User login
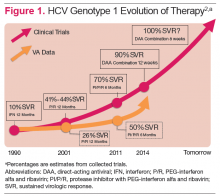
Hepatitis C virus (HCV) infection remains a significant problem in the VA system, with over 174,000 current actively infected patients.1 Despite the availability of antiviral treatment since the early 1990’s, only approximately 26% of patients have ever been treated. These treatments required the use of pegylated interferon alfa (PEG) and ribavirin (RBV), which are associated with significant adverse events (AEs) that prevented many from receiving the treatment. Of those treated, only a minority achieved a sustained virologic response (SVR), due to the limited efficacy of the treatments (Figure 1).2 With the advent of new direct-acting antiviral (DAA) treatments in 2011, treatment efficacy improved.
The first DAAs in use were the viral nonstructural protein 3/4A (NS3/4A) serine protease inhibitors (PIs) boceprevir and telaprevir, which were used with PEG and RBV for patients with HCV genotype 1 infection. This combination therapy improved SVR rates from about 26% to 50% in patients with HCV genotype 1 in the VA.3,4 However, due to the significant AEs with these combinations, relatively few patients were treated.
In late 2013, the FDA approved other DAAs, which allowed patients to be treated effectively without PEG. These included the nucleotide nonstructural protein 5B (NS5B) polymerase inhibitor sofosbuvir and a secondgeneration NS3/4A PI simeprevir.5-7 The first nucleotide analog NS5B polymerase inhibitor, sofosbuvir and the nonstructural protein 5A (NS5A) replication complex inhibitor, ledipasvir, was approved in October 2014.8-10 The recent developments in noninterferon treatments have been accompanied by revised treatment guidelines or recommendations by major professional societies. Current treatment recommendations will be reviewed here, but the recommendations will continue to evolve as new DAAs come to market.
DAA Sites of Action
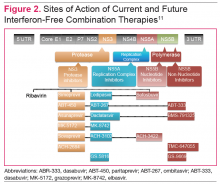
The HCV genome is a positive-stranded RNA molecule of about 9,500 nucleotides, which encodes a polyprotein of approximately 3,000 amino acids that form 10 individual viral proteins. These are composed of both structural and nonstructural (NS) proteins that are responsible for replication of the genome and formation of new viral particles. Understanding of the HCV-encoded proteins and their functions has permitted the development of different DAA therapies. In general, targeting a single protein is not effective, and combination therapy targeting 2 proteins is required for viral eradication (Figure 2).11 The 3 drug targets that are currently available include NS3/4A serine PIs (eg, simeprevir, boceprevir, telaprevir), NS5A replication complex inhibitors (eg, ledipasvir, daclatasvir), and NS5B RNA-dependent RNA polymerase inhibitors (eg, sofosbuvir).
Other DAA’s are in development that have targets in host rather than viral cells. These include cycolphilin A inhibitors and the micro-RNA (miR-122) antagonist miravirsen.12,13
The RNA-dependent RNA polymerase, encoded by the HCV NS5B is targeted by 2 classes of inhibitors: nucleoside or nucleotide analog inhibitors (NIs), and non-nucleoside inhibitors (NNIs).11 The only NI of the NS5B protein approved by the FDA is sofosbuvir. The resistance profiles of NIs and NNIs differ, because they bind to distinct sites on the NS5B protein. NIs are analogs of natural substrates and bind to the active site of the RNA polymerase, whereas NNIs are allosteric site inhibitors. NIs have activity in vitro against all HCV genotypes and have high barrier to resistance as the active site of NS5B polymerase is less tolerant of different amino acid substitutions.
In vitro studies have demonstrated that NIs are less likely to select for mutations compared with NNIs and PIs. The NNIs have limited genotypic coverage and have a lower barrier to resistance. Strategies for targeting HCV proteins include using a NI NS5B protein inhibitor as the backbone with a high barrier to resistance in combination with 1 or 2 other DAAs with lower barriers to resistance, or the combination of 3 DAAs with lower barriers to resistance.11 Ribavirin has broad-spectrum antiviral activity, one of which is anti-HCV activity. The mode of action of RBV against HCV is not well understood, but several mechanisms have been proposed, one of which is via inhibition of viral-dependent RNA polymerase.
