User login
Children who undergo radiotherapy, chemotherapy, or surgery for cancer face a risk of complications later in life, including pulmonary fibrosis and pneumonitis.
These long-term cancer survivors need systematic, lifelong surveillance, in a program that takes into account their individual risk (based on therapeutic exposures, genetic predisposition, lifestyle behaviors, and comorbid health conditions).1 Optimally, they would receive their care at multidisciplinary follow-up clinics organized by pediatric oncologists at tertiary care centers. However, access to such centers is limited, making this an option for relatively few. Consequently, as childhood cancer survivors age, internists and family practitioners may need to assume an increasing amount of responsibility for their follow-up care.
Because individual primary care providers are unlikely to follow more than a handful of survivors, specialists have developed guidelines for survivors of pediatric cancer. Working with established multidisciplinary clinics may help ensure appropriate follow-up for this population of patients.
This review summarizes the late effects of cancer therapy on the lungs and an approach to surveillance for the generalist or pulmonologist. We also review the quality of the evidence upon which these recommendations are based.
NUMBERS ON THE RISE
An estimated 1 of every 330 children develops cancer before age 19. With cure rates exceeding 75% for many pediatric malignancies, the number of survivors of childhood cancer, currently in excess of 270,000, will continue to increase.2
THE CHILDREN’S ONCOLOGY GROUP GUIDELINES
The Children’s Oncology Group (COG)3 released its first set of guidelines in 2003 for the follow-up care of patients treated for pediatric malignancies; the current version is available at www.survivorshipguidelines.org. The guidelines contain comprehensive screening recommendations, including those related to pulmonary toxicity, which can be used to standardize care.
Patient education materials accompany the guidelines, offering detailed information on guideline-specific topics in order to promote health maintenance.
HOW WE SEARCHED THE LITERATURE
We performed an extensive review of the literature via MEDLINE for the years 1975–2005. Key search terms were “childhood cancer,” “late effects,” and “pulmonary toxicity,” combined with keywords for each therapeutic exposure. References from selected articles were used to broaden the search. From several hundred citations, fewer than 30 were selected as best illustrating the relevant associations.
RISK IS THREE TIMES HIGHER IN CANCER SURVIVORS
The Childhood Cancer Survivor Study4 is the largest database of late effects, with more than 12,000 survivors of childhood cancer diagnosed between 1970 and 1986. Its data suggest that the risk of pulmonary conditions is more than three times higher in cancer survivors than in their siblings, as manifested by pulmonary signs (abnormal chest wall growth), symptoms (chronic cough, use of supplemental oxygen, exercise-induced shortness of breath), or specific diagnoses (lung fibrosis, recurrent pneumonia, pleurisy, bronchitis, recurrent sinus infection, or tonsillitis). Limitations: these data are retrospective, and the outcomes were detected by self-report and were not validated by review of medical records. Thus, the figures highlight the fact that pulmonary late effects are an important problem but do not give us a way to calculate risk exactly.
Other limitations of the literature: Treatments are constantly evolving, often in attempts to minimize late effects, and newer agents will need to be monitored for pulmonary toxicities. As noted, much of the available information is from studies of survivors of adult cancer; the potential for late effects of similar therapies in children is inferred. Most conclusions—and especially those based upon prospective serial evaluations—derive from small cohorts. For all treatments, the complications in the very long term remain undefined. What we know is summarized below.
CANCER THERAPY CAUSES FIBROSIS, PNEUMONITIS
The courses of these diseases are poorly characterized, since few longitudinal studies have been done. However, like most of the late effects of cancer therapy, pulmonary toxicity may first become apparent during the treatment and persist, or it may not appear until years later. Signs and symptoms may be static, progressive, or reversible.
ANGIOGENESIS MAY CONTRIBUTE TO FIBROSIS
On a microscopic level, pulmonary fibrosis is characterized by epithelial injury, fibroproliferation, and excessive extracellular matrix deposition.6–8
Evidence is mounting that these findings result in part from angiogenesis. Although this has not been studied in long-term cancer survivors, evidence of neovascularization was seen both in an animal model of lung fibrosis and in patients with idiopathic pulmonary fibrosis.6–8 High plasma concentrations of angiogenic cytokines (eg, tumor necrosis factor alpha, interleukin 8, and endothelin 1) have been found in these situations. Antiangiogenic agents and other immune modulators such as thalidomide may be beneficial in patients with lung fibrosis.7
On a macroscopic level, pulmonary fibrosis results in loss of lung volume in older children and in adults. In contrast, in younger children, interference with growth of both the lung and the chest wall may contribute to pulmonary dysfunction.
CANCER TYPES AND TREATMENTS VARY BY AGE
Cancers that commonly involve the thorax are listed in Table 2. Neuroblastoma, hepatoblastoma, extragonadal germ cell tumors, and Wilms tumor typically are diseases of young children; osteosarcoma, Ewing sarcoma, thyroid carcinoma, and Hodgkin disease are most common in older children and adolescents; soft tissue sarcoma and non-Hodgkin lymphoma span all age groups.
Surgery can, in some cases, control the cancer, as with mediastinal neuroblastoma and Ewing sarcoma of the chest wall.
Radiation to the chest remains a major component of treatment for Hodgkin disease, unresected thoracic Ewing sarcoma, soft tissue sarcoma with lung involvement or thyroid carcinoma, and Wilms tumor. Central nervous system tumors and leukemias, the most common pediatric malignancies, may require radiation to the spinal cord—with resulting radiation exposure of the lungs. Total-body irradiation is a component of many preparative regimens for stem cell transplantation.
Chemotherapy remains a mainstay for all types of tumors, and patients with germ cell tumors, Hodgkin disease, and brain tumors are at particular risk of pulmonary toxicity due to heavy reliance on bleomycin (Blenoxane) (for germ cell tumors, Hodgkin disease) and the nitrosoureas (for brain tumors).
RADIATION-INDUCED LUNG DAMAGE
The lungs are particularly sensitive to radiation, and pulmonary problems occur most often in patients with malignant diseases of the chest that are treated with radiation, ie, those involving the mediastinum, the lung parenchyma, or the chest wall.
Abnormal radiographic findings or restrictive changes on pulmonary function testing have been reported in more than 30% of patients who received radiation directly or indirectly to the lung.9–12 These changes have been detected months to years after radiation therapy, most often in patients who suffered radiation pneumonitis as an acute toxicity.
The amount of damage depends on the cumulative dose, how many treatments (“fractions”) this cumulative dose was divided into (dividing the radiation dose into smaller dose fractions can reduce toxicity), the volume of lung tissue involved, and the patient’s age (the younger, the worse) at the time of treatment.
Cumulative dose. Whole-lung irradiation is limited to 12 Gy, although localized areas of cancer can be treated with much higher doses.
Clinically apparent pneumonitis with cough, fever, or dyspnea generally occurs only in survivors who received more than 30 Gy in standard fractions to more than 50% of the lung. However, in 12 survivors of Wilms tumor who received median total doses of approximately 20 Gy to both lungs 7 to 14 years previously, 8 patients had dyspnea on exertion and radiographic evidence of interstitial and pleural thickening.13 Mean total lung volumes and the diffusing capacity of the lung for carbon monoxide (DLCO) were reduced in all patients to approximately 60% of predicted values.
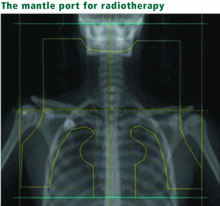
In a prospective study of adults with Hodgkin disease treated after 1980, 145 patients were examined 3 years after receiving more than 44 Gy limited to the mantle area.9 None were experiencing symptoms; however, 30% to 40% had a forced vital capacity (FVC) less than 80% of predicted, and 7% had a reduced DLCO. Some of these patients also had received bleomycin (see below), which may have exacerbated pulmonary toxicity. Asymptomatic restrictive and obstructive lung changes have been detected after lower doses of whole-lung radiation (11–14 Gy) were given for other malignant diseases.11,14
In a recent study of adults and older adolescents with stage I and IIA Hodgkin disease treated with radiation alone (40–45 Gy to involved fields, including the mediastinum), late pulmonary effects observed were minimal.10 While FVC, residual volume, forced expiratory volume in 1 second (FEV1), DLCO, and total lung capacity (TLC) were significantly lower at the end of radiation therapy than before treatment, all except DLCO returned nearly to normal within 1 year. The decrease in DLCO remained stable, but the forced expiratory flow rate between 25% and 75% of FVC (FEF 25%–75%) was significantly lower 3 years after treatment than at baseline.
The high doses of total lung irradiation cited in several of these reports are rarely used in today’s cancer protocols. For example, patients receiving radiation as adjunctive treatment for Hodgkin disease now receive lower doses (< 21 Gy), which are restricted to involved fields, and the pulmonary toxicity is less than in the past.
In one series, 159 children and adolescents with unfavorable Hodgkin disease treated from 1993 to 2000 received six cycles of chemotherapy followed by response-based, involved-field radiation therapy. Patients who achieved a complete response after the first two cycles of chemotherapy got 15 Gy, and those who achieved a partial response got 25.5 Gy to all sites of bulky lymphadenopathy.15 All patients underwent pulmonary function testing. Only 24 (30.8%) of them had pulmonary toxicity, which was limited to asymptomatic deficits of restriction and diffusion.
Nevertheless, the lungs receive some radiation even when they are not the target, such as in patients with malignant brain tumors, and this exposure can contribute to the development of lung disease, although these patients are likely to have no symptoms during day-to-day activities. Innovations in targeted radiation delivery (eg, conformal radiation) should further limit damage to normal lung tissue.
