User login
Nocardiosis is a rare human infection that has been reported worldwide but occurs more frequently in patients who reside in tropical areas. Although there is no predilection for age or ethnicity, nocardiosis is slightly more common in males than in females.1,2 The genus Nocardia belongs to the order Actinomycetales and includes more than 50 species of gram-positive, aerobic, filamentous, branching, partially acid-fast bacteria found ubiquitously in soil.3 The bacteria may be present in animals (eg, cattle, dogs),4 but transmission to humans is unusual. Clinical diagnosis of nocardiosis often is difficult because of its nonspecific manifestations and delays in its recognition, especially in western countries where the infection is consistently rare and probably underestimated.5-8 Cutaneous involvement generally manifests as 1 of 4 conditions: mycetoma, lymphocutaneous (sporotrichoid) infection, superficial skin infection, or systemic disease with cutaneous involvement.9 Systemic disseminated disease usually occurs in individuals with cellular immune deficiency, such as patients with human immunodeficiency virus; organ transplant recipients; or patients with a history of long-term use of corticosteroids, configuring an opportunistic infection.10-19 Incidence in these high-risk patients is 140- to 340-fold higher than in the general population.20Nocardia infections usually are acquired via dust inhalation, especially in dry environments. Focal pneumonitis is the first typical manifestation in immunosuppressed patients, followed by skin dissemination and central nervous system involvement.1
We report the case of a 65-year-old man who developed disseminated nocardiosis while undergoing long-term treatment with systemic corticosteroids for rheumatoid arthritis.
Case Report
A 65-year-old man presented to the dermatology department for evaluation of multiple papules and nodules with a puruloid discharge on the right leg. The first lesions had appeared on the right ankle approximately 1 month prior to presentation and were treated with systemic antibiotics by the patient’s general practitioner without remarkable benefits. The lesions progressed further on the right leg showing a sporotrichoid disposition.
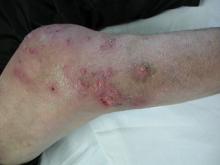
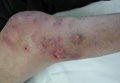
On physical examination major involvement was evident on the right knee with papules, subcutaneous nodules, and sinuses (Figure 1). Isolated lesions also were present on the right thigh. A few days later some lesions were present on the left elbow and arm. Involvement of the popliteal and inguinal lymph nodes was noted, with painful enlarged nodules covered by erythematous skin that were mobile on deep planes. The lesions had a sudden onset while the patient was in good health, causing progressive functional impotency of the leg without general malaise or fever. The patient’s history was remarkable for chronic rheumatoid arthritis of 20 years’ duration that was treated with hydroxychloroquine (400 mg daily) and methylprednisolone (16 mg daily).
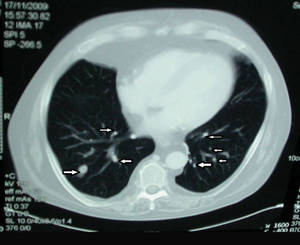
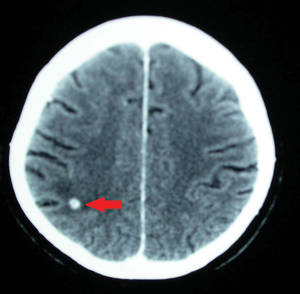
The patient was retired but reported daily tilling and manual labor on his farmland. He denied trauma or insect bites to the leg prior to the onset of lesions. Laboratory examination documented slight neutrophil leukocytosis (white blood cell count, 12.8×103/μL [reference range, 4.5–11.0×103/μL]; neutrophil count, 10.7×103/μL [reference range, 1.8–7.8×103/μL]; 85% neutrophils at formula count [reference range, 56%]), augmented C-reactive protein levels (15.8 mg/L [reference range, 0.08–3.1 mg/L]), a blood sedimentation rate of 74 mm/h (reference range, 0–20 mm/h), and elevated liver enzymes (aspartate aminotransferase, 52 U/L [reference range, 10–30 U/L]; alanine aminotransferase, 89 U/L [reference range, 10–40 U/L]; γ-glutamyltransferase, 68 U/L [reference range, 2–30 U/L]). Rheumatoid factor was 49.7 U/mL (reference range, 0–14.0 U/mL). Skin biopsy of a sample lesion suggested a chronic granulomatous suppuration. Tissue cultures and subsequent polymerase chain reaction assay identified Nocardia asteroides. A chest radiograph revealed multiple opaque nodules disseminated in both lungs, and a computed tomography (CT) scan confirmed multiple pulmonary lesions without involvement of the mediastinal lymph nodes (Figure 2). Computed tomography scans of the brain before and after contrast media perfusion showed the presence of an enhanced 8-mm mass among the right parietal and occipital lobes surrounded by an edematous halo (Figure 3). Neurologic examination was normal. During the radiologic assessment, the patient remembered having been hospitalized 1 year prior in a pulmonology unit at an outside institution for treatment of what was considered to be a multifocal nonspecific lung infection. A 1-month course of levofloxacin (500 mg daily) was administered at that time without any further follow-up. Review of the prior chest radiograph and CT scan confirmed the presence of the same radiologic findings as the current assessment, though of milder entity.
