User login
Data is sparse thus far, but there is concern in lung transplant medicine about the long-term risk of chronic lung allograft dysfunction (CLAD) and a potentially shortened longevity of transplanted lungs in recipients who become ill with COVID-19.
“My fear is that we’re potentially sitting on this iceberg worth of people who, come 6 months or a year from [the acute phase of] their COVID illness, will in fact have earlier and progressive, chronic rejection,” said Cameron R. Wolfe, MBBS, MPH, associate professor of medicine in transplant infectious disease at Duke University, Durham, N.C.
Lower respiratory viral infections have long been concerning for lung transplant recipients given their propensity to cause scarring, a decline in lung function, and a heightened risk of allograft rejection. Time will tell whether lung transplant recipients who survive COVID-19 follow a similar path, or one that is worse, he said.
Short-term data
Outcomes beyond hospitalization and acute illness for lung transplant recipients affected by COVID-19 have been reported in the literature by only a few lung transplant programs. These reports – as well as anecdotal experiences being informally shared among transplant programs – have raised the specter of more severe dysfunction following the acute phase and more early CLAD, said Tathagat Narula, MD, assistant professor of medicine at the Mayo Medical School, Rochester, Minn., and a consultant in lung transplantation at the Mayo Clinic’s Jacksonville program.
“The available data cover only 3-6 months out. We don’t know what will happen in the next 6 months and beyond,” Dr. Narula said in an interview.
The risks of COVID-19 in already-transplanted patients and issues relating to the inadequate antibody responses to vaccination are just some of the challenges of lung transplant medicine in the era of SARS-CoV-2. “COVID-19,” said Dr. Narula, “has completely changed the way we practice lung transplant medicine – the way we’re looking both at our recipients and our donors.”
Potential donors are being evaluated with lower respiratory SARS-CoV-2 testing and an abundance of caution. And patients with severe COVID-19 affecting their own lungs are roundly expected to drive up lung transplant volume in the near future. “The whole paradigm has changed,” Dr. Narula said.
Post-acute trajectories
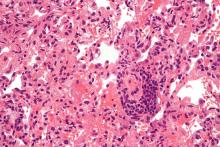
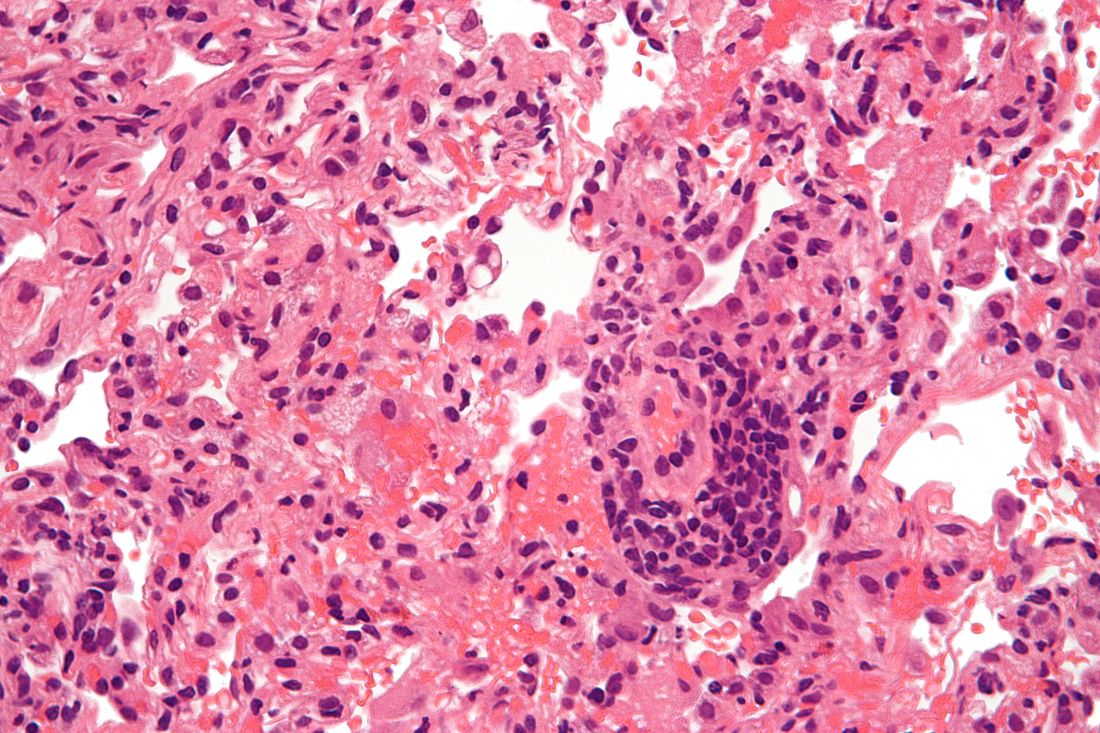
A chart review study published in October by the lung transplant team at the University of Texas Southwestern Medical Center, Dallas, covered 44 consecutive survivors at a median follow-up of 4.5 months from hospital discharge or acute illness (the survival rate was 83.3%). Patients had significantly impaired functional status, and 18 of the 44 (40.9%) had a significant and persistent loss of forced vital capacity or forced expiratory volume in 1 second (>10% from pre–COVID-19 baseline).
