- Centers for Disease Control and Prevention. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2013-2014. MMWR Recomm Rep. 2013;62:1-43.
- Rebora A. Hepatitis viruses and lichen planus. Arch Dermatol. 1994;130:1328-1329.
- Black MM. Lichen planus and lichenoid disorders. In: Rook A, Wilkinson DS, Ebling FJG, eds. Textbook of Dermatology. 6th ed. London, England: Blackwell Science Inc; 1998:1899-1890.
- Ghasri P, Roehmholdt BF, Young LC. A case of lichen planus following Tdap vaccination. J Drugs Dermatol. 2011;10:1067-1069.
- Tasanen K, Renko M, Kandelberg P, et al. Childhood lichen planus after simultaneous measles-mumps-rubella and diphtheria-tetanus-pertussis-polio vaccinations. Br J Dermatol. 2008;58:646-648.
- Shiohara T, Kano Y. Lichen planus and lichenoid dermatoses. In: Bolognia JL, Jorizzo J, Rapini RP, eds. Dermatology. 2nd ed. New York, NY: Mosby Elsevier; 2008:159-180.
- Miller CS, Epstein JB, Hall EH, et al. Changing oral care needs in the United States: the continuing need for oral medicine. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:34-44.
- Bouquot JE, Gorlin RJ. Leukoplakia, lichen planus, and other oral keratoses in 23,616 white Americans over the age of 35 years. Oral Surg Oral Med Oral Pathol. 1986;61:373-381.
- Axéll T, Rundquist L. Oral lichen planus—a demographic study. Community Dent Oral Epidemiol. 1987;15:52-56.
- Alabi GO, Akinsanya JB. Lichen planus in tropical Africa. Trop Geogr Med. 1981;33:143-147.
- Pannell RS, Fleming DM, Cross KW. The incidence of molluscum contagiosum, scabies and lichen planus. Epidemiol Infect. 2005;133:985-991.
- Ciaccio M, Rebora A. Lichen planus following HBV vaccination: a coincidence? Br J Dermatol. 1990;122:424.
- Sugerman PB, Satterwhite K, Bigby M. Autocytotoxic T-cell clones in lichen planus. Br J Dermatol. 2000;142:449-456.
- Yawalkar N, Pichler WJ. Mechanisms of cutaneous drug reactions [in German]. J Dtsch Dermatol Ges. 2004;2:1013-1023; quiz 1024-1026.
- Yawalkar N, Pichler WJ. Immunohistology of drug-induced exanthema: clues to pathogenesis. Curr Opin Allergy Clin Immunol. 2001;1:299-303.
- Yawalkar N, Egli F, Hari Y, et al. Infiltration of cytotoxic T cells in drug-induced cutaneous eruptions. Clin Exp Allergy. 2000;30:847-855.
- Yawalkar N, Shrikhande M, Hari Y, et al. Evidence for a role for IL-5 and eotaxin in activating and recruiting eosinophils in drug-induced cutaneous eruptions. J Allergy Clin Immunol. 2000;106:1171-1176.
- Grabenstein JD. Immu
noFacts 2013: Vaccines and Immunologic Drugs. St Louis, MO: Wolters Kluwer Health; 2012. - Drago F, Rebora A. Cutaneous immunologic reactions to hepatitis B virus vaccine. Ann Intern Med. 2002;136:780.
- Quintero OL, Amador-Patarroyo MJ, Montoya-Ortiz G, et al. Autoimmune disease and gender: plausible mechanisms for the female predominance of autoimmunity [published online November 12, 2011]. J Autoimmun. 2012;38:J109-J119.
- Geier DA, Geier MR. A case-control study of serious autoimmune adverse events following hepatitis B immunization. Autoimmunity. 2005;38:295-301.
- Geier DA, Geier MR. A case-control study of quadrivalent human papillomavirus vaccine-associated autoimmune adverse events. Clin Rheumatol. 2015;34:1225-1231.
