User login
Heart failure (HF) is one of the leading causes of hospitalizations and the most expensive Medicare diagnosis. Its prevalence continues to rise with a projected increase of 46% from 2012 to 2030 resulting in > 8 million people aged ≥ 18 years with HF in the United States. Despite improvements in therapy, mortality remains unacceptably high with a 50% mortality rate within 5 years. Early detection strategies are needed to identify patients at risk of developing HF to delay the disease course and improve survival.1,2
Emerging data indicates that natriuretic peptide biomarker-based screening using B-type natriuretic peptide (BNP) or N-terminal pro-B-type natriuretic peptide (NT-proBNP) and early intervention for patients at risk of HF could prevent development of left ventricular dysfunction or new-onset HF.3-5 The 2013 St. Vincent’s Screening to Prevent Heart Failure (STOP-HF) trial is the largest study to date to evaluate BNP as a screening tool for patients at risk for HF.4 Patients at risk of HF who did not have established left ventricular systolic dysfunction or symptomatic HF were assigned randomly to usual primary care or BNP screening. Patients with BNP levels ≥ 50 pg/mL underwent echocardiogram and were referred to a cardiovascular specialty service for management. The cardiovascular specialty clinic included a team of registered nurses, nurse practitioners, pharmacists, dieticians, palliative care specialists, and cardiologists. Individuals in the intervention group showed increased renin-angiotensin system (RAS) inhibitor use at follow-up (control, 49.6%; intervention, 59.6%; P = .01). All patients received coaching by a nurse who emphasized individual risk, importance of medication adherence, and healthy lifestyle behaviors. After a mean follow-up of 4.2 years, 59 of 677 participants (8.7%) in the control group and 37 of 697 (5.3%) in the intervention group (odds ratio [OR], 0.55; 95% CI, 0.37 to 0.82; P = .003) met the primary end point of left ventricular dysfunction with or without HF. BNP-based screening in conjunction with collaborative care reduced rates of left ventricular dysfunction and HF.
In the 2013 PONTIAC trial, patients with type 2 diabetes mellitus (T2DM) without cardiac disease but with NT-proBNP levels > 125 pg/mL were randomized to usual diabetes care or intensified care at a cardiac outpatient clinic for initiation and increase of RAS inhibitors and β blockers.5 After 2 years, patients randomized to the intensified care group showed a 65% risk reduction of the primary endpoint of hospitalization or death from cardiac disease (P = .04).
Based on this evidence, the 2017 focused update of the American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Failure Society of America (HFSA) guideline for managing HF added a IIa recommendation for natriuretic peptide biomarker screening in those at risk of developing HF.6 The guideline recommends biomarker screening in conjunction with team-based care, including a cardiovascular specialist, and guideline-directed management and therapy to prevent development of left ventricular dysfunction or new-onset HF.
Although ordering a natriuretic peptide biomarker laboratory test is straightforward, the variability of team-based care across institutions and health systems makes it difficult to standardize screening and interventions for patients at risk for HF. We developed and piloted a process using clinical pharmacists in primary care for natriuretic peptide biomarker screening and risk factor reduction within the established patient aligned care team (PACT) framework at a US Department of Veterans Affairs (VA) medical center. In this paper, we describe our implementation process including descriptive preliminary outcomes.
Methods
The PACT team-based approach in primary care clinics is similar to the patient-centered medical home framework. A PACT includes the veteran patient and an interdisciplinary team of health professionals composed of their primary care practitioner (PCP), registered nurse care manager, clinical pharmacist, and other clinical and administrative staff. The PACT clinical pharmacist has prescriptive authority within a scope of practice to provide postdiagnostic chronic disease state management including management of T2DM, hypertension, HF, chronic obstructive pulmonary disease, anticoagulation, tobacco cessation, and atherosclerotic cardiovascular disease (ASCVD) risk reduction. Clinical pharmacists can prescribe and adjust medications and order laboratory tests.
Our institution, Clement J. Zablocki VA Medical Center (CJZVAMC) in Milwaukee, Wisconsin, has a specialty HF clinic that primarily manages ACC/AHA Stage C HF patients. The HF clinic uses a team-based approach to collaborate and coordinate care for the veteran. The HF team is comprised of cardiology specialists, registered nurses, clinical pharmacists, dietitians, and administrative staff. Two PACT clinical pharmacists also staff the HF clinic at CJZVAMC and work collaboratively to initiate, adjust, and optimize veterans’ HF medication regimens.
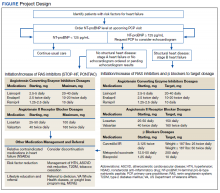
Two primary care PACT panels were selected for this project. Before implementation, a pharmacy resident and 3 PACT clinical pharmacists (2 of whom also staff the HF clinic) met with a HF cardiology specialist and 2 PACT PCPs to finalize the team-based process and workflow. PCPs were presented with the evidence-based background, purpose, and project design, which included patient identification, NT-proBNP laboratory test ordering, medication adjustment schedules, and protocol for ordering echocardiograms (Figure). Templated notes were created to allow for consistent documentation in patients’ electronic health record. A telephone script also was written for the initial telephone call to patients to explain in patient-friendly terms the implications of an elevated NT-proBNP level, the echocardiogram procedure, and recommendations for risk reduction.
Patient Selection
Patients aged ≥ 18 years with hypertension, taking antihypertensive medication for ≥ 1 month, or diagnosed with T2DM for ≥ 6 months were included. Using the parameters provided in the STOP-HF trial, patients with evidence or history of left ventricular dysfunction, defined as a left ventricular ejection fraction (EF) < 50% or an E/e’ ratio > 15 in the setting of normal EF, or symptomatic HF were excluded. Patients with a diagnosis causing life expectancy < 1 year were excluded, which was determined based on review of the patient’s chart or discussion with the PCP.
