User login
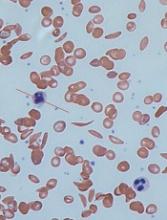
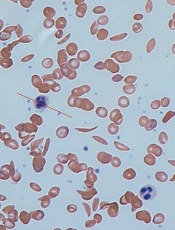
Computer models have revealed new details of what happens inside a red blood cell affected by sickle cell disease (SCD), according to research published in Biophysical Journal.
In patients with SCD, mutated hemoglobin can polymerize, assembling into long fibers that push against the membranes of red blood cells and force them out of shape.
“The goal of our work is to model both how these sickle hemoglobin fibers form as well as the mechanical properties of those fibers,” said study author Lu Lu, a PhD student at Brown University in Providence, Rhode Island.
“There had been separate models for each of these things individually developed by us, but this brings those together into one comprehensive model.”
The model uses detailed biomechanical data on how sickle hemoglobin molecules behave and bind with each other to simulate the assembly of a polymer fiber.
Prior to this work, the problem had been that, as the fiber grows, so does the amount of data the model must crunch. Modeling an entire polymer fiber at a cellular scale using the details of each molecule was simply too computationally expensive.
“Even the world’s fastest supercomputers wouldn’t be able to handle it,” said study author George Karniadakis, PhD, of Brown University.
“There’s just too much happening and no way to capture it all computationally. That’s what we were able to overcome with this work.”
The researchers’ solution was to apply what they call a mesoscopic adaptive resolution scheme (MARS).
The MARS model calculates the detailed dynamics of each individual hemoglobin molecule only at the end of polymer fibers, where new molecules are being recruited into the fiber.
Once 4 layers of a fiber have been established, the model automatically dials back the resolution at which it represents that section. The model retains the important information about how the fiber behaves mechanically but glosses over the fine details of each constituent molecule.
“By eliminating the fine details where we don’t need them, we develop a model that can simulate this whole process and its effects on a red blood cell,” Dr Karniadakis said.
Using the new MARS simulations, the researchers were able to show how different configurations of growing polymer fibers are able to produce cells with different shapes.
“We are able to produce a polymerization profile for each of the cell types associated with the disease,” Dr Karniadakis said. “Now, the goal is to use these models to look for ways of preventing the disease onset.”
Using these new models, Dr Karniadakis and his colleagues can run simulations that include fetal hemoglobin. Those simulations could be used to confirm the theory that fetal hemoglobin disrupts polymerization, as well as determine how much fetal hemoglobin is necessary.
That could help in establishing better dosage guidelines for hydroxyurea or in developing new and more effective drugs for SCD, according to the researchers.
“The models give us a way to do preliminary testing on new approaches to stopping this disease,” Dr Karniadakis said. “Now that we can simulate the entire polymerization process, we think the models will be much more useful.” ![]()
Computer models have revealed new details of what happens inside a red blood cell affected by sickle cell disease (SCD), according to research published in Biophysical Journal.
In patients with SCD, mutated hemoglobin can polymerize, assembling into long fibers that push against the membranes of red blood cells and force them out of shape.
“The goal of our work is to model both how these sickle hemoglobin fibers form as well as the mechanical properties of those fibers,” said study author Lu Lu, a PhD student at Brown University in Providence, Rhode Island.
“There had been separate models for each of these things individually developed by us, but this brings those together into one comprehensive model.”
The model uses detailed biomechanical data on how sickle hemoglobin molecules behave and bind with each other to simulate the assembly of a polymer fiber.
Prior to this work, the problem had been that, as the fiber grows, so does the amount of data the model must crunch. Modeling an entire polymer fiber at a cellular scale using the details of each molecule was simply too computationally expensive.
“Even the world’s fastest supercomputers wouldn’t be able to handle it,” said study author George Karniadakis, PhD, of Brown University.
“There’s just too much happening and no way to capture it all computationally. That’s what we were able to overcome with this work.”