Lichen planus (LP) is a chronic inflammatory dermatosis of unknown origin that involves the skin and mucous membranes, and lichenoid drug eruption (LDE) is an uncommon cutaneous adverse reaction to a medication.1 The manifestations resemble each other clinically, and sometimes it is difficult to differentiate between them on histology. The pathogenesis still is not well characterized, especially the key initiating event that leads to the development of LP or LDE postimmunization. There have been reports of LP or LDEs after certain vaccines, especially the hepatitis B and influenza vaccines.2-4 Both vaccines are routinely administered in the United States; more than 100 million individuals have received the hepatitis B vaccine in the United States since it became available in 1982,5 and the Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention (CDC) recommends that all individuals 6 months or older receive an influenza vaccine every year.6 Currently, influenza vaccine coverage among adults 18 years or older reaches approximately 40% annually in the United States.6
Although certain viral infections (eg, hepatitis C virus) seem to play a role in the development of LP,7,8 the link between LP and hepatitis B vaccination is less well recognized. Reports of LP and LDE after vaccination have been largely limited to case reports and case series.2-4,9,10 Therefore, we aimed to characterize and review cases of LP and LDE following vaccination by analyzing the Vaccine Adverse Event Reporting System (VAERS) database.
Methods
The VAERS is a national vaccine safety surveillance database maintained jointly by the CDC and the US Food and Drug Administration to analyze adverse events (AEs) following immunizations. Serious AEs and deaths recorded in the VAERS were followed up periodically by VAERS staff. Information on vaccine-associated LP or LDE was retrieved from the VAERS database using the CDC WONDER online interface (http://wonder.cdc.gov/vaers.html). To examine if LP or LDE after vaccination occurred more frequently in patients with certain demographic risk factors, all reported cases of LP and LDE associated with vaccines administered from July 1990 to November 2014 were identified in the symptoms section of the VAERS system using the search terms lichen planus, oral lichen planus, and lichenoid drug eruption. Characteristics such as age, gender, time to onset, type of vaccine, method of diagnosis, and clinical outcome were collected.
The statistical package for social sciences (SPSS version 22) was utilized for the descriptive analysis. Fisher exact and χ2 tests were used to evaluate statistical significance. A 2-sided P value of <.05 was considered statistically significant.
Results
There were 434,943 reported AEs following vaccination in the VAERS database from July 1990 to November 2014; among them, 33 cases involved LP or LDE. Of these vaccine-associated AEs, LP was diagnosed in 23 (69.7%) cases, while LDE and oral LP were diagnosed in 6 (18.2%) and 4 (12.1%) cases, respectively. Females represented slightly more than half (57.6% [19/33]) of the total cases. The median age of onset was 47 years. Approximately two-thirds of the identified cases were confirmed on skin biopsy and histology, while the rest were diagnosed either by a dermatologist or a primary care physician. The time to onset of symptoms ranged from 1 to 297 days after vaccination, with a median time of 14 days.
Patients with LP or LDE were significantly older compared to the reported AEs overall (P<.001); the median age of onset was 47 years for LP or LDE compared to 24 years for all reported AEs. Table 1 shows the various vaccines associated with LP or LDE. The hepatitis B, influenza, and herpes zoster vaccines were the 3 most common types of vaccines associated with these conditions. The hepatitis B vaccine accounted for 24.2% (8/33) of the reported events, followed by influenza (18.2% [6/33]) and herpes zoster (15.2% [5/33]) vaccines. In addition, there were 3 cases of cutaneous reaction after receiving the combination hepatitis A and hepatitis B vaccine. Table 2 presents details of the reported events associated with hepatitis B, influenza, herpes zoster, combination hepatitis A and hepatitis B, and hepatitis A vaccination.
Of 8 AEs associated with hepatitis B vaccination, 1 AE resulted in permanent disability and required hospitalization. O
Comment
The estimated prevalence of LP ranges from 0.22% to 5% worldwide,11-15 with an incidence of 0.032% to 0.037%.16 Although rare, LP and LDE can occur from certain medications or vaccines. Cases of LP have been reported after hepatitis B and influenza vaccinations. The first case of LP following hepatitis B vaccination was described by Ciaccio and Rebora17 in 1990. Since then, a total of 50 similar cases have been reported worldwide.2 There also have been reports of LP following influenza, tetanus-diphtheria-pertussis, measles-mumps-rubella, and inactivated polio vaccines.3,4,9,10 Table 3 summarizes cases of LP following various vaccinations.
The key initiating event of the pathogenesis for both LP and LDE is not completely understood. Both conditions share similar immunologic mechanisms of persistently activated CD8 autocytotoxic T lymphocytes against epidermal cells.18 These cells can induce apoptosis of basal epidermal keratinocytes and generate various cytokines (eg, IFN-γ, IL-5) to enhance expression of class II MHC molecules and antigen presentation to CD4 T cells.19-22 It is conceivable that one of the initiating factors may be related to components in vaccines.