Current HCV Treatment Recommendations
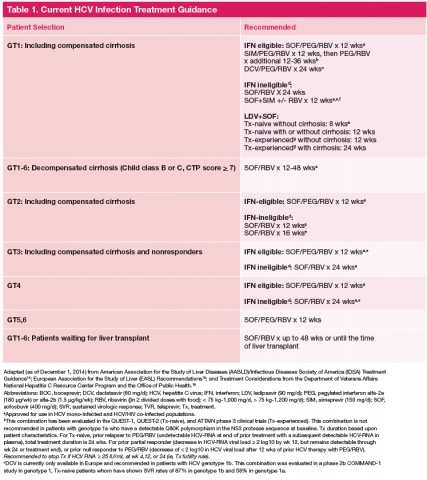
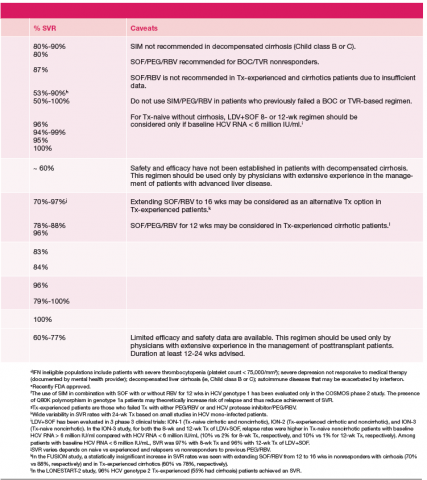
Current treatment recommendations are available from the American Association for the Study of the Liver Disease (AASLD) and the Infectious Diseases Society of America (IDSA) (http://www.hcvguidelines.org); the VA National Hepatitis C Resource Center Program and Office of Public Health (http://www.hepatitis.va.gov/pdf/2014hcv.pdf); and the European Association for the Study of the Liver (EASL) (http://www.easl.eu/_newsroom/latest-news/easl-recommendations-on-treatment-of-hepatitis-c-2014). These recommendations are updated frequently as new drugs enter the marketplace (Table 1).14-16
Since most patients are unable or unwilling to tolerate interferon, the majority of patients in treatment are currently receiving interferon-free combinations. Treatment of genotype 1 is currently dominated by the offlabel use of sofosbuvir in combination with simeprevir. Data have been published from a single phase II trial in patients with and without cirrhosis.7 Of note are emerging data from observational studies confirming the efficacy of over 80% SVR in patients with cirrhosis and genotype 1a with or without prior treatment.17 Treatment of patients with genotype 2 infection is dominated by the use of sofosbuvir and RBV combination. However, patients with cirrhosis do not respond as well as patients without cirrhosis, and it remains to be seen whether extending therapy is of any benefit.
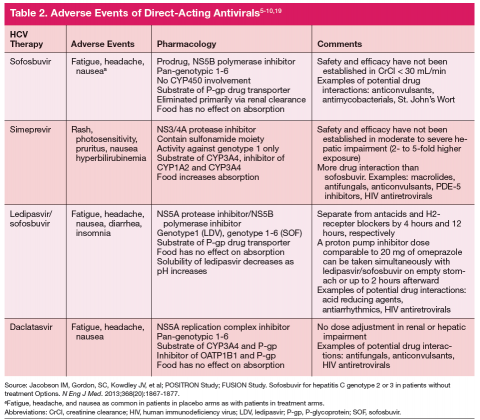
One exception for the use of PEG is the recommendation for patients with HCV genotype 3 and cirrhosis to consider the combination of PEG, RBV, and sofosbuvir for 12 weeks.18 This has the advantage of being more effective, less costly, and shorter treatment duration compared with the sofosbuvir and RBV association but is only appropriate for patients who can tolerate interferon. Common AEs and potential contraindications to treatment are listed in Table 2.5-10,19
New Treatment Options for HCV in 2015
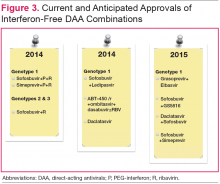
Figure 3 details DAA combinations currently in phase III trials that are expected to receive approval in 2015. This will expand the repertoire of drug combinations available and enable fine-tuning of regimens according to patient and viral characteristics and cost requirements.
Cost and Effectiveness
Cost-effectiveness issues are of immediate importance for health care systems and payors. The first generation PIs, boceprevir and telaprevir, were used in combination with PEG and RBV and entailed pharmacy costs on par with current noninterferon regimens.20,21 Studies generally demonstrated that these treatments are cost-effective, especially in patients with advanced fibrosis. Ollendorf and colleagues recently published an analysis of the costs of using interferon-free regimens for treatment of 540,000 patients with chronic HCV in California.22 Assuming that 50% of patients would present for treatment, the cost of the new DAAs would be immense and result in an increase in costs from $12 billion to $34 billion in the first year and net costs of $6 billion by the 20th year. If treatment were limited to patients with advanced cirrhosis, the first year costs would be increased by $7 billion, but at 20 years there would be approximately $1 billion in net cost savings.
For current treatments, a 12-week course of simeprevir/sofosbuvir has been shown to be more costeffective than 24 weeks of sofosbuvir/RBV for treatment
of genotype 1.23 Similarly, patients with genotype 1, no cirrhosis, and low viral load can be treated with 8 weeks of sofosbuvir/ledipasvir rather than other 12-week regimens, thereby reducing drug costs. The resources needed for upfront treatment of patients is of obvious concern, and various systems are struggling to determine how to provide access to these pharmaceuticals. Prioritizing patients according to risk for advanced fibrosis using noninvasive scoring systems should be used if there is limited access or resources.
