User login
Antipsychotic nonadherence is a known contributor to relapse risk among patients with schizophrenia.1 Because relapse episodes may be associated with antipsychotic treatment resistance, this must be avoided as much as possible by appropriate medication selection.2 Adverse effect burden is an important factor leading to oral antipsychotic nonadherence, with patient-derived data indicating that extrapyramidal symptoms (EPS) (odds ratio [OR] 0.57, P = .0007), sedation/cognitive adverse effects (OR 0.70, P = .033), prolactin/endocrine effects (OR 0.69, P = .0342), and metabolic adverse effects (OR 0.64, P = .0079) are all significantly related to lower rates of adherence.3 With this in mind, successive generations of antipsychotics have been released, with fewer tolerability issues present than seen with earlier compounds.1,4 Although these newer second-generation antipsychotics (SGAs) have not proven more effective for schizophrenia than those first marketed in the 1990s, they generally possess lower rates of EPS, hyperprolactinemia, anticholinergic and antihistaminic properties, metabolic adverse effects, and orthostasis.5 This improved adverse effect profile will hopefully increase the chances of antipsychotic acceptance in patients with schizophrenia, and thereby promote improved adherence.
Lumateperone (Caplyta) is a novel oral antipsychotic approved for the treatment of adult patients with schizophrenia (Table 1). It possesses some properties seen with other SGAs, including high affinity for serotonin 5HT2A receptors (Ki 0.54 nM) and lower affinity for dopamine D2 receptors (Ki 32 nM), along with low affinity for alpha1-adrenergic receptors (Ki 73 nM), and muscarinic and histaminergic receptors (Ki > 100 nM).6,7 However, there are some distinguishing features: the ratio of 5HT2A receptor affinity to D2 affinity is 60, greater than that of other SGAs such as risperidone (12), olanzapine (12.4) or aripiprazole (0.18)8; at steady state, the D2 occupancy remains <40% (Figure) and the corresponding rates of EPS/akathisia were only 6.7% for lumateperone vs 6.3% for placebo in short-term clinical trials.7,9
How it works
A unique aspect of lumateperone’s pharmacology may relate to differential actions at presynaptic and postsynaptic dopamine D2 receptors. Other antipsychotics possess comparable antagonist (or partial agonist) properties at postsynaptic D2 receptors (the D2 long isoform) and the presynaptic autoreceptor (the D2 short isoform). By blocking the presynaptic autoreceptor, feedback inhibition on dopamine release is removed; therefore, the required higher levels of postsynaptic D2 receptor occupancy needed for effective antipsychotic action (eg, 60% to 80% for antagonists, and 83% to 100% for partial agonists) may be a product of the need to oppose this increased presynaptic release of dopamine. In vitro assays show that lumateperone does not increase presynaptic dopamine release, indicating that it possesses agonist properties at the presynaptic D2 short receptor.10 That property may explain how lumateperone functions as an antipsychotic despite low levels of D2 receptor occupancy.10
Another hypothesis is based on our understanding of pimavanserin’s pharmacology. Pimavanserin is a selective 5HT2A antagonist FDA-approved for the treatment of Parkinson’s disease psychosis (PDP), with extremely high receptor affinity (Ki 0.087 nM) and no appreciable binding at dopamine receptors.5 Pimavanserin not only treats PDP, but is being evaluated in clinical trials for dementia-related psychosis, and has positive data for augmenting antipsychotics when there is a low level of D2 blockade.11,12 In a controlled trial, pimavanserin added to risperidone, 2 mg/d, was as effective as risperidone, 6 mg/d, illustrating the point that near-saturation of the 5HT2A receptor can increase antipsychotic efficacy when dopamine blockade is relatively low. For risperidone, 2 mg/d, the expected D2 occupancy is only 60%.13
Lumateperone also has moderate binding affinity for serotonin transporters (SERT) (Ki 33 nM). Serotonin transporter occupancy at the dose approved for schizophrenia (42 mg/d) is approximately 30%,14 below the ≥80% SERT occupancy seen with selective serotonin reuptake inhibitor (SSRI) antidepressants; nevertheless, there is evidence for antidepressant effects seen in preclinical assays, schizophrenia studies, and phase III trials for bipolar depression.8,15,16 It is hypothesized that near-saturation of the 5HT2A receptor might act synergistically with the modest extent of 5HT reuptake inhibition to promote downstream effects associated with effective antidepressant treatments.8 In vivo data also showed phosphorylation of N-methyl-
Clinical implications
Nonadherence with oral antipsychotics among patients with schizophrenia is often related to adverse effects.17 The SGAs marketed since 2000 generally have lower rates of sedation and metabolic and/or endocrine adverse events than earlier compounds, yet each has limitations:
- asenapine: sedation and weight gain
- the partial agonists (aripiprazole, brexpiprazole, cariprazine): akathisia
- lurasidone: dose-dependent EPS and akathisia
- iloperidone: orthostasis.18
Ziprasidone is an exception, because it had low rates of most adverse effects in schizophrenia trials, but the need to take it twice daily with a 500 kcal meal hampers its use. A meta-analysis of 32 oral antipsychotics, including first-generation agents, noted that the efficacy differences between medications are slight for patients without treatment-resistant schizophrenia, but “differences in side-effects are more marked.”18
Continue to: Until novel mechanisms are discovered...
Until novel mechanisms are discovered that increase schizophrenia response rates, the availability of newer antipsychotics with more favorable tolerability profiles presents clinicians and patients with added options when adverse effects interfere with prior treatment. In all phases of the adult schizophrenia trial program for lumateperone, 811 patients received short-term (4- to 6-week) exposure (dose range: 14 to 84 mg/d), while 329 had ≥6 months exposure and 108 had ≥1 year of exposure to the 42-mg/d dose. In these studies, there was no single adverse reaction leading to discontinuation that occurred at a rate >2%. The only adverse events that occurred at rates ≥5% and more than twice the rate of placebo were somnolence/sedation (lumateperone 24%, placebo 10%), and dry mouth (lumateperone 6%, placebo 2%). Nausea was present in 9% of the lumateperone group compared with 5% for placebo.7 In the short-term studies, the combined rate of EPS and akathisia was 6.7% for lumateperone and 6.3% for placebo.7 This difference translates to a number needed to harm of 250 for these neurologic adverse effects. The functional impact of lumateperone’s glutamatergic mechanisms is not well characterized within the schizophrenia population, but the antidepressant potential has been studied for patients with bipolar depression, with 1 positive phase III trial.19
Efficacy in adults with schizophrenia. The efficacy of lumateperone has been established in 2 pivotal, double-blind, placebo-controlled trials. The first was a 4-week, phase II trial (Study 005) in which 335 adults age 18 to 55 with an acute exacerbation of schizophrenia were randomized in a 1:1:1:1 manner to lumateperone, 42 mg/d (60 mg of lumateperone tosylate), lumateperone, 84 mg/d (120 mg of lumateperone tosylate), risperidone, 4 mg/d, or placebo, all taken once daily.20 For the 4 treatment arms, the least squares mean changes from baseline to the Day 28 endpoint on the primary outcome measure, Positive and Negative Syndrome Scale (PANSS) total score, were: lumateperone, 42 mg/d: −13.2 points; lumateperone, 84 mg/d: −8.3 points; risperidone, 4 mg/d: −13.4 points; and placebo: −7.4 points. Both lumateperone, 42 mg/d, and risperidone, 4 mg/d, were significantly different than placebo, and with identical moderate effect sizes of 0.4.20 Lumateperone, 84 mg/d, did not separate from placebo on the primary outcome. The responder analysis also indicated that a similar proportion of patients (40%) randomized to lumateperone, 42 mg/d, or risperidone, 4 mg/d, improved by ≥30% on PANSS total score.