Hepatitis B, influenza, and herpes zoster vaccines were the 3 most common vaccines implicated in postimmunization LP or LDEs in our study. The excipients of these vaccines were compared based on the product inserts to identify any common components. It was found that all 3 vaccines contain either yeast protein or egg protein with various forms of phosphate buffers, while the hepatitis A and herpes zoster vaccines share Medical Research Council cell strain 5 (human diploid) cells as well as other cellular components.23 Sato et al4 suggested that specific vaccine components, such as the vaccine itself or egg proteins, could have contributed to the development of LP following vaccination. It has been postulated that the protein S fraction of hepatitis B surface antigen plays a crucial role in the pathogenesis of both LP and LDE after hepatitis B vaccination.2,24 It is likely that protein S shares common epitopes on keratinocytes that are recognized by the immune system, thus activating cytotoxic T lymphocytes and inducing apoptosis.2,24
In this study, the median time to onset of vaccine-related LP was 14 days, which is consistent with a case series by Sato et al,4 suggesting that adverse reactions mainly occurred within 2 weeks after influenza vaccination. Onset of symptoms within 2 weeks of vaccination would therefore be a crucial clue for diagnosing possible vaccine-related LP or LDE. On the other hand, at least 4 patients in our study had onset of LP and LDE more than 1 month after vaccination; 2 of 4 cases even reported symptom onset at 175 and 297 days after hepatitis B vaccination, which were much longer than the 120 days reported by Tarakji et al.2 It is not known if these cases constitute true vaccine-associated LP or LDE or if unmeasured confounding factors such as concurrent medications or comorbidities may have contributed to the development of these AEs.
It also is interesting to note that LP and LDE affected mainly middle-aged women. An increased risk of autoimmunity in female adults partly explains this observation.25 Some vaccines, such as herpes zoster and influenza vaccines, generally are recommended for older adults who also are more likely to have multiple comorbidities or take multiple medications/supplements, which can potentially skew the prevalence of AEs toward an older age group. It should be noted, however, that LP and LDE were relatively uncommon AEs following vaccination in the current study. In this study, LP and LDE consisted of only 0.01% (N=42,230) of all AEs after hepatitis B vaccination, while the more common AEs such as pyrexia, nonspecific rashes, nonspecific gastrointestinal symptoms, and headache contributed to approximately 66.5% of all reported events.
One of the strengths of our study is that up to two-thirds of cases were confirmed histologically and all patients were seen and followed up by dermatologists or physicians. The VAERS is an easily accessible, up-to-date, and live reporting system that collects all AEs associated with vaccines in the United States. Important clinical and laboratory information usually is available in the database; however, the main limitation is that this study can only demonstrate a possible association but not a causal relationship between vaccination and LP or LDE. There can be various sources of biases such as underreporting, overreporting, or inaccurate reporting.26,27 Pertinent clinical information (eg, new medications, new dental fillings/implants) that could potentially misrepresent the actual relationship between vaccination and development of AEs also was not available in the VAERS database. A cohort study with long-term follow-up or a large-scale case-control study would be useful in evaluating such associations.
Conclusion
Lichen planus and LDE can occur, albeit rarely, after vaccination, especially following hepatitis B vaccination. When middle-aged adults present to the clinic with LP or LDE, it is important to inquire about recent vaccination history in addition to a detailed medication history.
Lichen planus (LP) is a chronic inflammatory dermatosis of unknown origin that involves the skin and mucous membranes, and lichenoid drug eruption (LDE) is an uncommon cutaneous adverse reaction to a medication.1 The manifestations resemble each other clinically, and sometimes it is difficult to differentiate between them on histology. The pathogenesis still is not well characterized, especially the key initiating event that leads to the development of LP or LDE postimmunization. There have been reports of LP or LDEs after certain vaccines, especially the hepatitis B and influenza vaccines.2-4 Both vaccines are routinely administered in the United States; more than 100 million individuals have received the hepatitis B vaccine in the United States since it became available in 1982,5 and the Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention (CDC) recommends that all individuals 6 months or older receive an influenza vaccine every year.6 Currently, influenza vaccine coverage among adults 18 years or older reaches approximately 40% annually in the United States.6
Although certain viral infections (eg, hepatitis C virus) seem to play a role in the development of LP,7,8 the link between LP and hepatitis B vaccination is less well recognized. Reports of LP and LDE after vaccination have been largely limited to case reports and case series.2-4,9,10 Therefore, we aimed to characterize and review cases of LP and LDE following vaccination by analyzing the Vaccine Adverse Event Reporting System (VAERS) database.
