User login
Von Willebrand disease (VWD) represents the most common inherited bleeding disorder, with a prevalence of approximately 1 in 1,000 people.
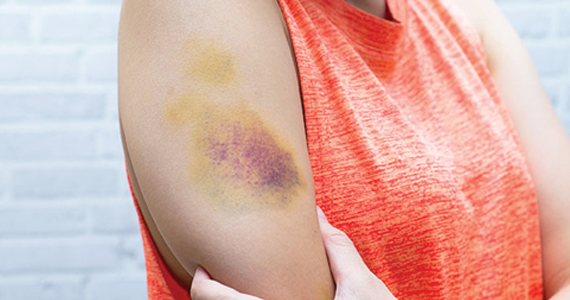
Bruising and mucocutaneous bleeding (epistaxis, gingival bleeding, and bleeding after dental extraction) are the most common presenting symptoms of VWD. Because VWD substantially increases the risk of heavy menstrual bleeding (HMB) and, to some extent, intrapartum bleeding complications, and postpartum hemorrhage, women experience a disproportionate burden from VWD. Thus, ObGyns are likely to be called on to make treatment recommendations in VWD patients with these concerns.1
In 2017, the American Society of Hematology, the International Society on Thrombosis and Haemostasis, the National Hemophilia Foundation, and the World Federation of Hemophilia determined that among clinical issues related to VWD, updating guidelines for women with VWD represented the highest priority.2 Accordingly, an international group of hematologists/coagulation specialists performed systematic literature reviews to address 3 questions faced by women with VWD and their clinicians:
- What are the most effective treatments for HMB?
- What is the safest approach for women desiring neuraxial analgesia for intrapartum pain?
- What is the impact of postpartum administration of tranexamic acid (TxA) on postpartum hemorrhage (PPH)?3
Evidence on management strategies for HMB in women with VWD
The prevalence of HMB in women with VWD ranges from 50% to 92%. Reports suggest that between 5% and 24% of women presenting with this symptom have VWD.3 However, the prevalence of VWD among women seeking care for HMB relates to referral patterns, with the prevalence of VWD substantially higher in patient populations who are referred to clinicians or centers that focus on care of patients with bleeding disorders.
The systematic review authors3 identified 2 comparative studies that assessed the treatment of HMB in women with VWD. One was a crossover trial that enrolled 116 VWD patients with HMB with a mean age of 36 years.4 All participants in this trial chose not to use combination oral contraceptives (COCs) as they had not experienced good results with prior COC use. Trial participants were randomly assigned to receive either intranasal desmopressin (DDAVP; a synthetic analog of the antidiuretic agent vasopressin, which stimulates the release of VWF from endothelial cells) or oral TxA therapy for 2 menstrual cycles. Participants then crossed over to the other drug for 2 additional cycles. Although both agents significantly reduced estimated menstrual blood loss, TxA was more effective in decreasing bleeding than intranasal DDAVP.4
In a retrospective cohort study, investigators compared COC use with intranasal DDAVP in 36 adolescents who had VWD and HMB.5 Participant follow-up ranged from 6 months to 4 years. The estimated efficacy of COCs and intranasal DDAVP was 86% and 77%, respectively, a difference that did not achieve statistical significance. Some of the adolescents who used intranasal DDAVP reported severe headaches and flushing.5
In addition, the systematic review authors3 identified 5 case series that described the use of the levonorgestrel (52 mg)-releasing intrauterine device (LNG 52 IUD) in women with VWD and HMB; 4 of these addressed the efficacy of progestin-releasing IUDs in reducing HMB in this patient population.6-9 Using different approaches to define HMB, the authors of these reports followed between 7 and 26 patients with bleeding disorders (most with confirmed VWD) and HMB for variable amounts of time after placement of an LNG 52 IUD. Many of the women described in these case series had tried other HMB treatments, including COCs, without success. Although these 4 reports assessed different outcomes, all reported that placement of the LNG 52 IUD substantially reduced menstrual blood loss, often resulting in amenorrhea. Several of these reports also noted important improvements in quality of life following LNG 52 IUD placement. One case series reported LNG 52 IUD placement in 13 adolescents with VWD and HMB. The mean time to achieve amenorrhea or occasional spotting was 94 days.6
The fifth report, which followed 20 women (median age, 31 years) with HMB associated with VWD or other bleeding disorders who underwent LNG 52 IUD placement, aimed to describe IUD expulsions and malpositioned IUDs in this population. In this small group of patients, 3 IUD expulsions and 2 malpositioned IUDs were observed. Furthermore, an additional 5 women had their device removed prematurely due to patient dissatisfaction. Accordingly, the IUD continuation rate in this case series was only 50%.10
Evidence on management of pregnancy, delivery, and the postpartum period
Heavy menstrual bleeding is not the only challenge for women with VWD. While pregnancy is accompanied by higher levels of VWF, potentially offsetting the risk of bleeding at the time of delivery, the levels do not achieve the same magnitude as they would in unaffected women.11 Women are at an increased risk of primary PPH12,13 and, importantly, since VWF levels fall exponentially after delivery when women are still experiencing lochia,11 they are at increased risk of secondary or delayed PPH.
Two questions arise frequently in the care of women with VWD at the time of delivery and during the postpartum period:
- What is the safest approach for women who desire neuraxial analgesia for intrapartum pain?
- What is the impact of postpartum administration of TxA on PPH?
The second systematic review the authors performed3 focused on VWF levels in women receiving neuraxial anesthesia during labor. After screening 27 studies, the authors included 5 case series, which did not describe outcomes based on VWF levels but rather described the outcomes of women with VWF levels of greater than 0.50 IU/mL (> 50% of normal compared with a normal standard).