 |
 |
The patient was transferred from the dermatology department to the infectious diseases unit. Fiberoptic bronchoscopy with bronchoalveolar lavage and transbronchial biopsy confirmed pulmonary nocardiosis. Treatment with a combination of endovenous carbapenem and fluoroquinolone antibiotics for 1 month led to complete resolution of both the cutaneous lesions and the single brain abscess. Improvement of pulmonary involvement was noted on radiology, but another course of endovenous treatment with antibiotics was required, followed by oral amoxicillin–clavulanic acid (2 g daily) for 3 months. The patient underwent maintenance antibiotic therapy for 1 year without relapse and is considered to be cured.
Comment
Cutaneous nocardiosis occurs either as part of a disseminated infection or as a primary skin inoculation, usually following trauma or exposure from working outdoors.2,9,21,22 Nocardial mycetoma, also known as actinomycetoma, is a chronic condition that mainly affects the lower extremities but also can affect the hands and forearms; it is frequently reported in tropical regions,23 but disseminated forms are rare and undervalued, especially in European countries. A certain rise in incidence has been reported in Europe as a consequence of immune suppression, and pulmonary disease is the most common presentation in these patients.1,2,6-12 One-third of patients have disseminated disease with high morbidity and a high mortality rate.20 Major case collections in Italy have been studied by a collaborative hospital network of 11 cities,10,11 confirming difficulty of diagnosis and underestimation, prevalence of N asteroides as a pathogen, resistance to several antimicrobials, and a high relapse index. Diagnosis remains challenging, as clinical suspicion is not frequently supported by histology and/or microbiology because of difficulty in bacteria isolation. Molecular methods are not routinely available in Italy, and only stringent efforts and cooperation from different university departments has allowed final identification of nocardiosis.
The sporotrichoid pattern of the lesions on the right leg was unique; it is considered the rarest presentation of cutaneous nocardiosis.24 Our first suspicion was sporotrichosis, an infection that is especially common in farmers in Italy,25,26 but the microbiologist’s evaluation of the bacterial pure cultures growing on skin specimens was negative for deep fungal infections. Further identification and confirmation by polymerase chain reaction assay of N asteroides required several weeks. Meanwhile, the chest radiograph followed by CT scans of the lungs and brain led to the diagnosis of a disseminated infection. Documentation of lung involvement 1 year prior to the development of the cutaneous lesions excluded primary cutaneous nocardiosis with secondary dissemination as reported in other immunocompromised patients, including those undergoing long-term treatment with corticosteroids.15-19 Continuous follow-up and physician awareness of possible unusual infections is mandatory in patients undergoing immunosuppressive therapy. Our patient had a 20-year history of rheumatoid arthritis but was otherwise healthy with no signs of lung distress, fever, or general malaise. His only concern was leg impairment following acute development of painful lesions discharging pus over 1 month.
Conclusion
This case report highlights the role of the dermatologist as the first-line physician involved in the diagnosis of rare and potentially severe infections. Cooperation with the microbiologist, pathologist, and other internal medicine specialists is crucial; however, sometimes it is just the clinical suspicion and the perseverance of the dermatologist that ultimately leads to the correct diagnosis, which often is otherwise unspecific, undervalued, and misdiagnosed as more common diseases.
1. Lerner PI. Nocardiosis. Clin Infect Dis. 1996;22:891-903.
2. Minero MV, Marin M, Cercenado E, et al. Nocardiosis at the turn of the century. Medicine (Baltimore). 2009;88:250-261.
3. McNeil MM, Brown JM. The medically important aerobic actinomycetes: epidemiology and microbiology. Clin Microbiol Rev. 1994;7:357-417.
4. Ribeiro MG, Salerno T, Mattos-Guaraldi AL, et al. Nocardiosis: an overview and additional report of 28 cases in cattle and dogs. Rev Inst Med Trop Sao Paulo. 2008;50:177-185.
5. Beaman BL, Burnside J, Edwards B, et al. Nocardial infections in the United States, 1972-1974. J Infect Dis. 1976;134:286-289.
6. Boiron P, Provost F, Chevrier G, et al. Review of nocardial infections in France 1987 to 1990. Eur J Clin Microbiol Infect Dis. 1992;11:709-714.
7. Farina C, Boiron P, Ferrari I, et al. Report of human nocardiosis in Italy between 1993 and 1997. Eur J Epidemiol. 2001;17:1019-1022.
8. Farina C, Boiron P, Goglio A, et al. Human nocardiosis in northern Italy from 1982 to 1992. Northern Italy Collaborative Group on nocardiosis. Scand J Infect Dis. 1995;27:23-27.