The second pivotal trial (Study 301) was a phase III, double-blind, placebo-controlled trial of 450 adults, age 18 to 60, with an acute exacerbation of schizophrenia who were randomized in 1:1:1 manner to receive lumateperone, 42 mg/d (lumateperone tosylate 60 mg), lumateperone, 28 mg/d (lumateperone tosylate 40 mg), or placebo once daily for 4 weeks.21 For the 3 treatment arms, the least squares mean changes on PANSS total score from baseline to the Day 28 endpoint were: lumateperone, 42 mg/d: −14.5 points; lumateperone, 28 mg/d: −12.9 points; and placebo: −10.3 points. Lumateperone, 28 mg/d, did not separate from placebo on the primary outcome. The responder analysis also indicated that 36.5% of those receiving lumateperone, 42 mg/d, and 36.3% of those receiving lumateperone, 28 mg/d, improved by ≥30% on PANSS total score, compared with 25.5% of patients treated with placebo.
Unlike the 2 positive trials in which placebo change in total PANSS scores were −7.4 and −10.3 points, respectively, in a phase III trial (Study 302) with 696 participants, placebo showed a 15.1-point decrease from baseline PANSS total score.19 Among the 3 treatment arms of this study (lumateperone, 14 mg/d, lumateperone, 42 mg/d, and risperidone, 4 mg/d), only risperidone was superior to placebo.
Adverse events
In the phase II pivotal study, completion rates among the 4 arms were comparable: lumateperone, 42 mg/d: 71%; lumateperone, 84 mg/d: 76%; risperidone, 4 mg/d: 77%; and placebo: 72%.20 There were no serious adverse events (SAEs) associated with lumateperone; the 2 SAEs that occurred involved worsening of schizophrenia/psychotic disorder for risperidone (n = 1) and for placebo (n = 1). Five participants discontinued the study due to an adverse event: 2 who were receiving lumateperone (1 due to dry mouth, and 1 due to worsening of schizophrenia) and 3 who were receiving risperidone (2 due to akathisia, and 1 due to blood creatine phosphokinase increase).20 The most frequent adverse event was somnolence/sedation (placebo: 13%; lumateperone, 42 mg/d: 17%; risperidone, 4 mg/d: 21%; and lumateperone, 84 mg/d: 32.5%). Neither dose of lumateperone was associated with increased rates of EPS. Median weight gain to Day 28 was 1 kg for placebo and for each dose of lumateperone, and 2.5 kg for risperidone. Compared with risperidone, lumateperone showed statistically significantly lower prolactin levels (lumateperone, 42 mg/d and 84 mg/d: P < .001), and metabolic parameters, including fasting glucose (lumateperone 42 mg/d: P = .007; lumateperone, 84 mg/d: P = .023), total cholesterol (lumateperone, 42 mg/d: P = .012; lumateperone, 84 mg/d: P = .004), and triglycerides (lumateperone, 42 mg/d: P = .074; lumateperone, 84 mg/d: P = .002).20 There was no significant impact on the corrected QT interval.
Continue to: In the phase III trial...
In the phase III trial, completion rates among the 3 arms were lumateperone, 42 mg/d: 85%; lumateperone, 28 mg/d: 80%; and placebo: 74%. There was 1 SAE in a patient receiving lumateperone, 28 mg/d. This individual had preexisting risk factors and a history of seizures, and experienced a seizure during the study. Adverse events that occurred in either lumateperone group at a rate ≥5% and more than twice the rate of placebo were somnolence (lumateperone, 42 mg/d: 17.3%; lumateperone, 28 mg/d: 11.3%; and placebo: 4.0%); sedation (lumateperone, 42 mg/d: 12.7%; lumateperone, 28 mg/d: 9.3%; and placebo: 5.4%); fatigue (lumateperone, 42 mg/d: 5.3%; lumateperone, 28 mg/d: 4.7%; and placebo: 1.3%); and constipation (lumateperone, 42 mg/d: 6.7%; lumateperone, 28 mg/d: 4.0%; and placebo: 2.7%).21 No EPS-related adverse events occurred in ≥5% patients in any treatment arm. Median change in weight from baseline to Day 28 was 0.9 kg for lumateperone, 42 mg/d, 0.6 kg for lumateperone, 28 mg/d, and 0.7 kg for placebo. There were no significant mean changes in metabolic parameters for any treatment arm, and none of the patients had a corrected QT interval (QTc) >500 ms or a change in QTc >60 ms from baseline.21
Pharmacologic profile
Lumateperone’s in vitro binding profile includes high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), lower affinity for dopamine D2 receptors (Ki 32 nM), moderate binding affinity for SERT (Ki 33 nM), and lower affinity for alpha 1-adrenergic receptors (Ki 73 nM) and muscarinic and histaminergic receptors (Ki >100 nM).6,7 As noted above, this 60-fold ratio of 5HT2A to D2 affinity is extremely high; moreover, imaging data reveal low D2 receptor occupancy, consistent with the lack of clinically significant EPS seen in the trials. In vitro assays also reveal impact on glutamate pathways, and pathways associated with antidepressant response.8 The clinical benefits of the glutamatergic properties remain theoretical, but the antidepressant benefit has been seen in a positive phase III trial for bipolar depression.19
Clinical considerations
Effect sizes in the 2 positive pivotal trials were 0.3 and 0.4, comparable with those for other antipsychotics approved within the last decade: brexpiprazole, 0.26; cariprazine, 0.34; and lurasidone, 0.36.21 The absence of clinically significant EPS, lack of impact on metabolic or endocrine parameters, and lack of titration are all appealing properties. That only 42 mg/d proved effective may reflect the fact that the other doses studied to date in randomized, fixed-dose studies were 14 mg/d (Study 302) and 84 mg/d (Study 005), evaluated in one study each. While those 2 doses might indeed be outside the therapeutic window, given the heterogeneity of schizophrenia, future studies might help further refine the therapeutic range for schizophrenia, especially for doses closer to 42 mg/d (eg, 28 mg/d, 63 mg/d). Should 42 mg/d not prove effective, there is no data for now to suggest whether a dose increase may be helpful. As there is only 1 marketed dose of lumateperone (42-mg capsules), and no easy way to modify this dose, lumateperone’s package insert includes cautionary language regarding situations where there will be less-than-expected drug exposure (use of cytochrome P450 [CYP] 3A4 inducers), greater-than-expected drug exposure (moderate or strong CYP 3A4 inhibitors), or use in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria (Child-Pugh B or C). These are not contraindications.
Unique properties of lumateperone include the lack of presynaptic dopamine D2 antagonism, low D2 receptor occupancy, and the absence of significant EPS and metabolic or endocrine adverse effects. In vitro data indicate glutamatergic effects, and human data indicate antidepressant effects in bipolar patients. Despite the absence of significant histamine H1 or muscarinic affinity, the rate of somnolence/sedation was twice that of placebo (lumateperone 24%, placebo 10%).7
Why Rx? Reasons to prescribe lumateperone for adult patients with schizophrenia include:
- Favorable tolerability profile, including no significant signal for EPS or endocrine or metabolic adverse effects, and no QT prolongation
- No need for titration.
