User login
VMS, also known as hot flashes, night sweats, or cold sweats, occur for the majority of perimenopausal and menopausal women.1 In one study, the mean duration of clinically significant VMS was 5 years, and one-third of participants continued to have bothersome hot flashes 10 or more years after the onset of menopause.2 VMS may contribute to disrupted sleep patterns and depressed mood.3
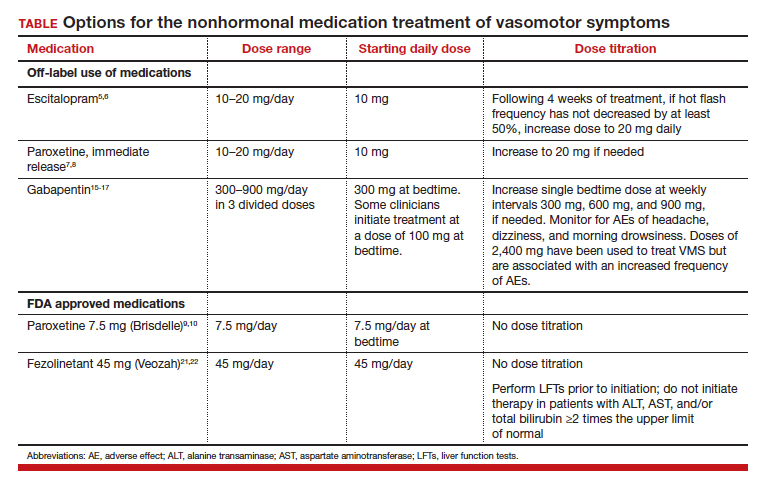
All obstetrician-gynecologists know that estradiol and other estrogens are highly effective in the treatment of bothersome VMS. A meta-analysis reported that the frequency of VMS was reduced by 60% to 80% with oral estradiol (1 mg/day), transdermal estradiol(0.05 mg/day), and conjugated estrogen (0.625 mg).4 Breast tenderness and irregular uterine bleeding are common side effects of estrogen treatment of VMS. Estrogen treatment is contraindicated in patients with estrogen-responsive cancers, coronary heart disease, myocardial infarction, stroke, venous thromboembolism, and some cases of inherited thrombophilia. For these patients, an important option is the nonhormonal treatment of VMS, and several nonhormonal medications have been demonstrated to be effective therapy (TABLE 1). In this editorial I will review the medication treatment of VMS with escitalopram, paroxetine, gabapentin, and fezolinetant.
Escitalopram and paroxetine
Escitalopram and paroxetine have been shown to reduce VMS more than placebo in multiple clinical trials.5-10 In addition, escitalopram and paroxetine, at the doses tested, may be more effective for the treatment of VMS than sertraline, citalopram, or fluoxetine.11 In one trial assessing the efficacy of escitalopram to treat VMS, 205 patients with VMS were randomly assigned to 8 weeks of treatment with placebo or escitalopram.5 The initial escitalopram dose was 10 mg daily. At week 4:
- if VMS frequency was reduced by ≥ 50%, the patient remained on the 10-mg dose
- if VMS frequency was reduced by < 50%, the escitalopram dose was increased to 20 mg daily.
Following 8 weeks of treatment, the frequency of VMS decreased for patients in the placebo and escitalopram groups by 33% and 47%, respectively. Similar results have been reported in other studies.6
Paroxetine at a dose of 7.5 mg/day administered at bedtime is approved by the US Food and Drug Administration (FDA) for the treatment of VMS. In a pivotal study, 1,112 patients with VMS were randomly assigned to receive a placebo or paroxetine 7.5 mg at bedtime.9 In the 12-week study the reported decrease in mean weekly frequency of VMS for patients in the placebo and paroxetine groups were -37 and -44, respectively.9 Paroxetine 7.5 mg also reduced awakenings per night attributed to VMS and increased nighttime sleep duration.10
Depressed mood is prevalent among perimenopausal and postmenopausal patients.12 Prescribing escitalopram or paroxetine for VMS also may improve mood. Venlafaxine and desvenlafaxine are effective for the treatment of VMS;13,14 however, I seldom prescribe these medications for VMS because in my experience they are associated with more bothersome side effects, including dry mouth, decreased appetite, nausea, and insomnia than escitalopram or low-dose paroxetine.