The researchers’ solution was to apply what they call a mesoscopic adaptive resolution scheme (MARS).
The MARS model calculates the detailed dynamics of each individual hemoglobin molecule only at the end of polymer fibers, where new molecules are being recruited into the fiber.
Once 4 layers of a fiber have been established, the model automatically dials back the resolution at which it represents that section. The model retains the important information about how the fiber behaves mechanically but glosses over the fine details of each constituent molecule.
“By eliminating the fine details where we don’t need them, we develop a model that can simulate this whole process and its effects on a red blood cell,” Dr Karniadakis said.
Using the new MARS simulations, the researchers were able to show how different configurations of growing polymer fibers are able to produce cells with different shapes.
“We are able to produce a polymerization profile for each of the cell types associated with the disease,” Dr Karniadakis said. “Now, the goal is to use these models to look for ways of preventing the disease onset.”
Using these new models, Dr Karniadakis and his colleagues can run simulations that include fetal hemoglobin. Those simulations could be used to confirm the theory that fetal hemoglobin disrupts polymerization, as well as determine how much fetal hemoglobin is necessary.
That could help in establishing better dosage guidelines for hydroxyurea or in developing new and more effective drugs for SCD, according to the researchers.
“The models give us a way to do preliminary testing on new approaches to stopping this disease,” Dr Karniadakis said. “Now that we can simulate the entire polymerization process, we think the models will be much more useful.” ![]()
Computer models have revealed new details of what happens inside a red blood cell affected by sickle cell disease (SCD), according to research published in Biophysical Journal.
In patients with SCD, mutated hemoglobin can polymerize, assembling into long fibers that push against the membranes of red blood cells and force them out of shape.
“The goal of our work is to model both how these sickle hemoglobin fibers form as well as the mechanical properties of those fibers,” said study author Lu Lu, a PhD student at Brown University in Providence, Rhode Island.
“There had been separate models for each of these things individually developed by us, but this brings those together into one comprehensive model.”
The model uses detailed biomechanical data on how sickle hemoglobin molecules behave and bind with each other to simulate the assembly of a polymer fiber.
Prior to this work, the problem had been that, as the fiber grows, so does the amount of data the model must crunch. Modeling an entire polymer fiber at a cellular scale using the details of each molecule was simply too computationally expensive.
“Even the world’s fastest supercomputers wouldn’t be able to handle it,” said study author George Karniadakis, PhD, of Brown University.
“There’s just too much happening and no way to capture it all computationally. That’s what we were able to overcome with this work.”
The researchers’ solution was to apply what they call a mesoscopic adaptive resolution scheme (MARS).
The MARS model calculates the detailed dynamics of each individual hemoglobin molecule only at the end of polymer fibers, where new molecules are being recruited into the fiber.
Once 4 layers of a fiber have been established, the model automatically dials back the resolution at which it represents that section. The model retains the important information about how the fiber behaves mechanically but glosses over the fine details of each constituent molecule.
“By eliminating the fine details where we don’t need them, we develop a model that can simulate this whole process and its effects on a red blood cell,” Dr Karniadakis said.
Using the new MARS simulations, the researchers were able to show how different configurations of growing polymer fibers are able to produce cells with different shapes.
“We are able to produce a polymerization profile for each of the cell types associated with the disease,” Dr Karniadakis said. “Now, the goal is to use these models to look for ways of preventing the disease onset.”
Using these new models, Dr Karniadakis and his colleagues can run simulations that include fetal hemoglobin. Those simulations could be used to confirm the theory that fetal hemoglobin disrupts polymerization, as well as determine how much fetal hemoglobin is necessary.
That could help in establishing better dosage guidelines for hydroxyurea or in developing new and more effective drugs for SCD, according to the researchers.
“The models give us a way to do preliminary testing on new approaches to stopping this disease,” Dr Karniadakis said. “Now that we can simulate the entire polymerization process, we think the models will be much more useful.” ![]()