Three patients met the criteria for new CLAD after COVID-19 infection, with all three classified as restrictive allograft syndrome (RAS) phenotype.
Moreover, the majority of COVID-19 survivors who had CT chest scans (22 of 28) showed persistent parenchymal opacities – a finding that, regardless of symptomatology, suggests persistent allograft injury, said Amit Banga, MD, associate professor of medicine and medical director of the ex vivo lung perfusion program in UT Southwestern’s lung transplant program.
“The implication is that there may be long-term consequences of COVID-19, perhaps related to some degree of ongoing inflammation and damage,” said Dr. Banga, a coauthor of the postinfection outcomes paper.
The UT Southwestern lung transplant program, which normally performs 60-80 transplants a year, began routine CT scanning 4-5 months into the pandemic, after “stumbling into a few patients who had no symptoms indicative of COVID pneumonia and no changes on an x-ray but significant involvement on a CT,” he said.
Without routine scanning in the general population of COVID-19 patients, Dr. Banga noted, “we’re limited in convincingly saying that COVID is uniquely doing this to lung transplant recipients.” Nor can they conclude that SARS-CoV-2 is unique from other respiratory viruses such as respiratory syncytial virus (RSV) in this regard. (The program has added CT scanning to its protocol for lung transplant recipients afflicted with other respiratory viruses to learn more.)
However, in the big picture, COVID-19 has proven to be far worse for lung transplant recipients than illness with other respiratory viruses, including RSV. “Patients have more frequent and greater loss of lung function, and worse debility from the acute illness,” Dr. Banga said.
“The cornerstones of treatment of both these viruses are very similar, but both the in-hospital course and the postdischarge outcomes are significantly different.”
In an initial paper published in September 2021, Dr. Banga and colleagues compared their first 25 lung transplant patients testing positive for SARS-CoV-2 with a historical cohort of 36 patients with RSV treated during 2016-2018.
Patients with COVID-19 had significantly worse morbidity and mortality, including worse postinfection lung function loss, functional decline, and 3-month survival.
More time, he said, will shed light on the risks of CLAD and the long-term potential for recovery of lung function. Currently, at UT Southwestern, it appears that patients who survive acute illness and the “first 3-6 months after COVID-19, when we’re seeing all the postinfection morbidity, may [enter] a period of stability,” Dr. Banga said.
Overall, he said, patients in their initial cohort are “holding steady” without unusual morbidity, readmissions, or “other setbacks to their allografts.”
At the Mayo Clinic in Jacksonville, which normally performs 40-50 lung transplants a year, transplant physicians have similarly observed significant declines in lung function beyond the acute phase of COVID-19. “Anecdotally, we’re seeing that some patients are beginning to recover some of their lung function, while others have not,” said Dr. Narula. “And we don’t have predictors as to who will progress to CLAD. It’s a big knowledge gap.”
Dr. Narula noted that patients with restrictive allograft syndrome, such as those reported by the UT Southwestern team, “have scarring of the lung and a much worse prognosis than the obstructive type of chronic rejection.” Whether there’s a role for antifibrotic therapy is a question worthy of research.
In UT Southwestern’s analysis, persistently lower absolute lymphocyte counts (< 600/dL) and higher ferritin levels (>150 ng/mL) at the time of hospital discharge were independently associated with significant lung function loss. This finding, reported in their October paper, has helped guide their management practices, Dr. Banga said.
“Persistently elevated ferritin may indicate ongoing inflammation at the allograft level,” he said. “We now send [such patients] home on a longer course of oral corticosteroids.”
At the front end of care for infected lung transplant recipients, Dr. Banga said that his team and physicians at other lung transplant programs are holding the cell-cycle inhibitor component of patients’ maintenance immunosuppression therapy (commonly mycophenolate or azathioprine) once infection is diagnosed to maximize chances of a better outcome.
“There may be variation on how long [the regimens are adjusted],” he said. “We changed our duration from 4 weeks to 2 due to patients developing a rebound worsening in the third and fourth week of acute illness.”
There is significant variation from institution to institution in how viral infections are managed in lung transplant recipients, he and Dr. Narula said. “Our numbers are so small in lung transplant, and we don’t have standardized protocols – it’s one of the biggest challenges in our field,” said Dr. Narula.