Conclusion
Since the VA has a large population of patients with HCV infection, the advancement in HCV treatment is of paramount importance. The advent of new DAAs in 2011 improved treatment efficacy for patients with HCV, but few patients could be treated due to AEs related to PEG. In 2013, new DAAs were introduced that did not require conjunctive therapy with PEG, providing a treatment option for patients who could not tolerate PEG. New DAA combinations are currently in trials and, upon approval, will provide more options for patients with HCV infection.
Author disclosures
Dr. Ho has received research and grant support from Genentech, Inc. and Gilead; he is on the speakers’ bureau for Prime Education, Inc. The other authors report no actual or potential conflicts of interest with regard to this article.
Grant Support
Funding provided by VA HSR&D grant IIR-13-052-2, VA HIV/HCV QUERI program, and the Research Service of the Department of Veterans Affairs.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Backus LI, Belperio PS, Loomis TP, Yip GH, Mole LA. Hepatitis C virus screening and prevalence among US veterans in Department of Veterans Affairs care. JAMA Intern Med. 2013;173(16):1549-1552.
2. Backus LI, Boothroyd DB, Phillips BR, Mole LA. Predictors of response of US veterans to treatment for the hepatitis C virus. Hepatology. 2007;46(1):37-47.
3. Backus LI, Belperio PS, Shahoumian TA, Cheung R, Mole LA. Comparative effectiveness of the hepatitis C virus protease inhibitors boceprevir and telaprevir in a large U.S. cohort. Aliment Pharmacol Ther. 2014;39(1):93-103.
4. Ioannou GN, Beste LA, Green PK. Similar effectiveness of boceprevir and telaprevir treatment regimens for hepatitis C virus infection on the basis of a nationwide study of veterans. Clin Gastroenterol Hepatol. 2014;12(8):1371-1380.
5. Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878-1887.
6. Jacobson IM, Gordon SC, Kowdley KV, et al; POSITRON Study; FUSION Study. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368(20):1867-1877.
7. Lawitz E, Sulkowski MS, Ghalib R, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384(9956):1756-1765.
8. Afdhal N, Reddy KR, Nelson DR, et al; ION-2 Investigators. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370(16):1483-1493.
9. Afdhal N, Zeuzem S, Kwo P, et al; ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889-1898
10. Kowdley KV, Gordon SC, Reddy KR, et al; ION-3 Investigators. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370(20):1879-1888.
11. Pawlotsky JM. New hepatitis C virus (HCV) drugs and the hope for a cure: Concepts in anti-HCV drug development. Semin Liver Dis. 2014;34(1):22-29.
12. Membreno FE, Espinales JC, Lawitz EJ. Cyclophilin inhibitors for hepatitis C therapy. Clin Liver Dis. 2013;17(1):129-139.
13. Janssen HL, Reesink HW, Lawitz EJ, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368(18):1685-1694.
14. American Association for the Study of Liver Diseases; Infectious Diseases Society of America. Recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org. Accessed November 25, 2014.
15. Department of Veterans Affairs. Chronic Hepatitis C Virus (HCV) Infection: Treatment considerations from the Department of Veterans Affairs National Hepatitis C Resource Center Program at the Office of Public Health. http://www.hepatitis.va.gov/pdf/2014hcv.pdf. Revised May 13, 2014. Accessed November 25, 2014.
16. European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C in 2014. http://www.easl.eu/_newsroom/latest-news/easl-recommendations-on-treatment-of-hepatitis-c-2014. Accessed November 25, 2014.
17. Dieterich D, Bacon BR, Flamm SL, et al. Evaluation of sofosbuvir and simeprevir-based regimens in the TRIO network: academic and community treatment of a real-world, heterogeneous population. Hepatology. 2014;60(suppl S1):220A. Abstract 46.
18. Lawitz EJ, Poordad F, Brainard D, et al. Sofosbuvir with peginterferon-ribavirin for 12 weeks in previously treated patients with hepatitis C genotype 2 or 3 and cirrhosis [published online ahead of print October 16, 2014]. Hepatology. 2014; doi:10.1002/hep.27567.
19. Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al; AI444040 Study Group. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370(3):211-221.
20. Liu S, Cipriano LE, Holodniy M, Owens DK, Goldhaber-Fiebert JD. New protease inhibitors for the treatment of chronic hepatitis C: a cost-effectiveness analysis. Ann Intern Med. 2012;156(4):279-290.
21. Chan K, Lai MN, Groessl EJ, et al. Cost effectiveness of direct-acting antiviral
therapy for treatment-naive patients with chronic HCV genotype 1 infection in the veterans health administration. Clin Gastroenterol Hepatol. 2013;11(11):1503-1510.
22. Ollendorf DA, Tice JA, Pearson SD. The comparative clinical effectiveness and value of simeprevir and sofosbuvir for chronic hepatitis C virus infection. JAMA Intern Med. 2014;174(7):1170-1171.
23. Hagan LM, Sulkowski MS, Schinazi RF. Cost analysis of sofosbuvir/ribavirin versus sofosbuvir/simeprevir for genotype 1 hepatitis C virus in interferon-ineligible/intolerant individuals. Hepatology. 2014;60(1):37-45.