Age at the time of treatment also may influence the type and incidence of pulmonary sequelae. In older children and adults, radiation for thoracic malignancy results in pulmonary fibrosis with loss of lung volume. Similar injury can occur in younger children, but pulmonary function may also be compromised by inhibited growth of the supportive structures and the chest wall. One report suggests that children younger than 3 years at the time of therapy experience more chronic toxicity.11
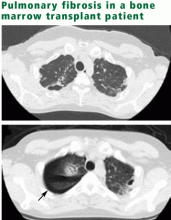
In contrast, after bone marrow transplantation, children seem to be at less risk of significant late pulmonary dysfunction than adults are, despite similar preparatory regimens.16 This may in part reflect a lower incidence of severe graft-vs-host disease involving the lung. Nonetheless, in a recent report of children treated with fractionated total-body irradiation between 1985 and 1993, restrictive pulmonary diseases were found in 30 of 42 patients at a median of 3.1 years after treatment (range 0.5–17 years).17 Most of these patients had asymptomatic mild restrictive disease, and the one patient with severe changes had previously received thoracic radiation for treatment of neuroblastoma. In about half of the cases, pulmonary function abnormalities were permanent although not progressive.
CHEMOTHERAPY-RELATED LUNG DAMAGE
A growing list of chemotherapeutic agents appears to cause pulmonary disease in long-term survivors.5
Bleomycin
Bleomycin toxicity is the prototype for chemotherapy-related lung injury: bleomycin was the first chemotherapy drug shown to cause lung injury, this effect is suggested by a large database, and the mechanism is typical.5,18 Preclinical studies have attributed bleomycin’s toxicity to its tendency to promote free radicals.
Although interstitial pneumonitis and pulmonary fibrosis have been reported in children, clinically apparent bleomycin pneumonopathy is most frequent in older adults. Usually, the abnormalities began within 3 months of therapy and persisted or progressed. Like the acute toxicity, it is dose-dependent and more common above a threshold cumulative dose of 400 units/m2. Above this dose, 10% of adult patients without other risk factors develop fibrosis; data are not available for these doses in children. At lower doses, fibrosis occurs sporadically in fewer than 5% of patients, with a 1% to 2% mortality rate. In some series, bleomycin toxicity was anticipated on the basis of DLCO abnormalities.
Bleomycin pulmonary toxicity is variably exacerbated by concurrent or previous radiation therapy.
Increased oxygen concentrations associated with general anesthesia have also been found to exacerbate prior bleomycin-induced pulmonary injury.19,20 In one instance (reviewed by Zaniboni et al20), the patient recovered with corticosteroid treatment.
Alkylating agents
Carmustine (also called BCNU; brand names BiCNU, Gliadel) and lomustine (CCNU; CeeNU) are thought to cause dose-related lung injury. When cumulative carmustine doses are greater than 1,500 mg/m2, more than 50% of patients develop symptoms.21
In a careful clinicopathologic review of 31 children with brain tumors, restrictive changes with lung fibrosis were reported up to 17 years after treatment, most often with carmustine 100 mg/m2 every 6 to 8 weeks for up to 2 years.22 Four of the 8 patients still alive at the time of study experienced shortness of breath and coughing; 6 showed a characteristic pattern of upper zone fibrosis on chest radiography and computed tomography; all 8 survivors had restrictive findings on pulmonary function testing, with vital capacities of about 50% of normal. Toxicity increases with more intensive dose-scheduling.
Cyclophosphamide (Cytoxan) may cause delayed-onset pulmonary fibrosis with severe restrictive lung disease in association with marked reductions in the anteroposterior diameter of the chest, although the evidence is less convincing than with carmustine and lomustine, coming from case reports and small series.23
Melphalan (Alkeran), generally in doses used in stem cell transplant conditioning regimens, is also thought to cause pulmonary fibrosis.24
Busulfan most predictably causes toxicity when it is used in transplantation doses (ie, more than 500 mg), and may be associated with a progressive, potentially fatal restrictive lung disease.25 A current trend is to adjust the dose on the basis of pharmacokinetic analysis, which we hope will reduce toxicity.
Other agents
Methotrexate (MTX; Trexall) also has been associated with chronic pneumonitis and fibrosis.26 This probably occurs with an incidence well below 1% and may be idiosyncratic and not dose-related. Asymptomatic changes in pulmonary function tests that do not predict clinically significant problems have most frequently been associated with low-dose oral administration during more than 3 years.27 This is a treatment approach used in patients with psoriasis or rheumatoid arthritis, but is now obsolete for pediatric cancer. Intravenous and, rarely, intrathecal administration also have been associated with pulmonary toxicity.28 A single report of two patients who developed diffuse interstitial pulmonary infiltrates and chronic pulmonary changes links vinblastine to these sequelae.29
LUNG INJURY AFTER BONE MARROW TRANSPLANTATION
Hematopoietic stem cell transplantation is associated with various late pulmonary complications. The factors that influence these complications are similar to those discussed earlier. However, the intensity of therapy in patients undergoing transplantation and the additive effects of previous therapies magnify the risks.
Patients undergoing total-body irradiation as part of their preparation for transplantation have a high incidence of late pulmonary complications.25,30–32 Busulfan, carmustine, bleomycin, and cyclophosphamide, also commonly used conditioning chemotherapies, are known to cause pneumonitis and fibrosis after transplantation.33
Some acute pulmonary toxicities can have long-standing effects, including serious pulmonary infections, idiopathic pneumonia syndrome, bronchiolitis obliterans, acute respiratory distress syndrome, or other damage related to graft-vs-host disease.
OTHER RISK FACTORS FOR LUNG DAMAGE
Additional factors contributing to chronic pulmonary toxicity include superimposed infection, underlying pneumonopathy (eg, asthma), cigarette use, respirator toxicity, chronic graft-vs-host disease, and the effects of chronic pulmonary involvement by tumor or reaction to tumor. For example, a subset of patients with Langerhans cell histiocytosis can develop histiocytic pulmonary infiltrates or honeycombing with severe chronic restrictive lung disease unrelated to therapy or the presence of active tumor.
Although not well documented, scuba diving also has been said to exacerbate pulmonary fibrosis through increased underwater pressures and high oxygen levels.34
Lung lobectomy during childhood appears to have no significant impact on long-term pulmonary function,35–37 but the effect of lung surgery for children with cancer is not well defined.
GET THE PATIENT’S TREATMENT SUMMARY
Regardless of the setting for follow-up, the first step in any evaluation is to obtain the patient’s medical history and especially a treatment summary. The treatment summary should outline the cancer diagnosis, involved sites of disease, age at diagnosis, specific treatments (surgery, chemotherapy, radiation), and other key interventions and events during and after cancer therapy. Sample forms for physicians and patients are available at www.survivorshipguidelines.org.
Before the long-term survivor of childhood cancer graduates from the care of a pediatric oncologist, this treatment record and possible long-term problems should be reviewed with the family and, in the case of an adolescent, with the patient. Correspondence between the pediatric oncologist and subsequent caregivers should also include a treatment summary. The treatment summary allows the survivor or his health care provider to interface with the COG guidelines to determine recommended follow-up care. The primary care physician and the patient both should have copies of this document.
We are developing an interactive Web-based version of a standardized summary form, designed to interface with an automated version of the COG guidelines in order to generate individualized follow-up recommendations.
ASK ABOUT LUNG SYMPTOMS
We recommend that health care providers investigate symptoms of pulmonary dysfunction, and specifically ask about chronic cough with or without fever, shortness of breath, and dyspnea on exertion during yearly health care visits.
Baseline pulmonary function testing (including DLCO and spirometry) and chest radiography are recommended 2 or more years after completion of therapy to document persistent deficits and determine the need for continued monitoring. Reevaluation of pulmonary function should be considered in patients with established deficits who require general anesthesia and for those treated with bleomycin.
Scuba diving remains controversial for long-term survivors. Consequently, patients with risk factors for lung disease should be encouraged to consult with a pulmonary specialist to determine if diving poses a health threat to their pulmonary status. If clinical pulmonary dysfunction is identified, referral to colleagues in pulmonology for additional evaluation and treatment is essential. Increasing familiarity of primary care providers with surveillance concepts is a key element in survivorship care.
Smoking cessation can enhance the health of all patients and is particularly important among long-term survivors, especially those who received treatments predisposing to pulmonary injury. Strategies for cessation and patient information can be found at www.cdc.gov/tobacco/how2quit.htm.
Clinicians can take advantage of every patient interaction to assess readiness for smoking cessation and assist patients in this goal. Following the principles of patient-centered counseling, physicians can guide patients into considering a change of behavior with advice and encouragement. Whenever possible, physicians should personalize the risks of smoking as well as the short-term and long-term benefits. As smokers prepare to quit, their physicians can assist in developing a plan that includes a quit date.
Many pharmacologic agents are available to assist patients, ie, nicotine inhalers, sprays, gum, and transdermal patches; the antidepressants bupropion (Wellbutrin) and nortriptiline (Pamelor); the alpha-2 adrenergic agonist clonidine (Catapres); and, most recently, the nicotine receptor partial agonist varenicline (Chantix).38
Follow-up to prevent relapse is an important part of this process.
Acknowledgment. This work was supported in part by the Swim Across America Foundation and the Campini Foundation.
- Hewitt M, Weiner S, Simone J. The epidemiology of childhood cancer. Washington DC: The National Academies Press, 2003:20–36.
- Ries L, Eisner M, Kosary C, et al. SEER cancer statistics review, 1975–2001. Bethesda, MD: National Cancer Institute; 2004.
- Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: The Children’s Oncology Group long-term follow-up guidelines from the Children’s Oncology Group Late Effects Committee and nursing discipline. J Clin Oncol 2004; 22:4979–4990.
- Mertens AC, Yasui Y, Liu Y, et al. Pulmonary complications in survivors of childhood and adolescent cancer. A report from the Childhood Cancer Survivor Study. Cancer 2002; 95:2431–2441.
