User login
The prevalence of diabetes mellitus (DM) is growing at epidemic proportions in the U.S. and has been reported as the most common reason for hospital admissions in western countries.1 There continues to be an alarmingly steady increase in the incidence of type 2 DM (T2DM), especially among the young and obese. Long-term diabetes-related complications also are likely to rise in prevalence. In particular, the diabetic foot is associated with morbidity and disability, leading to a significant impairment of quality of life.2 People with DM develop foot ulcers because of neuropathy (sensory, motor, and autonomic deficits), ischemia, or both.3 The initiating injury may be from acute mechanical or thermal trauma or from repetitively or continuously applied mechanical stress.4
From foot ulcerations to neuropathy to peripheral vascular disease, the challenges are significant and can result in amputations and even premature death. To address these challenges, early diagnosis and a multidisciplinary team approach should be employed. Managing the numerous comorbidities is essential for treatment.1,2,5
Due to the longevity of patients with DM, diabetes-associated complications are expected to rise in prevalence.6 The American Diabetes Association recently reported that T2DM accounts for about 90% to 95% of all persons with DM.7,8 Today, many hospitalizations for patients with DM are for lower extremity conditions, such as ulceration, infection, or gangrene. Diabetic foot ulcerations (DFUs) are painful and costly for both the patient and the health care system. Every year, more than 1 million people with DM worldwide lose a leg as a consequence of this disease.9 Most DM-related amputations are preceded by a foot ulcer.
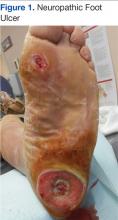
Diabetic foot ulcerations are the most common foot condition leading to lower extremity amputation (Figure 1).10 About 14 million individuals in the U.S. with diagnosed and undiagnosed DM will experience pathologic changes of their lower extremities that, when combined with minor trauma and infection, may lead to serious foot problems.11 Although the triad of vasculopathy, neuropathy, and susceptibility to infection are the primary permissive factors in its pathogenesis, DFU can also be attributed to other important risk factors. The presence of peripheral neuropathy and peripheral arterial disease (PAD) are considered to be the most significant risk factors for all types of diabetic foot complications.12
Related: A Combined Treatment Protocol for Patients With Diabetic Peripheral Neuropathy
Optimal care of foot ulceration depends on the treating physician’s understanding of the pathophysiology involved, familiarity with accepted principles of treatment, and the knowledge that a coordinated, multidisciplinary team approach will best accomplish the goal of limb salvage. All efforts should be made to prevent foot lesions, and when present, existing ulcers should be treated promptly and aggressively, which can often prevent an exacerbation of the problem and decrease the incidence of amputations. Even when ulcers have healed, patients with DM and a history of a lower extremity ulcer should consider it a lifelong condition that requires monitoring to prevent recurrence.13,14
This review provides a brief overview of DFU, including etiology, evaluation, treatment, and prevention, to provide clinicians with the clinical markers, evidence, and DFU treatment recommendations.
Etiologies
Multiple risk factors contribute to the development and pathogenesis of DFUs.5,6,15,16 Neuropathy and PAD are major factors in the pathogenesis of diabetic foot ulcers.17 However, there are several additional factors leading to the occurrence of foot complications. Reiber and colleagues have determined that 63% of their patients’ ulcers were attributed to the critical triad of peripheral sensory neuropathy, trauma, and deformity.15
Other factors also implicated in the causal pathway to ulceration were ischemia, callus, and edema. Infection was rarely implicated in the etiology of these lesions, although once an ulcer has developed, infection and PAD were found to be the major causes for amputation.10,18,19 Many of the risk factors for foot ulcer are also predisposing factors for amputation, because ulcers are primary antecedent events leading to amputation.20-23
Evaluation
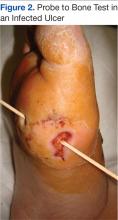
The clinical evaluation must include a thorough and systematic lower extremity examination when starting DFU treatment. It is important to have a thorough assessment of the ulcer’s size and depth, and the evaluation should include a description of its appearance and measurement of its diameter at each visit. Evaluation for the presence of local and systemic infection and potential for osteomyelitis, using a small sterile blunt probe, is critical in determining depth of penetration and tracking along tendon sheaths (Figure 2).
Peripheral arterial disease is directly linked to lower extremity disorders, such as intermittent claudication, pain on exertion, pain at rest, and, in severe cases, critical limb ischemia and gangrene.1 Bilateral lower extremity pulses should routinely be palpated. When dorsalis pedis or posterior tibial artery pulses are absent or diminished, Doppler segmental pressures to the toes, pulse volume recording, skin perfusion pressure, or transcutaneous oxygen evaluation is indicated, and vascular consultation should be sought.3 Ischemia is caused by peripheral arterial occlusive disease of larger vessels, not by microangiopathy.13 Poor arterial inflow is associated not only with impaired ulcer healing, but also subsequent infection, gangrene, and amputation.13
Diabetic peripheral neuropathy is characterized by loss of protective sensation, allowing ulceration in areas of high pressure. Peripheral sensory neuropathy as measured by vibration perception thresholds can impart a 3.4-fold to 32-fold risk of ulceration.19,21 Patients insensitive to a 10-g monofilament, commonly used to assess peripheral neuropathy, has been shown in several studies to convey a 2.2-fold to18-fold risk of ulceration.6,19,27,28 In the large, population-based North-West Diabetes Foot Care Study, loss of protective sensation to the 10-g monofilament increased the risk of ulceration 80%, whereas abnormal ankle reflexes increased this risk 55%.29
Peripheral neuropathy has been demonstrated as a strong risk factor for foot ulceration in many cross-sectional studies and is present in > 80% of affected patients.29 Recent studies suggested that impaired sensation makes the foot increasingly vulnerable to damage caused by mechanical, thermal, or pressure-related injury.30 Autonomic neuropathy by virtue of subsequent anhidrosis causes dryness of the skin and, therefore, vulnerability to fissuring.13
Unhealed cracks in the skin can easily lead to infection, especially in the presence of PAD. Neuropathy has an insidious and nonhomogeneous manifestation, making it difficult to identify its onset and a challenge for patients and clinicians.31,32
Sacco and colleagues reviewed current literature and the International Consensus on the Diabetic Foot recommendation and concluded that most attention is given to patients with imminent foot ulceration rather than attempting to develop and improve assessment techniques that detect early impairments.31,33 They propose that effort should be made that detect patients at risk of developing diabetic polyneuropathy. Although the 10-g monofilament pressure perception threshold is a common screening technique for early detection, tests of the vibration perception threshold may be more sensitive.
The authors propose that different monofilament sizes could probably better help determine the disease status, as the vibration tests do. In addition to the considerable subjectivity of both methods of assessing sensitivity, they are unquestionably clinical resources that can contribute to early detection of DPN. Future studies should focus on developing assessment strategies and tools that better detect early neuropathic changes. Early diagnosis of impending problems will aid in preventing further limb-threatening complications.
Treatment
The management of diabetic foot disease is focused primarily on avoiding lower extremity amputation and should be carried out through 3 main strategies: identification of the at risk foot, treatment of the acutely diseased foot, and prevention of further complications.34 The primary goal in the treatment of DFUs is to obtain wound closure. Prompt, aggressive treatment of DFUs can often prevent an exacerbation of the problem and the potential need for amputation. The aim of therapy, therefore, should be early intervention to allow prompt healing of the lesion and, once healed, prevent its recurrence.3,20,25,35
Management of the foot ulcer is largely determined by its severity (grade), vascularity, and presence of infection.3,14,36 A multidisciplinary team approach should be used due to the multifaceted nature of foot ulcers, as well as for managing the numerous comorbidities attendant with these patients. The choice of treatment methods is determined by patient and ulcer characteristics. Equally important is the ability of patients to comply with the treatment as well as with the location and severity of the ulcer.4
Rest, elevation, and removal of pressure (off-loading) are essential components of treatment and should be initiated at first presentation. Recent studies provided evidence that indicated proper off-loading promotes more rapid DFU healing.37,38 Ill-fitting footwear should be discarded and replaced with an appropriate off-loading device for mitigating pressure at the site of the ulceration. Although many off-loading modalities are currently in use, only a few studies describe the frequency and rate of wound healing associated with their use.
The total contact cast (TCC) is considered the superior standard therapy in management for neuropathic ulcers due to its proven ability to redistribute pressure, thereby promoting expeditious wound closure. Another inherent benefit is to ensure patient adherence with off-loading as well as reducing activity levels.24,39 Previous randomized controlled trials have demonstrated that patients treated with TCC healed a higher percentage of plantar ulcers at a faster rate than did patients in the control groups. One unique study demonstrated histologic evidence of more rapid angiogenesis with formation of granulation tissue in the casted group compared with the standard treatment group.40,41
Potential disadvantages of the TCC include the need for expertise in its proper application, the need for weekly cast changes, and related costs.24,35 Although a number of new devices have been introduced as alternatives to the TCC, only several clinical studies demonstrating their efficacy have been published.5,14,25,36 If nonweight bearing with crutches, wheelchair, or more effective devices are not feasible, even a pressure-attenuating insert can be used in a simple postoperative shoe until specialty referral is made.
Debridement of necrotic, callus, fibrous, and senescent tissues is a mainstay of ulcer therapy.42,43 It is considered the first and the most important therapeutic step leading to wound closure in patients with DFU.42-44 Unhealthy tissue must be sharply debrided back to bleeding tissue to fully visualize the extent of the ulcer as well as to detect any underlying abscesses or sinuses. It has been reported that regular (weekly) sharp debridement is associated with more rapid healing of ulcers compared with less frequent debridement.45-47 Wilcox and colleagues indicated that frequent debridement healed more wounds in a shorter time (P < .001).46 The more frequent the debridement, the better the healing outcome. There are different types of debridement methods, including surgical, enzymatic, autolytic, mechanical, and biologic.48 Surgical or sharp debridement can convert a chronic ulcer into an acute wound that is more likely to heal.24 Adequate debridement must always precede the application of topical wound healing agents, dressings, or wound closure procedures.24 Conversely, a wound that does not receive the necessary debridement is one that has not been adequately treated.
There are numerous types of dressings that have been developed over the past decade that promote wound healing. Few have undergone any formal clinical studies to determine efficacy or effectiveness to help guide clinicians in their use.
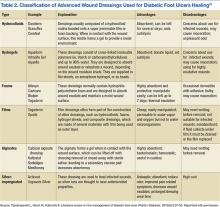
Yazdanpanah and colleagues argued that dressings should confer moisture balance, protease sequestration, growth factor stimulation, antimicrobial activity, oxygen permeability, and the capacity to promote autolytic debridement to facilitate the production of granulation tissues and the re-epithelialization process.24 In addition, it should have a prolonged time of action, high efficiency, and protection against contamination or infection.17 The group noted that no single dressing fulfills all the requirements of a diabetic patient with a foot ulcer. The choice of dressing is largely determined by the causes of DFU, wound location, depth, amount of scar or slough, exudates, condition of wound margins, presence of infection and pain, need for adhesiveness, and conformability of the dressing (Table 2).
Advanced Therapies
In 2003, Sheehan and colleagues reported that a 50% change in foot ulcer area after 4 weeks of observation is a robust predictor of healing at 12 weeks.49 In addition, wounds failing to achieve a 50% reduction in area after 4 weeks need to be reassessed and considered for advanced treatment modalities if there are no otherwise identified impediments to wound healing.6,9,38 These findings have served as a pivotal clinical decision point in the care of DFUs over the past several years for early identification of patients who may not respond to the standard of care. Today, most wound care protocols advocate use of standard therapies for at least 4 weeks before advanced therapies are considered.
Significant improvements have been achieved in the treatment of ulcerations, and today clinicians have several advanced therapeutic options for management of chronic DFUs. These new technologies have been shown to increase the probability of complete wound closure in difficult-to-heal foot ulcerations in patients with diabetes. Among these are recombinant platelet-derived growth factors, a human living skin equivalent, and a human fibroblast-derived dermal substitute.49-51 Tissue-engineered skin equivalent (Apligraf) and human dermis (Dermagraft) are types of biologically active dressings that are derived from fibroblasts of neonatal foreskins.