9. Kalb RE, Kaplan MH, Grossman ME. Cutaneous nocardiosis. case reports and review. J Am Acad Dermatol. 1985;13:125-133.
10. Fontana I, Gasloli G, Rossi AM, et al. Nocardiosis in a kidney-pancreas transplant. J Transplant. 2010;2010:573234.
11. Frank M, Woschnagg H, Mölzer G, et al. Cerebellar nocardiosis and myopathy from long-term corticosteroids for idiopathic thrombocytopenia. Yonsei Med J. 2010;51:131-137.
12. Devi KR, Singh LR, Devi NT, et al. Subcutaneous nocardial abscess in a post-renal transplant patient. Indian J Med Microbiol. 2007;25:279-281.
13. Iona E, Giannoni F, Brunori L, et al. Isolation of Nocardia asiatica from cutaneous ulcers of a human immunodeficiency virus-infected patient in Italy. J Clin Microbiol. 2007;45:2088-2089.
14. Pardo M, Bonifaz A, Valencia A, et al. Actinomycetoma by Nocardia brasiliensis in a girl with Down syndrome. Dermatol Online J. 2008;14:9.
15. Nenoff P, Kellermann S, Borte G, et al. Pulmonary nocardiosis with cutaneous involvement mimicking a metastasizing lung carcinoma in a patient with chronic myelogenous leukaemia. Eur J Dermatol. 2000;10:47-51.
16. Baldi BG, Santana AN, Takagaki TY. Pulmonary and cutaneous nocardiosis in a patient treated with corticosteroids [in English, Portuguese]. J Bras Penumol. 2006;32:592-595.
17. Singh SM, Rau NV, Cohen LB, et al. Cutaneous nocardiosis complicating management of Crohn’s disease with infliximab and prednisone. CMAJ. 2004;171:1063-1064.
18. Dekeyser S, Corroyer-Simovic B, Cachia M, et al. Nocardia otitidiscaviarum, cutaneous infection in a patient receiving long-term corticosteroid treatment [in French]. Ann Biol Clin (Paris). 2003;61:219-222.
19. Hashimoto Y, Hiruma M, Hisamichi K, et al. Primary cutaneous nocardiosis with multiple, subcutaneous abscesses in a patient with sarcoidosis. J Dermatolog Treat. 2002;13:201-203.
20. Ambrosioni J, Lew D, Garbino J. Nocardiosis: updated clinical review and experience at a tertiary center. Infection. 2010;38:89-97.
21. Patil SP, Gautam MM, Sodha AA, et al. Primary cutaneous nocardiosis with craniocerebral extension: a case report. Dermatol Online J. 2009;15:8.
22. Lakshmi V, Sundaram C, Meena AK, et al. Primary cutaneous nocardiosis with epidural abscess caused by Nocardia brasiliensis: a case report. Neurol India. 2002;50:90-92.
23. Bonifaz A, Flores P, Saúl A, et al. Treatment of actinomycetoma due to Nocardia spp. with amoxicillin-clavulanate. Br J Dermatol. 2007;156:308-311.
24. Baradkar VP, Mathur M, Kulkarni SD, et al. Sporotrichoid pattern of cutaneous nocardiasis due to Nocardia asteroids. Indian J Pathol Microbiol. 2008;51:432-434.
25. Barile F, Mastrolonardo M, Loconsole F, et al. Cutaneous sporotrichosis in the period 1978-1992 in the province of Bari, Apulia, Southern Italy. Mycoses. 1993;36:181-185.
26. Alberici F, Paties CT, Lombardi G, et al. Sporothrix schenckii var luriei as the cause of sporotrichosis in Italy. Eur J Epidemiol. 1989;5:173-177.
Nocardiosis is a rare human infection that has been reported worldwide but occurs more frequently in patients who reside in tropical areas. Although there is no predilection for age or ethnicity, nocardiosis is slightly more common in males than in females.1,2 The genus Nocardia belongs to the order Actinomycetales and includes more than 50 species of gram-positive, aerobic, filamentous, branching, partially acid-fast bacteria found ubiquitously in soil.3 The bacteria may be present in animals (eg, cattle, dogs),4 but transmission to humans is unusual. Clinical diagnosis of nocardiosis often is difficult because of its nonspecific manifestations and delays in its recognition, especially in western countries where the infection is consistently rare and probably underestimated.5-8 Cutaneous involvement generally manifests as 1 of 4 conditions: mycetoma, lymphocutaneous (sporotrichoid) infection, superficial skin infection, or systemic disease with cutaneous involvement.9 Systemic disseminated disease usually occurs in individuals with cellular immune deficiency, such as patients with human immunodeficiency virus; organ transplant recipients; or patients with a history of long-term use of corticosteroids, configuring an opportunistic infection.10-19 Incidence in these high-risk patients is 140- to 340-fold higher than in the general population.20Nocardia infections usually are acquired via dust inhalation, especially in dry environments. Focal pneumonitis is the first typical manifestation in immunosuppressed patients, followed by skin dissemination and central nervous system involvement.1
We report the case of a 65-year-old man who developed disseminated nocardiosis while undergoing long-term treatment with systemic corticosteroids for rheumatoid arthritis.