- Bhatia S, Blatt J, Meadows A. Late effects of childhood cancer and its treatment. In:Pizzo P, Poplack D. Principles and Practice of Pediatric Oncology. Philadelphia: Lippincott Williams & Wilkins, 2006.
- Anscher MS, Chen L, Rabbani Z, et al. Recent progress in defining mechanisms and potential targets for prevention of normal tissue injury after radiation therapy. Int J Radiat Oncol Biol Phys 2005; 62:255–259.
- Bhatt N, Baran CP, Allen J, et al. Promising pharmacologic innovations in treating pulmonary fibrosis. Curr Opin Pharmacol 2006; 6:284–292.
- Abid SH, Malhotra V, Perry MC. Radiation-induced and chemotherapy-induced pulmonary injury. Curr Opin Oncol 2001: 13:242–248.
- Horning SJ, Adhikari A, Rizk N, et al. Effect of treatment for Hodgkin’s disease on pulmonary function: results of a prospective study. J Clin Oncol 1994; 12:297–305.
- Villani F, Viviani S, Bonfante V, et al. Late pulmonary effects in favorable stage I and IIA Hodgkin’s disease treated with radiotherapy alone. Am J Clin Oncol 2000; 23:18–21.
- Miller RW, Fusner JE, Fink RJ, et al. Pulmonary function abnormalities in long-term survivors of childhood cancer. Med Pediatr Oncol 1986; 14:202–207.
- Weiner DJ, Maity A, Carlson CA, et al. Pulmonary function abnormalities in children treated with whole lung irradiation. Pediatr Blood Cancer 2006; 46:222–227.
- Wohl ME, Griscom NT, Traggis DG, et al. Effects of therapeutic irradiation delivered in early childhood upon subsequent lung function. Pediatrics 1975; 55:507–516.
- Hudson MM, Greenwald C, Thompson E, et al. Efficacy and toxicity of multiagent chemotherapy and low-dose involved-field radiotherapy in children and adolescents with Hodgkin’s disease. J Clin Oncol 1993; 11:100–108.
- Hudson MM, Krasin M, Link MP, et al. Risk-adapted, combined-modality therapy with VAMP/COP and response-based, involved-field radiation for unfavorable pediatric Hodgkin’s disease. J Clin Oncol 2004; 22:4541–4550.
- Quigley PM, Yeager AM, Loughlin GM. The effects of bone marrow transplantation on pulmonary function in children. Pediatr Pulmonol 1994; 18:361–367.
- Faraci M, Barra S, Cohen A, et al. Very late nonfatal consequences of fractionated TBI in children undergoing bone marrow transplant. Int J Radiat Oncol Biol Phys 2005; 63:1568–1575.
- Eigen H, Wyszomierski D. Bleomycin lung injury in children. Pathophysiology and guidelines for management. Am J Pediatr Hematol Oncol 1985; 7:71–78.
- Goldiner PL, Schweizer O. The hazards of anesthesia and surgery in bleomycin-treated patients. Semin Oncol 1979; 6:121–124.
- Zaniboni A, Prabhu S, Audisio RA. Chemotherapy and anaesthetic drugs: too little is known. Lancet Oncol 2005; 6:176–181.
- Aronin PA, Mahaley MS, Rudnick SA, et al. Prediction of BCNU pulmonary toxicity in patients with malignant gliomas: an assessment of risk factors. N Engl J Med 1980; 303:183–188.
- O’Driscoll BR, Hasleton PS, Taylor PM, et al. Active lung fibrosis up to 17 years after chemotherapy with carmustine (BCNU) in childhood. N Engl J Med 1990; 323:378–382.
- Alvarado CS, Boat TF, Newman AJ. Late-onset pulmonary fibrosis and chest deformity in two children treated with cyclophosphamide. J Pediatr 1978; 92:443–446.
- Codling BW, Chakera TM. Pulmonary fibrosis following therapy with melphalan for multiple myeloma. J Clin Pathol 1972; 25:668–673.
- Bruno B, Souillet G, Bertrand Y, et al. Effects of allogeneic bone marrow transplantation on pulmonary function in 80 children in a single paediatric centre. Bone Marrow Transplant 2004; 34:143–147.
- Lateef O, Shakoor N, Balk RA. Methotrexate pulmonary toxicity. Expert Opin Drug Saf 2005; 4:723–730.
- Cottin V, Tebob J, Massonnet B, et al. Pulmonary function in patients receiving long-term methotrexate. Chest 1996; 109:933–938.
- Gutin PH, Green MR, Bleyer WA, et al. Methotrexate pneumonitis induced by intrathecal methotrexate therapy: a case report with pharmacokinetic data. Cancer 1976; 38:1529–1534.
- Konits PH, Aisner J, Sutherland JC, et al. Possible pulmonary toxicity secondary to vinblastine. Cancer 1982; 50:2771–2774.
- Cerveri I, Zoia MC, Fulgoni P, et al. Late pulmonary sequelae after childhood bone marrow transplantation. Thorax 1999; 54:131–135.
- Cerveri I, Fulgoni P, Giorgiani G, et al. Lung function abnormalities after bone marrow transplantation in children: has the trend recently changed? Chest 2001; 120:1900–1906.
- Neve V, Foot AB, Michon J, et al. Longitudinal clinical and functional pulmonary follow-up after megatherapy, fractionated total body irradiation, and autologous bone marrow transplantation for metastatic neuroblastoma. Med Pediatr Oncol 1999; 32:170–176.
- Nenadov Beck M, Meresse V, Hartmann O, et al. Long-term pulmonary sequelae after autologous bone marrow transplantation in children without total body irradiation. Bone Marrow Transplant 1995; 16:771–775.
- Huls G, ten Bokkel Huinink D. Bleomycin and scuba diving: to dive or not to dive? Neth J Med 2003; 61:50–53.
- Kreisel D, Krupnick AS, Huddleston CB. Outcomes and late complications after pulmonary resections in the pediatric population. Semin Thorac Cardiovasc Surg 2004; 16:215–219.
- Lezama-del Valle Valle P, Blakely ML, Lobe TE. Physiologic consequences of pneumonectomy. Long-term consequences of pneumonectomy done in children. Chest Surg Clin North Am 1999; 9:485–495.
- Tobias JD, Bozeman PM, Mackert PW, et al. Postoperative outcome following thoracotomy in the pediatric oncology patient with diminished pulmonary function. J Surg Oncol 1993; 52:105–109.
- Drugs for Tobacco Dependence. Treatment Guidelines from the Medical Letter. June, 2003: 1(10):65–68.
Children who undergo radiotherapy, chemotherapy, or surgery for cancer face a risk of complications later in life, including pulmonary fibrosis and pneumonitis.
These long-term cancer survivors need systematic, lifelong surveillance, in a program that takes into account their individual risk (based on therapeutic exposures, genetic predisposition, lifestyle behaviors, and comorbid health conditions).1 Optimally, they would receive their care at multidisciplinary follow-up clinics organized by pediatric oncologists at tertiary care centers. However, access to such centers is limited, making this an option for relatively few. Consequently, as childhood cancer survivors age, internists and family practitioners may need to assume an increasing amount of responsibility for their follow-up care.
Because individual primary care providers are unlikely to follow more than a handful of survivors, specialists have developed guidelines for survivors of pediatric cancer. Working with established multidisciplinary clinics may help ensure appropriate follow-up for this population of patients.
This review summarizes the late effects of cancer therapy on the lungs and an approach to surveillance for the generalist or pulmonologist. We also review the quality of the evidence upon which these recommendations are based.
NUMBERS ON THE RISE
An estimated 1 of every 330 children develops cancer before age 19. With cure rates exceeding 75% for many pediatric malignancies, the number of survivors of childhood cancer, currently in excess of 270,000, will continue to increase.2
THE CHILDREN’S ONCOLOGY GROUP GUIDELINES
The Children’s Oncology Group (COG)3 released its first set of guidelines in 2003 for the follow-up care of patients treated for pediatric malignancies; the current version is available at www.survivorshipguidelines.org. The guidelines contain comprehensive screening recommendations, including those related to pulmonary toxicity, which can be used to standardize care.
Patient education materials accompany the guidelines, offering detailed information on guideline-specific topics in order to promote health maintenance.
HOW WE SEARCHED THE LITERATURE
We performed an extensive review of the literature via MEDLINE for the years 1975–2005. Key search terms were “childhood cancer,” “late effects,” and “pulmonary toxicity,” combined with keywords for each therapeutic exposure. References from selected articles were used to broaden the search. From several hundred citations, fewer than 30 were selected as best illustrating the relevant associations.
RISK IS THREE TIMES HIGHER IN CANCER SURVIVORS
The Childhood Cancer Survivor Study4 is the largest database of late effects, with more than 12,000 survivors of childhood cancer diagnosed between 1970 and 1986. Its data suggest that the risk of pulmonary conditions is more than three times higher in cancer survivors than in their siblings, as manifested by pulmonary signs (abnormal chest wall growth), symptoms (chronic cough, use of supplemental oxygen, exercise-induced shortness of breath), or specific diagnoses (lung fibrosis, recurrent pneumonia, pleurisy, bronchitis, recurrent sinus infection, or tonsillitis). Limitations: these data are retrospective, and the outcomes were detected by self-report and were not validated by review of medical records. Thus, the figures highlight the fact that pulmonary late effects are an important problem but do not give us a way to calculate risk exactly.
Other limitations of the literature: Treatments are constantly evolving, often in attempts to minimize late effects, and newer agents will need to be monitored for pulmonary toxicities. As noted, much of the available information is from studies of survivors of adult cancer; the potential for late effects of similar therapies in children is inferred. Most conclusions—and especially those based upon prospective serial evaluations—derive from small cohorts. For all treatments, the complications in the very long term remain undefined. What we know is summarized below.