Hepatitis C virus (HCV) infection remains a significant problem in the VA system, with over 174,000 current actively infected patients.1 Despite the availability of antiviral treatment since the early 1990’s, only approximately 26% of patients have ever been treated. These treatments required the use of pegylated interferon alfa (PEG) and ribavirin (RBV), which are associated with significant adverse events (AEs) that prevented many from receiving the treatment. Of those treated, only a minority achieved a sustained virologic response (SVR), due to the limited efficacy of the treatments (Figure 1).2 With the advent of new direct-acting antiviral (DAA) treatments in 2011, treatment efficacy improved.
The first DAAs in use were the viral nonstructural protein 3/4A (NS3/4A) serine protease inhibitors (PIs) boceprevir and telaprevir, which were used with PEG and RBV for patients with HCV genotype 1 infection. This combination therapy improved SVR rates from about 26% to 50% in patients with HCV genotype 1 in the VA.3,4 However, due to the significant AEs with these combinations, relatively few patients were treated.
In late 2013, the FDA approved other DAAs, which allowed patients to be treated effectively without PEG. These included the nucleotide nonstructural protein 5B (NS5B) polymerase inhibitor sofosbuvir and a secondgeneration NS3/4A PI simeprevir.5-7 The first nucleotide analog NS5B polymerase inhibitor, sofosbuvir and the nonstructural protein 5A (NS5A) replication complex inhibitor, ledipasvir, was approved in October 2014.8-10 The recent developments in noninterferon treatments have been accompanied by revised treatment guidelines or recommendations by major professional societies. Current treatment recommendations will be reviewed here, but the recommendations will continue to evolve as new DAAs come to market.
DAA Sites of Action
The HCV genome is a positive-stranded RNA molecule of about 9,500 nucleotides, which encodes a polyprotein of approximately 3,000 amino acids that form 10 individual viral proteins. These are composed of both structural and nonstructural (NS) proteins that are responsible for replication of the genome and formation of new viral particles. Understanding of the HCV-encoded proteins and their functions has permitted the development of different DAA therapies. In general, targeting a single protein is not effective, and combination therapy targeting 2 proteins is required for viral eradication (Figure 2).11 The 3 drug targets that are currently available include NS3/4A serine PIs (eg, simeprevir, boceprevir, telaprevir), NS5A replication complex inhibitors (eg, ledipasvir, daclatasvir), and NS5B RNA-dependent RNA polymerase inhibitors (eg, sofosbuvir).
Other DAA’s are in development that have targets in host rather than viral cells. These include cycolphilin A inhibitors and the micro-RNA (miR-122) antagonist miravirsen.12,13
The RNA-dependent RNA polymerase, encoded by the HCV NS5B is targeted by 2 classes of inhibitors: nucleoside or nucleotide analog inhibitors (NIs), and non-nucleoside inhibitors (NNIs).11 The only NI of the NS5B protein approved by the FDA is sofosbuvir. The resistance profiles of NIs and NNIs differ, because they bind to distinct sites on the NS5B protein. NIs are analogs of natural substrates and bind to the active site of the RNA polymerase, whereas NNIs are allosteric site inhibitors. NIs have activity in vitro against all HCV genotypes and have high barrier to resistance as the active site of NS5B polymerase is less tolerant of different amino acid substitutions.
In vitro studies have demonstrated that NIs are less likely to select for mutations compared with NNIs and PIs. The NNIs have limited genotypic coverage and have a lower barrier to resistance. Strategies for targeting HCV proteins include using a NI NS5B protein inhibitor as the backbone with a high barrier to resistance in combination with 1 or 2 other DAAs with lower barriers to resistance, or the combination of 3 DAAs with lower barriers to resistance.11 Ribavirin has broad-spectrum antiviral activity, one of which is anti-HCV activity. The mode of action of RBV against HCV is not well understood, but several mechanisms have been proposed, one of which is via inhibition of viral-dependent RNA polymerase.
Current HCV Treatment Recommendations
Current treatment recommendations are available from the American Association for the Study of the Liver Disease (AASLD) and the Infectious Diseases Society of America (IDSA) (http://www.hcvguidelines.org); the VA National Hepatitis C Resource Center Program and Office of Public Health (http://www.hepatitis.va.gov/pdf/2014hcv.pdf); and the European Association for the Study of the Liver (EASL) (http://www.easl.eu/_newsroom/latest-news/easl-recommendations-on-treatment-of-hepatitis-c-2014). These recommendations are updated frequently as new drugs enter the marketplace (Table 1).14-16
Since most patients are unable or unwilling to tolerate interferon, the majority of patients in treatment are currently receiving interferon-free combinations. Treatment of genotype 1 is currently dominated by the offlabel use of sofosbuvir in combination with simeprevir. Data have been published from a single phase II trial in patients with and without cirrhosis.7 Of note are emerging data from observational studies confirming the efficacy of over 80% SVR in patients with cirrhosis and genotype 1a with or without prior treatment.17 Treatment of patients with genotype 2 infection is dominated by the use of sofosbuvir and RBV combination. However, patients with cirrhosis do not respond as well as patients without cirrhosis, and it remains to be seen whether extending therapy is of any benefit.