The most recent advancements for wound care therapies is that of stem cell therapies, primarily bone marrow-derived and, most recently, placental-derived stem cells, including dehydrated human amnion chorion (Epifix) and amniotic matrix with mesenchymal stem cells (Grafix).52,53 Because of the expense of these products, they cannot be used universally in the treatment of DFUs but rather are used and reserved for difficult-to-heal wounds. In addition, negative pressure therapy has assumed a major role in the management of traumatic, acute, and chronic wounds and has shown efficacy in healing DFUs.54-57 Hyperbaric oxygen therapy and several biophysical modalities have been studied and found to be efficacious in healing a wide variety of chronic wounds over the past decade as well, although results vary by study, and no advanced modality has become universal in its application.58-64
Table 3 lists most of the wound care technologies commonly used in current clinical practice. Although randomized controlled trials have been published supporting the use of most of these modalities, a lack of strong data proving efficacy for use of such treatment options remains.
Treatment of any underlying ischemia is critical in achieving a successful outcome. Vascular surgical consultation should be obtained on presentation of an ischemic wound and in cases where ulcers show no sign of progress despite appropriate management.4,13 Revascularization is commonly performed in patients with critical limb ischemia and DFUs but is also performed in patients with less severe arteriopathy. The goal is to restore a palpable pulse on the affected foot.65 The postrevascularization ulcer-healing rate ranges from 46% to 91% at 1 year and seems to be improved in those patients with distal arterial reconstruction and restoration of pulsatile flow.66
Endovascular approaches are becoming increasingly common in patients whose arterial disease is more limited or morbidity is a significant concern.67,68 Studies report that the exact role of isolated endovascular procedures is still to be determined, although such interventions are frequently performed in concert with angiography preceding vascular reconstructive procedures.69,70 However, in many such studies, healing was often a secondary criterion, and there was no description of the initial wound or its management.71
Challenges
Within the VA setting there is a wide range of patient comorbidities that frequently present clinicians with unique challenges. Often these patients are older with many social and mental health conditions, including self-abuse, drug-abuse, nonadherence, psychological issues and lack of financial and/or educational resources or support. Many of these patients have comorbidities associated with diabetes that can delay healing of their ulcerations.
Systemwide VA mandates have implemented multidisciplinary foot care teams. The teams identify veterans at risk for lower limb complications; provide preventive care; track high-risk foot care across the continuum of outpatient, inpatient, and rehabilitative care; and provide education, orthoses, and social support.72,73 In the late 1990s, the VHA implemented a national program of foot risk screening and referral, conducted largely in primary care.29 By 1998 as determined from medical record reviews, 95% of veterans had a visual examination, 84% had palpation of pulses, and 78% had undergone a sensory examination. In addition, about 83% of patients had a monofilament examination, and 85% of individuals with risk factors were referred to foot specialists in 2004.72,74 Veterans at higher risk for lower extremity complications routinely receive subsequent preventive foot care, such as education or prescription of therapeutic shoes in the VHA.
Tseng and colleagues evaluated risk-adjusted trends in amputations among veterans with diabetes during a 5-year period and reported a decrease in amputation rates observed for all types of lower extremity amputations (LEA) and among all racial groups.74 Implementation of such universal programs for foot screening, tracked through performance measures, may have contributed to a decrease in LEAs and improved outcomes in the VA patient population.
Prevention
A healthy, intact diabetic foot is best maintained by a consistent and recurrent preventive treatment strategy. Prevention of ulcer recurrence remains to be a major clinical challenge. Andrews and colleagues demonstrated that recurrence rates range from 28% at 12 months to 100% at 40 months.75 They report that the highest incidence of reulceration is in the site of a previous ulceration, noting that a newly healed ulcer is covered with fragile skin and after complete healing, there is an area of higher density tissue (scar). Shearing between the different tissue densities often contributes to new ulcers.
After the ulcer heals, the patient and their caregivers must incorporate preventative measures in care plans to reduce the risk of wound reoccurrence. A study reported by Barshes and colleagues demonstrated that a majority of people with diabetes do not receive guideline-recommended foot care, including regular foot examinations.76 Identifying the patients with diabetes at risk for ulceration requires foot examination,including the vascular and neurologic systems, skin conditions, and foot structure.77 Among the complications of diabetes, lower limb amputation is considered to be preventable.78,79 Because there is a great beneficial effect of patient education on reducing LEAs, a flexible schedule for diabetes education, that offers education at any time for the maximum convenience of patients rather than focusing on health care provider’s convenience is critical.79,80 Conservative management of foot problems also has reduced the risk of amputation by simple procedures, such as appropriate foot wear, cleanliness, aggressive surgical debridement, and evidence-based ulcer management.34 This is best accomplished through a multidisciplinary approach involving a team of specialists and personnel who provide a coordinated process of care, including a patient motivated to ensure its success.6
Conclusions
The authors have described the components of assessment and treatment that can help ensure successful healing of foot ulcers in diabetic patients. These approaches should be used whenever feasible to reduce the high morbidity and risk of serious complications resulting from foot ulcers. Advances in treating chronic diabetic wounds are promising; however, the intrinsic pathophysiologic abnormalities that lead to ulcers in the first place cannot be ignored. No known therapy will be effective without concomitant management of ischemia, infection, and adequate off-loading.6,75
Not all diabetic foot complications can be prevented, but it is possible to dramatically reduce their incidence through appropriate management and prevention programs. The multidisciplinary team approach that combines the expertise of many types of health care providers for diabetic foot disorders has been demonstrated as the optimal method to achieve favorable rates of limb salvage in the high-risk diabetic patient.
1. Phillips A, Mehl AA. Diabetes mellitus and the increased risk of foot injuries. J Wound Care. 2015;24(5)(suppl 2):4-7.
2. Anichini R, Zecchini F, Cerretini I, et al. Improvement of diabetic foot care after the implementation of the International Consensus on the Diabetic Foot (ICDF): results of a 5-year prospective study. Diabetes Res Clin Pract. 2007;75(2):153-158.
3. Frykberg RG. Diabetic foot ulcers: current concepts. J Foot Ankle Surg. 1998;37(5):440-446.
4. Cavanagh PR, Ulbrecht JS, Caputo GM. New developments in the biomechanics of the diabetic foot. Diabetes Metab Res Rev. 2000;16(suppl 1):S6-S10.
5. Frykberg RG. Diabetic foot ulcers: pathogenesis and management. Am Fam Physician. 2002;66(9):1655-1662.
6. Frykberg RG, Zgonis T, Armstrong DG, et al. Diabetic foot disorders: a clinical practice guideline (2006 revision). J Foot Ankle Surg. 2006;45(5)(suppl 1):S1-S66.
7. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(suppl 1):S81-S90.
8. American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2015;38(suppl 1):S8-S16.
9. Bakker K, Schaper N; International Working Group on Diabetic Foot Editorial Board.The development of global consensus guidelines on the management and prevention of the diabetic foot 2011. Diabetes Metab Res Rev. 2012;28(suppl 1):116-118.
10. Armstrong DG, Nguyen HC, Lavery LA, van Schie CH, Boulton AJ, Harkless LB. Off-loading the diabetic foot wound: a randomized clinical trial. Diabetes Care. 2001;24(6):1019-1022.
11. Reiber GE, Vileikyte L, Boyko Ed, et al. Causal pathways for incident lower-extremity ulcers in patients with diabetes from two settings. Diabetes Care. 1999;22(1):157-162.
12. Al-Rubeaan K, Al Derwish M, et al. Diabetic foot complications and their risk factors from a large retrospective cohort study. PLoS One. 2015;10(5):e0124446.
13. Vuorisalo S, Venermo M, Lepantalo M. Treatment of diabetic foot ulcers. J Cardiovasc Surg (Torino). 2009;50(3):275-291.
14. Frykberg ER. Medical management of disasters and mass casualties from terrorist bombings: how can we cope? J Trauma. 2002;53(2):201-212.
15. Reiber GE, Boyko EJ, Smith DG. Lower extremity foot ulcers and amputations in diabetes. In: National Diabetes Data Group of the National Institute of Diabetes and Digestive and Kidney Diseases. Diabetes in America. 2nd ed. Bethesda, MD: National Institutes of Health; 1995:409-427.
16. Waaijman R, de Haart M, Arts ML, et al. Risk factors for plantar foot ulcer recurrence in neuropathic diabetic patients. Diabetes Care. 2014;37(6):1697-1705.
17. O'Loughlin A, McIntosh C, Dinneen SF, O'Brien T. Review paper: basic concepts to novel therapies: a review of the diabetic foot. Int J Low Extrem Wounds. 2010;9(2):90-102.
18. Jeffcoate WJ, Chipchase SY, Ince P, Game FL. Assessing the outcome of the management of diabetic foot ulcers using ulcer-related and person-related measures. Diabetes Care. 2006;29(8):1784-1787.
19. McNeely MJ, Boyko EJ, Ahroni JH, et al. The independent contributions of diabetic neuropathy and yasculopatny in foot ulceration: how great are the risks? Diabetes Care. 1995;18(2):216-219.
20. Frykberg RG. Diabetic foot ulcers: pathogenesis and management. Am Fam Physician. 2002;66(9):1655-1662.
21. Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation: basis for prevention. Diabetes Care. 1990;13(5):513-521.
22. Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Davignon DR, Smith DG. A prospective study of risk factors for diabetic foot ulcer. The Seattle Diabetic Foot Study. Diabetes Care. 1999;22(7):1036-1042.
23. Armstrong DG, Lavery LA. Diabetic foot ulcers: prevention, diagnosis and classification. Am Fam Physician. 1998;57(6):1325-1332, 1337-1328.
24. Yazdanpanah L, Nasiri M, Adarvishi S. Literature review on the management of diabetic foot ulcer. World J Diabetes. 2015;6(1):37-53.
25. Frykberg RG. Diabetic foot ulcerations. In: Frykberg RG, ed. The High Risk Foot in Diabetes Mellitus. New York, NY: Churchill Livingstone; 1991.
26. Grayson ML, Gibbons GW, Balogh K, Levin E, Karchmer AW. Probing to bone in infected pedal ulcers. A clinical sign of underlying osteomyelitis in diabetic patients. JAMA. 1995;273(9):721-723.
27. Mayfield JA, Reiber GE, Sanders LJ, Janisse D, Pogach LM. Preventive foot care in people with diabetes. Diabetes Care. 1998;21(12):2161-2177.
28. Kalani M, Brismar K, Fagrell B, Ostergren J, Jörneskog G. Transcutaneous oxygen tension and toe blood pressure as predictors for outcome of diabetic foot ulcers. Diabetes Care. 1999;22(1):147-151.
29. Abbott CA, Vileikyte L, Williamson S, Carrington AL, Boulton AJ. Multicenter study of the incidence of and predictive risk factors for diabetic neuropathic foot ulceration. Diabetes Care. 1998;21(7):1071-1075.
30. Tuttolomondo A, Maida C, Pinto A. Diabetic foot syndrome: immune-inflammatory features as possible cardiovascular markers in diabetes. World J Orthop. 2015;6(1):62-76.
31. Sacco IC, Suda EY, Vigneron V, Sartor CD. An 'importance' map of signs and symptoms to classify diabetic polyneuropathy: an exploratory data analysis. PLoS One. 2015;10(6):e0129763.
32. Asad A, Hameed MA, Khan UA, Ahmed N, Butt MU. Reliability of the neurological scores for assessment of sensorimotor neuropathy in type 2 diabetics. J Pak Med Assoc. 2010;60(3):166-170.
33. Dyck PJ, Albers JW, Andersen H, et al. Diabetic polyneuropathies: update on research definition, diagnostic criteria and estimation of severity. Diabetes Metab Res Rev. 2011;27(7):620-628.
34. Ahmad J. The diabetic foot. Diabetes Metab Syndr. 2015;pii: S1871-4021(15)00030-2. [Epub ahead of print.]
35. Prompers L, Schaper N, Apelqvist J, et al. Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia. 2008;51(5):747-755.
36. Frykberg RG. Team approach toward lower extremity amputation prevention in diabetes. J Am Podiatr Med Assoc. 1997;87(7):305-312.
37. Cavanagh PR, Bus SA. Off-loading the diabetic foot for ulcer prevention and healing. J Am Podiatr Med Assoc. 2010;100(5):360-368.