CANCER THERAPY CAUSES FIBROSIS, PNEUMONITIS
The courses of these diseases are poorly characterized, since few longitudinal studies have been done. However, like most of the late effects of cancer therapy, pulmonary toxicity may first become apparent during the treatment and persist, or it may not appear until years later. Signs and symptoms may be static, progressive, or reversible.
ANGIOGENESIS MAY CONTRIBUTE TO FIBROSIS
On a microscopic level, pulmonary fibrosis is characterized by epithelial injury, fibroproliferation, and excessive extracellular matrix deposition.6–8
Evidence is mounting that these findings result in part from angiogenesis. Although this has not been studied in long-term cancer survivors, evidence of neovascularization was seen both in an animal model of lung fibrosis and in patients with idiopathic pulmonary fibrosis.6–8 High plasma concentrations of angiogenic cytokines (eg, tumor necrosis factor alpha, interleukin 8, and endothelin 1) have been found in these situations. Antiangiogenic agents and other immune modulators such as thalidomide may be beneficial in patients with lung fibrosis.7
On a macroscopic level, pulmonary fibrosis results in loss of lung volume in older children and in adults. In contrast, in younger children, interference with growth of both the lung and the chest wall may contribute to pulmonary dysfunction.
CANCER TYPES AND TREATMENTS VARY BY AGE
Cancers that commonly involve the thorax are listed in Table 2. Neuroblastoma, hepatoblastoma, extragonadal germ cell tumors, and Wilms tumor typically are diseases of young children; osteosarcoma, Ewing sarcoma, thyroid carcinoma, and Hodgkin disease are most common in older children and adolescents; soft tissue sarcoma and non-Hodgkin lymphoma span all age groups.
Surgery can, in some cases, control the cancer, as with mediastinal neuroblastoma and Ewing sarcoma of the chest wall.
Radiation to the chest remains a major component of treatment for Hodgkin disease, unresected thoracic Ewing sarcoma, soft tissue sarcoma with lung involvement or thyroid carcinoma, and Wilms tumor. Central nervous system tumors and leukemias, the most common pediatric malignancies, may require radiation to the spinal cord—with resulting radiation exposure of the lungs. Total-body irradiation is a component of many preparative regimens for stem cell transplantation.
Chemotherapy remains a mainstay for all types of tumors, and patients with germ cell tumors, Hodgkin disease, and brain tumors are at particular risk of pulmonary toxicity due to heavy reliance on bleomycin (Blenoxane) (for germ cell tumors, Hodgkin disease) and the nitrosoureas (for brain tumors).
RADIATION-INDUCED LUNG DAMAGE
The lungs are particularly sensitive to radiation, and pulmonary problems occur most often in patients with malignant diseases of the chest that are treated with radiation, ie, those involving the mediastinum, the lung parenchyma, or the chest wall.
Abnormal radiographic findings or restrictive changes on pulmonary function testing have been reported in more than 30% of patients who received radiation directly or indirectly to the lung.9–12 These changes have been detected months to years after radiation therapy, most often in patients who suffered radiation pneumonitis as an acute toxicity.
The amount of damage depends on the cumulative dose, how many treatments (“fractions”) this cumulative dose was divided into (dividing the radiation dose into smaller dose fractions can reduce toxicity), the volume of lung tissue involved, and the patient’s age (the younger, the worse) at the time of treatment.
Cumulative dose. Whole-lung irradiation is limited to 12 Gy, although localized areas of cancer can be treated with much higher doses.
Clinically apparent pneumonitis with cough, fever, or dyspnea generally occurs only in survivors who received more than 30 Gy in standard fractions to more than 50% of the lung. However, in 12 survivors of Wilms tumor who received median total doses of approximately 20 Gy to both lungs 7 to 14 years previously, 8 patients had dyspnea on exertion and radiographic evidence of interstitial and pleural thickening.13 Mean total lung volumes and the diffusing capacity of the lung for carbon monoxide (DLCO) were reduced in all patients to approximately 60% of predicted values.
In a prospective study of adults with Hodgkin disease treated after 1980, 145 patients were examined 3 years after receiving more than 44 Gy limited to the mantle area.9 None were experiencing symptoms; however, 30% to 40% had a forced vital capacity (FVC) less than 80% of predicted, and 7% had a reduced DLCO. Some of these patients also had received bleomycin (see below), which may have exacerbated pulmonary toxicity. Asymptomatic restrictive and obstructive lung changes have been detected after lower doses of whole-lung radiation (11–14 Gy) were given for other malignant diseases.11,14
In a recent study of adults and older adolescents with stage I and IIA Hodgkin disease treated with radiation alone (40–45 Gy to involved fields, including the mediastinum), late pulmonary effects observed were minimal.10 While FVC, residual volume, forced expiratory volume in 1 second (FEV1), DLCO, and total lung capacity (TLC) were significantly lower at the end of radiation therapy than before treatment, all except DLCO returned nearly to normal within 1 year. The decrease in DLCO remained stable, but the forced expiratory flow rate between 25% and 75% of FVC (FEF 25%–75%) was significantly lower 3 years after treatment than at baseline.
The high doses of total lung irradiation cited in several of these reports are rarely used in today’s cancer protocols. For example, patients receiving radiation as adjunctive treatment for Hodgkin disease now receive lower doses (< 21 Gy), which are restricted to involved fields, and the pulmonary toxicity is less than in the past.
In one series, 159 children and adolescents with unfavorable Hodgkin disease treated from 1993 to 2000 received six cycles of chemotherapy followed by response-based, involved-field radiation therapy. Patients who achieved a complete response after the first two cycles of chemotherapy got 15 Gy, and those who achieved a partial response got 25.5 Gy to all sites of bulky lymphadenopathy.15 All patients underwent pulmonary function testing. Only 24 (30.8%) of them had pulmonary toxicity, which was limited to asymptomatic deficits of restriction and diffusion.
Nevertheless, the lungs receive some radiation even when they are not the target, such as in patients with malignant brain tumors, and this exposure can contribute to the development of lung disease, although these patients are likely to have no symptoms during day-to-day activities. Innovations in targeted radiation delivery (eg, conformal radiation) should further limit damage to normal lung tissue.
Age at the time of treatment also may influence the type and incidence of pulmonary sequelae. In older children and adults, radiation for thoracic malignancy results in pulmonary fibrosis with loss of lung volume. Similar injury can occur in younger children, but pulmonary function may also be compromised by inhibited growth of the supportive structures and the chest wall. One report suggests that children younger than 3 years at the time of therapy experience more chronic toxicity.11
In contrast, after bone marrow transplantation, children seem to be at less risk of significant late pulmonary dysfunction than adults are, despite similar preparatory regimens.16 This may in part reflect a lower incidence of severe graft-vs-host disease involving the lung. Nonetheless, in a recent report of children treated with fractionated total-body irradiation between 1985 and 1993, restrictive pulmonary diseases were found in 30 of 42 patients at a median of 3.1 years after treatment (range 0.5–17 years).17 Most of these patients had asymptomatic mild restrictive disease, and the one patient with severe changes had previously received thoracic radiation for treatment of neuroblastoma. In about half of the cases, pulmonary function abnormalities were permanent although not progressive.
CHEMOTHERAPY-RELATED LUNG DAMAGE
A growing list of chemotherapeutic agents appears to cause pulmonary disease in long-term survivors.5
Bleomycin
Bleomycin toxicity is the prototype for chemotherapy-related lung injury: bleomycin was the first chemotherapy drug shown to cause lung injury, this effect is suggested by a large database, and the mechanism is typical.5,18 Preclinical studies have attributed bleomycin’s toxicity to its tendency to promote free radicals.
Although interstitial pneumonitis and pulmonary fibrosis have been reported in children, clinically apparent bleomycin pneumonopathy is most frequent in older adults. Usually, the abnormalities began within 3 months of therapy and persisted or progressed. Like the acute toxicity, it is dose-dependent and more common above a threshold cumulative dose of 400 units/m2. Above this dose, 10% of adult patients without other risk factors develop fibrosis; data are not available for these doses in children. At lower doses, fibrosis occurs sporadically in fewer than 5% of patients, with a 1% to 2% mortality rate. In some series, bleomycin toxicity was anticipated on the basis of DLCO abnormalities.
Bleomycin pulmonary toxicity is variably exacerbated by concurrent or previous radiation therapy.
Increased oxygen concentrations associated with general anesthesia have also been found to exacerbate prior bleomycin-induced pulmonary injury.19,20 In one instance (reviewed by Zaniboni et al20), the patient recovered with corticosteroid treatment.
Alkylating agents
Carmustine (also called BCNU; brand names BiCNU, Gliadel) and lomustine (CCNU; CeeNU) are thought to cause dose-related lung injury. When cumulative carmustine doses are greater than 1,500 mg/m2, more than 50% of patients develop symptoms.21
In a careful clinicopathologic review of 31 children with brain tumors, restrictive changes with lung fibrosis were reported up to 17 years after treatment, most often with carmustine 100 mg/m2 every 6 to 8 weeks for up to 2 years.22 Four of the 8 patients still alive at the time of study experienced shortness of breath and coughing; 6 showed a characteristic pattern of upper zone fibrosis on chest radiography and computed tomography; all 8 survivors had restrictive findings on pulmonary function testing, with vital capacities of about 50% of normal. Toxicity increases with more intensive dose-scheduling.
Cyclophosphamide (Cytoxan) may cause delayed-onset pulmonary fibrosis with severe restrictive lung disease in association with marked reductions in the anteroposterior diameter of the chest, although the evidence is less convincing than with carmustine and lomustine, coming from case reports and small series.23
Melphalan (Alkeran), generally in doses used in stem cell transplant conditioning regimens, is also thought to cause pulmonary fibrosis.24
Busulfan most predictably causes toxicity when it is used in transplantation doses (ie, more than 500 mg), and may be associated with a progressive, potentially fatal restrictive lung disease.25 A current trend is to adjust the dose on the basis of pharmacokinetic analysis, which we hope will reduce toxicity.