One exception for the use of PEG is the recommendation for patients with HCV genotype 3 and cirrhosis to consider the combination of PEG, RBV, and sofosbuvir for 12 weeks.18 This has the advantage of being more effective, less costly, and shorter treatment duration compared with the sofosbuvir and RBV association but is only appropriate for patients who can tolerate interferon. Common AEs and potential contraindications to treatment are listed in Table 2.5-10,19
New Treatment Options for HCV in 2015
Figure 3 details DAA combinations currently in phase III trials that are expected to receive approval in 2015. This will expand the repertoire of drug combinations available and enable fine-tuning of regimens according to patient and viral characteristics and cost requirements.
Cost and Effectiveness
Cost-effectiveness issues are of immediate importance for health care systems and payors. The first generation PIs, boceprevir and telaprevir, were used in combination with PEG and RBV and entailed pharmacy costs on par with current noninterferon regimens.20,21 Studies generally demonstrated that these treatments are cost-effective, especially in patients with advanced fibrosis. Ollendorf and colleagues recently published an analysis of the costs of using interferon-free regimens for treatment of 540,000 patients with chronic HCV in California.22 Assuming that 50% of patients would present for treatment, the cost of the new DAAs would be immense and result in an increase in costs from $12 billion to $34 billion in the first year and net costs of $6 billion by the 20th year. If treatment were limited to patients with advanced cirrhosis, the first year costs would be increased by $7 billion, but at 20 years there would be approximately $1 billion in net cost savings.
For current treatments, a 12-week course of simeprevir/sofosbuvir has been shown to be more costeffective than 24 weeks of sofosbuvir/RBV for treatment
of genotype 1.23 Similarly, patients with genotype 1, no cirrhosis, and low viral load can be treated with 8 weeks of sofosbuvir/ledipasvir rather than other 12-week regimens, thereby reducing drug costs. The resources needed for upfront treatment of patients is of obvious concern, and various systems are struggling to determine how to provide access to these pharmaceuticals. Prioritizing patients according to risk for advanced fibrosis using noninvasive scoring systems should be used if there is limited access or resources.
Conclusion
Since the VA has a large population of patients with HCV infection, the advancement in HCV treatment is of paramount importance. The advent of new DAAs in 2011 improved treatment efficacy for patients with HCV, but few patients could be treated due to AEs related to PEG. In 2013, new DAAs were introduced that did not require conjunctive therapy with PEG, providing a treatment option for patients who could not tolerate PEG. New DAA combinations are currently in trials and, upon approval, will provide more options for patients with HCV infection.
Author disclosures
Dr. Ho has received research and grant support from Genentech, Inc. and Gilead; he is on the speakers’ bureau for Prime Education, Inc. The other authors report no actual or potential conflicts of interest with regard to this article.
Grant Support
Funding provided by VA HSR&D grant IIR-13-052-2, VA HIV/HCV QUERI program, and the Research Service of the Department of Veterans Affairs.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Hepatitis C virus (HCV) infection remains a significant problem in the VA system, with over 174,000 current actively infected patients.1 Despite the availability of antiviral treatment since the early 1990’s, only approximately 26% of patients have ever been treated. These treatments required the use of pegylated interferon alfa (PEG) and ribavirin (RBV), which are associated with significant adverse events (AEs) that prevented many from receiving the treatment. Of those treated, only a minority achieved a sustained virologic response (SVR), due to the limited efficacy of the treatments (Figure 1).2 With the advent of new direct-acting antiviral (DAA) treatments in 2011, treatment efficacy improved.
The first DAAs in use were the viral nonstructural protein 3/4A (NS3/4A) serine protease inhibitors (PIs) boceprevir and telaprevir, which were used with PEG and RBV for patients with HCV genotype 1 infection. This combination therapy improved SVR rates from about 26% to 50% in patients with HCV genotype 1 in the VA.3,4 However, due to the significant AEs with these combinations, relatively few patients were treated.
In late 2013, the FDA approved other DAAs, which allowed patients to be treated effectively without PEG. These included the nucleotide nonstructural protein 5B (NS5B) polymerase inhibitor sofosbuvir and a secondgeneration NS3/4A PI simeprevir.5-7 The first nucleotide analog NS5B polymerase inhibitor, sofosbuvir and the nonstructural protein 5A (NS5A) replication complex inhibitor, ledipasvir, was approved in October 2014.8-10 The recent developments in noninterferon treatments have been accompanied by revised treatment guidelines or recommendations by major professional societies. Current treatment recommendations will be reviewed here, but the recommendations will continue to evolve as new DAAs come to market.