38. Boulton A. The diabetic foot: from art to science. The 18th Camillo Golgi lecture. Diabetologia. 2004;47(8):1343-1353.
39. Boulton AJ. Pressure and the diabetic foot: clinical science and offloading techniques. Am J Surg. 2004;187(5)(suppl 1):S17-S24.
40. Mueller MJ, Diamond JE, Sinacore DR, et al. Total contact casting in treatment of diabetic plantar ulcers. Controlled clinical trial. Diabetes Care. 1989;12(6):384-388.
41. Piaggesi A, Viacava P, Rizzo L, et al. Semiquantitative analysis of the histopathological features of the neuropathic foot ulcer: effects of pressure relief. Diabetes Care. 2003;26(11):3123-3128.
42. Lebrun E, Tomic-Canic M, Kirsner RS. The role of surgical debridement in healing of diabetic foot ulcers. Wound Repair Regen. 2010;18(5):433-438.
43. Edwards J, Stapley S. Debridement of diabetic foot ulcers. Cochrane Database Syst Rev. 2010(1):CD003556.
44. Tallis A, Motley TA, Wunderlich RP, et al. Clinical and economic assessment of diabetic foot ulcer debridement with collagenase: results of a randomized controlled study. Clin Ther. 2013;35(11):1805-1820.
45. Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366(9498):1736-1743.
46. Warriner RA III, Wilcox JR, Carter MJ, Stewart DG. More frequent visits to wound care clinics result in faster times to close diabetic foot and venous leg ulcers. Adv Skin Wound Care. 2012;25(11):494-501.
47. Wilcox JR, Carter MJ, Covington S. Frequency of debridements and time to heal: a retrospective cohort study of 312 744 wounds. JAMA Dermatol. 2013;149(9):1050-1058.
48. Tiwari A, Jain S, Mehta S, Kumar R, Kapoor G, Kumar K. Limb salvage surgery for osteosarcoma: early results in Indian patients. Indian J Orthop. 2014;48(3):266-272.
49. Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Diabetes Care. 2003;26(6):1879-1882.
50. Wieman TJ, Smiell JM, Su Y. Efficacy and safely of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers: a phase III randomized placebo-controlled double-blind study. Diabetes Care. 1998;21(5):822-827.
51. Naughton G, Mansbridge J, Gentzkow G. A metabolically active human dermal replacement for the treatment of diabetic foot ulcers. Artif Organs.1997;21(11):1203-1210.
52. Zelen CM, Serena TE, Denoziere G, Fetterolf DE. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J. 2013;10(5):502-507.
53. Lavery LA, Fulmer J, Shebetka KA, et al. The efficacy and safety of Grafix® for the treatment of chronic diabetic foot ulcers: results of a multi-centre, controlled, randomised, blinded, clinical trial. Int Wound J. 2014;11(5):554-560.
54. Wolvos TA. Negative pressure wound therapy with instillation: the current state of the art. Surg Technol Int. 2014;24:53-62.
55. Andros G, Armstrong DG, Attinger CE, et al; Tucson Expert Consensus Conference. Consensus statement on negative pressure wound therapy (V.A.C. Therapy) for the management of diabetic foot wounds. Ostomy Wound Manage. 2006(suppl):1-32.
56. Armstrong DG, Lavery LA. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet. 2005;366(9498):1704-1710.
57. Armstrong DG, Marston WA, Reyzelman AM, Kirsner RS. Comparative effectiveness of mechanically and electrically powered negative pressure wound therapy devices: a multicenter randomized controlled trial. Wound Repair Regen. 2012;20(3):332-341.
58. Faglia E, Favales F, Aldeghi A, et al. Adjunctive systemic hyperbaric oxygen therapy in treatment of severe prevalently ischemic diabetic foot ulcer. A randomized study. Diabetes Care. 1996;19(12):1338-1343.
59. Fife CE, Buyukcakir C, Otto G, Sheffield P, Love T, Warriner R 3rd. Factors influencing the outcome of lower-extremity diabetic ulcers treated with hyperbaric oxygen therapy. Wound Repair Regen. 2007;15(3):322-331.
60. Kranke P, Bennett MH, Martyn-St. James M, Schnabel A, Debus SE. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2012;4:CD004123.
61. Frykberg R, Martin E, Tallis A, Tierney E. A case history of multimodal therapy in healing a complicated diabetic foot wound: negative pressure, dermal replacement and pulsed radio frequency energy therapies. Int Wound J. 2011;8(2):132-139.
62. Frykberg RG, Driver VR, Lavery LA, Armstrong DG, Isenberg RA. The use of pulsed radio frequency energy therapy in treating lower extremity wounds: results of a retrospective study of a wound registry. Ostomy Wound Manage. 2011;57(3):22-29.
63. Kloth LC. Electrical Stimulation Technologies for Wound Healing. Adv Wound Care. 2014;3(2):81-90.
64. Ennis WJ, Foremann P, Mozen N, Massey J, Conner-Kerr T, Meneses P. Ultrasound therapy for recalcitrant diabetic foot ulcers: results of a randomized, double-blind, controlled, multicenter study. Ostomy Wound Manage. 2005;51(8):24-39.
65. Mills JL Sr, Conte MS, Armstrong DG, et al. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg. 2014;59(1):220-234.e2.
66. Pomposelli FB, Kansal N, Hamdan AD, et al. A decade of experience with dorsalis pedis artery bypass: analysis of outcome in more than 1000 cases. J Vasc Surg. 2003;37(2):307-315.
67. Bradbury AW, Adam DJ, Bell J, et al. Multicentre randomised controlled trial of the clinical and cost-effectiveness of a bypass-surgery-first versus a balloon-angioplasty-first revascularisation strategy for severe limb ischaemia due to infrainguinal disease. The Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial. Health Technol Assess. 2010;14(14):1-210, iii-iv.
68. Conte MS. Challenges of distal bypass surgery in patients with diabetes: patient selection, techniques, and outcomes. J Am Podiatr Med Assoc. 2010;100(5):429-438.
69. Caputo GM, Cavanagh PR, Ulbrecht JS, Gibbons GW, Karchmer AW. Assessment and management of foot disease in patients with diabetes. N Engl J Med. 1994;331(13):854-860.
70. Dyet JF, Nicholson AA, Ettles DF. Vascular imaging and intervention in peripheral arteries in the diabetic patient. Diabetes Metab Res Rev. 2000;16(suppl):S16-S22.
71. Vouillarmet J, Bourron O, Gaudric J, Lermusiaux P, Millon A, Hartemann A. Lower-extremity arterial revascularization: is there any evidence for diabetic foot ulcer-healing? Diabetes Metab. 2015; pii: S1262-3636(15)00083-X. [Epub ahead of print.]
72. Pogach L, Charns MP, Wrobel JS, et al. Impact of policies and performance measurement on development of organizational coordinating strategies for chronic care delivery. Am J Manag Care. 2004;10(2, pt 2):171-180.
73. Longo WE, Cheadle W, Fink A, et al. The role of the Veterans Affairs Medical Centers in patient care, surgical education, research and faculty development. Am J Surg. 2005;190(5):662-675.
74. Tseng CL, Rajan M, Miller DR, Lafrance JP, Pogach L. Trends in initial lower extremity amputation rates among Veterans Health Administration health care System users from 2000 to 2004. Diabetes Care. 2011;34(5):1157-1163.
75. Andrews KL, Houdek MT, Kiemele LJ. Wound management of chronic diabetic foot ulcers: from the basics to regenerative medicine. Prostht Orthot Int. 2015;39(1):29-39.
76. Barshes NR, Sigireddi M, Wrobel JS, et al. The system of care for the diabetic foot: objectives, outcomes, and opportunities. Diabet Foot Ankle. 2013;4:10.3402/dfa.v4i0.21847.
77. Boulton AJ, Armstrong DG, Albert SF, et al. Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31(8):1679-1685.
78. Morey-Vargas OL, Smith SA. BE SMART: strategies for foot care and prevention of foot complications in patients with diabetes. Prosthet Orthot Int. 2015;39(1):48-60.
79. Chiwanga FS, Njelekela MA. Diabetic foot: prevalence, knowledge, and foot self-care practices among diabetic patients in Dar es Salaam, Tanzania-a cross-sectional study. J Foot Ankle Res. 2015;8:20.
80. Ward A, Metz L, Oddone EZ, Edelman D. Foot education improves knowledge and satisfaction among patients at high risk for diabetic foot ulcer. Diabetes Educ. 1999;25(4):560-567.
The prevalence of diabetes mellitus (DM) is growing at epidemic proportions in the U.S. and has been reported as the most common reason for hospital admissions in western countries.1 There continues to be an alarmingly steady increase in the incidence of type 2 DM (T2DM), especially among the young and obese. Long-term diabetes-related complications also are likely to rise in prevalence. In particular, the diabetic foot is associated with morbidity and disability, leading to a significant impairment of quality of life.2 People with DM develop foot ulcers because of neuropathy (sensory, motor, and autonomic deficits), ischemia, or both.3 The initiating injury may be from acute mechanical or thermal trauma or from repetitively or continuously applied mechanical stress.4
From foot ulcerations to neuropathy to peripheral vascular disease, the challenges are significant and can result in amputations and even premature death. To address these challenges, early diagnosis and a multidisciplinary team approach should be employed. Managing the numerous comorbidities is essential for treatment.1,2,5
Due to the longevity of patients with DM, diabetes-associated complications are expected to rise in prevalence.6 The American Diabetes Association recently reported that T2DM accounts for about 90% to 95% of all persons with DM.7,8 Today, many hospitalizations for patients with DM are for lower extremity conditions, such as ulceration, infection, or gangrene. Diabetic foot ulcerations (DFUs) are painful and costly for both the patient and the health care system. Every year, more than 1 million people with DM worldwide lose a leg as a consequence of this disease.9 Most DM-related amputations are preceded by a foot ulcer.
Diabetic foot ulcerations are the most common foot condition leading to lower extremity amputation (Figure 1).10 About 14 million individuals in the U.S. with diagnosed and undiagnosed DM will experience pathologic changes of their lower extremities that, when combined with minor trauma and infection, may lead to serious foot problems.11 Although the triad of vasculopathy, neuropathy, and susceptibility to infection are the primary permissive factors in its pathogenesis, DFU can also be attributed to other important risk factors. The presence of peripheral neuropathy and peripheral arterial disease (PAD) are considered to be the most significant risk factors for all types of diabetic foot complications.12
Related: A Combined Treatment Protocol for Patients With Diabetic Peripheral Neuropathy
Optimal care of foot ulceration depends on the treating physician’s understanding of the pathophysiology involved, familiarity with accepted principles of treatment, and the knowledge that a coordinated, multidisciplinary team approach will best accomplish the goal of limb salvage. All efforts should be made to prevent foot lesions, and when present, existing ulcers should be treated promptly and aggressively, which can often prevent an exacerbation of the problem and decrease the incidence of amputations. Even when ulcers have healed, patients with DM and a history of a lower extremity ulcer should consider it a lifelong condition that requires monitoring to prevent recurrence.13,14
This review provides a brief overview of DFU, including etiology, evaluation, treatment, and prevention, to provide clinicians with the clinical markers, evidence, and DFU treatment recommendations.
Etiologies
Multiple risk factors contribute to the development and pathogenesis of DFUs.5,6,15,16 Neuropathy and PAD are major factors in the pathogenesis of diabetic foot ulcers.17 However, there are several additional factors leading to the occurrence of foot complications. Reiber and colleagues have determined that 63% of their patients’ ulcers were attributed to the critical triad of peripheral sensory neuropathy, trauma, and deformity.15
Other factors also implicated in the causal pathway to ulceration were ischemia, callus, and edema. Infection was rarely implicated in the etiology of these lesions, although once an ulcer has developed, infection and PAD were found to be the major causes for amputation.10,18,19 Many of the risk factors for foot ulcer are also predisposing factors for amputation, because ulcers are primary antecedent events leading to amputation.20-23
Evaluation
The clinical evaluation must include a thorough and systematic lower extremity examination when starting DFU treatment. It is important to have a thorough assessment of the ulcer’s size and depth, and the evaluation should include a description of its appearance and measurement of its diameter at each visit. Evaluation for the presence of local and systemic infection and potential for osteomyelitis, using a small sterile blunt probe, is critical in determining depth of penetration and tracking along tendon sheaths (Figure 2).