Meta-analysis showed that the proportion of anesthesia complications was 6%, which sounds high, but the range of complications was what would be expected in any population (hypotension, accidental dural puncture, inadequate anesthesia, and bloody tap with no further complications). No spinal, subdural, or epidural hematomas were noted.3 Such hematomas are an extremely rare complication of neuraxial anesthesia, occurring in only 1 in 200,000 or 1 in 250,000 obstetric patients14,15; accordingly, an increase in the rate of hematomas among women with VWD could go undetected. The absence of hematomas among women with VWD as reported in the systematic review does not mean there is not an increase in the rate of hematomas in women with VWD. The relative risk is unknown and caution would be advised.
The third systematic review that the authors performed3 was on TxA treatment in the postpartum period. After screening 41 studies, the authors included 2 retrospective cohort studies.16,17 The majority of the participants had VWD. With very-low-certainty evidence, the authors found that TxA reduces the risk of:
- severe primary PPH (risk ratio [RR], 0.36; 95% confidence interval [CI], 0.05–2.59)
- primary PPH (RR, 0.25; 95% CI, 0.04–1.75)
- secondary PPH (RR, 0.42; 95% CI, 0.02–0.91—does not cross 1.0).
Note that the 95% confidence intervals for severe as well as primary PPH crossed 1.0 and therefore these reductions in risk did not achieve statistical significance. Additionally, there was very-low-certainty evidence on the effect of TxA on blood transfusions, vaginal hematomas, blood loss, and thrombotic complications.3
Continue to: Our recommendations for HMB management...
Our recommendations for HMB management
When first evaluating any woman with HMB, it is important to check a blood count and ferritin level, if not already done. If there is any suggestion of iron deficiency (with or without anemia), we recommend oral iron supplementation. This is best accomplished with slow-release iron supplement formulations (or less expensive generic or house brands that contain less than 65 mg of elemental iron per tablet) taken every other day. Such preparations may cause fewer gastrointestinal adverse effects than other oral iron formulations.18 Although it may appear counterintuitive, oral iron is better absorbed (and also may cause fewer gastrointestinal adverse effects) when taken every other day.19
Initial management of HMB, whether or not a bleeding disorder is present, often consists or oral hormonal management. If no contraindications are present, we recommend initiation of a COC with a short hormone-free interval (for example, a 24/4 formulation). If contraindications to contraceptive doses of estrogen are present, continuous use of norethindrone acetate 5-mg tablets or off-label use of combination tablets with 5 µg of ethinyl estradiol and 1 mg of norethindrone acetate (a formulation approved for the treatment of menopausal symptoms) is appropriate.20
Once a patient is established on oral hormonal management, placement of a levonorgestrel-releasing IUD should be considered. Given that expulsion rates may be higher in women with HMB, if feasible, consider using abdominal ultrasound guidance for IUD placement.
For women with VWD who fail first-line therapy (hormonal management) or are trying to become pregnant, TxA (two 650-mg tablets 3 times daily for up to 5 days during episodes of heavy flow) can reduce HMB.20,21
Our recommendations for management of pregnancy and delivery
The second and third systematic reviews discussed above provide very limited guidance on comprehensive management. The care of the pregnant patient with VWD starts with assessment of VWF levels and making an accurate diagnosis. This usually requires the input of a hematologist or other expert in hemostasis. If no recent VWF levels are available, the ObGyn can obtain a von Willebrand panel that includes VWF antigen, VWF activity (most commonly ristocetin cofactor), and factor VIII.
Levels should be reassessed around 36 weeks’ gestation in anticipation of delivery. VWF levels increase during pregnancy; accordingly, in mild, type 1 VWD, half the time treatment is not necessary.11 If VWF activity is less than 50 IU/dL (less than 50% of normal) at 36 weeks’ gestation, the patient should receive VWF concentrate (dosed in VWF units). This requires consultation with hematology and specialized pharmacy support.
For these reasons, the patient with a VWF level less than 50% should be delivered in a referral center with the necessary resources. Anesthesia should be aware of the patient. Unless
Due to the quantity of fluids administered during labor or at the time of delivery and the coexistent administration of oxytocin, desmopressin (synthetic vasopressin) should not be used without monitoring sodium levels, should not be dosed more than once, or should be avoided altogether due to the risk of water intoxication.
If the patient has sustained VWF and factor VIII levels greater than 50 IU/dL, she would be a candidate to deliver in her local hospital and receive neuraxial anesthesia.
Based on the best data we have for women with VWD, a patient with a VWF greater than 50 IU/dL is no more likely to experience PPH than other women.11 Intravenous TxA can be used for prevention or treatment of immediate postpartum bleeding per protocol (1 g after cord clamp and 1 g 30 minutes or more later).22 Oral TxA can be used for prevention or treatment of delayed postpartum bleeding as per HMB. Regardless of the outcome of any testing during pregnancy, nonsteroidal anti-inflammatory drugs should be avoided postpartum and the patient should be monitored closely for bleeding.
Neonatal care
As for the fetus/neonate, the parents should be aware that the infant has a 50% chance of inheriting VWD. If the baby’s father has no history of bleeding, it is unlikely that the infant would be any more affected than the patient herself. Nonetheless, cord blood (in one or more light blue top tubes) should be obtained at the time of delivery and sent for a von Willebrand panel. If the infant is male, a circumcision should be postponed until VWD is ruled out. In addition, fetal invasive procedures should be avoided during labor. Fetal scalp electrode placement should be avoided. Operative vaginal delivery also should be avoided. Cesarean delivery would be preferred to operative vaginal delivery, but if operative vaginal delivery is unavoidable, use of forceps is preferred to vacuum extraction. ●
- ACOG committee opinion no. 451: Von Willebrand disease in women. Obstet Gynecol. 2009;114:1439-1443. doi: 10.1097 /AOG.0b013e3181c6f975.
- Kalot MA, Al-Khatib M, Connell NT, et al; VWD Working Group. An international survey to inform priorities for new guidance on von Willebrand disease. Hemophilia. 2020;26:106-116. doi: 10.1111/hae.13881.
- Brignardello-Petersen R, El Alayli A, Husainat N, et al. Gynecologic and obstetric management of women with von Willebrand disease: summary of 3 systematic reviews of the literature. Blood Adv. 2022;6:228-237. doi: 10.1182 /bloodadvances.2021005589.
