User login
Eosinophilic granuloma (EG) is the most common benign form of Langerhans cell histiocytosis (LCH). Initially described by Lichtenstein in 1953, LCH encompasses a triad of proliferative granulomatous disorders primarily affecting children: EG, Hand-Schüller-Christian disease, and Letterer-Siwe disease.1 Lichtenstein first termed the disease histiocytosis X, after recognizing that the 3 syndromes had the same histology.1 The term was updated after the clonal proliferation of Langerhans cells in the pathogenesis of the disease was discovered.
As LCH is generally considered a pediatric disease, there is little in the literature regarding adult-onset LCH. The incidence of LCH in adults is reported as 1 to 2 cases per million, significantly lower than that in children.2,3 Two studies have reported the mean age at diagnosis in adults as the fourth decade of life, and have suggested a male predominance.4,5 The vast majority of adult LCH cases described are simple EG, with very few cases of multisystem disseminated disease reported.5
Adult patients with LCH typically present with solitary lesions in bone. Approximately 10% of cases have extraosseous involvement, with the lung being the most common site.6 Lesions tend to be unifocal, with fewer than 10 reports describing multifocal EG.1,7-13 The axial skeleton is most frequently involved, with the majority of lesions occurring in the skull, ribs, vertebrae, or mandible.14 While less common, the femur, humerus, and clavicle are most often involved when the appendicular skeleton is affected.5
In a literature review, a few case reports describe adult-onset EG of the skull. Only 5 case reports since the 1970s describe adult patients with EG of the femur. We present a rare case of multifocal EG in a 48-year-old woman with lesions of the femur and skull, as well as a review of the literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 48-year-old woman presented with progressive right knee pain that was exacerbated by weight-bearing. She denied trauma, fevers, fatigue, or weight change. Her history was significant for an EG of the skull, excised at an outside institution 2 years prior to presentation. The patient also admitted to recent onset of right-sided skull pain, near the region of her previous surgery.
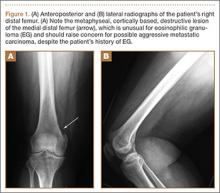
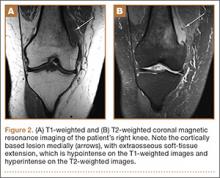
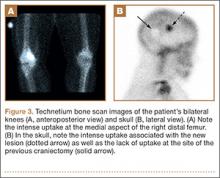
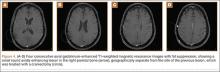
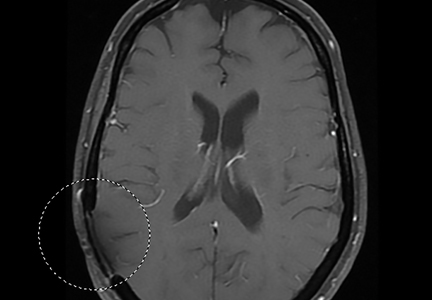
Physical examination demonstrated tenderness to palpation and fullness over the right medial distal femur and a normal neurovascular examination of the right lower extremity. Radiographs of the knee showed a cortically based, lytic, destructive lesion involving the medial femoral condyle, with soft-tissue extension (Figures 1A, 1B). Magnetic resonance imaging (MRI) of the right knee showed the lesion, with extraosseous soft-tissue extension (Figures 2A, 2B). The mass was isointense to muscle on T1-weighted images and hyperintense on T2-weighted images. Technetium bone scanning showed increased uptake in the right femur and the right skull (Figures 3A, 3B). MRI of the brain confirmed a new lesion in the right diploic space, distinct from the previous EG lesion site (Figures 4A-4D). An ultrasound-guided biopsy of the femur was performed and was consistent with EG.
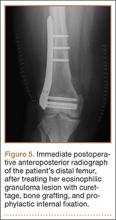
After reevaluation and clearance by her neurosurgeon, the patient underwent curettage and allografting of the femoral lesion, with prophylactic internal fixation using a titanium distal femoral locking plate (Figure 5). Intraoperative frozen section was consistent with EG, which was confirmed with additional immunohistochemical workup (Figures 6A-6D).
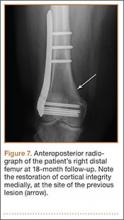
The patient recovered uneventfully and follow-up radiographs showed restoration of the bony cortex of the medial femoral condyle (Figure 7). The second skull lesion, which was also consistent with EG, was excised by her neurosurgeon.
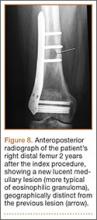
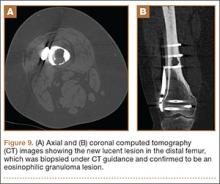
The patient remained asymptomatic until 2 years later, when she began experiencing mild pain in her right distal thigh and knee. Radiographs showed a new lytic focus in the right distal metadiaphysis (Figure 8) which was not present on her last radiograph 6 months prior. A computed tomography (CT) scan showed a lytic lesion involving the right distal femur medullary canal with cortical thinning and destruction, most pronounced posteriorly (Figures 9A, 9B). There was also an extraosseous soft-tissue component to the lesion. Bone scan showed increased uptake in the area of the new lesion. There was no increased uptake elsewhere, including the medial distal femur at the site of the old lesion, to suggest other lesions, and no increased uptake in the skull.
Given that the location of the lesion was distinct from the prior site of curettage and bone grafting, it was thought to be consistent with a new EG lesion. The patient underwent CT-guided biopsy, with simultaneous intralesional corticosteroid injection to treat the lesion when on-site pathology confirmed the etiology. Further surgical management was deemed unnecessary because internal fixation was present and spanned the new lesion. Final analysis of the fine-needle aspirate of the new lesion was positive for numerous eosinophils and histiocytes, consistent with EG.
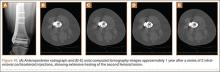
At 6-week follow-up after the intralesional steroid injection, the patient’s pain continued to abate, and she was ambulating with crutches. Repeat CT scan of the right distal femur showed improvement of the extraosseous soft-tissue component, while the lucency in the femur itself remained unchanged. The decision was made to proceed with a second intralesional corticosteroid injection under CT guidance. The patient’s symptoms continued to improve, and repeat imaging 1 year after her steroid injections showed substantial bony healing with reconstitution of her cortical bone (Figures 10A-10E).
The patient had had 4 distinct tumors consistent with EG and was referred to a medical oncologist for further workup. The patient began treatment with zoledronic acid to prevent development of further lesions. At most recent follow-up, the patient was 18 months out from her second intralesional corticosteroid injection and was doing very well. She reported being pain-free and was walking 3 to 4 miles per week without gait aids. There was no evidence of new disease. The medial distal femur lesion was completely healed, and the distal metaphyseal lesion was nearly healed, with very little residual evidence of lesions.
