User login
Accountable-care organizations are becoming more prominent in the United States, and therefore health care systems in the near future will be reimbursed on the basis of their ability to care for patient populations rather than individual patients. As a result, primary care physicians will need to be well versed in the care of patients with common chronic diseases such as chronic kidney disease (CKD). By one estimate, patients with CKD constitute 14% of the US population age 20 and older, or more than 31 million people.1
An earlier article in this journal reviewed how to identify patients with CKD and how to interpret the estimated glomerular filtration rate (GFR).2 This article examines the care of patients with advanced CKD, how to manage their health risks, and how to optimize their care by coordinating with nephrologists.
GOALS OF CKD CARE
CKD is defined either as renal damage (which is most commonly manifested by proteinuria, but which may include pathologic changes on biopsy or other markers of damage on serum, urine, or imaging studies), or as a GFR less than 60 mL/min/1.73 m2 for at least 3 months.3 It is divided into five stages (Table 1).
Since most patients with CKD never reach end-stage renal disease, much of their care is aimed at slowing the progression of renal dysfunction and addressing medical issues that arise as a result of CKD. To these ends, it is important to detect CKD early and refer these patients to a nephrology team in a timely manner. Their care can be separated into several important tasks:
- Identify the cause of CKD, if possible; address potentially reversible causes such as obstruction or medication-related causes. If a primarily glomerular process (marked by heavy proteinuria and dysmorphic red blood cells and red blood cell casts in the urine sediment) or interstitial nephritis (manifested by white blood cells in the urine) is suspected, refer to a nephrologist early.
- Provide treatment to correct the specific cause (if one is present) or slow the deterioration of renal function.
- Address cardiovascular risk factors.
- Address metabolic abnormalities related to CKD.
- If the CKD is advanced, educate the patient about end-stage renal disease and its treatment options, and guide the patient through the transition to end-stage renal disease.
WHEN SHOULD A NEPHROLOGIST BE CONSULTED?
The ideal timing of referral to a nephrologist is not well defined and depends on the comfort level of the primary care provider.
Treatments to slow the progression of CKD and decrease cardiovascular risk should begin early in CKD (ie, in stage 3) and can be managed by the primary care provider with guidance from a nephrologist. Patients referred to a nephrologist while in stage 3 have been shown to go longer without CKD progression than those referred in later stages.4 Early referral to a nephrologist has also been associated with a decreased mortality rate.5 The studies that found these trends, however, were limited by the fact that patients with stage 3 CKD are less likely to progress to end-stage renal disease or to die of cardiovascular disease than patients with stage 4 or 5 CKD.
Once stage 4 CKD develops, the nephrologist should take a more active role in the care plan. In this stage, cardiovascular risk rises, and the risk of developing end-stage renal disease rises dramatically.6 With comprehensive care in a CKD clinic, even patients with advanced CKD are more likely to have a stabilization of renal function.7 Kinchen et al8 found that patients referred to a nephrologist within 4 months of starting dialysis had a lower survival rate than those referred earlier. Therefore, if a nephrologist was not involved in the patient’s care prior to stage 4, then a referral must be made.
Recommendation. Patients with stage 3 CKD can be referred for an initial evaluation and development of a treatment plan, but most of the responsibility for their care can remain with the primary care provider. Once stage 4 CKD develops, the nephrologist should assume an increasing role. However, if glomerular disease is suspected, we recommend referral to a nephrologist regardless of the estimated GFR.
ELEVATED CARDIOVASCULAR RISK
Patients with stage 3 CKD are 20 times more likely to die of a cardiovascular event than to reach end-stage renal disease.6 This increased risk does not quite reach the status of a cardiovascular disease risk equivalent, as does diabetes,9,10 but cardiovascular risk reduction should be a primary focus of care for the CKD patient.
The cardiovascular risk in part is attributed to a high prevalence of traditional cardiovascular risk factors, including diabetes mellitus, hypertension, and hyperlipidemia.11,12 About two-thirds of CKD patients have metabolic syndrome, which is a risk factor for cardiovascular disease and is associated with more rapid progression of CKD.13 In addition, renal dysfunction, proteinuria, and hyperphosphatemia are also risk factors for cardiovascular disease.14–19
The risk of death from a cardiovascular event increases as kidney function declines, with reported 5-year death rates of 19.5% in stage 2, 24.3% in stage 3, and 45.7% in stage 4 CKD. However, imbalance between mortality risk and progression to end-stage renal disease may be age-dependent.20 Younger patients (age 45 and younger) are more likely to progress to end-stage renal disease, whereas in older patients (over age 65), the relative risk of dying of cardiovascular disease is higher.
Aggressive lipid management
Hyperlipidemia is a common risk factor for cardiovascular morbidity and mortality in CKD.21 However, until recently, all studies of outcomes of patients treated for hyperlipidemia excluded patients with CKD. Post hoc analyses of these studies 22–27 showed statins to be beneficial in primary and secondary cardiovascular prevention in patents with “normal” serum creatinine values but estimated GFR levels of 50 to 59 mL/min/1.73 m2.
The SHARP trial28 was the first prospective trial to study lipid-lowering therapy in patients with CKD. In this trial, patients with various stages of CKD, including advanced CKD, had fewer major vascular events if they received the combination of low-dose simvastatin (Zocor) and ezetimibe (Zetia). However, the evidence does not suggest that statin therapy slows the progression of CKD.28–31
Recommendation. Manage hyperlipidemia aggressively using statin therapy with or without ezetimibe, with a target low-density lipoprotein cholesterol level below 100 mg/dL.32
Manage other cardiovascular risk factors
Because hypertension and proteinuria are risk factors not only for cardiovascular disease but also for progression of CKD, they are discussed in the section below.
ATTEMPT TO PREVENT WORSENING OF RENAL FUNCTION
Medications to avoid
It is important to review a CKD patient’s medication list—prescription and over-the-counter drugs—to identify any that may contribute to a worsening of renal function. CKD patients need to be informed about avoiding medications such as nonsteroidal anti-inflammatory drugs, proton pump inhibitors, and herbal supplements because they can cause further renal injury. In addition, other medications (eg, metformin) are contraindicated in CKD because of side effects that may occur in CKD.
Patients should be encouraged to discuss any changes in their medications, including over-the-counter products, with their primary care physicians.
Manage hypertension aggressively
Many patients with CKD also have hypertension,33,34 possibly because they have a higher frequency of underlying essential hypertension or because CKD often worsens preexisting hypertension. Moreover, uncontrolled hypertension is associated with a further decline in renal function.35,36
The ACCORD trial37 found no benefit in lowering systolic blood pressure to less than 120 mm Hg compared with less than 140 mm Hg in patients with diabetes mellitus. (The patients in this study did not necessarily have CKD.)
A meta-analysis38 of trials of antihypertensive treatment in patients with CKD found that the optimal target systolic blood pressure for decreasing the progression of CKD was 110 to 129 mm Hg. The relative risk of progression of renal dysfunction was:
- 1.83 (95% confidence interval [CI] 0.97–3.44) at 130 mm to 139 mm Hg, vs
- 3.14 (95% CI 1.64–5.99) at 160 mm Hg or higher.
There is also evidence that blood pressure control can be relaxed as patients age. While the exact age differs among published guidelines, the evidence supports a goal blood pressure of less than 150/90 mm Hg once a patient reaches the age of 70, regardless of CKD or proteinuria.
Recommendation. Current evidence suggests the following blood pressure goals in CKD patients:
- With diabetes mellitus or proteinuria: < 130/80 mm Hg
- Without proteinuria: < 140/90 mm Hg
- Age 70 and older: <150/90 mm Hg.39
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) are the preferred antihypertensive drugs in patients with diabetes or proteinuria (see below).
Manage proteinuria
Proteinuria is also associated with progression of CKD. AASK,40 a study that included nondiabetic African American patients whose estimated GFRs were between 20 and 60 mL/min/1.73 m2, showed that higher levels of proteinuria were associated with a higher risk of decline in GFR and a higher risk of end-stage renal disease. Findings were similar to those in studies of other CKD populations.41–43 Proteinuria is also an independent risk factor for cardiovascular disease and death. Multiple large studies16,17,44,45 have found associations between higher levels of albumin excretion and risk of major cardiovascular events, cardiovascular death, and death from any cause in people with and without diabetes.
Reducing proteinuria has been shown to both slow progression of renal dysfunction and reduce the cardiovascular risk.44,45 In a substudy of the IDNT46 in patients with diabetic nephropathy, each 50% reduction in urinary protein excretion was associated with a 56% reduction in risk of progression of CKD. Similar effects have been shown in nondiabetic CKD patients.47
ACE inhibitors and ARBs are the preferred treatments for proteinuria in patients with CKD.48–50 Combination therapy with an ACE inhibitor and an ARB has been used,51–53 with a better response in proteinuria reduction. However, combination therapy with these drugs cannot currently be recommended, as the only prospective study of this regimen to date suggested worse renal and overall outcomes in patients at high cardiovascular risk.54 These drugs may also have renoprotective effects independent of their effects on blood pressure and proteinuria.38 Dietary salt restriction and diuretic therapy can further increase the efficacy of proteinuria reduction by ACE inhibitors or ARBs.55,56
On the other hand, stopping ACE inhibitors or ARBs may be beneficial as the patient nears end-stage renal disease. Ahmed et al57 demonstrated that stopping ACE inhibitors or ARBs in advanced stage 4 CKD (mean estimated GFR 16 mL/min/1.73 m2) was associated with improved GFR and delayed onset of renal replacement therapy. This improvement may be due to regaining the slight decrease in GFR that occurred when these medications were started.
Nondihydropyridine calcium channel blockers such as diltiazem (Cardizem) and verapamil (Calan) have also been shown to be useful for reducing proteinuria,58 whereas dihydropyridine calcium channel blockers such as amlodipine (Norvasc) and nifedipine (Procardia), when used without ACE inhibitors or ARBs, can worsen proteinuria.58,59
Correct metabolic acidosis
The kidneys play an important role in maintaining acid-base balance, keeping the blood from becoming too acidic both by reabsorbing bicarbonate filtered into the urine by the glomerulus and by excreting the daily acid load. Metabolic acidosis can develop when these functions break down at more advanced stages of CKD, most often when the estimated GFR declines to less than 20 mL/min/1.73 m2.
Bicarbonate levels of 22 mmol/L or less have been associated with a higher risk of worsening renal function.60 When such patients were treated with sodium bicarbonate to achieve a serum bicarbonate of at least 23 mmol/L, they had an 80% lower rate of progression to end-stage renal disease without any increase in edema, admission for congestive heart failure, or change in blood pressure.61
Susantitaphong et al62 reviewed six randomized trials of bicarbonate supplementation in CKD and found that it was associated with improved kidney function and a 79% lower rate of progression to end-stage renal disease.
The proposed mechanism behind this benefit lies in the increase in ammonia production that each surviving nephron must undertake to handle the daily acid load. The increased ammonia is thought to play a role in activating the alternative complement pathway,63 causing renal inflammation and injury.
Recommendation. Bicarbonate therapy should be used to maintain serum bicarbonate levels above 22 mmol/L in CKD.64
OTHER ASPECTS OF CKD CARE
Bone mineral disorders
Patients with CKD develop secondary hyperparathyroidism, hyperphosphatemia, and (in advanced CKD) hypocalcemia, all leading to disorders of bone mineral metabolism.
Traditionally, it has been thought that decreased production of 1,25-dihydroxyvitamin D by dysfunctional kidneys leads to decreased suppression of the parathyroid gland and to secondary hyperparathyroidism. The major long-term adverse effect of this is a weakened bone matrix resulting from increased calcium and phosphorus efflux from bones (renal osteodystrophy).
The discovery of fibroblast growth factor 23 (FGF-23) has improved our understanding of the physiology behind disordered bone mineral metabolism in CKD. FGF-23, produced by osteoblasts and osteocytes, acts directly on the kidney to increase renal phosphate excretion. It also suppresses 1,25-dihydroxyvitamin D levels by inhibiting 1-alpha-hydroxylase,65 and it stimulates parathyroid hormone secretion. FGF-23 levels rise much earlier in CKD than do parathyroid hormone levels, suggesting that abnormalities in phosphorus balance and FGF-23 may be the earliest pathophysiologic changes.66
The initial treatment of bone mineral disorders is to some extent guided by laboratory values. Phosphate levels higher than 3.5 or 4 mg/dL and elevated FGF-23 levels have been associated with increased mortality rates in CKD patients.18,19,67–69 All patients should also have their 1,25-dihydroxyvitamin D level checked and supplemented if deficient. In many patients with early stage 3 CKD, this may correct secondary hyperparathyroidism.70
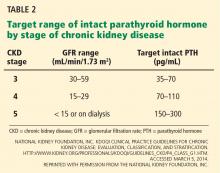
Serum phosphorus levels should be kept in the normal range in stage 3 and 4 CKD,71 either by restricting dietary phosphorus intake (< 800 or < 1,000 mg/day) or by using a phosphate binder, which is taken with meals to prevent phosphorus absorption from the gastrointestinal tract. Current US recommendations are to allow graded increases in parathyroid hormone based on the stage of CKD (Table 2).71 However, these targets are still an area of uncertainty, with some guidelines suggesting that wider variations in parathyroid hormone can be allowed, so there may be wider variation in clinical practice in this area.72 If the serum phosphorus level is in the goal range but parathyroid hormone levels are still high, an activated vitamin D analogue such as calcitriol is recommended, although with the emerging role of FGF-23, some experts also call for early use of a phosphate binder in this group.
The treatment of bone mineral disorders in CKD is fairly complex, and we recommend that it be done by or with the close direction of a nephrologist.
Recommendations on bone disorders
- Check levels of calcium, phosphorus, 25-hydroxyvitamin D, and parathyroid hormone in all patients whose estimated GFR is less than 60 mL/min/1.73 m2, with frequency of measurements based on the stage of CKD.71
- Replace vitamin D if deficient.
- Treat elevated phosphorus levels with a protein-restricted diet (nutrition referral) and a phosphate binder.
- Treat elevated hyperparathyroid hormone levels with a vitamin D analogue once phosphorus levels have been controlled.
- Refer patients with an elevated phosphorus or parathyroid hormone level to a nephrology service for consultation before initiating medical therapy.
Anemia is common, treatment controversial
The treatment of anemia attributed to CKD has been a topic of controversy over the past decade, and we recommend that it be done with the guidance of a nephrologist.