- Kouides PA, Byams VR, Philipp CS, et al. Multisite management study of menorrhagia with abnormal laboratory haemostasis: a prospective crossover study of intranasal desmopressin and oral tranexamic acid. Br J Haematol. 2009;145:212-220. doi: 10.1111/j.1365-2141.2009.07610.x.
- Amesse LS, Pfaff-Amesse T, Leonardi R, et al. Oral contraceptives and DDAVP nasal spray: patterns of use in managing vWD-associated menorrhagia: a single-institution study. J Pediatr Hematol Oncol. 2005;27:357-363. doi: 10.1097/01.mph.0000173175.95152.95.
- Adeyemi-Fowode OA, Santos XM, Dietrich JE, et al. Levonorgestrel-releasing intrauterine device use in female adolescents with heavy menstrual bleeding and bleeding disorders: single institution review. J Pediatr Adolesc Gynecol. 2017;30:479-483. doi: 10.1016/j.jpag.2016.04.001.
- Chi C, Huq FY, Kadir RA. Levonorgestrel-releasing intrauterine system for the management of heavy menstrual bleeding in women with inherited bleeding disorders: long-term follow-up. Contraception. 2011;83:242-247. doi: 10.1016/j.contraception.2010.07.010.
- Kingman CE, Kadir RA, Lee CA, et al. The use of levonorgestrel-releasing intrauterine system for treatment of menorrhagia in women with inherited bleeding disorders. BJOG. 2004;111:1425-1428. doi: 10.1111/j.1471-0528.2004.00305.x.
- Lukes AS, Reardon B, Arepally G. Use of the levonorgestrel-releasing intrauterine system in women with hemostatic disorders. Fertil Steril. 2008;90:673-677. doi: 10.1016 /j.fertnstert.2007.07.1315.
- Rimmer E, Jamieson MA, James P. Malposition and expulsion of the levonorgestrel intrauterine system among women with inherited bleeding disorders. Haemophilia. 2013;19:933-938. doi: 10.1111/hae.12184.
- James AH, Konkle BA, Kouides P, et al. Postpartum von Willebrand factor levels in women with and without von Willebrand disease and implications for prophylaxis. Haemophilia. 2015;21:81-87. doi: 10.1111/hae.12568.
- James AH, Jamison MG. Bleeding events and other complications during pregnancy and childbirth in women with von Willebrand disease. J Thromb Haemost. 2007;5: 1165-1169. doi: 10.1111/j.1538-7836.2007.02563.x.
- Al-Zirqi I, Vangen S, Forsen L, et al. Prevalence and risk factors of severe obstetric haemorrhage. BJOG. 2008;115:1265-1272. doi: 10.1111/j.1471-0528.2008.01859.x.
- Moen V, Dahlgren N, Irestedt L. Severe neurological complications after central neuraxial blockades in Sweden 1990-1999. Anesthesiology. 2004;101:950-959. doi: 10.1097/00000542-200410000-00021.
- D’Angelo R, Smiley RM, Riley ET, et al. Serious complications related to obstetric anesthesia: the serious complication repository project of the Society for Obstetric Anesthesia and Perinatology. Anesthesiology. 2014;120:1505-1512. doi: 10.1097/ALN.000000000000253.
- Govorov I, Lofgren S, Chaireti R, et al. Postpartum hemorrhage in women with von Willebrand disease—a retrospective observational study. PLos One. 2016;11:e0164683. doi: 10.1371/journal.pone.0164683.
- Hawke L, Grabell J, Sim W, et al. Obstetric bleeding among women with inherited bleeding disorders: a retrospective study. Haemophilia. 2016;22:906-911. doi: 10.1111/hae.13067.
- James AH. Iron deficiency anemia in pregnancy. Obstet Gynecol. 2021;138:663-674. doi:10.1097/AOG .000000000000.4559.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524-e533. doi: 10.1016/S2352-3026(17)30182-5.
- Kaunitz AM. Abnormal uterine bleeding in reproductiveage women. JAMA. 2019;321:2126-2127. doi: 10.1001 /jama.2019.5248.
- James AH, Kouides PA, Abdul-Kadir R, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. Am J Obstet Gynecol. 2009;201:12.e1-8. doi: 10.1016 /j.ajog.2009.04.024.
- WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105-2116. doi: 10.1016/S0140-6736(17)30638-4.
Von Willebrand disease (VWD) represents the most common inherited bleeding disorder, with a prevalence of approximately 1 in 1,000 people.
Bruising and mucocutaneous bleeding (epistaxis, gingival bleeding, and bleeding after dental extraction) are the most common presenting symptoms of VWD. Because VWD substantially increases the risk of heavy menstrual bleeding (HMB) and, to some extent, intrapartum bleeding complications, and postpartum hemorrhage, women experience a disproportionate burden from VWD. Thus, ObGyns are likely to be called on to make treatment recommendations in VWD patients with these concerns.1
In 2017, the American Society of Hematology, the International Society on Thrombosis and Haemostasis, the National Hemophilia Foundation, and the World Federation of Hemophilia determined that among clinical issues related to VWD, updating guidelines for women with VWD represented the highest priority.2 Accordingly, an international group of hematologists/coagulation specialists performed systematic literature reviews to address 3 questions faced by women with VWD and their clinicians:
- What are the most effective treatments for HMB?
- What is the safest approach for women desiring neuraxial analgesia for intrapartum pain?
- What is the impact of postpartum administration of tranexamic acid (TxA) on postpartum hemorrhage (PPH)?3
Evidence on management strategies for HMB in women with VWD
The prevalence of HMB in women with VWD ranges from 50% to 92%. Reports suggest that between 5% and 24% of women presenting with this symptom have VWD.3 However, the prevalence of VWD among women seeking care for HMB relates to referral patterns, with the prevalence of VWD substantially higher in patient populations who are referred to clinicians or centers that focus on care of patients with bleeding disorders.
