User login
Guidelines for treating diabetes mellitus are mostly based on clinical studies in middle-aged people, and recommendations tend to be the same for everyone, whether young and strong or elderly and frail. But diabetes management should be individualized, especially in the elderly, taking into account each patient’s medical history, functional ability, home care situation, and life expectancy. Aggressive glycemic control is less important than avoiding hypoglycemia and achieving a good quality of life.
This article reviews the general principles for recognizing and managing diabetes in elderly patients, focusing on the management of blood sugar per se. In a future issue of this journal, we will discuss some of the many complications of diabetes in the elderly.
DIABETES DIFFERS IN ELDERLY PATIENTS
“The elderly” is a heterogeneous group with widely varying physiologic profiles, functional capabilities, and life expectancy (on average, about 88 years for men and 90 years for women in the United States). Although the elderly are sometimes classified as “young-old” (age 65–80) and “old-old” (80+), this distinction is too simplistic for clinical decision-making.
Diabetes mellitus in the elderly also is heterogeneous. One distinction is the age at which the disease developed.
Aging is associated with declining beta-cell function and lower blood insulin levels independent of insulin resistance, and with insulin resistance itself. The risk of developing type 2 diabetes mellitus increases with obesity, lack of physical activity, and loss of muscle mass, all of which often develop with aging.1
Middle-aged patients with diabetes have increased fasting hepatic glucose production, increased insulin resistance, and an abnormal insulin response to a glucose load. On the other hand, patients who develop diabetes at an older age tend to have normal hepatic glucose production. Older patients who are lean secrete markedly less insulin in response to a glucose load but have relatively less insulin resistance.2 Patients who develop type 2 diabetes in old age are more likely to have near-normal fasting blood glucose levels but significant postprandial hyperglycemia.3,4 Elderly patients who developed diabetes during middle age have metabolic abnormalities more typical of middle-aged patients with type 2 diabetes.
DIABETES IS COMMON, AND INCREASING IN PREVALENCE
By age 75, 40% of people in the United States have either glucose intolerance or diabetes mellitus.5 Metabolic syndrome, which is the constellation of insulin resistance (type 2 diabetes mellitus), hyperlipidemia, hypertension, and obesity, is more prevalent in people age 65 to 74 years than in younger and older people.3
The National Diabetes Surveillance System of the US Centers for Disease Control and Prevention estimated that the prevalence of diabetes mellitus in people 65 to 74 years old in 2005 was 18.5%, about 12 times the prevalence among those younger than 45years.6 The prevalence has been gradually increasing and has nearly doubled over the past 25 years, with certain groups—native Americans, Hispanics, and African-Americans—at particularly high risk of developing the disease.
Although the prevalence of diabetes in people older than 75 years is lower than among people in the 65-to-74-year range, the elderly segment of our population is increasing, and the impact of diabetes and its associated burden of death and disease from vascular complications is enormous.
SYMPTOMS ARE OFTEN NONSPECIFIC
Unfortunately, diabetes is underdiagnosed and frequently undertreated, resulting in even more disease and death.7–9
Diabetes is often missed in the elderly because its presenting symptoms may be nonspecific, eg, failure to thrive, low energy, falls, dizziness, confusion, nocturia (with or without incontinence), and urinary tract infection.The classic symptoms of frequent urination (often leading to worsening incontinence), thirst, and increased hunger usually occur only when plasma glucose levels are above 200 mg/dL. Weight loss, blurred vision, and dehydration may also be present with high blood glucose levels. With lesser degrees of hyperglycemia, patients may have no symptoms or present with weight loss or signs and symptoms of chronic infection, especially of the genitourinary tract, skin, or mouth.
Hyperglycemia in elderly patients is also associated with reduced cognitive function (which may improve with blood glucose control).10
The American Diabetes Association recommends screening by measuring the fasting plasma glucose level every 3 years beginning at 45 years.11 However, some experts believe that this method is inadequate for the elderly12; some suggest that screening should be done more often in those with risk factors for diabetes, including obesity, inactivity, hypertension, and dyslipidemia, all of which are common in the elderly. Targeted screening in patients with hypertension may be the most cost-effective strategy.13
Screening with hemoglobin A1c levels is not recommended because of lack of standardization among laboratories.14
INDIVIDUALIZED MANAGEMENT IS BEST
Despite disease differences, the general goals for diabetes care are the same for all ages:
- To control hyperglycemia and its symptoms
- To prevent, evaluate, and treat macrovascular and microvascular complications
- To teach patients to manage themselves
- To maintain or improve the patient’s general health status.
Unfortunately, most specific recommendations are based on studies in younger people. Guidelines should ideally reflect the complexities of a particular clinical situation, but most recommendations are applied to the young and old alike, as well as to the relatively healthy and the frail and ill.15–17 Consideration should be given to a patient’s health beliefs, severity of vascular complications and other medical problems, economic situation, life expectancy ,functional status, and availability of support services. In addition, some patients prefer aggressive treatment, while others would rather compromise some aspects of care in order to maintain a certain quality of life, to save money, or to avoid having caregivers provide treatment.
Age-related changes in pharmacokinetics as well as polypharmacy increase the risk of drug interactions and adverse effects, especially drug-induced hypoglycemia. In addition, age-associated changes in cognitive, visual, and physical function, dentition, and taste perception can reduce a patient’s ability to carry out treatment. Frequent hospitalizations also disrupt outpatient regimens.
Comorbidities make treatment more challenging, but some conditions—such as hypertension, renal insufficiency and eye disorders—make doctors more likely to control hyperglycemia more aggressively, fearing that the loss of a little more function in an impaired organ may lead to failure.
The benefits of tight glycemic control should be weighed against the risks and the realities of an individual situation. Priority should be given to achieving the best quality of life possible.17 Recent guidelines from the California Health Foundation and the American Geriatrics Association focused on the major health threats to older patients and prioritizing care for each person.15 The guidelines recommend screening for geriatric syndromes that are more prevalent inpatients with diabetes or are strongly affected by the disease or its treatment. Diabetes care should be examined in the setting of common geriatric problems: depression, polypharmacy, cognitive impairment, urinary incontinence, falls, and pain.
Heart risk trumps glycemic control
The expert panel15 concluded that rates of disease and death can be reduced more by targeting cardiovascular risk factors than by intensively managing hyperglycemia. One rationale is that it takes 8 years for aggressive glycemic control to reduce the risk of diabetic retinopathy or renal disease but only 2 years of treating hypertension and dyslipidemia to reduce the risk of cardiovascular disease.15,17–21 A recent Japanese study found normal mortality rates in elderly patients under long-term, intensive multifactorial diabetes control.22 High-functioning, motivated patients could benefit from therapy aimed at achieving most or all of the recommended goals, but frail patients may suffer from applying all therapies and may benefit from only some of them.
If appropriate goals cannot be met, it may help to refer patients to a geriatric specialist to evaluate possible barriers to adherence such as depression or poor cognition, physical functioning, or support.
MANAGEMENT STRATEGIES
Weight loss and exercise help prevent diabetes
The Diabetes Prevention Program23 randomized 3,234 people (mean age 51 years) with impaired glucose tolerance to receive either metformin (Fortamet, Glucophage) 850 mg twice daily or placebo or to undertake lifestyle modifications with goals of at least a 7% weight loss and at least 150 minutes of physical activity per week. Compared with the placebo group, the lifestyle modification group had a 58% lower incidence of diabetes while those in the metformin group had only a 31% lower incidence. Among those older than 60 years, the advantage of lifestyle modification over metformin was even greater.
Control blood glucose, avoid hypoglycemia
- Hemoglobin A1c levels < 7.0%
- Preprandial blood glucose levels 90–130 mg/dL
- Bedtime blood glucose levels 110–150 mg/dL.
Guidelines from the Department of Veterans Affairs24 and the American Geriatrics Society15 are slightly different, and are based on randomized trials in younger patients, primarily the Diabetes Control and Complications Trial (DCCT)25 and the United Kingdom Prospective Diabetes Study(UKPDS).21,26 A recent position statement from the American College of Physicians, based on a review of all the major guidelines, recommends the following: “Statement 1: To prevent microvascular complication of diabetes, the goal for glycemic control should be as low as is feasible without undue risk for adverse events or an unacceptable burden on patients. Treatment goals should be based on a discussion of the benefits and harms of specific levels of glycemic control with the patient. A hemoglobin A1c level less than 7% based on individualized assessment is a reasonable goal for many but not all patients. Statement 2: The goal for hemoglobin A1c should be based on individualized assessment of risk for complication from diabetes, comorbidity, life expectancy, and patient preferences.”27
Although few data exist for elderly patients, these guidelines are the most current approach to treating diabetes in the elderly. Less stringent goals are appropriate for patients who have limited life expectancy, hypoglycemia unawareness (lack of autonomic warning symptoms of low blood sugar), seizures, dementia, psychiatric illness, or alcoholism. It is important to keep in mind the following as one strives for lower A1c levels: Although the relative risk reduction accomplished by lowering hemoglobin A1c is linear, the absolute risk reduction is log-linear—more benefit is gained by lowering hemoglobin A1c from 9% to 8% than from 8% to 7%.28
Hypoglycemia is a major limiting factor in glycemic control. Many risk factors for hypoglycemia are common in the elderly (Table 2). Hypoglycemia was a chief adverse event in both the DCCT and the UKPDS, with a twofold to threefold higher rate in patients who were intensively treated.29 Even mild hypoglycemia in the elderly can result in an injurious fall, which can lead to long-term functional decline. The rate of severe or fatal hypoglycemia—the major risk of tight glycemic treatment—increases exponentially with age.30–33
As people age, the mechanisms that regulate blood sugar are impaired: the glucagon response is diminished, which increases dependence on the epinephrine response to prevent hypoglycemia.34 Medications such as beta-blockers, which can suppress the symptoms of hypoglycemia, may further impair the response. Consequently, older patients may be less aware of hypoglycemia, and the symptoms may be less intense. Renal insufficiency may also exacerbate the problem by reducing clearance of oral agents. In addition, confused patients may take extra doses of medications.
Patients with type 2 diabetes treated with insulin, sulfonylureas, or meglitinides should be evaluated for symptoms of hypoglycemia. Older patients may have more neuroglycopenic symptoms (eg, dizziness, weakness, confusion, nightmares, violent behavior) than adrenergic symptoms (eg, sweating, palpitations, tremors), although both types should be asked about during an evaluation.2,32,33 Hypoglycemia may also present as transient hemiparesis, coma, or falls.35
We carefully evaluate the glycemic regimen and care environment of any elderly patient who presents with a blood glucose level below 100 mg/dL. The regimen should be altered for less strict control if the patient is cognitively impaired, is at risk of falling, or has an unstable care situation (eg, has irregular meals or needs assistance with daily activities and does not have a regular caregiver). Patients at significant risk of hypoglycemia should be encouraged to check their blood glucose level with a fingerstick before driving.