Vaccination issues, evaluation of donors
Whether or not immunosuppression regimens should be adjusted prior to vaccination is a controversial question, but is “an absolutely valid one” and is currently being studied in at least one National Institutes of Health–funded trial involving solid organ transplant recipients, said Dr. Wolfe.
“Some have jumped to the conclusion [based on some earlier data] that they should reduce immunosuppression regimens for everyone at the time of vaccination ... but I don’t know the answer yet,” he said. “Balancing staying rejection free with potentially gaining more immune response is complicated ... and it may depend on where the pandemic is going in your area and other factors.”
Reductions aside, Dr. Wolfe tells lung transplant recipients that, based on his approximation of a number of different studies in solid organ transplant recipients, approximately 40%-50% of patients who are immunized with two doses of the COVID-19 mRNA vaccines will develop meaningful antibody levels – and that this rises to 50%-60% after a third dose.
It is difficult to glean from available studies the level of vaccine response for lung transplant recipients specifically. But given that their level of maintenance immunosuppression is higher than for recipients of other donor organs, “as a broad sweep, lung transplant recipients tend to be lower in the pecking order of response,” he said.
Still, “there’s a lot to gain,” he said, pointing to a recent study from the Morbidity and Mortality Weekly Report (2021 Nov 5. doi: 10.15585/mmwr.mm7044e3) showing that effectiveness of mRNA vaccination against COVID-19–associated hospitalization was 77% among immunocompromised adults (compared with 90% in immunocompetent adults).
“This is good vindication to keep vaccinating,” he said, “and perhaps speaks to how difficult it is to assess the vaccine response [through measurement of antibody only].”
Neither Duke University’s transplant program, which performed 100-120 lung transplants a year pre-COVID, nor the programs at UT Southwestern or the Mayo Clinic in Jacksonville require that solid organ transplant candidates be vaccinated against SARS-CoV-2 in order to receive transplants, as some other transplant programs have done. (When asked about the issue, Dr. Banga and Dr. Narula each said that they have had no or little trouble convincing patients awaiting lung transplants of the need for COVID-19 vaccination.)
In an August statement, the American Society of Transplantation recommended vaccination for all solid organ transplant recipients, preferably prior to transplantation, and said that it “support[s] the development of institutional policies regarding pretransplant vaccination.”
The Society is not tracking centers’ vaccination policies. But Kaiser Health News reported in October that a growing number of transplant programs, such as UCHealth in Denver and UW Medicine in Seattle, have decided to either bar patients who refuse to be vaccinated from receiving transplants or give them lower priority on waitlists.
Potential lung donors, meanwhile, must be evaluated with lower respiratory COVID-19 testing, with results available prior to transplantation, according to policy developed by the Organ Procurement and Transplantation Network and effective in May 2021. The policy followed three published cases of donor-derived COVID-19 in lung transplant recipients, said Dr. Wolfe, who wrote about use of COVID-positive donors in an editorial published in October.
In each case, the donor had a negative COVID-19 nasopharyngeal swab at the time of organ procurement but was later found to have the virus on bronchoalveolar lavage, he said.
(The use of other organs from COVID-positive donors is appearing thus far to be safe, Dr. Wolfe noted. In the editorial, he references 13 cases of solid organ transplantation from SARS-CoV-2–infected donors into noninfected recipients; none of the 13 transplant recipients developed COVID-19).
Some questions remain, such as how many lower respiratory tests should be run, and how donors should be evaluated in cases of discordant results. Dr. Banga shared the case of a donor with one positive lower respiratory test result followed by two negative results. After internal debate, and consideration of potential false positives and other issues, the team at UT Southwestern decided to decline the donor, Dr. Banga said.
Other programs are likely making similar, appropriately cautious decisions, said Dr. Wolfe. “There’s no way in real-time donor evaluation to know whether the positive test is active virus that could infect the recipient and replicate ... or whether it’s [picking up] inactive or dead fragments of virus that was there several weeks ago. Our tests don’t differentiate that.”
Transplants in COVID-19 patients
Decision-making about lung transplant candidacy among patients with COVID-19 acute respiratory distress syndrome is complex and in need of a new paradigm.
“Some of these patients have the potential to recover, and they’re going to recover way later than what we’re used to,” said Dr. Banga. “We can’t extrapolate for COVID ARDS what we’ve learned for any other virus-related ARDS.”
Dr. Narula also has recently seen at least one COVID-19 patient on ECMO and under evaluation for transplantation recover. “We do not want to transplant too early,” he said, noting that there is consensus that lung transplant should be pursued only when the damage is deemed irreversible clinically and radiologically in the best judgment of the team. Still, “for many of these patients the only exit route will be lung transplants. For the next 12-24 months, a significant proportion of our lung transplant patients will have had post-COVID–related lung damage.”