DAA Sites of Action
The HCV genome is a positive-stranded RNA molecule of about 9,500 nucleotides, which encodes a polyprotein of approximately 3,000 amino acids that form 10 individual viral proteins. These are composed of both structural and nonstructural (NS) proteins that are responsible for replication of the genome and formation of new viral particles. Understanding of the HCV-encoded proteins and their functions has permitted the development of different DAA therapies. In general, targeting a single protein is not effective, and combination therapy targeting 2 proteins is required for viral eradication (Figure 2).11 The 3 drug targets that are currently available include NS3/4A serine PIs (eg, simeprevir, boceprevir, telaprevir), NS5A replication complex inhibitors (eg, ledipasvir, daclatasvir), and NS5B RNA-dependent RNA polymerase inhibitors (eg, sofosbuvir).
Other DAA’s are in development that have targets in host rather than viral cells. These include cycolphilin A inhibitors and the micro-RNA (miR-122) antagonist miravirsen.12,13
The RNA-dependent RNA polymerase, encoded by the HCV NS5B is targeted by 2 classes of inhibitors: nucleoside or nucleotide analog inhibitors (NIs), and non-nucleoside inhibitors (NNIs).11 The only NI of the NS5B protein approved by the FDA is sofosbuvir. The resistance profiles of NIs and NNIs differ, because they bind to distinct sites on the NS5B protein. NIs are analogs of natural substrates and bind to the active site of the RNA polymerase, whereas NNIs are allosteric site inhibitors. NIs have activity in vitro against all HCV genotypes and have high barrier to resistance as the active site of NS5B polymerase is less tolerant of different amino acid substitutions.
In vitro studies have demonstrated that NIs are less likely to select for mutations compared with NNIs and PIs. The NNIs have limited genotypic coverage and have a lower barrier to resistance. Strategies for targeting HCV proteins include using a NI NS5B protein inhibitor as the backbone with a high barrier to resistance in combination with 1 or 2 other DAAs with lower barriers to resistance, or the combination of 3 DAAs with lower barriers to resistance.11 Ribavirin has broad-spectrum antiviral activity, one of which is anti-HCV activity. The mode of action of RBV against HCV is not well understood, but several mechanisms have been proposed, one of which is via inhibition of viral-dependent RNA polymerase.
Current HCV Treatment Recommendations
Current treatment recommendations are available from the American Association for the Study of the Liver Disease (AASLD) and the Infectious Diseases Society of America (IDSA) (http://www.hcvguidelines.org); the VA National Hepatitis C Resource Center Program and Office of Public Health (http://www.hepatitis.va.gov/pdf/2014hcv.pdf); and the European Association for the Study of the Liver (EASL) (http://www.easl.eu/_newsroom/latest-news/easl-recommendations-on-treatment-of-hepatitis-c-2014). These recommendations are updated frequently as new drugs enter the marketplace (Table 1).14-16
Since most patients are unable or unwilling to tolerate interferon, the majority of patients in treatment are currently receiving interferon-free combinations. Treatment of genotype 1 is currently dominated by the offlabel use of sofosbuvir in combination with simeprevir. Data have been published from a single phase II trial in patients with and without cirrhosis.7 Of note are emerging data from observational studies confirming the efficacy of over 80% SVR in patients with cirrhosis and genotype 1a with or without prior treatment.17 Treatment of patients with genotype 2 infection is dominated by the use of sofosbuvir and RBV combination. However, patients with cirrhosis do not respond as well as patients without cirrhosis, and it remains to be seen whether extending therapy is of any benefit.
One exception for the use of PEG is the recommendation for patients with HCV genotype 3 and cirrhosis to consider the combination of PEG, RBV, and sofosbuvir for 12 weeks.18 This has the advantage of being more effective, less costly, and shorter treatment duration compared with the sofosbuvir and RBV association but is only appropriate for patients who can tolerate interferon. Common AEs and potential contraindications to treatment are listed in Table 2.5-10,19
New Treatment Options for HCV in 2015
Figure 3 details DAA combinations currently in phase III trials that are expected to receive approval in 2015. This will expand the repertoire of drug combinations available and enable fine-tuning of regimens according to patient and viral characteristics and cost requirements.
Cost and Effectiveness
Cost-effectiveness issues are of immediate importance for health care systems and payors. The first generation PIs, boceprevir and telaprevir, were used in combination with PEG and RBV and entailed pharmacy costs on par with current noninterferon regimens.20,21 Studies generally demonstrated that these treatments are cost-effective, especially in patients with advanced fibrosis. Ollendorf and colleagues recently published an analysis of the costs of using interferon-free regimens for treatment of 540,000 patients with chronic HCV in California.22 Assuming that 50% of patients would present for treatment, the cost of the new DAAs would be immense and result in an increase in costs from $12 billion to $34 billion in the first year and net costs of $6 billion by the 20th year. If treatment were limited to patients with advanced cirrhosis, the first year costs would be increased by $7 billion, but at 20 years there would be approximately $1 billion in net cost savings.