Tight control in the hospital is controversial
Glycemic control in the hospital has traditionally been designed primarily to maintain “safe” blood glucose levels, ie, to prevent hyperglycemia-induced dehydration and catabolism while avoiding hypoglycemia. Recent studies have suggested that tighter glycemic control may reduce the rates of complications and death perioperatively and in patients with myocardial infarction or who are seriously ill in the intensive care unit, although the evidence is mixed.36–38 Specific targets are controversial, and although studies have included some elderly patients, results cannot be generalized to this group.
DIABETES CARE TAKES A TEAM
Geriatric patients have complex problems. In the face of multiple comorbidities, difficult social situations, and polypharmacy, the physician can best address the drug therapy and lifestyle changes that diabetes management requires by working with a certified diabetes educator, dietitian, social worker, and pharmacist.
Nonpharmacologic therapy
The first step in therapy for glycemic control is diet and exercise, although such measures are often limited in the elderly.
Diet. Carbohydrate control can maintain euglycemia in some patients with type 2 diabetes. But for the elderly, especially those living in long-term health care facilities, malnutrition may be of more concern than obesity, making dietary restrictions harmful. Patients in danger of malnutrition should be given unrestricted menus with consistent amounts of carbohydrate at meals and snacks. Medications should be adjusted to control blood glucose levels if necessary.39
For patients living in the community, dietary therapy should be individualized by a dietitian. Medicare covers up to 10 hours of diabetes education with a certified diabetes educator or registered dietitian within a 12-month period if at least one of the following criteria are met: the patient is newly diagnosed with diabetes, the hemoglobin A1c level is higher than 8.5%, medication has been recently started, or the risk of complications is high.
Supplementation of vitamins and minerals is prudent. Supplemental magnesium, zinc, and vitamins C and E may improve glycemic control.40–44
Exercise reduces insulin resistance, weight, and blood pressure; increases muscle mass; and improves lipid levels. Both aerobic and nonaerobic activity are beneficial.45–47 The best time to exercise is 1 to 2 hours after a meal, when glucose levels tend to be highest. Either hypoglycemia or hyperglycemia may occur up to 24 hours following exercise, and medications may need to be adjusted.
Oral medications
Insulin
Insulin therapy is necessary if oral combination therapy proves insufficient. Insulin is generally required for patients with moderate or severe hyperglycemia, especially for those with renal or hepatic insufficiency.52,53 Before prescribing insulin therapy to elderly patients, we need to consider their visual acuity, manual dexterity and sensation, cognitive function, family support, and financial situation.However, several studies showed that quality of life improves in the year after starting insulin for patients whose blood sugar was previously poorly controlled with oral agents.54,55
An evening dose of neutral protamine Hagedorn (NPH) insulin is a good way to start. More complex regimens may be necessary, depending on glycemic goals.
A number of premixed preparations of various types of insulin with different durations of action are available. They may improve accuracy, acceptability, and ease of insulin administration, although glycemic control and the risk of hypoglycemia may not change or in fact may be worse.56 Some patients may not achieve adequate glucose control with fixed-dose regimens.57,58
Frequent, small, titrated doses of short-acting agents control hyperglycemia better, particularly postprandial hyperglycemia,resulting in less hypoglycemia. However, these regimens may be too complex for many elderly patients; a patient’s support system must be evaluated before recommending this type of therapy.
Most insulins are available in vials and in pens, the latter of which are quick and easy to use, provide precise doses, and can be managed by many elderly patients. Pens require the user to attach a needle, set the dose by a dial, and depress the plunger to inject the dose. Some are prefilled and disposable, others have refillable cartridges. Studies in patients older than 60 years have shown the pen systems to be more acceptable, safer, and more effective than conventional syringes.
If conventional syringes are used, low-dose syringes (30-unit or 50-unit), which have more visible unit markings, should be prescribed whenever possible rather than the 100-unit sizes. Magnifying devices that attach to a syringe are also available.
Studies have also shown that continuous subcutaneous insulin infusion is safe for selected elderly patients.
Incretin mimetics: Possibly well-suited
Incretins, such as glucagon-like peptide-1, are hormones released from the gastrointestinal tract in response to eating. They stimulate insulin secretion by non-glucose-related pathways.
Exenatide (Byetta), a 39-amino-acid peptide incretin mimetic, is a synthetic version of exendin-4, an incretin isolated from the saliva of the Gila monster. Recently approved for treating type 2 diabetes, it is given subcutaneously.59,60 Oral dipeptidyl peptidase-4 inhibitors (sitagliptin and vildagliptin) decrease the degradation of endogenous incretin and thus prolong its action.61 Because a decline in glucose-mediated beta-cell insulin secretion is a major contributor to the development of diabetes in the elderly, the drug may be especially helpful for this population.However, further clinical research and experience is needed before specific recommendations for elderly patients can be made.
SELF-MANAGEMENT IS IMPORTANT
Patient education is critical
Patient education is a cornerstone of diabetes self-management,62–66 and is especially important for patients who are cognitively impaired or who have limited language proficiency.Patient education is covered under Medicare Part B. Ample resources are available in print and electronic formats. Community resources can also be important.
Home glucose monitoring is simpler now
A patient’s insulin regimen should ideally be tailored according to home blood glucose level monitoring before and after meals and at bedtime. Medicare reimburses for once daily testing for patients who are not taking insulin and for three-times-daily testing for those taking insulin.
Elderly patients can be taught to reliably monitor their own blood glucose levels without diminishing their quality of life. Monitoring is now easier with new glucometers and test strips that use small amounts of blood. Testing can now also be performed on blood taken from the forearm, upper arm, thigh, or calf with the FreeStyle (TheraSense), One Touch Ultra (LifeScan) and Soft Tac (MediSense) meters. The Soft Tac meter lances skin and automatically transfers blood to the test strip, making use even easier. Talking glucometers are available for blind patients.
Coordination counts
A variety of models of chronic care delivery have been proposed. Regardless of which model is chosen, the complexities of management call for a multidisciplinary team approach, and coordination of care in order to ensure appropriate information flow becomes critical.
Editor’s note: In next month’s issue of this journal, Drs. Hornick and Aron will discuss the management of diabetic complications in the elderly, including coronary artery disease, neuropathy, and kidney disease.
- Edelstein SL, Knowler WC, Bain RP, et al. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes 1997; 46:701–710.
- Meneilly GS, Tessier D. Diabetes in elderly adults. J Gerontol A Biol Sci Med Sci 2001; 56:M5–M13.
- Rodriguez A, Muller DC, Engelhardt M, Andres R. Contribution of impaired glucose tolerance in subjects with the metabolic syndrome: Baltimore Longitudinal Study of Aging. Metabolism Clin Exper 2005; 54:542–547.
- Crandall J, Barzilai N. Treatment of diabetes mellitus in older people: oral therapy options. J Am Geriatr Soc 2003; 51:272–274.
- Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care 1998; 21:518–524.
- Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion. Diabetes Public Health Resource. National Diabetes Surveillance System. www.cdc.gov/diabetes/statistics/prev/national/figbyage.htm.
- Franse LV, Di Bari M, Shorr RI, et al; Health, Aging, and Body Composition Study Group. Type 2 diabetes in older well-functioning people: who is underdiagnosed? Data from the Health, Aging, and Body Composition study. Diabetes Care 2001; 24:2065–2070. Erratum in: Diabetes Care 2002; 25:413.
- Shorr RI, Franse LV, Resnick HE, Di Bari M, Johnson KC, Pahor M. Glycemic control of older adults with type 2 diabetes: findings from the Third National Health and Nutrition Examination Survey, 1988–1994. J Am Geriatr Soc 2000; 48:264–267.
- Smith NL, Savage PJ, Heckbert SR, et al. Glucose, blood pressure, and lipid control in older people with and without diabetes mellitus: the Cardiovascular Health Study. J Am Geriatr Soc 2002; 50:416–423.
- Meneilly GS, Cheung E, Tessier D, Yakura C, Tuokko H. The effect of improved glycemic control on cognitive functions in the elderly patient with diabetes. J Gerontol 1993; 48:M117–M121.
- American Diabetes Association. Standards of medical care in diabetes.Diabetes Care 2005; 28:S4–S36.
- Motta M, Bennati E, Ferlito L, Malaguarnera M. Diabetes mellitus in the elderly: diagnostic features. Arch Gerontol Geriatr 2006; 42:101–106.
- Hoerger TJ, Harris R, Hicks KA, Donahue K, Sorensen S, Engelgau M. Screening for type 2 diabetes mellitus: a cost effective analysis. Ann Intern Med 2004; 140:689–699.
- Harris RP, Lux LJ, Bunton AJ, et al. Screening for type 2 diabetes mellitus. Systematic Evidence Review Number 19. February 4, 2003.US Department of Health and Human Services, Agency for Healthcare Research and Quality. Rockville, MD. www.ahrq.gov/downloads/pub/prevent/pdfser/diabser.pdf.
- Brown AF, Mangione CM, Saliba D, Sarkisian CA; California Healthcare Foundation/American Geriatrics Society Panel on Improving Care for Elders with Diabetes. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc 2003; 51:S265–S280.
- VA/DoD Clinical Practice Guideline for the Management of Diabetes Mellitus in the Primary Care Setting 2003. www.oqp.med.va.gov/cpg/dm/DM3_cpg/content/introduction.htm.
- Durso SC. Using clinical guidelines designed for older adults with diabetes mellitus and complex health status. JAMA 2006; 295:1935–1940.
- Curb JD, Pressel SL, Cutler JA, et al. Effect of diuretic-based antihypertensive treatment on cardiovascular disease risk in older patients with isolated systolic hypertension. Systolic Hypertension in the Elderly Program Cooperative Research Group. JAMA 1996; 276:1886–1892. Erratum in: JAMA 1997; 277:1356.
- Goddijn PP, Bilo HJ, Feskens EJ, Groeniert KH, van der Zee KI, Meyboom-de Jong B. Longitudinal study on glycaemic control and quality of life in patients with Type 2 diabetes mellitus referred for intensified control. Diabet Med 1999; 16:23–30.
- Pyorala K, Pedersen TR, Kjekshus J, Faergeman O, Olsson AG, Thorgeirsson G. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care 1997; 20:614–620. Erratum in: Diabetes Care 1997; 20:1048.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853. Erratum in: Lancet 1999; 354:602.
- Katakura M, Naka M, Kondo T, et al, and the Nagano Elderly Diabetes Study Group. Normal mortality in the elderly with diabetes under strict glycemic and blood pressure control: outcome of 6-year prospective study. Diabetes Res Clin Practice 2007; 78:108–114.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346:393–403.
- Pogach LM, Brietzke SA, Cowan CL Jr, Conlin P, Walder DJ, Sawin CT; VA/DoD Diabetes Guideline Development Group. Development of evidence-based clinical practice guidelines for diabetes: the Department of Veterans Affairs/Department of Defense guidelines initiative. Diabetes Care 2004; 27:B82–B89.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317:703–713. Erratum in: BMJ 1999; 318:29.
- Qaseem A, Vijan S, Snow V, Cross JT, Weiss KB, Owens DK for the Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Glycemic control and type 2 diabetes mellitus: the optimal hemoglobin A1c targets. A guidance statement from the American College of Physicians. Ann Intern Med 2007; 147:417–422.