Methods
The VAERS is a national vaccine safety surveillance database maintained jointly by the CDC and the US Food and Drug Administration to analyze adverse events (AEs) following immunizations. Serious AEs and deaths recorded in the VAERS were followed up periodically by VAERS staff. Information on vaccine-associated LP or LDE was retrieved from the VAERS database using the CDC WONDER online interface (http://wonder.cdc.gov/vaers.html). To examine if LP or LDE after vaccination occurred more frequently in patients with certain demographic risk factors, all reported cases of LP and LDE associated with vaccines administered from July 1990 to November 2014 were identified in the symptoms section of the VAERS system using the search terms lichen planus, oral lichen planus, and lichenoid drug eruption. Characteristics such as age, gender, time to onset, type of vaccine, method of diagnosis, and clinical outcome were collected.
The statistical package for social sciences (SPSS version 22) was utilized for the descriptive analysis. Fisher exact and χ2 tests were used to evaluate statistical significance. A 2-sided P value of <.05 was considered statistically significant.
Results
There were 434,943 reported AEs following vaccination in the VAERS database from July 1990 to November 2014; among them, 33 cases involved LP or LDE. Of these vaccine-associated AEs, LP was diagnosed in 23 (69.7%) cases, while LDE and oral LP were diagnosed in 6 (18.2%) and 4 (12.1%) cases, respectively. Females represented slightly more than half (57.6% [19/33]) of the total cases. The median age of onset was 47 years. Approximately two-thirds of the identified cases were confirmed on skin biopsy and histology, while the rest were diagnosed either by a dermatologist or a primary care physician. The time to onset of symptoms ranged from 1 to 297 days after vaccination, with a median time of 14 days.
Patients with LP or LDE were significantly older compared to the reported AEs overall (P<.001); the median age of onset was 47 years for LP or LDE compared to 24 years for all reported AEs. Table 1 shows the various vaccines associated with LP or LDE. The hepatitis B, influenza, and herpes zoster vaccines were the 3 most common types of vaccines associated with these conditions. The hepatitis B vaccine accounted for 24.2% (8/33) of the reported events, followed by influenza (18.2% [6/33]) and herpes zoster (15.2% [5/33]) vaccines. In addition, there were 3 cases of cutaneous reaction after receiving the combination hepatitis A and hepatitis B vaccine. Table 2 presents details of the reported events associated with hepatitis B, influenza, herpes zoster, combination hepatitis A and hepatitis B, and hepatitis A vaccination.
Of 8 AEs associated with hepatitis B vaccination, 1 AE resulted in permanent disability and required hospitalization. O
Comment
The estimated prevalence of LP ranges from 0.22% to 5% worldwide,11-15 with an incidence of 0.032% to 0.037%.16 Although rare, LP and LDE can occur from certain medications or vaccines. Cases of LP have been reported after hepatitis B and influenza vaccinations. The first case of LP following hepatitis B vaccination was described by Ciaccio and Rebora17 in 1990. Since then, a total of 50 similar cases have been reported worldwide.2 There also have been reports of LP following influenza, tetanus-diphtheria-pertussis, measles-mumps-rubella, and inactivated polio vaccines.3,4,9,10 Table 3 summarizes cases of LP following various vaccinations.
The key initiating event of the pathogenesis for both LP and LDE is not completely understood. Both conditions share similar immunologic mechanisms of persistently activated CD8 autocytotoxic T lymphocytes against epidermal cells.18 These cells can induce apoptosis of basal epidermal keratinocytes and generate various cytokines (eg, IFN-γ, IL-5) to enhance expression of class II MHC molecules and antigen presentation to CD4 T cells.19-22 It is conceivable that one of the initiating factors may be related to components in vaccines.