Anemia is common in CKD, and declining kidney function is an independent predictor of anemia.73 Anemia is a risk factor for left ventricular hypertrophy, cardiovascular disease,74 and death in CKD.75
The anemia of CKD is attributed to relative erythropoietin deficiency and bone marrow resistance to erythropoietin, but this is a diagnosis of exclusion, and other causes of anemia must be ruled out. Iron deficiency is a common cause of anemia in CKD, and treatment of iron deficiency may correct anemia in more than one-third of these patients.76,77
Erythropoiesis-stimulating agents such as epoetin alfa (Procrit) and darbepoetin (Aranesp) are used to treat renal anemia. However, the target hemoglobin level has been a subject of debate. Three prospective trials78–80 found no benefit in raising the hemoglobin level to normal ranges using these agents, and several found an association with higher rates of stroke and venous thrombosis. The US Food and Drug Administration suggests that the only role for these agents in CKD is to avoid the need for transfusions. They should not be used to normalize the hemoglobin level. The target, although not explicitly specified, is suggested to be around 10 g/dL.81
PREPARE FOR END-STAGE RENAL DISEASE
Discuss the options
Because the risk of developing end-stage renal disease rises dramatically once CKD reaches stage 4, all such patients should have a discussion about renal replacement therapy. They should be educated about their options for treatment (hemodialysis, peritoneal dialysis, and transplantation, as well as not proceeding with renal replacement therapy), often in a formal class. They should then be actively engaged in the decision about how to proceed. Survival and quality of life should be discussed, particularly with patients who are over age 80, who are severely ill, or who are living in a nursing facility, as these groups get limited survival benefit from starting dialysis, and quality of life may actually decrease with dialysis.82,83
The Renal Physicians Association has created clinical practice guidelines for shared decision-making, consisting of 10 practice recommendations that outline a systematic approach to patients needing renal replacement therapy.84
Consider preemptive kidney transplantation
Any patient thought to be a suitable candidate for renal transplantation should be referred to a transplantation center for evaluation. Studies have shown that kidney transplantation offers a survival advantage compared with chronic dialysis and should preferably be done preemptively, ie, before dialysis is required.85–90 Therefore, patients with estimated GFRs in the low 20s should be referred for a transplantation evaluation.
If a living donor is available, the transplantation team usually waits to perform the procedure until the patient is closer to needing dialysis, often when the estimated GFR is around 15 to 16 mL/min/1.73 m2. If no living donor is available, the patient can earn time on the deceased-donor waiting list once his or her estimated GFR falls to below 20 mL/min/1.7 m2.
Plan for dialysis access
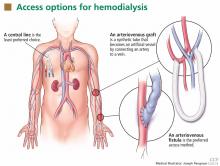
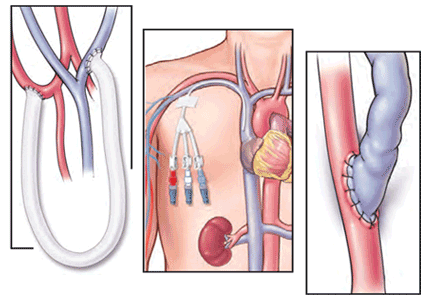
Patients starting hemodialysis first need to undergo a procedure to provide access to the blood. The three options are an arteriovenous fistula, an arteriovenous graft, and a central venous catheter (Figure 1).
An arteriovenous fistula is the best option, being the most durable, followed by a graft and then a catheter.91 Arteriovenous fistulas also have the lowest rates of infection,92 thrombosis,93 and intervention to maintain patency.93
The fistula is created by ligating a vein draining an extremity, most often the nondominant arm, and anastomosing the vein to an artery. The higher arterial pressure causes the vein to dilate and thicken (“arterialize”), thus making it able to withstand repeated cannulation necessary for hemodialysis.
An arteriovenous fistula typically takes 1 to 3 months to “mature” to the point where it can be used,94,95 and, depending on the patient and experience of the vascular surgeon, a significant number may never mature. Thus, it is important to discuss hemodialysis access before the patient reaches end-stage renal disease so that he or she can be referred to a vascular surgeon early, when the estimated GFR is about 20 mL/min/1.73 m2.
An arteriovenous graft. Not all patients have suitable vessels for creation of an arteriovenous fistula. In such patients, an arteriovenous graft, typically made of polytetrafluoroethylene, is the next best option. The graft is typically ready to use in 2 weeks and thus does not require as much advance planning. Grafts tend to narrow more often than fistulas and require more procedures to keep them patent.
A central venous catheter is most often inserted into the internal jugular vein and tunneled under the skin to exit in an area covered by the patient’s shirt.
Tunneled dialysis catheters are associated with higher rates of infection, thrombosis, and overall mortality and are therefore the least preferred choice. They are reserved for patients who have not had advance planning for end-stage renal disease, who do not have acceptable vessels for an arteriovenous fistula or graft, or who have refused surgical access.
Protect the fistula arm. It is recommended that venipuncture, intravenous lines, and blood pressure measurements be avoided in the nondominant upper arm of patients with stage 4 and 5 CKD to protect those veins for the potential creation of an arteriovenous fistula.96 For the same reason, peripherally inserted central catheter lines and subclavian catheters should be avoided in these patients. If an arteriovenous fistula has already been placed, this arm must be protected from such procedures at all times.
Studies have shown that late referral to a nephrologist is associated with a lower incidence of starting dialysis with a permanent vascular access.97,98
If the patient wishes to start peritoneal dialysis, the peritoneal dialysis catheter can usually be used 2 weeks after being inserted.
Starting dialysis
The appropriate time for starting dialysis remains controversial, especially in elderly patients with multiple comorbid conditions.
The IDEAL study99 found no benefit in starting dialysis at a GFR of 10 to 14 mL/min compared with 5 to 7 mL/min. Thus, there is no single estimated GFR at which dialysis should be started. Rather, the development of early uremic symptoms and the patient’s quality of life should guide this decision.82,83,99–101
Hemodialysis involves three sessions per week, each taking about 4 hours. Evidence suggests that longer sessions or more sessions per week may offer benefits, especially in terms of blood pressure, volume, and dietary management. This has led to an increase in the popularity of home and in-center nocturnal hemodialysis programs across the United States.
Peritoneal dialysis?
Peritoneal dialysis is an excellent choice for patients who are motivated, can care for themselves at home, and have a support system available to assist them if needed. It allows for daily dialysis, less fluid restriction, and less dietary restriction, and it gives the patient an opportunity to stay independent. It also spares the veins in the arms, which may be needed for vascular access later in life if hemodialysis is needed.
Recommendation. We recommend that peritoneal dialysis be offered to any suitable patient who is approaching end-stage renal disease.
A COMPREHENSIVE, COLLABORATIVE APPROACH
Chronic kidney disease is a multisystem disorder, and its management requires a comprehensive approach (Table 3). Early detection and interventions are key to reducing cardiovascular events and progression to kidney failure.
Early referral to a nephrologist and team collaboration between the primary care provider, the nephrologist, and other health care providers are essential. Early in the course of CKD, it may be appropriate for a nephrologist to evaluate the patient and recommend a set of treatment goals. Follow-up may be infrequent or unnecessary.
As CKD progresses, especially as the patient reaches an estimated GFR of 30 mL/min/1.73 m2, the nephrologist will take a more active role in the patient’s care and medical decision-making. In some circumstances, it may even be appropriate for the nephrologist to be the patient’s source of primary care, with the primary care provider as a consultant.
Caring for patients with CKD includes not only strategies to preserve renal function and prolong survival, but also making critical decisions about starting dialysis and about the need for transplantation. Early involvement of a nephrologist and early preparation for end-stage renal disease with preemptive transplantation and arteriovenous fistula placement are associated with better patient outcomes. Key to this is collaboration between the primary care provider and the nephrologist, with levels of responsibility for patient care that adapt to the patient’s degree of renal dysfunction and other comorbidities. Such strategies to select patients for timely nephrology referral may help improve outcomes in this vulnerable population.
- United States Renal Data System (USRDS). Identification and care of patients with CKD. http://www.usrds.org/2012/pdf/v1_ch2_12.pdf. Accessed March 5, 2014.
- Simon J, Amde M, Poggio ED. Interpreting the estimated glomerular filtration rate in primary care: benefits and pitfalls. Cleve Clin J Med 2011; 78:189–195.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. http://www.kidney.org/professionals/kdoqi/guidelines_ckd/p4_class_g1.htm. Accessed March 5, 2014.
- Orlando LA, Owen WF, Matchar DB. Relationship between nephrologist care and progression of chronic kidney disease. N C Med J 2007; 68:9–16.
- Tseng CL, Kern EF, Miller DR, et al. Survival benefit of nephrologic care in patients with diabetes mellitus and chronic kidney disease. Arch Intern Med 2008; 168:55–62.
- Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 2004; 164:659–663.
- Serrano A, Huang J, Ghossein C, et al. Stabilization of glomerular filtration rate in advanced chronic kidney disease: a two-year follow-up of a cohort of chronic kidney disease patients stages 4 and 5. Adv Chronic Kidney Dis 2007; 14:105–112.
- Kinchen KS, Sadler J, Fink N, et al. The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med 2002; 137:479–486.
- Tonelli M, Muntner P, Lloyd A, et al; Alberta Kidney Disease Network. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet 2012; 380:807–814.
- Wattanakit K, Coresh J, Muntner P, Marsh J, Folsom AR. Cardiovascular risk among adults with chronic kidney disease, with or without prior myocardial infarction. J Am Coll Cardiol 2006; 48:1183–1189.
- Foley RN, Wang C, Collins AJ. Cardiovascular risk factor profiles and kidney function stage in the US general population: the NHANES III study. Mayo Clin Proc 2005; 80:1270–1277.
- Muntner P, He J, Astor BC, Folsom AR, Coresh J. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the Atherosclerosis Risk in Communities Study. J Am Soc Nephrol 2005; 16:529–538.
- Navaneethan SD, Schold JD, Kirwan JP, et al. Metabolic syndrome, ESRD, and death in CKD. Clin J Am Soc Nephrol 2013; 8:945–952.
- Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 2005; 16:489–495.
- Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol 2004; 15:1307–1315.
- Gerstein HC, Mann JF, Yi Q, et al; HOPE Study Investigators. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001; 286:421–426.
- Hillege HL, Fidler V, Diercks GF, et al; Prevention of Renal and Vascular End Stage Disease (PREVEND) Study Group. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 2002; 106:1777–1782.
- Foley RN, Collins AJ, Ishani A, Kalra PA. Calcium-phosphate levels and cardiovascular disease in community-dwelling adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 2008; 156:556–563.
- Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G; Cholesterol And Recurrent Events Trial Investigators. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 2005; 112:2627–2633.
- Menon V, Wang X, Sarnak MJ, et al. Long-term outcomes in nondiabetic chronic kidney disease. Kidney Int 2008; 73:1310–1315.
- Kasiske BL. Hyperlipidemia in patients with chronic renal disease. Am J Kidney Dis 1998; 32(suppl 3):S142–S156.
- Kendrick J, Shlipak MG, Targher G, Cook T, Lindenfeld J, Chonchol M. Effect of lovastatin on primary prevention of cardiovascular events in mild CKD and kidney function loss: a post hoc analysis of the Air Force/Texas Coronary Atherosclerosis Prevention Study. Am J Kidney Dis 2010; 55:42–49.
- Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS Investigators. Effects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes: an analysis from the Collaborative Atorvastatin Diabetes Study (CARDS). Am J Kidney Dis 2009; 54:810–819.
- Koren MJ, Davidson MH, Wilson DJ, Fayyad RS, Zuckerman A, Reed DP; ALLIANCE Investigators. Focused atorvastatin therapy in managed-care patients with coronary heart disease and CKD. Am J Kidney Dis 2009; 53:741–750.
- Fellström BC, Jardine AG, Schmieder RE, et al; AURORA Study Group. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 2009; 360:1395–1407.
- Chonchol M, Cook T, Kjekshus J, Pedersen TR, Lindenfeld J. Simvastatin for secondary prevention of all-cause mortality and major coronary events in patients with mild chronic renal insufficiency. Am J Kidney Dis 2007; 49:373–382.
- Ridker PM, MacFadyen J, Cressman M, Glynn RJ. Efficacy of rosuvastatin among men and women with moderate chronic kidney disease and elevated high-sensitivity C-reactive protein: a secondary analysis from the JUPITER (Justification for the Use of Statins in Prevention-an Intervention Trial Evaluating Rosuvastatin) trial. J Am Coll Cardiol 2010; 55:1266–1273.
- Baigent C, Landray MJ, Reith C, et al; SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011; 377:2181–2192.
- Shepherd J, Kastelein JJ, Bittner V, et al; Treating to New Targets Investigators. Effect of intensive lipid lowering with atorvastatin on renal function in patients with coronary heart disease: the Treating to New Targets (TNT) study. Clin J Am Soc Nephrol 2007; 2:1131–1139.
- Tonelli M, Isles C, Craven T, et al. Effect of pravastatin on rate of kidney function loss in people with or at risk for coronary disease. Circulation 2005; 112:171–178.
- Palmer SC, Craig JC, Navaneethan SD, Tonelli M, Pellegrini F, Strippoli GF. Benefits and harms of statin therapy for persons with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med 2012; 157:263–275.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Managing Dyslipidemias in Chronic Kidney Disease. http://www.kidney.org/professionals/kdoqi/guidelines_lipids/. Accessed March 5, 2014.
- Buckalew VM, Berg RL, Wang SR, Porush JG, Rauch S, Schulman G. Prevalence of hypertension in 1,795 subjects with chronic renal disease: the modification of diet in renal disease study baseline cohort. Modification of Diet in Renal Disease Study Group. Am J Kidney Dis 1996; 28:811–821.
- Coresh J, Wei GL, McQuillan G, et al. Prevalence of high blood pressure and elevated serum creatinine level in the United States: findings from the third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med 2001; 161:1207–1216.
- Klag MJ, Whelton PK, Randall BL, et al. Blood pressure and end-stage renal disease in men. N Engl J Med 1996; 334:13–18.
- Locatelli F, Marcelli D, Comelli M, et al. Proteinuria and blood pressure as causal components of progression to end-stage renal failure. Northern Italian Cooperative Study Group. Nephrol Dial Transplant 1996; 11:461–467.
- ACCORD Study Group; Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- Jafar TH, Stark PC, Schmid CH, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med 2003; 139:244–252.
- Khosla N, Bakris G. Lessons learned from recent hypertension trials about kidney disease. Clin J Am Soc Nephrol 2006; 1:229–235.