Case Report
A 65-year-old man presented to the dermatology department for evaluation of multiple papules and nodules with a puruloid discharge on the right leg. The first lesions had appeared on the right ankle approximately 1 month prior to presentation and were treated with systemic antibiotics by the patient’s general practitioner without remarkable benefits. The lesions progressed further on the right leg showing a sporotrichoid disposition.
On physical examination major involvement was evident on the right knee with papules, subcutaneous nodules, and sinuses (Figure 1). Isolated lesions also were present on the right thigh. A few days later some lesions were present on the left elbow and arm. Involvement of the popliteal and inguinal lymph nodes was noted, with painful enlarged nodules covered by erythematous skin that were mobile on deep planes. The lesions had a sudden onset while the patient was in good health, causing progressive functional impotency of the leg without general malaise or fever. The patient’s history was remarkable for chronic rheumatoid arthritis of 20 years’ duration that was treated with hydroxychloroquine (400 mg daily) and methylprednisolone (16 mg daily).
The patient was retired but reported daily tilling and manual labor on his farmland. He denied trauma or insect bites to the leg prior to the onset of lesions. Laboratory examination documented slight neutrophil leukocytosis (white blood cell count, 12.8×103/μL [reference range, 4.5–11.0×103/μL]; neutrophil count, 10.7×103/μL [reference range, 1.8–7.8×103/μL]; 85% neutrophils at formula count [reference range, 56%]), augmented C-reactive protein levels (15.8 mg/L [reference range, 0.08–3.1 mg/L]), a blood sedimentation rate of 74 mm/h (reference range, 0–20 mm/h), and elevated liver enzymes (aspartate aminotransferase, 52 U/L [reference range, 10–30 U/L]; alanine aminotransferase, 89 U/L [reference range, 10–40 U/L]; γ-glutamyltransferase, 68 U/L [reference range, 2–30 U/L]). Rheumatoid factor was 49.7 U/mL (reference range, 0–14.0 U/mL). Skin biopsy of a sample lesion suggested a chronic granulomatous suppuration. Tissue cultures and subsequent polymerase chain reaction assay identified Nocardia asteroides. A chest radiograph revealed multiple opaque nodules disseminated in both lungs, and a computed tomography (CT) scan confirmed multiple pulmonary lesions without involvement of the mediastinal lymph nodes (Figure 2). Computed tomography scans of the brain before and after contrast media perfusion showed the presence of an enhanced 8-mm mass among the right parietal and occipital lobes surrounded by an edematous halo (Figure 3). Neurologic examination was normal. During the radiologic assessment, the patient remembered having been hospitalized 1 year prior in a pulmonology unit at an outside institution for treatment of what was considered to be a multifocal nonspecific lung infection. A 1-month course of levofloxacin (500 mg daily) was administered at that time without any further follow-up. Review of the prior chest radiograph and CT scan confirmed the presence of the same radiologic findings as the current assessment, though of milder entity.
 |
 |
The patient was transferred from the dermatology department to the infectious diseases unit. Fiberoptic bronchoscopy with bronchoalveolar lavage and transbronchial biopsy confirmed pulmonary nocardiosis. Treatment with a combination of endovenous carbapenem and fluoroquinolone antibiotics for 1 month led to complete resolution of both the cutaneous lesions and the single brain abscess. Improvement of pulmonary involvement was noted on radiology, but another course of endovenous treatment with antibiotics was required, followed by oral amoxicillin–clavulanic acid (2 g daily) for 3 months. The patient underwent maintenance antibiotic therapy for 1 year without relapse and is considered to be cured.
Comment
Cutaneous nocardiosis occurs either as part of a disseminated infection or as a primary skin inoculation, usually following trauma or exposure from working outdoors.2,9,21,22 Nocardial mycetoma, also known as actinomycetoma, is a chronic condition that mainly affects the lower extremities but also can affect the hands and forearms; it is frequently reported in tropical regions,23 but disseminated forms are rare and undervalued, especially in European countries. A certain rise in incidence has been reported in Europe as a consequence of immune suppression, and pulmonary disease is the most common presentation in these patients.1,2,6-12 One-third of patients have disseminated disease with high morbidity and a high mortality rate.20 Major case collections in Italy have been studied by a collaborative hospital network of 11 cities,10,11 confirming difficulty of diagnosis and underestimation, prevalence of N asteroides as a pathogen, resistance to several antimicrobials, and a high relapse index. Diagnosis remains challenging, as clinical suspicion is not frequently supported by histology and/or microbiology because of difficulty in bacteria isolation. Molecular methods are not routinely available in Italy, and only stringent efforts and cooperation from different university departments has allowed final identification of nocardiosis.