- Pogach L, Engelgau M, Aron D. Measuring progress toward achieving hemoglobin A1c goals in diabetes care: pass/fail or partial credit. JAMA 2007; 297:520–523.
- Egger M, Davey Smith G, Stettler C, Diem P. Risk of adverse effects of intensified treatment in insulin-dependent diabetes mellitus: ameta-analysis. Diabet Med 1997; 14:919–928.
- Burge MR, Schmitz-Fiorentino K, Fischette C, Qualls CR, Schade DS. A prospective trial of risk factors for sulfonylurea-induced hypoglycemia in type 2 diabetes mellitus. JAMA 1998; 279:137–143.
- Ben-Ami H, Nagachandran P, Mendelson A, Edoute Y. Drug-induced hypoglycemic coma in 102 diabetic patients. Arch Intern Med 1999; 159:281–284.
- Shorr RI, Ray WA, Daugherty JR, Griffin MR. Individual sulfonylureas and serious hypoglycemia in older people. J Am Geriatr Soc 1996; 44:751–755.
- Shorr RI, Ray WA, Daugherty JR, Griffin MR. Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas. Arch Intern Med 1997; 157:1681–1685.
- Burge MR, Kamin JR, Timm CT, Qualls CR, Schade DS. Low-dose epinephrine supports plasma glucose in fasted elderly patients with type 2 diabetes. Metabolism 2000; 49:195–202.
- Alagiakrishnan K, Lechelt K, McCracken P, Torrible S, Sclater A.Atypical presentation of silent nocturnal hypoglycemia in an olderperson. J Am Geriatr Soc 2001; 49:1577–1578.
- Malmberg K, Norhammar A, Wedel H, Ryden L. Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation 1999; 99:2626–2632.
- van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med 2001; 345:1359–1367.
- Metchick LN, Petit WA Jr, Inzucchi SE. Inpatient management of diabetes mellitus. Am J Med 2002; 113:317–323.
- Coulston AM, Mandelbaum D, Reaven GM. Dietary management of nursing home residents with non-insulin-dependent diabetes mellitus. Am J Clin Nutr 1990; 51:67–71.
- Song MK, Rosenthal MJ, Naliboff BD, Phanumas L, Kang KW. Effects of bovine prostate powder on zinc, glucose and insulin metabolism in old patients with non-insulin-dependent diabetes mellitus. Metabolism 1998; 47:39–43.
- Paolisso G, D’Amore A, Galzerano D, et al. Daily vitamin E supplements improve metabolic control but not insulin secretion in elderly type II diabetic patients. Diabetes Care 1993; 16:1433–1437.
- Paolisso G, Scheen A, Cozzolino D, et al. Changes in glucose turnover parameters and improvement of glucose oxidation after 4-week magnesium administration in elderly noninsulin-dependent (type II) diabetic patients. J Clin Endocrinol Metab 1994; 78:1510–1514.
- Paolisso G, Passariello N, Pizza G, et al. Dietary magnesium supplements improve B-cell response to glucose and arginine in elderly non-insulin dependent diabetic subjects. Acta Endocrinol (Copenh) 1989; 121:16–20.
- Paolisso G, D’Amore A, Balbi V, et al. Plasma vitamin C affects glucose homeostasis in healthy subjects and in non-insulin-dependent diabetics. Am J Physiol 1994; 266:E261–E268. Erratum in: Am J Physiol 1994; 267:section E following table of contents.
- Agurs-Collins TD, Kumanyika SK, Ten Have TR, Adams-Campbell LL. A randomized controlled trail of weight reduction and exercise for diabetes management in older African-American subjects. Diabetes Care 1997; 200:1503–1511.
- Skarfors ET, Wegener TA, Lithell H, Selinus I. Physical training as treatment for type 2 (non-insulin-dependent) diabetes in elderly men. A feasibility study over 2 years. Diabetologia 1987; 30:930–933.
- Raz I, Hauser E, Bursztyn M. Moderate exercise improves glucose metabolism in uncontrolled elderly patients with non-insulin-dependent diabetes mellitus. Isr J Med Sci 1994; 30:766–770.
- Brodows RG. Benefits and risks with glyburide and glipizide in elderly NIDDM patients. Diabetes Care 1992; 15:75–80.
- Landgraf R. Meglitinide analogues in the treatment of type 2 diabetes mellitus. Drugs Aging 2000; 17:411–425.
- Ron Y, Wainstein J, Leibovitz A, et al. The effect of acarbose on the colonic transit time of elderly long-term care patients with type 2 diabetes mellitus. J Gerontol A Biol Sci Med Sci 2002; 57:M111–M114.
- Bolen S, Feldman L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med 2007; 147:386–399.
- Rosenstock J. Management of type 2 diabetes mellitus in the elderly: special considerations. Drugs Aging 2001; 18:31–44.
- Gerstein HC, Haynes RB, eds. Evidence-Based Diabetes Management. Hamilton, Ont.; London: B.C. Dekker; 2001.
- Tovi J, Engfeldt P. Well being and symptoms in elderly type 2 diabetes patients with poor metabolic control: effect of insulin treatment. Practical Diabetes Int 1998; 15:73–77.
- Reza M, Taylor CD, Towse K, Ward JD, Hendra TJ. Insulin improves well-being for selected elderly type 2 diabetic subjects. Diabetes Res Clin Pract 2002; 55:201–207.
- Janka HU, Plewe G, Busch K. Combination of oral antidiabetic agents with basal insulin versus premixed insulin alone in randomized elderly patients with type 2 diabetes mellitus. J Am Geriatrics Soc 2007; 55:182–188.
- Coscelli C, Calabrese G, Fedele D, et al. Use of premixed insulin among the elderly. Reduction of errors in patient preparation of mixtures. Diabetes Care 1992; 15:1628–1630.
- Rolla AR, Rakel RE. Practical approaches to insulin therapy for type 2 diabetes mellitus with premixed insulin analogues. Clin Ther 2005; 27:1113–1125.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28:1083–1091.
- Mathieu C, Bollaerts K. Antihyperglycaemic therapy in elderly patients with type 2 diabetes: potential role of incretin mimetics and DPP-4 inhibitors. Int J Clin Pract 2007; 61(suppl 154):29–37.
- Bernbaum M, Albert SG, McGinnis J, Brusca S, Mooradian AD. The reliability of self blood glucose monitoring in elderly diabetic patients. J Am Geriatr Soc 1994; 42:779–781.
- Gilden JL, Hendryx M, Casia C, Singh SP. The effectiveness of diabetes education programs for older patients and their spouses. J Am Geriatr Soc 1989; 37:1023–1030.
- Glasgow RE, Toobert DJ, Hampson SE, Brown JE, Lewinsohn PM, Donnelly J. Improving self-care among older patients with type II diabetes: the ‘Sixty Something…’ Study. Patient Educ Couns 1992; 19:61–74.
- Huang ES, Gorawara-Bhat R, Chin MH. Self-reported goals of older patients with type 2 diabetes mellitus. J Am Geriatr Soc 2005; 53:306–311.
- Langa KM, Vijan S, Hayward RA, et al. Informal caregiving for diabetes and diabetic complications among elderly Americans. J Gerontol B Psychol Sci Soc Sci 2002; 57:S177–S186.
Guidelines for treating diabetes mellitus are mostly based on clinical studies in middle-aged people, and recommendations tend to be the same for everyone, whether young and strong or elderly and frail. But diabetes management should be individualized, especially in the elderly, taking into account each patient’s medical history, functional ability, home care situation, and life expectancy. Aggressive glycemic control is less important than avoiding hypoglycemia and achieving a good quality of life.
This article reviews the general principles for recognizing and managing diabetes in elderly patients, focusing on the management of blood sugar per se. In a future issue of this journal, we will discuss some of the many complications of diabetes in the elderly.
DIABETES DIFFERS IN ELDERLY PATIENTS
“The elderly” is a heterogeneous group with widely varying physiologic profiles, functional capabilities, and life expectancy (on average, about 88 years for men and 90 years for women in the United States). Although the elderly are sometimes classified as “young-old” (age 65–80) and “old-old” (80+), this distinction is too simplistic for clinical decision-making.
Diabetes mellitus in the elderly also is heterogeneous. One distinction is the age at which the disease developed.
Aging is associated with declining beta-cell function and lower blood insulin levels independent of insulin resistance, and with insulin resistance itself. The risk of developing type 2 diabetes mellitus increases with obesity, lack of physical activity, and loss of muscle mass, all of which often develop with aging.1
Middle-aged patients with diabetes have increased fasting hepatic glucose production, increased insulin resistance, and an abnormal insulin response to a glucose load. On the other hand, patients who develop diabetes at an older age tend to have normal hepatic glucose production. Older patients who are lean secrete markedly less insulin in response to a glucose load but have relatively less insulin resistance.2 Patients who develop type 2 diabetes in old age are more likely to have near-normal fasting blood glucose levels but significant postprandial hyperglycemia.3,4 Elderly patients who developed diabetes during middle age have metabolic abnormalities more typical of middle-aged patients with type 2 diabetes.
DIABETES IS COMMON, AND INCREASING IN PREVALENCE
By age 75, 40% of people in the United States have either glucose intolerance or diabetes mellitus.5 Metabolic syndrome, which is the constellation of insulin resistance (type 2 diabetes mellitus), hyperlipidemia, hypertension, and obesity, is more prevalent in people age 65 to 74 years than in younger and older people.3
The National Diabetes Surveillance System of the US Centers for Disease Control and Prevention estimated that the prevalence of diabetes mellitus in people 65 to 74 years old in 2005 was 18.5%, about 12 times the prevalence among those younger than 45years.6 The prevalence has been gradually increasing and has nearly doubled over the past 25 years, with certain groups—native Americans, Hispanics, and African-Americans—at particularly high risk of developing the disease.
Although the prevalence of diabetes in people older than 75 years is lower than among people in the 65-to-74-year range, the elderly segment of our population is increasing, and the impact of diabetes and its associated burden of death and disease from vascular complications is enormous.
SYMPTOMS ARE OFTEN NONSPECIFIC
Unfortunately, diabetes is underdiagnosed and frequently undertreated, resulting in even more disease and death.7–9
Diabetes is often missed in the elderly because its presenting symptoms may be nonspecific, eg, failure to thrive, low energy, falls, dizziness, confusion, nocturia (with or without incontinence), and urinary tract infection.The classic symptoms of frequent urination (often leading to worsening incontinence), thirst, and increased hunger usually occur only when plasma glucose levels are above 200 mg/dL. Weight loss, blurred vision, and dehydration may also be present with high blood glucose levels. With lesser degrees of hyperglycemia, patients may have no symptoms or present with weight loss or signs and symptoms of chronic infection, especially of the genitourinary tract, skin, or mouth.