Discussion
Adult-onset multifocal EG is a rare entity. Most affected patients develop lesions in the axial skeleton, with the skull, mandible, and vertebrae most commonly involved.14 Only 5 cases of femoral EG have been reported, one of which was multifocal.11,14-17
Of these patients, 3 were between the ages of 33 and 53 years and had insidious onset of hip pain that failed conservative management.14,15,17 Further imaging and biopsy revealed unifocal EG in the proximal femur in each case. Each patient received a different form of treatment, including curettage and radiation, radiofrequency ablation, and/or physical therapy. At the time of publication, all patients had reported improvement in their clinical symptoms.14,15,17 The fourth patient was a man with human immunodeficiency virus (HIV) with 3 months of progressive thigh pain. Further evaluation found an isolated EG of the femoral diaphysis that progressed to pathologic fracture. He was treated with curettage and intramedullary nailing, and had improved symptoms and radiographic signs of healing at 30-month follow-up.16
An interesting case by Kerzl and colleagues11 reported a 63-year-old woman with a 24-year history of multiple symmetric lesions of the femora, leading to multiple pathologic fractures. Like our patient, her initial lesion was in the skull. Initial pathology specimens led to the diagnosis of EG. However, as the patient aged, she developed symptoms of diabetes insipidus and xanthelasma, which led to reevaluation of histology from 3 bony lesions. The patient was determined to have multifocal EG of the skull and femur, with simultaneous occurrence of Erdheim-Chester disease, which also causes bone lesions in addition to diabetes insipidus and xanthelasma.11
Though LCH was initially described more than 50 years ago, many aspects of LCH remain an enigma, especially in adults. The etiology of the disease is poorly understood. Controversy exists regarding whether LCH is primarily an immunoregulatory, neoplastic, or reactive disorder. The vast majority of adult cases described in the literature are EG, with very few cases of multisystem disseminated disease reported.5
The spectrum of disorders constituting LCH is heterogenous. Eosinophilic granuloma is the most common form, reportedly accounting for 60% to 70% of all cases, usually presenting as solitary bone lesions.6 Eosinophilic granuloma refers to the localized form of LCH, in which the disease is limited to bone or lung.18 This is the least aggressive form of the disease, with the most favorable prognosis. Hand- Schüller-Christian disease is a chronic, recurring form of LCH, with disseminated disease, affecting both bone and extraskeletal sites. Hand-Schüller-Christian disease is known for the classic triad of diabetes insipidus, exophthalmos, and destructive bone lesions. Patients may also present with otitis media or neurologic complaints from pathologic vertebral fractures. Letterer-Siwe disease refers to the acute, disseminated, fulminant form of LCH. This is the least common form of LCH and is predominately described in young children. Patients present with hepatosplenomegaly, lymphadenopathy, skin rash, fever, anemia, and thrombocytopenia.19 It is rapidly progressive, leading to multiorgan dysfunction and death within 1 to 2 years.18
The classification of LCH follows the Histiocyte Society guidelines developed from multicenter randomized trials in children.3 Classification is based on affected organs and is divided into 2 categories: single-system disease or multisystem disease. Single-system disease may be single site (bone, skin, or solitary lymph node) or multisite (multifocal bone disease or multiple lymph nodes). Multisystem disease is further classified into low-risk or risk groups. The low-risk group involves disseminated disease without involvement of risk organs (lungs, liver, spleen, and hematopoietic system). Involvement of 1 or more risk organs places the patient in the risk group, associated with the least favorable prognosis.3
In adults, the most common presenting symptoms are local pain from bony involvement, weight loss, and fever. Bony lesions most often occur in the skull, especially in the jaw. Long bones are less frequently involved, with lesions occurring in the long bones in approximately 17% of patients.3 The rib has also been reported as a common site of involvement in adults.5 Similar to children, diabetes insipidus remains a classic manifestation of LCH because of pituitary gland involvement. Other common symptoms of LCH in adults are cough, dyspnea, and chest pain from pulmonary involvement. Up to 20% to 30% of adult LCH patients have isolated pulmonary lesions, although pulmonary LCH may also occur as part of multisystem disease (risk group).3,4,20
Eosinophilic granuloma bone lesions have a variety of radiographic appearances but most commonly appear as lytic lesions. They often mimic aggressive lesions with permeative bone destruction, periostitis, ill-defined borders, and cortical erosion. Most lesions arise in the medullary space but can present as a destructive, cortically based lesion, as it did in our patient’s first femoral lesion. The differential diagnosis for a lytic medullary bone lesion includes benign entities, such as nonossifying fibromas, bone cysts, or osteomyelitis, but also includes malignant tumors, such as metastases, Ewing sarcoma, and lymphoma. A destructive, cortically based lesion in an adult should raise a very high suspicion for metastatic carcinoma until proven otherwise. Other diagnostic considerations for a cortically based lesion include chondromyxoid fibroma and surface bone lesions, such as surface chondroma and osteoma, or osteosarcoma (parosteal and periosteal). In the skull, lesions commonly erode the outer table more than the inner table (the typical “beveled-edge” appearance). Skull lesions also may have a small, central, dense focus within the lytic lesion (“button sequestrum”).
Bone scanning is often not as sensitive in detecting EG lesions compared with other bone tumors, although in our patient the bone scan was positive. In patients with a negative bone scan but a high index of suspicion, a radiographic skeletal survey should be obtained to rule out other lesions. MRI typically shows T2-hyperintense, T1-hypointense lesions with surrounding bone marrow edema and variable contrast enhancement, which is relatively nonspecific. The high sensitivity of MRI allows accurate delineation of the extent of the lesions and evaluates for the presence of an extraosseous soft-tissue component. Biopsy is generally necessary to establish a definitive histologic diagnosis. In our patient, despite her history of biopsy-proven EG, the aggressive appearance of a destructive, cortically based lesion made obtaining a biopsy critical to establish a definitive diagnosis in this case.
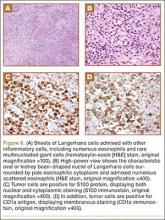
The histopathologic examination of the tissue from our patient was typical of that seen in patients with EG. It revealed tissue fragments with diffuse sheets of histiocytes displaying nuclear grooves, admixed numerous eosinophils with eosinophilic microabscesses, and scattered lymphocytes (Figures 6A, 6B). There were areas of necrosis, raising the possibility of osteomyelitis. However, the presence of classic histomorphologic features of LCH in the majority of the tissue fragments, along with CD1a- and S100-positivity in the histiocytes, confirmed the diagnosis of LCH (Figures 6C, 6D). Although not highly specific, a positive CD1a immunostain with the described histomorphologic findings in the proper clinical setting is often considered sufficient for LCH diagnosis. S100 is an important adjunct immunostain in the evaluation of histiocytic disorders. A positive S100 immunostain helps identify histiocytes, which are also CD1a-positive, because the latter immunostain can also be positive in some lymphomas and thymomas.21
After diagnosis of LCH has been confirmed, staging includes radiographs of any suspicious bone lesions, chest radiograph, bone scan, abdominal ultrasound, routine laboratory studies, and chest CT if pulmonary LCH is suspected.
The optimal treatment strategy for adult patients has not been clearly defined, and current strategies for LCH vary depending on organ involvement and extent of disease. Therapeutic options include observation, local treatment with steroids, local excision with curettage with or without bone grafting, chemotherapy, immunomodulation, irradiation, and stem cell transplantation in advanced disease. In general, patients who benefit from systemic therapy, such as chemotherapy or immunomodulation, include those with multisystem disease, refractory or recurrent lesions, and multifocal skeletal involvement.22
Patients with more limited disease, such as EG of bone, may undergo observation or local intralesional treatment. Eosinophilic granuloma of bone may resolve spontaneously and commonly does so when it is located in the pediatric spine. However, the therapeutic approach in adults with EG is controversial, given that spontaneous resolution is less likely to occur in the skeletally mature. Plasschaert and colleagues23 reported a recurrence rate of 26% in skeletally mature patients with EG of bone treated with biopsy followed by curettage with or without grafting. In the skeletally immature group, there were no clinical or radiographic signs of recurrence in the 2-year follow-up period.23 Thus, treatment in the adult population must be considered separate from the skeletally immature and in the appropriate clinical context. Depending on the location of the lesion, patients may become symptomatic or be at risk for pathologic fracture. In such circumstances, curettage with or without bone grafting and prophylactic internal fixation may be indicated. Other treatments, such as intralesional infiltration with corticosteroids, have been reported, but the role of such treatment in adults is undetermined.24,25 Radiation is typically not recommended in single-system disease unless a vital organ is threatened.26 Overall, patients with single-system disease have an excellent prognosis, and treatment should be determined on an individual basis.3
Eosinophilic granuloma represents less than 1% of all bone tumors, and adult presentation is very rare. The differential diagnosis of lytic bone lesions is broad and includes metastatic carcinoma, lymphoma/myeloma, osteomyelitis, osteoblastoma, aneurysmal bone cyst, and Ewing sarcoma. While EG is more common and easily diagnosed in children, it should be considered in the differential diagnosis in adults, so that the appropriate diagnostic workup and treatment can be performed.