A clinical pharmacist screened patients with an upcoming PCP appointment between September 2019 and January 2020 for eligibility. For patients who met criteria, the clinical pharmacist ordered a NT-proBNP laboratory test to their already scheduled tests and entered a templated note into the patient’s chart to alert the PCP of the test. NT-proBNP was used rather than BNP because it was the natriuretic peptide laboratory test available at CJZVAMC during this time. Patients with NT-proBNP < 125 pg/mL received usual care from their PCPs. Patients with NT-proBNP ≥ 125 pg/mL received a follow-up phone call from a clinical pharmacist to discuss the laboratory test result with recommendations for initiation or increase of RAS inhibitors and an echocardiogram. If the patient agreed to an echocardiogram, the PCP was notified to order the test. For patients aged > 80 years with elevated NT-proBNP, risk vs benefit and patient-specific goals of care were discussed with the PCP. For patients whose echocardiograms revealed left ventricular dysfunction, initiation or adjustment of β blockers was considered. During RAS inhibitor increase, the clinical pharmacists provided a review of the patient’s risk factors and optimized management of hypertension, T2DM, ASCVD risk reduction, oral nonsteroidal anti-inflammatory drug (NSAID) reduction, and tobacco cessation.
Outcome Measures
Outcome measures included the percentage of patients who met inclusion/exclusion criteria and had an elevated NT-proBNP level, percent change in RAS inhibitor prescriptions and optimized dosing after intervention, frequency of left ventricular dysfunction visualized with echocardiograms, and quantification of pharmacist interventions in disease state management. Descriptive statistics were used to analyze demographic data, RAS inhibitors prescriptions before and after intervention, echocardiogram results, pharmacist recommendations, and acceptance rates of disease state management.
Results
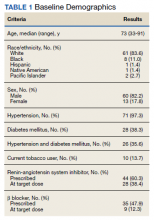
Between September 2019 and January 2020, 570 patients from 2 PACT teams were screened. Of the 570 patients, 246 met inclusion criteria with upcoming appointments. Of these, 24 were excluded, 10 for EF < 50%, 13 for E/e’ > 15 in setting of normal EF, and 1 for hypertension diagnosis without an antihypertensive regimen or elevated blood pressure. The remaining 222 patients had an NT-proBNP level ordered and drawn and 73 (32.9%) patients had an NT-proBNP ≥ 125 pg/mL. Baseline characteristics are described in Table 1.
Data was collected through March 2020 (due to COVID-19) found that among the 73 patients with elevated NT-proBNP: 14 had an echocardiogram within the past year without evidence of left ventricular dysfunction; 39 had echocardiograms ordered; and 19 had echocardiograms completed by March 2020. Among the 19 echocardiograms, 16 (84%) showed no evidence of left ventricular dysfunction, 2 (11%) revealed mildly reduced EF (40% to 50%), and 1 (5%) revealed a reduced EF (< 40%). These patients were identified early in the disease course before symptom onset and received intervention with RAS inhibitors and disease state management.
Patients prescribed RAS inhibitors increased from 44 to 50. The number of patients who were able to have their RAS inhibitor dosage adjusted increased from 28 to 31. For the 3 patients with mildly reduced or reduced EF, management with β blockers was based on RAS inhibitor adjustment toleration. One patient with mildly reduced EF was switched from metoprolol tartrate to metoprolol succinate.
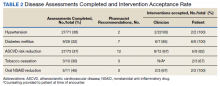
Clinical pharmacists completed disease state assessments to optimize management of hypertension, T2DM, ASCVD risk reduction, oral NSAID reduction, and tobacco cessation (Table 2). Interventions clinical pharmacists recommended for hypertension, in addition to RAS inhibitor management, included initiation and adjustment of amlodipine. For T2DM, interventions included initiation of metformin and initiation or adjustment of empagliflozin. For ASCVD risk reduction, interventions included starting a statin or adjusting statin therapies to appropriate intensities based on clinical ASCVD 10-year risk. Tobacco cessation interventions included pharmacotherapies, counseling, and education with written materials. Pharmacists counseled patients to minimize or eliminate NSAID use and, when appropriate, discontinued active oral NSAID prescriptions.
Discussion
We included patients diagnosed with T2DM and hypertension for several reasons. Most patients (62%) studied in the STOP-HF trial were diagnosed with hypertension. Also, T2DM represented the patient population enrolled in the PONTIAC trial. Guidance from the European Society of Cardiology recommends use of natriuretic peptides in high-risk populations, such as patients with DM and hypertension, to help target initiation of preventive measures.7 Lastly, T2DM and hypertension patients were easily identified using population management software available at the VA.
The percentage of patients in this project with risk factors for HF and an elevated NT-proBNP were similar to the elevated levels described in the STOP-HF trial. In our project, 32.9% of patients had elevated NT-proBNP levels, similar to the 41.6% of patients in STOP-HF. Among the completed echocardiograms, 16% revealed mildly reduced or reduced EF. These patients were identified early in the disease course before symptom onset and received intervention with RAS inhibitors and disease state management.
In addition to early identification of reduced EF, this project allowed a targeted approach to identifying patients for risk factor reduction. Between the 2 PACT teams, 246 patients with T2DM and/or hypertension were seen from September 2019 to January 2020. By using natriuretic peptide screening, the clinical pharmacists were able to prioritize and focus risk factor management on patients at higher risk. Pharmacists were then able to intervene for all risk factors assessed: hypertension, T2DM, ASCVD risk reduction, NSAID use reduction, and tobacco cessation.
During the implementation period, VA criteria of use of the angiotensin receptor-neprilysin inhibitor, sacubitril/valsartan, was restricted to VA cardiology. For patients with reduced EF, it was up to the PCP’s discretion to consult cardiology for further follow-up. In November 2020, the VA removed the restriction to cardiology and PCPs were able to order sacubitril/valsartan. Although not included in the Figure at the time of project implementation, the clinical pharmacist could now transition a patient with reduced EF from a RAS inhibitor to sacubitril/valsartan and adjust to target dosages.