Other agents
Methotrexate (MTX; Trexall) also has been associated with chronic pneumonitis and fibrosis.26 This probably occurs with an incidence well below 1% and may be idiosyncratic and not dose-related. Asymptomatic changes in pulmonary function tests that do not predict clinically significant problems have most frequently been associated with low-dose oral administration during more than 3 years.27 This is a treatment approach used in patients with psoriasis or rheumatoid arthritis, but is now obsolete for pediatric cancer. Intravenous and, rarely, intrathecal administration also have been associated with pulmonary toxicity.28 A single report of two patients who developed diffuse interstitial pulmonary infiltrates and chronic pulmonary changes links vinblastine to these sequelae.29
LUNG INJURY AFTER BONE MARROW TRANSPLANTATION
Hematopoietic stem cell transplantation is associated with various late pulmonary complications. The factors that influence these complications are similar to those discussed earlier. However, the intensity of therapy in patients undergoing transplantation and the additive effects of previous therapies magnify the risks.
Patients undergoing total-body irradiation as part of their preparation for transplantation have a high incidence of late pulmonary complications.25,30–32 Busulfan, carmustine, bleomycin, and cyclophosphamide, also commonly used conditioning chemotherapies, are known to cause pneumonitis and fibrosis after transplantation.33
Some acute pulmonary toxicities can have long-standing effects, including serious pulmonary infections, idiopathic pneumonia syndrome, bronchiolitis obliterans, acute respiratory distress syndrome, or other damage related to graft-vs-host disease.
OTHER RISK FACTORS FOR LUNG DAMAGE
Additional factors contributing to chronic pulmonary toxicity include superimposed infection, underlying pneumonopathy (eg, asthma), cigarette use, respirator toxicity, chronic graft-vs-host disease, and the effects of chronic pulmonary involvement by tumor or reaction to tumor. For example, a subset of patients with Langerhans cell histiocytosis can develop histiocytic pulmonary infiltrates or honeycombing with severe chronic restrictive lung disease unrelated to therapy or the presence of active tumor.
Although not well documented, scuba diving also has been said to exacerbate pulmonary fibrosis through increased underwater pressures and high oxygen levels.34
Lung lobectomy during childhood appears to have no significant impact on long-term pulmonary function,35–37 but the effect of lung surgery for children with cancer is not well defined.
GET THE PATIENT’S TREATMENT SUMMARY
Regardless of the setting for follow-up, the first step in any evaluation is to obtain the patient’s medical history and especially a treatment summary. The treatment summary should outline the cancer diagnosis, involved sites of disease, age at diagnosis, specific treatments (surgery, chemotherapy, radiation), and other key interventions and events during and after cancer therapy. Sample forms for physicians and patients are available at www.survivorshipguidelines.org.
Before the long-term survivor of childhood cancer graduates from the care of a pediatric oncologist, this treatment record and possible long-term problems should be reviewed with the family and, in the case of an adolescent, with the patient. Correspondence between the pediatric oncologist and subsequent caregivers should also include a treatment summary. The treatment summary allows the survivor or his health care provider to interface with the COG guidelines to determine recommended follow-up care. The primary care physician and the patient both should have copies of this document.
We are developing an interactive Web-based version of a standardized summary form, designed to interface with an automated version of the COG guidelines in order to generate individualized follow-up recommendations.
ASK ABOUT LUNG SYMPTOMS
We recommend that health care providers investigate symptoms of pulmonary dysfunction, and specifically ask about chronic cough with or without fever, shortness of breath, and dyspnea on exertion during yearly health care visits.
Baseline pulmonary function testing (including DLCO and spirometry) and chest radiography are recommended 2 or more years after completion of therapy to document persistent deficits and determine the need for continued monitoring. Reevaluation of pulmonary function should be considered in patients with established deficits who require general anesthesia and for those treated with bleomycin.
Scuba diving remains controversial for long-term survivors. Consequently, patients with risk factors for lung disease should be encouraged to consult with a pulmonary specialist to determine if diving poses a health threat to their pulmonary status. If clinical pulmonary dysfunction is identified, referral to colleagues in pulmonology for additional evaluation and treatment is essential. Increasing familiarity of primary care providers with surveillance concepts is a key element in survivorship care.
Smoking cessation can enhance the health of all patients and is particularly important among long-term survivors, especially those who received treatments predisposing to pulmonary injury. Strategies for cessation and patient information can be found at www.cdc.gov/tobacco/how2quit.htm.
Clinicians can take advantage of every patient interaction to assess readiness for smoking cessation and assist patients in this goal. Following the principles of patient-centered counseling, physicians can guide patients into considering a change of behavior with advice and encouragement. Whenever possible, physicians should personalize the risks of smoking as well as the short-term and long-term benefits. As smokers prepare to quit, their physicians can assist in developing a plan that includes a quit date.
Many pharmacologic agents are available to assist patients, ie, nicotine inhalers, sprays, gum, and transdermal patches; the antidepressants bupropion (Wellbutrin) and nortriptiline (Pamelor); the alpha-2 adrenergic agonist clonidine (Catapres); and, most recently, the nicotine receptor partial agonist varenicline (Chantix).38
Follow-up to prevent relapse is an important part of this process.
Acknowledgment. This work was supported in part by the Swim Across America Foundation and the Campini Foundation.
Children who undergo radiotherapy, chemotherapy, or surgery for cancer face a risk of complications later in life, including pulmonary fibrosis and pneumonitis.
These long-term cancer survivors need systematic, lifelong surveillance, in a program that takes into account their individual risk (based on therapeutic exposures, genetic predisposition, lifestyle behaviors, and comorbid health conditions).1 Optimally, they would receive their care at multidisciplinary follow-up clinics organized by pediatric oncologists at tertiary care centers. However, access to such centers is limited, making this an option for relatively few. Consequently, as childhood cancer survivors age, internists and family practitioners may need to assume an increasing amount of responsibility for their follow-up care.
Because individual primary care providers are unlikely to follow more than a handful of survivors, specialists have developed guidelines for survivors of pediatric cancer. Working with established multidisciplinary clinics may help ensure appropriate follow-up for this population of patients.
This review summarizes the late effects of cancer therapy on the lungs and an approach to surveillance for the generalist or pulmonologist. We also review the quality of the evidence upon which these recommendations are based.
NUMBERS ON THE RISE
An estimated 1 of every 330 children develops cancer before age 19. With cure rates exceeding 75% for many pediatric malignancies, the number of survivors of childhood cancer, currently in excess of 270,000, will continue to increase.2
THE CHILDREN’S ONCOLOGY GROUP GUIDELINES
The Children’s Oncology Group (COG)3 released its first set of guidelines in 2003 for the follow-up care of patients treated for pediatric malignancies; the current version is available at www.survivorshipguidelines.org. The guidelines contain comprehensive screening recommendations, including those related to pulmonary toxicity, which can be used to standardize care.
Patient education materials accompany the guidelines, offering detailed information on guideline-specific topics in order to promote health maintenance.
HOW WE SEARCHED THE LITERATURE
We performed an extensive review of the literature via MEDLINE for the years 1975–2005. Key search terms were “childhood cancer,” “late effects,” and “pulmonary toxicity,” combined with keywords for each therapeutic exposure. References from selected articles were used to broaden the search. From several hundred citations, fewer than 30 were selected as best illustrating the relevant associations.
RISK IS THREE TIMES HIGHER IN CANCER SURVIVORS
The Childhood Cancer Survivor Study4 is the largest database of late effects, with more than 12,000 survivors of childhood cancer diagnosed between 1970 and 1986. Its data suggest that the risk of pulmonary conditions is more than three times higher in cancer survivors than in their siblings, as manifested by pulmonary signs (abnormal chest wall growth), symptoms (chronic cough, use of supplemental oxygen, exercise-induced shortness of breath), or specific diagnoses (lung fibrosis, recurrent pneumonia, pleurisy, bronchitis, recurrent sinus infection, or tonsillitis). Limitations: these data are retrospective, and the outcomes were detected by self-report and were not validated by review of medical records. Thus, the figures highlight the fact that pulmonary late effects are an important problem but do not give us a way to calculate risk exactly.
Other limitations of the literature: Treatments are constantly evolving, often in attempts to minimize late effects, and newer agents will need to be monitored for pulmonary toxicities. As noted, much of the available information is from studies of survivors of adult cancer; the potential for late effects of similar therapies in children is inferred. Most conclusions—and especially those based upon prospective serial evaluations—derive from small cohorts. For all treatments, the complications in the very long term remain undefined. What we know is summarized below.
CANCER THERAPY CAUSES FIBROSIS, PNEUMONITIS
The courses of these diseases are poorly characterized, since few longitudinal studies have been done. However, like most of the late effects of cancer therapy, pulmonary toxicity may first become apparent during the treatment and persist, or it may not appear until years later. Signs and symptoms may be static, progressive, or reversible.
ANGIOGENESIS MAY CONTRIBUTE TO FIBROSIS
On a microscopic level, pulmonary fibrosis is characterized by epithelial injury, fibroproliferation, and excessive extracellular matrix deposition.6–8
Evidence is mounting that these findings result in part from angiogenesis. Although this has not been studied in long-term cancer survivors, evidence of neovascularization was seen both in an animal model of lung fibrosis and in patients with idiopathic pulmonary fibrosis.6–8 High plasma concentrations of angiogenic cytokines (eg, tumor necrosis factor alpha, interleukin 8, and endothelin 1) have been found in these situations. Antiangiogenic agents and other immune modulators such as thalidomide may be beneficial in patients with lung fibrosis.7
On a macroscopic level, pulmonary fibrosis results in loss of lung volume in older children and in adults. In contrast, in younger children, interference with growth of both the lung and the chest wall may contribute to pulmonary dysfunction.
