User login
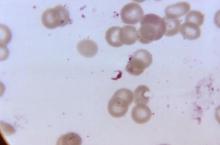
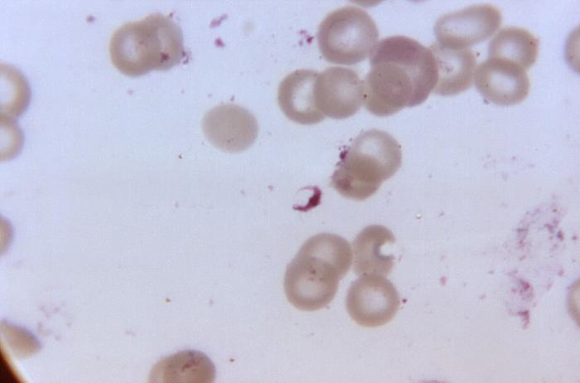
Recent research suggests the novel antimalarial agent KAF156 is effective without visible safety concerns in adults with uncomplicated Plasmodium vivax or P. falciparum malaria, according to a study published in the New England Journal of Medicine.
From March to August 2013, 21 adults with acute uncomplicated malaria (11 with P. vivax malaria and 10 with P. falciparum malaria) were enrolled in multiple-dose cohorts (400 mg of KAF156 given once daily for 3 days). A third cohort of patients was treated with a single 800-mg dose of KAF156 in order to assess the cure rate at 28 days and the potential for a single-dose cure.
Among the 21 patients with P. falciparum malaria who received the single 800-mg dose and were followed for 28 days, 1 had reinfection and 7 had recrudescent infections (cure rate, 67%). Gametocytemia was detected in two of the patients with P. vivax malaria at baseline and cleared in both patients within 16 hours after receipt of KAF156. In the patients with P. falciparum malaria, one patient had gametocytemia from baseline to 54 hours after receiving the dose and one had intermittent gametocytemia from baseline until 72 hours after dose administration.
The investigators also reported two patients had posttreatment gametocytemia – one had a single positive reading at 24 hours, and the other had positive readings from 12 to 96 hours, at which time sampling finished. Most patients had at least one adverse event, although no grade 4 or serious adverse events were noted. Overall, there were more adverse events after the single 800-mg dose than after multiple 400-mg doses.
“New antimalarial drugs are needed as artemisinin resistance spreads in Southeast Asia and partner-drug resistance follows.” the researchers concluded. “Our study showed that KAF156, a new antimalarial drug, has activity against vivax and falciparum malaria, including artemisinin-resistant parasites.”
Read the full study in the New England Journal of Medicine (2016 Sep. 21. doi: 10.1056/NEJMoa1602250).
Recent research suggests the novel antimalarial agent KAF156 is effective without visible safety concerns in adults with uncomplicated Plasmodium vivax or P. falciparum malaria, according to a study published in the New England Journal of Medicine.
From March to August 2013, 21 adults with acute uncomplicated malaria (11 with P. vivax malaria and 10 with P. falciparum malaria) were enrolled in multiple-dose cohorts (400 mg of KAF156 given once daily for 3 days). A third cohort of patients was treated with a single 800-mg dose of KAF156 in order to assess the cure rate at 28 days and the potential for a single-dose cure.
Among the 21 patients with P. falciparum malaria who received the single 800-mg dose and were followed for 28 days, 1 had reinfection and 7 had recrudescent infections (cure rate, 67%). Gametocytemia was detected in two of the patients with P. vivax malaria at baseline and cleared in both patients within 16 hours after receipt of KAF156. In the patients with P. falciparum malaria, one patient had gametocytemia from baseline to 54 hours after receiving the dose and one had intermittent gametocytemia from baseline until 72 hours after dose administration.
The investigators also reported two patients had posttreatment gametocytemia – one had a single positive reading at 24 hours, and the other had positive readings from 12 to 96 hours, at which time sampling finished. Most patients had at least one adverse event, although no grade 4 or serious adverse events were noted. Overall, there were more adverse events after the single 800-mg dose than after multiple 400-mg doses.
“New antimalarial drugs are needed as artemisinin resistance spreads in Southeast Asia and partner-drug resistance follows.” the researchers concluded. “Our study showed that KAF156, a new antimalarial drug, has activity against vivax and falciparum malaria, including artemisinin-resistant parasites.”
Read the full study in the New England Journal of Medicine (2016 Sep. 21. doi: 10.1056/NEJMoa1602250).
Recent research suggests the novel antimalarial agent KAF156 is effective without visible safety concerns in adults with uncomplicated Plasmodium vivax or P. falciparum malaria, according to a study published in the New England Journal of Medicine.
From March to August 2013, 21 adults with acute uncomplicated malaria (11 with P. vivax malaria and 10 with P. falciparum malaria) were enrolled in multiple-dose cohorts (400 mg of KAF156 given once daily for 3 days). A third cohort of patients was treated with a single 800-mg dose of KAF156 in order to assess the cure rate at 28 days and the potential for a single-dose cure.
Among the 21 patients with P. falciparum malaria who received the single 800-mg dose and were followed for 28 days, 1 had reinfection and 7 had recrudescent infections (cure rate, 67%). Gametocytemia was detected in two of the patients with P. vivax malaria at baseline and cleared in both patients within 16 hours after receipt of KAF156. In the patients with P. falciparum malaria, one patient had gametocytemia from baseline to 54 hours after receiving the dose and one had intermittent gametocytemia from baseline until 72 hours after dose administration.
The investigators also reported two patients had posttreatment gametocytemia – one had a single positive reading at 24 hours, and the other had positive readings from 12 to 96 hours, at which time sampling finished. Most patients had at least one adverse event, although no grade 4 or serious adverse events were noted. Overall, there were more adverse events after the single 800-mg dose than after multiple 400-mg doses.
“New antimalarial drugs are needed as artemisinin resistance spreads in Southeast Asia and partner-drug resistance follows.” the researchers concluded. “Our study showed that KAF156, a new antimalarial drug, has activity against vivax and falciparum malaria, including artemisinin-resistant parasites.”
Read the full study in the New England Journal of Medicine (2016 Sep. 21. doi: 10.1056/NEJMoa1602250).