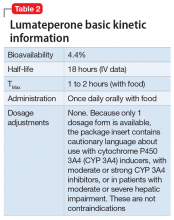
Dosing. There is only 1 dose available for lumateperone, 42-mg capsules (Table 2). As the dose cannot be modified, the package insert contains cautionary language regarding situations with less-than-expected drug exposure (use of CYP 3A4 inducers), greater-than-expected drug exposure (moderate or strong CYP 3A4 inhibitors), or use in patients with moderate or severe hepatic impairment as defined by Child-Pugh criteria (Child-Pugh B or C). These are not contraindications.
Contraindications. The only contraindication is known hypersensitivity to lumateperone.
Continue to: Bottom Line
Bottom Line
Lumateperone is a novel oral antipsychotic indicated for treating adults with schizophrenia. Its unique properties include the lack of presynaptic dopamine D2 antagonism, low D2 receptor occupancy, and the absence of significant extrapyramidal symptoms and metabolic or endocrine adverse effects. In clinical trials, the most frequent adverse event was somnolence/sedation.
Related Resource
- Fulton D. FDA approves Caplyta to treat schizophrenenia in adults. https://www.mdedge.com/psychiatry/article/214733/schizophrenia-other-psychotic-disorders/fda-approves-caplyta-treat.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Iloperidone • Fanapt
Lumateperone • Caplyta
Lurasidone • Latuda
Olanzapine • Zyprexa
Pimavanserin • Nuplazid
Risperidone • Risperdal
Ziprasidone • Geodon
1. Dufort A, Zipursky RB. Understanding and managing treatment adherence in schizophrenia [published online January 3, 2019]. Clin Schizophr Relat Psychoses. 2019. doi: 10.3371/CSRP.ADRZ.121218.
2. Takeuchi H, Siu C, Remington G, et al. Does relapse contribute to treatment resistance? Antipsychotic response in first- vs. second-episode schizophrenia. Neuropsychopharmacology. 2019;44(6):1036-1042.
3. Dibonaventura M, Gabriel S, Dupclay L, et al. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry. 2012;12:20.
4. Kurokawa S, Kishimoto T, Su K-P, et al. Psychiatrists’ perceptions of medication adherence among patients with schizophrenia: an international survey. Schizophr Res. 2019;211:105-107.
5. Meyer JM. Pharmacotherapy of psychosis and mania. In: Brunton LL, Hilal-Dandan R, Knollmann BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. Chicago, Illinois: McGraw-Hill; 2018:279-302.
6. Davis RE, Correll CU. ITI-007 in the treatment of schizophrenia: from novel pharmacology to clinical outcomes. Expert Rev Neurother. 2016;16(6):601-614.
7. Caplyta [package Insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2019.
8. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232(3):605-621.
9. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
10. Zhang L, Hendrick JP. The presynaptic D2 partial agonist lumateperone acts as a postsynaptic D2 antagonist. Matters. 2018. doi: 10.19185/matters.201712000006.
11. Meltzer HY, Elkis H, Vanover K, et al. Pimavanserin, a selective serotonin (5-HT)2A-inverse agonist, enhances the efficacy and safety of risperidone, 2mg/day, but does not enhance efficacy of haloperidol, 2mg/day: comparison with reference dose risperidone, 6mg/day. Schizophr Res. 2012;141(2-3):144-152.
12. Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist. Schizophr Res. 2019;208:217-220.
13. Remington G, Mamo D, Labelle A, et al. A PET study evaluating dopamine D2 receptor occupancy for long-acting injectable risperidone. Am J Psychiatry. 2006;163(3):396-401.
14. Davis RE, Vanover KE, Zhou Y, et al. ITI-007 demonstrates brain occupancy at serotonin 5-HT2A and dopamine D2 receptors and serotonin transporters using positron emission tomography in healthy volunteers. Psychopharmacology (Berl). 2015;232(15):2863-2872.
15. Kumar B, Kuhad A, Kuhad A. Lumateperone: a new treatment approach for neuropsychiatric disorders. Drugs Today (Barc). 2018;54(12):713-719.
16. Vanover K, Glass S, Kozauer S, et al. 30 lumateperone (ITI-007) for the treatment of schizophrenia: overview of placebo-controlled clinical trials and an open-label safety switching study. CNS Spectr. 2019;24(1):190-191.
17. Young SL, Taylor M, Lawrie SM. “First do no harm.” A systematic review of the prevalence and management of antipsychotic adverse effects. J Psychopharmacol. 2015;29(4):353-362.
18. Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939-951.
19. Vyas P, Hwang BJ, Brašic ´ JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2019;1-7.
20. Lieberman JA, Davis RE, Correll CU, et al. ITI-007 for the treatment of schizophrenia: a 4-week randomized, double-blind, controlled trial. Biol Psychiatry. 2016;79(12):952-961.
21. Correll CU, Davis RE, Weingart M, et al. Efficacy and safety of lumateperone for treatment of schizophrenia [published online January 8, 2020]. JAMA Psychiatry. 2020;E1-E10.
Antipsychotic nonadherence is a known contributor to relapse risk among patients with schizophrenia.1 Because relapse episodes may be associated with antipsychotic treatment resistance, this must be avoided as much as possible by appropriate medication selection.2 Adverse effect burden is an important factor leading to oral antipsychotic nonadherence, with patient-derived data indicating that extrapyramidal symptoms (EPS) (odds ratio [OR] 0.57, P = .0007), sedation/cognitive adverse effects (OR 0.70, P = .033), prolactin/endocrine effects (OR 0.69, P = .0342), and metabolic adverse effects (OR 0.64, P = .0079) are all significantly related to lower rates of adherence.3 With this in mind, successive generations of antipsychotics have been released, with fewer tolerability issues present than seen with earlier compounds.1,4 Although these newer second-generation antipsychotics (SGAs) have not proven more effective for schizophrenia than those first marketed in the 1990s, they generally possess lower rates of EPS, hyperprolactinemia, anticholinergic and antihistaminic properties, metabolic adverse effects, and orthostasis.5 This improved adverse effect profile will hopefully increase the chances of antipsychotic acceptance in patients with schizophrenia, and thereby promote improved adherence.