CANCER TYPES AND TREATMENTS VARY BY AGE
Cancers that commonly involve the thorax are listed in Table 2. Neuroblastoma, hepatoblastoma, extragonadal germ cell tumors, and Wilms tumor typically are diseases of young children; osteosarcoma, Ewing sarcoma, thyroid carcinoma, and Hodgkin disease are most common in older children and adolescents; soft tissue sarcoma and non-Hodgkin lymphoma span all age groups.
Surgery can, in some cases, control the cancer, as with mediastinal neuroblastoma and Ewing sarcoma of the chest wall.
Radiation to the chest remains a major component of treatment for Hodgkin disease, unresected thoracic Ewing sarcoma, soft tissue sarcoma with lung involvement or thyroid carcinoma, and Wilms tumor. Central nervous system tumors and leukemias, the most common pediatric malignancies, may require radiation to the spinal cord—with resulting radiation exposure of the lungs. Total-body irradiation is a component of many preparative regimens for stem cell transplantation.
Chemotherapy remains a mainstay for all types of tumors, and patients with germ cell tumors, Hodgkin disease, and brain tumors are at particular risk of pulmonary toxicity due to heavy reliance on bleomycin (Blenoxane) (for germ cell tumors, Hodgkin disease) and the nitrosoureas (for brain tumors).
RADIATION-INDUCED LUNG DAMAGE
The lungs are particularly sensitive to radiation, and pulmonary problems occur most often in patients with malignant diseases of the chest that are treated with radiation, ie, those involving the mediastinum, the lung parenchyma, or the chest wall.
Abnormal radiographic findings or restrictive changes on pulmonary function testing have been reported in more than 30% of patients who received radiation directly or indirectly to the lung.9–12 These changes have been detected months to years after radiation therapy, most often in patients who suffered radiation pneumonitis as an acute toxicity.
The amount of damage depends on the cumulative dose, how many treatments (“fractions”) this cumulative dose was divided into (dividing the radiation dose into smaller dose fractions can reduce toxicity), the volume of lung tissue involved, and the patient’s age (the younger, the worse) at the time of treatment.
Cumulative dose. Whole-lung irradiation is limited to 12 Gy, although localized areas of cancer can be treated with much higher doses.
Clinically apparent pneumonitis with cough, fever, or dyspnea generally occurs only in survivors who received more than 30 Gy in standard fractions to more than 50% of the lung. However, in 12 survivors of Wilms tumor who received median total doses of approximately 20 Gy to both lungs 7 to 14 years previously, 8 patients had dyspnea on exertion and radiographic evidence of interstitial and pleural thickening.13 Mean total lung volumes and the diffusing capacity of the lung for carbon monoxide (DLCO) were reduced in all patients to approximately 60% of predicted values.
In a prospective study of adults with Hodgkin disease treated after 1980, 145 patients were examined 3 years after receiving more than 44 Gy limited to the mantle area.9 None were experiencing symptoms; however, 30% to 40% had a forced vital capacity (FVC) less than 80% of predicted, and 7% had a reduced DLCO. Some of these patients also had received bleomycin (see below), which may have exacerbated pulmonary toxicity. Asymptomatic restrictive and obstructive lung changes have been detected after lower doses of whole-lung radiation (11–14 Gy) were given for other malignant diseases.11,14
In a recent study of adults and older adolescents with stage I and IIA Hodgkin disease treated with radiation alone (40–45 Gy to involved fields, including the mediastinum), late pulmonary effects observed were minimal.10 While FVC, residual volume, forced expiratory volume in 1 second (FEV1), DLCO, and total lung capacity (TLC) were significantly lower at the end of radiation therapy than before treatment, all except DLCO returned nearly to normal within 1 year. The decrease in DLCO remained stable, but the forced expiratory flow rate between 25% and 75% of FVC (FEF 25%–75%) was significantly lower 3 years after treatment than at baseline.
The high doses of total lung irradiation cited in several of these reports are rarely used in today’s cancer protocols. For example, patients receiving radiation as adjunctive treatment for Hodgkin disease now receive lower doses (< 21 Gy), which are restricted to involved fields, and the pulmonary toxicity is less than in the past.
In one series, 159 children and adolescents with unfavorable Hodgkin disease treated from 1993 to 2000 received six cycles of chemotherapy followed by response-based, involved-field radiation therapy. Patients who achieved a complete response after the first two cycles of chemotherapy got 15 Gy, and those who achieved a partial response got 25.5 Gy to all sites of bulky lymphadenopathy.15 All patients underwent pulmonary function testing. Only 24 (30.8%) of them had pulmonary toxicity, which was limited to asymptomatic deficits of restriction and diffusion.
Nevertheless, the lungs receive some radiation even when they are not the target, such as in patients with malignant brain tumors, and this exposure can contribute to the development of lung disease, although these patients are likely to have no symptoms during day-to-day activities. Innovations in targeted radiation delivery (eg, conformal radiation) should further limit damage to normal lung tissue.
Age at the time of treatment also may influence the type and incidence of pulmonary sequelae. In older children and adults, radiation for thoracic malignancy results in pulmonary fibrosis with loss of lung volume. Similar injury can occur in younger children, but pulmonary function may also be compromised by inhibited growth of the supportive structures and the chest wall. One report suggests that children younger than 3 years at the time of therapy experience more chronic toxicity.11
In contrast, after bone marrow transplantation, children seem to be at less risk of significant late pulmonary dysfunction than adults are, despite similar preparatory regimens.16 This may in part reflect a lower incidence of severe graft-vs-host disease involving the lung. Nonetheless, in a recent report of children treated with fractionated total-body irradiation between 1985 and 1993, restrictive pulmonary diseases were found in 30 of 42 patients at a median of 3.1 years after treatment (range 0.5–17 years).17 Most of these patients had asymptomatic mild restrictive disease, and the one patient with severe changes had previously received thoracic radiation for treatment of neuroblastoma. In about half of the cases, pulmonary function abnormalities were permanent although not progressive.
CHEMOTHERAPY-RELATED LUNG DAMAGE
A growing list of chemotherapeutic agents appears to cause pulmonary disease in long-term survivors.5
Bleomycin
Bleomycin toxicity is the prototype for chemotherapy-related lung injury: bleomycin was the first chemotherapy drug shown to cause lung injury, this effect is suggested by a large database, and the mechanism is typical.5,18 Preclinical studies have attributed bleomycin’s toxicity to its tendency to promote free radicals.
Although interstitial pneumonitis and pulmonary fibrosis have been reported in children, clinically apparent bleomycin pneumonopathy is most frequent in older adults. Usually, the abnormalities began within 3 months of therapy and persisted or progressed. Like the acute toxicity, it is dose-dependent and more common above a threshold cumulative dose of 400 units/m2. Above this dose, 10% of adult patients without other risk factors develop fibrosis; data are not available for these doses in children. At lower doses, fibrosis occurs sporadically in fewer than 5% of patients, with a 1% to 2% mortality rate. In some series, bleomycin toxicity was anticipated on the basis of DLCO abnormalities.
Bleomycin pulmonary toxicity is variably exacerbated by concurrent or previous radiation therapy.
Increased oxygen concentrations associated with general anesthesia have also been found to exacerbate prior bleomycin-induced pulmonary injury.19,20 In one instance (reviewed by Zaniboni et al20), the patient recovered with corticosteroid treatment.
Alkylating agents
Carmustine (also called BCNU; brand names BiCNU, Gliadel) and lomustine (CCNU; CeeNU) are thought to cause dose-related lung injury. When cumulative carmustine doses are greater than 1,500 mg/m2, more than 50% of patients develop symptoms.21
In a careful clinicopathologic review of 31 children with brain tumors, restrictive changes with lung fibrosis were reported up to 17 years after treatment, most often with carmustine 100 mg/m2 every 6 to 8 weeks for up to 2 years.22 Four of the 8 patients still alive at the time of study experienced shortness of breath and coughing; 6 showed a characteristic pattern of upper zone fibrosis on chest radiography and computed tomography; all 8 survivors had restrictive findings on pulmonary function testing, with vital capacities of about 50% of normal. Toxicity increases with more intensive dose-scheduling.
Cyclophosphamide (Cytoxan) may cause delayed-onset pulmonary fibrosis with severe restrictive lung disease in association with marked reductions in the anteroposterior diameter of the chest, although the evidence is less convincing than with carmustine and lomustine, coming from case reports and small series.23
Melphalan (Alkeran), generally in doses used in stem cell transplant conditioning regimens, is also thought to cause pulmonary fibrosis.24
Busulfan most predictably causes toxicity when it is used in transplantation doses (ie, more than 500 mg), and may be associated with a progressive, potentially fatal restrictive lung disease.25 A current trend is to adjust the dose on the basis of pharmacokinetic analysis, which we hope will reduce toxicity.
Other agents
Methotrexate (MTX; Trexall) also has been associated with chronic pneumonitis and fibrosis.26 This probably occurs with an incidence well below 1% and may be idiosyncratic and not dose-related. Asymptomatic changes in pulmonary function tests that do not predict clinically significant problems have most frequently been associated with low-dose oral administration during more than 3 years.27 This is a treatment approach used in patients with psoriasis or rheumatoid arthritis, but is now obsolete for pediatric cancer. Intravenous and, rarely, intrathecal administration also have been associated with pulmonary toxicity.28 A single report of two patients who developed diffuse interstitial pulmonary infiltrates and chronic pulmonary changes links vinblastine to these sequelae.29
LUNG INJURY AFTER BONE MARROW TRANSPLANTATION
Hematopoietic stem cell transplantation is associated with various late pulmonary complications. The factors that influence these complications are similar to those discussed earlier. However, the intensity of therapy in patients undergoing transplantation and the additive effects of previous therapies magnify the risks.