The systematic review authors3 identified 2 comparative studies that assessed the treatment of HMB in women with VWD. One was a crossover trial that enrolled 116 VWD patients with HMB with a mean age of 36 years.4 All participants in this trial chose not to use combination oral contraceptives (COCs) as they had not experienced good results with prior COC use. Trial participants were randomly assigned to receive either intranasal desmopressin (DDAVP; a synthetic analog of the antidiuretic agent vasopressin, which stimulates the release of VWF from endothelial cells) or oral TxA therapy for 2 menstrual cycles. Participants then crossed over to the other drug for 2 additional cycles. Although both agents significantly reduced estimated menstrual blood loss, TxA was more effective in decreasing bleeding than intranasal DDAVP.4
In a retrospective cohort study, investigators compared COC use with intranasal DDAVP in 36 adolescents who had VWD and HMB.5 Participant follow-up ranged from 6 months to 4 years. The estimated efficacy of COCs and intranasal DDAVP was 86% and 77%, respectively, a difference that did not achieve statistical significance. Some of the adolescents who used intranasal DDAVP reported severe headaches and flushing.5
In addition, the systematic review authors3 identified 5 case series that described the use of the levonorgestrel (52 mg)-releasing intrauterine device (LNG 52 IUD) in women with VWD and HMB; 4 of these addressed the efficacy of progestin-releasing IUDs in reducing HMB in this patient population.6-9 Using different approaches to define HMB, the authors of these reports followed between 7 and 26 patients with bleeding disorders (most with confirmed VWD) and HMB for variable amounts of time after placement of an LNG 52 IUD. Many of the women described in these case series had tried other HMB treatments, including COCs, without success. Although these 4 reports assessed different outcomes, all reported that placement of the LNG 52 IUD substantially reduced menstrual blood loss, often resulting in amenorrhea. Several of these reports also noted important improvements in quality of life following LNG 52 IUD placement. One case series reported LNG 52 IUD placement in 13 adolescents with VWD and HMB. The mean time to achieve amenorrhea or occasional spotting was 94 days.6
The fifth report, which followed 20 women (median age, 31 years) with HMB associated with VWD or other bleeding disorders who underwent LNG 52 IUD placement, aimed to describe IUD expulsions and malpositioned IUDs in this population. In this small group of patients, 3 IUD expulsions and 2 malpositioned IUDs were observed. Furthermore, an additional 5 women had their device removed prematurely due to patient dissatisfaction. Accordingly, the IUD continuation rate in this case series was only 50%.10
Evidence on management of pregnancy, delivery, and the postpartum period
Heavy menstrual bleeding is not the only challenge for women with VWD. While pregnancy is accompanied by higher levels of VWF, potentially offsetting the risk of bleeding at the time of delivery, the levels do not achieve the same magnitude as they would in unaffected women.11 Women are at an increased risk of primary PPH12,13 and, importantly, since VWF levels fall exponentially after delivery when women are still experiencing lochia,11 they are at increased risk of secondary or delayed PPH.
Two questions arise frequently in the care of women with VWD at the time of delivery and during the postpartum period:
- What is the safest approach for women who desire neuraxial analgesia for intrapartum pain?
- What is the impact of postpartum administration of TxA on PPH?
The second systematic review the authors performed3 focused on VWF levels in women receiving neuraxial anesthesia during labor. After screening 27 studies, the authors included 5 case series, which did not describe outcomes based on VWF levels but rather described the outcomes of women with VWF levels of greater than 0.50 IU/mL (> 50% of normal compared with a normal standard).
Meta-analysis showed that the proportion of anesthesia complications was 6%, which sounds high, but the range of complications was what would be expected in any population (hypotension, accidental dural puncture, inadequate anesthesia, and bloody tap with no further complications). No spinal, subdural, or epidural hematomas were noted.3 Such hematomas are an extremely rare complication of neuraxial anesthesia, occurring in only 1 in 200,000 or 1 in 250,000 obstetric patients14,15; accordingly, an increase in the rate of hematomas among women with VWD could go undetected. The absence of hematomas among women with VWD as reported in the systematic review does not mean there is not an increase in the rate of hematomas in women with VWD. The relative risk is unknown and caution would be advised.
The third systematic review that the authors performed3 was on TxA treatment in the postpartum period. After screening 41 studies, the authors included 2 retrospective cohort studies.16,17 The majority of the participants had VWD. With very-low-certainty evidence, the authors found that TxA reduces the risk of:
- severe primary PPH (risk ratio [RR], 0.36; 95% confidence interval [CI], 0.05–2.59)
- primary PPH (RR, 0.25; 95% CI, 0.04–1.75)
- secondary PPH (RR, 0.42; 95% CI, 0.02–0.91—does not cross 1.0).
Note that the 95% confidence intervals for severe as well as primary PPH crossed 1.0 and therefore these reductions in risk did not achieve statistical significance. Additionally, there was very-low-certainty evidence on the effect of TxA on blood transfusions, vaginal hematomas, blood loss, and thrombotic complications.3
Continue to: Our recommendations for HMB management...
Our recommendations for HMB management
When first evaluating any woman with HMB, it is important to check a blood count and ferritin level, if not already done. If there is any suggestion of iron deficiency (with or without anemia), we recommend oral iron supplementation. This is best accomplished with slow-release iron supplement formulations (or less expensive generic or house brands that contain less than 65 mg of elemental iron per tablet) taken every other day. Such preparations may cause fewer gastrointestinal adverse effects than other oral iron formulations.18 Although it may appear counterintuitive, oral iron is better absorbed (and also may cause fewer gastrointestinal adverse effects) when taken every other day.19
Initial management of HMB, whether or not a bleeding disorder is present, often consists or oral hormonal management. If no contraindications are present, we recommend initiation of a COC with a short hormone-free interval (for example, a 24/4 formulation). If contraindications to contraceptive doses of estrogen are present, continuous use of norethindrone acetate 5-mg tablets or off-label use of combination tablets with 5 µg of ethinyl estradiol and 1 mg of norethindrone acetate (a formulation approved for the treatment of menopausal symptoms) is appropriate.20
Once a patient is established on oral hormonal management, placement of a levonorgestrel-releasing IUD should be considered. Given that expulsion rates may be higher in women with HMB, if feasible, consider using abdominal ultrasound guidance for IUD placement.