1. Lahiani D, Hammami BK, Maâloul I, et al. Multifocal Langerhans cell histiocytosis of bone: late revelation in a 76-year-old woman. Rev Med Interne. 2008;29(3):249-251.
2. Baumgartner I, von Hochstetter A, Baumert B, Luetolf U, Follath F. Langerhans’-cell histiocytosis in adults. Med Pediatr Oncol. 1997;28(1):9-14.
3. Stockschlaeder M, Sucker C. Adult Langerhans cell histiocytosis. Eur J Haematol. 2006;76(5):363-368.
4. Aricò M, Girschikofsky M, Généreau T, et al. Langerhans cell histiocytosis in adults. Report from the International Registry of the Histiocyte Society. Eur J Cancer. 2003;39(16):2341-2348.
5. Islinger RB, Kuklo TR, Owens BD, et al. Langerhans’ cell histiocytosis in patients older than 21 years. Clin Orthop Relat Res. 2000;379:231-235.
6. Key SJ, O’Brien CJ, Silvester KC, Crean SJ. Eosinophilic granuloma: resolution of maxillofacial bony lesions following minimal intervention. Report of three cases and a review of the literature. J Craniomaxillofac Surg. 2004;32(3):170-175.
7. Bodner G, Kreczy A, Rachbauer F, Baechter O, Peer S. Eosinophilic granuloma of the bone: ultrasonographic imaging. Australas Radiol. 2002;46(4):418-421.
8. Boutsen Y, Esselinckx W, Delos M, Nisolle JF. Adult onset of multifocal eosinophilic granuloma of bone: a long-term follow-up with evaluation of various treatment options and spontaneous healing. Clin Rheumatol. 1999;18(1):69-73.
9. Corti F, Valicenti A, Bertolucci D, Bruno J, Gustinucci R. Multifocal Langerhans cell granulomatosis. Report of a clinical case. Minerva Med. 1994;85(7-8):413-416.
10. Demirci I. Adult eosinophilic granuloma of the lumbar spine with atypical dissemination. Case report: a long-term follow-up. Zentralbl Neurochir. 2004;65(2):84-87.
11. Kerzl R, Eyerich K, Eberlein B, et al. Parallel occurrence of Erdheim-Chester disease and eosinophilic granuloma in the same patient. J Eur Acad Dermatol Venereol. 2009;23(2):224-226.
12. Nguyen BD, Roarke MC, Chivers SF. Multifocal Langerhans cell histiocytosis with infiltrative pelvic lesions: PET/CT imaging. Clin Nucl Med. 2010;35(10): 824-826.
13. Scolozzi P, Lombardi T, Monnier P, Jaques B. Multisystem Langerhans’ cell histiocytosis (Hand-Schuller-Christian disease) in an adult: a case report and review of the literature. Eur Arch Otorhinolaryngol. 2004;261(6):326-330.
14. King JJ, Melvin JS, Iwenofu OH, Fox EJ. Thigh pain in a 53-year-old woman. Clin Orthop Relat Res. 2009;467(6):1652-1657.
15. Hair LC, Deyle GD. Eosinophilic granuloma in a patient with hip pain. J Orthop Sports Phys Ther. 2011;41(2):119.
16. Panayiotakopoulos GD, Sipsas NV, Kontos A, et al. Eosinophilic granuloma of the femur in an HIV-1 positive patient. AIDS Patient Care STDS. 2002;16(3):103-106.
17. Rodrigues RJ, Lewis HH. Eosinophilic granuloma of bone. Review of literature and case presentation. Clin Orthop Relat Res. 1971;77:183-192.
18. Stull MA, Kransdorf MJ, Devaney KO. Langerhans cell histiocytosis of bone. Radiographics. 1992;12(4):801-823.
19. Lichtenstein L. Histiocytosis X (eosinophilic granuloma of bone, Letterer-Siwe disease, and Schueller-Christian disease). Further observations of pathological and clinical importance. J Bone Joint Surg Am. 1964;46:76-90.
20. Götz G, Fichter J. Langerhans’-cell histiocytosis in 58 adults. Eur J Med Res. 2004;9(11):510-514.
21. Cheng KL, Glu PG, Weiss LM. Hematopoeitic tumors. In: Peiguo C, Weiss L, eds. Modern Immunohistochemistry. New York, NY: Cambridge University Press; 2009:503.
22. Broadbent V, Gadner H. Current therapy for Langerhans cell histiocytosis. Hematol Oncol Clin North Am. 1998;12(2):327-338.
23. Plasschaert F, Craig C, Bell R, Cole WG, Wunder JS, Alman BA. Eosinophilic granuloma. A different behaviour in children than in adults. J Bone Joint Surg Br. 2002;84(6):870-872.
24. Capanna R, Springfield DS, Ruggieri P, et al. Direct cortisone injection in osinophilic granuloma of bone: a preliminary report on 11 patients. J Pediatr Orthop. 1985;5(3):339-342.
25. Egeler RM, Thompson RC Jr, Voûte PA, Nesbit ME Jr. Intralesional infiltration of corticosteroids in localized Langerhans’ cell histiocytosis. J Pediatr Orthop. 1992;12(6):811-814.
26. Ladisch S, Gadner H. Treatment of Langerhans cell histiocytosis–evolution and current approaches. Br J Cancer Suppl. 1994;23:S41-S46.
Eosinophilic granuloma (EG) is the most common benign form of Langerhans cell histiocytosis (LCH). Initially described by Lichtenstein in 1953, LCH encompasses a triad of proliferative granulomatous disorders primarily affecting children: EG, Hand-Schüller-Christian disease, and Letterer-Siwe disease.1 Lichtenstein first termed the disease histiocytosis X, after recognizing that the 3 syndromes had the same histology.1 The term was updated after the clonal proliferation of Langerhans cells in the pathogenesis of the disease was discovered.
As LCH is generally considered a pediatric disease, there is little in the literature regarding adult-onset LCH. The incidence of LCH in adults is reported as 1 to 2 cases per million, significantly lower than that in children.2,3 Two studies have reported the mean age at diagnosis in adults as the fourth decade of life, and have suggested a male predominance.4,5 The vast majority of adult LCH cases described are simple EG, with very few cases of multisystem disseminated disease reported.5
Adult patients with LCH typically present with solitary lesions in bone. Approximately 10% of cases have extraosseous involvement, with the lung being the most common site.6 Lesions tend to be unifocal, with fewer than 10 reports describing multifocal EG.1,7-13 The axial skeleton is most frequently involved, with the majority of lesions occurring in the skull, ribs, vertebrae, or mandible.14 While less common, the femur, humerus, and clavicle are most often involved when the appendicular skeleton is affected.5
In a literature review, a few case reports describe adult-onset EG of the skull. Only 5 case reports since the 1970s describe adult patients with EG of the femur. We present a rare case of multifocal EG in a 48-year-old woman with lesions of the femur and skull, as well as a review of the literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 48-year-old woman presented with progressive right knee pain that was exacerbated by weight-bearing. She denied trauma, fevers, fatigue, or weight change. Her history was significant for an EG of the skull, excised at an outside institution 2 years prior to presentation. The patient also admitted to recent onset of right-sided skull pain, near the region of her previous surgery.