As of October 2021, 233 lung transplants had been performed in the United States in recipients whose primary diagnosis was reported as COVID related, said Anne Paschke, media relations specialist with the United Network for Organ Sharing.
Dr. Banga, Dr. Wolfe, and Dr. Narula reported that they have no relevant disclosures.
Data is sparse thus far, but there is concern in lung transplant medicine about the long-term risk of chronic lung allograft dysfunction (CLAD) and a potentially shortened longevity of transplanted lungs in recipients who become ill with COVID-19.
“My fear is that we’re potentially sitting on this iceberg worth of people who, come 6 months or a year from [the acute phase of] their COVID illness, will in fact have earlier and progressive, chronic rejection,” said Cameron R. Wolfe, MBBS, MPH, associate professor of medicine in transplant infectious disease at Duke University, Durham, N.C.
Lower respiratory viral infections have long been concerning for lung transplant recipients given their propensity to cause scarring, a decline in lung function, and a heightened risk of allograft rejection. Time will tell whether lung transplant recipients who survive COVID-19 follow a similar path, or one that is worse, he said.
Short-term data
Outcomes beyond hospitalization and acute illness for lung transplant recipients affected by COVID-19 have been reported in the literature by only a few lung transplant programs. These reports – as well as anecdotal experiences being informally shared among transplant programs – have raised the specter of more severe dysfunction following the acute phase and more early CLAD, said Tathagat Narula, MD, assistant professor of medicine at the Mayo Medical School, Rochester, Minn., and a consultant in lung transplantation at the Mayo Clinic’s Jacksonville program.
“The available data cover only 3-6 months out. We don’t know what will happen in the next 6 months and beyond,” Dr. Narula said in an interview.
The risks of COVID-19 in already-transplanted patients and issues relating to the inadequate antibody responses to vaccination are just some of the challenges of lung transplant medicine in the era of SARS-CoV-2. “COVID-19,” said Dr. Narula, “has completely changed the way we practice lung transplant medicine – the way we’re looking both at our recipients and our donors.”
Potential donors are being evaluated with lower respiratory SARS-CoV-2 testing and an abundance of caution. And patients with severe COVID-19 affecting their own lungs are roundly expected to drive up lung transplant volume in the near future. “The whole paradigm has changed,” Dr. Narula said.
Post-acute trajectories
A chart review study published in October by the lung transplant team at the University of Texas Southwestern Medical Center, Dallas, covered 44 consecutive survivors at a median follow-up of 4.5 months from hospital discharge or acute illness (the survival rate was 83.3%). Patients had significantly impaired functional status, and 18 of the 44 (40.9%) had a significant and persistent loss of forced vital capacity or forced expiratory volume in 1 second (>10% from pre–COVID-19 baseline).
Three patients met the criteria for new CLAD after COVID-19 infection, with all three classified as restrictive allograft syndrome (RAS) phenotype.
Moreover, the majority of COVID-19 survivors who had CT chest scans (22 of 28) showed persistent parenchymal opacities – a finding that, regardless of symptomatology, suggests persistent allograft injury, said Amit Banga, MD, associate professor of medicine and medical director of the ex vivo lung perfusion program in UT Southwestern’s lung transplant program.
“The implication is that there may be long-term consequences of COVID-19, perhaps related to some degree of ongoing inflammation and damage,” said Dr. Banga, a coauthor of the postinfection outcomes paper.
The UT Southwestern lung transplant program, which normally performs 60-80 transplants a year, began routine CT scanning 4-5 months into the pandemic, after “stumbling into a few patients who had no symptoms indicative of COVID pneumonia and no changes on an x-ray but significant involvement on a CT,” he said.
Without routine scanning in the general population of COVID-19 patients, Dr. Banga noted, “we’re limited in convincingly saying that COVID is uniquely doing this to lung transplant recipients.” Nor can they conclude that SARS-CoV-2 is unique from other respiratory viruses such as respiratory syncytial virus (RSV) in this regard. (The program has added CT scanning to its protocol for lung transplant recipients afflicted with other respiratory viruses to learn more.)
However, in the big picture, COVID-19 has proven to be far worse for lung transplant recipients than illness with other respiratory viruses, including RSV. “Patients have more frequent and greater loss of lung function, and worse debility from the acute illness,” Dr. Banga said.
“The cornerstones of treatment of both these viruses are very similar, but both the in-hospital course and the postdischarge outcomes are significantly different.”
In an initial paper published in September 2021, Dr. Banga and colleagues compared their first 25 lung transplant patients testing positive for SARS-CoV-2 with a historical cohort of 36 patients with RSV treated during 2016-2018.
Patients with COVID-19 had significantly worse morbidity and mortality, including worse postinfection lung function loss, functional decline, and 3-month survival.