Hyperglycemia in elderly patients is also associated with reduced cognitive function (which may improve with blood glucose control).10
The American Diabetes Association recommends screening by measuring the fasting plasma glucose level every 3 years beginning at 45 years.11 However, some experts believe that this method is inadequate for the elderly12; some suggest that screening should be done more often in those with risk factors for diabetes, including obesity, inactivity, hypertension, and dyslipidemia, all of which are common in the elderly. Targeted screening in patients with hypertension may be the most cost-effective strategy.13
Screening with hemoglobin A1c levels is not recommended because of lack of standardization among laboratories.14
INDIVIDUALIZED MANAGEMENT IS BEST
Despite disease differences, the general goals for diabetes care are the same for all ages:
- To control hyperglycemia and its symptoms
- To prevent, evaluate, and treat macrovascular and microvascular complications
- To teach patients to manage themselves
- To maintain or improve the patient’s general health status.
Unfortunately, most specific recommendations are based on studies in younger people. Guidelines should ideally reflect the complexities of a particular clinical situation, but most recommendations are applied to the young and old alike, as well as to the relatively healthy and the frail and ill.15–17 Consideration should be given to a patient’s health beliefs, severity of vascular complications and other medical problems, economic situation, life expectancy ,functional status, and availability of support services. In addition, some patients prefer aggressive treatment, while others would rather compromise some aspects of care in order to maintain a certain quality of life, to save money, or to avoid having caregivers provide treatment.
Age-related changes in pharmacokinetics as well as polypharmacy increase the risk of drug interactions and adverse effects, especially drug-induced hypoglycemia. In addition, age-associated changes in cognitive, visual, and physical function, dentition, and taste perception can reduce a patient’s ability to carry out treatment. Frequent hospitalizations also disrupt outpatient regimens.
Comorbidities make treatment more challenging, but some conditions—such as hypertension, renal insufficiency and eye disorders—make doctors more likely to control hyperglycemia more aggressively, fearing that the loss of a little more function in an impaired organ may lead to failure.
The benefits of tight glycemic control should be weighed against the risks and the realities of an individual situation. Priority should be given to achieving the best quality of life possible.17 Recent guidelines from the California Health Foundation and the American Geriatrics Association focused on the major health threats to older patients and prioritizing care for each person.15 The guidelines recommend screening for geriatric syndromes that are more prevalent inpatients with diabetes or are strongly affected by the disease or its treatment. Diabetes care should be examined in the setting of common geriatric problems: depression, polypharmacy, cognitive impairment, urinary incontinence, falls, and pain.
Heart risk trumps glycemic control
The expert panel15 concluded that rates of disease and death can be reduced more by targeting cardiovascular risk factors than by intensively managing hyperglycemia. One rationale is that it takes 8 years for aggressive glycemic control to reduce the risk of diabetic retinopathy or renal disease but only 2 years of treating hypertension and dyslipidemia to reduce the risk of cardiovascular disease.15,17–21 A recent Japanese study found normal mortality rates in elderly patients under long-term, intensive multifactorial diabetes control.22 High-functioning, motivated patients could benefit from therapy aimed at achieving most or all of the recommended goals, but frail patients may suffer from applying all therapies and may benefit from only some of them.
If appropriate goals cannot be met, it may help to refer patients to a geriatric specialist to evaluate possible barriers to adherence such as depression or poor cognition, physical functioning, or support.
MANAGEMENT STRATEGIES
Weight loss and exercise help prevent diabetes
The Diabetes Prevention Program23 randomized 3,234 people (mean age 51 years) with impaired glucose tolerance to receive either metformin (Fortamet, Glucophage) 850 mg twice daily or placebo or to undertake lifestyle modifications with goals of at least a 7% weight loss and at least 150 minutes of physical activity per week. Compared with the placebo group, the lifestyle modification group had a 58% lower incidence of diabetes while those in the metformin group had only a 31% lower incidence. Among those older than 60 years, the advantage of lifestyle modification over metformin was even greater.
Control blood glucose, avoid hypoglycemia
- Hemoglobin A1c levels < 7.0%
- Preprandial blood glucose levels 90–130 mg/dL
- Bedtime blood glucose levels 110–150 mg/dL.
Guidelines from the Department of Veterans Affairs24 and the American Geriatrics Society15 are slightly different, and are based on randomized trials in younger patients, primarily the Diabetes Control and Complications Trial (DCCT)25 and the United Kingdom Prospective Diabetes Study(UKPDS).21,26 A recent position statement from the American College of Physicians, based on a review of all the major guidelines, recommends the following: “Statement 1: To prevent microvascular complication of diabetes, the goal for glycemic control should be as low as is feasible without undue risk for adverse events or an unacceptable burden on patients. Treatment goals should be based on a discussion of the benefits and harms of specific levels of glycemic control with the patient. A hemoglobin A1c level less than 7% based on individualized assessment is a reasonable goal for many but not all patients. Statement 2: The goal for hemoglobin A1c should be based on individualized assessment of risk for complication from diabetes, comorbidity, life expectancy, and patient preferences.”27
Although few data exist for elderly patients, these guidelines are the most current approach to treating diabetes in the elderly. Less stringent goals are appropriate for patients who have limited life expectancy, hypoglycemia unawareness (lack of autonomic warning symptoms of low blood sugar), seizures, dementia, psychiatric illness, or alcoholism. It is important to keep in mind the following as one strives for lower A1c levels: Although the relative risk reduction accomplished by lowering hemoglobin A1c is linear, the absolute risk reduction is log-linear—more benefit is gained by lowering hemoglobin A1c from 9% to 8% than from 8% to 7%.28
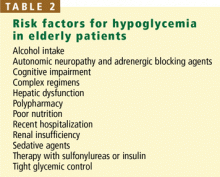
Hypoglycemia is a major limiting factor in glycemic control. Many risk factors for hypoglycemia are common in the elderly (Table 2). Hypoglycemia was a chief adverse event in both the DCCT and the UKPDS, with a twofold to threefold higher rate in patients who were intensively treated.29 Even mild hypoglycemia in the elderly can result in an injurious fall, which can lead to long-term functional decline. The rate of severe or fatal hypoglycemia—the major risk of tight glycemic treatment—increases exponentially with age.30–33
As people age, the mechanisms that regulate blood sugar are impaired: the glucagon response is diminished, which increases dependence on the epinephrine response to prevent hypoglycemia.34 Medications such as beta-blockers, which can suppress the symptoms of hypoglycemia, may further impair the response. Consequently, older patients may be less aware of hypoglycemia, and the symptoms may be less intense. Renal insufficiency may also exacerbate the problem by reducing clearance of oral agents. In addition, confused patients may take extra doses of medications.
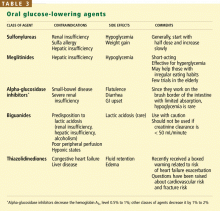
Patients with type 2 diabetes treated with insulin, sulfonylureas, or meglitinides should be evaluated for symptoms of hypoglycemia. Older patients may have more neuroglycopenic symptoms (eg, dizziness, weakness, confusion, nightmares, violent behavior) than adrenergic symptoms (eg, sweating, palpitations, tremors), although both types should be asked about during an evaluation.2,32,33 Hypoglycemia may also present as transient hemiparesis, coma, or falls.35
We carefully evaluate the glycemic regimen and care environment of any elderly patient who presents with a blood glucose level below 100 mg/dL. The regimen should be altered for less strict control if the patient is cognitively impaired, is at risk of falling, or has an unstable care situation (eg, has irregular meals or needs assistance with daily activities and does not have a regular caregiver). Patients at significant risk of hypoglycemia should be encouraged to check their blood glucose level with a fingerstick before driving.
Tight control in the hospital is controversial
Glycemic control in the hospital has traditionally been designed primarily to maintain “safe” blood glucose levels, ie, to prevent hyperglycemia-induced dehydration and catabolism while avoiding hypoglycemia. Recent studies have suggested that tighter glycemic control may reduce the rates of complications and death perioperatively and in patients with myocardial infarction or who are seriously ill in the intensive care unit, although the evidence is mixed.36–38 Specific targets are controversial, and although studies have included some elderly patients, results cannot be generalized to this group.
DIABETES CARE TAKES A TEAM
Geriatric patients have complex problems. In the face of multiple comorbidities, difficult social situations, and polypharmacy, the physician can best address the drug therapy and lifestyle changes that diabetes management requires by working with a certified diabetes educator, dietitian, social worker, and pharmacist.
Nonpharmacologic therapy
The first step in therapy for glycemic control is diet and exercise, although such measures are often limited in the elderly.
Diet. Carbohydrate control can maintain euglycemia in some patients with type 2 diabetes. But for the elderly, especially those living in long-term health care facilities, malnutrition may be of more concern than obesity, making dietary restrictions harmful. Patients in danger of malnutrition should be given unrestricted menus with consistent amounts of carbohydrate at meals and snacks. Medications should be adjusted to control blood glucose levels if necessary.39
For patients living in the community, dietary therapy should be individualized by a dietitian. Medicare covers up to 10 hours of diabetes education with a certified diabetes educator or registered dietitian within a 12-month period if at least one of the following criteria are met: the patient is newly diagnosed with diabetes, the hemoglobin A1c level is higher than 8.5%, medication has been recently started, or the risk of complications is high.
Supplementation of vitamins and minerals is prudent. Supplemental magnesium, zinc, and vitamins C and E may improve glycemic control.40–44
Exercise reduces insulin resistance, weight, and blood pressure; increases muscle mass; and improves lipid levels. Both aerobic and nonaerobic activity are beneficial.45–47 The best time to exercise is 1 to 2 hours after a meal, when glucose levels tend to be highest. Either hypoglycemia or hyperglycemia may occur up to 24 hours following exercise, and medications may need to be adjusted.
Oral medications
Insulin
Insulin therapy is necessary if oral combination therapy proves insufficient. Insulin is generally required for patients with moderate or severe hyperglycemia, especially for those with renal or hepatic insufficiency.52,53 Before prescribing insulin therapy to elderly patients, we need to consider their visual acuity, manual dexterity and sensation, cognitive function, family support, and financial situation.However, several studies showed that quality of life improves in the year after starting insulin for patients whose blood sugar was previously poorly controlled with oral agents.54,55
An evening dose of neutral protamine Hagedorn (NPH) insulin is a good way to start. More complex regimens may be necessary, depending on glycemic goals.
A number of premixed preparations of various types of insulin with different durations of action are available. They may improve accuracy, acceptability, and ease of insulin administration, although glycemic control and the risk of hypoglycemia may not change or in fact may be worse.56 Some patients may not achieve adequate glucose control with fixed-dose regimens.57,58
Frequent, small, titrated doses of short-acting agents control hyperglycemia better, particularly postprandial hyperglycemia,resulting in less hypoglycemia. However, these regimens may be too complex for many elderly patients; a patient’s support system must be evaluated before recommending this type of therapy.
Most insulins are available in vials and in pens, the latter of which are quick and easy to use, provide precise doses, and can be managed by many elderly patients. Pens require the user to attach a needle, set the dose by a dial, and depress the plunger to inject the dose. Some are prefilled and disposable, others have refillable cartridges. Studies in patients older than 60 years have shown the pen systems to be more acceptable, safer, and more effective than conventional syringes.
If conventional syringes are used, low-dose syringes (30-unit or 50-unit), which have more visible unit markings, should be prescribed whenever possible rather than the 100-unit sizes. Magnifying devices that attach to a syringe are also available.