Hepatitis B, influenza, and herpes zoster vaccines were the 3 most common vaccines implicated in postimmunization LP or LDEs in our study. The excipients of these vaccines were compared based on the product inserts to identify any common components. It was found that all 3 vaccines contain either yeast protein or egg protein with various forms of phosphate buffers, while the hepatitis A and herpes zoster vaccines share Medical Research Council cell strain 5 (human diploid) cells as well as other cellular components.23 Sato et al4 suggested that specific vaccine components, such as the vaccine itself or egg proteins, could have contributed to the development of LP following vaccination. It has been postulated that the protein S fraction of hepatitis B surface antigen plays a crucial role in the pathogenesis of both LP and LDE after hepatitis B vaccination.2,24 It is likely that protein S shares common epitopes on keratinocytes that are recognized by the immune system, thus activating cytotoxic T lymphocytes and inducing apoptosis.2,24
In this study, the median time to onset of vaccine-related LP was 14 days, which is consistent with a case series by Sato et al,4 suggesting that adverse reactions mainly occurred within 2 weeks after influenza vaccination. Onset of symptoms within 2 weeks of vaccination would therefore be a crucial clue for diagnosing possible vaccine-related LP or LDE. On the other hand, at least 4 patients in our study had onset of LP and LDE more than 1 month after vaccination; 2 of 4 cases even reported symptom onset at 175 and 297 days after hepatitis B vaccination, which were much longer than the 120 days reported by Tarakji et al.2 It is not known if these cases constitute true vaccine-associated LP or LDE or if unmeasured confounding factors such as concurrent medications or comorbidities may have contributed to the development of these AEs.
It also is interesting to note that LP and LDE affected mainly middle-aged women. An increased risk of autoimmunity in female adults partly explains this observation.25 Some vaccines, such as herpes zoster and influenza vaccines, generally are recommended for older adults who also are more likely to have multiple comorbidities or take multiple medications/supplements, which can potentially skew the prevalence of AEs toward an older age group. It should be noted, however, that LP and LDE were relatively uncommon AEs following vaccination in the current study. In this study, LP and LDE consisted of only 0.01% (N=42,230) of all AEs after hepatitis B vaccination, while the more common AEs such as pyrexia, nonspecific rashes, nonspecific gastrointestinal symptoms, and headache contributed to approximately 66.5% of all reported events.
One of the strengths of our study is that up to two-thirds of cases were confirmed histologically and all patients were seen and followed up by dermatologists or physicians. The VAERS is an easily accessible, up-to-date, and live reporting system that collects all AEs associated with vaccines in the United States. Important clinical and laboratory information usually is available in the database; however, the main limitation is that this study can only demonstrate a possible association but not a causal relationship between vaccination and LP or LDE. There can be various sources of biases such as underreporting, overreporting, or inaccurate reporting.26,27 Pertinent clinical information (eg, new medications, new dental fillings/implants) that could potentially misrepresent the actual relationship between vaccination and development of AEs also was not available in the VAERS database. A cohort study with long-term follow-up or a large-scale case-control study would be useful in evaluating such associations.
Conclusion
Lichen planus and LDE can occur, albeit rarely, after vaccination, especially following hepatitis B vaccination. When middle-aged adults present to the clinic with LP or LDE, it is important to inquire about recent vaccination history in addition to a detailed medication history.
- Asarch A, Gottlieb AB, Lee J, et al. Lichen planus-like eruptions: an emerging side effect of tumor necrosis factor-alpha antagonists. J Am Acad Dermatol. 2009;61:104-111.
- Tarakji B, Ashok N, Alakeel R, et al. Hepatitis B vaccination and associated oral manifestations: a non-systemic review of literature and case reports. Ann Med Health Sci Res. 2014;4:829-836.
- Akay BN, Arslan A, Cekirge S, et al. The first reported case of lichen planus following inactivated influenza vaccination. J Drugs Dermatol. 2007;6:536-538.
- Sato NA, Kano Y, Shiohara T. Lichen planus occurring after influenza vaccination: report of three cases and review of the literature. Dermatology. 2010;221:296-299.
- Centers for Disease Control and Prevention. Hepatitis B FAQs for the public. https://www.cdc.gov/hepatitis/hbv/bfaq.htm. Updated May 23, 2016. Accessed April 4, 2017.
- Centers for Disease Control and Prevention. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2013-2014. MMWR Recomm Rep. 2013;62:1-43.
- Rebora A. Hepatitis viruses and lichen planus. Arch Dermatol. 1994;130:1328-1329.
- Black MM. Lichen planus and lichenoid disorders. In: Rook A, Wilkinson DS, Ebling FJG, eds. Textbook of Dermatology. 6th ed. London, England: Blackwell Science Inc; 1998:1899-1890.