Gabapentin
Numerous randomized clinical trials have reported that gabapentin is superior to placebo for the treatment of VMS.15 In one trial, 420 patients with breast cancer and VMS were randomly assigned to 8 weeks of treatment with placebo, gabapentin 300 mg/day (G300), or gabapentin 900 mg/day (G900) in 3 divided doses.16 Following 8 weeks of treatment, reduction in hot-flash severity score among patients receiving placebo, G300, or G900 was 15%, 31%, and 46%, respectively. Fatigue and somnolence were reported more frequently among patients taking gabapentin 900 mg/day. In a small trial, 60 patients with VMS were randomized to receive placebo, conjugated estrogen (0.2625 mg/day),or gabapentin (target dose of 2,400 mg/day in 3 divided doses).17 Following 12 weeks of treatment, the patient-reported decrease in VMS for those taking placebo, estrogen, or gabapentin was 54%, 72%, and 71%, respectively.
High-dose gabapentin treatment was associated with side effects of headache and dizziness more often than placebo or estrogen. Although gabapentin is not a treatment for insomnia, in my practice if a menopausal patient has prominent and bothersome symptoms of sleep disturbance and mild VMS symptoms, I will consider a trial of low-dose gabapentin. Some experts recommend initiating gabapentin at a dose of 100 mgdaily before bedtime to assess the effectiveness of a low dose that seldom causes significant side effects.

Fezolinetant
In a study of genetic variation associated with VMS, investigators discovered that nucleic acid variation in the neurokinin 3 (NK3) receptor was strongly associated with the prevalence of VMS, suggesting that this receptor is in the causal pathway to menopausal VMS.18 Additional research demonstrated that the kisspeptin/neurokinin B/dynorphin (KNDy) neurons, which are involved in the control of hypothalamic thermoregulation, are stimulated by neurokinin B, acting through the NK3 receptor, and suppressed by estradiol. A reduction in hypothalamic estrogen results in unopposed neurokinin B activity, which stimulates KNDy neurons, destabilizing the hypothalamic thermoregulatory center, causing vasodilation, which is perceived as hot flashes and sweating followed by chills.19
Fezolinetant is a high-affinity NK3 receptor antagonist that blocks the activity of neurokinin B, stabilizing the hypothalamic thermoregulatory center, thereby suppressing hot flashes. It is approved by the FDA for the treatment of moderate to severe VMS due to menopause using a fixed dose of 45 mg daily.20 In one clinical trial, 500 menopausal patients with bothersome VMS were randomly assigned to 12 weeks of treatment with placebo, fezolinetant 30 mg/day, or fezolinetant 45 mg/day. Following 12 weeks of treatment, the reported frequency rates of VMS among patients in the placebo, F30, and F45 groups were reduced by 43%, 61%, and 64%, respectively.21 In addition, following 12 weeks of treatment, the severity of VMS rates among patients in the placebo, F30, and F45 groups were reduced by 20%, 26%, and 32%, respectively.
Fezolinetant improved the quality of sleep and was associated with an improvement in patient-reported quality of life. Following 12 weeks of treatment, sleep quality among patients in the placebo, F30, and F45 groups was reported to be “much or moderately better” in 34%, 45%, and 54% of the patients, respectively.21 Similar results were reported in a companion study.22
Fezolinetant is contraindicated for patients with liver cirrhosis or severe renal impairment (estimated glomerular filtration rate of < 30 mL/min/1.73 m2). Before initiating treatment, serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and bilirubin (total and direct). Fezolinetant should not be prescribed if any of these tests are greater than twice the upper limit of normal. These tests should be repeated at 3, 6, and 9 months, and if the patient reports symptoms or signs of liver injury (nausea, vomiting, jaundice). Fezolinetant is metabolized by CYP1A2 and should not be prescribed to patients taking strong CYP1A2 inhibitors. The most common side effects associated with fezolinetant treatment are abdominal pain (4.3%), diarrhea (3.9%), insomnia (3.9%), back pain (3.0%), and hepatic transaminase elevation (2.3%). Fezolinetant has not been thoroughly evaluated in patients older than age 65. Following an oral dose of the medication, the median maximum concentration is reached in 1.5 hours, and the half-life is estimated to be 10 hours.20 Of all the medications discussed in this editorial, fezolinetant is the most expensive.
Effective VMS treatment improves overall health
Estrogen therapy is the gold standard treatment of VMS. However, many menopausal patients with bothersome VMS prefer not to take estrogen, and some have a medical condition that is a contraindication to estrogen treatment. The nonhormonal medication options for the treatment of VMS include escitalopram, paroxetine, gabapentin, and fezolinetant. Patients value the ability to choose the treatment they prefer, among all available hormonal and nonhormonal medication options. For mid-life women, effectively treating bothersome VMS is only one of many interventions that improves health. Optimal health is best achieved with23:
- high-quality diet
- daily physical activity
- appropriate body mass index
- nicotine avoidance
- a healthy sleep schedule
- normal blood pressure, lipid, and glucose levels.