More time, he said, will shed light on the risks of CLAD and the long-term potential for recovery of lung function. Currently, at UT Southwestern, it appears that patients who survive acute illness and the “first 3-6 months after COVID-19, when we’re seeing all the postinfection morbidity, may [enter] a period of stability,” Dr. Banga said.
Overall, he said, patients in their initial cohort are “holding steady” without unusual morbidity, readmissions, or “other setbacks to their allografts.”
At the Mayo Clinic in Jacksonville, which normally performs 40-50 lung transplants a year, transplant physicians have similarly observed significant declines in lung function beyond the acute phase of COVID-19. “Anecdotally, we’re seeing that some patients are beginning to recover some of their lung function, while others have not,” said Dr. Narula. “And we don’t have predictors as to who will progress to CLAD. It’s a big knowledge gap.”
Dr. Narula noted that patients with restrictive allograft syndrome, such as those reported by the UT Southwestern team, “have scarring of the lung and a much worse prognosis than the obstructive type of chronic rejection.” Whether there’s a role for antifibrotic therapy is a question worthy of research.
In UT Southwestern’s analysis, persistently lower absolute lymphocyte counts (< 600/dL) and higher ferritin levels (>150 ng/mL) at the time of hospital discharge were independently associated with significant lung function loss. This finding, reported in their October paper, has helped guide their management practices, Dr. Banga said.
“Persistently elevated ferritin may indicate ongoing inflammation at the allograft level,” he said. “We now send [such patients] home on a longer course of oral corticosteroids.”
At the front end of care for infected lung transplant recipients, Dr. Banga said that his team and physicians at other lung transplant programs are holding the cell-cycle inhibitor component of patients’ maintenance immunosuppression therapy (commonly mycophenolate or azathioprine) once infection is diagnosed to maximize chances of a better outcome.
“There may be variation on how long [the regimens are adjusted],” he said. “We changed our duration from 4 weeks to 2 due to patients developing a rebound worsening in the third and fourth week of acute illness.”
There is significant variation from institution to institution in how viral infections are managed in lung transplant recipients, he and Dr. Narula said. “Our numbers are so small in lung transplant, and we don’t have standardized protocols – it’s one of the biggest challenges in our field,” said Dr. Narula.
Vaccination issues, evaluation of donors
Whether or not immunosuppression regimens should be adjusted prior to vaccination is a controversial question, but is “an absolutely valid one” and is currently being studied in at least one National Institutes of Health–funded trial involving solid organ transplant recipients, said Dr. Wolfe.
“Some have jumped to the conclusion [based on some earlier data] that they should reduce immunosuppression regimens for everyone at the time of vaccination ... but I don’t know the answer yet,” he said. “Balancing staying rejection free with potentially gaining more immune response is complicated ... and it may depend on where the pandemic is going in your area and other factors.”
Reductions aside, Dr. Wolfe tells lung transplant recipients that, based on his approximation of a number of different studies in solid organ transplant recipients, approximately 40%-50% of patients who are immunized with two doses of the COVID-19 mRNA vaccines will develop meaningful antibody levels – and that this rises to 50%-60% after a third dose.
It is difficult to glean from available studies the level of vaccine response for lung transplant recipients specifically. But given that their level of maintenance immunosuppression is higher than for recipients of other donor organs, “as a broad sweep, lung transplant recipients tend to be lower in the pecking order of response,” he said.
Still, “there’s a lot to gain,” he said, pointing to a recent study from the Morbidity and Mortality Weekly Report (2021 Nov 5. doi: 10.15585/mmwr.mm7044e3) showing that effectiveness of mRNA vaccination against COVID-19–associated hospitalization was 77% among immunocompromised adults (compared with 90% in immunocompetent adults).
“This is good vindication to keep vaccinating,” he said, “and perhaps speaks to how difficult it is to assess the vaccine response [through measurement of antibody only].”
Neither Duke University’s transplant program, which performed 100-120 lung transplants a year pre-COVID, nor the programs at UT Southwestern or the Mayo Clinic in Jacksonville require that solid organ transplant candidates be vaccinated against SARS-CoV-2 in order to receive transplants, as some other transplant programs have done. (When asked about the issue, Dr. Banga and Dr. Narula each said that they have had no or little trouble convincing patients awaiting lung transplants of the need for COVID-19 vaccination.)
In an August statement, the American Society of Transplantation recommended vaccination for all solid organ transplant recipients, preferably prior to transplantation, and said that it “support[s] the development of institutional policies regarding pretransplant vaccination.”
The Society is not tracking centers’ vaccination policies. But Kaiser Health News reported in October that a growing number of transplant programs, such as UCHealth in Denver and UW Medicine in Seattle, have decided to either bar patients who refuse to be vaccinated from receiving transplants or give them lower priority on waitlists.