- Norris KC, Greene T, Kopple J, et al. Baseline predictors of renal disease progression in the African American Study of Hypertension and Kidney Disease. J Am Soc Nephrol 2006; 17:2928–2936.
- Keane WF, Brenner BM, de Zeeuw D, et al; RENAAL Study Investigators. The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: the RENAAL study. Kidney Int 2003; 63:1499–1507.
- Ruggenenti P, Perna A, Mosconi L, et al. Proteinuria predicts end-stage renal failure in non-diabetic chronic nephropathies. The “Gruppo Italiano di Studi Epidemiologici in Nefrologia” (GISEN). Kidney Int Suppl 1997; 63:S54–S57.
- de Goeij MC, Liem M, de Jager DJ, et al; PREPARE-1 Study Group. Proteinuria as a risk marker for the progression of chronic kidney disease in patients on predialysis care and the role of angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker treatment. Nephron Clin Pract 2012; 121:c73–c82.
- de Zeeuw D, Remuzzi G, Parving HH, et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation 2004; 110:921–927.
- Ibsen H, Olsen MH, Wachtell K, et al. Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: losartan intervention for endpoint reduction in hypertension study. Hypertension 2005; 45:198–202.
- Atkins RC, Briganti EM, Lewis JB, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis 2005; 45:281–287.
- Jafar TH, Stark PC, Schmid CH, et al; AIPRD Study Group; Angiotensin-Converting Enzyme Inhibition and Progression of Renal Disease. Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int 2001; 60:1131–1140.
- ACE Inhibitors in Diabetic Nephropathy Trialist Group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors? A meta-analysis of individual patient data. Ann Intern Med 2001; 134:370–379.
- Casas JP, Chua W, Loukogeorgakis S, et al. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet 2005; 366:2026–2033.
- Strippoli GF, Craig M, Deeks JJ, Schena FP, Craig JC. Effects of angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists on mortality and renal outcomes in diabetic nephropathy: systematic review. BMJ 2004; 329:828.
- MacKinnon M, Shurraw S, Akbari A, Knoll GA, Jaffey J, Clark HD. Combination therapy with an angiotensin receptor blocker and an ACE inhibitor in proteinuric renal disease: a systematic review of the efficacy and safety data. Am J Kidney Dis 2006; 48:8–20.
- Kunz R, Friedrich C, Wolbers M, Mann JF. Meta-analysis: effect of mono-therapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med 2008; 148:30–48.
- Ruggenenti P, Perticucci E, Cravedi P, et al. Role of remission clinics in the longitudinal treatment of CKD. J Am Soc Nephrol 2008; 19:1213–1224.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Esnault VL, Ekhlas A, Delcroix C, Moutel MG, Nguyen JM. Diuretic and enhanced sodium restriction results in improved antiproteinuric response to RAS blocking agents. J Am Soc Nephrol 2005; 16:474–481.
- Vogt L, Waanders F, Boomsma F, de Zeeuw D, Navis G. Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J Am Soc Nephrol 2008; 19:999–1007.
- Ahmed AK, Kamath NS, El Kossi M, El Nahas AM. The impact of stopping inhibitors of the renin-angiotensin system in patients with advanced chronic kidney disease. Nephrol Dial Transplant 2010; 25:3977–3982.
- Bakris GL, Weir MR, Secic M, Campbell B, Weis-McNulty A. Differential effects of calcium antagonist subclasses on markers of nephropathy progression. Kidney Int 2004; 65:1991–2002.
- Kloke HJ, Wetzels JF, Koene RA, Huysmans FT. Effects of low-dose nifedipine on urinary protein excretion rate in patients with renal disease. Nephrol Dial Transplant 1998; 13:646–650.
- Shah SN, Abramowitz M, Hostetter TH, Melamed ML. Serum bicarbonate levels and the progression of kidney disease: a cohort study. Am J Kidney Dis 2009; 54:270–277.
- de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 2009; 20:2075–2084.
- Susantitaphong P, Sewaralthahab K, Balk EM, Jaber BL, Madias NE. Short- and long-term effects of alkali therapy in chronic kidney disease: a systematic review. Am J Nephrol 2012; 35:540–547.
- Nath KA, Hostetter MK, Hostetter TH. Ammonia-complement interaction in the pathogenesis of progressive renal injury. Kidney Int Suppl 1989; 27:S52–S54.
- Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis 2000; 35(suppl 2):S1–S140.
- Shimada T, Yamazaki Y, Takahashi M, et al. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol 2005; 289:F1088–F1095.
- Hasegawa H, Nagano N, Urakawa I, et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int 2010; 78:975–980.
- de Boer IH, Rue TC, Kestenbaum B. Serum phosphorus concentrations in the third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis 2009; 53:399–407.
- Kendrick J, Cheung AK, Kaufman JS, et al; HOST Investigators. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol 2011; 22:1913–1922.
- Palmer SC, Hayen A, Macaskill P, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA. 2011; 305:1119–1127.
- Kooienga L, Fried L, Scragg R, Kendrick J, Smits G, Chonchol M. The effect of combined calcium and vitamin D3 supplementation on serum intact parathyroid hormone in moderate CKD. Am J Kidney Dis 2009; 53:408–416.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease. www.kidney.org/professionals/kdoqi/guidelines_bone/guide1.htm#table15. Accessed March 5, 2014.
- Kidney International. KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). http://kdigo.org/home/mineral-bone-disorder. Accessed March 5, 2014.
- Kazmi WH, Kausz AT, Khan S, et al. Anemia: an early complication of chronic renal insufficiency. Am J Kidney Dis 2001; 38:803–812.
- Sarnak MJ, Tighiouart H, Manjunath G, et al. Anemia as a risk factor for cardiovascular disease in the Atherosclerosis Risk in Communities (ARIC) study. J Am Coll Cardiol 2002; 40:27–33.
- Thorp ML, Johnson ES, Yang X, Petrik AF, Platt R, Smith DH. Effect of anaemia on mortality, cardiovascular hospitalizations and end-stage renal disease among patients with chronic kidney disease. Nephrology (Carlton) 2009; 14:240–246.
- Mircescu G, Gârneata L, Capusa C, Ursea N. Intravenous iron supplementation for the treatment of anaemia in pre-dialyzed chronic renal failure patients. Nephrol Dial Transplant 2006; 21:120–124.
- Silverberg DS, Iaina A, Peer G, et al. Intravenous iron supplementation for the treatment of the anemia of moderate to severe chronic renal failure patients not receiving dialysis. Am J Kidney Dis 1996; 27:234–238.
- Singh AK, Szczech L, Tang KL, et al; CHOIR Investigators. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355:2085–2098.
- Drüeke TB, Locatelli F, Clyne N, et al; CREATE Investigators. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355:2071–2084.
- Pfeffer MA, Burdmann EA, Chen CY, et al; TREAT Investigators. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361:2019–2032.
- US Food and Drug Administration (FDA). FDA Drug Safety Communication: modified dosing recommendations to improve the safe use of erythropoiesis-stimulating agents (ESAs) in chronic kidney disease. http://www.fda.gov/drugs/drugsafety/ucm259639.htm. Accessed March 5, 2014.
- Kurella M, Covinsky KE, Collins AJ, Chertow GM. Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med 2007; 146:177–183.
- Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 2009; 361:1539–1547.
- Renal Physicians Association. Clinical Practice Guideline. Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis. 2nd ed.
- Vollmer WM, Wahl PW, Blagg CR. Survival with dialysis and transplantation in patients with end-stage renal disease. N Engl J Med 1983; 308:1553–1558.
- Port FK, Wolfe RA, Mauger EA, Berling DP, Jiang K. Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA 1993; 270:1339–1343.
- Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999; 341:1725–1730.
- Cosio FG, Alamir A, Yim S, et al. Patient survival after renal transplantation: I. The impact of dialysis pre-transplant. Kidney Int 1998; 53:767–772.
- Meier-Kriesche HU, Port FK, Ojo AO, et al. Effect of waiting time on renal transplant outcome. Kidney Int 2000; 58:1311–1317.
- Mange KC, Joffe MM, Feldman HI. Effect of the use or nonuse of long-term dialysis on the subsequent survival of renal transplants from living donors. N Engl J Med 2001; 344:726–731.
- Dhingra RK, Young EW, Hulbert-Shearon TE, Leavey SF, Port FK. Type of vascular access and mortality in US hemodialysis patients. Kidney Int 2001; 60:1443–1451.
- Nassar GM, Ayus JC. Infectious complications of the hemodialysis access. Kidney Int 2001; 60:1–13.
- Perera GB, Mueller MP, Kubaska SM, Wilson SE, Lawrence PF, Fujitani RM. Superiority of autogenous arteriovenous hemodialysis access: maintenance of function with fewer secondary interventions. Ann Vasc Surg 2004; 18:66–73.
- Basile C, Casucci F, Lomonte C. Timing of first cannulation of arteriovenous fistula: time matters, but there is also something else. Nephrol Dial Transplant 2005; 20:1519–1520.
- Biuckians A, Scott EC, Meier GH, Panneton JM, Glickman MH. The natural history of autologous fistulas as first-time dialysis access in the KDOQI era. J Vasc Surg 2008; 47:415–421.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Vascular Access. http://www.kidney.org/professionals/KDOQI/guideline_upHD_PD_VA/va_guide1.htm. Accessed March 5, 2014.
- Arora P, Obrador GT, Ruthazer R, et al. Prevalence, predictors, and consequences of late nephrology referral at a tertiary care center. J Am Soc Nephrol 1999; 10:1281–1286.
- Gøransson LG, Bergrem H. Consequences of late referral of patients with end-stage renal disease. J Intern Med 2001; 250:154–159.
- Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med 2010; 363:609–619.
- Carson RC, Juszczak M, Davenport A, Burns A. Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin J Am Soc Nephrol 2009; 4:1611–1619.
- Murtagh FE, Marsh JE, Donohoe P, Ekbal NJ, Sheerin NS, Harris FE. Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant 2007; 22:1955–1962.
Accountable-care organizations are becoming more prominent in the United States, and therefore health care systems in the near future will be reimbursed on the basis of their ability to care for patient populations rather than individual patients. As a result, primary care physicians will need to be well versed in the care of patients with common chronic diseases such as chronic kidney disease (CKD). By one estimate, patients with CKD constitute 14% of the US population age 20 and older, or more than 31 million people.1
An earlier article in this journal reviewed how to identify patients with CKD and how to interpret the estimated glomerular filtration rate (GFR).2 This article examines the care of patients with advanced CKD, how to manage their health risks, and how to optimize their care by coordinating with nephrologists.
GOALS OF CKD CARE
CKD is defined either as renal damage (which is most commonly manifested by proteinuria, but which may include pathologic changes on biopsy or other markers of damage on serum, urine, or imaging studies), or as a GFR less than 60 mL/min/1.73 m2 for at least 3 months.3 It is divided into five stages (Table 1).
Since most patients with CKD never reach end-stage renal disease, much of their care is aimed at slowing the progression of renal dysfunction and addressing medical issues that arise as a result of CKD. To these ends, it is important to detect CKD early and refer these patients to a nephrology team in a timely manner. Their care can be separated into several important tasks:
- Identify the cause of CKD, if possible; address potentially reversible causes such as obstruction or medication-related causes. If a primarily glomerular process (marked by heavy proteinuria and dysmorphic red blood cells and red blood cell casts in the urine sediment) or interstitial nephritis (manifested by white blood cells in the urine) is suspected, refer to a nephrologist early.
- Provide treatment to correct the specific cause (if one is present) or slow the deterioration of renal function.
- Address cardiovascular risk factors.
- Address metabolic abnormalities related to CKD.
- If the CKD is advanced, educate the patient about end-stage renal disease and its treatment options, and guide the patient through the transition to end-stage renal disease.
WHEN SHOULD A NEPHROLOGIST BE CONSULTED?
The ideal timing of referral to a nephrologist is not well defined and depends on the comfort level of the primary care provider.
Treatments to slow the progression of CKD and decrease cardiovascular risk should begin early in CKD (ie, in stage 3) and can be managed by the primary care provider with guidance from a nephrologist. Patients referred to a nephrologist while in stage 3 have been shown to go longer without CKD progression than those referred in later stages.4 Early referral to a nephrologist has also been associated with a decreased mortality rate.5 The studies that found these trends, however, were limited by the fact that patients with stage 3 CKD are less likely to progress to end-stage renal disease or to die of cardiovascular disease than patients with stage 4 or 5 CKD.
Once stage 4 CKD develops, the nephrologist should take a more active role in the care plan. In this stage, cardiovascular risk rises, and the risk of developing end-stage renal disease rises dramatically.6 With comprehensive care in a CKD clinic, even patients with advanced CKD are more likely to have a stabilization of renal function.7 Kinchen et al8 found that patients referred to a nephrologist within 4 months of starting dialysis had a lower survival rate than those referred earlier. Therefore, if a nephrologist was not involved in the patient’s care prior to stage 4, then a referral must be made.
Recommendation. Patients with stage 3 CKD can be referred for an initial evaluation and development of a treatment plan, but most of the responsibility for their care can remain with the primary care provider. Once stage 4 CKD develops, the nephrologist should assume an increasing role. However, if glomerular disease is suspected, we recommend referral to a nephrologist regardless of the estimated GFR.
ELEVATED CARDIOVASCULAR RISK
Patients with stage 3 CKD are 20 times more likely to die of a cardiovascular event than to reach end-stage renal disease.6 This increased risk does not quite reach the status of a cardiovascular disease risk equivalent, as does diabetes,9,10 but cardiovascular risk reduction should be a primary focus of care for the CKD patient.
The cardiovascular risk in part is attributed to a high prevalence of traditional cardiovascular risk factors, including diabetes mellitus, hypertension, and hyperlipidemia.11,12 About two-thirds of CKD patients have metabolic syndrome, which is a risk factor for cardiovascular disease and is associated with more rapid progression of CKD.13 In addition, renal dysfunction, proteinuria, and hyperphosphatemia are also risk factors for cardiovascular disease.14–19
The risk of death from a cardiovascular event increases as kidney function declines, with reported 5-year death rates of 19.5% in stage 2, 24.3% in stage 3, and 45.7% in stage 4 CKD. However, imbalance between mortality risk and progression to end-stage renal disease may be age-dependent.20 Younger patients (age 45 and younger) are more likely to progress to end-stage renal disease, whereas in older patients (over age 65), the relative risk of dying of cardiovascular disease is higher.