Studies have also shown that continuous subcutaneous insulin infusion is safe for selected elderly patients.
Incretin mimetics: Possibly well-suited
Incretins, such as glucagon-like peptide-1, are hormones released from the gastrointestinal tract in response to eating. They stimulate insulin secretion by non-glucose-related pathways.
Exenatide (Byetta), a 39-amino-acid peptide incretin mimetic, is a synthetic version of exendin-4, an incretin isolated from the saliva of the Gila monster. Recently approved for treating type 2 diabetes, it is given subcutaneously.59,60 Oral dipeptidyl peptidase-4 inhibitors (sitagliptin and vildagliptin) decrease the degradation of endogenous incretin and thus prolong its action.61 Because a decline in glucose-mediated beta-cell insulin secretion is a major contributor to the development of diabetes in the elderly, the drug may be especially helpful for this population.However, further clinical research and experience is needed before specific recommendations for elderly patients can be made.
SELF-MANAGEMENT IS IMPORTANT
Patient education is critical
Patient education is a cornerstone of diabetes self-management,62–66 and is especially important for patients who are cognitively impaired or who have limited language proficiency.Patient education is covered under Medicare Part B. Ample resources are available in print and electronic formats. Community resources can also be important.
Home glucose monitoring is simpler now
A patient’s insulin regimen should ideally be tailored according to home blood glucose level monitoring before and after meals and at bedtime. Medicare reimburses for once daily testing for patients who are not taking insulin and for three-times-daily testing for those taking insulin.
Elderly patients can be taught to reliably monitor their own blood glucose levels without diminishing their quality of life. Monitoring is now easier with new glucometers and test strips that use small amounts of blood. Testing can now also be performed on blood taken from the forearm, upper arm, thigh, or calf with the FreeStyle (TheraSense), One Touch Ultra (LifeScan) and Soft Tac (MediSense) meters. The Soft Tac meter lances skin and automatically transfers blood to the test strip, making use even easier. Talking glucometers are available for blind patients.
Coordination counts
A variety of models of chronic care delivery have been proposed. Regardless of which model is chosen, the complexities of management call for a multidisciplinary team approach, and coordination of care in order to ensure appropriate information flow becomes critical.
Editor’s note: In next month’s issue of this journal, Drs. Hornick and Aron will discuss the management of diabetic complications in the elderly, including coronary artery disease, neuropathy, and kidney disease.
Guidelines for treating diabetes mellitus are mostly based on clinical studies in middle-aged people, and recommendations tend to be the same for everyone, whether young and strong or elderly and frail. But diabetes management should be individualized, especially in the elderly, taking into account each patient’s medical history, functional ability, home care situation, and life expectancy. Aggressive glycemic control is less important than avoiding hypoglycemia and achieving a good quality of life.
This article reviews the general principles for recognizing and managing diabetes in elderly patients, focusing on the management of blood sugar per se. In a future issue of this journal, we will discuss some of the many complications of diabetes in the elderly.
DIABETES DIFFERS IN ELDERLY PATIENTS
“The elderly” is a heterogeneous group with widely varying physiologic profiles, functional capabilities, and life expectancy (on average, about 88 years for men and 90 years for women in the United States). Although the elderly are sometimes classified as “young-old” (age 65–80) and “old-old” (80+), this distinction is too simplistic for clinical decision-making.
Diabetes mellitus in the elderly also is heterogeneous. One distinction is the age at which the disease developed.
Aging is associated with declining beta-cell function and lower blood insulin levels independent of insulin resistance, and with insulin resistance itself. The risk of developing type 2 diabetes mellitus increases with obesity, lack of physical activity, and loss of muscle mass, all of which often develop with aging.1
Middle-aged patients with diabetes have increased fasting hepatic glucose production, increased insulin resistance, and an abnormal insulin response to a glucose load. On the other hand, patients who develop diabetes at an older age tend to have normal hepatic glucose production. Older patients who are lean secrete markedly less insulin in response to a glucose load but have relatively less insulin resistance.2 Patients who develop type 2 diabetes in old age are more likely to have near-normal fasting blood glucose levels but significant postprandial hyperglycemia.3,4 Elderly patients who developed diabetes during middle age have metabolic abnormalities more typical of middle-aged patients with type 2 diabetes.
DIABETES IS COMMON, AND INCREASING IN PREVALENCE
By age 75, 40% of people in the United States have either glucose intolerance or diabetes mellitus.5 Metabolic syndrome, which is the constellation of insulin resistance (type 2 diabetes mellitus), hyperlipidemia, hypertension, and obesity, is more prevalent in people age 65 to 74 years than in younger and older people.3
The National Diabetes Surveillance System of the US Centers for Disease Control and Prevention estimated that the prevalence of diabetes mellitus in people 65 to 74 years old in 2005 was 18.5%, about 12 times the prevalence among those younger than 45years.6 The prevalence has been gradually increasing and has nearly doubled over the past 25 years, with certain groups—native Americans, Hispanics, and African-Americans—at particularly high risk of developing the disease.
Although the prevalence of diabetes in people older than 75 years is lower than among people in the 65-to-74-year range, the elderly segment of our population is increasing, and the impact of diabetes and its associated burden of death and disease from vascular complications is enormous.
SYMPTOMS ARE OFTEN NONSPECIFIC
Unfortunately, diabetes is underdiagnosed and frequently undertreated, resulting in even more disease and death.7–9
Diabetes is often missed in the elderly because its presenting symptoms may be nonspecific, eg, failure to thrive, low energy, falls, dizziness, confusion, nocturia (with or without incontinence), and urinary tract infection.The classic symptoms of frequent urination (often leading to worsening incontinence), thirst, and increased hunger usually occur only when plasma glucose levels are above 200 mg/dL. Weight loss, blurred vision, and dehydration may also be present with high blood glucose levels. With lesser degrees of hyperglycemia, patients may have no symptoms or present with weight loss or signs and symptoms of chronic infection, especially of the genitourinary tract, skin, or mouth.
Hyperglycemia in elderly patients is also associated with reduced cognitive function (which may improve with blood glucose control).10
The American Diabetes Association recommends screening by measuring the fasting plasma glucose level every 3 years beginning at 45 years.11 However, some experts believe that this method is inadequate for the elderly12; some suggest that screening should be done more often in those with risk factors for diabetes, including obesity, inactivity, hypertension, and dyslipidemia, all of which are common in the elderly. Targeted screening in patients with hypertension may be the most cost-effective strategy.13
Screening with hemoglobin A1c levels is not recommended because of lack of standardization among laboratories.14
INDIVIDUALIZED MANAGEMENT IS BEST
Despite disease differences, the general goals for diabetes care are the same for all ages:
- To control hyperglycemia and its symptoms
- To prevent, evaluate, and treat macrovascular and microvascular complications
- To teach patients to manage themselves
- To maintain or improve the patient’s general health status.
Unfortunately, most specific recommendations are based on studies in younger people. Guidelines should ideally reflect the complexities of a particular clinical situation, but most recommendations are applied to the young and old alike, as well as to the relatively healthy and the frail and ill.15–17 Consideration should be given to a patient’s health beliefs, severity of vascular complications and other medical problems, economic situation, life expectancy ,functional status, and availability of support services. In addition, some patients prefer aggressive treatment, while others would rather compromise some aspects of care in order to maintain a certain quality of life, to save money, or to avoid having caregivers provide treatment.
Age-related changes in pharmacokinetics as well as polypharmacy increase the risk of drug interactions and adverse effects, especially drug-induced hypoglycemia. In addition, age-associated changes in cognitive, visual, and physical function, dentition, and taste perception can reduce a patient’s ability to carry out treatment. Frequent hospitalizations also disrupt outpatient regimens.
Comorbidities make treatment more challenging, but some conditions—such as hypertension, renal insufficiency and eye disorders—make doctors more likely to control hyperglycemia more aggressively, fearing that the loss of a little more function in an impaired organ may lead to failure.
The benefits of tight glycemic control should be weighed against the risks and the realities of an individual situation. Priority should be given to achieving the best quality of life possible.17 Recent guidelines from the California Health Foundation and the American Geriatrics Association focused on the major health threats to older patients and prioritizing care for each person.15 The guidelines recommend screening for geriatric syndromes that are more prevalent inpatients with diabetes or are strongly affected by the disease or its treatment. Diabetes care should be examined in the setting of common geriatric problems: depression, polypharmacy, cognitive impairment, urinary incontinence, falls, and pain.
Heart risk trumps glycemic control
The expert panel15 concluded that rates of disease and death can be reduced more by targeting cardiovascular risk factors than by intensively managing hyperglycemia. One rationale is that it takes 8 years for aggressive glycemic control to reduce the risk of diabetic retinopathy or renal disease but only 2 years of treating hypertension and dyslipidemia to reduce the risk of cardiovascular disease.15,17–21 A recent Japanese study found normal mortality rates in elderly patients under long-term, intensive multifactorial diabetes control.22 High-functioning, motivated patients could benefit from therapy aimed at achieving most or all of the recommended goals, but frail patients may suffer from applying all therapies and may benefit from only some of them.
If appropriate goals cannot be met, it may help to refer patients to a geriatric specialist to evaluate possible barriers to adherence such as depression or poor cognition, physical functioning, or support.
MANAGEMENT STRATEGIES
Weight loss and exercise help prevent diabetes
The Diabetes Prevention Program23 randomized 3,234 people (mean age 51 years) with impaired glucose tolerance to receive either metformin (Fortamet, Glucophage) 850 mg twice daily or placebo or to undertake lifestyle modifications with goals of at least a 7% weight loss and at least 150 minutes of physical activity per week. Compared with the placebo group, the lifestyle modification group had a 58% lower incidence of diabetes while those in the metformin group had only a 31% lower incidence. Among those older than 60 years, the advantage of lifestyle modification over metformin was even greater.
Control blood glucose, avoid hypoglycemia
- Hemoglobin A1c levels < 7.0%
- Preprandial blood glucose levels 90–130 mg/dL
- Bedtime blood glucose levels 110–150 mg/dL.