For women with VWD who fail first-line therapy (hormonal management) or are trying to become pregnant, TxA (two 650-mg tablets 3 times daily for up to 5 days during episodes of heavy flow) can reduce HMB.20,21
Our recommendations for management of pregnancy and delivery
The second and third systematic reviews discussed above provide very limited guidance on comprehensive management. The care of the pregnant patient with VWD starts with assessment of VWF levels and making an accurate diagnosis. This usually requires the input of a hematologist or other expert in hemostasis. If no recent VWF levels are available, the ObGyn can obtain a von Willebrand panel that includes VWF antigen, VWF activity (most commonly ristocetin cofactor), and factor VIII.
Levels should be reassessed around 36 weeks’ gestation in anticipation of delivery. VWF levels increase during pregnancy; accordingly, in mild, type 1 VWD, half the time treatment is not necessary.11 If VWF activity is less than 50 IU/dL (less than 50% of normal) at 36 weeks’ gestation, the patient should receive VWF concentrate (dosed in VWF units). This requires consultation with hematology and specialized pharmacy support.
For these reasons, the patient with a VWF level less than 50% should be delivered in a referral center with the necessary resources. Anesthesia should be aware of the patient. Unless
Due to the quantity of fluids administered during labor or at the time of delivery and the coexistent administration of oxytocin, desmopressin (synthetic vasopressin) should not be used without monitoring sodium levels, should not be dosed more than once, or should be avoided altogether due to the risk of water intoxication.
If the patient has sustained VWF and factor VIII levels greater than 50 IU/dL, she would be a candidate to deliver in her local hospital and receive neuraxial anesthesia.
Based on the best data we have for women with VWD, a patient with a VWF greater than 50 IU/dL is no more likely to experience PPH than other women.11 Intravenous TxA can be used for prevention or treatment of immediate postpartum bleeding per protocol (1 g after cord clamp and 1 g 30 minutes or more later).22 Oral TxA can be used for prevention or treatment of delayed postpartum bleeding as per HMB. Regardless of the outcome of any testing during pregnancy, nonsteroidal anti-inflammatory drugs should be avoided postpartum and the patient should be monitored closely for bleeding.
Neonatal care
As for the fetus/neonate, the parents should be aware that the infant has a 50% chance of inheriting VWD. If the baby’s father has no history of bleeding, it is unlikely that the infant would be any more affected than the patient herself. Nonetheless, cord blood (in one or more light blue top tubes) should be obtained at the time of delivery and sent for a von Willebrand panel. If the infant is male, a circumcision should be postponed until VWD is ruled out. In addition, fetal invasive procedures should be avoided during labor. Fetal scalp electrode placement should be avoided. Operative vaginal delivery also should be avoided. Cesarean delivery would be preferred to operative vaginal delivery, but if operative vaginal delivery is unavoidable, use of forceps is preferred to vacuum extraction. ●
Von Willebrand disease (VWD) represents the most common inherited bleeding disorder, with a prevalence of approximately 1 in 1,000 people.
Bruising and mucocutaneous bleeding (epistaxis, gingival bleeding, and bleeding after dental extraction) are the most common presenting symptoms of VWD. Because VWD substantially increases the risk of heavy menstrual bleeding (HMB) and, to some extent, intrapartum bleeding complications, and postpartum hemorrhage, women experience a disproportionate burden from VWD. Thus, ObGyns are likely to be called on to make treatment recommendations in VWD patients with these concerns.1
In 2017, the American Society of Hematology, the International Society on Thrombosis and Haemostasis, the National Hemophilia Foundation, and the World Federation of Hemophilia determined that among clinical issues related to VWD, updating guidelines for women with VWD represented the highest priority.2 Accordingly, an international group of hematologists/coagulation specialists performed systematic literature reviews to address 3 questions faced by women with VWD and their clinicians:
- What are the most effective treatments for HMB?
- What is the safest approach for women desiring neuraxial analgesia for intrapartum pain?
- What is the impact of postpartum administration of tranexamic acid (TxA) on postpartum hemorrhage (PPH)?3
Evidence on management strategies for HMB in women with VWD
The prevalence of HMB in women with VWD ranges from 50% to 92%. Reports suggest that between 5% and 24% of women presenting with this symptom have VWD.3 However, the prevalence of VWD among women seeking care for HMB relates to referral patterns, with the prevalence of VWD substantially higher in patient populations who are referred to clinicians or centers that focus on care of patients with bleeding disorders.