Potential lung donors, meanwhile, must be evaluated with lower respiratory COVID-19 testing, with results available prior to transplantation, according to policy developed by the Organ Procurement and Transplantation Network and effective in May 2021. The policy followed three published cases of donor-derived COVID-19 in lung transplant recipients, said Dr. Wolfe, who wrote about use of COVID-positive donors in an editorial published in October.
In each case, the donor had a negative COVID-19 nasopharyngeal swab at the time of organ procurement but was later found to have the virus on bronchoalveolar lavage, he said.
(The use of other organs from COVID-positive donors is appearing thus far to be safe, Dr. Wolfe noted. In the editorial, he references 13 cases of solid organ transplantation from SARS-CoV-2–infected donors into noninfected recipients; none of the 13 transplant recipients developed COVID-19).
Some questions remain, such as how many lower respiratory tests should be run, and how donors should be evaluated in cases of discordant results. Dr. Banga shared the case of a donor with one positive lower respiratory test result followed by two negative results. After internal debate, and consideration of potential false positives and other issues, the team at UT Southwestern decided to decline the donor, Dr. Banga said.
Other programs are likely making similar, appropriately cautious decisions, said Dr. Wolfe. “There’s no way in real-time donor evaluation to know whether the positive test is active virus that could infect the recipient and replicate ... or whether it’s [picking up] inactive or dead fragments of virus that was there several weeks ago. Our tests don’t differentiate that.”
Transplants in COVID-19 patients
Decision-making about lung transplant candidacy among patients with COVID-19 acute respiratory distress syndrome is complex and in need of a new paradigm.
“Some of these patients have the potential to recover, and they’re going to recover way later than what we’re used to,” said Dr. Banga. “We can’t extrapolate for COVID ARDS what we’ve learned for any other virus-related ARDS.”
Dr. Narula also has recently seen at least one COVID-19 patient on ECMO and under evaluation for transplantation recover. “We do not want to transplant too early,” he said, noting that there is consensus that lung transplant should be pursued only when the damage is deemed irreversible clinically and radiologically in the best judgment of the team. Still, “for many of these patients the only exit route will be lung transplants. For the next 12-24 months, a significant proportion of our lung transplant patients will have had post-COVID–related lung damage.”
As of October 2021, 233 lung transplants had been performed in the United States in recipients whose primary diagnosis was reported as COVID related, said Anne Paschke, media relations specialist with the United Network for Organ Sharing.
Dr. Banga, Dr. Wolfe, and Dr. Narula reported that they have no relevant disclosures.
Data is sparse thus far, but there is concern in lung transplant medicine about the long-term risk of chronic lung allograft dysfunction (CLAD) and a potentially shortened longevity of transplanted lungs in recipients who become ill with COVID-19.
“My fear is that we’re potentially sitting on this iceberg worth of people who, come 6 months or a year from [the acute phase of] their COVID illness, will in fact have earlier and progressive, chronic rejection,” said Cameron R. Wolfe, MBBS, MPH, associate professor of medicine in transplant infectious disease at Duke University, Durham, N.C.
Lower respiratory viral infections have long been concerning for lung transplant recipients given their propensity to cause scarring, a decline in lung function, and a heightened risk of allograft rejection. Time will tell whether lung transplant recipients who survive COVID-19 follow a similar path, or one that is worse, he said.
Short-term data
Outcomes beyond hospitalization and acute illness for lung transplant recipients affected by COVID-19 have been reported in the literature by only a few lung transplant programs. These reports – as well as anecdotal experiences being informally shared among transplant programs – have raised the specter of more severe dysfunction following the acute phase and more early CLAD, said Tathagat Narula, MD, assistant professor of medicine at the Mayo Medical School, Rochester, Minn., and a consultant in lung transplantation at the Mayo Clinic’s Jacksonville program.
“The available data cover only 3-6 months out. We don’t know what will happen in the next 6 months and beyond,” Dr. Narula said in an interview.
The risks of COVID-19 in already-transplanted patients and issues relating to the inadequate antibody responses to vaccination are just some of the challenges of lung transplant medicine in the era of SARS-CoV-2. “COVID-19,” said Dr. Narula, “has completely changed the way we practice lung transplant medicine – the way we’re looking both at our recipients and our donors.”
Potential donors are being evaluated with lower respiratory SARS-CoV-2 testing and an abundance of caution. And patients with severe COVID-19 affecting their own lungs are roundly expected to drive up lung transplant volume in the near future. “The whole paradigm has changed,” Dr. Narula said.
Post-acute trajectories
A chart review study published in October by the lung transplant team at the University of Texas Southwestern Medical Center, Dallas, covered 44 consecutive survivors at a median follow-up of 4.5 months from hospital discharge or acute illness (the survival rate was 83.3%). Patients had significantly impaired functional status, and 18 of the 44 (40.9%) had a significant and persistent loss of forced vital capacity or forced expiratory volume in 1 second (>10% from pre–COVID-19 baseline).