Guidelines from the Department of Veterans Affairs24 and the American Geriatrics Society15 are slightly different, and are based on randomized trials in younger patients, primarily the Diabetes Control and Complications Trial (DCCT)25 and the United Kingdom Prospective Diabetes Study(UKPDS).21,26 A recent position statement from the American College of Physicians, based on a review of all the major guidelines, recommends the following: “Statement 1: To prevent microvascular complication of diabetes, the goal for glycemic control should be as low as is feasible without undue risk for adverse events or an unacceptable burden on patients. Treatment goals should be based on a discussion of the benefits and harms of specific levels of glycemic control with the patient. A hemoglobin A1c level less than 7% based on individualized assessment is a reasonable goal for many but not all patients. Statement 2: The goal for hemoglobin A1c should be based on individualized assessment of risk for complication from diabetes, comorbidity, life expectancy, and patient preferences.”27
Although few data exist for elderly patients, these guidelines are the most current approach to treating diabetes in the elderly. Less stringent goals are appropriate for patients who have limited life expectancy, hypoglycemia unawareness (lack of autonomic warning symptoms of low blood sugar), seizures, dementia, psychiatric illness, or alcoholism. It is important to keep in mind the following as one strives for lower A1c levels: Although the relative risk reduction accomplished by lowering hemoglobin A1c is linear, the absolute risk reduction is log-linear—more benefit is gained by lowering hemoglobin A1c from 9% to 8% than from 8% to 7%.28
Hypoglycemia is a major limiting factor in glycemic control. Many risk factors for hypoglycemia are common in the elderly (Table 2). Hypoglycemia was a chief adverse event in both the DCCT and the UKPDS, with a twofold to threefold higher rate in patients who were intensively treated.29 Even mild hypoglycemia in the elderly can result in an injurious fall, which can lead to long-term functional decline. The rate of severe or fatal hypoglycemia—the major risk of tight glycemic treatment—increases exponentially with age.30–33
As people age, the mechanisms that regulate blood sugar are impaired: the glucagon response is diminished, which increases dependence on the epinephrine response to prevent hypoglycemia.34 Medications such as beta-blockers, which can suppress the symptoms of hypoglycemia, may further impair the response. Consequently, older patients may be less aware of hypoglycemia, and the symptoms may be less intense. Renal insufficiency may also exacerbate the problem by reducing clearance of oral agents. In addition, confused patients may take extra doses of medications.
Patients with type 2 diabetes treated with insulin, sulfonylureas, or meglitinides should be evaluated for symptoms of hypoglycemia. Older patients may have more neuroglycopenic symptoms (eg, dizziness, weakness, confusion, nightmares, violent behavior) than adrenergic symptoms (eg, sweating, palpitations, tremors), although both types should be asked about during an evaluation.2,32,33 Hypoglycemia may also present as transient hemiparesis, coma, or falls.35
We carefully evaluate the glycemic regimen and care environment of any elderly patient who presents with a blood glucose level below 100 mg/dL. The regimen should be altered for less strict control if the patient is cognitively impaired, is at risk of falling, or has an unstable care situation (eg, has irregular meals or needs assistance with daily activities and does not have a regular caregiver). Patients at significant risk of hypoglycemia should be encouraged to check their blood glucose level with a fingerstick before driving.
Tight control in the hospital is controversial
Glycemic control in the hospital has traditionally been designed primarily to maintain “safe” blood glucose levels, ie, to prevent hyperglycemia-induced dehydration and catabolism while avoiding hypoglycemia. Recent studies have suggested that tighter glycemic control may reduce the rates of complications and death perioperatively and in patients with myocardial infarction or who are seriously ill in the intensive care unit, although the evidence is mixed.36–38 Specific targets are controversial, and although studies have included some elderly patients, results cannot be generalized to this group.
DIABETES CARE TAKES A TEAM
Geriatric patients have complex problems. In the face of multiple comorbidities, difficult social situations, and polypharmacy, the physician can best address the drug therapy and lifestyle changes that diabetes management requires by working with a certified diabetes educator, dietitian, social worker, and pharmacist.
Nonpharmacologic therapy
The first step in therapy for glycemic control is diet and exercise, although such measures are often limited in the elderly.
Diet. Carbohydrate control can maintain euglycemia in some patients with type 2 diabetes. But for the elderly, especially those living in long-term health care facilities, malnutrition may be of more concern than obesity, making dietary restrictions harmful. Patients in danger of malnutrition should be given unrestricted menus with consistent amounts of carbohydrate at meals and snacks. Medications should be adjusted to control blood glucose levels if necessary.39
For patients living in the community, dietary therapy should be individualized by a dietitian. Medicare covers up to 10 hours of diabetes education with a certified diabetes educator or registered dietitian within a 12-month period if at least one of the following criteria are met: the patient is newly diagnosed with diabetes, the hemoglobin A1c level is higher than 8.5%, medication has been recently started, or the risk of complications is high.
Supplementation of vitamins and minerals is prudent. Supplemental magnesium, zinc, and vitamins C and E may improve glycemic control.40–44
Exercise reduces insulin resistance, weight, and blood pressure; increases muscle mass; and improves lipid levels. Both aerobic and nonaerobic activity are beneficial.45–47 The best time to exercise is 1 to 2 hours after a meal, when glucose levels tend to be highest. Either hypoglycemia or hyperglycemia may occur up to 24 hours following exercise, and medications may need to be adjusted.
Oral medications
Insulin
Insulin therapy is necessary if oral combination therapy proves insufficient. Insulin is generally required for patients with moderate or severe hyperglycemia, especially for those with renal or hepatic insufficiency.52,53 Before prescribing insulin therapy to elderly patients, we need to consider their visual acuity, manual dexterity and sensation, cognitive function, family support, and financial situation.However, several studies showed that quality of life improves in the year after starting insulin for patients whose blood sugar was previously poorly controlled with oral agents.54,55
An evening dose of neutral protamine Hagedorn (NPH) insulin is a good way to start. More complex regimens may be necessary, depending on glycemic goals.
A number of premixed preparations of various types of insulin with different durations of action are available. They may improve accuracy, acceptability, and ease of insulin administration, although glycemic control and the risk of hypoglycemia may not change or in fact may be worse.56 Some patients may not achieve adequate glucose control with fixed-dose regimens.57,58
Frequent, small, titrated doses of short-acting agents control hyperglycemia better, particularly postprandial hyperglycemia,resulting in less hypoglycemia. However, these regimens may be too complex for many elderly patients; a patient’s support system must be evaluated before recommending this type of therapy.
Most insulins are available in vials and in pens, the latter of which are quick and easy to use, provide precise doses, and can be managed by many elderly patients. Pens require the user to attach a needle, set the dose by a dial, and depress the plunger to inject the dose. Some are prefilled and disposable, others have refillable cartridges. Studies in patients older than 60 years have shown the pen systems to be more acceptable, safer, and more effective than conventional syringes.
If conventional syringes are used, low-dose syringes (30-unit or 50-unit), which have more visible unit markings, should be prescribed whenever possible rather than the 100-unit sizes. Magnifying devices that attach to a syringe are also available.
Studies have also shown that continuous subcutaneous insulin infusion is safe for selected elderly patients.
Incretin mimetics: Possibly well-suited
Incretins, such as glucagon-like peptide-1, are hormones released from the gastrointestinal tract in response to eating. They stimulate insulin secretion by non-glucose-related pathways.
Exenatide (Byetta), a 39-amino-acid peptide incretin mimetic, is a synthetic version of exendin-4, an incretin isolated from the saliva of the Gila monster. Recently approved for treating type 2 diabetes, it is given subcutaneously.59,60 Oral dipeptidyl peptidase-4 inhibitors (sitagliptin and vildagliptin) decrease the degradation of endogenous incretin and thus prolong its action.61 Because a decline in glucose-mediated beta-cell insulin secretion is a major contributor to the development of diabetes in the elderly, the drug may be especially helpful for this population.However, further clinical research and experience is needed before specific recommendations for elderly patients can be made.
SELF-MANAGEMENT IS IMPORTANT
Patient education is critical
Patient education is a cornerstone of diabetes self-management,62–66 and is especially important for patients who are cognitively impaired or who have limited language proficiency.Patient education is covered under Medicare Part B. Ample resources are available in print and electronic formats. Community resources can also be important.
Home glucose monitoring is simpler now
A patient’s insulin regimen should ideally be tailored according to home blood glucose level monitoring before and after meals and at bedtime. Medicare reimburses for once daily testing for patients who are not taking insulin and for three-times-daily testing for those taking insulin.
Elderly patients can be taught to reliably monitor their own blood glucose levels without diminishing their quality of life. Monitoring is now easier with new glucometers and test strips that use small amounts of blood. Testing can now also be performed on blood taken from the forearm, upper arm, thigh, or calf with the FreeStyle (TheraSense), One Touch Ultra (LifeScan) and Soft Tac (MediSense) meters. The Soft Tac meter lances skin and automatically transfers blood to the test strip, making use even easier. Talking glucometers are available for blind patients.
Coordination counts
A variety of models of chronic care delivery have been proposed. Regardless of which model is chosen, the complexities of management call for a multidisciplinary team approach, and coordination of care in order to ensure appropriate information flow becomes critical.
Editor’s note: In next month’s issue of this journal, Drs. Hornick and Aron will discuss the management of diabetic complications in the elderly, including coronary artery disease, neuropathy, and kidney disease.
- Edelstein SL, Knowler WC, Bain RP, et al. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes 1997; 46:701–710.
- Meneilly GS, Tessier D. Diabetes in elderly adults. J Gerontol A Biol Sci Med Sci 2001; 56:M5–M13.
- Rodriguez A, Muller DC, Engelhardt M, Andres R. Contribution of impaired glucose tolerance in subjects with the metabolic syndrome: Baltimore Longitudinal Study of Aging. Metabolism Clin Exper 2005; 54:542–547.
- Crandall J, Barzilai N. Treatment of diabetes mellitus in older people: oral therapy options. J Am Geriatr Soc 2003; 51:272–274.
- Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care 1998; 21:518–524.
- Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion. Diabetes Public Health Resource. National Diabetes Surveillance System. www.cdc.gov/diabetes/statistics/prev/national/figbyage.htm.
- Franse LV, Di Bari M, Shorr RI, et al; Health, Aging, and Body Composition Study Group. Type 2 diabetes in older well-functioning people: who is underdiagnosed? Data from the Health, Aging, and Body Composition study. Diabetes Care 2001; 24:2065–2070. Erratum in: Diabetes Care 2002; 25:413.
- Shorr RI, Franse LV, Resnick HE, Di Bari M, Johnson KC, Pahor M. Glycemic control of older adults with type 2 diabetes: findings from the Third National Health and Nutrition Examination Survey, 1988–1994. J Am Geriatr Soc 2000; 48:264–267.
- Smith NL, Savage PJ, Heckbert SR, et al. Glucose, blood pressure, and lipid control in older people with and without diabetes mellitus: the Cardiovascular Health Study. J Am Geriatr Soc 2002; 50:416–423.
- Meneilly GS, Cheung E, Tessier D, Yakura C, Tuokko H. The effect of improved glycemic control on cognitive functions in the elderly patient with diabetes. J Gerontol 1993; 48:M117–M121.
- American Diabetes Association. Standards of medical care in diabetes.Diabetes Care 2005; 28:S4–S36.
- Motta M, Bennati E, Ferlito L, Malaguarnera M. Diabetes mellitus in the elderly: diagnostic features. Arch Gerontol Geriatr 2006; 42:101–106.
- Hoerger TJ, Harris R, Hicks KA, Donahue K, Sorensen S, Engelgau M. Screening for type 2 diabetes mellitus: a cost effective analysis. Ann Intern Med 2004; 140:689–699.
- Harris RP, Lux LJ, Bunton AJ, et al. Screening for type 2 diabetes mellitus. Systematic Evidence Review Number 19. February 4, 2003.US Department of Health and Human Services, Agency for Healthcare Research and Quality. Rockville, MD. www.ahrq.gov/downloads/pub/prevent/pdfser/diabser.pdf.