Aggressive lipid management
Hyperlipidemia is a common risk factor for cardiovascular morbidity and mortality in CKD.21 However, until recently, all studies of outcomes of patients treated for hyperlipidemia excluded patients with CKD. Post hoc analyses of these studies 22–27 showed statins to be beneficial in primary and secondary cardiovascular prevention in patents with “normal” serum creatinine values but estimated GFR levels of 50 to 59 mL/min/1.73 m2.
The SHARP trial28 was the first prospective trial to study lipid-lowering therapy in patients with CKD. In this trial, patients with various stages of CKD, including advanced CKD, had fewer major vascular events if they received the combination of low-dose simvastatin (Zocor) and ezetimibe (Zetia). However, the evidence does not suggest that statin therapy slows the progression of CKD.28–31
Recommendation. Manage hyperlipidemia aggressively using statin therapy with or without ezetimibe, with a target low-density lipoprotein cholesterol level below 100 mg/dL.32
Manage other cardiovascular risk factors
Because hypertension and proteinuria are risk factors not only for cardiovascular disease but also for progression of CKD, they are discussed in the section below.
ATTEMPT TO PREVENT WORSENING OF RENAL FUNCTION
Medications to avoid
It is important to review a CKD patient’s medication list—prescription and over-the-counter drugs—to identify any that may contribute to a worsening of renal function. CKD patients need to be informed about avoiding medications such as nonsteroidal anti-inflammatory drugs, proton pump inhibitors, and herbal supplements because they can cause further renal injury. In addition, other medications (eg, metformin) are contraindicated in CKD because of side effects that may occur in CKD.
Patients should be encouraged to discuss any changes in their medications, including over-the-counter products, with their primary care physicians.
Manage hypertension aggressively
Many patients with CKD also have hypertension,33,34 possibly because they have a higher frequency of underlying essential hypertension or because CKD often worsens preexisting hypertension. Moreover, uncontrolled hypertension is associated with a further decline in renal function.35,36
The ACCORD trial37 found no benefit in lowering systolic blood pressure to less than 120 mm Hg compared with less than 140 mm Hg in patients with diabetes mellitus. (The patients in this study did not necessarily have CKD.)
A meta-analysis38 of trials of antihypertensive treatment in patients with CKD found that the optimal target systolic blood pressure for decreasing the progression of CKD was 110 to 129 mm Hg. The relative risk of progression of renal dysfunction was:
- 1.83 (95% confidence interval [CI] 0.97–3.44) at 130 mm to 139 mm Hg, vs
- 3.14 (95% CI 1.64–5.99) at 160 mm Hg or higher.
There is also evidence that blood pressure control can be relaxed as patients age. While the exact age differs among published guidelines, the evidence supports a goal blood pressure of less than 150/90 mm Hg once a patient reaches the age of 70, regardless of CKD or proteinuria.
Recommendation. Current evidence suggests the following blood pressure goals in CKD patients:
- With diabetes mellitus or proteinuria: < 130/80 mm Hg
- Without proteinuria: < 140/90 mm Hg
- Age 70 and older: <150/90 mm Hg.39
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) are the preferred antihypertensive drugs in patients with diabetes or proteinuria (see below).
Manage proteinuria
Proteinuria is also associated with progression of CKD. AASK,40 a study that included nondiabetic African American patients whose estimated GFRs were between 20 and 60 mL/min/1.73 m2, showed that higher levels of proteinuria were associated with a higher risk of decline in GFR and a higher risk of end-stage renal disease. Findings were similar to those in studies of other CKD populations.41–43 Proteinuria is also an independent risk factor for cardiovascular disease and death. Multiple large studies16,17,44,45 have found associations between higher levels of albumin excretion and risk of major cardiovascular events, cardiovascular death, and death from any cause in people with and without diabetes.
Reducing proteinuria has been shown to both slow progression of renal dysfunction and reduce the cardiovascular risk.44,45 In a substudy of the IDNT46 in patients with diabetic nephropathy, each 50% reduction in urinary protein excretion was associated with a 56% reduction in risk of progression of CKD. Similar effects have been shown in nondiabetic CKD patients.47
ACE inhibitors and ARBs are the preferred treatments for proteinuria in patients with CKD.48–50 Combination therapy with an ACE inhibitor and an ARB has been used,51–53 with a better response in proteinuria reduction. However, combination therapy with these drugs cannot currently be recommended, as the only prospective study of this regimen to date suggested worse renal and overall outcomes in patients at high cardiovascular risk.54 These drugs may also have renoprotective effects independent of their effects on blood pressure and proteinuria.38 Dietary salt restriction and diuretic therapy can further increase the efficacy of proteinuria reduction by ACE inhibitors or ARBs.55,56
On the other hand, stopping ACE inhibitors or ARBs may be beneficial as the patient nears end-stage renal disease. Ahmed et al57 demonstrated that stopping ACE inhibitors or ARBs in advanced stage 4 CKD (mean estimated GFR 16 mL/min/1.73 m2) was associated with improved GFR and delayed onset of renal replacement therapy. This improvement may be due to regaining the slight decrease in GFR that occurred when these medications were started.
Nondihydropyridine calcium channel blockers such as diltiazem (Cardizem) and verapamil (Calan) have also been shown to be useful for reducing proteinuria,58 whereas dihydropyridine calcium channel blockers such as amlodipine (Norvasc) and nifedipine (Procardia), when used without ACE inhibitors or ARBs, can worsen proteinuria.58,59
Correct metabolic acidosis
The kidneys play an important role in maintaining acid-base balance, keeping the blood from becoming too acidic both by reabsorbing bicarbonate filtered into the urine by the glomerulus and by excreting the daily acid load. Metabolic acidosis can develop when these functions break down at more advanced stages of CKD, most often when the estimated GFR declines to less than 20 mL/min/1.73 m2.
Bicarbonate levels of 22 mmol/L or less have been associated with a higher risk of worsening renal function.60 When such patients were treated with sodium bicarbonate to achieve a serum bicarbonate of at least 23 mmol/L, they had an 80% lower rate of progression to end-stage renal disease without any increase in edema, admission for congestive heart failure, or change in blood pressure.61
Susantitaphong et al62 reviewed six randomized trials of bicarbonate supplementation in CKD and found that it was associated with improved kidney function and a 79% lower rate of progression to end-stage renal disease.
The proposed mechanism behind this benefit lies in the increase in ammonia production that each surviving nephron must undertake to handle the daily acid load. The increased ammonia is thought to play a role in activating the alternative complement pathway,63 causing renal inflammation and injury.
Recommendation. Bicarbonate therapy should be used to maintain serum bicarbonate levels above 22 mmol/L in CKD.64
OTHER ASPECTS OF CKD CARE
Bone mineral disorders
Patients with CKD develop secondary hyperparathyroidism, hyperphosphatemia, and (in advanced CKD) hypocalcemia, all leading to disorders of bone mineral metabolism.
Traditionally, it has been thought that decreased production of 1,25-dihydroxyvitamin D by dysfunctional kidneys leads to decreased suppression of the parathyroid gland and to secondary hyperparathyroidism. The major long-term adverse effect of this is a weakened bone matrix resulting from increased calcium and phosphorus efflux from bones (renal osteodystrophy).
The discovery of fibroblast growth factor 23 (FGF-23) has improved our understanding of the physiology behind disordered bone mineral metabolism in CKD. FGF-23, produced by osteoblasts and osteocytes, acts directly on the kidney to increase renal phosphate excretion. It also suppresses 1,25-dihydroxyvitamin D levels by inhibiting 1-alpha-hydroxylase,65 and it stimulates parathyroid hormone secretion. FGF-23 levels rise much earlier in CKD than do parathyroid hormone levels, suggesting that abnormalities in phosphorus balance and FGF-23 may be the earliest pathophysiologic changes.66
The initial treatment of bone mineral disorders is to some extent guided by laboratory values. Phosphate levels higher than 3.5 or 4 mg/dL and elevated FGF-23 levels have been associated with increased mortality rates in CKD patients.18,19,67–69 All patients should also have their 1,25-dihydroxyvitamin D level checked and supplemented if deficient. In many patients with early stage 3 CKD, this may correct secondary hyperparathyroidism.70
Serum phosphorus levels should be kept in the normal range in stage 3 and 4 CKD,71 either by restricting dietary phosphorus intake (< 800 or < 1,000 mg/day) or by using a phosphate binder, which is taken with meals to prevent phosphorus absorption from the gastrointestinal tract. Current US recommendations are to allow graded increases in parathyroid hormone based on the stage of CKD (Table 2).71 However, these targets are still an area of uncertainty, with some guidelines suggesting that wider variations in parathyroid hormone can be allowed, so there may be wider variation in clinical practice in this area.72 If the serum phosphorus level is in the goal range but parathyroid hormone levels are still high, an activated vitamin D analogue such as calcitriol is recommended, although with the emerging role of FGF-23, some experts also call for early use of a phosphate binder in this group.
The treatment of bone mineral disorders in CKD is fairly complex, and we recommend that it be done by or with the close direction of a nephrologist.
Recommendations on bone disorders
- Check levels of calcium, phosphorus, 25-hydroxyvitamin D, and parathyroid hormone in all patients whose estimated GFR is less than 60 mL/min/1.73 m2, with frequency of measurements based on the stage of CKD.71
- Replace vitamin D if deficient.
- Treat elevated phosphorus levels with a protein-restricted diet (nutrition referral) and a phosphate binder.
- Treat elevated hyperparathyroid hormone levels with a vitamin D analogue once phosphorus levels have been controlled.
- Refer patients with an elevated phosphorus or parathyroid hormone level to a nephrology service for consultation before initiating medical therapy.
Anemia is common, treatment controversial
The treatment of anemia attributed to CKD has been a topic of controversy over the past decade, and we recommend that it be done with the guidance of a nephrologist.
Anemia is common in CKD, and declining kidney function is an independent predictor of anemia.73 Anemia is a risk factor for left ventricular hypertrophy, cardiovascular disease,74 and death in CKD.75
The anemia of CKD is attributed to relative erythropoietin deficiency and bone marrow resistance to erythropoietin, but this is a diagnosis of exclusion, and other causes of anemia must be ruled out. Iron deficiency is a common cause of anemia in CKD, and treatment of iron deficiency may correct anemia in more than one-third of these patients.76,77
Erythropoiesis-stimulating agents such as epoetin alfa (Procrit) and darbepoetin (Aranesp) are used to treat renal anemia. However, the target hemoglobin level has been a subject of debate. Three prospective trials78–80 found no benefit in raising the hemoglobin level to normal ranges using these agents, and several found an association with higher rates of stroke and venous thrombosis. The US Food and Drug Administration suggests that the only role for these agents in CKD is to avoid the need for transfusions. They should not be used to normalize the hemoglobin level. The target, although not explicitly specified, is suggested to be around 10 g/dL.81
PREPARE FOR END-STAGE RENAL DISEASE
Discuss the options
Because the risk of developing end-stage renal disease rises dramatically once CKD reaches stage 4, all such patients should have a discussion about renal replacement therapy. They should be educated about their options for treatment (hemodialysis, peritoneal dialysis, and transplantation, as well as not proceeding with renal replacement therapy), often in a formal class. They should then be actively engaged in the decision about how to proceed. Survival and quality of life should be discussed, particularly with patients who are over age 80, who are severely ill, or who are living in a nursing facility, as these groups get limited survival benefit from starting dialysis, and quality of life may actually decrease with dialysis.82,83
The Renal Physicians Association has created clinical practice guidelines for shared decision-making, consisting of 10 practice recommendations that outline a systematic approach to patients needing renal replacement therapy.84
Consider preemptive kidney transplantation
Any patient thought to be a suitable candidate for renal transplantation should be referred to a transplantation center for evaluation. Studies have shown that kidney transplantation offers a survival advantage compared with chronic dialysis and should preferably be done preemptively, ie, before dialysis is required.85–90 Therefore, patients with estimated GFRs in the low 20s should be referred for a transplantation evaluation.
If a living donor is available, the transplantation team usually waits to perform the procedure until the patient is closer to needing dialysis, often when the estimated GFR is around 15 to 16 mL/min/1.73 m2. If no living donor is available, the patient can earn time on the deceased-donor waiting list once his or her estimated GFR falls to below 20 mL/min/1.7 m2.
Plan for dialysis access
Patients starting hemodialysis first need to undergo a procedure to provide access to the blood. The three options are an arteriovenous fistula, an arteriovenous graft, and a central venous catheter (Figure 1).
An arteriovenous fistula is the best option, being the most durable, followed by a graft and then a catheter.91 Arteriovenous fistulas also have the lowest rates of infection,92 thrombosis,93 and intervention to maintain patency.93
The fistula is created by ligating a vein draining an extremity, most often the nondominant arm, and anastomosing the vein to an artery. The higher arterial pressure causes the vein to dilate and thicken (“arterialize”), thus making it able to withstand repeated cannulation necessary for hemodialysis.
An arteriovenous fistula typically takes 1 to 3 months to “mature” to the point where it can be used,94,95 and, depending on the patient and experience of the vascular surgeon, a significant number may never mature. Thus, it is important to discuss hemodialysis access before the patient reaches end-stage renal disease so that he or she can be referred to a vascular surgeon early, when the estimated GFR is about 20 mL/min/1.73 m2.
An arteriovenous graft. Not all patients have suitable vessels for creation of an arteriovenous fistula. In such patients, an arteriovenous graft, typically made of polytetrafluoroethylene, is the next best option. The graft is typically ready to use in 2 weeks and thus does not require as much advance planning. Grafts tend to narrow more often than fistulas and require more procedures to keep them patent.
A central venous catheter is most often inserted into the internal jugular vein and tunneled under the skin to exit in an area covered by the patient’s shirt.
Tunneled dialysis catheters are associated with higher rates of infection, thrombosis, and overall mortality and are therefore the least preferred choice. They are reserved for patients who have not had advance planning for end-stage renal disease, who do not have acceptable vessels for an arteriovenous fistula or graft, or who have refused surgical access.
Protect the fistula arm. It is recommended that venipuncture, intravenous lines, and blood pressure measurements be avoided in the nondominant upper arm of patients with stage 4 and 5 CKD to protect those veins for the potential creation of an arteriovenous fistula.96 For the same reason, peripherally inserted central catheter lines and subclavian catheters should be avoided in these patients. If an arteriovenous fistula has already been placed, this arm must be protected from such procedures at all times.
Studies have shown that late referral to a nephrologist is associated with a lower incidence of starting dialysis with a permanent vascular access.97,98
If the patient wishes to start peritoneal dialysis, the peritoneal dialysis catheter can usually be used 2 weeks after being inserted.