Lumateperone (Caplyta) is a novel oral antipsychotic approved for the treatment of adult patients with schizophrenia (Table 1). It possesses some properties seen with other SGAs, including high affinity for serotonin 5HT2A receptors (Ki 0.54 nM) and lower affinity for dopamine D2 receptors (Ki 32 nM), along with low affinity for alpha1-adrenergic receptors (Ki 73 nM), and muscarinic and histaminergic receptors (Ki > 100 nM).6,7 However, there are some distinguishing features: the ratio of 5HT2A receptor affinity to D2 affinity is 60, greater than that of other SGAs such as risperidone (12), olanzapine (12.4) or aripiprazole (0.18)8; at steady state, the D2 occupancy remains <40% (Figure) and the corresponding rates of EPS/akathisia were only 6.7% for lumateperone vs 6.3% for placebo in short-term clinical trials.7,9
How it works
A unique aspect of lumateperone’s pharmacology may relate to differential actions at presynaptic and postsynaptic dopamine D2 receptors. Other antipsychotics possess comparable antagonist (or partial agonist) properties at postsynaptic D2 receptors (the D2 long isoform) and the presynaptic autoreceptor (the D2 short isoform). By blocking the presynaptic autoreceptor, feedback inhibition on dopamine release is removed; therefore, the required higher levels of postsynaptic D2 receptor occupancy needed for effective antipsychotic action (eg, 60% to 80% for antagonists, and 83% to 100% for partial agonists) may be a product of the need to oppose this increased presynaptic release of dopamine. In vitro assays show that lumateperone does not increase presynaptic dopamine release, indicating that it possesses agonist properties at the presynaptic D2 short receptor.10 That property may explain how lumateperone functions as an antipsychotic despite low levels of D2 receptor occupancy.10
Another hypothesis is based on our understanding of pimavanserin’s pharmacology. Pimavanserin is a selective 5HT2A antagonist FDA-approved for the treatment of Parkinson’s disease psychosis (PDP), with extremely high receptor affinity (Ki 0.087 nM) and no appreciable binding at dopamine receptors.5 Pimavanserin not only treats PDP, but is being evaluated in clinical trials for dementia-related psychosis, and has positive data for augmenting antipsychotics when there is a low level of D2 blockade.11,12 In a controlled trial, pimavanserin added to risperidone, 2 mg/d, was as effective as risperidone, 6 mg/d, illustrating the point that near-saturation of the 5HT2A receptor can increase antipsychotic efficacy when dopamine blockade is relatively low. For risperidone, 2 mg/d, the expected D2 occupancy is only 60%.13
Lumateperone also has moderate binding affinity for serotonin transporters (SERT) (Ki 33 nM). Serotonin transporter occupancy at the dose approved for schizophrenia (42 mg/d) is approximately 30%,14 below the ≥80% SERT occupancy seen with selective serotonin reuptake inhibitor (SSRI) antidepressants; nevertheless, there is evidence for antidepressant effects seen in preclinical assays, schizophrenia studies, and phase III trials for bipolar depression.8,15,16 It is hypothesized that near-saturation of the 5HT2A receptor might act synergistically with the modest extent of 5HT reuptake inhibition to promote downstream effects associated with effective antidepressant treatments.8 In vivo data also showed phosphorylation of N-methyl-
Clinical implications
Nonadherence with oral antipsychotics among patients with schizophrenia is often related to adverse effects.17 The SGAs marketed since 2000 generally have lower rates of sedation and metabolic and/or endocrine adverse events than earlier compounds, yet each has limitations:
- asenapine: sedation and weight gain
- the partial agonists (aripiprazole, brexpiprazole, cariprazine): akathisia
- lurasidone: dose-dependent EPS and akathisia
- iloperidone: orthostasis.18
Ziprasidone is an exception, because it had low rates of most adverse effects in schizophrenia trials, but the need to take it twice daily with a 500 kcal meal hampers its use. A meta-analysis of 32 oral antipsychotics, including first-generation agents, noted that the efficacy differences between medications are slight for patients without treatment-resistant schizophrenia, but “differences in side-effects are more marked.”18
Continue to: Until novel mechanisms are discovered...
Until novel mechanisms are discovered that increase schizophrenia response rates, the availability of newer antipsychotics with more favorable tolerability profiles presents clinicians and patients with added options when adverse effects interfere with prior treatment. In all phases of the adult schizophrenia trial program for lumateperone, 811 patients received short-term (4- to 6-week) exposure (dose range: 14 to 84 mg/d), while 329 had ≥6 months exposure and 108 had ≥1 year of exposure to the 42-mg/d dose. In these studies, there was no single adverse reaction leading to discontinuation that occurred at a rate >2%. The only adverse events that occurred at rates ≥5% and more than twice the rate of placebo were somnolence/sedation (lumateperone 24%, placebo 10%), and dry mouth (lumateperone 6%, placebo 2%). Nausea was present in 9% of the lumateperone group compared with 5% for placebo.7 In the short-term studies, the combined rate of EPS and akathisia was 6.7% for lumateperone and 6.3% for placebo.7 This difference translates to a number needed to harm of 250 for these neurologic adverse effects. The functional impact of lumateperone’s glutamatergic mechanisms is not well characterized within the schizophrenia population, but the antidepressant potential has been studied for patients with bipolar depression, with 1 positive phase III trial.19
Efficacy in adults with schizophrenia. The efficacy of lumateperone has been established in 2 pivotal, double-blind, placebo-controlled trials. The first was a 4-week, phase II trial (Study 005) in which 335 adults age 18 to 55 with an acute exacerbation of schizophrenia were randomized in a 1:1:1:1 manner to lumateperone, 42 mg/d (60 mg of lumateperone tosylate), lumateperone, 84 mg/d (120 mg of lumateperone tosylate), risperidone, 4 mg/d, or placebo, all taken once daily.20 For the 4 treatment arms, the least squares mean changes from baseline to the Day 28 endpoint on the primary outcome measure, Positive and Negative Syndrome Scale (PANSS) total score, were: lumateperone, 42 mg/d: −13.2 points; lumateperone, 84 mg/d: −8.3 points; risperidone, 4 mg/d: −13.4 points; and placebo: −7.4 points. Both lumateperone, 42 mg/d, and risperidone, 4 mg/d, were significantly different than placebo, and with identical moderate effect sizes of 0.4.20 Lumateperone, 84 mg/d, did not separate from placebo on the primary outcome. The responder analysis also indicated that a similar proportion of patients (40%) randomized to lumateperone, 42 mg/d, or risperidone, 4 mg/d, improved by ≥30% on PANSS total score.
The second pivotal trial (Study 301) was a phase III, double-blind, placebo-controlled trial of 450 adults, age 18 to 60, with an acute exacerbation of schizophrenia who were randomized in 1:1:1 manner to receive lumateperone, 42 mg/d (lumateperone tosylate 60 mg), lumateperone, 28 mg/d (lumateperone tosylate 40 mg), or placebo once daily for 4 weeks.21 For the 3 treatment arms, the least squares mean changes on PANSS total score from baseline to the Day 28 endpoint were: lumateperone, 42 mg/d: −14.5 points; lumateperone, 28 mg/d: −12.9 points; and placebo: −10.3 points. Lumateperone, 28 mg/d, did not separate from placebo on the primary outcome. The responder analysis also indicated that 36.5% of those receiving lumateperone, 42 mg/d, and 36.3% of those receiving lumateperone, 28 mg/d, improved by ≥30% on PANSS total score, compared with 25.5% of patients treated with placebo.
Unlike the 2 positive trials in which placebo change in total PANSS scores were −7.4 and −10.3 points, respectively, in a phase III trial (Study 302) with 696 participants, placebo showed a 15.1-point decrease from baseline PANSS total score.19 Among the 3 treatment arms of this study (lumateperone, 14 mg/d, lumateperone, 42 mg/d, and risperidone, 4 mg/d), only risperidone was superior to placebo.