Clinical pharmacists involved in this project had established working relationships with each of the PACT members before project initiation. The PACT employed the clinical pharmacists regularly for chronic disease state management. This facilitated adoption of the natriuretic peptide screening process and PCP buy-in and support. The PCPs agreed to discuss adding a NT-proBNP laboratory test with the patient, when possible, during their in-person appointment and informed the patient that a pharmacist would call if the result was elevated. This warm hand-off facilitated the patient’s reception to the clinical pharmacists’ recommendations after an elevated NT-proBNP result. We also reported PCPs’ high acceptance rate of pharmacist recommendations and interventions for disease state management. These high acceptance rates reflect the established working relationships between clinical pharmacists and the PACT.
Development of templated notes, medication adjustment schedules, and telephone script allowed for consistent implementation into the PACT panels. This process could be duplicated and adopted into other PACTs who want to use a clinical pharmacist to facilitate natriuretic peptide screening and risk factor reduction. The findings from this project can be extrapolated to other team-based care such as the patient-centered medical home model because these programs exhibit many similarities. Both health care models centralize patient care and use interdisciplinary care teams to promote continuity, care coordination, and access to achieve optimized patient outcomes.
Cost was an important factor to consider when implementing this project. With an increase in prescriptions and elective, outpatient echocardiograms, higher outpatient cost is expected. A cost-effectiveness analysis in the STOP-HF trial found an overall cost benefit by reducing the number of patients diagnosed with left ventricular dysfunction or HF and emergency hospitalizations for cardiac events in those who received collaborative care after natriuretic peptide testing.8 These cost savings offset increased outpatient costs.
Limitations
Participants were identified initially through a computer-generated list of patients with hypertension or T2DM without a HF diagnosis documented in their problem list. This problem list is manually updated by PCPs. Although we reviewed records for exclusion criteria, eligible patients might have been excluded. The use and interpretation of an NT-proBNP level is not specific to cardiac disease. Elevations can be seen with increased age, kidney dysfunction, and pulmonary disease. Additionally, an NT-proBNP level might be falsely low in patients who are overweight or obese. Because of the relatively short period of time, we could not analyze associations with HF diagnosis or progression, hospitalizations due to HF, or mortality. Regarding external validity, because of the pre-established interdisciplinary clinic settings and VA pharmacists’ scope of practice with prescriptive authority, implementing this project might have been better received by PCPs and allowed for higher acceptance rates of pharmacist interventions at the VA compared with a community setting.
Conclusions
The ACC/AHA/HFSA guidelines recommended use of natriuretic peptide biomarker screening in conjunction with team-based care for those at risk of developing HF. We describe our process for implementing team-based care using clinical pharmacists in primary care. Our process provides a targeted approach to identifying patients for risk factor reduction through comprehensive medication management and could be replicated by other primary care clinics using a patient-centered medical home model.
Acknowledgments
We would like to acknowledge Dr. Sara Hariman, Dr. Payal Sanghani, and Dr. Cecilia Scholcoff for their support and collaboration with the project.
1. Braunwald E. Heart failure. J Am Coll Cardiol HF. 2013;1(1):1-20. doi: 10.1016/j.jchf.2012.10.002
2. Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606-619. doi:10.1161/HHF.0b013e318291329a
3. Doust J, Lehman R, Glasziou P. The role of BNP testing in heart failure. Am Fam Physician. 2006;74(11):1893-1900.
4. Ledwidge M, Gallagher J, Conlon C, et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA. 2013;310(1):66-74. doi:10.1001/jama.2013.7588
5. Huelsmann M, Neuhold S, Resl M, et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol. 2013;62(15):1365-1372. doi:10.1016/j.jacc.2013.05.069
6. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776-803. doi:10.1016/j.jacc.2017.04.025
7. Mueller C, McDonald K, de Boer RA, et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eu J Heart Fail. 2019;21:715-731. doi:10.1002/ejhf.1494
8. Ledwidge MT, O’Connell E, Gallagher J, et al; Heart Failure Association of the European Society of Cardiology. Cost-effectiveness of natriuretic peptide-based screening and collaborative care: a report from the STOP-HF (St. Vincent’s Screening to Prevent Heart Failure) study. Eur J Heart Fail. 2015;17(7):672-679.
Heart failure (HF) is one of the leading causes of hospitalizations and the most expensive Medicare diagnosis. Its prevalence continues to rise with a projected increase of 46% from 2012 to 2030 resulting in > 8 million people aged ≥ 18 years with HF in the United States. Despite improvements in therapy, mortality remains unacceptably high with a 50% mortality rate within 5 years. Early detection strategies are needed to identify patients at risk of developing HF to delay the disease course and improve survival.1,2
Emerging data indicates that natriuretic peptide biomarker-based screening using B-type natriuretic peptide (BNP) or N-terminal pro-B-type natriuretic peptide (NT-proBNP) and early intervention for patients at risk of HF could prevent development of left ventricular dysfunction or new-onset HF.3-5 The 2013 St. Vincent’s Screening to Prevent Heart Failure (STOP-HF) trial is the largest study to date to evaluate BNP as a screening tool for patients at risk for HF.4 Patients at risk of HF who did not have established left ventricular systolic dysfunction or symptomatic HF were assigned randomly to usual primary care or BNP screening. Patients with BNP levels ≥ 50 pg/mL underwent echocardiogram and were referred to a cardiovascular specialty service for management. The cardiovascular specialty clinic included a team of registered nurses, nurse practitioners, pharmacists, dieticians, palliative care specialists, and cardiologists. Individuals in the intervention group showed increased renin-angiotensin system (RAS) inhibitor use at follow-up (control, 49.6%; intervention, 59.6%; P = .01). All patients received coaching by a nurse who emphasized individual risk, importance of medication adherence, and healthy lifestyle behaviors. After a mean follow-up of 4.2 years, 59 of 677 participants (8.7%) in the control group and 37 of 697 (5.3%) in the intervention group (odds ratio [OR], 0.55; 95% CI, 0.37 to 0.82; P = .003) met the primary end point of left ventricular dysfunction with or without HF. BNP-based screening in conjunction with collaborative care reduced rates of left ventricular dysfunction and HF.