Physical examination demonstrated tenderness to palpation and fullness over the right medial distal femur and a normal neurovascular examination of the right lower extremity. Radiographs of the knee showed a cortically based, lytic, destructive lesion involving the medial femoral condyle, with soft-tissue extension (Figures 1A, 1B). Magnetic resonance imaging (MRI) of the right knee showed the lesion, with extraosseous soft-tissue extension (Figures 2A, 2B). The mass was isointense to muscle on T1-weighted images and hyperintense on T2-weighted images. Technetium bone scanning showed increased uptake in the right femur and the right skull (Figures 3A, 3B). MRI of the brain confirmed a new lesion in the right diploic space, distinct from the previous EG lesion site (Figures 4A-4D). An ultrasound-guided biopsy of the femur was performed and was consistent with EG.
After reevaluation and clearance by her neurosurgeon, the patient underwent curettage and allografting of the femoral lesion, with prophylactic internal fixation using a titanium distal femoral locking plate (Figure 5). Intraoperative frozen section was consistent with EG, which was confirmed with additional immunohistochemical workup (Figures 6A-6D).
The patient recovered uneventfully and follow-up radiographs showed restoration of the bony cortex of the medial femoral condyle (Figure 7). The second skull lesion, which was also consistent with EG, was excised by her neurosurgeon.
The patient remained asymptomatic until 2 years later, when she began experiencing mild pain in her right distal thigh and knee. Radiographs showed a new lytic focus in the right distal metadiaphysis (Figure 8) which was not present on her last radiograph 6 months prior. A computed tomography (CT) scan showed a lytic lesion involving the right distal femur medullary canal with cortical thinning and destruction, most pronounced posteriorly (Figures 9A, 9B). There was also an extraosseous soft-tissue component to the lesion. Bone scan showed increased uptake in the area of the new lesion. There was no increased uptake elsewhere, including the medial distal femur at the site of the old lesion, to suggest other lesions, and no increased uptake in the skull.
Given that the location of the lesion was distinct from the prior site of curettage and bone grafting, it was thought to be consistent with a new EG lesion. The patient underwent CT-guided biopsy, with simultaneous intralesional corticosteroid injection to treat the lesion when on-site pathology confirmed the etiology. Further surgical management was deemed unnecessary because internal fixation was present and spanned the new lesion. Final analysis of the fine-needle aspirate of the new lesion was positive for numerous eosinophils and histiocytes, consistent with EG.
At 6-week follow-up after the intralesional steroid injection, the patient’s pain continued to abate, and she was ambulating with crutches. Repeat CT scan of the right distal femur showed improvement of the extraosseous soft-tissue component, while the lucency in the femur itself remained unchanged. The decision was made to proceed with a second intralesional corticosteroid injection under CT guidance. The patient’s symptoms continued to improve, and repeat imaging 1 year after her steroid injections showed substantial bony healing with reconstitution of her cortical bone (Figures 10A-10E).
The patient had had 4 distinct tumors consistent with EG and was referred to a medical oncologist for further workup. The patient began treatment with zoledronic acid to prevent development of further lesions. At most recent follow-up, the patient was 18 months out from her second intralesional corticosteroid injection and was doing very well. She reported being pain-free and was walking 3 to 4 miles per week without gait aids. There was no evidence of new disease. The medial distal femur lesion was completely healed, and the distal metaphyseal lesion was nearly healed, with very little residual evidence of lesions.
Discussion
Adult-onset multifocal EG is a rare entity. Most affected patients develop lesions in the axial skeleton, with the skull, mandible, and vertebrae most commonly involved.14 Only 5 cases of femoral EG have been reported, one of which was multifocal.11,14-17
Of these patients, 3 were between the ages of 33 and 53 years and had insidious onset of hip pain that failed conservative management.14,15,17 Further imaging and biopsy revealed unifocal EG in the proximal femur in each case. Each patient received a different form of treatment, including curettage and radiation, radiofrequency ablation, and/or physical therapy. At the time of publication, all patients had reported improvement in their clinical symptoms.14,15,17 The fourth patient was a man with human immunodeficiency virus (HIV) with 3 months of progressive thigh pain. Further evaluation found an isolated EG of the femoral diaphysis that progressed to pathologic fracture. He was treated with curettage and intramedullary nailing, and had improved symptoms and radiographic signs of healing at 30-month follow-up.16
An interesting case by Kerzl and colleagues11 reported a 63-year-old woman with a 24-year history of multiple symmetric lesions of the femora, leading to multiple pathologic fractures. Like our patient, her initial lesion was in the skull. Initial pathology specimens led to the diagnosis of EG. However, as the patient aged, she developed symptoms of diabetes insipidus and xanthelasma, which led to reevaluation of histology from 3 bony lesions. The patient was determined to have multifocal EG of the skull and femur, with simultaneous occurrence of Erdheim-Chester disease, which also causes bone lesions in addition to diabetes insipidus and xanthelasma.11
Though LCH was initially described more than 50 years ago, many aspects of LCH remain an enigma, especially in adults. The etiology of the disease is poorly understood. Controversy exists regarding whether LCH is primarily an immunoregulatory, neoplastic, or reactive disorder. The vast majority of adult cases described in the literature are EG, with very few cases of multisystem disseminated disease reported.5
The spectrum of disorders constituting LCH is heterogenous. Eosinophilic granuloma is the most common form, reportedly accounting for 60% to 70% of all cases, usually presenting as solitary bone lesions.6 Eosinophilic granuloma refers to the localized form of LCH, in which the disease is limited to bone or lung.18 This is the least aggressive form of the disease, with the most favorable prognosis. Hand- Schüller-Christian disease is a chronic, recurring form of LCH, with disseminated disease, affecting both bone and extraskeletal sites. Hand-Schüller-Christian disease is known for the classic triad of diabetes insipidus, exophthalmos, and destructive bone lesions. Patients may also present with otitis media or neurologic complaints from pathologic vertebral fractures. Letterer-Siwe disease refers to the acute, disseminated, fulminant form of LCH. This is the least common form of LCH and is predominately described in young children. Patients present with hepatosplenomegaly, lymphadenopathy, skin rash, fever, anemia, and thrombocytopenia.19 It is rapidly progressive, leading to multiorgan dysfunction and death within 1 to 2 years.18
The classification of LCH follows the Histiocyte Society guidelines developed from multicenter randomized trials in children.3 Classification is based on affected organs and is divided into 2 categories: single-system disease or multisystem disease. Single-system disease may be single site (bone, skin, or solitary lymph node) or multisite (multifocal bone disease or multiple lymph nodes). Multisystem disease is further classified into low-risk or risk groups. The low-risk group involves disseminated disease without involvement of risk organs (lungs, liver, spleen, and hematopoietic system). Involvement of 1 or more risk organs places the patient in the risk group, associated with the least favorable prognosis.3
In adults, the most common presenting symptoms are local pain from bony involvement, weight loss, and fever. Bony lesions most often occur in the skull, especially in the jaw. Long bones are less frequently involved, with lesions occurring in the long bones in approximately 17% of patients.3 The rib has also been reported as a common site of involvement in adults.5 Similar to children, diabetes insipidus remains a classic manifestation of LCH because of pituitary gland involvement. Other common symptoms of LCH in adults are cough, dyspnea, and chest pain from pulmonary involvement. Up to 20% to 30% of adult LCH patients have isolated pulmonary lesions, although pulmonary LCH may also occur as part of multisystem disease (risk group).3,4,20
Eosinophilic granuloma bone lesions have a variety of radiographic appearances but most commonly appear as lytic lesions. They often mimic aggressive lesions with permeative bone destruction, periostitis, ill-defined borders, and cortical erosion. Most lesions arise in the medullary space but can present as a destructive, cortically based lesion, as it did in our patient’s first femoral lesion. The differential diagnosis for a lytic medullary bone lesion includes benign entities, such as nonossifying fibromas, bone cysts, or osteomyelitis, but also includes malignant tumors, such as metastases, Ewing sarcoma, and lymphoma. A destructive, cortically based lesion in an adult should raise a very high suspicion for metastatic carcinoma until proven otherwise. Other diagnostic considerations for a cortically based lesion include chondromyxoid fibroma and surface bone lesions, such as surface chondroma and osteoma, or osteosarcoma (parosteal and periosteal). In the skull, lesions commonly erode the outer table more than the inner table (the typical “beveled-edge” appearance). Skull lesions also may have a small, central, dense focus within the lytic lesion (“button sequestrum”).