The systematic review authors3 identified 2 comparative studies that assessed the treatment of HMB in women with VWD. One was a crossover trial that enrolled 116 VWD patients with HMB with a mean age of 36 years.4 All participants in this trial chose not to use combination oral contraceptives (COCs) as they had not experienced good results with prior COC use. Trial participants were randomly assigned to receive either intranasal desmopressin (DDAVP; a synthetic analog of the antidiuretic agent vasopressin, which stimulates the release of VWF from endothelial cells) or oral TxA therapy for 2 menstrual cycles. Participants then crossed over to the other drug for 2 additional cycles. Although both agents significantly reduced estimated menstrual blood loss, TxA was more effective in decreasing bleeding than intranasal DDAVP.4
In a retrospective cohort study, investigators compared COC use with intranasal DDAVP in 36 adolescents who had VWD and HMB.5 Participant follow-up ranged from 6 months to 4 years. The estimated efficacy of COCs and intranasal DDAVP was 86% and 77%, respectively, a difference that did not achieve statistical significance. Some of the adolescents who used intranasal DDAVP reported severe headaches and flushing.5
In addition, the systematic review authors3 identified 5 case series that described the use of the levonorgestrel (52 mg)-releasing intrauterine device (LNG 52 IUD) in women with VWD and HMB; 4 of these addressed the efficacy of progestin-releasing IUDs in reducing HMB in this patient population.6-9 Using different approaches to define HMB, the authors of these reports followed between 7 and 26 patients with bleeding disorders (most with confirmed VWD) and HMB for variable amounts of time after placement of an LNG 52 IUD. Many of the women described in these case series had tried other HMB treatments, including COCs, without success. Although these 4 reports assessed different outcomes, all reported that placement of the LNG 52 IUD substantially reduced menstrual blood loss, often resulting in amenorrhea. Several of these reports also noted important improvements in quality of life following LNG 52 IUD placement. One case series reported LNG 52 IUD placement in 13 adolescents with VWD and HMB. The mean time to achieve amenorrhea or occasional spotting was 94 days.6
The fifth report, which followed 20 women (median age, 31 years) with HMB associated with VWD or other bleeding disorders who underwent LNG 52 IUD placement, aimed to describe IUD expulsions and malpositioned IUDs in this population. In this small group of patients, 3 IUD expulsions and 2 malpositioned IUDs were observed. Furthermore, an additional 5 women had their device removed prematurely due to patient dissatisfaction. Accordingly, the IUD continuation rate in this case series was only 50%.10
Evidence on management of pregnancy, delivery, and the postpartum period
Heavy menstrual bleeding is not the only challenge for women with VWD. While pregnancy is accompanied by higher levels of VWF, potentially offsetting the risk of bleeding at the time of delivery, the levels do not achieve the same magnitude as they would in unaffected women.11 Women are at an increased risk of primary PPH12,13 and, importantly, since VWF levels fall exponentially after delivery when women are still experiencing lochia,11 they are at increased risk of secondary or delayed PPH.
Two questions arise frequently in the care of women with VWD at the time of delivery and during the postpartum period:
- What is the safest approach for women who desire neuraxial analgesia for intrapartum pain?
- What is the impact of postpartum administration of TxA on PPH?
The second systematic review the authors performed3 focused on VWF levels in women receiving neuraxial anesthesia during labor. After screening 27 studies, the authors included 5 case series, which did not describe outcomes based on VWF levels but rather described the outcomes of women with VWF levels of greater than 0.50 IU/mL (> 50% of normal compared with a normal standard).
Meta-analysis showed that the proportion of anesthesia complications was 6%, which sounds high, but the range of complications was what would be expected in any population (hypotension, accidental dural puncture, inadequate anesthesia, and bloody tap with no further complications). No spinal, subdural, or epidural hematomas were noted.3 Such hematomas are an extremely rare complication of neuraxial anesthesia, occurring in only 1 in 200,000 or 1 in 250,000 obstetric patients14,15; accordingly, an increase in the rate of hematomas among women with VWD could go undetected. The absence of hematomas among women with VWD as reported in the systematic review does not mean there is not an increase in the rate of hematomas in women with VWD. The relative risk is unknown and caution would be advised.
The third systematic review that the authors performed3 was on TxA treatment in the postpartum period. After screening 41 studies, the authors included 2 retrospective cohort studies.16,17 The majority of the participants had VWD. With very-low-certainty evidence, the authors found that TxA reduces the risk of:
- severe primary PPH (risk ratio [RR], 0.36; 95% confidence interval [CI], 0.05–2.59)
- primary PPH (RR, 0.25; 95% CI, 0.04–1.75)
- secondary PPH (RR, 0.42; 95% CI, 0.02–0.91—does not cross 1.0).
Note that the 95% confidence intervals for severe as well as primary PPH crossed 1.0 and therefore these reductions in risk did not achieve statistical significance. Additionally, there was very-low-certainty evidence on the effect of TxA on blood transfusions, vaginal hematomas, blood loss, and thrombotic complications.3
Continue to: Our recommendations for HMB management...
Our recommendations for HMB management
When first evaluating any woman with HMB, it is important to check a blood count and ferritin level, if not already done. If there is any suggestion of iron deficiency (with or without anemia), we recommend oral iron supplementation. This is best accomplished with slow-release iron supplement formulations (or less expensive generic or house brands that contain less than 65 mg of elemental iron per tablet) taken every other day. Such preparations may cause fewer gastrointestinal adverse effects than other oral iron formulations.18 Although it may appear counterintuitive, oral iron is better absorbed (and also may cause fewer gastrointestinal adverse effects) when taken every other day.19
Initial management of HMB, whether or not a bleeding disorder is present, often consists or oral hormonal management. If no contraindications are present, we recommend initiation of a COC with a short hormone-free interval (for example, a 24/4 formulation). If contraindications to contraceptive doses of estrogen are present, continuous use of norethindrone acetate 5-mg tablets or off-label use of combination tablets with 5 µg of ethinyl estradiol and 1 mg of norethindrone acetate (a formulation approved for the treatment of menopausal symptoms) is appropriate.20
Once a patient is established on oral hormonal management, placement of a levonorgestrel-releasing IUD should be considered. Given that expulsion rates may be higher in women with HMB, if feasible, consider using abdominal ultrasound guidance for IUD placement.
For women with VWD who fail first-line therapy (hormonal management) or are trying to become pregnant, TxA (two 650-mg tablets 3 times daily for up to 5 days during episodes of heavy flow) can reduce HMB.20,21
Our recommendations for management of pregnancy and delivery
The second and third systematic reviews discussed above provide very limited guidance on comprehensive management. The care of the pregnant patient with VWD starts with assessment of VWF levels and making an accurate diagnosis. This usually requires the input of a hematologist or other expert in hemostasis. If no recent VWF levels are available, the ObGyn can obtain a von Willebrand panel that includes VWF antigen, VWF activity (most commonly ristocetin cofactor), and factor VIII.
Levels should be reassessed around 36 weeks’ gestation in anticipation of delivery. VWF levels increase during pregnancy; accordingly, in mild, type 1 VWD, half the time treatment is not necessary.11 If VWF activity is less than 50 IU/dL (less than 50% of normal) at 36 weeks’ gestation, the patient should receive VWF concentrate (dosed in VWF units). This requires consultation with hematology and specialized pharmacy support.