Peripheral arterial disease is directly linked to lower extremity disorders, such as intermittent claudication, pain on exertion, pain at rest, and, in severe cases, critical limb ischemia and gangrene.1 Bilateral lower extremity pulses should routinely be palpated. When dorsalis pedis or posterior tibial artery pulses are absent or diminished, Doppler segmental pressures to the toes, pulse volume recording, skin perfusion pressure, or transcutaneous oxygen evaluation is indicated, and vascular consultation should be sought.3 Ischemia is caused by peripheral arterial occlusive disease of larger vessels, not by microangiopathy.13 Poor arterial inflow is associated not only with impaired ulcer healing, but also subsequent infection, gangrene, and amputation.13
Diabetic peripheral neuropathy is characterized by loss of protective sensation, allowing ulceration in areas of high pressure. Peripheral sensory neuropathy as measured by vibration perception thresholds can impart a 3.4-fold to 32-fold risk of ulceration.19,21 Patients insensitive to a 10-g monofilament, commonly used to assess peripheral neuropathy, has been shown in several studies to convey a 2.2-fold to18-fold risk of ulceration.6,19,27,28 In the large, population-based North-West Diabetes Foot Care Study, loss of protective sensation to the 10-g monofilament increased the risk of ulceration 80%, whereas abnormal ankle reflexes increased this risk 55%.29
Peripheral neuropathy has been demonstrated as a strong risk factor for foot ulceration in many cross-sectional studies and is present in > 80% of affected patients.29 Recent studies suggested that impaired sensation makes the foot increasingly vulnerable to damage caused by mechanical, thermal, or pressure-related injury.30 Autonomic neuropathy by virtue of subsequent anhidrosis causes dryness of the skin and, therefore, vulnerability to fissuring.13
Unhealed cracks in the skin can easily lead to infection, especially in the presence of PAD. Neuropathy has an insidious and nonhomogeneous manifestation, making it difficult to identify its onset and a challenge for patients and clinicians.31,32
Sacco and colleagues reviewed current literature and the International Consensus on the Diabetic Foot recommendation and concluded that most attention is given to patients with imminent foot ulceration rather than attempting to develop and improve assessment techniques that detect early impairments.31,33 They propose that effort should be made that detect patients at risk of developing diabetic polyneuropathy. Although the 10-g monofilament pressure perception threshold is a common screening technique for early detection, tests of the vibration perception threshold may be more sensitive.
The authors propose that different monofilament sizes could probably better help determine the disease status, as the vibration tests do. In addition to the considerable subjectivity of both methods of assessing sensitivity, they are unquestionably clinical resources that can contribute to early detection of DPN. Future studies should focus on developing assessment strategies and tools that better detect early neuropathic changes. Early diagnosis of impending problems will aid in preventing further limb-threatening complications.
Treatment
The management of diabetic foot disease is focused primarily on avoiding lower extremity amputation and should be carried out through 3 main strategies: identification of the at risk foot, treatment of the acutely diseased foot, and prevention of further complications.34 The primary goal in the treatment of DFUs is to obtain wound closure. Prompt, aggressive treatment of DFUs can often prevent an exacerbation of the problem and the potential need for amputation. The aim of therapy, therefore, should be early intervention to allow prompt healing of the lesion and, once healed, prevent its recurrence.3,20,25,35
Management of the foot ulcer is largely determined by its severity (grade), vascularity, and presence of infection.3,14,36 A multidisciplinary team approach should be used due to the multifaceted nature of foot ulcers, as well as for managing the numerous comorbidities attendant with these patients. The choice of treatment methods is determined by patient and ulcer characteristics. Equally important is the ability of patients to comply with the treatment as well as with the location and severity of the ulcer.4
Rest, elevation, and removal of pressure (off-loading) are essential components of treatment and should be initiated at first presentation. Recent studies provided evidence that indicated proper off-loading promotes more rapid DFU healing.37,38 Ill-fitting footwear should be discarded and replaced with an appropriate off-loading device for mitigating pressure at the site of the ulceration. Although many off-loading modalities are currently in use, only a few studies describe the frequency and rate of wound healing associated with their use.
The total contact cast (TCC) is considered the superior standard therapy in management for neuropathic ulcers due to its proven ability to redistribute pressure, thereby promoting expeditious wound closure. Another inherent benefit is to ensure patient adherence with off-loading as well as reducing activity levels.24,39 Previous randomized controlled trials have demonstrated that patients treated with TCC healed a higher percentage of plantar ulcers at a faster rate than did patients in the control groups. One unique study demonstrated histologic evidence of more rapid angiogenesis with formation of granulation tissue in the casted group compared with the standard treatment group.40,41
Potential disadvantages of the TCC include the need for expertise in its proper application, the need for weekly cast changes, and related costs.24,35 Although a number of new devices have been introduced as alternatives to the TCC, only several clinical studies demonstrating their efficacy have been published.5,14,25,36 If nonweight bearing with crutches, wheelchair, or more effective devices are not feasible, even a pressure-attenuating insert can be used in a simple postoperative shoe until specialty referral is made.
Debridement of necrotic, callus, fibrous, and senescent tissues is a mainstay of ulcer therapy.42,43 It is considered the first and the most important therapeutic step leading to wound closure in patients with DFU.42-44 Unhealthy tissue must be sharply debrided back to bleeding tissue to fully visualize the extent of the ulcer as well as to detect any underlying abscesses or sinuses. It has been reported that regular (weekly) sharp debridement is associated with more rapid healing of ulcers compared with less frequent debridement.45-47 Wilcox and colleagues indicated that frequent debridement healed more wounds in a shorter time (P < .001).46 The more frequent the debridement, the better the healing outcome. There are different types of debridement methods, including surgical, enzymatic, autolytic, mechanical, and biologic.48 Surgical or sharp debridement can convert a chronic ulcer into an acute wound that is more likely to heal.24 Adequate debridement must always precede the application of topical wound healing agents, dressings, or wound closure procedures.24 Conversely, a wound that does not receive the necessary debridement is one that has not been adequately treated.
There are numerous types of dressings that have been developed over the past decade that promote wound healing. Few have undergone any formal clinical studies to determine efficacy or effectiveness to help guide clinicians in their use.
Yazdanpanah and colleagues argued that dressings should confer moisture balance, protease sequestration, growth factor stimulation, antimicrobial activity, oxygen permeability, and the capacity to promote autolytic debridement to facilitate the production of granulation tissues and the re-epithelialization process.24 In addition, it should have a prolonged time of action, high efficiency, and protection against contamination or infection.17 The group noted that no single dressing fulfills all the requirements of a diabetic patient with a foot ulcer. The choice of dressing is largely determined by the causes of DFU, wound location, depth, amount of scar or slough, exudates, condition of wound margins, presence of infection and pain, need for adhesiveness, and conformability of the dressing (Table 2).
Advanced Therapies
In 2003, Sheehan and colleagues reported that a 50% change in foot ulcer area after 4 weeks of observation is a robust predictor of healing at 12 weeks.49 In addition, wounds failing to achieve a 50% reduction in area after 4 weeks need to be reassessed and considered for advanced treatment modalities if there are no otherwise identified impediments to wound healing.6,9,38 These findings have served as a pivotal clinical decision point in the care of DFUs over the past several years for early identification of patients who may not respond to the standard of care. Today, most wound care protocols advocate use of standard therapies for at least 4 weeks before advanced therapies are considered.
Significant improvements have been achieved in the treatment of ulcerations, and today clinicians have several advanced therapeutic options for management of chronic DFUs. These new technologies have been shown to increase the probability of complete wound closure in difficult-to-heal foot ulcerations in patients with diabetes. Among these are recombinant platelet-derived growth factors, a human living skin equivalent, and a human fibroblast-derived dermal substitute.49-51 Tissue-engineered skin equivalent (Apligraf) and human dermis (Dermagraft) are types of biologically active dressings that are derived from fibroblasts of neonatal foreskins.
The most recent advancements for wound care therapies is that of stem cell therapies, primarily bone marrow-derived and, most recently, placental-derived stem cells, including dehydrated human amnion chorion (Epifix) and amniotic matrix with mesenchymal stem cells (Grafix).52,53 Because of the expense of these products, they cannot be used universally in the treatment of DFUs but rather are used and reserved for difficult-to-heal wounds. In addition, negative pressure therapy has assumed a major role in the management of traumatic, acute, and chronic wounds and has shown efficacy in healing DFUs.54-57 Hyperbaric oxygen therapy and several biophysical modalities have been studied and found to be efficacious in healing a wide variety of chronic wounds over the past decade as well, although results vary by study, and no advanced modality has become universal in its application.58-64
Table 3 lists most of the wound care technologies commonly used in current clinical practice. Although randomized controlled trials have been published supporting the use of most of these modalities, a lack of strong data proving efficacy for use of such treatment options remains.
Treatment of any underlying ischemia is critical in achieving a successful outcome. Vascular surgical consultation should be obtained on presentation of an ischemic wound and in cases where ulcers show no sign of progress despite appropriate management.4,13 Revascularization is commonly performed in patients with critical limb ischemia and DFUs but is also performed in patients with less severe arteriopathy. The goal is to restore a palpable pulse on the affected foot.65 The postrevascularization ulcer-healing rate ranges from 46% to 91% at 1 year and seems to be improved in those patients with distal arterial reconstruction and restoration of pulsatile flow.66
Endovascular approaches are becoming increasingly common in patients whose arterial disease is more limited or morbidity is a significant concern.67,68 Studies report that the exact role of isolated endovascular procedures is still to be determined, although such interventions are frequently performed in concert with angiography preceding vascular reconstructive procedures.69,70 However, in many such studies, healing was often a secondary criterion, and there was no description of the initial wound or its management.71
Challenges
Within the VA setting there is a wide range of patient comorbidities that frequently present clinicians with unique challenges. Often these patients are older with many social and mental health conditions, including self-abuse, drug-abuse, nonadherence, psychological issues and lack of financial and/or educational resources or support. Many of these patients have comorbidities associated with diabetes that can delay healing of their ulcerations.
Systemwide VA mandates have implemented multidisciplinary foot care teams. The teams identify veterans at risk for lower limb complications; provide preventive care; track high-risk foot care across the continuum of outpatient, inpatient, and rehabilitative care; and provide education, orthoses, and social support.72,73 In the late 1990s, the VHA implemented a national program of foot risk screening and referral, conducted largely in primary care.29 By 1998 as determined from medical record reviews, 95% of veterans had a visual examination, 84% had palpation of pulses, and 78% had undergone a sensory examination. In addition, about 83% of patients had a monofilament examination, and 85% of individuals with risk factors were referred to foot specialists in 2004.72,74 Veterans at higher risk for lower extremity complications routinely receive subsequent preventive foot care, such as education or prescription of therapeutic shoes in the VHA.
Tseng and colleagues evaluated risk-adjusted trends in amputations among veterans with diabetes during a 5-year period and reported a decrease in amputation rates observed for all types of lower extremity amputations (LEA) and among all racial groups.74 Implementation of such universal programs for foot screening, tracked through performance measures, may have contributed to a decrease in LEAs and improved outcomes in the VA patient population.
Prevention
A healthy, intact diabetic foot is best maintained by a consistent and recurrent preventive treatment strategy. Prevention of ulcer recurrence remains to be a major clinical challenge. Andrews and colleagues demonstrated that recurrence rates range from 28% at 12 months to 100% at 40 months.75 They report that the highest incidence of reulceration is in the site of a previous ulceration, noting that a newly healed ulcer is covered with fragile skin and after complete healing, there is an area of higher density tissue (scar). Shearing between the different tissue densities often contributes to new ulcers.