Bone scanning is often not as sensitive in detecting EG lesions compared with other bone tumors, although in our patient the bone scan was positive. In patients with a negative bone scan but a high index of suspicion, a radiographic skeletal survey should be obtained to rule out other lesions. MRI typically shows T2-hyperintense, T1-hypointense lesions with surrounding bone marrow edema and variable contrast enhancement, which is relatively nonspecific. The high sensitivity of MRI allows accurate delineation of the extent of the lesions and evaluates for the presence of an extraosseous soft-tissue component. Biopsy is generally necessary to establish a definitive histologic diagnosis. In our patient, despite her history of biopsy-proven EG, the aggressive appearance of a destructive, cortically based lesion made obtaining a biopsy critical to establish a definitive diagnosis in this case.
The histopathologic examination of the tissue from our patient was typical of that seen in patients with EG. It revealed tissue fragments with diffuse sheets of histiocytes displaying nuclear grooves, admixed numerous eosinophils with eosinophilic microabscesses, and scattered lymphocytes (Figures 6A, 6B). There were areas of necrosis, raising the possibility of osteomyelitis. However, the presence of classic histomorphologic features of LCH in the majority of the tissue fragments, along with CD1a- and S100-positivity in the histiocytes, confirmed the diagnosis of LCH (Figures 6C, 6D). Although not highly specific, a positive CD1a immunostain with the described histomorphologic findings in the proper clinical setting is often considered sufficient for LCH diagnosis. S100 is an important adjunct immunostain in the evaluation of histiocytic disorders. A positive S100 immunostain helps identify histiocytes, which are also CD1a-positive, because the latter immunostain can also be positive in some lymphomas and thymomas.21
After diagnosis of LCH has been confirmed, staging includes radiographs of any suspicious bone lesions, chest radiograph, bone scan, abdominal ultrasound, routine laboratory studies, and chest CT if pulmonary LCH is suspected.
The optimal treatment strategy for adult patients has not been clearly defined, and current strategies for LCH vary depending on organ involvement and extent of disease. Therapeutic options include observation, local treatment with steroids, local excision with curettage with or without bone grafting, chemotherapy, immunomodulation, irradiation, and stem cell transplantation in advanced disease. In general, patients who benefit from systemic therapy, such as chemotherapy or immunomodulation, include those with multisystem disease, refractory or recurrent lesions, and multifocal skeletal involvement.22
Patients with more limited disease, such as EG of bone, may undergo observation or local intralesional treatment. Eosinophilic granuloma of bone may resolve spontaneously and commonly does so when it is located in the pediatric spine. However, the therapeutic approach in adults with EG is controversial, given that spontaneous resolution is less likely to occur in the skeletally mature. Plasschaert and colleagues23 reported a recurrence rate of 26% in skeletally mature patients with EG of bone treated with biopsy followed by curettage with or without grafting. In the skeletally immature group, there were no clinical or radiographic signs of recurrence in the 2-year follow-up period.23 Thus, treatment in the adult population must be considered separate from the skeletally immature and in the appropriate clinical context. Depending on the location of the lesion, patients may become symptomatic or be at risk for pathologic fracture. In such circumstances, curettage with or without bone grafting and prophylactic internal fixation may be indicated. Other treatments, such as intralesional infiltration with corticosteroids, have been reported, but the role of such treatment in adults is undetermined.24,25 Radiation is typically not recommended in single-system disease unless a vital organ is threatened.26 Overall, patients with single-system disease have an excellent prognosis, and treatment should be determined on an individual basis.3
Eosinophilic granuloma represents less than 1% of all bone tumors, and adult presentation is very rare. The differential diagnosis of lytic bone lesions is broad and includes metastatic carcinoma, lymphoma/myeloma, osteomyelitis, osteoblastoma, aneurysmal bone cyst, and Ewing sarcoma. While EG is more common and easily diagnosed in children, it should be considered in the differential diagnosis in adults, so that the appropriate diagnostic workup and treatment can be performed.
Eosinophilic granuloma (EG) is the most common benign form of Langerhans cell histiocytosis (LCH). Initially described by Lichtenstein in 1953, LCH encompasses a triad of proliferative granulomatous disorders primarily affecting children: EG, Hand-Schüller-Christian disease, and Letterer-Siwe disease.1 Lichtenstein first termed the disease histiocytosis X, after recognizing that the 3 syndromes had the same histology.1 The term was updated after the clonal proliferation of Langerhans cells in the pathogenesis of the disease was discovered.
As LCH is generally considered a pediatric disease, there is little in the literature regarding adult-onset LCH. The incidence of LCH in adults is reported as 1 to 2 cases per million, significantly lower than that in children.2,3 Two studies have reported the mean age at diagnosis in adults as the fourth decade of life, and have suggested a male predominance.4,5 The vast majority of adult LCH cases described are simple EG, with very few cases of multisystem disseminated disease reported.5
Adult patients with LCH typically present with solitary lesions in bone. Approximately 10% of cases have extraosseous involvement, with the lung being the most common site.6 Lesions tend to be unifocal, with fewer than 10 reports describing multifocal EG.1,7-13 The axial skeleton is most frequently involved, with the majority of lesions occurring in the skull, ribs, vertebrae, or mandible.14 While less common, the femur, humerus, and clavicle are most often involved when the appendicular skeleton is affected.5
In a literature review, a few case reports describe adult-onset EG of the skull. Only 5 case reports since the 1970s describe adult patients with EG of the femur. We present a rare case of multifocal EG in a 48-year-old woman with lesions of the femur and skull, as well as a review of the literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 48-year-old woman presented with progressive right knee pain that was exacerbated by weight-bearing. She denied trauma, fevers, fatigue, or weight change. Her history was significant for an EG of the skull, excised at an outside institution 2 years prior to presentation. The patient also admitted to recent onset of right-sided skull pain, near the region of her previous surgery.