For these reasons, the patient with a VWF level less than 50% should be delivered in a referral center with the necessary resources. Anesthesia should be aware of the patient. Unless
Due to the quantity of fluids administered during labor or at the time of delivery and the coexistent administration of oxytocin, desmopressin (synthetic vasopressin) should not be used without monitoring sodium levels, should not be dosed more than once, or should be avoided altogether due to the risk of water intoxication.
If the patient has sustained VWF and factor VIII levels greater than 50 IU/dL, she would be a candidate to deliver in her local hospital and receive neuraxial anesthesia.
Based on the best data we have for women with VWD, a patient with a VWF greater than 50 IU/dL is no more likely to experience PPH than other women.11 Intravenous TxA can be used for prevention or treatment of immediate postpartum bleeding per protocol (1 g after cord clamp and 1 g 30 minutes or more later).22 Oral TxA can be used for prevention or treatment of delayed postpartum bleeding as per HMB. Regardless of the outcome of any testing during pregnancy, nonsteroidal anti-inflammatory drugs should be avoided postpartum and the patient should be monitored closely for bleeding.
Neonatal care
As for the fetus/neonate, the parents should be aware that the infant has a 50% chance of inheriting VWD. If the baby’s father has no history of bleeding, it is unlikely that the infant would be any more affected than the patient herself. Nonetheless, cord blood (in one or more light blue top tubes) should be obtained at the time of delivery and sent for a von Willebrand panel. If the infant is male, a circumcision should be postponed until VWD is ruled out. In addition, fetal invasive procedures should be avoided during labor. Fetal scalp electrode placement should be avoided. Operative vaginal delivery also should be avoided. Cesarean delivery would be preferred to operative vaginal delivery, but if operative vaginal delivery is unavoidable, use of forceps is preferred to vacuum extraction. ●
- ACOG committee opinion no. 451: Von Willebrand disease in women. Obstet Gynecol. 2009;114:1439-1443. doi: 10.1097 /AOG.0b013e3181c6f975.
- Kalot MA, Al-Khatib M, Connell NT, et al; VWD Working Group. An international survey to inform priorities for new guidance on von Willebrand disease. Hemophilia. 2020;26:106-116. doi: 10.1111/hae.13881.
- Brignardello-Petersen R, El Alayli A, Husainat N, et al. Gynecologic and obstetric management of women with von Willebrand disease: summary of 3 systematic reviews of the literature. Blood Adv. 2022;6:228-237. doi: 10.1182 /bloodadvances.2021005589.
- Kouides PA, Byams VR, Philipp CS, et al. Multisite management study of menorrhagia with abnormal laboratory haemostasis: a prospective crossover study of intranasal desmopressin and oral tranexamic acid. Br J Haematol. 2009;145:212-220. doi: 10.1111/j.1365-2141.2009.07610.x.
- Amesse LS, Pfaff-Amesse T, Leonardi R, et al. Oral contraceptives and DDAVP nasal spray: patterns of use in managing vWD-associated menorrhagia: a single-institution study. J Pediatr Hematol Oncol. 2005;27:357-363. doi: 10.1097/01.mph.0000173175.95152.95.
- Adeyemi-Fowode OA, Santos XM, Dietrich JE, et al. Levonorgestrel-releasing intrauterine device use in female adolescents with heavy menstrual bleeding and bleeding disorders: single institution review. J Pediatr Adolesc Gynecol. 2017;30:479-483. doi: 10.1016/j.jpag.2016.04.001.
- Chi C, Huq FY, Kadir RA. Levonorgestrel-releasing intrauterine system for the management of heavy menstrual bleeding in women with inherited bleeding disorders: long-term follow-up. Contraception. 2011;83:242-247. doi: 10.1016/j.contraception.2010.07.010.
- Kingman CE, Kadir RA, Lee CA, et al. The use of levonorgestrel-releasing intrauterine system for treatment of menorrhagia in women with inherited bleeding disorders. BJOG. 2004;111:1425-1428. doi: 10.1111/j.1471-0528.2004.00305.x.
- Lukes AS, Reardon B, Arepally G. Use of the levonorgestrel-releasing intrauterine system in women with hemostatic disorders. Fertil Steril. 2008;90:673-677. doi: 10.1016 /j.fertnstert.2007.07.1315.
- Rimmer E, Jamieson MA, James P. Malposition and expulsion of the levonorgestrel intrauterine system among women with inherited bleeding disorders. Haemophilia. 2013;19:933-938. doi: 10.1111/hae.12184.
- James AH, Konkle BA, Kouides P, et al. Postpartum von Willebrand factor levels in women with and without von Willebrand disease and implications for prophylaxis. Haemophilia. 2015;21:81-87. doi: 10.1111/hae.12568.
- James AH, Jamison MG. Bleeding events and other complications during pregnancy and childbirth in women with von Willebrand disease. J Thromb Haemost. 2007;5: 1165-1169. doi: 10.1111/j.1538-7836.2007.02563.x.
- Al-Zirqi I, Vangen S, Forsen L, et al. Prevalence and risk factors of severe obstetric haemorrhage. BJOG. 2008;115:1265-1272. doi: 10.1111/j.1471-0528.2008.01859.x.
- Moen V, Dahlgren N, Irestedt L. Severe neurological complications after central neuraxial blockades in Sweden 1990-1999. Anesthesiology. 2004;101:950-959. doi: 10.1097/00000542-200410000-00021.
- D’Angelo R, Smiley RM, Riley ET, et al. Serious complications related to obstetric anesthesia: the serious complication repository project of the Society for Obstetric Anesthesia and Perinatology. Anesthesiology. 2014;120:1505-1512. doi: 10.1097/ALN.000000000000253.
- Govorov I, Lofgren S, Chaireti R, et al. Postpartum hemorrhage in women with von Willebrand disease—a retrospective observational study. PLos One. 2016;11:e0164683. doi: 10.1371/journal.pone.0164683.