Adverse events
In the phase II pivotal study, completion rates among the 4 arms were comparable: lumateperone, 42 mg/d: 71%; lumateperone, 84 mg/d: 76%; risperidone, 4 mg/d: 77%; and placebo: 72%.20 There were no serious adverse events (SAEs) associated with lumateperone; the 2 SAEs that occurred involved worsening of schizophrenia/psychotic disorder for risperidone (n = 1) and for placebo (n = 1). Five participants discontinued the study due to an adverse event: 2 who were receiving lumateperone (1 due to dry mouth, and 1 due to worsening of schizophrenia) and 3 who were receiving risperidone (2 due to akathisia, and 1 due to blood creatine phosphokinase increase).20 The most frequent adverse event was somnolence/sedation (placebo: 13%; lumateperone, 42 mg/d: 17%; risperidone, 4 mg/d: 21%; and lumateperone, 84 mg/d: 32.5%). Neither dose of lumateperone was associated with increased rates of EPS. Median weight gain to Day 28 was 1 kg for placebo and for each dose of lumateperone, and 2.5 kg for risperidone. Compared with risperidone, lumateperone showed statistically significantly lower prolactin levels (lumateperone, 42 mg/d and 84 mg/d: P < .001), and metabolic parameters, including fasting glucose (lumateperone 42 mg/d: P = .007; lumateperone, 84 mg/d: P = .023), total cholesterol (lumateperone, 42 mg/d: P = .012; lumateperone, 84 mg/d: P = .004), and triglycerides (lumateperone, 42 mg/d: P = .074; lumateperone, 84 mg/d: P = .002).20 There was no significant impact on the corrected QT interval.
Continue to: In the phase III trial...
In the phase III trial, completion rates among the 3 arms were lumateperone, 42 mg/d: 85%; lumateperone, 28 mg/d: 80%; and placebo: 74%. There was 1 SAE in a patient receiving lumateperone, 28 mg/d. This individual had preexisting risk factors and a history of seizures, and experienced a seizure during the study. Adverse events that occurred in either lumateperone group at a rate ≥5% and more than twice the rate of placebo were somnolence (lumateperone, 42 mg/d: 17.3%; lumateperone, 28 mg/d: 11.3%; and placebo: 4.0%); sedation (lumateperone, 42 mg/d: 12.7%; lumateperone, 28 mg/d: 9.3%; and placebo: 5.4%); fatigue (lumateperone, 42 mg/d: 5.3%; lumateperone, 28 mg/d: 4.7%; and placebo: 1.3%); and constipation (lumateperone, 42 mg/d: 6.7%; lumateperone, 28 mg/d: 4.0%; and placebo: 2.7%).21 No EPS-related adverse events occurred in ≥5% patients in any treatment arm. Median change in weight from baseline to Day 28 was 0.9 kg for lumateperone, 42 mg/d, 0.6 kg for lumateperone, 28 mg/d, and 0.7 kg for placebo. There were no significant mean changes in metabolic parameters for any treatment arm, and none of the patients had a corrected QT interval (QTc) >500 ms or a change in QTc >60 ms from baseline.21
Pharmacologic profile
Lumateperone’s in vitro binding profile includes high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), lower affinity for dopamine D2 receptors (Ki 32 nM), moderate binding affinity for SERT (Ki 33 nM), and lower affinity for alpha 1-adrenergic receptors (Ki 73 nM) and muscarinic and histaminergic receptors (Ki >100 nM).6,7 As noted above, this 60-fold ratio of 5HT2A to D2 affinity is extremely high; moreover, imaging data reveal low D2 receptor occupancy, consistent with the lack of clinically significant EPS seen in the trials. In vitro assays also reveal impact on glutamate pathways, and pathways associated with antidepressant response.8 The clinical benefits of the glutamatergic properties remain theoretical, but the antidepressant benefit has been seen in a positive phase III trial for bipolar depression.19
Clinical considerations
Effect sizes in the 2 positive pivotal trials were 0.3 and 0.4, comparable with those for other antipsychotics approved within the last decade: brexpiprazole, 0.26; cariprazine, 0.34; and lurasidone, 0.36.21 The absence of clinically significant EPS, lack of impact on metabolic or endocrine parameters, and lack of titration are all appealing properties. That only 42 mg/d proved effective may reflect the fact that the other doses studied to date in randomized, fixed-dose studies were 14 mg/d (Study 302) and 84 mg/d (Study 005), evaluated in one study each. While those 2 doses might indeed be outside the therapeutic window, given the heterogeneity of schizophrenia, future studies might help further refine the therapeutic range for schizophrenia, especially for doses closer to 42 mg/d (eg, 28 mg/d, 63 mg/d). Should 42 mg/d not prove effective, there is no data for now to suggest whether a dose increase may be helpful. As there is only 1 marketed dose of lumateperone (42-mg capsules), and no easy way to modify this dose, lumateperone’s package insert includes cautionary language regarding situations where there will be less-than-expected drug exposure (use of cytochrome P450 [CYP] 3A4 inducers), greater-than-expected drug exposure (moderate or strong CYP 3A4 inhibitors), or use in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria (Child-Pugh B or C). These are not contraindications.
Unique properties of lumateperone include the lack of presynaptic dopamine D2 antagonism, low D2 receptor occupancy, and the absence of significant EPS and metabolic or endocrine adverse effects. In vitro data indicate glutamatergic effects, and human data indicate antidepressant effects in bipolar patients. Despite the absence of significant histamine H1 or muscarinic affinity, the rate of somnolence/sedation was twice that of placebo (lumateperone 24%, placebo 10%).7
Why Rx? Reasons to prescribe lumateperone for adult patients with schizophrenia include:
- Favorable tolerability profile, including no significant signal for EPS or endocrine or metabolic adverse effects, and no QT prolongation
- No need for titration.
Dosing. There is only 1 dose available for lumateperone, 42-mg capsules (Table 2). As the dose cannot be modified, the package insert contains cautionary language regarding situations with less-than-expected drug exposure (use of CYP 3A4 inducers), greater-than-expected drug exposure (moderate or strong CYP 3A4 inhibitors), or use in patients with moderate or severe hepatic impairment as defined by Child-Pugh criteria (Child-Pugh B or C). These are not contraindications.
Contraindications. The only contraindication is known hypersensitivity to lumateperone.
Continue to: Bottom Line
Bottom Line
Lumateperone is a novel oral antipsychotic indicated for treating adults with schizophrenia. Its unique properties include the lack of presynaptic dopamine D2 antagonism, low D2 receptor occupancy, and the absence of significant extrapyramidal symptoms and metabolic or endocrine adverse effects. In clinical trials, the most frequent adverse event was somnolence/sedation.
Related Resource
- Fulton D. FDA approves Caplyta to treat schizophrenenia in adults. https://www.mdedge.com/psychiatry/article/214733/schizophrenia-other-psychotic-disorders/fda-approves-caplyta-treat.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Iloperidone • Fanapt
Lumateperone • Caplyta
Lurasidone • Latuda
Olanzapine • Zyprexa
Pimavanserin • Nuplazid
Risperidone • Risperdal
Ziprasidone • Geodon
Antipsychotic nonadherence is a known contributor to relapse risk among patients with schizophrenia.1 Because relapse episodes may be associated with antipsychotic treatment resistance, this must be avoided as much as possible by appropriate medication selection.2 Adverse effect burden is an important factor leading to oral antipsychotic nonadherence, with patient-derived data indicating that extrapyramidal symptoms (EPS) (odds ratio [OR] 0.57, P = .0007), sedation/cognitive adverse effects (OR 0.70, P = .033), prolactin/endocrine effects (OR 0.69, P = .0342), and metabolic adverse effects (OR 0.64, P = .0079) are all significantly related to lower rates of adherence.3 With this in mind, successive generations of antipsychotics have been released, with fewer tolerability issues present than seen with earlier compounds.1,4 Although these newer second-generation antipsychotics (SGAs) have not proven more effective for schizophrenia than those first marketed in the 1990s, they generally possess lower rates of EPS, hyperprolactinemia, anticholinergic and antihistaminic properties, metabolic adverse effects, and orthostasis.5 This improved adverse effect profile will hopefully increase the chances of antipsychotic acceptance in patients with schizophrenia, and thereby promote improved adherence.