The sporotrichoid pattern of the lesions on the right leg was unique; it is considered the rarest presentation of cutaneous nocardiosis.24 Our first suspicion was sporotrichosis, an infection that is especially common in farmers in Italy,25,26 but the microbiologist’s evaluation of the bacterial pure cultures growing on skin specimens was negative for deep fungal infections. Further identification and confirmation by polymerase chain reaction assay of N asteroides required several weeks. Meanwhile, the chest radiograph followed by CT scans of the lungs and brain led to the diagnosis of a disseminated infection. Documentation of lung involvement 1 year prior to the development of the cutaneous lesions excluded primary cutaneous nocardiosis with secondary dissemination as reported in other immunocompromised patients, including those undergoing long-term treatment with corticosteroids.15-19 Continuous follow-up and physician awareness of possible unusual infections is mandatory in patients undergoing immunosuppressive therapy. Our patient had a 20-year history of rheumatoid arthritis but was otherwise healthy with no signs of lung distress, fever, or general malaise. His only concern was leg impairment following acute development of painful lesions discharging pus over 1 month.
Conclusion
This case report highlights the role of the dermatologist as the first-line physician involved in the diagnosis of rare and potentially severe infections. Cooperation with the microbiologist, pathologist, and other internal medicine specialists is crucial; however, sometimes it is just the clinical suspicion and the perseverance of the dermatologist that ultimately leads to the correct diagnosis, which often is otherwise unspecific, undervalued, and misdiagnosed as more common diseases.
Nocardiosis is a rare human infection that has been reported worldwide but occurs more frequently in patients who reside in tropical areas. Although there is no predilection for age or ethnicity, nocardiosis is slightly more common in males than in females.1,2 The genus Nocardia belongs to the order Actinomycetales and includes more than 50 species of gram-positive, aerobic, filamentous, branching, partially acid-fast bacteria found ubiquitously in soil.3 The bacteria may be present in animals (eg, cattle, dogs),4 but transmission to humans is unusual. Clinical diagnosis of nocardiosis often is difficult because of its nonspecific manifestations and delays in its recognition, especially in western countries where the infection is consistently rare and probably underestimated.5-8 Cutaneous involvement generally manifests as 1 of 4 conditions: mycetoma, lymphocutaneous (sporotrichoid) infection, superficial skin infection, or systemic disease with cutaneous involvement.9 Systemic disseminated disease usually occurs in individuals with cellular immune deficiency, such as patients with human immunodeficiency virus; organ transplant recipients; or patients with a history of long-term use of corticosteroids, configuring an opportunistic infection.10-19 Incidence in these high-risk patients is 140- to 340-fold higher than in the general population.20Nocardia infections usually are acquired via dust inhalation, especially in dry environments. Focal pneumonitis is the first typical manifestation in immunosuppressed patients, followed by skin dissemination and central nervous system involvement.1
We report the case of a 65-year-old man who developed disseminated nocardiosis while undergoing long-term treatment with systemic corticosteroids for rheumatoid arthritis.
Case Report
A 65-year-old man presented to the dermatology department for evaluation of multiple papules and nodules with a puruloid discharge on the right leg. The first lesions had appeared on the right ankle approximately 1 month prior to presentation and were treated with systemic antibiotics by the patient’s general practitioner without remarkable benefits. The lesions progressed further on the right leg showing a sporotrichoid disposition.
On physical examination major involvement was evident on the right knee with papules, subcutaneous nodules, and sinuses (Figure 1). Isolated lesions also were present on the right thigh. A few days later some lesions were present on the left elbow and arm. Involvement of the popliteal and inguinal lymph nodes was noted, with painful enlarged nodules covered by erythematous skin that were mobile on deep planes. The lesions had a sudden onset while the patient was in good health, causing progressive functional impotency of the leg without general malaise or fever. The patient’s history was remarkable for chronic rheumatoid arthritis of 20 years’ duration that was treated with hydroxychloroquine (400 mg daily) and methylprednisolone (16 mg daily).
The patient was retired but reported daily tilling and manual labor on his farmland. He denied trauma or insect bites to the leg prior to the onset of lesions. Laboratory examination documented slight neutrophil leukocytosis (white blood cell count, 12.8×103/μL [reference range, 4.5–11.0×103/μL]; neutrophil count, 10.7×103/μL [reference range, 1.8–7.8×103/μL]; 85% neutrophils at formula count [reference range, 56%]), augmented C-reactive protein levels (15.8 mg/L [reference range, 0.08–3.1 mg/L]), a blood sedimentation rate of 74 mm/h (reference range, 0–20 mm/h), and elevated liver enzymes (aspartate aminotransferase, 52 U/L [reference range, 10–30 U/L]; alanine aminotransferase, 89 U/L [reference range, 10–40 U/L]; γ-glutamyltransferase, 68 U/L [reference range, 2–30 U/L]). Rheumatoid factor was 49.7 U/mL (reference range, 0–14.0 U/mL). Skin biopsy of a sample lesion suggested a chronic granulomatous suppuration. Tissue cultures and subsequent polymerase chain reaction assay identified Nocardia asteroides. A chest radiograph revealed multiple opaque nodules disseminated in both lungs, and a computed tomography (CT) scan confirmed multiple pulmonary lesions without involvement of the mediastinal lymph nodes (Figure 2). Computed tomography scans of the brain before and after contrast media perfusion showed the presence of an enhanced 8-mm mass among the right parietal and occipital lobes surrounded by an edematous halo (Figure 3). Neurologic examination was normal. During the radiologic assessment, the patient remembered having been hospitalized 1 year prior in a pulmonology unit at an outside institution for treatment of what was considered to be a multifocal nonspecific lung infection. A 1-month course of levofloxacin (500 mg daily) was administered at that time without any further follow-up. Review of the prior chest radiograph and CT scan confirmed the presence of the same radiologic findings as the current assessment, though of milder entity.