After the ulcer heals, the patient and their caregivers must incorporate preventative measures in care plans to reduce the risk of wound reoccurrence. A study reported by Barshes and colleagues demonstrated that a majority of people with diabetes do not receive guideline-recommended foot care, including regular foot examinations.76 Identifying the patients with diabetes at risk for ulceration requires foot examination,including the vascular and neurologic systems, skin conditions, and foot structure.77 Among the complications of diabetes, lower limb amputation is considered to be preventable.78,79 Because there is a great beneficial effect of patient education on reducing LEAs, a flexible schedule for diabetes education, that offers education at any time for the maximum convenience of patients rather than focusing on health care provider’s convenience is critical.79,80 Conservative management of foot problems also has reduced the risk of amputation by simple procedures, such as appropriate foot wear, cleanliness, aggressive surgical debridement, and evidence-based ulcer management.34 This is best accomplished through a multidisciplinary approach involving a team of specialists and personnel who provide a coordinated process of care, including a patient motivated to ensure its success.6
Conclusions
The authors have described the components of assessment and treatment that can help ensure successful healing of foot ulcers in diabetic patients. These approaches should be used whenever feasible to reduce the high morbidity and risk of serious complications resulting from foot ulcers. Advances in treating chronic diabetic wounds are promising; however, the intrinsic pathophysiologic abnormalities that lead to ulcers in the first place cannot be ignored. No known therapy will be effective without concomitant management of ischemia, infection, and adequate off-loading.6,75
Not all diabetic foot complications can be prevented, but it is possible to dramatically reduce their incidence through appropriate management and prevention programs. The multidisciplinary team approach that combines the expertise of many types of health care providers for diabetic foot disorders has been demonstrated as the optimal method to achieve favorable rates of limb salvage in the high-risk diabetic patient.
The prevalence of diabetes mellitus (DM) is growing at epidemic proportions in the U.S. and has been reported as the most common reason for hospital admissions in western countries.1 There continues to be an alarmingly steady increase in the incidence of type 2 DM (T2DM), especially among the young and obese. Long-term diabetes-related complications also are likely to rise in prevalence. In particular, the diabetic foot is associated with morbidity and disability, leading to a significant impairment of quality of life.2 People with DM develop foot ulcers because of neuropathy (sensory, motor, and autonomic deficits), ischemia, or both.3 The initiating injury may be from acute mechanical or thermal trauma or from repetitively or continuously applied mechanical stress.4
From foot ulcerations to neuropathy to peripheral vascular disease, the challenges are significant and can result in amputations and even premature death. To address these challenges, early diagnosis and a multidisciplinary team approach should be employed. Managing the numerous comorbidities is essential for treatment.1,2,5
Due to the longevity of patients with DM, diabetes-associated complications are expected to rise in prevalence.6 The American Diabetes Association recently reported that T2DM accounts for about 90% to 95% of all persons with DM.7,8 Today, many hospitalizations for patients with DM are for lower extremity conditions, such as ulceration, infection, or gangrene. Diabetic foot ulcerations (DFUs) are painful and costly for both the patient and the health care system. Every year, more than 1 million people with DM worldwide lose a leg as a consequence of this disease.9 Most DM-related amputations are preceded by a foot ulcer.
Diabetic foot ulcerations are the most common foot condition leading to lower extremity amputation (Figure 1).10 About 14 million individuals in the U.S. with diagnosed and undiagnosed DM will experience pathologic changes of their lower extremities that, when combined with minor trauma and infection, may lead to serious foot problems.11 Although the triad of vasculopathy, neuropathy, and susceptibility to infection are the primary permissive factors in its pathogenesis, DFU can also be attributed to other important risk factors. The presence of peripheral neuropathy and peripheral arterial disease (PAD) are considered to be the most significant risk factors for all types of diabetic foot complications.12
Related: A Combined Treatment Protocol for Patients With Diabetic Peripheral Neuropathy
Optimal care of foot ulceration depends on the treating physician’s understanding of the pathophysiology involved, familiarity with accepted principles of treatment, and the knowledge that a coordinated, multidisciplinary team approach will best accomplish the goal of limb salvage. All efforts should be made to prevent foot lesions, and when present, existing ulcers should be treated promptly and aggressively, which can often prevent an exacerbation of the problem and decrease the incidence of amputations. Even when ulcers have healed, patients with DM and a history of a lower extremity ulcer should consider it a lifelong condition that requires monitoring to prevent recurrence.13,14
This review provides a brief overview of DFU, including etiology, evaluation, treatment, and prevention, to provide clinicians with the clinical markers, evidence, and DFU treatment recommendations.
Etiologies
Multiple risk factors contribute to the development and pathogenesis of DFUs.5,6,15,16 Neuropathy and PAD are major factors in the pathogenesis of diabetic foot ulcers.17 However, there are several additional factors leading to the occurrence of foot complications. Reiber and colleagues have determined that 63% of their patients’ ulcers were attributed to the critical triad of peripheral sensory neuropathy, trauma, and deformity.15
Other factors also implicated in the causal pathway to ulceration were ischemia, callus, and edema. Infection was rarely implicated in the etiology of these lesions, although once an ulcer has developed, infection and PAD were found to be the major causes for amputation.10,18,19 Many of the risk factors for foot ulcer are also predisposing factors for amputation, because ulcers are primary antecedent events leading to amputation.20-23
Evaluation
The clinical evaluation must include a thorough and systematic lower extremity examination when starting DFU treatment. It is important to have a thorough assessment of the ulcer’s size and depth, and the evaluation should include a description of its appearance and measurement of its diameter at each visit. Evaluation for the presence of local and systemic infection and potential for osteomyelitis, using a small sterile blunt probe, is critical in determining depth of penetration and tracking along tendon sheaths (Figure 2).
Peripheral arterial disease is directly linked to lower extremity disorders, such as intermittent claudication, pain on exertion, pain at rest, and, in severe cases, critical limb ischemia and gangrene.1 Bilateral lower extremity pulses should routinely be palpated. When dorsalis pedis or posterior tibial artery pulses are absent or diminished, Doppler segmental pressures to the toes, pulse volume recording, skin perfusion pressure, or transcutaneous oxygen evaluation is indicated, and vascular consultation should be sought.3 Ischemia is caused by peripheral arterial occlusive disease of larger vessels, not by microangiopathy.13 Poor arterial inflow is associated not only with impaired ulcer healing, but also subsequent infection, gangrene, and amputation.13
Diabetic peripheral neuropathy is characterized by loss of protective sensation, allowing ulceration in areas of high pressure. Peripheral sensory neuropathy as measured by vibration perception thresholds can impart a 3.4-fold to 32-fold risk of ulceration.19,21 Patients insensitive to a 10-g monofilament, commonly used to assess peripheral neuropathy, has been shown in several studies to convey a 2.2-fold to18-fold risk of ulceration.6,19,27,28 In the large, population-based North-West Diabetes Foot Care Study, loss of protective sensation to the 10-g monofilament increased the risk of ulceration 80%, whereas abnormal ankle reflexes increased this risk 55%.29
Peripheral neuropathy has been demonstrated as a strong risk factor for foot ulceration in many cross-sectional studies and is present in > 80% of affected patients.29 Recent studies suggested that impaired sensation makes the foot increasingly vulnerable to damage caused by mechanical, thermal, or pressure-related injury.30 Autonomic neuropathy by virtue of subsequent anhidrosis causes dryness of the skin and, therefore, vulnerability to fissuring.13
Unhealed cracks in the skin can easily lead to infection, especially in the presence of PAD. Neuropathy has an insidious and nonhomogeneous manifestation, making it difficult to identify its onset and a challenge for patients and clinicians.31,32
Sacco and colleagues reviewed current literature and the International Consensus on the Diabetic Foot recommendation and concluded that most attention is given to patients with imminent foot ulceration rather than attempting to develop and improve assessment techniques that detect early impairments.31,33 They propose that effort should be made that detect patients at risk of developing diabetic polyneuropathy. Although the 10-g monofilament pressure perception threshold is a common screening technique for early detection, tests of the vibration perception threshold may be more sensitive.
The authors propose that different monofilament sizes could probably better help determine the disease status, as the vibration tests do. In addition to the considerable subjectivity of both methods of assessing sensitivity, they are unquestionably clinical resources that can contribute to early detection of DPN. Future studies should focus on developing assessment strategies and tools that better detect early neuropathic changes. Early diagnosis of impending problems will aid in preventing further limb-threatening complications.
Treatment
The management of diabetic foot disease is focused primarily on avoiding lower extremity amputation and should be carried out through 3 main strategies: identification of the at risk foot, treatment of the acutely diseased foot, and prevention of further complications.34 The primary goal in the treatment of DFUs is to obtain wound closure. Prompt, aggressive treatment of DFUs can often prevent an exacerbation of the problem and the potential need for amputation. The aim of therapy, therefore, should be early intervention to allow prompt healing of the lesion and, once healed, prevent its recurrence.3,20,25,35
Management of the foot ulcer is largely determined by its severity (grade), vascularity, and presence of infection.3,14,36 A multidisciplinary team approach should be used due to the multifaceted nature of foot ulcers, as well as for managing the numerous comorbidities attendant with these patients. The choice of treatment methods is determined by patient and ulcer characteristics. Equally important is the ability of patients to comply with the treatment as well as with the location and severity of the ulcer.4
Rest, elevation, and removal of pressure (off-loading) are essential components of treatment and should be initiated at first presentation. Recent studies provided evidence that indicated proper off-loading promotes more rapid DFU healing.37,38 Ill-fitting footwear should be discarded and replaced with an appropriate off-loading device for mitigating pressure at the site of the ulceration. Although many off-loading modalities are currently in use, only a few studies describe the frequency and rate of wound healing associated with their use.
The total contact cast (TCC) is considered the superior standard therapy in management for neuropathic ulcers due to its proven ability to redistribute pressure, thereby promoting expeditious wound closure. Another inherent benefit is to ensure patient adherence with off-loading as well as reducing activity levels.24,39 Previous randomized controlled trials have demonstrated that patients treated with TCC healed a higher percentage of plantar ulcers at a faster rate than did patients in the control groups. One unique study demonstrated histologic evidence of more rapid angiogenesis with formation of granulation tissue in the casted group compared with the standard treatment group.40,41
Potential disadvantages of the TCC include the need for expertise in its proper application, the need for weekly cast changes, and related costs.24,35 Although a number of new devices have been introduced as alternatives to the TCC, only several clinical studies demonstrating their efficacy have been published.5,14,25,36 If nonweight bearing with crutches, wheelchair, or more effective devices are not feasible, even a pressure-attenuating insert can be used in a simple postoperative shoe until specialty referral is made.
Debridement of necrotic, callus, fibrous, and senescent tissues is a mainstay of ulcer therapy.42,43 It is considered the first and the most important therapeutic step leading to wound closure in patients with DFU.42-44 Unhealthy tissue must be sharply debrided back to bleeding tissue to fully visualize the extent of the ulcer as well as to detect any underlying abscesses or sinuses. It has been reported that regular (weekly) sharp debridement is associated with more rapid healing of ulcers compared with less frequent debridement.45-47 Wilcox and colleagues indicated that frequent debridement healed more wounds in a shorter time (P < .001).46 The more frequent the debridement, the better the healing outcome. There are different types of debridement methods, including surgical, enzymatic, autolytic, mechanical, and biologic.48 Surgical or sharp debridement can convert a chronic ulcer into an acute wound that is more likely to heal.24 Adequate debridement must always precede the application of topical wound healing agents, dressings, or wound closure procedures.24 Conversely, a wound that does not receive the necessary debridement is one that has not been adequately treated.
There are numerous types of dressings that have been developed over the past decade that promote wound healing. Few have undergone any formal clinical studies to determine efficacy or effectiveness to help guide clinicians in their use.
Yazdanpanah and colleagues argued that dressings should confer moisture balance, protease sequestration, growth factor stimulation, antimicrobial activity, oxygen permeability, and the capacity to promote autolytic debridement to facilitate the production of granulation tissues and the re-epithelialization process.24 In addition, it should have a prolonged time of action, high efficiency, and protection against contamination or infection.17 The group noted that no single dressing fulfills all the requirements of a diabetic patient with a foot ulcer. The choice of dressing is largely determined by the causes of DFU, wound location, depth, amount of scar or slough, exudates, condition of wound margins, presence of infection and pain, need for adhesiveness, and conformability of the dressing (Table 2).
Advanced Therapies
In 2003, Sheehan and colleagues reported that a 50% change in foot ulcer area after 4 weeks of observation is a robust predictor of healing at 12 weeks.49 In addition, wounds failing to achieve a 50% reduction in area after 4 weeks need to be reassessed and considered for advanced treatment modalities if there are no otherwise identified impediments to wound healing.6,9,38 These findings have served as a pivotal clinical decision point in the care of DFUs over the past several years for early identification of patients who may not respond to the standard of care. Today, most wound care protocols advocate use of standard therapies for at least 4 weeks before advanced therapies are considered.