Lumateperone (Caplyta) is a novel oral antipsychotic approved for the treatment of adult patients with schizophrenia (Table 1). It possesses some properties seen with other SGAs, including high affinity for serotonin 5HT2A receptors (Ki 0.54 nM) and lower affinity for dopamine D2 receptors (Ki 32 nM), along with low affinity for alpha1-adrenergic receptors (Ki 73 nM), and muscarinic and histaminergic receptors (Ki > 100 nM).6,7 However, there are some distinguishing features: the ratio of 5HT2A receptor affinity to D2 affinity is 60, greater than that of other SGAs such as risperidone (12), olanzapine (12.4) or aripiprazole (0.18)8; at steady state, the D2 occupancy remains <40% (Figure) and the corresponding rates of EPS/akathisia were only 6.7% for lumateperone vs 6.3% for placebo in short-term clinical trials.7,9
How it works
A unique aspect of lumateperone’s pharmacology may relate to differential actions at presynaptic and postsynaptic dopamine D2 receptors. Other antipsychotics possess comparable antagonist (or partial agonist) properties at postsynaptic D2 receptors (the D2 long isoform) and the presynaptic autoreceptor (the D2 short isoform). By blocking the presynaptic autoreceptor, feedback inhibition on dopamine release is removed; therefore, the required higher levels of postsynaptic D2 receptor occupancy needed for effective antipsychotic action (eg, 60% to 80% for antagonists, and 83% to 100% for partial agonists) may be a product of the need to oppose this increased presynaptic release of dopamine. In vitro assays show that lumateperone does not increase presynaptic dopamine release, indicating that it possesses agonist properties at the presynaptic D2 short receptor.10 That property may explain how lumateperone functions as an antipsychotic despite low levels of D2 receptor occupancy.10
Another hypothesis is based on our understanding of pimavanserin’s pharmacology. Pimavanserin is a selective 5HT2A antagonist FDA-approved for the treatment of Parkinson’s disease psychosis (PDP), with extremely high receptor affinity (Ki 0.087 nM) and no appreciable binding at dopamine receptors.5 Pimavanserin not only treats PDP, but is being evaluated in clinical trials for dementia-related psychosis, and has positive data for augmenting antipsychotics when there is a low level of D2 blockade.11,12 In a controlled trial, pimavanserin added to risperidone, 2 mg/d, was as effective as risperidone, 6 mg/d, illustrating the point that near-saturation of the 5HT2A receptor can increase antipsychotic efficacy when dopamine blockade is relatively low. For risperidone, 2 mg/d, the expected D2 occupancy is only 60%.13
Lumateperone also has moderate binding affinity for serotonin transporters (SERT) (Ki 33 nM). Serotonin transporter occupancy at the dose approved for schizophrenia (42 mg/d) is approximately 30%,14 below the ≥80% SERT occupancy seen with selective serotonin reuptake inhibitor (SSRI) antidepressants; nevertheless, there is evidence for antidepressant effects seen in preclinical assays, schizophrenia studies, and phase III trials for bipolar depression.8,15,16 It is hypothesized that near-saturation of the 5HT2A receptor might act synergistically with the modest extent of 5HT reuptake inhibition to promote downstream effects associated with effective antidepressant treatments.8 In vivo data also showed phosphorylation of N-methyl-
Clinical implications
Nonadherence with oral antipsychotics among patients with schizophrenia is often related to adverse effects.17 The SGAs marketed since 2000 generally have lower rates of sedation and metabolic and/or endocrine adverse events than earlier compounds, yet each has limitations:
- asenapine: sedation and weight gain
- the partial agonists (aripiprazole, brexpiprazole, cariprazine): akathisia
- lurasidone: dose-dependent EPS and akathisia
- iloperidone: orthostasis.18
Ziprasidone is an exception, because it had low rates of most adverse effects in schizophrenia trials, but the need to take it twice daily with a 500 kcal meal hampers its use. A meta-analysis of 32 oral antipsychotics, including first-generation agents, noted that the efficacy differences between medications are slight for patients without treatment-resistant schizophrenia, but “differences in side-effects are more marked.”18
Continue to: Until novel mechanisms are discovered...
Until novel mechanisms are discovered that increase schizophrenia response rates, the availability of newer antipsychotics with more favorable tolerability profiles presents clinicians and patients with added options when adverse effects interfere with prior treatment. In all phases of the adult schizophrenia trial program for lumateperone, 811 patients received short-term (4- to 6-week) exposure (dose range: 14 to 84 mg/d), while 329 had ≥6 months exposure and 108 had ≥1 year of exposure to the 42-mg/d dose. In these studies, there was no single adverse reaction leading to discontinuation that occurred at a rate >2%. The only adverse events that occurred at rates ≥5% and more than twice the rate of placebo were somnolence/sedation (lumateperone 24%, placebo 10%), and dry mouth (lumateperone 6%, placebo 2%). Nausea was present in 9% of the lumateperone group compared with 5% for placebo.7 In the short-term studies, the combined rate of EPS and akathisia was 6.7% for lumateperone and 6.3% for placebo.7 This difference translates to a number needed to harm of 250 for these neurologic adverse effects. The functional impact of lumateperone’s glutamatergic mechanisms is not well characterized within the schizophrenia population, but the antidepressant potential has been studied for patients with bipolar depression, with 1 positive phase III trial.19
Efficacy in adults with schizophrenia. The efficacy of lumateperone has been established in 2 pivotal, double-blind, placebo-controlled trials. The first was a 4-week, phase II trial (Study 005) in which 335 adults age 18 to 55 with an acute exacerbation of schizophrenia were randomized in a 1:1:1:1 manner to lumateperone, 42 mg/d (60 mg of lumateperone tosylate), lumateperone, 84 mg/d (120 mg of lumateperone tosylate), risperidone, 4 mg/d, or placebo, all taken once daily.20 For the 4 treatment arms, the least squares mean changes from baseline to the Day 28 endpoint on the primary outcome measure, Positive and Negative Syndrome Scale (PANSS) total score, were: lumateperone, 42 mg/d: −13.2 points; lumateperone, 84 mg/d: −8.3 points; risperidone, 4 mg/d: −13.4 points; and placebo: −7.4 points. Both lumateperone, 42 mg/d, and risperidone, 4 mg/d, were significantly different than placebo, and with identical moderate effect sizes of 0.4.20 Lumateperone, 84 mg/d, did not separate from placebo on the primary outcome. The responder analysis also indicated that a similar proportion of patients (40%) randomized to lumateperone, 42 mg/d, or risperidone, 4 mg/d, improved by ≥30% on PANSS total score.
The second pivotal trial (Study 301) was a phase III, double-blind, placebo-controlled trial of 450 adults, age 18 to 60, with an acute exacerbation of schizophrenia who were randomized in 1:1:1 manner to receive lumateperone, 42 mg/d (lumateperone tosylate 60 mg), lumateperone, 28 mg/d (lumateperone tosylate 40 mg), or placebo once daily for 4 weeks.21 For the 3 treatment arms, the least squares mean changes on PANSS total score from baseline to the Day 28 endpoint were: lumateperone, 42 mg/d: −14.5 points; lumateperone, 28 mg/d: −12.9 points; and placebo: −10.3 points. Lumateperone, 28 mg/d, did not separate from placebo on the primary outcome. The responder analysis also indicated that 36.5% of those receiving lumateperone, 42 mg/d, and 36.3% of those receiving lumateperone, 28 mg/d, improved by ≥30% on PANSS total score, compared with 25.5% of patients treated with placebo.