Starting dialysis
The appropriate time for starting dialysis remains controversial, especially in elderly patients with multiple comorbid conditions.
The IDEAL study99 found no benefit in starting dialysis at a GFR of 10 to 14 mL/min compared with 5 to 7 mL/min. Thus, there is no single estimated GFR at which dialysis should be started. Rather, the development of early uremic symptoms and the patient’s quality of life should guide this decision.82,83,99–101
Hemodialysis involves three sessions per week, each taking about 4 hours. Evidence suggests that longer sessions or more sessions per week may offer benefits, especially in terms of blood pressure, volume, and dietary management. This has led to an increase in the popularity of home and in-center nocturnal hemodialysis programs across the United States.
Peritoneal dialysis?
Peritoneal dialysis is an excellent choice for patients who are motivated, can care for themselves at home, and have a support system available to assist them if needed. It allows for daily dialysis, less fluid restriction, and less dietary restriction, and it gives the patient an opportunity to stay independent. It also spares the veins in the arms, which may be needed for vascular access later in life if hemodialysis is needed.
Recommendation. We recommend that peritoneal dialysis be offered to any suitable patient who is approaching end-stage renal disease.
A COMPREHENSIVE, COLLABORATIVE APPROACH
Chronic kidney disease is a multisystem disorder, and its management requires a comprehensive approach (Table 3). Early detection and interventions are key to reducing cardiovascular events and progression to kidney failure.
Early referral to a nephrologist and team collaboration between the primary care provider, the nephrologist, and other health care providers are essential. Early in the course of CKD, it may be appropriate for a nephrologist to evaluate the patient and recommend a set of treatment goals. Follow-up may be infrequent or unnecessary.
As CKD progresses, especially as the patient reaches an estimated GFR of 30 mL/min/1.73 m2, the nephrologist will take a more active role in the patient’s care and medical decision-making. In some circumstances, it may even be appropriate for the nephrologist to be the patient’s source of primary care, with the primary care provider as a consultant.
Caring for patients with CKD includes not only strategies to preserve renal function and prolong survival, but also making critical decisions about starting dialysis and about the need for transplantation. Early involvement of a nephrologist and early preparation for end-stage renal disease with preemptive transplantation and arteriovenous fistula placement are associated with better patient outcomes. Key to this is collaboration between the primary care provider and the nephrologist, with levels of responsibility for patient care that adapt to the patient’s degree of renal dysfunction and other comorbidities. Such strategies to select patients for timely nephrology referral may help improve outcomes in this vulnerable population.
Accountable-care organizations are becoming more prominent in the United States, and therefore health care systems in the near future will be reimbursed on the basis of their ability to care for patient populations rather than individual patients. As a result, primary care physicians will need to be well versed in the care of patients with common chronic diseases such as chronic kidney disease (CKD). By one estimate, patients with CKD constitute 14% of the US population age 20 and older, or more than 31 million people.1
An earlier article in this journal reviewed how to identify patients with CKD and how to interpret the estimated glomerular filtration rate (GFR).2 This article examines the care of patients with advanced CKD, how to manage their health risks, and how to optimize their care by coordinating with nephrologists.
GOALS OF CKD CARE
CKD is defined either as renal damage (which is most commonly manifested by proteinuria, but which may include pathologic changes on biopsy or other markers of damage on serum, urine, or imaging studies), or as a GFR less than 60 mL/min/1.73 m2 for at least 3 months.3 It is divided into five stages (Table 1).
Since most patients with CKD never reach end-stage renal disease, much of their care is aimed at slowing the progression of renal dysfunction and addressing medical issues that arise as a result of CKD. To these ends, it is important to detect CKD early and refer these patients to a nephrology team in a timely manner. Their care can be separated into several important tasks:
- Identify the cause of CKD, if possible; address potentially reversible causes such as obstruction or medication-related causes. If a primarily glomerular process (marked by heavy proteinuria and dysmorphic red blood cells and red blood cell casts in the urine sediment) or interstitial nephritis (manifested by white blood cells in the urine) is suspected, refer to a nephrologist early.
- Provide treatment to correct the specific cause (if one is present) or slow the deterioration of renal function.
- Address cardiovascular risk factors.
- Address metabolic abnormalities related to CKD.
- If the CKD is advanced, educate the patient about end-stage renal disease and its treatment options, and guide the patient through the transition to end-stage renal disease.
WHEN SHOULD A NEPHROLOGIST BE CONSULTED?
The ideal timing of referral to a nephrologist is not well defined and depends on the comfort level of the primary care provider.
Treatments to slow the progression of CKD and decrease cardiovascular risk should begin early in CKD (ie, in stage 3) and can be managed by the primary care provider with guidance from a nephrologist. Patients referred to a nephrologist while in stage 3 have been shown to go longer without CKD progression than those referred in later stages.4 Early referral to a nephrologist has also been associated with a decreased mortality rate.5 The studies that found these trends, however, were limited by the fact that patients with stage 3 CKD are less likely to progress to end-stage renal disease or to die of cardiovascular disease than patients with stage 4 or 5 CKD.
Once stage 4 CKD develops, the nephrologist should take a more active role in the care plan. In this stage, cardiovascular risk rises, and the risk of developing end-stage renal disease rises dramatically.6 With comprehensive care in a CKD clinic, even patients with advanced CKD are more likely to have a stabilization of renal function.7 Kinchen et al8 found that patients referred to a nephrologist within 4 months of starting dialysis had a lower survival rate than those referred earlier. Therefore, if a nephrologist was not involved in the patient’s care prior to stage 4, then a referral must be made.
Recommendation. Patients with stage 3 CKD can be referred for an initial evaluation and development of a treatment plan, but most of the responsibility for their care can remain with the primary care provider. Once stage 4 CKD develops, the nephrologist should assume an increasing role. However, if glomerular disease is suspected, we recommend referral to a nephrologist regardless of the estimated GFR.
ELEVATED CARDIOVASCULAR RISK
Patients with stage 3 CKD are 20 times more likely to die of a cardiovascular event than to reach end-stage renal disease.6 This increased risk does not quite reach the status of a cardiovascular disease risk equivalent, as does diabetes,9,10 but cardiovascular risk reduction should be a primary focus of care for the CKD patient.
The cardiovascular risk in part is attributed to a high prevalence of traditional cardiovascular risk factors, including diabetes mellitus, hypertension, and hyperlipidemia.11,12 About two-thirds of CKD patients have metabolic syndrome, which is a risk factor for cardiovascular disease and is associated with more rapid progression of CKD.13 In addition, renal dysfunction, proteinuria, and hyperphosphatemia are also risk factors for cardiovascular disease.14–19
The risk of death from a cardiovascular event increases as kidney function declines, with reported 5-year death rates of 19.5% in stage 2, 24.3% in stage 3, and 45.7% in stage 4 CKD. However, imbalance between mortality risk and progression to end-stage renal disease may be age-dependent.20 Younger patients (age 45 and younger) are more likely to progress to end-stage renal disease, whereas in older patients (over age 65), the relative risk of dying of cardiovascular disease is higher.
Aggressive lipid management
Hyperlipidemia is a common risk factor for cardiovascular morbidity and mortality in CKD.21 However, until recently, all studies of outcomes of patients treated for hyperlipidemia excluded patients with CKD. Post hoc analyses of these studies 22–27 showed statins to be beneficial in primary and secondary cardiovascular prevention in patents with “normal” serum creatinine values but estimated GFR levels of 50 to 59 mL/min/1.73 m2.
The SHARP trial28 was the first prospective trial to study lipid-lowering therapy in patients with CKD. In this trial, patients with various stages of CKD, including advanced CKD, had fewer major vascular events if they received the combination of low-dose simvastatin (Zocor) and ezetimibe (Zetia). However, the evidence does not suggest that statin therapy slows the progression of CKD.28–31
Recommendation. Manage hyperlipidemia aggressively using statin therapy with or without ezetimibe, with a target low-density lipoprotein cholesterol level below 100 mg/dL.32
Manage other cardiovascular risk factors
Because hypertension and proteinuria are risk factors not only for cardiovascular disease but also for progression of CKD, they are discussed in the section below.
ATTEMPT TO PREVENT WORSENING OF RENAL FUNCTION
Medications to avoid
It is important to review a CKD patient’s medication list—prescription and over-the-counter drugs—to identify any that may contribute to a worsening of renal function. CKD patients need to be informed about avoiding medications such as nonsteroidal anti-inflammatory drugs, proton pump inhibitors, and herbal supplements because they can cause further renal injury. In addition, other medications (eg, metformin) are contraindicated in CKD because of side effects that may occur in CKD.
Patients should be encouraged to discuss any changes in their medications, including over-the-counter products, with their primary care physicians.
Manage hypertension aggressively
Many patients with CKD also have hypertension,33,34 possibly because they have a higher frequency of underlying essential hypertension or because CKD often worsens preexisting hypertension. Moreover, uncontrolled hypertension is associated with a further decline in renal function.35,36
The ACCORD trial37 found no benefit in lowering systolic blood pressure to less than 120 mm Hg compared with less than 140 mm Hg in patients with diabetes mellitus. (The patients in this study did not necessarily have CKD.)
A meta-analysis38 of trials of antihypertensive treatment in patients with CKD found that the optimal target systolic blood pressure for decreasing the progression of CKD was 110 to 129 mm Hg. The relative risk of progression of renal dysfunction was:
- 1.83 (95% confidence interval [CI] 0.97–3.44) at 130 mm to 139 mm Hg, vs
- 3.14 (95% CI 1.64–5.99) at 160 mm Hg or higher.
There is also evidence that blood pressure control can be relaxed as patients age. While the exact age differs among published guidelines, the evidence supports a goal blood pressure of less than 150/90 mm Hg once a patient reaches the age of 70, regardless of CKD or proteinuria.
Recommendation. Current evidence suggests the following blood pressure goals in CKD patients:
- With diabetes mellitus or proteinuria: < 130/80 mm Hg
- Without proteinuria: < 140/90 mm Hg
- Age 70 and older: <150/90 mm Hg.39
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) are the preferred antihypertensive drugs in patients with diabetes or proteinuria (see below).
Manage proteinuria
Proteinuria is also associated with progression of CKD. AASK,40 a study that included nondiabetic African American patients whose estimated GFRs were between 20 and 60 mL/min/1.73 m2, showed that higher levels of proteinuria were associated with a higher risk of decline in GFR and a higher risk of end-stage renal disease. Findings were similar to those in studies of other CKD populations.41–43 Proteinuria is also an independent risk factor for cardiovascular disease and death. Multiple large studies16,17,44,45 have found associations between higher levels of albumin excretion and risk of major cardiovascular events, cardiovascular death, and death from any cause in people with and without diabetes.
Reducing proteinuria has been shown to both slow progression of renal dysfunction and reduce the cardiovascular risk.44,45 In a substudy of the IDNT46 in patients with diabetic nephropathy, each 50% reduction in urinary protein excretion was associated with a 56% reduction in risk of progression of CKD. Similar effects have been shown in nondiabetic CKD patients.47
ACE inhibitors and ARBs are the preferred treatments for proteinuria in patients with CKD.48–50 Combination therapy with an ACE inhibitor and an ARB has been used,51–53 with a better response in proteinuria reduction. However, combination therapy with these drugs cannot currently be recommended, as the only prospective study of this regimen to date suggested worse renal and overall outcomes in patients at high cardiovascular risk.54 These drugs may also have renoprotective effects independent of their effects on blood pressure and proteinuria.38 Dietary salt restriction and diuretic therapy can further increase the efficacy of proteinuria reduction by ACE inhibitors or ARBs.55,56
On the other hand, stopping ACE inhibitors or ARBs may be beneficial as the patient nears end-stage renal disease. Ahmed et al57 demonstrated that stopping ACE inhibitors or ARBs in advanced stage 4 CKD (mean estimated GFR 16 mL/min/1.73 m2) was associated with improved GFR and delayed onset of renal replacement therapy. This improvement may be due to regaining the slight decrease in GFR that occurred when these medications were started.
Nondihydropyridine calcium channel blockers such as diltiazem (Cardizem) and verapamil (Calan) have also been shown to be useful for reducing proteinuria,58 whereas dihydropyridine calcium channel blockers such as amlodipine (Norvasc) and nifedipine (Procardia), when used without ACE inhibitors or ARBs, can worsen proteinuria.58,59
Correct metabolic acidosis
The kidneys play an important role in maintaining acid-base balance, keeping the blood from becoming too acidic both by reabsorbing bicarbonate filtered into the urine by the glomerulus and by excreting the daily acid load. Metabolic acidosis can develop when these functions break down at more advanced stages of CKD, most often when the estimated GFR declines to less than 20 mL/min/1.73 m2.
Bicarbonate levels of 22 mmol/L or less have been associated with a higher risk of worsening renal function.60 When such patients were treated with sodium bicarbonate to achieve a serum bicarbonate of at least 23 mmol/L, they had an 80% lower rate of progression to end-stage renal disease without any increase in edema, admission for congestive heart failure, or change in blood pressure.61
Susantitaphong et al62 reviewed six randomized trials of bicarbonate supplementation in CKD and found that it was associated with improved kidney function and a 79% lower rate of progression to end-stage renal disease.
The proposed mechanism behind this benefit lies in the increase in ammonia production that each surviving nephron must undertake to handle the daily acid load. The increased ammonia is thought to play a role in activating the alternative complement pathway,63 causing renal inflammation and injury.
Recommendation. Bicarbonate therapy should be used to maintain serum bicarbonate levels above 22 mmol/L in CKD.64
OTHER ASPECTS OF CKD CARE
Bone mineral disorders
Patients with CKD develop secondary hyperparathyroidism, hyperphosphatemia, and (in advanced CKD) hypocalcemia, all leading to disorders of bone mineral metabolism.
Traditionally, it has been thought that decreased production of 1,25-dihydroxyvitamin D by dysfunctional kidneys leads to decreased suppression of the parathyroid gland and to secondary hyperparathyroidism. The major long-term adverse effect of this is a weakened bone matrix resulting from increased calcium and phosphorus efflux from bones (renal osteodystrophy).