In the 2013 PONTIAC trial, patients with type 2 diabetes mellitus (T2DM) without cardiac disease but with NT-proBNP levels > 125 pg/mL were randomized to usual diabetes care or intensified care at a cardiac outpatient clinic for initiation and increase of RAS inhibitors and β blockers.5 After 2 years, patients randomized to the intensified care group showed a 65% risk reduction of the primary endpoint of hospitalization or death from cardiac disease (P = .04).
Based on this evidence, the 2017 focused update of the American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Failure Society of America (HFSA) guideline for managing HF added a IIa recommendation for natriuretic peptide biomarker screening in those at risk of developing HF.6 The guideline recommends biomarker screening in conjunction with team-based care, including a cardiovascular specialist, and guideline-directed management and therapy to prevent development of left ventricular dysfunction or new-onset HF.
Although ordering a natriuretic peptide biomarker laboratory test is straightforward, the variability of team-based care across institutions and health systems makes it difficult to standardize screening and interventions for patients at risk for HF. We developed and piloted a process using clinical pharmacists in primary care for natriuretic peptide biomarker screening and risk factor reduction within the established patient aligned care team (PACT) framework at a US Department of Veterans Affairs (VA) medical center. In this paper, we describe our implementation process including descriptive preliminary outcomes.
Methods
The PACT team-based approach in primary care clinics is similar to the patient-centered medical home framework. A PACT includes the veteran patient and an interdisciplinary team of health professionals composed of their primary care practitioner (PCP), registered nurse care manager, clinical pharmacist, and other clinical and administrative staff. The PACT clinical pharmacist has prescriptive authority within a scope of practice to provide postdiagnostic chronic disease state management including management of T2DM, hypertension, HF, chronic obstructive pulmonary disease, anticoagulation, tobacco cessation, and atherosclerotic cardiovascular disease (ASCVD) risk reduction. Clinical pharmacists can prescribe and adjust medications and order laboratory tests.
Our institution, Clement J. Zablocki VA Medical Center (CJZVAMC) in Milwaukee, Wisconsin, has a specialty HF clinic that primarily manages ACC/AHA Stage C HF patients. The HF clinic uses a team-based approach to collaborate and coordinate care for the veteran. The HF team is comprised of cardiology specialists, registered nurses, clinical pharmacists, dietitians, and administrative staff. Two PACT clinical pharmacists also staff the HF clinic at CJZVAMC and work collaboratively to initiate, adjust, and optimize veterans’ HF medication regimens.
Two primary care PACT panels were selected for this project. Before implementation, a pharmacy resident and 3 PACT clinical pharmacists (2 of whom also staff the HF clinic) met with a HF cardiology specialist and 2 PACT PCPs to finalize the team-based process and workflow. PCPs were presented with the evidence-based background, purpose, and project design, which included patient identification, NT-proBNP laboratory test ordering, medication adjustment schedules, and protocol for ordering echocardiograms (Figure). Templated notes were created to allow for consistent documentation in patients’ electronic health record. A telephone script also was written for the initial telephone call to patients to explain in patient-friendly terms the implications of an elevated NT-proBNP level, the echocardiogram procedure, and recommendations for risk reduction.
Patient Selection
Patients aged ≥ 18 years with hypertension, taking antihypertensive medication for ≥ 1 month, or diagnosed with T2DM for ≥ 6 months were included. Using the parameters provided in the STOP-HF trial, patients with evidence or history of left ventricular dysfunction, defined as a left ventricular ejection fraction (EF) < 50% or an E/e’ ratio > 15 in the setting of normal EF, or symptomatic HF were excluded. Patients with a diagnosis causing life expectancy < 1 year were excluded, which was determined based on review of the patient’s chart or discussion with the PCP.
A clinical pharmacist screened patients with an upcoming PCP appointment between September 2019 and January 2020 for eligibility. For patients who met criteria, the clinical pharmacist ordered a NT-proBNP laboratory test to their already scheduled tests and entered a templated note into the patient’s chart to alert the PCP of the test. NT-proBNP was used rather than BNP because it was the natriuretic peptide laboratory test available at CJZVAMC during this time. Patients with NT-proBNP < 125 pg/mL received usual care from their PCPs. Patients with NT-proBNP ≥ 125 pg/mL received a follow-up phone call from a clinical pharmacist to discuss the laboratory test result with recommendations for initiation or increase of RAS inhibitors and an echocardiogram. If the patient agreed to an echocardiogram, the PCP was notified to order the test. For patients aged > 80 years with elevated NT-proBNP, risk vs benefit and patient-specific goals of care were discussed with the PCP. For patients whose echocardiograms revealed left ventricular dysfunction, initiation or adjustment of β blockers was considered. During RAS inhibitor increase, the clinical pharmacists provided a review of the patient’s risk factors and optimized management of hypertension, T2DM, ASCVD risk reduction, oral nonsteroidal anti-inflammatory drug (NSAID) reduction, and tobacco cessation.
Outcome Measures
Outcome measures included the percentage of patients who met inclusion/exclusion criteria and had an elevated NT-proBNP level, percent change in RAS inhibitor prescriptions and optimized dosing after intervention, frequency of left ventricular dysfunction visualized with echocardiograms, and quantification of pharmacist interventions in disease state management. Descriptive statistics were used to analyze demographic data, RAS inhibitors prescriptions before and after intervention, echocardiogram results, pharmacist recommendations, and acceptance rates of disease state management.
Results
Between September 2019 and January 2020, 570 patients from 2 PACT teams were screened. Of the 570 patients, 246 met inclusion criteria with upcoming appointments. Of these, 24 were excluded, 10 for EF < 50%, 13 for E/e’ > 15 in setting of normal EF, and 1 for hypertension diagnosis without an antihypertensive regimen or elevated blood pressure. The remaining 222 patients had an NT-proBNP level ordered and drawn and 73 (32.9%) patients had an NT-proBNP ≥ 125 pg/mL. Baseline characteristics are described in Table 1.