Patients undergoing total-body irradiation as part of their preparation for transplantation have a high incidence of late pulmonary complications.25,30–32 Busulfan, carmustine, bleomycin, and cyclophosphamide, also commonly used conditioning chemotherapies, are known to cause pneumonitis and fibrosis after transplantation.33
Some acute pulmonary toxicities can have long-standing effects, including serious pulmonary infections, idiopathic pneumonia syndrome, bronchiolitis obliterans, acute respiratory distress syndrome, or other damage related to graft-vs-host disease.
OTHER RISK FACTORS FOR LUNG DAMAGE
Additional factors contributing to chronic pulmonary toxicity include superimposed infection, underlying pneumonopathy (eg, asthma), cigarette use, respirator toxicity, chronic graft-vs-host disease, and the effects of chronic pulmonary involvement by tumor or reaction to tumor. For example, a subset of patients with Langerhans cell histiocytosis can develop histiocytic pulmonary infiltrates or honeycombing with severe chronic restrictive lung disease unrelated to therapy or the presence of active tumor.
Although not well documented, scuba diving also has been said to exacerbate pulmonary fibrosis through increased underwater pressures and high oxygen levels.34
Lung lobectomy during childhood appears to have no significant impact on long-term pulmonary function,35–37 but the effect of lung surgery for children with cancer is not well defined.
GET THE PATIENT’S TREATMENT SUMMARY
Regardless of the setting for follow-up, the first step in any evaluation is to obtain the patient’s medical history and especially a treatment summary. The treatment summary should outline the cancer diagnosis, involved sites of disease, age at diagnosis, specific treatments (surgery, chemotherapy, radiation), and other key interventions and events during and after cancer therapy. Sample forms for physicians and patients are available at www.survivorshipguidelines.org.
Before the long-term survivor of childhood cancer graduates from the care of a pediatric oncologist, this treatment record and possible long-term problems should be reviewed with the family and, in the case of an adolescent, with the patient. Correspondence between the pediatric oncologist and subsequent caregivers should also include a treatment summary. The treatment summary allows the survivor or his health care provider to interface with the COG guidelines to determine recommended follow-up care. The primary care physician and the patient both should have copies of this document.
We are developing an interactive Web-based version of a standardized summary form, designed to interface with an automated version of the COG guidelines in order to generate individualized follow-up recommendations.
ASK ABOUT LUNG SYMPTOMS
We recommend that health care providers investigate symptoms of pulmonary dysfunction, and specifically ask about chronic cough with or without fever, shortness of breath, and dyspnea on exertion during yearly health care visits.
Baseline pulmonary function testing (including DLCO and spirometry) and chest radiography are recommended 2 or more years after completion of therapy to document persistent deficits and determine the need for continued monitoring. Reevaluation of pulmonary function should be considered in patients with established deficits who require general anesthesia and for those treated with bleomycin.
Scuba diving remains controversial for long-term survivors. Consequently, patients with risk factors for lung disease should be encouraged to consult with a pulmonary specialist to determine if diving poses a health threat to their pulmonary status. If clinical pulmonary dysfunction is identified, referral to colleagues in pulmonology for additional evaluation and treatment is essential. Increasing familiarity of primary care providers with surveillance concepts is a key element in survivorship care.
Smoking cessation can enhance the health of all patients and is particularly important among long-term survivors, especially those who received treatments predisposing to pulmonary injury. Strategies for cessation and patient information can be found at www.cdc.gov/tobacco/how2quit.htm.
Clinicians can take advantage of every patient interaction to assess readiness for smoking cessation and assist patients in this goal. Following the principles of patient-centered counseling, physicians can guide patients into considering a change of behavior with advice and encouragement. Whenever possible, physicians should personalize the risks of smoking as well as the short-term and long-term benefits. As smokers prepare to quit, their physicians can assist in developing a plan that includes a quit date.
Many pharmacologic agents are available to assist patients, ie, nicotine inhalers, sprays, gum, and transdermal patches; the antidepressants bupropion (Wellbutrin) and nortriptiline (Pamelor); the alpha-2 adrenergic agonist clonidine (Catapres); and, most recently, the nicotine receptor partial agonist varenicline (Chantix).38
Follow-up to prevent relapse is an important part of this process.
Acknowledgment. This work was supported in part by the Swim Across America Foundation and the Campini Foundation.
- Hewitt M, Weiner S, Simone J. The epidemiology of childhood cancer. Washington DC: The National Academies Press, 2003:20–36.
- Ries L, Eisner M, Kosary C, et al. SEER cancer statistics review, 1975–2001. Bethesda, MD: National Cancer Institute; 2004.
- Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: The Children’s Oncology Group long-term follow-up guidelines from the Children’s Oncology Group Late Effects Committee and nursing discipline. J Clin Oncol 2004; 22:4979–4990.
- Mertens AC, Yasui Y, Liu Y, et al. Pulmonary complications in survivors of childhood and adolescent cancer. A report from the Childhood Cancer Survivor Study. Cancer 2002; 95:2431–2441.
- Bhatia S, Blatt J, Meadows A. Late effects of childhood cancer and its treatment. In:Pizzo P, Poplack D. Principles and Practice of Pediatric Oncology. Philadelphia: Lippincott Williams & Wilkins, 2006.
- Anscher MS, Chen L, Rabbani Z, et al. Recent progress in defining mechanisms and potential targets for prevention of normal tissue injury after radiation therapy. Int J Radiat Oncol Biol Phys 2005; 62:255–259.
- Bhatt N, Baran CP, Allen J, et al. Promising pharmacologic innovations in treating pulmonary fibrosis. Curr Opin Pharmacol 2006; 6:284–292.
- Abid SH, Malhotra V, Perry MC. Radiation-induced and chemotherapy-induced pulmonary injury. Curr Opin Oncol 2001: 13:242–248.
- Horning SJ, Adhikari A, Rizk N, et al. Effect of treatment for Hodgkin’s disease on pulmonary function: results of a prospective study. J Clin Oncol 1994; 12:297–305.
- Villani F, Viviani S, Bonfante V, et al. Late pulmonary effects in favorable stage I and IIA Hodgkin’s disease treated with radiotherapy alone. Am J Clin Oncol 2000; 23:18–21.
- Miller RW, Fusner JE, Fink RJ, et al. Pulmonary function abnormalities in long-term survivors of childhood cancer. Med Pediatr Oncol 1986; 14:202–207.
- Weiner DJ, Maity A, Carlson CA, et al. Pulmonary function abnormalities in children treated with whole lung irradiation. Pediatr Blood Cancer 2006; 46:222–227.
- Wohl ME, Griscom NT, Traggis DG, et al. Effects of therapeutic irradiation delivered in early childhood upon subsequent lung function. Pediatrics 1975; 55:507–516.
- Hudson MM, Greenwald C, Thompson E, et al. Efficacy and toxicity of multiagent chemotherapy and low-dose involved-field radiotherapy in children and adolescents with Hodgkin’s disease. J Clin Oncol 1993; 11:100–108.
- Hudson MM, Krasin M, Link MP, et al. Risk-adapted, combined-modality therapy with VAMP/COP and response-based, involved-field radiation for unfavorable pediatric Hodgkin’s disease. J Clin Oncol 2004; 22:4541–4550.
- Quigley PM, Yeager AM, Loughlin GM. The effects of bone marrow transplantation on pulmonary function in children. Pediatr Pulmonol 1994; 18:361–367.
- Faraci M, Barra S, Cohen A, et al. Very late nonfatal consequences of fractionated TBI in children undergoing bone marrow transplant. Int J Radiat Oncol Biol Phys 2005; 63:1568–1575.
- Eigen H, Wyszomierski D. Bleomycin lung injury in children. Pathophysiology and guidelines for management. Am J Pediatr Hematol Oncol 1985; 7:71–78.
- Goldiner PL, Schweizer O. The hazards of anesthesia and surgery in bleomycin-treated patients. Semin Oncol 1979; 6:121–124.
- Zaniboni A, Prabhu S, Audisio RA. Chemotherapy and anaesthetic drugs: too little is known. Lancet Oncol 2005; 6:176–181.
- Aronin PA, Mahaley MS, Rudnick SA, et al. Prediction of BCNU pulmonary toxicity in patients with malignant gliomas: an assessment of risk factors. N Engl J Med 1980; 303:183–188.
- O’Driscoll BR, Hasleton PS, Taylor PM, et al. Active lung fibrosis up to 17 years after chemotherapy with carmustine (BCNU) in childhood. N Engl J Med 1990; 323:378–382.
- Alvarado CS, Boat TF, Newman AJ. Late-onset pulmonary fibrosis and chest deformity in two children treated with cyclophosphamide. J Pediatr 1978; 92:443–446.
- Codling BW, Chakera TM. Pulmonary fibrosis following therapy with melphalan for multiple myeloma. J Clin Pathol 1972; 25:668–673.
- Bruno B, Souillet G, Bertrand Y, et al. Effects of allogeneic bone marrow transplantation on pulmonary function in 80 children in a single paediatric centre. Bone Marrow Transplant 2004; 34:143–147.
- Lateef O, Shakoor N, Balk RA. Methotrexate pulmonary toxicity. Expert Opin Drug Saf 2005; 4:723–730.
- Cottin V, Tebob J, Massonnet B, et al. Pulmonary function in patients receiving long-term methotrexate. Chest 1996; 109:933–938.
- Gutin PH, Green MR, Bleyer WA, et al. Methotrexate pneumonitis induced by intrathecal methotrexate therapy: a case report with pharmacokinetic data. Cancer 1976; 38:1529–1534.