 |
 |
The patient was transferred from the dermatology department to the infectious diseases unit. Fiberoptic bronchoscopy with bronchoalveolar lavage and transbronchial biopsy confirmed pulmonary nocardiosis. Treatment with a combination of endovenous carbapenem and fluoroquinolone antibiotics for 1 month led to complete resolution of both the cutaneous lesions and the single brain abscess. Improvement of pulmonary involvement was noted on radiology, but another course of endovenous treatment with antibiotics was required, followed by oral amoxicillin–clavulanic acid (2 g daily) for 3 months. The patient underwent maintenance antibiotic therapy for 1 year without relapse and is considered to be cured.
Comment
Cutaneous nocardiosis occurs either as part of a disseminated infection or as a primary skin inoculation, usually following trauma or exposure from working outdoors.2,9,21,22 Nocardial mycetoma, also known as actinomycetoma, is a chronic condition that mainly affects the lower extremities but also can affect the hands and forearms; it is frequently reported in tropical regions,23 but disseminated forms are rare and undervalued, especially in European countries. A certain rise in incidence has been reported in Europe as a consequence of immune suppression, and pulmonary disease is the most common presentation in these patients.1,2,6-12 One-third of patients have disseminated disease with high morbidity and a high mortality rate.20 Major case collections in Italy have been studied by a collaborative hospital network of 11 cities,10,11 confirming difficulty of diagnosis and underestimation, prevalence of N asteroides as a pathogen, resistance to several antimicrobials, and a high relapse index. Diagnosis remains challenging, as clinical suspicion is not frequently supported by histology and/or microbiology because of difficulty in bacteria isolation. Molecular methods are not routinely available in Italy, and only stringent efforts and cooperation from different university departments has allowed final identification of nocardiosis.
The sporotrichoid pattern of the lesions on the right leg was unique; it is considered the rarest presentation of cutaneous nocardiosis.24 Our first suspicion was sporotrichosis, an infection that is especially common in farmers in Italy,25,26 but the microbiologist’s evaluation of the bacterial pure cultures growing on skin specimens was negative for deep fungal infections. Further identification and confirmation by polymerase chain reaction assay of N asteroides required several weeks. Meanwhile, the chest radiograph followed by CT scans of the lungs and brain led to the diagnosis of a disseminated infection. Documentation of lung involvement 1 year prior to the development of the cutaneous lesions excluded primary cutaneous nocardiosis with secondary dissemination as reported in other immunocompromised patients, including those undergoing long-term treatment with corticosteroids.15-19 Continuous follow-up and physician awareness of possible unusual infections is mandatory in patients undergoing immunosuppressive therapy. Our patient had a 20-year history of rheumatoid arthritis but was otherwise healthy with no signs of lung distress, fever, or general malaise. His only concern was leg impairment following acute development of painful lesions discharging pus over 1 month.
Conclusion
This case report highlights the role of the dermatologist as the first-line physician involved in the diagnosis of rare and potentially severe infections. Cooperation with the microbiologist, pathologist, and other internal medicine specialists is crucial; however, sometimes it is just the clinical suspicion and the perseverance of the dermatologist that ultimately leads to the correct diagnosis, which often is otherwise unspecific, undervalued, and misdiagnosed as more common diseases.
1. Lerner PI. Nocardiosis. Clin Infect Dis. 1996;22:891-903.
2. Minero MV, Marin M, Cercenado E, et al. Nocardiosis at the turn of the century. Medicine (Baltimore). 2009;88:250-261.
3. McNeil MM, Brown JM. The medically important aerobic actinomycetes: epidemiology and microbiology. Clin Microbiol Rev. 1994;7:357-417.
4. Ribeiro MG, Salerno T, Mattos-Guaraldi AL, et al. Nocardiosis: an overview and additional report of 28 cases in cattle and dogs. Rev Inst Med Trop Sao Paulo. 2008;50:177-185.
5. Beaman BL, Burnside J, Edwards B, et al. Nocardial infections in the United States, 1972-1974. J Infect Dis. 1976;134:286-289.