Data was collected through March 2020 (due to COVID-19) found that among the 73 patients with elevated NT-proBNP: 14 had an echocardiogram within the past year without evidence of left ventricular dysfunction; 39 had echocardiograms ordered; and 19 had echocardiograms completed by March 2020. Among the 19 echocardiograms, 16 (84%) showed no evidence of left ventricular dysfunction, 2 (11%) revealed mildly reduced EF (40% to 50%), and 1 (5%) revealed a reduced EF (< 40%). These patients were identified early in the disease course before symptom onset and received intervention with RAS inhibitors and disease state management.
Patients prescribed RAS inhibitors increased from 44 to 50. The number of patients who were able to have their RAS inhibitor dosage adjusted increased from 28 to 31. For the 3 patients with mildly reduced or reduced EF, management with β blockers was based on RAS inhibitor adjustment toleration. One patient with mildly reduced EF was switched from metoprolol tartrate to metoprolol succinate.
Clinical pharmacists completed disease state assessments to optimize management of hypertension, T2DM, ASCVD risk reduction, oral NSAID reduction, and tobacco cessation (Table 2). Interventions clinical pharmacists recommended for hypertension, in addition to RAS inhibitor management, included initiation and adjustment of amlodipine. For T2DM, interventions included initiation of metformin and initiation or adjustment of empagliflozin. For ASCVD risk reduction, interventions included starting a statin or adjusting statin therapies to appropriate intensities based on clinical ASCVD 10-year risk. Tobacco cessation interventions included pharmacotherapies, counseling, and education with written materials. Pharmacists counseled patients to minimize or eliminate NSAID use and, when appropriate, discontinued active oral NSAID prescriptions.
Discussion
We included patients diagnosed with T2DM and hypertension for several reasons. Most patients (62%) studied in the STOP-HF trial were diagnosed with hypertension. Also, T2DM represented the patient population enrolled in the PONTIAC trial. Guidance from the European Society of Cardiology recommends use of natriuretic peptides in high-risk populations, such as patients with DM and hypertension, to help target initiation of preventive measures.7 Lastly, T2DM and hypertension patients were easily identified using population management software available at the VA.
The percentage of patients in this project with risk factors for HF and an elevated NT-proBNP were similar to the elevated levels described in the STOP-HF trial. In our project, 32.9% of patients had elevated NT-proBNP levels, similar to the 41.6% of patients in STOP-HF. Among the completed echocardiograms, 16% revealed mildly reduced or reduced EF. These patients were identified early in the disease course before symptom onset and received intervention with RAS inhibitors and disease state management.
In addition to early identification of reduced EF, this project allowed a targeted approach to identifying patients for risk factor reduction. Between the 2 PACT teams, 246 patients with T2DM and/or hypertension were seen from September 2019 to January 2020. By using natriuretic peptide screening, the clinical pharmacists were able to prioritize and focus risk factor management on patients at higher risk. Pharmacists were then able to intervene for all risk factors assessed: hypertension, T2DM, ASCVD risk reduction, NSAID use reduction, and tobacco cessation.
During the implementation period, VA criteria of use of the angiotensin receptor-neprilysin inhibitor, sacubitril/valsartan, was restricted to VA cardiology. For patients with reduced EF, it was up to the PCP’s discretion to consult cardiology for further follow-up. In November 2020, the VA removed the restriction to cardiology and PCPs were able to order sacubitril/valsartan. Although not included in the Figure at the time of project implementation, the clinical pharmacist could now transition a patient with reduced EF from a RAS inhibitor to sacubitril/valsartan and adjust to target dosages.
Clinical pharmacists involved in this project had established working relationships with each of the PACT members before project initiation. The PACT employed the clinical pharmacists regularly for chronic disease state management. This facilitated adoption of the natriuretic peptide screening process and PCP buy-in and support. The PCPs agreed to discuss adding a NT-proBNP laboratory test with the patient, when possible, during their in-person appointment and informed the patient that a pharmacist would call if the result was elevated. This warm hand-off facilitated the patient’s reception to the clinical pharmacists’ recommendations after an elevated NT-proBNP result. We also reported PCPs’ high acceptance rate of pharmacist recommendations and interventions for disease state management. These high acceptance rates reflect the established working relationships between clinical pharmacists and the PACT.
Development of templated notes, medication adjustment schedules, and telephone script allowed for consistent implementation into the PACT panels. This process could be duplicated and adopted into other PACTs who want to use a clinical pharmacist to facilitate natriuretic peptide screening and risk factor reduction. The findings from this project can be extrapolated to other team-based care such as the patient-centered medical home model because these programs exhibit many similarities. Both health care models centralize patient care and use interdisciplinary care teams to promote continuity, care coordination, and access to achieve optimized patient outcomes.
Cost was an important factor to consider when implementing this project. With an increase in prescriptions and elective, outpatient echocardiograms, higher outpatient cost is expected. A cost-effectiveness analysis in the STOP-HF trial found an overall cost benefit by reducing the number of patients diagnosed with left ventricular dysfunction or HF and emergency hospitalizations for cardiac events in those who received collaborative care after natriuretic peptide testing.8 These cost savings offset increased outpatient costs.
Limitations
Participants were identified initially through a computer-generated list of patients with hypertension or T2DM without a HF diagnosis documented in their problem list. This problem list is manually updated by PCPs. Although we reviewed records for exclusion criteria, eligible patients might have been excluded. The use and interpretation of an NT-proBNP level is not specific to cardiac disease. Elevations can be seen with increased age, kidney dysfunction, and pulmonary disease. Additionally, an NT-proBNP level might be falsely low in patients who are overweight or obese. Because of the relatively short period of time, we could not analyze associations with HF diagnosis or progression, hospitalizations due to HF, or mortality. Regarding external validity, because of the pre-established interdisciplinary clinic settings and VA pharmacists’ scope of practice with prescriptive authority, implementing this project might have been better received by PCPs and allowed for higher acceptance rates of pharmacist interventions at the VA compared with a community setting.