- Ghasri P, Roehmholdt BF, Young LC. A case of lichen planus following Tdap vaccination. J Drugs Dermatol. 2011;10:1067-1069.
- Tasanen K, Renko M, Kandelberg P, et al. Childhood lichen planus after simultaneous measles-mumps-rubella and diphtheria-tetanus-pertussis-polio vaccinations. Br J Dermatol. 2008;58:646-648.
- Shiohara T, Kano Y. Lichen planus and lichenoid dermatoses. In: Bolognia JL, Jorizzo J, Rapini RP, eds. Dermatology. 2nd ed. New York, NY: Mosby Elsevier; 2008:159-180.
- Miller CS, Epstein JB, Hall EH, et al. Changing oral care needs in the United States: the continuing need for oral medicine. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:34-44.
- Bouquot JE, Gorlin RJ. Leukoplakia, lichen planus, and other oral keratoses in 23,616 white Americans over the age of 35 years. Oral Surg Oral Med Oral Pathol. 1986;61:373-381.
- Axéll T, Rundquist L. Oral lichen planus—a demographic study. Community Dent Oral Epidemiol. 1987;15:52-56.
- Alabi GO, Akinsanya JB. Lichen planus in tropical Africa. Trop Geogr Med. 1981;33:143-147.
- Pannell RS, Fleming DM, Cross KW. The incidence of molluscum contagiosum, scabies and lichen planus. Epidemiol Infect. 2005;133:985-991.
- Ciaccio M, Rebora A. Lichen planus following HBV vaccination: a coincidence? Br J Dermatol. 1990;122:424.
- Sugerman PB, Satterwhite K, Bigby M. Autocytotoxic T-cell clones in lichen planus. Br J Dermatol. 2000;142:449-456.
- Yawalkar N, Pichler WJ. Mechanisms of cutaneous drug reactions [in German]. J Dtsch Dermatol Ges. 2004;2:1013-1023; quiz 1024-1026.
- Yawalkar N, Pichler WJ. Immunohistology of drug-induced exanthema: clues to pathogenesis. Curr Opin Allergy Clin Immunol. 2001;1:299-303.
- Yawalkar N, Egli F, Hari Y, et al. Infiltration of cytotoxic T cells in drug-induced cutaneous eruptions. Clin Exp Allergy. 2000;30:847-855.
- Yawalkar N, Shrikhande M, Hari Y, et al. Evidence for a role for IL-5 and eotaxin in activating and recruiting eosinophils in drug-induced cutaneous eruptions. J Allergy Clin Immunol. 2000;106:1171-1176.
- Grabenstein JD. Immu
noFacts 2013: Vaccines and Immunologic Drugs. St Louis, MO: Wolters Kluwer Health; 2012. - Drago F, Rebora A. Cutaneous immunologic reactions to hepatitis B virus vaccine. Ann Intern Med. 2002;136:780.
- Quintero OL, Amador-Patarroyo MJ, Montoya-Ortiz G, et al. Autoimmune disease and gender: plausible mechanisms for the female predominance of autoimmunity [published online November 12, 2011]. J Autoimmun. 2012;38:J109-J119.
- Geier DA, Geier MR. A case-control study of serious autoimmune adverse events following hepatitis B immunization. Autoimmunity. 2005;38:295-301.
- Geier DA, Geier MR. A case-control study of quadrivalent human papillomavirus vaccine-associated autoimmune adverse events. Clin Rheumatol. 2015;34:1225-1231.
- Asarch A, Gottlieb AB, Lee J, et al. Lichen planus-like eruptions: an emerging side effect of tumor necrosis factor-alpha antagonists. J Am Acad Dermatol. 2009;61:104-111.
- Tarakji B, Ashok N, Alakeel R, et al. Hepatitis B vaccination and associated oral manifestations: a non-systemic review of literature and case reports. Ann Med Health Sci Res. 2014;4:829-836.
- Akay BN, Arslan A, Cekirge S, et al. The first reported case of lichen planus following inactivated influenza vaccination. J Drugs Dermatol. 2007;6:536-538.
- Sato NA, Kano Y, Shiohara T. Lichen planus occurring after influenza vaccination: report of three cases and review of the literature. Dermatology. 2010;221:296-299.