Three patients met the criteria for new CLAD after COVID-19 infection, with all three classified as restrictive allograft syndrome (RAS) phenotype.
Moreover, the majority of COVID-19 survivors who had CT chest scans (22 of 28) showed persistent parenchymal opacities – a finding that, regardless of symptomatology, suggests persistent allograft injury, said Amit Banga, MD, associate professor of medicine and medical director of the ex vivo lung perfusion program in UT Southwestern’s lung transplant program.
“The implication is that there may be long-term consequences of COVID-19, perhaps related to some degree of ongoing inflammation and damage,” said Dr. Banga, a coauthor of the postinfection outcomes paper.
The UT Southwestern lung transplant program, which normally performs 60-80 transplants a year, began routine CT scanning 4-5 months into the pandemic, after “stumbling into a few patients who had no symptoms indicative of COVID pneumonia and no changes on an x-ray but significant involvement on a CT,” he said.
Without routine scanning in the general population of COVID-19 patients, Dr. Banga noted, “we’re limited in convincingly saying that COVID is uniquely doing this to lung transplant recipients.” Nor can they conclude that SARS-CoV-2 is unique from other respiratory viruses such as respiratory syncytial virus (RSV) in this regard. (The program has added CT scanning to its protocol for lung transplant recipients afflicted with other respiratory viruses to learn more.)
However, in the big picture, COVID-19 has proven to be far worse for lung transplant recipients than illness with other respiratory viruses, including RSV. “Patients have more frequent and greater loss of lung function, and worse debility from the acute illness,” Dr. Banga said.
“The cornerstones of treatment of both these viruses are very similar, but both the in-hospital course and the postdischarge outcomes are significantly different.”
In an initial paper published in September 2021, Dr. Banga and colleagues compared their first 25 lung transplant patients testing positive for SARS-CoV-2 with a historical cohort of 36 patients with RSV treated during 2016-2018.
Patients with COVID-19 had significantly worse morbidity and mortality, including worse postinfection lung function loss, functional decline, and 3-month survival.
More time, he said, will shed light on the risks of CLAD and the long-term potential for recovery of lung function. Currently, at UT Southwestern, it appears that patients who survive acute illness and the “first 3-6 months after COVID-19, when we’re seeing all the postinfection morbidity, may [enter] a period of stability,” Dr. Banga said.
Overall, he said, patients in their initial cohort are “holding steady” without unusual morbidity, readmissions, or “other setbacks to their allografts.”
At the Mayo Clinic in Jacksonville, which normally performs 40-50 lung transplants a year, transplant physicians have similarly observed significant declines in lung function beyond the acute phase of COVID-19. “Anecdotally, we’re seeing that some patients are beginning to recover some of their lung function, while others have not,” said Dr. Narula. “And we don’t have predictors as to who will progress to CLAD. It’s a big knowledge gap.”
Dr. Narula noted that patients with restrictive allograft syndrome, such as those reported by the UT Southwestern team, “have scarring of the lung and a much worse prognosis than the obstructive type of chronic rejection.” Whether there’s a role for antifibrotic therapy is a question worthy of research.
In UT Southwestern’s analysis, persistently lower absolute lymphocyte counts (< 600/dL) and higher ferritin levels (>150 ng/mL) at the time of hospital discharge were independently associated with significant lung function loss. This finding, reported in their October paper, has helped guide their management practices, Dr. Banga said.
“Persistently elevated ferritin may indicate ongoing inflammation at the allograft level,” he said. “We now send [such patients] home on a longer course of oral corticosteroids.”
At the front end of care for infected lung transplant recipients, Dr. Banga said that his team and physicians at other lung transplant programs are holding the cell-cycle inhibitor component of patients’ maintenance immunosuppression therapy (commonly mycophenolate or azathioprine) once infection is diagnosed to maximize chances of a better outcome.
“There may be variation on how long [the regimens are adjusted],” he said. “We changed our duration from 4 weeks to 2 due to patients developing a rebound worsening in the third and fourth week of acute illness.”
There is significant variation from institution to institution in how viral infections are managed in lung transplant recipients, he and Dr. Narula said. “Our numbers are so small in lung transplant, and we don’t have standardized protocols – it’s one of the biggest challenges in our field,” said Dr. Narula.
Vaccination issues, evaluation of donors
Whether or not immunosuppression regimens should be adjusted prior to vaccination is a controversial question, but is “an absolutely valid one” and is currently being studied in at least one National Institutes of Health–funded trial involving solid organ transplant recipients, said Dr. Wolfe.