Significant improvements have been achieved in the treatment of ulcerations, and today clinicians have several advanced therapeutic options for management of chronic DFUs. These new technologies have been shown to increase the probability of complete wound closure in difficult-to-heal foot ulcerations in patients with diabetes. Among these are recombinant platelet-derived growth factors, a human living skin equivalent, and a human fibroblast-derived dermal substitute.49-51 Tissue-engineered skin equivalent (Apligraf) and human dermis (Dermagraft) are types of biologically active dressings that are derived from fibroblasts of neonatal foreskins.
The most recent advancements for wound care therapies is that of stem cell therapies, primarily bone marrow-derived and, most recently, placental-derived stem cells, including dehydrated human amnion chorion (Epifix) and amniotic matrix with mesenchymal stem cells (Grafix).52,53 Because of the expense of these products, they cannot be used universally in the treatment of DFUs but rather are used and reserved for difficult-to-heal wounds. In addition, negative pressure therapy has assumed a major role in the management of traumatic, acute, and chronic wounds and has shown efficacy in healing DFUs.54-57 Hyperbaric oxygen therapy and several biophysical modalities have been studied and found to be efficacious in healing a wide variety of chronic wounds over the past decade as well, although results vary by study, and no advanced modality has become universal in its application.58-64
Table 3 lists most of the wound care technologies commonly used in current clinical practice. Although randomized controlled trials have been published supporting the use of most of these modalities, a lack of strong data proving efficacy for use of such treatment options remains.
Treatment of any underlying ischemia is critical in achieving a successful outcome. Vascular surgical consultation should be obtained on presentation of an ischemic wound and in cases where ulcers show no sign of progress despite appropriate management.4,13 Revascularization is commonly performed in patients with critical limb ischemia and DFUs but is also performed in patients with less severe arteriopathy. The goal is to restore a palpable pulse on the affected foot.65 The postrevascularization ulcer-healing rate ranges from 46% to 91% at 1 year and seems to be improved in those patients with distal arterial reconstruction and restoration of pulsatile flow.66
Endovascular approaches are becoming increasingly common in patients whose arterial disease is more limited or morbidity is a significant concern.67,68 Studies report that the exact role of isolated endovascular procedures is still to be determined, although such interventions are frequently performed in concert with angiography preceding vascular reconstructive procedures.69,70 However, in many such studies, healing was often a secondary criterion, and there was no description of the initial wound or its management.71
Challenges
Within the VA setting there is a wide range of patient comorbidities that frequently present clinicians with unique challenges. Often these patients are older with many social and mental health conditions, including self-abuse, drug-abuse, nonadherence, psychological issues and lack of financial and/or educational resources or support. Many of these patients have comorbidities associated with diabetes that can delay healing of their ulcerations.
Systemwide VA mandates have implemented multidisciplinary foot care teams. The teams identify veterans at risk for lower limb complications; provide preventive care; track high-risk foot care across the continuum of outpatient, inpatient, and rehabilitative care; and provide education, orthoses, and social support.72,73 In the late 1990s, the VHA implemented a national program of foot risk screening and referral, conducted largely in primary care.29 By 1998 as determined from medical record reviews, 95% of veterans had a visual examination, 84% had palpation of pulses, and 78% had undergone a sensory examination. In addition, about 83% of patients had a monofilament examination, and 85% of individuals with risk factors were referred to foot specialists in 2004.72,74 Veterans at higher risk for lower extremity complications routinely receive subsequent preventive foot care, such as education or prescription of therapeutic shoes in the VHA.
Tseng and colleagues evaluated risk-adjusted trends in amputations among veterans with diabetes during a 5-year period and reported a decrease in amputation rates observed for all types of lower extremity amputations (LEA) and among all racial groups.74 Implementation of such universal programs for foot screening, tracked through performance measures, may have contributed to a decrease in LEAs and improved outcomes in the VA patient population.
Prevention
A healthy, intact diabetic foot is best maintained by a consistent and recurrent preventive treatment strategy. Prevention of ulcer recurrence remains to be a major clinical challenge. Andrews and colleagues demonstrated that recurrence rates range from 28% at 12 months to 100% at 40 months.75 They report that the highest incidence of reulceration is in the site of a previous ulceration, noting that a newly healed ulcer is covered with fragile skin and after complete healing, there is an area of higher density tissue (scar). Shearing between the different tissue densities often contributes to new ulcers.
After the ulcer heals, the patient and their caregivers must incorporate preventative measures in care plans to reduce the risk of wound reoccurrence. A study reported by Barshes and colleagues demonstrated that a majority of people with diabetes do not receive guideline-recommended foot care, including regular foot examinations.76 Identifying the patients with diabetes at risk for ulceration requires foot examination,including the vascular and neurologic systems, skin conditions, and foot structure.77 Among the complications of diabetes, lower limb amputation is considered to be preventable.78,79 Because there is a great beneficial effect of patient education on reducing LEAs, a flexible schedule for diabetes education, that offers education at any time for the maximum convenience of patients rather than focusing on health care provider’s convenience is critical.79,80 Conservative management of foot problems also has reduced the risk of amputation by simple procedures, such as appropriate foot wear, cleanliness, aggressive surgical debridement, and evidence-based ulcer management.34 This is best accomplished through a multidisciplinary approach involving a team of specialists and personnel who provide a coordinated process of care, including a patient motivated to ensure its success.6
Conclusions
The authors have described the components of assessment and treatment that can help ensure successful healing of foot ulcers in diabetic patients. These approaches should be used whenever feasible to reduce the high morbidity and risk of serious complications resulting from foot ulcers. Advances in treating chronic diabetic wounds are promising; however, the intrinsic pathophysiologic abnormalities that lead to ulcers in the first place cannot be ignored. No known therapy will be effective without concomitant management of ischemia, infection, and adequate off-loading.6,75
Not all diabetic foot complications can be prevented, but it is possible to dramatically reduce their incidence through appropriate management and prevention programs. The multidisciplinary team approach that combines the expertise of many types of health care providers for diabetic foot disorders has been demonstrated as the optimal method to achieve favorable rates of limb salvage in the high-risk diabetic patient.
1. Phillips A, Mehl AA. Diabetes mellitus and the increased risk of foot injuries. J Wound Care. 2015;24(5)(suppl 2):4-7.
2. Anichini R, Zecchini F, Cerretini I, et al. Improvement of diabetic foot care after the implementation of the International Consensus on the Diabetic Foot (ICDF): results of a 5-year prospective study. Diabetes Res Clin Pract. 2007;75(2):153-158.
3. Frykberg RG. Diabetic foot ulcers: current concepts. J Foot Ankle Surg. 1998;37(5):440-446.
4. Cavanagh PR, Ulbrecht JS, Caputo GM. New developments in the biomechanics of the diabetic foot. Diabetes Metab Res Rev. 2000;16(suppl 1):S6-S10.
5. Frykberg RG. Diabetic foot ulcers: pathogenesis and management. Am Fam Physician. 2002;66(9):1655-1662.
6. Frykberg RG, Zgonis T, Armstrong DG, et al. Diabetic foot disorders: a clinical practice guideline (2006 revision). J Foot Ankle Surg. 2006;45(5)(suppl 1):S1-S66.
7. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(suppl 1):S81-S90.
8. American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2015;38(suppl 1):S8-S16.
9. Bakker K, Schaper N; International Working Group on Diabetic Foot Editorial Board.The development of global consensus guidelines on the management and prevention of the diabetic foot 2011. Diabetes Metab Res Rev. 2012;28(suppl 1):116-118.
10. Armstrong DG, Nguyen HC, Lavery LA, van Schie CH, Boulton AJ, Harkless LB. Off-loading the diabetic foot wound: a randomized clinical trial. Diabetes Care. 2001;24(6):1019-1022.
11. Reiber GE, Vileikyte L, Boyko Ed, et al. Causal pathways for incident lower-extremity ulcers in patients with diabetes from two settings. Diabetes Care. 1999;22(1):157-162.
12. Al-Rubeaan K, Al Derwish M, et al. Diabetic foot complications and their risk factors from a large retrospective cohort study. PLoS One. 2015;10(5):e0124446.
13. Vuorisalo S, Venermo M, Lepantalo M. Treatment of diabetic foot ulcers. J Cardiovasc Surg (Torino). 2009;50(3):275-291.
14. Frykberg ER. Medical management of disasters and mass casualties from terrorist bombings: how can we cope? J Trauma. 2002;53(2):201-212.
15. Reiber GE, Boyko EJ, Smith DG. Lower extremity foot ulcers and amputations in diabetes. In: National Diabetes Data Group of the National Institute of Diabetes and Digestive and Kidney Diseases. Diabetes in America. 2nd ed. Bethesda, MD: National Institutes of Health; 1995:409-427.
16. Waaijman R, de Haart M, Arts ML, et al. Risk factors for plantar foot ulcer recurrence in neuropathic diabetic patients. Diabetes Care. 2014;37(6):1697-1705.
17. O'Loughlin A, McIntosh C, Dinneen SF, O'Brien T. Review paper: basic concepts to novel therapies: a review of the diabetic foot. Int J Low Extrem Wounds. 2010;9(2):90-102.
18. Jeffcoate WJ, Chipchase SY, Ince P, Game FL. Assessing the outcome of the management of diabetic foot ulcers using ulcer-related and person-related measures. Diabetes Care. 2006;29(8):1784-1787.
19. McNeely MJ, Boyko EJ, Ahroni JH, et al. The independent contributions of diabetic neuropathy and yasculopatny in foot ulceration: how great are the risks? Diabetes Care. 1995;18(2):216-219.
20. Frykberg RG. Diabetic foot ulcers: pathogenesis and management. Am Fam Physician. 2002;66(9):1655-1662.
21. Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation: basis for prevention. Diabetes Care. 1990;13(5):513-521.
22. Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Davignon DR, Smith DG. A prospective study of risk factors for diabetic foot ulcer. The Seattle Diabetic Foot Study. Diabetes Care. 1999;22(7):1036-1042.
23. Armstrong DG, Lavery LA. Diabetic foot ulcers: prevention, diagnosis and classification. Am Fam Physician. 1998;57(6):1325-1332, 1337-1328.
24. Yazdanpanah L, Nasiri M, Adarvishi S. Literature review on the management of diabetic foot ulcer. World J Diabetes. 2015;6(1):37-53.
25. Frykberg RG. Diabetic foot ulcerations. In: Frykberg RG, ed. The High Risk Foot in Diabetes Mellitus. New York, NY: Churchill Livingstone; 1991.
26. Grayson ML, Gibbons GW, Balogh K, Levin E, Karchmer AW. Probing to bone in infected pedal ulcers. A clinical sign of underlying osteomyelitis in diabetic patients. JAMA. 1995;273(9):721-723.
27. Mayfield JA, Reiber GE, Sanders LJ, Janisse D, Pogach LM. Preventive foot care in people with diabetes. Diabetes Care. 1998;21(12):2161-2177.
28. Kalani M, Brismar K, Fagrell B, Ostergren J, Jörneskog G. Transcutaneous oxygen tension and toe blood pressure as predictors for outcome of diabetic foot ulcers. Diabetes Care. 1999;22(1):147-151.
29. Abbott CA, Vileikyte L, Williamson S, Carrington AL, Boulton AJ. Multicenter study of the incidence of and predictive risk factors for diabetic neuropathic foot ulceration. Diabetes Care. 1998;21(7):1071-1075.
30. Tuttolomondo A, Maida C, Pinto A. Diabetic foot syndrome: immune-inflammatory features as possible cardiovascular markers in diabetes. World J Orthop. 2015;6(1):62-76.
31. Sacco IC, Suda EY, Vigneron V, Sartor CD. An 'importance' map of signs and symptoms to classify diabetic polyneuropathy: an exploratory data analysis. PLoS One. 2015;10(6):e0129763.
32. Asad A, Hameed MA, Khan UA, Ahmed N, Butt MU. Reliability of the neurological scores for assessment of sensorimotor neuropathy in type 2 diabetics. J Pak Med Assoc. 2010;60(3):166-170.