- Brown AF, Mangione CM, Saliba D, Sarkisian CA; California Healthcare Foundation/American Geriatrics Society Panel on Improving Care for Elders with Diabetes. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc 2003; 51:S265–S280.
- VA/DoD Clinical Practice Guideline for the Management of Diabetes Mellitus in the Primary Care Setting 2003. www.oqp.med.va.gov/cpg/dm/DM3_cpg/content/introduction.htm.
- Durso SC. Using clinical guidelines designed for older adults with diabetes mellitus and complex health status. JAMA 2006; 295:1935–1940.
- Curb JD, Pressel SL, Cutler JA, et al. Effect of diuretic-based antihypertensive treatment on cardiovascular disease risk in older patients with isolated systolic hypertension. Systolic Hypertension in the Elderly Program Cooperative Research Group. JAMA 1996; 276:1886–1892. Erratum in: JAMA 1997; 277:1356.
- Goddijn PP, Bilo HJ, Feskens EJ, Groeniert KH, van der Zee KI, Meyboom-de Jong B. Longitudinal study on glycaemic control and quality of life in patients with Type 2 diabetes mellitus referred for intensified control. Diabet Med 1999; 16:23–30.
- Pyorala K, Pedersen TR, Kjekshus J, Faergeman O, Olsson AG, Thorgeirsson G. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care 1997; 20:614–620. Erratum in: Diabetes Care 1997; 20:1048.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853. Erratum in: Lancet 1999; 354:602.
- Katakura M, Naka M, Kondo T, et al, and the Nagano Elderly Diabetes Study Group. Normal mortality in the elderly with diabetes under strict glycemic and blood pressure control: outcome of 6-year prospective study. Diabetes Res Clin Practice 2007; 78:108–114.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346:393–403.
- Pogach LM, Brietzke SA, Cowan CL Jr, Conlin P, Walder DJ, Sawin CT; VA/DoD Diabetes Guideline Development Group. Development of evidence-based clinical practice guidelines for diabetes: the Department of Veterans Affairs/Department of Defense guidelines initiative. Diabetes Care 2004; 27:B82–B89.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317:703–713. Erratum in: BMJ 1999; 318:29.
- Qaseem A, Vijan S, Snow V, Cross JT, Weiss KB, Owens DK for the Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Glycemic control and type 2 diabetes mellitus: the optimal hemoglobin A1c targets. A guidance statement from the American College of Physicians. Ann Intern Med 2007; 147:417–422.
- Pogach L, Engelgau M, Aron D. Measuring progress toward achieving hemoglobin A1c goals in diabetes care: pass/fail or partial credit. JAMA 2007; 297:520–523.
- Egger M, Davey Smith G, Stettler C, Diem P. Risk of adverse effects of intensified treatment in insulin-dependent diabetes mellitus: ameta-analysis. Diabet Med 1997; 14:919–928.
- Burge MR, Schmitz-Fiorentino K, Fischette C, Qualls CR, Schade DS. A prospective trial of risk factors for sulfonylurea-induced hypoglycemia in type 2 diabetes mellitus. JAMA 1998; 279:137–143.
- Ben-Ami H, Nagachandran P, Mendelson A, Edoute Y. Drug-induced hypoglycemic coma in 102 diabetic patients. Arch Intern Med 1999; 159:281–284.
- Shorr RI, Ray WA, Daugherty JR, Griffin MR. Individual sulfonylureas and serious hypoglycemia in older people. J Am Geriatr Soc 1996; 44:751–755.
- Shorr RI, Ray WA, Daugherty JR, Griffin MR. Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas. Arch Intern Med 1997; 157:1681–1685.
- Burge MR, Kamin JR, Timm CT, Qualls CR, Schade DS. Low-dose epinephrine supports plasma glucose in fasted elderly patients with type 2 diabetes. Metabolism 2000; 49:195–202.
- Alagiakrishnan K, Lechelt K, McCracken P, Torrible S, Sclater A.Atypical presentation of silent nocturnal hypoglycemia in an olderperson. J Am Geriatr Soc 2001; 49:1577–1578.
- Malmberg K, Norhammar A, Wedel H, Ryden L. Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation 1999; 99:2626–2632.
- van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med 2001; 345:1359–1367.
- Metchick LN, Petit WA Jr, Inzucchi SE. Inpatient management of diabetes mellitus. Am J Med 2002; 113:317–323.
- Coulston AM, Mandelbaum D, Reaven GM. Dietary management of nursing home residents with non-insulin-dependent diabetes mellitus. Am J Clin Nutr 1990; 51:67–71.
- Song MK, Rosenthal MJ, Naliboff BD, Phanumas L, Kang KW. Effects of bovine prostate powder on zinc, glucose and insulin metabolism in old patients with non-insulin-dependent diabetes mellitus. Metabolism 1998; 47:39–43.
- Paolisso G, D’Amore A, Galzerano D, et al. Daily vitamin E supplements improve metabolic control but not insulin secretion in elderly type II diabetic patients. Diabetes Care 1993; 16:1433–1437.
- Paolisso G, Scheen A, Cozzolino D, et al. Changes in glucose turnover parameters and improvement of glucose oxidation after 4-week magnesium administration in elderly noninsulin-dependent (type II) diabetic patients. J Clin Endocrinol Metab 1994; 78:1510–1514.
- Paolisso G, Passariello N, Pizza G, et al. Dietary magnesium supplements improve B-cell response to glucose and arginine in elderly non-insulin dependent diabetic subjects. Acta Endocrinol (Copenh) 1989; 121:16–20.
- Paolisso G, D’Amore A, Balbi V, et al. Plasma vitamin C affects glucose homeostasis in healthy subjects and in non-insulin-dependent diabetics. Am J Physiol 1994; 266:E261–E268. Erratum in: Am J Physiol 1994; 267:section E following table of contents.
- Agurs-Collins TD, Kumanyika SK, Ten Have TR, Adams-Campbell LL. A randomized controlled trail of weight reduction and exercise for diabetes management in older African-American subjects. Diabetes Care 1997; 200:1503–1511.
- Skarfors ET, Wegener TA, Lithell H, Selinus I. Physical training as treatment for type 2 (non-insulin-dependent) diabetes in elderly men. A feasibility study over 2 years. Diabetologia 1987; 30:930–933.
- Raz I, Hauser E, Bursztyn M. Moderate exercise improves glucose metabolism in uncontrolled elderly patients with non-insulin-dependent diabetes mellitus. Isr J Med Sci 1994; 30:766–770.
- Brodows RG. Benefits and risks with glyburide and glipizide in elderly NIDDM patients. Diabetes Care 1992; 15:75–80.
- Landgraf R. Meglitinide analogues in the treatment of type 2 diabetes mellitus. Drugs Aging 2000; 17:411–425.
- Ron Y, Wainstein J, Leibovitz A, et al. The effect of acarbose on the colonic transit time of elderly long-term care patients with type 2 diabetes mellitus. J Gerontol A Biol Sci Med Sci 2002; 57:M111–M114.
- Bolen S, Feldman L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med 2007; 147:386–399.
- Rosenstock J. Management of type 2 diabetes mellitus in the elderly: special considerations. Drugs Aging 2001; 18:31–44.
- Gerstein HC, Haynes RB, eds. Evidence-Based Diabetes Management. Hamilton, Ont.; London: B.C. Dekker; 2001.
- Tovi J, Engfeldt P. Well being and symptoms in elderly type 2 diabetes patients with poor metabolic control: effect of insulin treatment. Practical Diabetes Int 1998; 15:73–77.
- Reza M, Taylor CD, Towse K, Ward JD, Hendra TJ. Insulin improves well-being for selected elderly type 2 diabetic subjects. Diabetes Res Clin Pract 2002; 55:201–207.
- Janka HU, Plewe G, Busch K. Combination of oral antidiabetic agents with basal insulin versus premixed insulin alone in randomized elderly patients with type 2 diabetes mellitus. J Am Geriatrics Soc 2007; 55:182–188.
- Coscelli C, Calabrese G, Fedele D, et al. Use of premixed insulin among the elderly. Reduction of errors in patient preparation of mixtures. Diabetes Care 1992; 15:1628–1630.
- Rolla AR, Rakel RE. Practical approaches to insulin therapy for type 2 diabetes mellitus with premixed insulin analogues. Clin Ther 2005; 27:1113–1125.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28:1083–1091.
- Mathieu C, Bollaerts K. Antihyperglycaemic therapy in elderly patients with type 2 diabetes: potential role of incretin mimetics and DPP-4 inhibitors. Int J Clin Pract 2007; 61(suppl 154):29–37.
- Bernbaum M, Albert SG, McGinnis J, Brusca S, Mooradian AD. The reliability of self blood glucose monitoring in elderly diabetic patients. J Am Geriatr Soc 1994; 42:779–781.
- Gilden JL, Hendryx M, Casia C, Singh SP. The effectiveness of diabetes education programs for older patients and their spouses. J Am Geriatr Soc 1989; 37:1023–1030.
- Glasgow RE, Toobert DJ, Hampson SE, Brown JE, Lewinsohn PM, Donnelly J. Improving self-care among older patients with type II diabetes: the ‘Sixty Something…’ Study. Patient Educ Couns 1992; 19:61–74.
- Huang ES, Gorawara-Bhat R, Chin MH. Self-reported goals of older patients with type 2 diabetes mellitus. J Am Geriatr Soc 2005; 53:306–311.
- Langa KM, Vijan S, Hayward RA, et al. Informal caregiving for diabetes and diabetic complications among elderly Americans. J Gerontol B Psychol Sci Soc Sci 2002; 57:S177–S186.
- Edelstein SL, Knowler WC, Bain RP, et al. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes 1997; 46:701–710.
- Meneilly GS, Tessier D. Diabetes in elderly adults. J Gerontol A Biol Sci Med Sci 2001; 56:M5–M13.
- Rodriguez A, Muller DC, Engelhardt M, Andres R. Contribution of impaired glucose tolerance in subjects with the metabolic syndrome: Baltimore Longitudinal Study of Aging. Metabolism Clin Exper 2005; 54:542–547.
- Crandall J, Barzilai N. Treatment of diabetes mellitus in older people: oral therapy options. J Am Geriatr Soc 2003; 51:272–274.
- Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care 1998; 21:518–524.
- Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion. Diabetes Public Health Resource. National Diabetes Surveillance System. www.cdc.gov/diabetes/statistics/prev/national/figbyage.htm.
- Franse LV, Di Bari M, Shorr RI, et al; Health, Aging, and Body Composition Study Group. Type 2 diabetes in older well-functioning people: who is underdiagnosed? Data from the Health, Aging, and Body Composition study. Diabetes Care 2001; 24:2065–2070. Erratum in: Diabetes Care 2002; 25:413.
- Shorr RI, Franse LV, Resnick HE, Di Bari M, Johnson KC, Pahor M. Glycemic control of older adults with type 2 diabetes: findings from the Third National Health and Nutrition Examination Survey, 1988–1994. J Am Geriatr Soc 2000; 48:264–267.
- Smith NL, Savage PJ, Heckbert SR, et al. Glucose, blood pressure, and lipid control in older people with and without diabetes mellitus: the Cardiovascular Health Study. J Am Geriatr Soc 2002; 50:416–423.