- Hawke L, Grabell J, Sim W, et al. Obstetric bleeding among women with inherited bleeding disorders: a retrospective study. Haemophilia. 2016;22:906-911. doi: 10.1111/hae.13067.
- James AH. Iron deficiency anemia in pregnancy. Obstet Gynecol. 2021;138:663-674. doi:10.1097/AOG .000000000000.4559.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524-e533. doi: 10.1016/S2352-3026(17)30182-5.
- Kaunitz AM. Abnormal uterine bleeding in reproductiveage women. JAMA. 2019;321:2126-2127. doi: 10.1001 /jama.2019.5248.
- James AH, Kouides PA, Abdul-Kadir R, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. Am J Obstet Gynecol. 2009;201:12.e1-8. doi: 10.1016 /j.ajog.2009.04.024.
- WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105-2116. doi: 10.1016/S0140-6736(17)30638-4.
- ACOG committee opinion no. 451: Von Willebrand disease in women. Obstet Gynecol. 2009;114:1439-1443. doi: 10.1097 /AOG.0b013e3181c6f975.
- Kalot MA, Al-Khatib M, Connell NT, et al; VWD Working Group. An international survey to inform priorities for new guidance on von Willebrand disease. Hemophilia. 2020;26:106-116. doi: 10.1111/hae.13881.
- Brignardello-Petersen R, El Alayli A, Husainat N, et al. Gynecologic and obstetric management of women with von Willebrand disease: summary of 3 systematic reviews of the literature. Blood Adv. 2022;6:228-237. doi: 10.1182 /bloodadvances.2021005589.
- Kouides PA, Byams VR, Philipp CS, et al. Multisite management study of menorrhagia with abnormal laboratory haemostasis: a prospective crossover study of intranasal desmopressin and oral tranexamic acid. Br J Haematol. 2009;145:212-220. doi: 10.1111/j.1365-2141.2009.07610.x.
- Amesse LS, Pfaff-Amesse T, Leonardi R, et al. Oral contraceptives and DDAVP nasal spray: patterns of use in managing vWD-associated menorrhagia: a single-institution study. J Pediatr Hematol Oncol. 2005;27:357-363. doi: 10.1097/01.mph.0000173175.95152.95.
- Adeyemi-Fowode OA, Santos XM, Dietrich JE, et al. Levonorgestrel-releasing intrauterine device use in female adolescents with heavy menstrual bleeding and bleeding disorders: single institution review. J Pediatr Adolesc Gynecol. 2017;30:479-483. doi: 10.1016/j.jpag.2016.04.001.
- Chi C, Huq FY, Kadir RA. Levonorgestrel-releasing intrauterine system for the management of heavy menstrual bleeding in women with inherited bleeding disorders: long-term follow-up. Contraception. 2011;83:242-247. doi: 10.1016/j.contraception.2010.07.010.
- Kingman CE, Kadir RA, Lee CA, et al. The use of levonorgestrel-releasing intrauterine system for treatment of menorrhagia in women with inherited bleeding disorders. BJOG. 2004;111:1425-1428. doi: 10.1111/j.1471-0528.2004.00305.x.
- Lukes AS, Reardon B, Arepally G. Use of the levonorgestrel-releasing intrauterine system in women with hemostatic disorders. Fertil Steril. 2008;90:673-677. doi: 10.1016 /j.fertnstert.2007.07.1315.
- Rimmer E, Jamieson MA, James P. Malposition and expulsion of the levonorgestrel intrauterine system among women with inherited bleeding disorders. Haemophilia. 2013;19:933-938. doi: 10.1111/hae.12184.
- James AH, Konkle BA, Kouides P, et al. Postpartum von Willebrand factor levels in women with and without von Willebrand disease and implications for prophylaxis. Haemophilia. 2015;21:81-87. doi: 10.1111/hae.12568.
- James AH, Jamison MG. Bleeding events and other complications during pregnancy and childbirth in women with von Willebrand disease. J Thromb Haemost. 2007;5: 1165-1169. doi: 10.1111/j.1538-7836.2007.02563.x.
- Al-Zirqi I, Vangen S, Forsen L, et al. Prevalence and risk factors of severe obstetric haemorrhage. BJOG. 2008;115:1265-1272. doi: 10.1111/j.1471-0528.2008.01859.x.
- Moen V, Dahlgren N, Irestedt L. Severe neurological complications after central neuraxial blockades in Sweden 1990-1999. Anesthesiology. 2004;101:950-959. doi: 10.1097/00000542-200410000-00021.
- D’Angelo R, Smiley RM, Riley ET, et al. Serious complications related to obstetric anesthesia: the serious complication repository project of the Society for Obstetric Anesthesia and Perinatology. Anesthesiology. 2014;120:1505-1512. doi: 10.1097/ALN.000000000000253.
- Govorov I, Lofgren S, Chaireti R, et al. Postpartum hemorrhage in women with von Willebrand disease—a retrospective observational study. PLos One. 2016;11:e0164683. doi: 10.1371/journal.pone.0164683.
- Hawke L, Grabell J, Sim W, et al. Obstetric bleeding among women with inherited bleeding disorders: a retrospective study. Haemophilia. 2016;22:906-911. doi: 10.1111/hae.13067.
- James AH. Iron deficiency anemia in pregnancy. Obstet Gynecol. 2021;138:663-674. doi:10.1097/AOG .000000000000.4559.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524-e533. doi: 10.1016/S2352-3026(17)30182-5.
- Kaunitz AM. Abnormal uterine bleeding in reproductiveage women. JAMA. 2019;321:2126-2127. doi: 10.1001 /jama.2019.5248.
- James AH, Kouides PA, Abdul-Kadir R, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. Am J Obstet Gynecol. 2009;201:12.e1-8. doi: 10.1016 /j.ajog.2009.04.024.
- WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105-2116. doi: 10.1016/S0140-6736(17)30638-4.