Conclusions
The ACC/AHA/HFSA guidelines recommended use of natriuretic peptide biomarker screening in conjunction with team-based care for those at risk of developing HF. We describe our process for implementing team-based care using clinical pharmacists in primary care. Our process provides a targeted approach to identifying patients for risk factor reduction through comprehensive medication management and could be replicated by other primary care clinics using a patient-centered medical home model.
Acknowledgments
We would like to acknowledge Dr. Sara Hariman, Dr. Payal Sanghani, and Dr. Cecilia Scholcoff for their support and collaboration with the project.
Heart failure (HF) is one of the leading causes of hospitalizations and the most expensive Medicare diagnosis. Its prevalence continues to rise with a projected increase of 46% from 2012 to 2030 resulting in > 8 million people aged ≥ 18 years with HF in the United States. Despite improvements in therapy, mortality remains unacceptably high with a 50% mortality rate within 5 years. Early detection strategies are needed to identify patients at risk of developing HF to delay the disease course and improve survival.1,2
Emerging data indicates that natriuretic peptide biomarker-based screening using B-type natriuretic peptide (BNP) or N-terminal pro-B-type natriuretic peptide (NT-proBNP) and early intervention for patients at risk of HF could prevent development of left ventricular dysfunction or new-onset HF.3-5 The 2013 St. Vincent’s Screening to Prevent Heart Failure (STOP-HF) trial is the largest study to date to evaluate BNP as a screening tool for patients at risk for HF.4 Patients at risk of HF who did not have established left ventricular systolic dysfunction or symptomatic HF were assigned randomly to usual primary care or BNP screening. Patients with BNP levels ≥ 50 pg/mL underwent echocardiogram and were referred to a cardiovascular specialty service for management. The cardiovascular specialty clinic included a team of registered nurses, nurse practitioners, pharmacists, dieticians, palliative care specialists, and cardiologists. Individuals in the intervention group showed increased renin-angiotensin system (RAS) inhibitor use at follow-up (control, 49.6%; intervention, 59.6%; P = .01). All patients received coaching by a nurse who emphasized individual risk, importance of medication adherence, and healthy lifestyle behaviors. After a mean follow-up of 4.2 years, 59 of 677 participants (8.7%) in the control group and 37 of 697 (5.3%) in the intervention group (odds ratio [OR], 0.55; 95% CI, 0.37 to 0.82; P = .003) met the primary end point of left ventricular dysfunction with or without HF. BNP-based screening in conjunction with collaborative care reduced rates of left ventricular dysfunction and HF.
In the 2013 PONTIAC trial, patients with type 2 diabetes mellitus (T2DM) without cardiac disease but with NT-proBNP levels > 125 pg/mL were randomized to usual diabetes care or intensified care at a cardiac outpatient clinic for initiation and increase of RAS inhibitors and β blockers.5 After 2 years, patients randomized to the intensified care group showed a 65% risk reduction of the primary endpoint of hospitalization or death from cardiac disease (P = .04).
Based on this evidence, the 2017 focused update of the American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Failure Society of America (HFSA) guideline for managing HF added a IIa recommendation for natriuretic peptide biomarker screening in those at risk of developing HF.6 The guideline recommends biomarker screening in conjunction with team-based care, including a cardiovascular specialist, and guideline-directed management and therapy to prevent development of left ventricular dysfunction or new-onset HF.
Although ordering a natriuretic peptide biomarker laboratory test is straightforward, the variability of team-based care across institutions and health systems makes it difficult to standardize screening and interventions for patients at risk for HF. We developed and piloted a process using clinical pharmacists in primary care for natriuretic peptide biomarker screening and risk factor reduction within the established patient aligned care team (PACT) framework at a US Department of Veterans Affairs (VA) medical center. In this paper, we describe our implementation process including descriptive preliminary outcomes.
Methods
The PACT team-based approach in primary care clinics is similar to the patient-centered medical home framework. A PACT includes the veteran patient and an interdisciplinary team of health professionals composed of their primary care practitioner (PCP), registered nurse care manager, clinical pharmacist, and other clinical and administrative staff. The PACT clinical pharmacist has prescriptive authority within a scope of practice to provide postdiagnostic chronic disease state management including management of T2DM, hypertension, HF, chronic obstructive pulmonary disease, anticoagulation, tobacco cessation, and atherosclerotic cardiovascular disease (ASCVD) risk reduction. Clinical pharmacists can prescribe and adjust medications and order laboratory tests.
Our institution, Clement J. Zablocki VA Medical Center (CJZVAMC) in Milwaukee, Wisconsin, has a specialty HF clinic that primarily manages ACC/AHA Stage C HF patients. The HF clinic uses a team-based approach to collaborate and coordinate care for the veteran. The HF team is comprised of cardiology specialists, registered nurses, clinical pharmacists, dietitians, and administrative staff. Two PACT clinical pharmacists also staff the HF clinic at CJZVAMC and work collaboratively to initiate, adjust, and optimize veterans’ HF medication regimens.
Two primary care PACT panels were selected for this project. Before implementation, a pharmacy resident and 3 PACT clinical pharmacists (2 of whom also staff the HF clinic) met with a HF cardiology specialist and 2 PACT PCPs to finalize the team-based process and workflow. PCPs were presented with the evidence-based background, purpose, and project design, which included patient identification, NT-proBNP laboratory test ordering, medication adjustment schedules, and protocol for ordering echocardiograms (Figure). Templated notes were created to allow for consistent documentation in patients’ electronic health record. A telephone script also was written for the initial telephone call to patients to explain in patient-friendly terms the implications of an elevated NT-proBNP level, the echocardiogram procedure, and recommendations for risk reduction.