- Konits PH, Aisner J, Sutherland JC, et al. Possible pulmonary toxicity secondary to vinblastine. Cancer 1982; 50:2771–2774.
- Cerveri I, Zoia MC, Fulgoni P, et al. Late pulmonary sequelae after childhood bone marrow transplantation. Thorax 1999; 54:131–135.
- Cerveri I, Fulgoni P, Giorgiani G, et al. Lung function abnormalities after bone marrow transplantation in children: has the trend recently changed? Chest 2001; 120:1900–1906.
- Neve V, Foot AB, Michon J, et al. Longitudinal clinical and functional pulmonary follow-up after megatherapy, fractionated total body irradiation, and autologous bone marrow transplantation for metastatic neuroblastoma. Med Pediatr Oncol 1999; 32:170–176.
- Nenadov Beck M, Meresse V, Hartmann O, et al. Long-term pulmonary sequelae after autologous bone marrow transplantation in children without total body irradiation. Bone Marrow Transplant 1995; 16:771–775.
- Huls G, ten Bokkel Huinink D. Bleomycin and scuba diving: to dive or not to dive? Neth J Med 2003; 61:50–53.
- Kreisel D, Krupnick AS, Huddleston CB. Outcomes and late complications after pulmonary resections in the pediatric population. Semin Thorac Cardiovasc Surg 2004; 16:215–219.
- Lezama-del Valle Valle P, Blakely ML, Lobe TE. Physiologic consequences of pneumonectomy. Long-term consequences of pneumonectomy done in children. Chest Surg Clin North Am 1999; 9:485–495.
- Tobias JD, Bozeman PM, Mackert PW, et al. Postoperative outcome following thoracotomy in the pediatric oncology patient with diminished pulmonary function. J Surg Oncol 1993; 52:105–109.
- Drugs for Tobacco Dependence. Treatment Guidelines from the Medical Letter. June, 2003: 1(10):65–68.
- Hewitt M, Weiner S, Simone J. The epidemiology of childhood cancer. Washington DC: The National Academies Press, 2003:20–36.
- Ries L, Eisner M, Kosary C, et al. SEER cancer statistics review, 1975–2001. Bethesda, MD: National Cancer Institute; 2004.
- Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: The Children’s Oncology Group long-term follow-up guidelines from the Children’s Oncology Group Late Effects Committee and nursing discipline. J Clin Oncol 2004; 22:4979–4990.
- Mertens AC, Yasui Y, Liu Y, et al. Pulmonary complications in survivors of childhood and adolescent cancer. A report from the Childhood Cancer Survivor Study. Cancer 2002; 95:2431–2441.
- Bhatia S, Blatt J, Meadows A. Late effects of childhood cancer and its treatment. In:Pizzo P, Poplack D. Principles and Practice of Pediatric Oncology. Philadelphia: Lippincott Williams & Wilkins, 2006.
- Anscher MS, Chen L, Rabbani Z, et al. Recent progress in defining mechanisms and potential targets for prevention of normal tissue injury after radiation therapy. Int J Radiat Oncol Biol Phys 2005; 62:255–259.
- Bhatt N, Baran CP, Allen J, et al. Promising pharmacologic innovations in treating pulmonary fibrosis. Curr Opin Pharmacol 2006; 6:284–292.
- Abid SH, Malhotra V, Perry MC. Radiation-induced and chemotherapy-induced pulmonary injury. Curr Opin Oncol 2001: 13:242–248.
- Horning SJ, Adhikari A, Rizk N, et al. Effect of treatment for Hodgkin’s disease on pulmonary function: results of a prospective study. J Clin Oncol 1994; 12:297–305.
- Villani F, Viviani S, Bonfante V, et al. Late pulmonary effects in favorable stage I and IIA Hodgkin’s disease treated with radiotherapy alone. Am J Clin Oncol 2000; 23:18–21.
- Miller RW, Fusner JE, Fink RJ, et al. Pulmonary function abnormalities in long-term survivors of childhood cancer. Med Pediatr Oncol 1986; 14:202–207.
- Weiner DJ, Maity A, Carlson CA, et al. Pulmonary function abnormalities in children treated with whole lung irradiation. Pediatr Blood Cancer 2006; 46:222–227.
- Wohl ME, Griscom NT, Traggis DG, et al. Effects of therapeutic irradiation delivered in early childhood upon subsequent lung function. Pediatrics 1975; 55:507–516.
- Hudson MM, Greenwald C, Thompson E, et al. Efficacy and toxicity of multiagent chemotherapy and low-dose involved-field radiotherapy in children and adolescents with Hodgkin’s disease. J Clin Oncol 1993; 11:100–108.
- Hudson MM, Krasin M, Link MP, et al. Risk-adapted, combined-modality therapy with VAMP/COP and response-based, involved-field radiation for unfavorable pediatric Hodgkin’s disease. J Clin Oncol 2004; 22:4541–4550.
- Quigley PM, Yeager AM, Loughlin GM. The effects of bone marrow transplantation on pulmonary function in children. Pediatr Pulmonol 1994; 18:361–367.
- Faraci M, Barra S, Cohen A, et al. Very late nonfatal consequences of fractionated TBI in children undergoing bone marrow transplant. Int J Radiat Oncol Biol Phys 2005; 63:1568–1575.
- Eigen H, Wyszomierski D. Bleomycin lung injury in children. Pathophysiology and guidelines for management. Am J Pediatr Hematol Oncol 1985; 7:71–78.
- Goldiner PL, Schweizer O. The hazards of anesthesia and surgery in bleomycin-treated patients. Semin Oncol 1979; 6:121–124.
- Zaniboni A, Prabhu S, Audisio RA. Chemotherapy and anaesthetic drugs: too little is known. Lancet Oncol 2005; 6:176–181.
- Aronin PA, Mahaley MS, Rudnick SA, et al. Prediction of BCNU pulmonary toxicity in patients with malignant gliomas: an assessment of risk factors. N Engl J Med 1980; 303:183–188.
- O’Driscoll BR, Hasleton PS, Taylor PM, et al. Active lung fibrosis up to 17 years after chemotherapy with carmustine (BCNU) in childhood. N Engl J Med 1990; 323:378–382.
- Alvarado CS, Boat TF, Newman AJ. Late-onset pulmonary fibrosis and chest deformity in two children treated with cyclophosphamide. J Pediatr 1978; 92:443–446.
- Codling BW, Chakera TM. Pulmonary fibrosis following therapy with melphalan for multiple myeloma. J Clin Pathol 1972; 25:668–673.
- Bruno B, Souillet G, Bertrand Y, et al. Effects of allogeneic bone marrow transplantation on pulmonary function in 80 children in a single paediatric centre. Bone Marrow Transplant 2004; 34:143–147.
- Lateef O, Shakoor N, Balk RA. Methotrexate pulmonary toxicity. Expert Opin Drug Saf 2005; 4:723–730.
- Cottin V, Tebob J, Massonnet B, et al. Pulmonary function in patients receiving long-term methotrexate. Chest 1996; 109:933–938.
- Gutin PH, Green MR, Bleyer WA, et al. Methotrexate pneumonitis induced by intrathecal methotrexate therapy: a case report with pharmacokinetic data. Cancer 1976; 38:1529–1534.
- Konits PH, Aisner J, Sutherland JC, et al. Possible pulmonary toxicity secondary to vinblastine. Cancer 1982; 50:2771–2774.
- Cerveri I, Zoia MC, Fulgoni P, et al. Late pulmonary sequelae after childhood bone marrow transplantation. Thorax 1999; 54:131–135.
- Cerveri I, Fulgoni P, Giorgiani G, et al. Lung function abnormalities after bone marrow transplantation in children: has the trend recently changed? Chest 2001; 120:1900–1906.
- Neve V, Foot AB, Michon J, et al. Longitudinal clinical and functional pulmonary follow-up after megatherapy, fractionated total body irradiation, and autologous bone marrow transplantation for metastatic neuroblastoma. Med Pediatr Oncol 1999; 32:170–176.
- Nenadov Beck M, Meresse V, Hartmann O, et al. Long-term pulmonary sequelae after autologous bone marrow transplantation in children without total body irradiation. Bone Marrow Transplant 1995; 16:771–775.
- Huls G, ten Bokkel Huinink D. Bleomycin and scuba diving: to dive or not to dive? Neth J Med 2003; 61:50–53.
- Kreisel D, Krupnick AS, Huddleston CB. Outcomes and late complications after pulmonary resections in the pediatric population. Semin Thorac Cardiovasc Surg 2004; 16:215–219.
- Lezama-del Valle Valle P, Blakely ML, Lobe TE. Physiologic consequences of pneumonectomy. Long-term consequences of pneumonectomy done in children. Chest Surg Clin North Am 1999; 9:485–495.
- Tobias JD, Bozeman PM, Mackert PW, et al. Postoperative outcome following thoracotomy in the pediatric oncology patient with diminished pulmonary function. J Surg Oncol 1993; 52:105–109.
- Drugs for Tobacco Dependence. Treatment Guidelines from the Medical Letter. June, 2003: 1(10):65–68.
KEY POINTS
- Radiation therapy causes pulmonary fibrosis, interstitial pneumonitis, and restrictive or obstructive lung disease. The risk is dose-dependent and increases with concomitant chemotherapy, younger age at treatment, atopic history, and smoking.
- Alkylating agents cause pulmonary fibrosis. Bleomycin can cause interstitial pneumonitis, pulmonary fibrosis, or, very rarely, acute respiratory distress syndrome.
- Cancer survivors should have a yearly history and physical examination, plus pulmonary function testing and radiography at baseline and repeated as clinically indicated.
- All patients who smoke should be encouraged to quit.