The discovery of fibroblast growth factor 23 (FGF-23) has improved our understanding of the physiology behind disordered bone mineral metabolism in CKD. FGF-23, produced by osteoblasts and osteocytes, acts directly on the kidney to increase renal phosphate excretion. It also suppresses 1,25-dihydroxyvitamin D levels by inhibiting 1-alpha-hydroxylase,65 and it stimulates parathyroid hormone secretion. FGF-23 levels rise much earlier in CKD than do parathyroid hormone levels, suggesting that abnormalities in phosphorus balance and FGF-23 may be the earliest pathophysiologic changes.66
The initial treatment of bone mineral disorders is to some extent guided by laboratory values. Phosphate levels higher than 3.5 or 4 mg/dL and elevated FGF-23 levels have been associated with increased mortality rates in CKD patients.18,19,67–69 All patients should also have their 1,25-dihydroxyvitamin D level checked and supplemented if deficient. In many patients with early stage 3 CKD, this may correct secondary hyperparathyroidism.70
Serum phosphorus levels should be kept in the normal range in stage 3 and 4 CKD,71 either by restricting dietary phosphorus intake (< 800 or < 1,000 mg/day) or by using a phosphate binder, which is taken with meals to prevent phosphorus absorption from the gastrointestinal tract. Current US recommendations are to allow graded increases in parathyroid hormone based on the stage of CKD (Table 2).71 However, these targets are still an area of uncertainty, with some guidelines suggesting that wider variations in parathyroid hormone can be allowed, so there may be wider variation in clinical practice in this area.72 If the serum phosphorus level is in the goal range but parathyroid hormone levels are still high, an activated vitamin D analogue such as calcitriol is recommended, although with the emerging role of FGF-23, some experts also call for early use of a phosphate binder in this group.
The treatment of bone mineral disorders in CKD is fairly complex, and we recommend that it be done by or with the close direction of a nephrologist.
Recommendations on bone disorders
- Check levels of calcium, phosphorus, 25-hydroxyvitamin D, and parathyroid hormone in all patients whose estimated GFR is less than 60 mL/min/1.73 m2, with frequency of measurements based on the stage of CKD.71
- Replace vitamin D if deficient.
- Treat elevated phosphorus levels with a protein-restricted diet (nutrition referral) and a phosphate binder.
- Treat elevated hyperparathyroid hormone levels with a vitamin D analogue once phosphorus levels have been controlled.
- Refer patients with an elevated phosphorus or parathyroid hormone level to a nephrology service for consultation before initiating medical therapy.
Anemia is common, treatment controversial
The treatment of anemia attributed to CKD has been a topic of controversy over the past decade, and we recommend that it be done with the guidance of a nephrologist.
Anemia is common in CKD, and declining kidney function is an independent predictor of anemia.73 Anemia is a risk factor for left ventricular hypertrophy, cardiovascular disease,74 and death in CKD.75
The anemia of CKD is attributed to relative erythropoietin deficiency and bone marrow resistance to erythropoietin, but this is a diagnosis of exclusion, and other causes of anemia must be ruled out. Iron deficiency is a common cause of anemia in CKD, and treatment of iron deficiency may correct anemia in more than one-third of these patients.76,77
Erythropoiesis-stimulating agents such as epoetin alfa (Procrit) and darbepoetin (Aranesp) are used to treat renal anemia. However, the target hemoglobin level has been a subject of debate. Three prospective trials78–80 found no benefit in raising the hemoglobin level to normal ranges using these agents, and several found an association with higher rates of stroke and venous thrombosis. The US Food and Drug Administration suggests that the only role for these agents in CKD is to avoid the need for transfusions. They should not be used to normalize the hemoglobin level. The target, although not explicitly specified, is suggested to be around 10 g/dL.81
PREPARE FOR END-STAGE RENAL DISEASE
Discuss the options
Because the risk of developing end-stage renal disease rises dramatically once CKD reaches stage 4, all such patients should have a discussion about renal replacement therapy. They should be educated about their options for treatment (hemodialysis, peritoneal dialysis, and transplantation, as well as not proceeding with renal replacement therapy), often in a formal class. They should then be actively engaged in the decision about how to proceed. Survival and quality of life should be discussed, particularly with patients who are over age 80, who are severely ill, or who are living in a nursing facility, as these groups get limited survival benefit from starting dialysis, and quality of life may actually decrease with dialysis.82,83
The Renal Physicians Association has created clinical practice guidelines for shared decision-making, consisting of 10 practice recommendations that outline a systematic approach to patients needing renal replacement therapy.84
Consider preemptive kidney transplantation
Any patient thought to be a suitable candidate for renal transplantation should be referred to a transplantation center for evaluation. Studies have shown that kidney transplantation offers a survival advantage compared with chronic dialysis and should preferably be done preemptively, ie, before dialysis is required.85–90 Therefore, patients with estimated GFRs in the low 20s should be referred for a transplantation evaluation.
If a living donor is available, the transplantation team usually waits to perform the procedure until the patient is closer to needing dialysis, often when the estimated GFR is around 15 to 16 mL/min/1.73 m2. If no living donor is available, the patient can earn time on the deceased-donor waiting list once his or her estimated GFR falls to below 20 mL/min/1.7 m2.
Plan for dialysis access
Patients starting hemodialysis first need to undergo a procedure to provide access to the blood. The three options are an arteriovenous fistula, an arteriovenous graft, and a central venous catheter (Figure 1).
An arteriovenous fistula is the best option, being the most durable, followed by a graft and then a catheter.91 Arteriovenous fistulas also have the lowest rates of infection,92 thrombosis,93 and intervention to maintain patency.93
The fistula is created by ligating a vein draining an extremity, most often the nondominant arm, and anastomosing the vein to an artery. The higher arterial pressure causes the vein to dilate and thicken (“arterialize”), thus making it able to withstand repeated cannulation necessary for hemodialysis.
An arteriovenous fistula typically takes 1 to 3 months to “mature” to the point where it can be used,94,95 and, depending on the patient and experience of the vascular surgeon, a significant number may never mature. Thus, it is important to discuss hemodialysis access before the patient reaches end-stage renal disease so that he or she can be referred to a vascular surgeon early, when the estimated GFR is about 20 mL/min/1.73 m2.
An arteriovenous graft. Not all patients have suitable vessels for creation of an arteriovenous fistula. In such patients, an arteriovenous graft, typically made of polytetrafluoroethylene, is the next best option. The graft is typically ready to use in 2 weeks and thus does not require as much advance planning. Grafts tend to narrow more often than fistulas and require more procedures to keep them patent.
A central venous catheter is most often inserted into the internal jugular vein and tunneled under the skin to exit in an area covered by the patient’s shirt.
Tunneled dialysis catheters are associated with higher rates of infection, thrombosis, and overall mortality and are therefore the least preferred choice. They are reserved for patients who have not had advance planning for end-stage renal disease, who do not have acceptable vessels for an arteriovenous fistula or graft, or who have refused surgical access.
Protect the fistula arm. It is recommended that venipuncture, intravenous lines, and blood pressure measurements be avoided in the nondominant upper arm of patients with stage 4 and 5 CKD to protect those veins for the potential creation of an arteriovenous fistula.96 For the same reason, peripherally inserted central catheter lines and subclavian catheters should be avoided in these patients. If an arteriovenous fistula has already been placed, this arm must be protected from such procedures at all times.
Studies have shown that late referral to a nephrologist is associated with a lower incidence of starting dialysis with a permanent vascular access.97,98
If the patient wishes to start peritoneal dialysis, the peritoneal dialysis catheter can usually be used 2 weeks after being inserted.
Starting dialysis
The appropriate time for starting dialysis remains controversial, especially in elderly patients with multiple comorbid conditions.
The IDEAL study99 found no benefit in starting dialysis at a GFR of 10 to 14 mL/min compared with 5 to 7 mL/min. Thus, there is no single estimated GFR at which dialysis should be started. Rather, the development of early uremic symptoms and the patient’s quality of life should guide this decision.82,83,99–101
Hemodialysis involves three sessions per week, each taking about 4 hours. Evidence suggests that longer sessions or more sessions per week may offer benefits, especially in terms of blood pressure, volume, and dietary management. This has led to an increase in the popularity of home and in-center nocturnal hemodialysis programs across the United States.
Peritoneal dialysis?
Peritoneal dialysis is an excellent choice for patients who are motivated, can care for themselves at home, and have a support system available to assist them if needed. It allows for daily dialysis, less fluid restriction, and less dietary restriction, and it gives the patient an opportunity to stay independent. It also spares the veins in the arms, which may be needed for vascular access later in life if hemodialysis is needed.
Recommendation. We recommend that peritoneal dialysis be offered to any suitable patient who is approaching end-stage renal disease.
A COMPREHENSIVE, COLLABORATIVE APPROACH
Chronic kidney disease is a multisystem disorder, and its management requires a comprehensive approach (Table 3). Early detection and interventions are key to reducing cardiovascular events and progression to kidney failure.
Early referral to a nephrologist and team collaboration between the primary care provider, the nephrologist, and other health care providers are essential. Early in the course of CKD, it may be appropriate for a nephrologist to evaluate the patient and recommend a set of treatment goals. Follow-up may be infrequent or unnecessary.
As CKD progresses, especially as the patient reaches an estimated GFR of 30 mL/min/1.73 m2, the nephrologist will take a more active role in the patient’s care and medical decision-making. In some circumstances, it may even be appropriate for the nephrologist to be the patient’s source of primary care, with the primary care provider as a consultant.
Caring for patients with CKD includes not only strategies to preserve renal function and prolong survival, but also making critical decisions about starting dialysis and about the need for transplantation. Early involvement of a nephrologist and early preparation for end-stage renal disease with preemptive transplantation and arteriovenous fistula placement are associated with better patient outcomes. Key to this is collaboration between the primary care provider and the nephrologist, with levels of responsibility for patient care that adapt to the patient’s degree of renal dysfunction and other comorbidities. Such strategies to select patients for timely nephrology referral may help improve outcomes in this vulnerable population.
- United States Renal Data System (USRDS). Identification and care of patients with CKD. http://www.usrds.org/2012/pdf/v1_ch2_12.pdf. Accessed March 5, 2014.
- Simon J, Amde M, Poggio ED. Interpreting the estimated glomerular filtration rate in primary care: benefits and pitfalls. Cleve Clin J Med 2011; 78:189–195.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. http://www.kidney.org/professionals/kdoqi/guidelines_ckd/p4_class_g1.htm. Accessed March 5, 2014.
- Orlando LA, Owen WF, Matchar DB. Relationship between nephrologist care and progression of chronic kidney disease. N C Med J 2007; 68:9–16.
- Tseng CL, Kern EF, Miller DR, et al. Survival benefit of nephrologic care in patients with diabetes mellitus and chronic kidney disease. Arch Intern Med 2008; 168:55–62.
- Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 2004; 164:659–663.
- Serrano A, Huang J, Ghossein C, et al. Stabilization of glomerular filtration rate in advanced chronic kidney disease: a two-year follow-up of a cohort of chronic kidney disease patients stages 4 and 5. Adv Chronic Kidney Dis 2007; 14:105–112.
- Kinchen KS, Sadler J, Fink N, et al. The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med 2002; 137:479–486.
- Tonelli M, Muntner P, Lloyd A, et al; Alberta Kidney Disease Network. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet 2012; 380:807–814.
- Wattanakit K, Coresh J, Muntner P, Marsh J, Folsom AR. Cardiovascular risk among adults with chronic kidney disease, with or without prior myocardial infarction. J Am Coll Cardiol 2006; 48:1183–1189.
- Foley RN, Wang C, Collins AJ. Cardiovascular risk factor profiles and kidney function stage in the US general population: the NHANES III study. Mayo Clin Proc 2005; 80:1270–1277.
- Muntner P, He J, Astor BC, Folsom AR, Coresh J. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the Atherosclerosis Risk in Communities Study. J Am Soc Nephrol 2005; 16:529–538.
- Navaneethan SD, Schold JD, Kirwan JP, et al. Metabolic syndrome, ESRD, and death in CKD. Clin J Am Soc Nephrol 2013; 8:945–952.
- Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 2005; 16:489–495.
- Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol 2004; 15:1307–1315.
- Gerstein HC, Mann JF, Yi Q, et al; HOPE Study Investigators. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001; 286:421–426.
- Hillege HL, Fidler V, Diercks GF, et al; Prevention of Renal and Vascular End Stage Disease (PREVEND) Study Group. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 2002; 106:1777–1782.
- Foley RN, Collins AJ, Ishani A, Kalra PA. Calcium-phosphate levels and cardiovascular disease in community-dwelling adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 2008; 156:556–563.
- Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G; Cholesterol And Recurrent Events Trial Investigators. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 2005; 112:2627–2633.
- Menon V, Wang X, Sarnak MJ, et al. Long-term outcomes in nondiabetic chronic kidney disease. Kidney Int 2008; 73:1310–1315.
- Kasiske BL. Hyperlipidemia in patients with chronic renal disease. Am J Kidney Dis 1998; 32(suppl 3):S142–S156.
- Kendrick J, Shlipak MG, Targher G, Cook T, Lindenfeld J, Chonchol M. Effect of lovastatin on primary prevention of cardiovascular events in mild CKD and kidney function loss: a post hoc analysis of the Air Force/Texas Coronary Atherosclerosis Prevention Study. Am J Kidney Dis 2010; 55:42–49.
- Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS Investigators. Effects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes: an analysis from the Collaborative Atorvastatin Diabetes Study (CARDS). Am J Kidney Dis 2009; 54:810–819.
- Koren MJ, Davidson MH, Wilson DJ, Fayyad RS, Zuckerman A, Reed DP; ALLIANCE Investigators. Focused atorvastatin therapy in managed-care patients with coronary heart disease and CKD. Am J Kidney Dis 2009; 53:741–750.
- Fellström BC, Jardine AG, Schmieder RE, et al; AURORA Study Group. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 2009; 360:1395–1407.
- Chonchol M, Cook T, Kjekshus J, Pedersen TR, Lindenfeld J. Simvastatin for secondary prevention of all-cause mortality and major coronary events in patients with mild chronic renal insufficiency. Am J Kidney Dis 2007; 49:373–382.
- Ridker PM, MacFadyen J, Cressman M, Glynn RJ. Efficacy of rosuvastatin among men and women with moderate chronic kidney disease and elevated high-sensitivity C-reactive protein: a secondary analysis from the JUPITER (Justification for the Use of Statins in Prevention-an Intervention Trial Evaluating Rosuvastatin) trial. J Am Coll Cardiol 2010; 55:1266–1273.
- Baigent C, Landray MJ, Reith C, et al; SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011; 377:2181–2192.