For current treatments, a 12-week course of simeprevir/sofosbuvir has been shown to be more costeffective than 24 weeks of sofosbuvir/RBV for treatment
of genotype 1.23 Similarly, patients with genotype 1, no cirrhosis, and low viral load can be treated with 8 weeks of sofosbuvir/ledipasvir rather than other 12-week regimens, thereby reducing drug costs. The resources needed for upfront treatment of patients is of obvious concern, and various systems are struggling to determine how to provide access to these pharmaceuticals. Prioritizing patients according to risk for advanced fibrosis using noninvasive scoring systems should be used if there is limited access or resources.
Conclusion
Since the VA has a large population of patients with HCV infection, the advancement in HCV treatment is of paramount importance. The advent of new DAAs in 2011 improved treatment efficacy for patients with HCV, but few patients could be treated due to AEs related to PEG. In 2013, new DAAs were introduced that did not require conjunctive therapy with PEG, providing a treatment option for patients who could not tolerate PEG. New DAA combinations are currently in trials and, upon approval, will provide more options for patients with HCV infection.
Author disclosures
Dr. Ho has received research and grant support from Genentech, Inc. and Gilead; he is on the speakers’ bureau for Prime Education, Inc. The other authors report no actual or potential conflicts of interest with regard to this article.
Grant Support
Funding provided by VA HSR&D grant IIR-13-052-2, VA HIV/HCV QUERI program, and the Research Service of the Department of Veterans Affairs.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Backus LI, Belperio PS, Loomis TP, Yip GH, Mole LA. Hepatitis C virus screening and prevalence among US veterans in Department of Veterans Affairs care. JAMA Intern Med. 2013;173(16):1549-1552.
2. Backus LI, Boothroyd DB, Phillips BR, Mole LA. Predictors of response of US veterans to treatment for the hepatitis C virus. Hepatology. 2007;46(1):37-47.
3. Backus LI, Belperio PS, Shahoumian TA, Cheung R, Mole LA. Comparative effectiveness of the hepatitis C virus protease inhibitors boceprevir and telaprevir in a large U.S. cohort. Aliment Pharmacol Ther. 2014;39(1):93-103.
4. Ioannou GN, Beste LA, Green PK. Similar effectiveness of boceprevir and telaprevir treatment regimens for hepatitis C virus infection on the basis of a nationwide study of veterans. Clin Gastroenterol Hepatol. 2014;12(8):1371-1380.
5. Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878-1887.
6. Jacobson IM, Gordon SC, Kowdley KV, et al; POSITRON Study; FUSION Study. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368(20):1867-1877.
7. Lawitz E, Sulkowski MS, Ghalib R, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384(9956):1756-1765.
8. Afdhal N, Reddy KR, Nelson DR, et al; ION-2 Investigators. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370(16):1483-1493.
9. Afdhal N, Zeuzem S, Kwo P, et al; ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889-1898
10. Kowdley KV, Gordon SC, Reddy KR, et al; ION-3 Investigators. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370(20):1879-1888.
11. Pawlotsky JM. New hepatitis C virus (HCV) drugs and the hope for a cure: Concepts in anti-HCV drug development. Semin Liver Dis. 2014;34(1):22-29.
12. Membreno FE, Espinales JC, Lawitz EJ. Cyclophilin inhibitors for hepatitis C therapy. Clin Liver Dis. 2013;17(1):129-139.
13. Janssen HL, Reesink HW, Lawitz EJ, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368(18):1685-1694.
14. American Association for the Study of Liver Diseases; Infectious Diseases Society of America. Recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org. Accessed November 25, 2014.
15. Department of Veterans Affairs. Chronic Hepatitis C Virus (HCV) Infection: Treatment considerations from the Department of Veterans Affairs National Hepatitis C Resource Center Program at the Office of Public Health. http://www.hepatitis.va.gov/pdf/2014hcv.pdf. Revised May 13, 2014. Accessed November 25, 2014.
16. European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C in 2014. http://www.easl.eu/_newsroom/latest-news/easl-recommendations-on-treatment-of-hepatitis-c-2014. Accessed November 25, 2014.
17. Dieterich D, Bacon BR, Flamm SL, et al. Evaluation of sofosbuvir and simeprevir-based regimens in the TRIO network: academic and community treatment of a real-world, heterogeneous population. Hepatology. 2014;60(suppl S1):220A. Abstract 46.
18. Lawitz EJ, Poordad F, Brainard D, et al. Sofosbuvir with peginterferon-ribavirin for 12 weeks in previously treated patients with hepatitis C genotype 2 or 3 and cirrhosis [published online ahead of print October 16, 2014]. Hepatology. 2014; doi:10.1002/hep.27567.
19. Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al; AI444040 Study Group. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370(3):211-221.
20. Liu S, Cipriano LE, Holodniy M, Owens DK, Goldhaber-Fiebert JD. New protease inhibitors for the treatment of chronic hepatitis C: a cost-effectiveness analysis. Ann Intern Med. 2012;156(4):279-290.
21. Chan K, Lai MN, Groessl EJ, et al. Cost effectiveness of direct-acting antiviral
therapy for treatment-naive patients with chronic HCV genotype 1 infection in the veterans health administration. Clin Gastroenterol Hepatol. 2013;11(11):1503-1510.