Patient Selection
Patients aged ≥ 18 years with hypertension, taking antihypertensive medication for ≥ 1 month, or diagnosed with T2DM for ≥ 6 months were included. Using the parameters provided in the STOP-HF trial, patients with evidence or history of left ventricular dysfunction, defined as a left ventricular ejection fraction (EF) < 50% or an E/e’ ratio > 15 in the setting of normal EF, or symptomatic HF were excluded. Patients with a diagnosis causing life expectancy < 1 year were excluded, which was determined based on review of the patient’s chart or discussion with the PCP.
A clinical pharmacist screened patients with an upcoming PCP appointment between September 2019 and January 2020 for eligibility. For patients who met criteria, the clinical pharmacist ordered a NT-proBNP laboratory test to their already scheduled tests and entered a templated note into the patient’s chart to alert the PCP of the test. NT-proBNP was used rather than BNP because it was the natriuretic peptide laboratory test available at CJZVAMC during this time. Patients with NT-proBNP < 125 pg/mL received usual care from their PCPs. Patients with NT-proBNP ≥ 125 pg/mL received a follow-up phone call from a clinical pharmacist to discuss the laboratory test result with recommendations for initiation or increase of RAS inhibitors and an echocardiogram. If the patient agreed to an echocardiogram, the PCP was notified to order the test. For patients aged > 80 years with elevated NT-proBNP, risk vs benefit and patient-specific goals of care were discussed with the PCP. For patients whose echocardiograms revealed left ventricular dysfunction, initiation or adjustment of β blockers was considered. During RAS inhibitor increase, the clinical pharmacists provided a review of the patient’s risk factors and optimized management of hypertension, T2DM, ASCVD risk reduction, oral nonsteroidal anti-inflammatory drug (NSAID) reduction, and tobacco cessation.
Outcome Measures
Outcome measures included the percentage of patients who met inclusion/exclusion criteria and had an elevated NT-proBNP level, percent change in RAS inhibitor prescriptions and optimized dosing after intervention, frequency of left ventricular dysfunction visualized with echocardiograms, and quantification of pharmacist interventions in disease state management. Descriptive statistics were used to analyze demographic data, RAS inhibitors prescriptions before and after intervention, echocardiogram results, pharmacist recommendations, and acceptance rates of disease state management.
Results
Between September 2019 and January 2020, 570 patients from 2 PACT teams were screened. Of the 570 patients, 246 met inclusion criteria with upcoming appointments. Of these, 24 were excluded, 10 for EF < 50%, 13 for E/e’ > 15 in setting of normal EF, and 1 for hypertension diagnosis without an antihypertensive regimen or elevated blood pressure. The remaining 222 patients had an NT-proBNP level ordered and drawn and 73 (32.9%) patients had an NT-proBNP ≥ 125 pg/mL. Baseline characteristics are described in Table 1.
Data was collected through March 2020 (due to COVID-19) found that among the 73 patients with elevated NT-proBNP: 14 had an echocardiogram within the past year without evidence of left ventricular dysfunction; 39 had echocardiograms ordered; and 19 had echocardiograms completed by March 2020. Among the 19 echocardiograms, 16 (84%) showed no evidence of left ventricular dysfunction, 2 (11%) revealed mildly reduced EF (40% to 50%), and 1 (5%) revealed a reduced EF (< 40%). These patients were identified early in the disease course before symptom onset and received intervention with RAS inhibitors and disease state management.
Patients prescribed RAS inhibitors increased from 44 to 50. The number of patients who were able to have their RAS inhibitor dosage adjusted increased from 28 to 31. For the 3 patients with mildly reduced or reduced EF, management with β blockers was based on RAS inhibitor adjustment toleration. One patient with mildly reduced EF was switched from metoprolol tartrate to metoprolol succinate.
Clinical pharmacists completed disease state assessments to optimize management of hypertension, T2DM, ASCVD risk reduction, oral NSAID reduction, and tobacco cessation (Table 2). Interventions clinical pharmacists recommended for hypertension, in addition to RAS inhibitor management, included initiation and adjustment of amlodipine. For T2DM, interventions included initiation of metformin and initiation or adjustment of empagliflozin. For ASCVD risk reduction, interventions included starting a statin or adjusting statin therapies to appropriate intensities based on clinical ASCVD 10-year risk. Tobacco cessation interventions included pharmacotherapies, counseling, and education with written materials. Pharmacists counseled patients to minimize or eliminate NSAID use and, when appropriate, discontinued active oral NSAID prescriptions.
Discussion
We included patients diagnosed with T2DM and hypertension for several reasons. Most patients (62%) studied in the STOP-HF trial were diagnosed with hypertension. Also, T2DM represented the patient population enrolled in the PONTIAC trial. Guidance from the European Society of Cardiology recommends use of natriuretic peptides in high-risk populations, such as patients with DM and hypertension, to help target initiation of preventive measures.7 Lastly, T2DM and hypertension patients were easily identified using population management software available at the VA.
The percentage of patients in this project with risk factors for HF and an elevated NT-proBNP were similar to the elevated levels described in the STOP-HF trial. In our project, 32.9% of patients had elevated NT-proBNP levels, similar to the 41.6% of patients in STOP-HF. Among the completed echocardiograms, 16% revealed mildly reduced or reduced EF. These patients were identified early in the disease course before symptom onset and received intervention with RAS inhibitors and disease state management.
In addition to early identification of reduced EF, this project allowed a targeted approach to identifying patients for risk factor reduction. Between the 2 PACT teams, 246 patients with T2DM and/or hypertension were seen from September 2019 to January 2020. By using natriuretic peptide screening, the clinical pharmacists were able to prioritize and focus risk factor management on patients at higher risk. Pharmacists were then able to intervene for all risk factors assessed: hypertension, T2DM, ASCVD risk reduction, NSAID use reduction, and tobacco cessation.