Physical examination demonstrated tenderness to palpation and fullness over the right medial distal femur and a normal neurovascular examination of the right lower extremity. Radiographs of the knee showed a cortically based, lytic, destructive lesion involving the medial femoral condyle, with soft-tissue extension (Figures 1A, 1B). Magnetic resonance imaging (MRI) of the right knee showed the lesion, with extraosseous soft-tissue extension (Figures 2A, 2B). The mass was isointense to muscle on T1-weighted images and hyperintense on T2-weighted images. Technetium bone scanning showed increased uptake in the right femur and the right skull (Figures 3A, 3B). MRI of the brain confirmed a new lesion in the right diploic space, distinct from the previous EG lesion site (Figures 4A-4D). An ultrasound-guided biopsy of the femur was performed and was consistent with EG.
After reevaluation and clearance by her neurosurgeon, the patient underwent curettage and allografting of the femoral lesion, with prophylactic internal fixation using a titanium distal femoral locking plate (Figure 5). Intraoperative frozen section was consistent with EG, which was confirmed with additional immunohistochemical workup (Figures 6A-6D).
The patient recovered uneventfully and follow-up radiographs showed restoration of the bony cortex of the medial femoral condyle (Figure 7). The second skull lesion, which was also consistent with EG, was excised by her neurosurgeon.
The patient remained asymptomatic until 2 years later, when she began experiencing mild pain in her right distal thigh and knee. Radiographs showed a new lytic focus in the right distal metadiaphysis (Figure 8) which was not present on her last radiograph 6 months prior. A computed tomography (CT) scan showed a lytic lesion involving the right distal femur medullary canal with cortical thinning and destruction, most pronounced posteriorly (Figures 9A, 9B). There was also an extraosseous soft-tissue component to the lesion. Bone scan showed increased uptake in the area of the new lesion. There was no increased uptake elsewhere, including the medial distal femur at the site of the old lesion, to suggest other lesions, and no increased uptake in the skull.
Given that the location of the lesion was distinct from the prior site of curettage and bone grafting, it was thought to be consistent with a new EG lesion. The patient underwent CT-guided biopsy, with simultaneous intralesional corticosteroid injection to treat the lesion when on-site pathology confirmed the etiology. Further surgical management was deemed unnecessary because internal fixation was present and spanned the new lesion. Final analysis of the fine-needle aspirate of the new lesion was positive for numerous eosinophils and histiocytes, consistent with EG.
At 6-week follow-up after the intralesional steroid injection, the patient’s pain continued to abate, and she was ambulating with crutches. Repeat CT scan of the right distal femur showed improvement of the extraosseous soft-tissue component, while the lucency in the femur itself remained unchanged. The decision was made to proceed with a second intralesional corticosteroid injection under CT guidance. The patient’s symptoms continued to improve, and repeat imaging 1 year after her steroid injections showed substantial bony healing with reconstitution of her cortical bone (Figures 10A-10E).
The patient had had 4 distinct tumors consistent with EG and was referred to a medical oncologist for further workup. The patient began treatment with zoledronic acid to prevent development of further lesions. At most recent follow-up, the patient was 18 months out from her second intralesional corticosteroid injection and was doing very well. She reported being pain-free and was walking 3 to 4 miles per week without gait aids. There was no evidence of new disease. The medial distal femur lesion was completely healed, and the distal metaphyseal lesion was nearly healed, with very little residual evidence of lesions.
Discussion
Adult-onset multifocal EG is a rare entity. Most affected patients develop lesions in the axial skeleton, with the skull, mandible, and vertebrae most commonly involved.14 Only 5 cases of femoral EG have been reported, one of which was multifocal.11,14-17
Of these patients, 3 were between the ages of 33 and 53 years and had insidious onset of hip pain that failed conservative management.14,15,17 Further imaging and biopsy revealed unifocal EG in the proximal femur in each case. Each patient received a different form of treatment, including curettage and radiation, radiofrequency ablation, and/or physical therapy. At the time of publication, all patients had reported improvement in their clinical symptoms.14,15,17 The fourth patient was a man with human immunodeficiency virus (HIV) with 3 months of progressive thigh pain. Further evaluation found an isolated EG of the femoral diaphysis that progressed to pathologic fracture. He was treated with curettage and intramedullary nailing, and had improved symptoms and radiographic signs of healing at 30-month follow-up.16
An interesting case by Kerzl and colleagues11 reported a 63-year-old woman with a 24-year history of multiple symmetric lesions of the femora, leading to multiple pathologic fractures. Like our patient, her initial lesion was in the skull. Initial pathology specimens led to the diagnosis of EG. However, as the patient aged, she developed symptoms of diabetes insipidus and xanthelasma, which led to reevaluation of histology from 3 bony lesions. The patient was determined to have multifocal EG of the skull and femur, with simultaneous occurrence of Erdheim-Chester disease, which also causes bone lesions in addition to diabetes insipidus and xanthelasma.11
Though LCH was initially described more than 50 years ago, many aspects of LCH remain an enigma, especially in adults. The etiology of the disease is poorly understood. Controversy exists regarding whether LCH is primarily an immunoregulatory, neoplastic, or reactive disorder. The vast majority of adult cases described in the literature are EG, with very few cases of multisystem disseminated disease reported.5
The spectrum of disorders constituting LCH is heterogenous. Eosinophilic granuloma is the most common form, reportedly accounting for 60% to 70% of all cases, usually presenting as solitary bone lesions.6 Eosinophilic granuloma refers to the localized form of LCH, in which the disease is limited to bone or lung.18 This is the least aggressive form of the disease, with the most favorable prognosis. Hand- Schüller-Christian disease is a chronic, recurring form of LCH, with disseminated disease, affecting both bone and extraskeletal sites. Hand-Schüller-Christian disease is known for the classic triad of diabetes insipidus, exophthalmos, and destructive bone lesions. Patients may also present with otitis media or neurologic complaints from pathologic vertebral fractures. Letterer-Siwe disease refers to the acute, disseminated, fulminant form of LCH. This is the least common form of LCH and is predominately described in young children. Patients present with hepatosplenomegaly, lymphadenopathy, skin rash, fever, anemia, and thrombocytopenia.19 It is rapidly progressive, leading to multiorgan dysfunction and death within 1 to 2 years.18
The classification of LCH follows the Histiocyte Society guidelines developed from multicenter randomized trials in children.3 Classification is based on affected organs and is divided into 2 categories: single-system disease or multisystem disease. Single-system disease may be single site (bone, skin, or solitary lymph node) or multisite (multifocal bone disease or multiple lymph nodes). Multisystem disease is further classified into low-risk or risk groups. The low-risk group involves disseminated disease without involvement of risk organs (lungs, liver, spleen, and hematopoietic system). Involvement of 1 or more risk organs places the patient in the risk group, associated with the least favorable prognosis.3
In adults, the most common presenting symptoms are local pain from bony involvement, weight loss, and fever. Bony lesions most often occur in the skull, especially in the jaw. Long bones are less frequently involved, with lesions occurring in the long bones in approximately 17% of patients.3 The rib has also been reported as a common site of involvement in adults.5 Similar to children, diabetes insipidus remains a classic manifestation of LCH because of pituitary gland involvement. Other common symptoms of LCH in adults are cough, dyspnea, and chest pain from pulmonary involvement. Up to 20% to 30% of adult LCH patients have isolated pulmonary lesions, although pulmonary LCH may also occur as part of multisystem disease (risk group).3,4,20
Eosinophilic granuloma bone lesions have a variety of radiographic appearances but most commonly appear as lytic lesions. They often mimic aggressive lesions with permeative bone destruction, periostitis, ill-defined borders, and cortical erosion. Most lesions arise in the medullary space but can present as a destructive, cortically based lesion, as it did in our patient’s first femoral lesion. The differential diagnosis for a lytic medullary bone lesion includes benign entities, such as nonossifying fibromas, bone cysts, or osteomyelitis, but also includes malignant tumors, such as metastases, Ewing sarcoma, and lymphoma. A destructive, cortically based lesion in an adult should raise a very high suspicion for metastatic carcinoma until proven otherwise. Other diagnostic considerations for a cortically based lesion include chondromyxoid fibroma and surface bone lesions, such as surface chondroma and osteoma, or osteosarcoma (parosteal and periosteal). In the skull, lesions commonly erode the outer table more than the inner table (the typical “beveled-edge” appearance). Skull lesions also may have a small, central, dense focus within the lytic lesion (“button sequestrum”).