22. Ollendorf DA, Tice JA, Pearson SD. The comparative clinical effectiveness and value of simeprevir and sofosbuvir for chronic hepatitis C virus infection. JAMA Intern Med. 2014;174(7):1170-1171.
23. Hagan LM, Sulkowski MS, Schinazi RF. Cost analysis of sofosbuvir/ribavirin versus sofosbuvir/simeprevir for genotype 1 hepatitis C virus in interferon-ineligible/intolerant individuals. Hepatology. 2014;60(1):37-45.
1. Backus LI, Belperio PS, Loomis TP, Yip GH, Mole LA. Hepatitis C virus screening and prevalence among US veterans in Department of Veterans Affairs care. JAMA Intern Med. 2013;173(16):1549-1552.
2. Backus LI, Boothroyd DB, Phillips BR, Mole LA. Predictors of response of US veterans to treatment for the hepatitis C virus. Hepatology. 2007;46(1):37-47.
3. Backus LI, Belperio PS, Shahoumian TA, Cheung R, Mole LA. Comparative effectiveness of the hepatitis C virus protease inhibitors boceprevir and telaprevir in a large U.S. cohort. Aliment Pharmacol Ther. 2014;39(1):93-103.
4. Ioannou GN, Beste LA, Green PK. Similar effectiveness of boceprevir and telaprevir treatment regimens for hepatitis C virus infection on the basis of a nationwide study of veterans. Clin Gastroenterol Hepatol. 2014;12(8):1371-1380.
5. Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878-1887.
6. Jacobson IM, Gordon SC, Kowdley KV, et al; POSITRON Study; FUSION Study. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368(20):1867-1877.
7. Lawitz E, Sulkowski MS, Ghalib R, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384(9956):1756-1765.
8. Afdhal N, Reddy KR, Nelson DR, et al; ION-2 Investigators. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370(16):1483-1493.
9. Afdhal N, Zeuzem S, Kwo P, et al; ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889-1898
10. Kowdley KV, Gordon SC, Reddy KR, et al; ION-3 Investigators. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370(20):1879-1888.
11. Pawlotsky JM. New hepatitis C virus (HCV) drugs and the hope for a cure: Concepts in anti-HCV drug development. Semin Liver Dis. 2014;34(1):22-29.
12. Membreno FE, Espinales JC, Lawitz EJ. Cyclophilin inhibitors for hepatitis C therapy. Clin Liver Dis. 2013;17(1):129-139.
13. Janssen HL, Reesink HW, Lawitz EJ, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368(18):1685-1694.
14. American Association for the Study of Liver Diseases; Infectious Diseases Society of America. Recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org. Accessed November 25, 2014.
15. Department of Veterans Affairs. Chronic Hepatitis C Virus (HCV) Infection: Treatment considerations from the Department of Veterans Affairs National Hepatitis C Resource Center Program at the Office of Public Health. http://www.hepatitis.va.gov/pdf/2014hcv.pdf. Revised May 13, 2014. Accessed November 25, 2014.
16. European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C in 2014. http://www.easl.eu/_newsroom/latest-news/easl-recommendations-on-treatment-of-hepatitis-c-2014. Accessed November 25, 2014.
17. Dieterich D, Bacon BR, Flamm SL, et al. Evaluation of sofosbuvir and simeprevir-based regimens in the TRIO network: academic and community treatment of a real-world, heterogeneous population. Hepatology. 2014;60(suppl S1):220A. Abstract 46.
18. Lawitz EJ, Poordad F, Brainard D, et al. Sofosbuvir with peginterferon-ribavirin for 12 weeks in previously treated patients with hepatitis C genotype 2 or 3 and cirrhosis [published online ahead of print October 16, 2014]. Hepatology. 2014; doi:10.1002/hep.27567.
19. Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al; AI444040 Study Group. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370(3):211-221.
20. Liu S, Cipriano LE, Holodniy M, Owens DK, Goldhaber-Fiebert JD. New protease inhibitors for the treatment of chronic hepatitis C: a cost-effectiveness analysis. Ann Intern Med. 2012;156(4):279-290.
21. Chan K, Lai MN, Groessl EJ, et al. Cost effectiveness of direct-acting antiviral
therapy for treatment-naive patients with chronic HCV genotype 1 infection in the veterans health administration. Clin Gastroenterol Hepatol. 2013;11(11):1503-1510.
22. Ollendorf DA, Tice JA, Pearson SD. The comparative clinical effectiveness and value of simeprevir and sofosbuvir for chronic hepatitis C virus infection. JAMA Intern Med. 2014;174(7):1170-1171.
23. Hagan LM, Sulkowski MS, Schinazi RF. Cost analysis of sofosbuvir/ribavirin versus sofosbuvir/simeprevir for genotype 1 hepatitis C virus in interferon-ineligible/intolerant individuals. Hepatology. 2014;60(1):37-45.