- Centers for Disease Control and Prevention. Hepatitis B FAQs for the public. https://www.cdc.gov/hepatitis/hbv/bfaq.htm. Updated May 23, 2016. Accessed April 4, 2017.
- Centers for Disease Control and Prevention. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2013-2014. MMWR Recomm Rep. 2013;62:1-43.
- Rebora A. Hepatitis viruses and lichen planus. Arch Dermatol. 1994;130:1328-1329.
- Black MM. Lichen planus and lichenoid disorders. In: Rook A, Wilkinson DS, Ebling FJG, eds. Textbook of Dermatology. 6th ed. London, England: Blackwell Science Inc; 1998:1899-1890.
- Ghasri P, Roehmholdt BF, Young LC. A case of lichen planus following Tdap vaccination. J Drugs Dermatol. 2011;10:1067-1069.
- Tasanen K, Renko M, Kandelberg P, et al. Childhood lichen planus after simultaneous measles-mumps-rubella and diphtheria-tetanus-pertussis-polio vaccinations. Br J Dermatol. 2008;58:646-648.
- Shiohara T, Kano Y. Lichen planus and lichenoid dermatoses. In: Bolognia JL, Jorizzo J, Rapini RP, eds. Dermatology. 2nd ed. New York, NY: Mosby Elsevier; 2008:159-180.
- Miller CS, Epstein JB, Hall EH, et al. Changing oral care needs in the United States: the continuing need for oral medicine. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:34-44.
- Bouquot JE, Gorlin RJ. Leukoplakia, lichen planus, and other oral keratoses in 23,616 white Americans over the age of 35 years. Oral Surg Oral Med Oral Pathol. 1986;61:373-381.
- Axéll T, Rundquist L. Oral lichen planus—a demographic study. Community Dent Oral Epidemiol. 1987;15:52-56.
- Alabi GO, Akinsanya JB. Lichen planus in tropical Africa. Trop Geogr Med. 1981;33:143-147.
- Pannell RS, Fleming DM, Cross KW. The incidence of molluscum contagiosum, scabies and lichen planus. Epidemiol Infect. 2005;133:985-991.
- Ciaccio M, Rebora A. Lichen planus following HBV vaccination: a coincidence? Br J Dermatol. 1990;122:424.
- Sugerman PB, Satterwhite K, Bigby M. Autocytotoxic T-cell clones in lichen planus. Br J Dermatol. 2000;142:449-456.
- Yawalkar N, Pichler WJ. Mechanisms of cutaneous drug reactions [in German]. J Dtsch Dermatol Ges. 2004;2:1013-1023; quiz 1024-1026.
- Yawalkar N, Pichler WJ. Immunohistology of drug-induced exanthema: clues to pathogenesis. Curr Opin Allergy Clin Immunol. 2001;1:299-303.
- Yawalkar N, Egli F, Hari Y, et al. Infiltration of cytotoxic T cells in drug-induced cutaneous eruptions. Clin Exp Allergy. 2000;30:847-855.
- Yawalkar N, Shrikhande M, Hari Y, et al. Evidence for a role for IL-5 and eotaxin in activating and recruiting eosinophils in drug-induced cutaneous eruptions. J Allergy Clin Immunol. 2000;106:1171-1176.
- Grabenstein JD. Immu
noFacts 2013: Vaccines and Immunologic Drugs. St Louis, MO: Wolters Kluwer Health; 2012. - Drago F, Rebora A. Cutaneous immunologic reactions to hepatitis B virus vaccine. Ann Intern Med. 2002;136:780.
- Quintero OL, Amador-Patarroyo MJ, Montoya-Ortiz G, et al. Autoimmune disease and gender: plausible mechanisms for the female predominance of autoimmunity [published online November 12, 2011]. J Autoimmun. 2012;38:J109-J119.
- Geier DA, Geier MR. A case-control study of serious autoimmune adverse events following hepatitis B immunization. Autoimmunity. 2005;38:295-301.
- Geier DA, Geier MR. A case-control study of quadrivalent human papillomavirus vaccine-associated autoimmune adverse events. Clin Rheumatol. 2015;34:1225-1231.
Practice Points
- Lichen planus (LP) and lichenoid drug eruptions (LDEs) can uncommonly occur after vaccination.
- Common vaccines associated with LP and LDEs include hepatitis B and influenza vaccinations.
- It is important to be cognizant of such reactions, especially in patients who have recently received these common vaccines.