“Some have jumped to the conclusion [based on some earlier data] that they should reduce immunosuppression regimens for everyone at the time of vaccination ... but I don’t know the answer yet,” he said. “Balancing staying rejection free with potentially gaining more immune response is complicated ... and it may depend on where the pandemic is going in your area and other factors.”
Reductions aside, Dr. Wolfe tells lung transplant recipients that, based on his approximation of a number of different studies in solid organ transplant recipients, approximately 40%-50% of patients who are immunized with two doses of the COVID-19 mRNA vaccines will develop meaningful antibody levels – and that this rises to 50%-60% after a third dose.
It is difficult to glean from available studies the level of vaccine response for lung transplant recipients specifically. But given that their level of maintenance immunosuppression is higher than for recipients of other donor organs, “as a broad sweep, lung transplant recipients tend to be lower in the pecking order of response,” he said.
Still, “there’s a lot to gain,” he said, pointing to a recent study from the Morbidity and Mortality Weekly Report (2021 Nov 5. doi: 10.15585/mmwr.mm7044e3) showing that effectiveness of mRNA vaccination against COVID-19–associated hospitalization was 77% among immunocompromised adults (compared with 90% in immunocompetent adults).
“This is good vindication to keep vaccinating,” he said, “and perhaps speaks to how difficult it is to assess the vaccine response [through measurement of antibody only].”
Neither Duke University’s transplant program, which performed 100-120 lung transplants a year pre-COVID, nor the programs at UT Southwestern or the Mayo Clinic in Jacksonville require that solid organ transplant candidates be vaccinated against SARS-CoV-2 in order to receive transplants, as some other transplant programs have done. (When asked about the issue, Dr. Banga and Dr. Narula each said that they have had no or little trouble convincing patients awaiting lung transplants of the need for COVID-19 vaccination.)
In an August statement, the American Society of Transplantation recommended vaccination for all solid organ transplant recipients, preferably prior to transplantation, and said that it “support[s] the development of institutional policies regarding pretransplant vaccination.”
The Society is not tracking centers’ vaccination policies. But Kaiser Health News reported in October that a growing number of transplant programs, such as UCHealth in Denver and UW Medicine in Seattle, have decided to either bar patients who refuse to be vaccinated from receiving transplants or give them lower priority on waitlists.
Potential lung donors, meanwhile, must be evaluated with lower respiratory COVID-19 testing, with results available prior to transplantation, according to policy developed by the Organ Procurement and Transplantation Network and effective in May 2021. The policy followed three published cases of donor-derived COVID-19 in lung transplant recipients, said Dr. Wolfe, who wrote about use of COVID-positive donors in an editorial published in October.
In each case, the donor had a negative COVID-19 nasopharyngeal swab at the time of organ procurement but was later found to have the virus on bronchoalveolar lavage, he said.
(The use of other organs from COVID-positive donors is appearing thus far to be safe, Dr. Wolfe noted. In the editorial, he references 13 cases of solid organ transplantation from SARS-CoV-2–infected donors into noninfected recipients; none of the 13 transplant recipients developed COVID-19).
Some questions remain, such as how many lower respiratory tests should be run, and how donors should be evaluated in cases of discordant results. Dr. Banga shared the case of a donor with one positive lower respiratory test result followed by two negative results. After internal debate, and consideration of potential false positives and other issues, the team at UT Southwestern decided to decline the donor, Dr. Banga said.
Other programs are likely making similar, appropriately cautious decisions, said Dr. Wolfe. “There’s no way in real-time donor evaluation to know whether the positive test is active virus that could infect the recipient and replicate ... or whether it’s [picking up] inactive or dead fragments of virus that was there several weeks ago. Our tests don’t differentiate that.”
Transplants in COVID-19 patients
Decision-making about lung transplant candidacy among patients with COVID-19 acute respiratory distress syndrome is complex and in need of a new paradigm.
“Some of these patients have the potential to recover, and they’re going to recover way later than what we’re used to,” said Dr. Banga. “We can’t extrapolate for COVID ARDS what we’ve learned for any other virus-related ARDS.”
Dr. Narula also has recently seen at least one COVID-19 patient on ECMO and under evaluation for transplantation recover. “We do not want to transplant too early,” he said, noting that there is consensus that lung transplant should be pursued only when the damage is deemed irreversible clinically and radiologically in the best judgment of the team. Still, “for many of these patients the only exit route will be lung transplants. For the next 12-24 months, a significant proportion of our lung transplant patients will have had post-COVID–related lung damage.”
As of October 2021, 233 lung transplants had been performed in the United States in recipients whose primary diagnosis was reported as COVID related, said Anne Paschke, media relations specialist with the United Network for Organ Sharing.
Dr. Banga, Dr. Wolfe, and Dr. Narula reported that they have no relevant disclosures.