- Shepherd J, Kastelein JJ, Bittner V, et al; Treating to New Targets Investigators. Effect of intensive lipid lowering with atorvastatin on renal function in patients with coronary heart disease: the Treating to New Targets (TNT) study. Clin J Am Soc Nephrol 2007; 2:1131–1139.
- Tonelli M, Isles C, Craven T, et al. Effect of pravastatin on rate of kidney function loss in people with or at risk for coronary disease. Circulation 2005; 112:171–178.
- Palmer SC, Craig JC, Navaneethan SD, Tonelli M, Pellegrini F, Strippoli GF. Benefits and harms of statin therapy for persons with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med 2012; 157:263–275.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Managing Dyslipidemias in Chronic Kidney Disease. http://www.kidney.org/professionals/kdoqi/guidelines_lipids/. Accessed March 5, 2014.
- Buckalew VM, Berg RL, Wang SR, Porush JG, Rauch S, Schulman G. Prevalence of hypertension in 1,795 subjects with chronic renal disease: the modification of diet in renal disease study baseline cohort. Modification of Diet in Renal Disease Study Group. Am J Kidney Dis 1996; 28:811–821.
- Coresh J, Wei GL, McQuillan G, et al. Prevalence of high blood pressure and elevated serum creatinine level in the United States: findings from the third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med 2001; 161:1207–1216.
- Klag MJ, Whelton PK, Randall BL, et al. Blood pressure and end-stage renal disease in men. N Engl J Med 1996; 334:13–18.
- Locatelli F, Marcelli D, Comelli M, et al. Proteinuria and blood pressure as causal components of progression to end-stage renal failure. Northern Italian Cooperative Study Group. Nephrol Dial Transplant 1996; 11:461–467.
- ACCORD Study Group; Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- Jafar TH, Stark PC, Schmid CH, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med 2003; 139:244–252.
- Khosla N, Bakris G. Lessons learned from recent hypertension trials about kidney disease. Clin J Am Soc Nephrol 2006; 1:229–235.
- Norris KC, Greene T, Kopple J, et al. Baseline predictors of renal disease progression in the African American Study of Hypertension and Kidney Disease. J Am Soc Nephrol 2006; 17:2928–2936.
- Keane WF, Brenner BM, de Zeeuw D, et al; RENAAL Study Investigators. The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: the RENAAL study. Kidney Int 2003; 63:1499–1507.
- Ruggenenti P, Perna A, Mosconi L, et al. Proteinuria predicts end-stage renal failure in non-diabetic chronic nephropathies. The “Gruppo Italiano di Studi Epidemiologici in Nefrologia” (GISEN). Kidney Int Suppl 1997; 63:S54–S57.
- de Goeij MC, Liem M, de Jager DJ, et al; PREPARE-1 Study Group. Proteinuria as a risk marker for the progression of chronic kidney disease in patients on predialysis care and the role of angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker treatment. Nephron Clin Pract 2012; 121:c73–c82.
- de Zeeuw D, Remuzzi G, Parving HH, et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation 2004; 110:921–927.
- Ibsen H, Olsen MH, Wachtell K, et al. Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: losartan intervention for endpoint reduction in hypertension study. Hypertension 2005; 45:198–202.
- Atkins RC, Briganti EM, Lewis JB, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis 2005; 45:281–287.
- Jafar TH, Stark PC, Schmid CH, et al; AIPRD Study Group; Angiotensin-Converting Enzyme Inhibition and Progression of Renal Disease. Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int 2001; 60:1131–1140.
- ACE Inhibitors in Diabetic Nephropathy Trialist Group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors? A meta-analysis of individual patient data. Ann Intern Med 2001; 134:370–379.
- Casas JP, Chua W, Loukogeorgakis S, et al. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet 2005; 366:2026–2033.
- Strippoli GF, Craig M, Deeks JJ, Schena FP, Craig JC. Effects of angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists on mortality and renal outcomes in diabetic nephropathy: systematic review. BMJ 2004; 329:828.
- MacKinnon M, Shurraw S, Akbari A, Knoll GA, Jaffey J, Clark HD. Combination therapy with an angiotensin receptor blocker and an ACE inhibitor in proteinuric renal disease: a systematic review of the efficacy and safety data. Am J Kidney Dis 2006; 48:8–20.
- Kunz R, Friedrich C, Wolbers M, Mann JF. Meta-analysis: effect of mono-therapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med 2008; 148:30–48.
- Ruggenenti P, Perticucci E, Cravedi P, et al. Role of remission clinics in the longitudinal treatment of CKD. J Am Soc Nephrol 2008; 19:1213–1224.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Esnault VL, Ekhlas A, Delcroix C, Moutel MG, Nguyen JM. Diuretic and enhanced sodium restriction results in improved antiproteinuric response to RAS blocking agents. J Am Soc Nephrol 2005; 16:474–481.
- Vogt L, Waanders F, Boomsma F, de Zeeuw D, Navis G. Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J Am Soc Nephrol 2008; 19:999–1007.
- Ahmed AK, Kamath NS, El Kossi M, El Nahas AM. The impact of stopping inhibitors of the renin-angiotensin system in patients with advanced chronic kidney disease. Nephrol Dial Transplant 2010; 25:3977–3982.
- Bakris GL, Weir MR, Secic M, Campbell B, Weis-McNulty A. Differential effects of calcium antagonist subclasses on markers of nephropathy progression. Kidney Int 2004; 65:1991–2002.
- Kloke HJ, Wetzels JF, Koene RA, Huysmans FT. Effects of low-dose nifedipine on urinary protein excretion rate in patients with renal disease. Nephrol Dial Transplant 1998; 13:646–650.
- Shah SN, Abramowitz M, Hostetter TH, Melamed ML. Serum bicarbonate levels and the progression of kidney disease: a cohort study. Am J Kidney Dis 2009; 54:270–277.
- de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 2009; 20:2075–2084.
- Susantitaphong P, Sewaralthahab K, Balk EM, Jaber BL, Madias NE. Short- and long-term effects of alkali therapy in chronic kidney disease: a systematic review. Am J Nephrol 2012; 35:540–547.
- Nath KA, Hostetter MK, Hostetter TH. Ammonia-complement interaction in the pathogenesis of progressive renal injury. Kidney Int Suppl 1989; 27:S52–S54.
- Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis 2000; 35(suppl 2):S1–S140.
- Shimada T, Yamazaki Y, Takahashi M, et al. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol 2005; 289:F1088–F1095.
- Hasegawa H, Nagano N, Urakawa I, et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int 2010; 78:975–980.
- de Boer IH, Rue TC, Kestenbaum B. Serum phosphorus concentrations in the third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis 2009; 53:399–407.
- Kendrick J, Cheung AK, Kaufman JS, et al; HOST Investigators. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol 2011; 22:1913–1922.
- Palmer SC, Hayen A, Macaskill P, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA. 2011; 305:1119–1127.
- Kooienga L, Fried L, Scragg R, Kendrick J, Smits G, Chonchol M. The effect of combined calcium and vitamin D3 supplementation on serum intact parathyroid hormone in moderate CKD. Am J Kidney Dis 2009; 53:408–416.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease. www.kidney.org/professionals/kdoqi/guidelines_bone/guide1.htm#table15. Accessed March 5, 2014.
- Kidney International. KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). http://kdigo.org/home/mineral-bone-disorder. Accessed March 5, 2014.
- Kazmi WH, Kausz AT, Khan S, et al. Anemia: an early complication of chronic renal insufficiency. Am J Kidney Dis 2001; 38:803–812.
- Sarnak MJ, Tighiouart H, Manjunath G, et al. Anemia as a risk factor for cardiovascular disease in the Atherosclerosis Risk in Communities (ARIC) study. J Am Coll Cardiol 2002; 40:27–33.
- Thorp ML, Johnson ES, Yang X, Petrik AF, Platt R, Smith DH. Effect of anaemia on mortality, cardiovascular hospitalizations and end-stage renal disease among patients with chronic kidney disease. Nephrology (Carlton) 2009; 14:240–246.
- Mircescu G, Gârneata L, Capusa C, Ursea N. Intravenous iron supplementation for the treatment of anaemia in pre-dialyzed chronic renal failure patients. Nephrol Dial Transplant 2006; 21:120–124.
- Silverberg DS, Iaina A, Peer G, et al. Intravenous iron supplementation for the treatment of the anemia of moderate to severe chronic renal failure patients not receiving dialysis. Am J Kidney Dis 1996; 27:234–238.
- Singh AK, Szczech L, Tang KL, et al; CHOIR Investigators. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355:2085–2098.
- Drüeke TB, Locatelli F, Clyne N, et al; CREATE Investigators. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355:2071–2084.
- Pfeffer MA, Burdmann EA, Chen CY, et al; TREAT Investigators. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361:2019–2032.
- US Food and Drug Administration (FDA). FDA Drug Safety Communication: modified dosing recommendations to improve the safe use of erythropoiesis-stimulating agents (ESAs) in chronic kidney disease. http://www.fda.gov/drugs/drugsafety/ucm259639.htm. Accessed March 5, 2014.
- Kurella M, Covinsky KE, Collins AJ, Chertow GM. Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med 2007; 146:177–183.
- Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 2009; 361:1539–1547.
- Renal Physicians Association. Clinical Practice Guideline. Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis. 2nd ed.
- Vollmer WM, Wahl PW, Blagg CR. Survival with dialysis and transplantation in patients with end-stage renal disease. N Engl J Med 1983; 308:1553–1558.
- Port FK, Wolfe RA, Mauger EA, Berling DP, Jiang K. Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA 1993; 270:1339–1343.
- Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999; 341:1725–1730.
- Cosio FG, Alamir A, Yim S, et al. Patient survival after renal transplantation: I. The impact of dialysis pre-transplant. Kidney Int 1998; 53:767–772.
- Meier-Kriesche HU, Port FK, Ojo AO, et al. Effect of waiting time on renal transplant outcome. Kidney Int 2000; 58:1311–1317.
- Mange KC, Joffe MM, Feldman HI. Effect of the use or nonuse of long-term dialysis on the subsequent survival of renal transplants from living donors. N Engl J Med 2001; 344:726–731.
- Dhingra RK, Young EW, Hulbert-Shearon TE, Leavey SF, Port FK. Type of vascular access and mortality in US hemodialysis patients. Kidney Int 2001; 60:1443–1451.
- Nassar GM, Ayus JC. Infectious complications of the hemodialysis access. Kidney Int 2001; 60:1–13.
- Perera GB, Mueller MP, Kubaska SM, Wilson SE, Lawrence PF, Fujitani RM. Superiority of autogenous arteriovenous hemodialysis access: maintenance of function with fewer secondary interventions. Ann Vasc Surg 2004; 18:66–73.
- Basile C, Casucci F, Lomonte C. Timing of first cannulation of arteriovenous fistula: time matters, but there is also something else. Nephrol Dial Transplant 2005; 20:1519–1520.
- Biuckians A, Scott EC, Meier GH, Panneton JM, Glickman MH. The natural history of autologous fistulas as first-time dialysis access in the KDOQI era. J Vasc Surg 2008; 47:415–421.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Vascular Access. http://www.kidney.org/professionals/KDOQI/guideline_upHD_PD_VA/va_guide1.htm. Accessed March 5, 2014.
- Arora P, Obrador GT, Ruthazer R, et al. Prevalence, predictors, and consequences of late nephrology referral at a tertiary care center. J Am Soc Nephrol 1999; 10:1281–1286.
- Gøransson LG, Bergrem H. Consequences of late referral of patients with end-stage renal disease. J Intern Med 2001; 250:154–159.
- Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med 2010; 363:609–619.
- Carson RC, Juszczak M, Davenport A, Burns A. Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin J Am Soc Nephrol 2009; 4:1611–1619.
- Murtagh FE, Marsh JE, Donohoe P, Ekbal NJ, Sheerin NS, Harris FE. Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant 2007; 22:1955–1962.
- United States Renal Data System (USRDS). Identification and care of patients with CKD. http://www.usrds.org/2012/pdf/v1_ch2_12.pdf. Accessed March 5, 2014.
- Simon J, Amde M, Poggio ED. Interpreting the estimated glomerular filtration rate in primary care: benefits and pitfalls. Cleve Clin J Med 2011; 78:189–195.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. http://www.kidney.org/professionals/kdoqi/guidelines_ckd/p4_class_g1.htm. Accessed March 5, 2014.
- Orlando LA, Owen WF, Matchar DB. Relationship between nephrologist care and progression of chronic kidney disease. N C Med J 2007; 68:9–16.
- Tseng CL, Kern EF, Miller DR, et al. Survival benefit of nephrologic care in patients with diabetes mellitus and chronic kidney disease. Arch Intern Med 2008; 168:55–62.
- Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 2004; 164:659–663.
- Serrano A, Huang J, Ghossein C, et al. Stabilization of glomerular filtration rate in advanced chronic kidney disease: a two-year follow-up of a cohort of chronic kidney disease patients stages 4 and 5. Adv Chronic Kidney Dis 2007; 14:105–112.
- Kinchen KS, Sadler J, Fink N, et al. The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med 2002; 137:479–486.
- Tonelli M, Muntner P, Lloyd A, et al; Alberta Kidney Disease Network. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet 2012; 380:807–814.
- Wattanakit K, Coresh J, Muntner P, Marsh J, Folsom AR. Cardiovascular risk among adults with chronic kidney disease, with or without prior myocardial infarction. J Am Coll Cardiol 2006; 48:1183–1189.
- Foley RN, Wang C, Collins AJ. Cardiovascular risk factor profiles and kidney function stage in the US general population: the NHANES III study. Mayo Clin Proc 2005; 80:1270–1277.
- Muntner P, He J, Astor BC, Folsom AR, Coresh J. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the Atherosclerosis Risk in Communities Study. J Am Soc Nephrol 2005; 16:529–538.
- Navaneethan SD, Schold JD, Kirwan JP, et al. Metabolic syndrome, ESRD, and death in CKD. Clin J Am Soc Nephrol 2013; 8:945–952.
- Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 2005; 16:489–495.
- Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol 2004; 15:1307–1315.
- Gerstein HC, Mann JF, Yi Q, et al; HOPE Study Investigators. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001; 286:421–426.
- Hillege HL, Fidler V, Diercks GF, et al; Prevention of Renal and Vascular End Stage Disease (PREVEND) Study Group. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 2002; 106:1777–1782.
- Foley RN, Collins AJ, Ishani A, Kalra PA. Calcium-phosphate levels and cardiovascular disease in community-dwelling adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 2008; 156:556–563.
- Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G; Cholesterol And Recurrent Events Trial Investigators. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 2005; 112:2627–2633.