- Meneilly GS, Cheung E, Tessier D, Yakura C, Tuokko H. The effect of improved glycemic control on cognitive functions in the elderly patient with diabetes. J Gerontol 1993; 48:M117–M121.
- American Diabetes Association. Standards of medical care in diabetes.Diabetes Care 2005; 28:S4–S36.
- Motta M, Bennati E, Ferlito L, Malaguarnera M. Diabetes mellitus in the elderly: diagnostic features. Arch Gerontol Geriatr 2006; 42:101–106.
- Hoerger TJ, Harris R, Hicks KA, Donahue K, Sorensen S, Engelgau M. Screening for type 2 diabetes mellitus: a cost effective analysis. Ann Intern Med 2004; 140:689–699.
- Harris RP, Lux LJ, Bunton AJ, et al. Screening for type 2 diabetes mellitus. Systematic Evidence Review Number 19. February 4, 2003.US Department of Health and Human Services, Agency for Healthcare Research and Quality. Rockville, MD. www.ahrq.gov/downloads/pub/prevent/pdfser/diabser.pdf.
- Brown AF, Mangione CM, Saliba D, Sarkisian CA; California Healthcare Foundation/American Geriatrics Society Panel on Improving Care for Elders with Diabetes. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc 2003; 51:S265–S280.
- VA/DoD Clinical Practice Guideline for the Management of Diabetes Mellitus in the Primary Care Setting 2003. www.oqp.med.va.gov/cpg/dm/DM3_cpg/content/introduction.htm.
- Durso SC. Using clinical guidelines designed for older adults with diabetes mellitus and complex health status. JAMA 2006; 295:1935–1940.
- Curb JD, Pressel SL, Cutler JA, et al. Effect of diuretic-based antihypertensive treatment on cardiovascular disease risk in older patients with isolated systolic hypertension. Systolic Hypertension in the Elderly Program Cooperative Research Group. JAMA 1996; 276:1886–1892. Erratum in: JAMA 1997; 277:1356.
- Goddijn PP, Bilo HJ, Feskens EJ, Groeniert KH, van der Zee KI, Meyboom-de Jong B. Longitudinal study on glycaemic control and quality of life in patients with Type 2 diabetes mellitus referred for intensified control. Diabet Med 1999; 16:23–30.
- Pyorala K, Pedersen TR, Kjekshus J, Faergeman O, Olsson AG, Thorgeirsson G. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care 1997; 20:614–620. Erratum in: Diabetes Care 1997; 20:1048.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853. Erratum in: Lancet 1999; 354:602.
- Katakura M, Naka M, Kondo T, et al, and the Nagano Elderly Diabetes Study Group. Normal mortality in the elderly with diabetes under strict glycemic and blood pressure control: outcome of 6-year prospective study. Diabetes Res Clin Practice 2007; 78:108–114.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346:393–403.
- Pogach LM, Brietzke SA, Cowan CL Jr, Conlin P, Walder DJ, Sawin CT; VA/DoD Diabetes Guideline Development Group. Development of evidence-based clinical practice guidelines for diabetes: the Department of Veterans Affairs/Department of Defense guidelines initiative. Diabetes Care 2004; 27:B82–B89.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317:703–713. Erratum in: BMJ 1999; 318:29.
- Qaseem A, Vijan S, Snow V, Cross JT, Weiss KB, Owens DK for the Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Glycemic control and type 2 diabetes mellitus: the optimal hemoglobin A1c targets. A guidance statement from the American College of Physicians. Ann Intern Med 2007; 147:417–422.
- Pogach L, Engelgau M, Aron D. Measuring progress toward achieving hemoglobin A1c goals in diabetes care: pass/fail or partial credit. JAMA 2007; 297:520–523.
- Egger M, Davey Smith G, Stettler C, Diem P. Risk of adverse effects of intensified treatment in insulin-dependent diabetes mellitus: ameta-analysis. Diabet Med 1997; 14:919–928.
- Burge MR, Schmitz-Fiorentino K, Fischette C, Qualls CR, Schade DS. A prospective trial of risk factors for sulfonylurea-induced hypoglycemia in type 2 diabetes mellitus. JAMA 1998; 279:137–143.
- Ben-Ami H, Nagachandran P, Mendelson A, Edoute Y. Drug-induced hypoglycemic coma in 102 diabetic patients. Arch Intern Med 1999; 159:281–284.
- Shorr RI, Ray WA, Daugherty JR, Griffin MR. Individual sulfonylureas and serious hypoglycemia in older people. J Am Geriatr Soc 1996; 44:751–755.
- Shorr RI, Ray WA, Daugherty JR, Griffin MR. Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas. Arch Intern Med 1997; 157:1681–1685.
- Burge MR, Kamin JR, Timm CT, Qualls CR, Schade DS. Low-dose epinephrine supports plasma glucose in fasted elderly patients with type 2 diabetes. Metabolism 2000; 49:195–202.
- Alagiakrishnan K, Lechelt K, McCracken P, Torrible S, Sclater A.Atypical presentation of silent nocturnal hypoglycemia in an olderperson. J Am Geriatr Soc 2001; 49:1577–1578.
- Malmberg K, Norhammar A, Wedel H, Ryden L. Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation 1999; 99:2626–2632.
- van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med 2001; 345:1359–1367.
- Metchick LN, Petit WA Jr, Inzucchi SE. Inpatient management of diabetes mellitus. Am J Med 2002; 113:317–323.
- Coulston AM, Mandelbaum D, Reaven GM. Dietary management of nursing home residents with non-insulin-dependent diabetes mellitus. Am J Clin Nutr 1990; 51:67–71.
- Song MK, Rosenthal MJ, Naliboff BD, Phanumas L, Kang KW. Effects of bovine prostate powder on zinc, glucose and insulin metabolism in old patients with non-insulin-dependent diabetes mellitus. Metabolism 1998; 47:39–43.
- Paolisso G, D’Amore A, Galzerano D, et al. Daily vitamin E supplements improve metabolic control but not insulin secretion in elderly type II diabetic patients. Diabetes Care 1993; 16:1433–1437.
- Paolisso G, Scheen A, Cozzolino D, et al. Changes in glucose turnover parameters and improvement of glucose oxidation after 4-week magnesium administration in elderly noninsulin-dependent (type II) diabetic patients. J Clin Endocrinol Metab 1994; 78:1510–1514.
- Paolisso G, Passariello N, Pizza G, et al. Dietary magnesium supplements improve B-cell response to glucose and arginine in elderly non-insulin dependent diabetic subjects. Acta Endocrinol (Copenh) 1989; 121:16–20.
- Paolisso G, D’Amore A, Balbi V, et al. Plasma vitamin C affects glucose homeostasis in healthy subjects and in non-insulin-dependent diabetics. Am J Physiol 1994; 266:E261–E268. Erratum in: Am J Physiol 1994; 267:section E following table of contents.
- Agurs-Collins TD, Kumanyika SK, Ten Have TR, Adams-Campbell LL. A randomized controlled trail of weight reduction and exercise for diabetes management in older African-American subjects. Diabetes Care 1997; 200:1503–1511.
- Skarfors ET, Wegener TA, Lithell H, Selinus I. Physical training as treatment for type 2 (non-insulin-dependent) diabetes in elderly men. A feasibility study over 2 years. Diabetologia 1987; 30:930–933.
- Raz I, Hauser E, Bursztyn M. Moderate exercise improves glucose metabolism in uncontrolled elderly patients with non-insulin-dependent diabetes mellitus. Isr J Med Sci 1994; 30:766–770.
- Brodows RG. Benefits and risks with glyburide and glipizide in elderly NIDDM patients. Diabetes Care 1992; 15:75–80.
- Landgraf R. Meglitinide analogues in the treatment of type 2 diabetes mellitus. Drugs Aging 2000; 17:411–425.
- Ron Y, Wainstein J, Leibovitz A, et al. The effect of acarbose on the colonic transit time of elderly long-term care patients with type 2 diabetes mellitus. J Gerontol A Biol Sci Med Sci 2002; 57:M111–M114.
- Bolen S, Feldman L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med 2007; 147:386–399.
- Rosenstock J. Management of type 2 diabetes mellitus in the elderly: special considerations. Drugs Aging 2001; 18:31–44.
- Gerstein HC, Haynes RB, eds. Evidence-Based Diabetes Management. Hamilton, Ont.; London: B.C. Dekker; 2001.
- Tovi J, Engfeldt P. Well being and symptoms in elderly type 2 diabetes patients with poor metabolic control: effect of insulin treatment. Practical Diabetes Int 1998; 15:73–77.
- Reza M, Taylor CD, Towse K, Ward JD, Hendra TJ. Insulin improves well-being for selected elderly type 2 diabetic subjects. Diabetes Res Clin Pract 2002; 55:201–207.
- Janka HU, Plewe G, Busch K. Combination of oral antidiabetic agents with basal insulin versus premixed insulin alone in randomized elderly patients with type 2 diabetes mellitus. J Am Geriatrics Soc 2007; 55:182–188.
- Coscelli C, Calabrese G, Fedele D, et al. Use of premixed insulin among the elderly. Reduction of errors in patient preparation of mixtures. Diabetes Care 1992; 15:1628–1630.
- Rolla AR, Rakel RE. Practical approaches to insulin therapy for type 2 diabetes mellitus with premixed insulin analogues. Clin Ther 2005; 27:1113–1125.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28:1083–1091.
- Mathieu C, Bollaerts K. Antihyperglycaemic therapy in elderly patients with type 2 diabetes: potential role of incretin mimetics and DPP-4 inhibitors. Int J Clin Pract 2007; 61(suppl 154):29–37.
- Bernbaum M, Albert SG, McGinnis J, Brusca S, Mooradian AD. The reliability of self blood glucose monitoring in elderly diabetic patients. J Am Geriatr Soc 1994; 42:779–781.
- Gilden JL, Hendryx M, Casia C, Singh SP. The effectiveness of diabetes education programs for older patients and their spouses. J Am Geriatr Soc 1989; 37:1023–1030.
- Glasgow RE, Toobert DJ, Hampson SE, Brown JE, Lewinsohn PM, Donnelly J. Improving self-care among older patients with type II diabetes: the ‘Sixty Something…’ Study. Patient Educ Couns 1992; 19:61–74.
- Huang ES, Gorawara-Bhat R, Chin MH. Self-reported goals of older patients with type 2 diabetes mellitus. J Am Geriatr Soc 2005; 53:306–311.
- Langa KM, Vijan S, Hayward RA, et al. Informal caregiving for diabetes and diabetic complications among elderly Americans. J Gerontol B Psychol Sci Soc Sci 2002; 57:S177–S186.
KEY POINTS
- The diagnosis of diabetes in the elderly is often missed because its symptoms, such as dizziness, confusion, and nocturia, are often common and nonspecific.
- Elderly people at risk of malnutrition should have unrestricted meals and snacks; medications should be adjusted as necessary to control blood glucose levels.
- Tight control of blood glucose reduces the risk of death and diabetes-related complications but poses the risk of hypoglycemia.