33. Dyck PJ, Albers JW, Andersen H, et al. Diabetic polyneuropathies: update on research definition, diagnostic criteria and estimation of severity. Diabetes Metab Res Rev. 2011;27(7):620-628.
34. Ahmad J. The diabetic foot. Diabetes Metab Syndr. 2015;pii: S1871-4021(15)00030-2. [Epub ahead of print.]
35. Prompers L, Schaper N, Apelqvist J, et al. Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia. 2008;51(5):747-755.
36. Frykberg RG. Team approach toward lower extremity amputation prevention in diabetes. J Am Podiatr Med Assoc. 1997;87(7):305-312.
37. Cavanagh PR, Bus SA. Off-loading the diabetic foot for ulcer prevention and healing. J Am Podiatr Med Assoc. 2010;100(5):360-368.
38. Boulton A. The diabetic foot: from art to science. The 18th Camillo Golgi lecture. Diabetologia. 2004;47(8):1343-1353.
39. Boulton AJ. Pressure and the diabetic foot: clinical science and offloading techniques. Am J Surg. 2004;187(5)(suppl 1):S17-S24.
40. Mueller MJ, Diamond JE, Sinacore DR, et al. Total contact casting in treatment of diabetic plantar ulcers. Controlled clinical trial. Diabetes Care. 1989;12(6):384-388.
41. Piaggesi A, Viacava P, Rizzo L, et al. Semiquantitative analysis of the histopathological features of the neuropathic foot ulcer: effects of pressure relief. Diabetes Care. 2003;26(11):3123-3128.
42. Lebrun E, Tomic-Canic M, Kirsner RS. The role of surgical debridement in healing of diabetic foot ulcers. Wound Repair Regen. 2010;18(5):433-438.
43. Edwards J, Stapley S. Debridement of diabetic foot ulcers. Cochrane Database Syst Rev. 2010(1):CD003556.
44. Tallis A, Motley TA, Wunderlich RP, et al. Clinical and economic assessment of diabetic foot ulcer debridement with collagenase: results of a randomized controlled study. Clin Ther. 2013;35(11):1805-1820.
45. Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366(9498):1736-1743.
46. Warriner RA III, Wilcox JR, Carter MJ, Stewart DG. More frequent visits to wound care clinics result in faster times to close diabetic foot and venous leg ulcers. Adv Skin Wound Care. 2012;25(11):494-501.
47. Wilcox JR, Carter MJ, Covington S. Frequency of debridements and time to heal: a retrospective cohort study of 312 744 wounds. JAMA Dermatol. 2013;149(9):1050-1058.
48. Tiwari A, Jain S, Mehta S, Kumar R, Kapoor G, Kumar K. Limb salvage surgery for osteosarcoma: early results in Indian patients. Indian J Orthop. 2014;48(3):266-272.
49. Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Diabetes Care. 2003;26(6):1879-1882.
50. Wieman TJ, Smiell JM, Su Y. Efficacy and safely of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers: a phase III randomized placebo-controlled double-blind study. Diabetes Care. 1998;21(5):822-827.
51. Naughton G, Mansbridge J, Gentzkow G. A metabolically active human dermal replacement for the treatment of diabetic foot ulcers. Artif Organs.1997;21(11):1203-1210.
52. Zelen CM, Serena TE, Denoziere G, Fetterolf DE. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J. 2013;10(5):502-507.
53. Lavery LA, Fulmer J, Shebetka KA, et al. The efficacy and safety of Grafix® for the treatment of chronic diabetic foot ulcers: results of a multi-centre, controlled, randomised, blinded, clinical trial. Int Wound J. 2014;11(5):554-560.
54. Wolvos TA. Negative pressure wound therapy with instillation: the current state of the art. Surg Technol Int. 2014;24:53-62.
55. Andros G, Armstrong DG, Attinger CE, et al; Tucson Expert Consensus Conference. Consensus statement on negative pressure wound therapy (V.A.C. Therapy) for the management of diabetic foot wounds. Ostomy Wound Manage. 2006(suppl):1-32.
56. Armstrong DG, Lavery LA. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet. 2005;366(9498):1704-1710.
57. Armstrong DG, Marston WA, Reyzelman AM, Kirsner RS. Comparative effectiveness of mechanically and electrically powered negative pressure wound therapy devices: a multicenter randomized controlled trial. Wound Repair Regen. 2012;20(3):332-341.
58. Faglia E, Favales F, Aldeghi A, et al. Adjunctive systemic hyperbaric oxygen therapy in treatment of severe prevalently ischemic diabetic foot ulcer. A randomized study. Diabetes Care. 1996;19(12):1338-1343.
59. Fife CE, Buyukcakir C, Otto G, Sheffield P, Love T, Warriner R 3rd. Factors influencing the outcome of lower-extremity diabetic ulcers treated with hyperbaric oxygen therapy. Wound Repair Regen. 2007;15(3):322-331.
60. Kranke P, Bennett MH, Martyn-St. James M, Schnabel A, Debus SE. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2012;4:CD004123.
61. Frykberg R, Martin E, Tallis A, Tierney E. A case history of multimodal therapy in healing a complicated diabetic foot wound: negative pressure, dermal replacement and pulsed radio frequency energy therapies. Int Wound J. 2011;8(2):132-139.
62. Frykberg RG, Driver VR, Lavery LA, Armstrong DG, Isenberg RA. The use of pulsed radio frequency energy therapy in treating lower extremity wounds: results of a retrospective study of a wound registry. Ostomy Wound Manage. 2011;57(3):22-29.
63. Kloth LC. Electrical Stimulation Technologies for Wound Healing. Adv Wound Care. 2014;3(2):81-90.
64. Ennis WJ, Foremann P, Mozen N, Massey J, Conner-Kerr T, Meneses P. Ultrasound therapy for recalcitrant diabetic foot ulcers: results of a randomized, double-blind, controlled, multicenter study. Ostomy Wound Manage. 2005;51(8):24-39.
65. Mills JL Sr, Conte MS, Armstrong DG, et al. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg. 2014;59(1):220-234.e2.
66. Pomposelli FB, Kansal N, Hamdan AD, et al. A decade of experience with dorsalis pedis artery bypass: analysis of outcome in more than 1000 cases. J Vasc Surg. 2003;37(2):307-315.
67. Bradbury AW, Adam DJ, Bell J, et al. Multicentre randomised controlled trial of the clinical and cost-effectiveness of a bypass-surgery-first versus a balloon-angioplasty-first revascularisation strategy for severe limb ischaemia due to infrainguinal disease. The Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial. Health Technol Assess. 2010;14(14):1-210, iii-iv.
68. Conte MS. Challenges of distal bypass surgery in patients with diabetes: patient selection, techniques, and outcomes. J Am Podiatr Med Assoc. 2010;100(5):429-438.
69. Caputo GM, Cavanagh PR, Ulbrecht JS, Gibbons GW, Karchmer AW. Assessment and management of foot disease in patients with diabetes. N Engl J Med. 1994;331(13):854-860.
70. Dyet JF, Nicholson AA, Ettles DF. Vascular imaging and intervention in peripheral arteries in the diabetic patient. Diabetes Metab Res Rev. 2000;16(suppl):S16-S22.
71. Vouillarmet J, Bourron O, Gaudric J, Lermusiaux P, Millon A, Hartemann A. Lower-extremity arterial revascularization: is there any evidence for diabetic foot ulcer-healing? Diabetes Metab. 2015; pii: S1262-3636(15)00083-X. [Epub ahead of print.]
72. Pogach L, Charns MP, Wrobel JS, et al. Impact of policies and performance measurement on development of organizational coordinating strategies for chronic care delivery. Am J Manag Care. 2004;10(2, pt 2):171-180.
73. Longo WE, Cheadle W, Fink A, et al. The role of the Veterans Affairs Medical Centers in patient care, surgical education, research and faculty development. Am J Surg. 2005;190(5):662-675.
74. Tseng CL, Rajan M, Miller DR, Lafrance JP, Pogach L. Trends in initial lower extremity amputation rates among Veterans Health Administration health care System users from 2000 to 2004. Diabetes Care. 2011;34(5):1157-1163.
75. Andrews KL, Houdek MT, Kiemele LJ. Wound management of chronic diabetic foot ulcers: from the basics to regenerative medicine. Prostht Orthot Int. 2015;39(1):29-39.
76. Barshes NR, Sigireddi M, Wrobel JS, et al. The system of care for the diabetic foot: objectives, outcomes, and opportunities. Diabet Foot Ankle. 2013;4:10.3402/dfa.v4i0.21847.
77. Boulton AJ, Armstrong DG, Albert SF, et al. Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31(8):1679-1685.
78. Morey-Vargas OL, Smith SA. BE SMART: strategies for foot care and prevention of foot complications in patients with diabetes. Prosthet Orthot Int. 2015;39(1):48-60.
79. Chiwanga FS, Njelekela MA. Diabetic foot: prevalence, knowledge, and foot self-care practices among diabetic patients in Dar es Salaam, Tanzania-a cross-sectional study. J Foot Ankle Res. 2015;8:20.
80. Ward A, Metz L, Oddone EZ, Edelman D. Foot education improves knowledge and satisfaction among patients at high risk for diabetic foot ulcer. Diabetes Educ. 1999;25(4):560-567.
1. Phillips A, Mehl AA. Diabetes mellitus and the increased risk of foot injuries. J Wound Care. 2015;24(5)(suppl 2):4-7.
2. Anichini R, Zecchini F, Cerretini I, et al. Improvement of diabetic foot care after the implementation of the International Consensus on the Diabetic Foot (ICDF): results of a 5-year prospective study. Diabetes Res Clin Pract. 2007;75(2):153-158.
3. Frykberg RG. Diabetic foot ulcers: current concepts. J Foot Ankle Surg. 1998;37(5):440-446.
4. Cavanagh PR, Ulbrecht JS, Caputo GM. New developments in the biomechanics of the diabetic foot. Diabetes Metab Res Rev. 2000;16(suppl 1):S6-S10.
5. Frykberg RG. Diabetic foot ulcers: pathogenesis and management. Am Fam Physician. 2002;66(9):1655-1662.
6. Frykberg RG, Zgonis T, Armstrong DG, et al. Diabetic foot disorders: a clinical practice guideline (2006 revision). J Foot Ankle Surg. 2006;45(5)(suppl 1):S1-S66.
7. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(suppl 1):S81-S90.
8. American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2015;38(suppl 1):S8-S16.
9. Bakker K, Schaper N; International Working Group on Diabetic Foot Editorial Board.The development of global consensus guidelines on the management and prevention of the diabetic foot 2011. Diabetes Metab Res Rev. 2012;28(suppl 1):116-118.
10. Armstrong DG, Nguyen HC, Lavery LA, van Schie CH, Boulton AJ, Harkless LB. Off-loading the diabetic foot wound: a randomized clinical trial. Diabetes Care. 2001;24(6):1019-1022.
11. Reiber GE, Vileikyte L, Boyko Ed, et al. Causal pathways for incident lower-extremity ulcers in patients with diabetes from two settings. Diabetes Care. 1999;22(1):157-162.
12. Al-Rubeaan K, Al Derwish M, et al. Diabetic foot complications and their risk factors from a large retrospective cohort study. PLoS One. 2015;10(5):e0124446.
13. Vuorisalo S, Venermo M, Lepantalo M. Treatment of diabetic foot ulcers. J Cardiovasc Surg (Torino). 2009;50(3):275-291.
14. Frykberg ER. Medical management of disasters and mass casualties from terrorist bombings: how can we cope? J Trauma. 2002;53(2):201-212.
15. Reiber GE, Boyko EJ, Smith DG. Lower extremity foot ulcers and amputations in diabetes. In: National Diabetes Data Group of the National Institute of Diabetes and Digestive and Kidney Diseases. Diabetes in America. 2nd ed. Bethesda, MD: National Institutes of Health; 1995:409-427.
16. Waaijman R, de Haart M, Arts ML, et al. Risk factors for plantar foot ulcer recurrence in neuropathic diabetic patients. Diabetes Care. 2014;37(6):1697-1705.
17. O'Loughlin A, McIntosh C, Dinneen SF, O'Brien T. Review paper: basic concepts to novel therapies: a review of the diabetic foot. Int J Low Extrem Wounds. 2010;9(2):90-102.