6. Boiron P, Provost F, Chevrier G, et al. Review of nocardial infections in France 1987 to 1990. Eur J Clin Microbiol Infect Dis. 1992;11:709-714.
7. Farina C, Boiron P, Ferrari I, et al. Report of human nocardiosis in Italy between 1993 and 1997. Eur J Epidemiol. 2001;17:1019-1022.
8. Farina C, Boiron P, Goglio A, et al. Human nocardiosis in northern Italy from 1982 to 1992. Northern Italy Collaborative Group on nocardiosis. Scand J Infect Dis. 1995;27:23-27.
9. Kalb RE, Kaplan MH, Grossman ME. Cutaneous nocardiosis. case reports and review. J Am Acad Dermatol. 1985;13:125-133.
10. Fontana I, Gasloli G, Rossi AM, et al. Nocardiosis in a kidney-pancreas transplant. J Transplant. 2010;2010:573234.
11. Frank M, Woschnagg H, Mölzer G, et al. Cerebellar nocardiosis and myopathy from long-term corticosteroids for idiopathic thrombocytopenia. Yonsei Med J. 2010;51:131-137.
12. Devi KR, Singh LR, Devi NT, et al. Subcutaneous nocardial abscess in a post-renal transplant patient. Indian J Med Microbiol. 2007;25:279-281.
13. Iona E, Giannoni F, Brunori L, et al. Isolation of Nocardia asiatica from cutaneous ulcers of a human immunodeficiency virus-infected patient in Italy. J Clin Microbiol. 2007;45:2088-2089.
14. Pardo M, Bonifaz A, Valencia A, et al. Actinomycetoma by Nocardia brasiliensis in a girl with Down syndrome. Dermatol Online J. 2008;14:9.
15. Nenoff P, Kellermann S, Borte G, et al. Pulmonary nocardiosis with cutaneous involvement mimicking a metastasizing lung carcinoma in a patient with chronic myelogenous leukaemia. Eur J Dermatol. 2000;10:47-51.
16. Baldi BG, Santana AN, Takagaki TY. Pulmonary and cutaneous nocardiosis in a patient treated with corticosteroids [in English, Portuguese]. J Bras Penumol. 2006;32:592-595.
17. Singh SM, Rau NV, Cohen LB, et al. Cutaneous nocardiosis complicating management of Crohn’s disease with infliximab and prednisone. CMAJ. 2004;171:1063-1064.
18. Dekeyser S, Corroyer-Simovic B, Cachia M, et al. Nocardia otitidiscaviarum, cutaneous infection in a patient receiving long-term corticosteroid treatment [in French]. Ann Biol Clin (Paris). 2003;61:219-222.
19. Hashimoto Y, Hiruma M, Hisamichi K, et al. Primary cutaneous nocardiosis with multiple, subcutaneous abscesses in a patient with sarcoidosis. J Dermatolog Treat. 2002;13:201-203.
20. Ambrosioni J, Lew D, Garbino J. Nocardiosis: updated clinical review and experience at a tertiary center. Infection. 2010;38:89-97.
21. Patil SP, Gautam MM, Sodha AA, et al. Primary cutaneous nocardiosis with craniocerebral extension: a case report. Dermatol Online J. 2009;15:8.
22. Lakshmi V, Sundaram C, Meena AK, et al. Primary cutaneous nocardiosis with epidural abscess caused by Nocardia brasiliensis: a case report. Neurol India. 2002;50:90-92.
23. Bonifaz A, Flores P, Saúl A, et al. Treatment of actinomycetoma due to Nocardia spp. with amoxicillin-clavulanate. Br J Dermatol. 2007;156:308-311.
24. Baradkar VP, Mathur M, Kulkarni SD, et al. Sporotrichoid pattern of cutaneous nocardiasis due to Nocardia asteroids. Indian J Pathol Microbiol. 2008;51:432-434.
25. Barile F, Mastrolonardo M, Loconsole F, et al. Cutaneous sporotrichosis in the period 1978-1992 in the province of Bari, Apulia, Southern Italy. Mycoses. 1993;36:181-185.
26. Alberici F, Paties CT, Lombardi G, et al. Sporothrix schenckii var luriei as the cause of sporotrichosis in Italy. Eur J Epidemiol. 1989;5:173-177.
1. Lerner PI. Nocardiosis. Clin Infect Dis. 1996;22:891-903.
2. Minero MV, Marin M, Cercenado E, et al. Nocardiosis at the turn of the century. Medicine (Baltimore). 2009;88:250-261.
3. McNeil MM, Brown JM. The medically important aerobic actinomycetes: epidemiology and microbiology. Clin Microbiol Rev. 1994;7:357-417.
4. Ribeiro MG, Salerno T, Mattos-Guaraldi AL, et al. Nocardiosis: an overview and additional report of 28 cases in cattle and dogs. Rev Inst Med Trop Sao Paulo. 2008;50:177-185.