- Menon V, Wang X, Sarnak MJ, et al. Long-term outcomes in nondiabetic chronic kidney disease. Kidney Int 2008; 73:1310–1315.
- Kasiske BL. Hyperlipidemia in patients with chronic renal disease. Am J Kidney Dis 1998; 32(suppl 3):S142–S156.
- Kendrick J, Shlipak MG, Targher G, Cook T, Lindenfeld J, Chonchol M. Effect of lovastatin on primary prevention of cardiovascular events in mild CKD and kidney function loss: a post hoc analysis of the Air Force/Texas Coronary Atherosclerosis Prevention Study. Am J Kidney Dis 2010; 55:42–49.
- Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS Investigators. Effects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes: an analysis from the Collaborative Atorvastatin Diabetes Study (CARDS). Am J Kidney Dis 2009; 54:810–819.
- Koren MJ, Davidson MH, Wilson DJ, Fayyad RS, Zuckerman A, Reed DP; ALLIANCE Investigators. Focused atorvastatin therapy in managed-care patients with coronary heart disease and CKD. Am J Kidney Dis 2009; 53:741–750.
- Fellström BC, Jardine AG, Schmieder RE, et al; AURORA Study Group. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 2009; 360:1395–1407.
- Chonchol M, Cook T, Kjekshus J, Pedersen TR, Lindenfeld J. Simvastatin for secondary prevention of all-cause mortality and major coronary events in patients with mild chronic renal insufficiency. Am J Kidney Dis 2007; 49:373–382.
- Ridker PM, MacFadyen J, Cressman M, Glynn RJ. Efficacy of rosuvastatin among men and women with moderate chronic kidney disease and elevated high-sensitivity C-reactive protein: a secondary analysis from the JUPITER (Justification for the Use of Statins in Prevention-an Intervention Trial Evaluating Rosuvastatin) trial. J Am Coll Cardiol 2010; 55:1266–1273.
- Baigent C, Landray MJ, Reith C, et al; SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011; 377:2181–2192.
- Shepherd J, Kastelein JJ, Bittner V, et al; Treating to New Targets Investigators. Effect of intensive lipid lowering with atorvastatin on renal function in patients with coronary heart disease: the Treating to New Targets (TNT) study. Clin J Am Soc Nephrol 2007; 2:1131–1139.
- Tonelli M, Isles C, Craven T, et al. Effect of pravastatin on rate of kidney function loss in people with or at risk for coronary disease. Circulation 2005; 112:171–178.
- Palmer SC, Craig JC, Navaneethan SD, Tonelli M, Pellegrini F, Strippoli GF. Benefits and harms of statin therapy for persons with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med 2012; 157:263–275.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Managing Dyslipidemias in Chronic Kidney Disease. http://www.kidney.org/professionals/kdoqi/guidelines_lipids/. Accessed March 5, 2014.
- Buckalew VM, Berg RL, Wang SR, Porush JG, Rauch S, Schulman G. Prevalence of hypertension in 1,795 subjects with chronic renal disease: the modification of diet in renal disease study baseline cohort. Modification of Diet in Renal Disease Study Group. Am J Kidney Dis 1996; 28:811–821.
- Coresh J, Wei GL, McQuillan G, et al. Prevalence of high blood pressure and elevated serum creatinine level in the United States: findings from the third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med 2001; 161:1207–1216.
- Klag MJ, Whelton PK, Randall BL, et al. Blood pressure and end-stage renal disease in men. N Engl J Med 1996; 334:13–18.
- Locatelli F, Marcelli D, Comelli M, et al. Proteinuria and blood pressure as causal components of progression to end-stage renal failure. Northern Italian Cooperative Study Group. Nephrol Dial Transplant 1996; 11:461–467.
- ACCORD Study Group; Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- Jafar TH, Stark PC, Schmid CH, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med 2003; 139:244–252.
- Khosla N, Bakris G. Lessons learned from recent hypertension trials about kidney disease. Clin J Am Soc Nephrol 2006; 1:229–235.
- Norris KC, Greene T, Kopple J, et al. Baseline predictors of renal disease progression in the African American Study of Hypertension and Kidney Disease. J Am Soc Nephrol 2006; 17:2928–2936.
- Keane WF, Brenner BM, de Zeeuw D, et al; RENAAL Study Investigators. The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: the RENAAL study. Kidney Int 2003; 63:1499–1507.
- Ruggenenti P, Perna A, Mosconi L, et al. Proteinuria predicts end-stage renal failure in non-diabetic chronic nephropathies. The “Gruppo Italiano di Studi Epidemiologici in Nefrologia” (GISEN). Kidney Int Suppl 1997; 63:S54–S57.
- de Goeij MC, Liem M, de Jager DJ, et al; PREPARE-1 Study Group. Proteinuria as a risk marker for the progression of chronic kidney disease in patients on predialysis care and the role of angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker treatment. Nephron Clin Pract 2012; 121:c73–c82.
- de Zeeuw D, Remuzzi G, Parving HH, et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation 2004; 110:921–927.
- Ibsen H, Olsen MH, Wachtell K, et al. Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: losartan intervention for endpoint reduction in hypertension study. Hypertension 2005; 45:198–202.
- Atkins RC, Briganti EM, Lewis JB, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis 2005; 45:281–287.
- Jafar TH, Stark PC, Schmid CH, et al; AIPRD Study Group; Angiotensin-Converting Enzyme Inhibition and Progression of Renal Disease. Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int 2001; 60:1131–1140.
- ACE Inhibitors in Diabetic Nephropathy Trialist Group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors? A meta-analysis of individual patient data. Ann Intern Med 2001; 134:370–379.
- Casas JP, Chua W, Loukogeorgakis S, et al. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet 2005; 366:2026–2033.
- Strippoli GF, Craig M, Deeks JJ, Schena FP, Craig JC. Effects of angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists on mortality and renal outcomes in diabetic nephropathy: systematic review. BMJ 2004; 329:828.
- MacKinnon M, Shurraw S, Akbari A, Knoll GA, Jaffey J, Clark HD. Combination therapy with an angiotensin receptor blocker and an ACE inhibitor in proteinuric renal disease: a systematic review of the efficacy and safety data. Am J Kidney Dis 2006; 48:8–20.
- Kunz R, Friedrich C, Wolbers M, Mann JF. Meta-analysis: effect of mono-therapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med 2008; 148:30–48.
- Ruggenenti P, Perticucci E, Cravedi P, et al. Role of remission clinics in the longitudinal treatment of CKD. J Am Soc Nephrol 2008; 19:1213–1224.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Esnault VL, Ekhlas A, Delcroix C, Moutel MG, Nguyen JM. Diuretic and enhanced sodium restriction results in improved antiproteinuric response to RAS blocking agents. J Am Soc Nephrol 2005; 16:474–481.
- Vogt L, Waanders F, Boomsma F, de Zeeuw D, Navis G. Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J Am Soc Nephrol 2008; 19:999–1007.
- Ahmed AK, Kamath NS, El Kossi M, El Nahas AM. The impact of stopping inhibitors of the renin-angiotensin system in patients with advanced chronic kidney disease. Nephrol Dial Transplant 2010; 25:3977–3982.
- Bakris GL, Weir MR, Secic M, Campbell B, Weis-McNulty A. Differential effects of calcium antagonist subclasses on markers of nephropathy progression. Kidney Int 2004; 65:1991–2002.
- Kloke HJ, Wetzels JF, Koene RA, Huysmans FT. Effects of low-dose nifedipine on urinary protein excretion rate in patients with renal disease. Nephrol Dial Transplant 1998; 13:646–650.
- Shah SN, Abramowitz M, Hostetter TH, Melamed ML. Serum bicarbonate levels and the progression of kidney disease: a cohort study. Am J Kidney Dis 2009; 54:270–277.
- de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 2009; 20:2075–2084.
- Susantitaphong P, Sewaralthahab K, Balk EM, Jaber BL, Madias NE. Short- and long-term effects of alkali therapy in chronic kidney disease: a systematic review. Am J Nephrol 2012; 35:540–547.
- Nath KA, Hostetter MK, Hostetter TH. Ammonia-complement interaction in the pathogenesis of progressive renal injury. Kidney Int Suppl 1989; 27:S52–S54.
- Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis 2000; 35(suppl 2):S1–S140.
- Shimada T, Yamazaki Y, Takahashi M, et al. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol 2005; 289:F1088–F1095.
- Hasegawa H, Nagano N, Urakawa I, et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int 2010; 78:975–980.
- de Boer IH, Rue TC, Kestenbaum B. Serum phosphorus concentrations in the third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis 2009; 53:399–407.
- Kendrick J, Cheung AK, Kaufman JS, et al; HOST Investigators. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol 2011; 22:1913–1922.
- Palmer SC, Hayen A, Macaskill P, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA. 2011; 305:1119–1127.
- Kooienga L, Fried L, Scragg R, Kendrick J, Smits G, Chonchol M. The effect of combined calcium and vitamin D3 supplementation on serum intact parathyroid hormone in moderate CKD. Am J Kidney Dis 2009; 53:408–416.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease. www.kidney.org/professionals/kdoqi/guidelines_bone/guide1.htm#table15. Accessed March 5, 2014.
- Kidney International. KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). http://kdigo.org/home/mineral-bone-disorder. Accessed March 5, 2014.
- Kazmi WH, Kausz AT, Khan S, et al. Anemia: an early complication of chronic renal insufficiency. Am J Kidney Dis 2001; 38:803–812.
- Sarnak MJ, Tighiouart H, Manjunath G, et al. Anemia as a risk factor for cardiovascular disease in the Atherosclerosis Risk in Communities (ARIC) study. J Am Coll Cardiol 2002; 40:27–33.
- Thorp ML, Johnson ES, Yang X, Petrik AF, Platt R, Smith DH. Effect of anaemia on mortality, cardiovascular hospitalizations and end-stage renal disease among patients with chronic kidney disease. Nephrology (Carlton) 2009; 14:240–246.
- Mircescu G, Gârneata L, Capusa C, Ursea N. Intravenous iron supplementation for the treatment of anaemia in pre-dialyzed chronic renal failure patients. Nephrol Dial Transplant 2006; 21:120–124.
- Silverberg DS, Iaina A, Peer G, et al. Intravenous iron supplementation for the treatment of the anemia of moderate to severe chronic renal failure patients not receiving dialysis. Am J Kidney Dis 1996; 27:234–238.
- Singh AK, Szczech L, Tang KL, et al; CHOIR Investigators. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355:2085–2098.
- Drüeke TB, Locatelli F, Clyne N, et al; CREATE Investigators. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355:2071–2084.
- Pfeffer MA, Burdmann EA, Chen CY, et al; TREAT Investigators. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361:2019–2032.
- US Food and Drug Administration (FDA). FDA Drug Safety Communication: modified dosing recommendations to improve the safe use of erythropoiesis-stimulating agents (ESAs) in chronic kidney disease. http://www.fda.gov/drugs/drugsafety/ucm259639.htm. Accessed March 5, 2014.
- Kurella M, Covinsky KE, Collins AJ, Chertow GM. Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med 2007; 146:177–183.
- Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 2009; 361:1539–1547.
- Renal Physicians Association. Clinical Practice Guideline. Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis. 2nd ed.
- Vollmer WM, Wahl PW, Blagg CR. Survival with dialysis and transplantation in patients with end-stage renal disease. N Engl J Med 1983; 308:1553–1558.
- Port FK, Wolfe RA, Mauger EA, Berling DP, Jiang K. Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA 1993; 270:1339–1343.
- Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999; 341:1725–1730.
- Cosio FG, Alamir A, Yim S, et al. Patient survival after renal transplantation: I. The impact of dialysis pre-transplant. Kidney Int 1998; 53:767–772.
- Meier-Kriesche HU, Port FK, Ojo AO, et al. Effect of waiting time on renal transplant outcome. Kidney Int 2000; 58:1311–1317.
- Mange KC, Joffe MM, Feldman HI. Effect of the use or nonuse of long-term dialysis on the subsequent survival of renal transplants from living donors. N Engl J Med 2001; 344:726–731.
- Dhingra RK, Young EW, Hulbert-Shearon TE, Leavey SF, Port FK. Type of vascular access and mortality in US hemodialysis patients. Kidney Int 2001; 60:1443–1451.
- Nassar GM, Ayus JC. Infectious complications of the hemodialysis access. Kidney Int 2001; 60:1–13.
- Perera GB, Mueller MP, Kubaska SM, Wilson SE, Lawrence PF, Fujitani RM. Superiority of autogenous arteriovenous hemodialysis access: maintenance of function with fewer secondary interventions. Ann Vasc Surg 2004; 18:66–73.
- Basile C, Casucci F, Lomonte C. Timing of first cannulation of arteriovenous fistula: time matters, but there is also something else. Nephrol Dial Transplant 2005; 20:1519–1520.
- Biuckians A, Scott EC, Meier GH, Panneton JM, Glickman MH. The natural history of autologous fistulas as first-time dialysis access in the KDOQI era. J Vasc Surg 2008; 47:415–421.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Vascular Access. http://www.kidney.org/professionals/KDOQI/guideline_upHD_PD_VA/va_guide1.htm. Accessed March 5, 2014.
- Arora P, Obrador GT, Ruthazer R, et al. Prevalence, predictors, and consequences of late nephrology referral at a tertiary care center. J Am Soc Nephrol 1999; 10:1281–1286.
- Gøransson LG, Bergrem H. Consequences of late referral of patients with end-stage renal disease. J Intern Med 2001; 250:154–159.
- Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med 2010; 363:609–619.
- Carson RC, Juszczak M, Davenport A, Burns A. Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin J Am Soc Nephrol 2009; 4:1611–1619.
- Murtagh FE, Marsh JE, Donohoe P, Ekbal NJ, Sheerin NS, Harris FE. Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant 2007; 22:1955–1962.
KEY POINTS
- Steps to stabilize renal function include blood pressure and diabetes control.
- Patients have a very high risk of cardiovascular disease, and one should try to reduce modifiable risk factors such as hypertension (which is also a risk factor for the progression of CKD) and hyperlipidemia.
- In addition to controlling blood pressure, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers reduce proteinuria, a risk factor for progression of CKD.
- Patients with CKD develop secondary hyperparathyroidism, hyperphosphatemia, and, in advanced CKD, hypocalcemia, all leading to disorders of bone mineral metabolism. Low vitamin D levels should be raised with supplements, and high phosphorus levels should be lowered with dietary restriction and phosphate binders.