18. Jeffcoate WJ, Chipchase SY, Ince P, Game FL. Assessing the outcome of the management of diabetic foot ulcers using ulcer-related and person-related measures. Diabetes Care. 2006;29(8):1784-1787.
19. McNeely MJ, Boyko EJ, Ahroni JH, et al. The independent contributions of diabetic neuropathy and yasculopatny in foot ulceration: how great are the risks? Diabetes Care. 1995;18(2):216-219.
20. Frykberg RG. Diabetic foot ulcers: pathogenesis and management. Am Fam Physician. 2002;66(9):1655-1662.
21. Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation: basis for prevention. Diabetes Care. 1990;13(5):513-521.
22. Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Davignon DR, Smith DG. A prospective study of risk factors for diabetic foot ulcer. The Seattle Diabetic Foot Study. Diabetes Care. 1999;22(7):1036-1042.
23. Armstrong DG, Lavery LA. Diabetic foot ulcers: prevention, diagnosis and classification. Am Fam Physician. 1998;57(6):1325-1332, 1337-1328.
24. Yazdanpanah L, Nasiri M, Adarvishi S. Literature review on the management of diabetic foot ulcer. World J Diabetes. 2015;6(1):37-53.
25. Frykberg RG. Diabetic foot ulcerations. In: Frykberg RG, ed. The High Risk Foot in Diabetes Mellitus. New York, NY: Churchill Livingstone; 1991.
26. Grayson ML, Gibbons GW, Balogh K, Levin E, Karchmer AW. Probing to bone in infected pedal ulcers. A clinical sign of underlying osteomyelitis in diabetic patients. JAMA. 1995;273(9):721-723.
27. Mayfield JA, Reiber GE, Sanders LJ, Janisse D, Pogach LM. Preventive foot care in people with diabetes. Diabetes Care. 1998;21(12):2161-2177.
28. Kalani M, Brismar K, Fagrell B, Ostergren J, Jörneskog G. Transcutaneous oxygen tension and toe blood pressure as predictors for outcome of diabetic foot ulcers. Diabetes Care. 1999;22(1):147-151.
29. Abbott CA, Vileikyte L, Williamson S, Carrington AL, Boulton AJ. Multicenter study of the incidence of and predictive risk factors for diabetic neuropathic foot ulceration. Diabetes Care. 1998;21(7):1071-1075.
30. Tuttolomondo A, Maida C, Pinto A. Diabetic foot syndrome: immune-inflammatory features as possible cardiovascular markers in diabetes. World J Orthop. 2015;6(1):62-76.
31. Sacco IC, Suda EY, Vigneron V, Sartor CD. An 'importance' map of signs and symptoms to classify diabetic polyneuropathy: an exploratory data analysis. PLoS One. 2015;10(6):e0129763.
32. Asad A, Hameed MA, Khan UA, Ahmed N, Butt MU. Reliability of the neurological scores for assessment of sensorimotor neuropathy in type 2 diabetics. J Pak Med Assoc. 2010;60(3):166-170.
33. Dyck PJ, Albers JW, Andersen H, et al. Diabetic polyneuropathies: update on research definition, diagnostic criteria and estimation of severity. Diabetes Metab Res Rev. 2011;27(7):620-628.
34. Ahmad J. The diabetic foot. Diabetes Metab Syndr. 2015;pii: S1871-4021(15)00030-2. [Epub ahead of print.]
35. Prompers L, Schaper N, Apelqvist J, et al. Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia. 2008;51(5):747-755.
36. Frykberg RG. Team approach toward lower extremity amputation prevention in diabetes. J Am Podiatr Med Assoc. 1997;87(7):305-312.
37. Cavanagh PR, Bus SA. Off-loading the diabetic foot for ulcer prevention and healing. J Am Podiatr Med Assoc. 2010;100(5):360-368.
38. Boulton A. The diabetic foot: from art to science. The 18th Camillo Golgi lecture. Diabetologia. 2004;47(8):1343-1353.
39. Boulton AJ. Pressure and the diabetic foot: clinical science and offloading techniques. Am J Surg. 2004;187(5)(suppl 1):S17-S24.
40. Mueller MJ, Diamond JE, Sinacore DR, et al. Total contact casting in treatment of diabetic plantar ulcers. Controlled clinical trial. Diabetes Care. 1989;12(6):384-388.
41. Piaggesi A, Viacava P, Rizzo L, et al. Semiquantitative analysis of the histopathological features of the neuropathic foot ulcer: effects of pressure relief. Diabetes Care. 2003;26(11):3123-3128.
42. Lebrun E, Tomic-Canic M, Kirsner RS. The role of surgical debridement in healing of diabetic foot ulcers. Wound Repair Regen. 2010;18(5):433-438.
43. Edwards J, Stapley S. Debridement of diabetic foot ulcers. Cochrane Database Syst Rev. 2010(1):CD003556.
44. Tallis A, Motley TA, Wunderlich RP, et al. Clinical and economic assessment of diabetic foot ulcer debridement with collagenase: results of a randomized controlled study. Clin Ther. 2013;35(11):1805-1820.
45. Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366(9498):1736-1743.
46. Warriner RA III, Wilcox JR, Carter MJ, Stewart DG. More frequent visits to wound care clinics result in faster times to close diabetic foot and venous leg ulcers. Adv Skin Wound Care. 2012;25(11):494-501.
47. Wilcox JR, Carter MJ, Covington S. Frequency of debridements and time to heal: a retrospective cohort study of 312 744 wounds. JAMA Dermatol. 2013;149(9):1050-1058.
48. Tiwari A, Jain S, Mehta S, Kumar R, Kapoor G, Kumar K. Limb salvage surgery for osteosarcoma: early results in Indian patients. Indian J Orthop. 2014;48(3):266-272.
49. Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Diabetes Care. 2003;26(6):1879-1882.
50. Wieman TJ, Smiell JM, Su Y. Efficacy and safely of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers: a phase III randomized placebo-controlled double-blind study. Diabetes Care. 1998;21(5):822-827.
51. Naughton G, Mansbridge J, Gentzkow G. A metabolically active human dermal replacement for the treatment of diabetic foot ulcers. Artif Organs.1997;21(11):1203-1210.
52. Zelen CM, Serena TE, Denoziere G, Fetterolf DE. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J. 2013;10(5):502-507.
53. Lavery LA, Fulmer J, Shebetka KA, et al. The efficacy and safety of Grafix® for the treatment of chronic diabetic foot ulcers: results of a multi-centre, controlled, randomised, blinded, clinical trial. Int Wound J. 2014;11(5):554-560.
54. Wolvos TA. Negative pressure wound therapy with instillation: the current state of the art. Surg Technol Int. 2014;24:53-62.
55. Andros G, Armstrong DG, Attinger CE, et al; Tucson Expert Consensus Conference. Consensus statement on negative pressure wound therapy (V.A.C. Therapy) for the management of diabetic foot wounds. Ostomy Wound Manage. 2006(suppl):1-32.
56. Armstrong DG, Lavery LA. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet. 2005;366(9498):1704-1710.
57. Armstrong DG, Marston WA, Reyzelman AM, Kirsner RS. Comparative effectiveness of mechanically and electrically powered negative pressure wound therapy devices: a multicenter randomized controlled trial. Wound Repair Regen. 2012;20(3):332-341.
58. Faglia E, Favales F, Aldeghi A, et al. Adjunctive systemic hyperbaric oxygen therapy in treatment of severe prevalently ischemic diabetic foot ulcer. A randomized study. Diabetes Care. 1996;19(12):1338-1343.
59. Fife CE, Buyukcakir C, Otto G, Sheffield P, Love T, Warriner R 3rd. Factors influencing the outcome of lower-extremity diabetic ulcers treated with hyperbaric oxygen therapy. Wound Repair Regen. 2007;15(3):322-331.
60. Kranke P, Bennett MH, Martyn-St. James M, Schnabel A, Debus SE. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2012;4:CD004123.
61. Frykberg R, Martin E, Tallis A, Tierney E. A case history of multimodal therapy in healing a complicated diabetic foot wound: negative pressure, dermal replacement and pulsed radio frequency energy therapies. Int Wound J. 2011;8(2):132-139.
62. Frykberg RG, Driver VR, Lavery LA, Armstrong DG, Isenberg RA. The use of pulsed radio frequency energy therapy in treating lower extremity wounds: results of a retrospective study of a wound registry. Ostomy Wound Manage. 2011;57(3):22-29.
63. Kloth LC. Electrical Stimulation Technologies for Wound Healing. Adv Wound Care. 2014;3(2):81-90.
64. Ennis WJ, Foremann P, Mozen N, Massey J, Conner-Kerr T, Meneses P. Ultrasound therapy for recalcitrant diabetic foot ulcers: results of a randomized, double-blind, controlled, multicenter study. Ostomy Wound Manage. 2005;51(8):24-39.
65. Mills JL Sr, Conte MS, Armstrong DG, et al. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg. 2014;59(1):220-234.e2.
66. Pomposelli FB, Kansal N, Hamdan AD, et al. A decade of experience with dorsalis pedis artery bypass: analysis of outcome in more than 1000 cases. J Vasc Surg. 2003;37(2):307-315.
67. Bradbury AW, Adam DJ, Bell J, et al. Multicentre randomised controlled trial of the clinical and cost-effectiveness of a bypass-surgery-first versus a balloon-angioplasty-first revascularisation strategy for severe limb ischaemia due to infrainguinal disease. The Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial. Health Technol Assess. 2010;14(14):1-210, iii-iv.
68. Conte MS. Challenges of distal bypass surgery in patients with diabetes: patient selection, techniques, and outcomes. J Am Podiatr Med Assoc. 2010;100(5):429-438.
69. Caputo GM, Cavanagh PR, Ulbrecht JS, Gibbons GW, Karchmer AW. Assessment and management of foot disease in patients with diabetes. N Engl J Med. 1994;331(13):854-860.
70. Dyet JF, Nicholson AA, Ettles DF. Vascular imaging and intervention in peripheral arteries in the diabetic patient. Diabetes Metab Res Rev. 2000;16(suppl):S16-S22.
71. Vouillarmet J, Bourron O, Gaudric J, Lermusiaux P, Millon A, Hartemann A. Lower-extremity arterial revascularization: is there any evidence for diabetic foot ulcer-healing? Diabetes Metab. 2015; pii: S1262-3636(15)00083-X. [Epub ahead of print.]
72. Pogach L, Charns MP, Wrobel JS, et al. Impact of policies and performance measurement on development of organizational coordinating strategies for chronic care delivery. Am J Manag Care. 2004;10(2, pt 2):171-180.
73. Longo WE, Cheadle W, Fink A, et al. The role of the Veterans Affairs Medical Centers in patient care, surgical education, research and faculty development. Am J Surg. 2005;190(5):662-675.
74. Tseng CL, Rajan M, Miller DR, Lafrance JP, Pogach L. Trends in initial lower extremity amputation rates among Veterans Health Administration health care System users from 2000 to 2004. Diabetes Care. 2011;34(5):1157-1163.
75. Andrews KL, Houdek MT, Kiemele LJ. Wound management of chronic diabetic foot ulcers: from the basics to regenerative medicine. Prostht Orthot Int. 2015;39(1):29-39.
76. Barshes NR, Sigireddi M, Wrobel JS, et al. The system of care for the diabetic foot: objectives, outcomes, and opportunities. Diabet Foot Ankle. 2013;4:10.3402/dfa.v4i0.21847.
77. Boulton AJ, Armstrong DG, Albert SF, et al. Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31(8):1679-1685.
78. Morey-Vargas OL, Smith SA. BE SMART: strategies for foot care and prevention of foot complications in patients with diabetes. Prosthet Orthot Int. 2015;39(1):48-60.
79. Chiwanga FS, Njelekela MA. Diabetic foot: prevalence, knowledge, and foot self-care practices among diabetic patients in Dar es Salaam, Tanzania-a cross-sectional study. J Foot Ankle Res. 2015;8:20.
80. Ward A, Metz L, Oddone EZ, Edelman D. Foot education improves knowledge and satisfaction among patients at high risk for diabetic foot ulcer. Diabetes Educ. 1999;25(4):560-567.