5. Beaman BL, Burnside J, Edwards B, et al. Nocardial infections in the United States, 1972-1974. J Infect Dis. 1976;134:286-289.
6. Boiron P, Provost F, Chevrier G, et al. Review of nocardial infections in France 1987 to 1990. Eur J Clin Microbiol Infect Dis. 1992;11:709-714.
7. Farina C, Boiron P, Ferrari I, et al. Report of human nocardiosis in Italy between 1993 and 1997. Eur J Epidemiol. 2001;17:1019-1022.
8. Farina C, Boiron P, Goglio A, et al. Human nocardiosis in northern Italy from 1982 to 1992. Northern Italy Collaborative Group on nocardiosis. Scand J Infect Dis. 1995;27:23-27.
9. Kalb RE, Kaplan MH, Grossman ME. Cutaneous nocardiosis. case reports and review. J Am Acad Dermatol. 1985;13:125-133.
10. Fontana I, Gasloli G, Rossi AM, et al. Nocardiosis in a kidney-pancreas transplant. J Transplant. 2010;2010:573234.
11. Frank M, Woschnagg H, Mölzer G, et al. Cerebellar nocardiosis and myopathy from long-term corticosteroids for idiopathic thrombocytopenia. Yonsei Med J. 2010;51:131-137.
12. Devi KR, Singh LR, Devi NT, et al. Subcutaneous nocardial abscess in a post-renal transplant patient. Indian J Med Microbiol. 2007;25:279-281.
13. Iona E, Giannoni F, Brunori L, et al. Isolation of Nocardia asiatica from cutaneous ulcers of a human immunodeficiency virus-infected patient in Italy. J Clin Microbiol. 2007;45:2088-2089.
14. Pardo M, Bonifaz A, Valencia A, et al. Actinomycetoma by Nocardia brasiliensis in a girl with Down syndrome. Dermatol Online J. 2008;14:9.
15. Nenoff P, Kellermann S, Borte G, et al. Pulmonary nocardiosis with cutaneous involvement mimicking a metastasizing lung carcinoma in a patient with chronic myelogenous leukaemia. Eur J Dermatol. 2000;10:47-51.
16. Baldi BG, Santana AN, Takagaki TY. Pulmonary and cutaneous nocardiosis in a patient treated with corticosteroids [in English, Portuguese]. J Bras Penumol. 2006;32:592-595.
17. Singh SM, Rau NV, Cohen LB, et al. Cutaneous nocardiosis complicating management of Crohn’s disease with infliximab and prednisone. CMAJ. 2004;171:1063-1064.
18. Dekeyser S, Corroyer-Simovic B, Cachia M, et al. Nocardia otitidiscaviarum, cutaneous infection in a patient receiving long-term corticosteroid treatment [in French]. Ann Biol Clin (Paris). 2003;61:219-222.
19. Hashimoto Y, Hiruma M, Hisamichi K, et al. Primary cutaneous nocardiosis with multiple, subcutaneous abscesses in a patient with sarcoidosis. J Dermatolog Treat. 2002;13:201-203.
20. Ambrosioni J, Lew D, Garbino J. Nocardiosis: updated clinical review and experience at a tertiary center. Infection. 2010;38:89-97.
21. Patil SP, Gautam MM, Sodha AA, et al. Primary cutaneous nocardiosis with craniocerebral extension: a case report. Dermatol Online J. 2009;15:8.
22. Lakshmi V, Sundaram C, Meena AK, et al. Primary cutaneous nocardiosis with epidural abscess caused by Nocardia brasiliensis: a case report. Neurol India. 2002;50:90-92.
23. Bonifaz A, Flores P, Saúl A, et al. Treatment of actinomycetoma due to Nocardia spp. with amoxicillin-clavulanate. Br J Dermatol. 2007;156:308-311.
24. Baradkar VP, Mathur M, Kulkarni SD, et al. Sporotrichoid pattern of cutaneous nocardiasis due to Nocardia asteroids. Indian J Pathol Microbiol. 2008;51:432-434.
25. Barile F, Mastrolonardo M, Loconsole F, et al. Cutaneous sporotrichosis in the period 1978-1992 in the province of Bari, Apulia, Southern Italy. Mycoses. 1993;36:181-185.
26. Alberici F, Paties CT, Lombardi G, et al. Sporothrix schenckii var luriei as the cause of sporotrichosis in Italy. Eur J Epidemiol. 1989;5:173-177.
- A high index of suspicion by the dermatologist is needed to alert the pathologist, microbiologist, and other clinicians involved in the assessment and diagnosis of nocardiosis.
- Because cultures from skin lesions of nocardiosis often show negative results, several specimens should be collected for analysis.
- Histopathologic analysis of skin biopsies often is necessary to exclude other inflammatory conditions and implicate an infectious process.
- Treatment with empirical broad-spectrum antibiotics should be promptly initiated and adjusted based on antibiotic susceptibility test results.