Unlike the 2 positive trials in which placebo change in total PANSS scores were −7.4 and −10.3 points, respectively, in a phase III trial (Study 302) with 696 participants, placebo showed a 15.1-point decrease from baseline PANSS total score.19 Among the 3 treatment arms of this study (lumateperone, 14 mg/d, lumateperone, 42 mg/d, and risperidone, 4 mg/d), only risperidone was superior to placebo.
Adverse events
In the phase II pivotal study, completion rates among the 4 arms were comparable: lumateperone, 42 mg/d: 71%; lumateperone, 84 mg/d: 76%; risperidone, 4 mg/d: 77%; and placebo: 72%.20 There were no serious adverse events (SAEs) associated with lumateperone; the 2 SAEs that occurred involved worsening of schizophrenia/psychotic disorder for risperidone (n = 1) and for placebo (n = 1). Five participants discontinued the study due to an adverse event: 2 who were receiving lumateperone (1 due to dry mouth, and 1 due to worsening of schizophrenia) and 3 who were receiving risperidone (2 due to akathisia, and 1 due to blood creatine phosphokinase increase).20 The most frequent adverse event was somnolence/sedation (placebo: 13%; lumateperone, 42 mg/d: 17%; risperidone, 4 mg/d: 21%; and lumateperone, 84 mg/d: 32.5%). Neither dose of lumateperone was associated with increased rates of EPS. Median weight gain to Day 28 was 1 kg for placebo and for each dose of lumateperone, and 2.5 kg for risperidone. Compared with risperidone, lumateperone showed statistically significantly lower prolactin levels (lumateperone, 42 mg/d and 84 mg/d: P < .001), and metabolic parameters, including fasting glucose (lumateperone 42 mg/d: P = .007; lumateperone, 84 mg/d: P = .023), total cholesterol (lumateperone, 42 mg/d: P = .012; lumateperone, 84 mg/d: P = .004), and triglycerides (lumateperone, 42 mg/d: P = .074; lumateperone, 84 mg/d: P = .002).20 There was no significant impact on the corrected QT interval.
Continue to: In the phase III trial...
In the phase III trial, completion rates among the 3 arms were lumateperone, 42 mg/d: 85%; lumateperone, 28 mg/d: 80%; and placebo: 74%. There was 1 SAE in a patient receiving lumateperone, 28 mg/d. This individual had preexisting risk factors and a history of seizures, and experienced a seizure during the study. Adverse events that occurred in either lumateperone group at a rate ≥5% and more than twice the rate of placebo were somnolence (lumateperone, 42 mg/d: 17.3%; lumateperone, 28 mg/d: 11.3%; and placebo: 4.0%); sedation (lumateperone, 42 mg/d: 12.7%; lumateperone, 28 mg/d: 9.3%; and placebo: 5.4%); fatigue (lumateperone, 42 mg/d: 5.3%; lumateperone, 28 mg/d: 4.7%; and placebo: 1.3%); and constipation (lumateperone, 42 mg/d: 6.7%; lumateperone, 28 mg/d: 4.0%; and placebo: 2.7%).21 No EPS-related adverse events occurred in ≥5% patients in any treatment arm. Median change in weight from baseline to Day 28 was 0.9 kg for lumateperone, 42 mg/d, 0.6 kg for lumateperone, 28 mg/d, and 0.7 kg for placebo. There were no significant mean changes in metabolic parameters for any treatment arm, and none of the patients had a corrected QT interval (QTc) >500 ms or a change in QTc >60 ms from baseline.21
Pharmacologic profile
Lumateperone’s in vitro binding profile includes high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), lower affinity for dopamine D2 receptors (Ki 32 nM), moderate binding affinity for SERT (Ki 33 nM), and lower affinity for alpha 1-adrenergic receptors (Ki 73 nM) and muscarinic and histaminergic receptors (Ki >100 nM).6,7 As noted above, this 60-fold ratio of 5HT2A to D2 affinity is extremely high; moreover, imaging data reveal low D2 receptor occupancy, consistent with the lack of clinically significant EPS seen in the trials. In vitro assays also reveal impact on glutamate pathways, and pathways associated with antidepressant response.8 The clinical benefits of the glutamatergic properties remain theoretical, but the antidepressant benefit has been seen in a positive phase III trial for bipolar depression.19
Clinical considerations
Effect sizes in the 2 positive pivotal trials were 0.3 and 0.4, comparable with those for other antipsychotics approved within the last decade: brexpiprazole, 0.26; cariprazine, 0.34; and lurasidone, 0.36.21 The absence of clinically significant EPS, lack of impact on metabolic or endocrine parameters, and lack of titration are all appealing properties. That only 42 mg/d proved effective may reflect the fact that the other doses studied to date in randomized, fixed-dose studies were 14 mg/d (Study 302) and 84 mg/d (Study 005), evaluated in one study each. While those 2 doses might indeed be outside the therapeutic window, given the heterogeneity of schizophrenia, future studies might help further refine the therapeutic range for schizophrenia, especially for doses closer to 42 mg/d (eg, 28 mg/d, 63 mg/d). Should 42 mg/d not prove effective, there is no data for now to suggest whether a dose increase may be helpful. As there is only 1 marketed dose of lumateperone (42-mg capsules), and no easy way to modify this dose, lumateperone’s package insert includes cautionary language regarding situations where there will be less-than-expected drug exposure (use of cytochrome P450 [CYP] 3A4 inducers), greater-than-expected drug exposure (moderate or strong CYP 3A4 inhibitors), or use in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria (Child-Pugh B or C). These are not contraindications.
Unique properties of lumateperone include the lack of presynaptic dopamine D2 antagonism, low D2 receptor occupancy, and the absence of significant EPS and metabolic or endocrine adverse effects. In vitro data indicate glutamatergic effects, and human data indicate antidepressant effects in bipolar patients. Despite the absence of significant histamine H1 or muscarinic affinity, the rate of somnolence/sedation was twice that of placebo (lumateperone 24%, placebo 10%).7
Why Rx? Reasons to prescribe lumateperone for adult patients with schizophrenia include:
- Favorable tolerability profile, including no significant signal for EPS or endocrine or metabolic adverse effects, and no QT prolongation
- No need for titration.
Dosing. There is only 1 dose available for lumateperone, 42-mg capsules (Table 2). As the dose cannot be modified, the package insert contains cautionary language regarding situations with less-than-expected drug exposure (use of CYP 3A4 inducers), greater-than-expected drug exposure (moderate or strong CYP 3A4 inhibitors), or use in patients with moderate or severe hepatic impairment as defined by Child-Pugh criteria (Child-Pugh B or C). These are not contraindications.
Contraindications. The only contraindication is known hypersensitivity to lumateperone.
Continue to: Bottom Line
Bottom Line
Lumateperone is a novel oral antipsychotic indicated for treating adults with schizophrenia. Its unique properties include the lack of presynaptic dopamine D2 antagonism, low D2 receptor occupancy, and the absence of significant extrapyramidal symptoms and metabolic or endocrine adverse effects. In clinical trials, the most frequent adverse event was somnolence/sedation.