Bone scanning is often not as sensitive in detecting EG lesions compared with other bone tumors, although in our patient the bone scan was positive. In patients with a negative bone scan but a high index of suspicion, a radiographic skeletal survey should be obtained to rule out other lesions. MRI typically shows T2-hyperintense, T1-hypointense lesions with surrounding bone marrow edema and variable contrast enhancement, which is relatively nonspecific. The high sensitivity of MRI allows accurate delineation of the extent of the lesions and evaluates for the presence of an extraosseous soft-tissue component. Biopsy is generally necessary to establish a definitive histologic diagnosis. In our patient, despite her history of biopsy-proven EG, the aggressive appearance of a destructive, cortically based lesion made obtaining a biopsy critical to establish a definitive diagnosis in this case.
The histopathologic examination of the tissue from our patient was typical of that seen in patients with EG. It revealed tissue fragments with diffuse sheets of histiocytes displaying nuclear grooves, admixed numerous eosinophils with eosinophilic microabscesses, and scattered lymphocytes (Figures 6A, 6B). There were areas of necrosis, raising the possibility of osteomyelitis. However, the presence of classic histomorphologic features of LCH in the majority of the tissue fragments, along with CD1a- and S100-positivity in the histiocytes, confirmed the diagnosis of LCH (Figures 6C, 6D). Although not highly specific, a positive CD1a immunostain with the described histomorphologic findings in the proper clinical setting is often considered sufficient for LCH diagnosis. S100 is an important adjunct immunostain in the evaluation of histiocytic disorders. A positive S100 immunostain helps identify histiocytes, which are also CD1a-positive, because the latter immunostain can also be positive in some lymphomas and thymomas.21
After diagnosis of LCH has been confirmed, staging includes radiographs of any suspicious bone lesions, chest radiograph, bone scan, abdominal ultrasound, routine laboratory studies, and chest CT if pulmonary LCH is suspected.
The optimal treatment strategy for adult patients has not been clearly defined, and current strategies for LCH vary depending on organ involvement and extent of disease. Therapeutic options include observation, local treatment with steroids, local excision with curettage with or without bone grafting, chemotherapy, immunomodulation, irradiation, and stem cell transplantation in advanced disease. In general, patients who benefit from systemic therapy, such as chemotherapy or immunomodulation, include those with multisystem disease, refractory or recurrent lesions, and multifocal skeletal involvement.22
Patients with more limited disease, such as EG of bone, may undergo observation or local intralesional treatment. Eosinophilic granuloma of bone may resolve spontaneously and commonly does so when it is located in the pediatric spine. However, the therapeutic approach in adults with EG is controversial, given that spontaneous resolution is less likely to occur in the skeletally mature. Plasschaert and colleagues23 reported a recurrence rate of 26% in skeletally mature patients with EG of bone treated with biopsy followed by curettage with or without grafting. In the skeletally immature group, there were no clinical or radiographic signs of recurrence in the 2-year follow-up period.23 Thus, treatment in the adult population must be considered separate from the skeletally immature and in the appropriate clinical context. Depending on the location of the lesion, patients may become symptomatic or be at risk for pathologic fracture. In such circumstances, curettage with or without bone grafting and prophylactic internal fixation may be indicated. Other treatments, such as intralesional infiltration with corticosteroids, have been reported, but the role of such treatment in adults is undetermined.24,25 Radiation is typically not recommended in single-system disease unless a vital organ is threatened.26 Overall, patients with single-system disease have an excellent prognosis, and treatment should be determined on an individual basis.3
Eosinophilic granuloma represents less than 1% of all bone tumors, and adult presentation is very rare. The differential diagnosis of lytic bone lesions is broad and includes metastatic carcinoma, lymphoma/myeloma, osteomyelitis, osteoblastoma, aneurysmal bone cyst, and Ewing sarcoma. While EG is more common and easily diagnosed in children, it should be considered in the differential diagnosis in adults, so that the appropriate diagnostic workup and treatment can be performed.
1. Lahiani D, Hammami BK, Maâloul I, et al. Multifocal Langerhans cell histiocytosis of bone: late revelation in a 76-year-old woman. Rev Med Interne. 2008;29(3):249-251.
2. Baumgartner I, von Hochstetter A, Baumert B, Luetolf U, Follath F. Langerhans’-cell histiocytosis in adults. Med Pediatr Oncol. 1997;28(1):9-14.
3. Stockschlaeder M, Sucker C. Adult Langerhans cell histiocytosis. Eur J Haematol. 2006;76(5):363-368.
4. Aricò M, Girschikofsky M, Généreau T, et al. Langerhans cell histiocytosis in adults. Report from the International Registry of the Histiocyte Society. Eur J Cancer. 2003;39(16):2341-2348.
5. Islinger RB, Kuklo TR, Owens BD, et al. Langerhans’ cell histiocytosis in patients older than 21 years. Clin Orthop Relat Res. 2000;379:231-235.
6. Key SJ, O’Brien CJ, Silvester KC, Crean SJ. Eosinophilic granuloma: resolution of maxillofacial bony lesions following minimal intervention. Report of three cases and a review of the literature. J Craniomaxillofac Surg. 2004;32(3):170-175.
7. Bodner G, Kreczy A, Rachbauer F, Baechter O, Peer S. Eosinophilic granuloma of the bone: ultrasonographic imaging. Australas Radiol. 2002;46(4):418-421.
8. Boutsen Y, Esselinckx W, Delos M, Nisolle JF. Adult onset of multifocal eosinophilic granuloma of bone: a long-term follow-up with evaluation of various treatment options and spontaneous healing. Clin Rheumatol. 1999;18(1):69-73.
9. Corti F, Valicenti A, Bertolucci D, Bruno J, Gustinucci R. Multifocal Langerhans cell granulomatosis. Report of a clinical case. Minerva Med. 1994;85(7-8):413-416.
10. Demirci I. Adult eosinophilic granuloma of the lumbar spine with atypical dissemination. Case report: a long-term follow-up. Zentralbl Neurochir. 2004;65(2):84-87.
11. Kerzl R, Eyerich K, Eberlein B, et al. Parallel occurrence of Erdheim-Chester disease and eosinophilic granuloma in the same patient. J Eur Acad Dermatol Venereol. 2009;23(2):224-226.
12. Nguyen BD, Roarke MC, Chivers SF. Multifocal Langerhans cell histiocytosis with infiltrative pelvic lesions: PET/CT imaging. Clin Nucl Med. 2010;35(10): 824-826.
13. Scolozzi P, Lombardi T, Monnier P, Jaques B. Multisystem Langerhans’ cell histiocytosis (Hand-Schuller-Christian disease) in an adult: a case report and review of the literature. Eur Arch Otorhinolaryngol. 2004;261(6):326-330.
14. King JJ, Melvin JS, Iwenofu OH, Fox EJ. Thigh pain in a 53-year-old woman. Clin Orthop Relat Res. 2009;467(6):1652-1657.