During the implementation period, VA criteria of use of the angiotensin receptor-neprilysin inhibitor, sacubitril/valsartan, was restricted to VA cardiology. For patients with reduced EF, it was up to the PCP’s discretion to consult cardiology for further follow-up. In November 2020, the VA removed the restriction to cardiology and PCPs were able to order sacubitril/valsartan. Although not included in the Figure at the time of project implementation, the clinical pharmacist could now transition a patient with reduced EF from a RAS inhibitor to sacubitril/valsartan and adjust to target dosages.
Clinical pharmacists involved in this project had established working relationships with each of the PACT members before project initiation. The PACT employed the clinical pharmacists regularly for chronic disease state management. This facilitated adoption of the natriuretic peptide screening process and PCP buy-in and support. The PCPs agreed to discuss adding a NT-proBNP laboratory test with the patient, when possible, during their in-person appointment and informed the patient that a pharmacist would call if the result was elevated. This warm hand-off facilitated the patient’s reception to the clinical pharmacists’ recommendations after an elevated NT-proBNP result. We also reported PCPs’ high acceptance rate of pharmacist recommendations and interventions for disease state management. These high acceptance rates reflect the established working relationships between clinical pharmacists and the PACT.
Development of templated notes, medication adjustment schedules, and telephone script allowed for consistent implementation into the PACT panels. This process could be duplicated and adopted into other PACTs who want to use a clinical pharmacist to facilitate natriuretic peptide screening and risk factor reduction. The findings from this project can be extrapolated to other team-based care such as the patient-centered medical home model because these programs exhibit many similarities. Both health care models centralize patient care and use interdisciplinary care teams to promote continuity, care coordination, and access to achieve optimized patient outcomes.
Cost was an important factor to consider when implementing this project. With an increase in prescriptions and elective, outpatient echocardiograms, higher outpatient cost is expected. A cost-effectiveness analysis in the STOP-HF trial found an overall cost benefit by reducing the number of patients diagnosed with left ventricular dysfunction or HF and emergency hospitalizations for cardiac events in those who received collaborative care after natriuretic peptide testing.8 These cost savings offset increased outpatient costs.
Limitations
Participants were identified initially through a computer-generated list of patients with hypertension or T2DM without a HF diagnosis documented in their problem list. This problem list is manually updated by PCPs. Although we reviewed records for exclusion criteria, eligible patients might have been excluded. The use and interpretation of an NT-proBNP level is not specific to cardiac disease. Elevations can be seen with increased age, kidney dysfunction, and pulmonary disease. Additionally, an NT-proBNP level might be falsely low in patients who are overweight or obese. Because of the relatively short period of time, we could not analyze associations with HF diagnosis or progression, hospitalizations due to HF, or mortality. Regarding external validity, because of the pre-established interdisciplinary clinic settings and VA pharmacists’ scope of practice with prescriptive authority, implementing this project might have been better received by PCPs and allowed for higher acceptance rates of pharmacist interventions at the VA compared with a community setting.
Conclusions
The ACC/AHA/HFSA guidelines recommended use of natriuretic peptide biomarker screening in conjunction with team-based care for those at risk of developing HF. We describe our process for implementing team-based care using clinical pharmacists in primary care. Our process provides a targeted approach to identifying patients for risk factor reduction through comprehensive medication management and could be replicated by other primary care clinics using a patient-centered medical home model.
Acknowledgments
We would like to acknowledge Dr. Sara Hariman, Dr. Payal Sanghani, and Dr. Cecilia Scholcoff for their support and collaboration with the project.
1. Braunwald E. Heart failure. J Am Coll Cardiol HF. 2013;1(1):1-20. doi: 10.1016/j.jchf.2012.10.002
2. Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606-619. doi:10.1161/HHF.0b013e318291329a
3. Doust J, Lehman R, Glasziou P. The role of BNP testing in heart failure. Am Fam Physician. 2006;74(11):1893-1900.
4. Ledwidge M, Gallagher J, Conlon C, et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA. 2013;310(1):66-74. doi:10.1001/jama.2013.7588
5. Huelsmann M, Neuhold S, Resl M, et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol. 2013;62(15):1365-1372. doi:10.1016/j.jacc.2013.05.069
6. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776-803. doi:10.1016/j.jacc.2017.04.025
7. Mueller C, McDonald K, de Boer RA, et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eu J Heart Fail. 2019;21:715-731. doi:10.1002/ejhf.1494
8. Ledwidge MT, O’Connell E, Gallagher J, et al; Heart Failure Association of the European Society of Cardiology. Cost-effectiveness of natriuretic peptide-based screening and collaborative care: a report from the STOP-HF (St. Vincent’s Screening to Prevent Heart Failure) study. Eur J Heart Fail. 2015;17(7):672-679.
1. Braunwald E. Heart failure. J Am Coll Cardiol HF. 2013;1(1):1-20. doi: 10.1016/j.jchf.2012.10.002
2. Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606-619. doi:10.1161/HHF.0b013e318291329a
3. Doust J, Lehman R, Glasziou P. The role of BNP testing in heart failure. Am Fam Physician. 2006;74(11):1893-1900.
4. Ledwidge M, Gallagher J, Conlon C, et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA. 2013;310(1):66-74. doi:10.1001/jama.2013.7588
5. Huelsmann M, Neuhold S, Resl M, et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol. 2013;62(15):1365-1372. doi:10.1016/j.jacc.2013.05.069
6. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776-803. doi:10.1016/j.jacc.2017.04.025
7. Mueller C, McDonald K, de Boer RA, et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eu J Heart Fail. 2019;21:715-731. doi:10.1002/ejhf.1494
8. Ledwidge MT, O’Connell E, Gallagher J, et al; Heart Failure Association of the European Society of Cardiology. Cost-effectiveness of natriuretic peptide-based screening and collaborative care: a report from the STOP-HF (St. Vincent’s Screening to Prevent Heart Failure) study. Eur J Heart Fail. 2015;17(7):672-679.