Related Resource
- Fulton D. FDA approves Caplyta to treat schizophrenenia in adults. https://www.mdedge.com/psychiatry/article/214733/schizophrenia-other-psychotic-disorders/fda-approves-caplyta-treat.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Iloperidone • Fanapt
Lumateperone • Caplyta
Lurasidone • Latuda
Olanzapine • Zyprexa
Pimavanserin • Nuplazid
Risperidone • Risperdal
Ziprasidone • Geodon
1. Dufort A, Zipursky RB. Understanding and managing treatment adherence in schizophrenia [published online January 3, 2019]. Clin Schizophr Relat Psychoses. 2019. doi: 10.3371/CSRP.ADRZ.121218.
2. Takeuchi H, Siu C, Remington G, et al. Does relapse contribute to treatment resistance? Antipsychotic response in first- vs. second-episode schizophrenia. Neuropsychopharmacology. 2019;44(6):1036-1042.
3. Dibonaventura M, Gabriel S, Dupclay L, et al. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry. 2012;12:20.
4. Kurokawa S, Kishimoto T, Su K-P, et al. Psychiatrists’ perceptions of medication adherence among patients with schizophrenia: an international survey. Schizophr Res. 2019;211:105-107.
5. Meyer JM. Pharmacotherapy of psychosis and mania. In: Brunton LL, Hilal-Dandan R, Knollmann BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. Chicago, Illinois: McGraw-Hill; 2018:279-302.
6. Davis RE, Correll CU. ITI-007 in the treatment of schizophrenia: from novel pharmacology to clinical outcomes. Expert Rev Neurother. 2016;16(6):601-614.
7. Caplyta [package Insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2019.
8. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232(3):605-621.
9. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
10. Zhang L, Hendrick JP. The presynaptic D2 partial agonist lumateperone acts as a postsynaptic D2 antagonist. Matters. 2018. doi: 10.19185/matters.201712000006.
11. Meltzer HY, Elkis H, Vanover K, et al. Pimavanserin, a selective serotonin (5-HT)2A-inverse agonist, enhances the efficacy and safety of risperidone, 2mg/day, but does not enhance efficacy of haloperidol, 2mg/day: comparison with reference dose risperidone, 6mg/day. Schizophr Res. 2012;141(2-3):144-152.
12. Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist. Schizophr Res. 2019;208:217-220.
13. Remington G, Mamo D, Labelle A, et al. A PET study evaluating dopamine D2 receptor occupancy for long-acting injectable risperidone. Am J Psychiatry. 2006;163(3):396-401.
14. Davis RE, Vanover KE, Zhou Y, et al. ITI-007 demonstrates brain occupancy at serotonin 5-HT2A and dopamine D2 receptors and serotonin transporters using positron emission tomography in healthy volunteers. Psychopharmacology (Berl). 2015;232(15):2863-2872.
15. Kumar B, Kuhad A, Kuhad A. Lumateperone: a new treatment approach for neuropsychiatric disorders. Drugs Today (Barc). 2018;54(12):713-719.
16. Vanover K, Glass S, Kozauer S, et al. 30 lumateperone (ITI-007) for the treatment of schizophrenia: overview of placebo-controlled clinical trials and an open-label safety switching study. CNS Spectr. 2019;24(1):190-191.
17. Young SL, Taylor M, Lawrie SM. “First do no harm.” A systematic review of the prevalence and management of antipsychotic adverse effects. J Psychopharmacol. 2015;29(4):353-362.
18. Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939-951.
19. Vyas P, Hwang BJ, Brašic ´ JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2019;1-7.
20. Lieberman JA, Davis RE, Correll CU, et al. ITI-007 for the treatment of schizophrenia: a 4-week randomized, double-blind, controlled trial. Biol Psychiatry. 2016;79(12):952-961.
21. Correll CU, Davis RE, Weingart M, et al. Efficacy and safety of lumateperone for treatment of schizophrenia [published online January 8, 2020]. JAMA Psychiatry. 2020;E1-E10.
1. Dufort A, Zipursky RB. Understanding and managing treatment adherence in schizophrenia [published online January 3, 2019]. Clin Schizophr Relat Psychoses. 2019. doi: 10.3371/CSRP.ADRZ.121218.
2. Takeuchi H, Siu C, Remington G, et al. Does relapse contribute to treatment resistance? Antipsychotic response in first- vs. second-episode schizophrenia. Neuropsychopharmacology. 2019;44(6):1036-1042.
3. Dibonaventura M, Gabriel S, Dupclay L, et al. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry. 2012;12:20.
4. Kurokawa S, Kishimoto T, Su K-P, et al. Psychiatrists’ perceptions of medication adherence among patients with schizophrenia: an international survey. Schizophr Res. 2019;211:105-107.
5. Meyer JM. Pharmacotherapy of psychosis and mania. In: Brunton LL, Hilal-Dandan R, Knollmann BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. Chicago, Illinois: McGraw-Hill; 2018:279-302.
6. Davis RE, Correll CU. ITI-007 in the treatment of schizophrenia: from novel pharmacology to clinical outcomes. Expert Rev Neurother. 2016;16(6):601-614.
7. Caplyta [package Insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2019.
8. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232(3):605-621.
9. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
10. Zhang L, Hendrick JP. The presynaptic D2 partial agonist lumateperone acts as a postsynaptic D2 antagonist. Matters. 2018. doi: 10.19185/matters.201712000006.
11. Meltzer HY, Elkis H, Vanover K, et al. Pimavanserin, a selective serotonin (5-HT)2A-inverse agonist, enhances the efficacy and safety of risperidone, 2mg/day, but does not enhance efficacy of haloperidol, 2mg/day: comparison with reference dose risperidone, 6mg/day. Schizophr Res. 2012;141(2-3):144-152.
12. Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist. Schizophr Res. 2019;208:217-220.
13. Remington G, Mamo D, Labelle A, et al. A PET study evaluating dopamine D2 receptor occupancy for long-acting injectable risperidone. Am J Psychiatry. 2006;163(3):396-401.
14. Davis RE, Vanover KE, Zhou Y, et al. ITI-007 demonstrates brain occupancy at serotonin 5-HT2A and dopamine D2 receptors and serotonin transporters using positron emission tomography in healthy volunteers. Psychopharmacology (Berl). 2015;232(15):2863-2872.
15. Kumar B, Kuhad A, Kuhad A. Lumateperone: a new treatment approach for neuropsychiatric disorders. Drugs Today (Barc). 2018;54(12):713-719.
16. Vanover K, Glass S, Kozauer S, et al. 30 lumateperone (ITI-007) for the treatment of schizophrenia: overview of placebo-controlled clinical trials and an open-label safety switching study. CNS Spectr. 2019;24(1):190-191.
17. Young SL, Taylor M, Lawrie SM. “First do no harm.” A systematic review of the prevalence and management of antipsychotic adverse effects. J Psychopharmacol. 2015;29(4):353-362.
18. Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939-951.
19. Vyas P, Hwang BJ, Brašic ´ JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2019;1-7.
20. Lieberman JA, Davis RE, Correll CU, et al. ITI-007 for the treatment of schizophrenia: a 4-week randomized, double-blind, controlled trial. Biol Psychiatry. 2016;79(12):952-961.
21. Correll CU, Davis RE, Weingart M, et al. Efficacy and safety of lumateperone for treatment of schizophrenia [published online January 8, 2020]. JAMA Psychiatry. 2020;E1-E10.