15. Hair LC, Deyle GD. Eosinophilic granuloma in a patient with hip pain. J Orthop Sports Phys Ther. 2011;41(2):119.
16. Panayiotakopoulos GD, Sipsas NV, Kontos A, et al. Eosinophilic granuloma of the femur in an HIV-1 positive patient. AIDS Patient Care STDS. 2002;16(3):103-106.
17. Rodrigues RJ, Lewis HH. Eosinophilic granuloma of bone. Review of literature and case presentation. Clin Orthop Relat Res. 1971;77:183-192.
18. Stull MA, Kransdorf MJ, Devaney KO. Langerhans cell histiocytosis of bone. Radiographics. 1992;12(4):801-823.
19. Lichtenstein L. Histiocytosis X (eosinophilic granuloma of bone, Letterer-Siwe disease, and Schueller-Christian disease). Further observations of pathological and clinical importance. J Bone Joint Surg Am. 1964;46:76-90.
20. Götz G, Fichter J. Langerhans’-cell histiocytosis in 58 adults. Eur J Med Res. 2004;9(11):510-514.
21. Cheng KL, Glu PG, Weiss LM. Hematopoeitic tumors. In: Peiguo C, Weiss L, eds. Modern Immunohistochemistry. New York, NY: Cambridge University Press; 2009:503.
22. Broadbent V, Gadner H. Current therapy for Langerhans cell histiocytosis. Hematol Oncol Clin North Am. 1998;12(2):327-338.
23. Plasschaert F, Craig C, Bell R, Cole WG, Wunder JS, Alman BA. Eosinophilic granuloma. A different behaviour in children than in adults. J Bone Joint Surg Br. 2002;84(6):870-872.
24. Capanna R, Springfield DS, Ruggieri P, et al. Direct cortisone injection in osinophilic granuloma of bone: a preliminary report on 11 patients. J Pediatr Orthop. 1985;5(3):339-342.
25. Egeler RM, Thompson RC Jr, Voûte PA, Nesbit ME Jr. Intralesional infiltration of corticosteroids in localized Langerhans’ cell histiocytosis. J Pediatr Orthop. 1992;12(6):811-814.
26. Ladisch S, Gadner H. Treatment of Langerhans cell histiocytosis–evolution and current approaches. Br J Cancer Suppl. 1994;23:S41-S46.
1. Lahiani D, Hammami BK, Maâloul I, et al. Multifocal Langerhans cell histiocytosis of bone: late revelation in a 76-year-old woman. Rev Med Interne. 2008;29(3):249-251.
2. Baumgartner I, von Hochstetter A, Baumert B, Luetolf U, Follath F. Langerhans’-cell histiocytosis in adults. Med Pediatr Oncol. 1997;28(1):9-14.
3. Stockschlaeder M, Sucker C. Adult Langerhans cell histiocytosis. Eur J Haematol. 2006;76(5):363-368.
4. Aricò M, Girschikofsky M, Généreau T, et al. Langerhans cell histiocytosis in adults. Report from the International Registry of the Histiocyte Society. Eur J Cancer. 2003;39(16):2341-2348.
5. Islinger RB, Kuklo TR, Owens BD, et al. Langerhans’ cell histiocytosis in patients older than 21 years. Clin Orthop Relat Res. 2000;379:231-235.
6. Key SJ, O’Brien CJ, Silvester KC, Crean SJ. Eosinophilic granuloma: resolution of maxillofacial bony lesions following minimal intervention. Report of three cases and a review of the literature. J Craniomaxillofac Surg. 2004;32(3):170-175.
7. Bodner G, Kreczy A, Rachbauer F, Baechter O, Peer S. Eosinophilic granuloma of the bone: ultrasonographic imaging. Australas Radiol. 2002;46(4):418-421.
8. Boutsen Y, Esselinckx W, Delos M, Nisolle JF. Adult onset of multifocal eosinophilic granuloma of bone: a long-term follow-up with evaluation of various treatment options and spontaneous healing. Clin Rheumatol. 1999;18(1):69-73.
9. Corti F, Valicenti A, Bertolucci D, Bruno J, Gustinucci R. Multifocal Langerhans cell granulomatosis. Report of a clinical case. Minerva Med. 1994;85(7-8):413-416.
10. Demirci I. Adult eosinophilic granuloma of the lumbar spine with atypical dissemination. Case report: a long-term follow-up. Zentralbl Neurochir. 2004;65(2):84-87.
11. Kerzl R, Eyerich K, Eberlein B, et al. Parallel occurrence of Erdheim-Chester disease and eosinophilic granuloma in the same patient. J Eur Acad Dermatol Venereol. 2009;23(2):224-226.
12. Nguyen BD, Roarke MC, Chivers SF. Multifocal Langerhans cell histiocytosis with infiltrative pelvic lesions: PET/CT imaging. Clin Nucl Med. 2010;35(10): 824-826.
13. Scolozzi P, Lombardi T, Monnier P, Jaques B. Multisystem Langerhans’ cell histiocytosis (Hand-Schuller-Christian disease) in an adult: a case report and review of the literature. Eur Arch Otorhinolaryngol. 2004;261(6):326-330.
14. King JJ, Melvin JS, Iwenofu OH, Fox EJ. Thigh pain in a 53-year-old woman. Clin Orthop Relat Res. 2009;467(6):1652-1657.
15. Hair LC, Deyle GD. Eosinophilic granuloma in a patient with hip pain. J Orthop Sports Phys Ther. 2011;41(2):119.
16. Panayiotakopoulos GD, Sipsas NV, Kontos A, et al. Eosinophilic granuloma of the femur in an HIV-1 positive patient. AIDS Patient Care STDS. 2002;16(3):103-106.
17. Rodrigues RJ, Lewis HH. Eosinophilic granuloma of bone. Review of literature and case presentation. Clin Orthop Relat Res. 1971;77:183-192.
18. Stull MA, Kransdorf MJ, Devaney KO. Langerhans cell histiocytosis of bone. Radiographics. 1992;12(4):801-823.
19. Lichtenstein L. Histiocytosis X (eosinophilic granuloma of bone, Letterer-Siwe disease, and Schueller-Christian disease). Further observations of pathological and clinical importance. J Bone Joint Surg Am. 1964;46:76-90.
20. Götz G, Fichter J. Langerhans’-cell histiocytosis in 58 adults. Eur J Med Res. 2004;9(11):510-514.
21. Cheng KL, Glu PG, Weiss LM. Hematopoeitic tumors. In: Peiguo C, Weiss L, eds. Modern Immunohistochemistry. New York, NY: Cambridge University Press; 2009:503.
22. Broadbent V, Gadner H. Current therapy for Langerhans cell histiocytosis. Hematol Oncol Clin North Am. 1998;12(2):327-338.
23. Plasschaert F, Craig C, Bell R, Cole WG, Wunder JS, Alman BA. Eosinophilic granuloma. A different behaviour in children than in adults. J Bone Joint Surg Br. 2002;84(6):870-872.
24. Capanna R, Springfield DS, Ruggieri P, et al. Direct cortisone injection in osinophilic granuloma of bone: a preliminary report on 11 patients. J Pediatr Orthop. 1985;5(3):339-342.
25. Egeler RM, Thompson RC Jr, Voûte PA, Nesbit ME Jr. Intralesional infiltration of corticosteroids in localized Langerhans’ cell histiocytosis. J Pediatr Orthop. 1992;12(6):811-814.
26. Ladisch S, Gadner H. Treatment of Langerhans cell histiocytosis–evolution and current approaches. Br J Cancer Suppl. 1994;23:S41-S46.