Women who have a high-quality diet; daily physical activity; an appropriate body mass index; and normal blood pressure, cholesterol, and glucose levels are estimated to live 9 disease-free years longer than other women.24 ●
- Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopause transition: study of women’s health across the nation. Am J Pub Health. 2006;1226-1235.
- Freeman EW, Sammel MD, Sanders RJ. Risk of long-term hot flashes after natural menopause: evidence from the Penn Ovarian Aging Study cohort. Menopause. 2014;21:924-932.
- Hatcher KM, Smith RL, Chiang C, et al. Nocturnal hot flashes, but not serum hormone concentrations as a predictor of insomnia in menopausal women: results from the Midlife Women’s Health Study. J Women’s Health. 2023;32:94-101.
- Nelson HD. Commonly used types of postmenopausal estrogen for treatment of hot flashes: scientific review. JAMA. 2004;291:1610.
- Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011;305:267-227.
- Carpenter JS, Guthrie KA, Larson JC, et al. Effect of escitalopram on hot flash interference: a randomized, controlled trial. Fertil Steril. 2012;97:1399-1404.e1.
- Slaton RM, Champion MN, Palmore KB. A review of paroxetine for the treatment of vasomotor symptoms. J Pharm Pract. 2015;28:266-274.
- Stearns V, Slack R, Greep N, et al. Paroxetine is an effective treatment for hot flashes: results from a prospective randomized clinical trial. J Clin Oncol. 2005;23:6919-6930.
- Simon JA, Portman DJ, Kaunitz AM, et al. Lowdose paroxetine 7.5 mg for menopausal vasomotor symptoms: two randomized controlled trials. Menopause. 2013;20:1027-1035.
- Pinkerton JV, Joffe H, Kazempour K, et al. Lowdose paroxetine (7.5 mg) improves sleep in women with vasomotor symptoms associated with menopause. Menopause. 2015;22:50-58.
- Shams T, Firwana B, Habib F, et al. SSRIs for hot flashes: a systematic review and metaanalysis of randomized trials. J Gen Intern Med. 2014;29:204-213.
- Freeman EW. Depression in the menopause transition: risks in the changing hormone milieu as observed in the general population. Womens Midlife Health. 2015;1:2.
- Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet. 2000;356:2059-2063.
- Sun Z, Hao Y, Zhang M. Efficacy and safety of desvenlafaxine treatment for hot flashes associated with menopause: a meta-analysis of randomized controlled trials. Gynecol Obstet Invest. 2013;75:255-262.
- Toulis KA, Tzellos T, Kouvelas D, et al. Gabapentin for the treatment of hot flashes in women with natural or tamoxifen-induced menopause: a systematic review and meta-analysis. Clin Ther. 2009;31:221-235.
- Pandya KJ, Morrow GR, Roscoe JA, et al. Gabapentin for hot flashes in 420 women with breast cancer: a randomized double-blind placebocontrolled trial. Lancet. 2005;366:818-824.
- Reddy SY, Warner H, Guttuso T Jr, et al. Gabapentin, estrogen, and placebo for treating hot flushes: a randomized controlled trial. Obstet Gynecol. 2006;108:41-48.
- Crandall CJ, Manson JE, Hohensee C, et al. Association of genetic variation in the tachykinin receptor 3 locus with hot flashes and night sweats in the Women’s Health Initiative Study. Menopause. 2017;24:252.
- Rance NE, Dacks PA, Mittelman-Smith MA, et al. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neurendocrinol. 2013;34:211-227.
- Veozah (package insert). Astellas Pharma; Northbrook, Illinois. May 2023.
- Johnson KA, Martin N, Nappi RE, et al. Efficacy and safety of fezolinetant in moderate-to-severe vasomotor symptoms associated with menopause: a Phase 3 RCT. J Clin Endocrinol Metab. 2023;108:1981-1997.
- Lederman S, Ottery FD, Cano A, et al. Fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause (SKYLIGHT 1): a phase 3 randomised controlled study. Lancet. 2023;401:1091-1102.
- Lloyd-Jones DM, Allen NB, Anderson CAM, et al. Life’s essential 8: updating and enhancing the American Heart Association’s construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. 2022;146:e18-43.
- Wang X, Ma H, Li X, et al. Association of cardiovascular health with life expectancy free of cardiovascular disease, diabetes, cancer, and dementia in U.K. adults. JAMA Int Med. 2023;183:340-349.
VMS, also known as hot flashes, night sweats, or cold sweats, occur for the majority of perimenopausal and menopausal women.1 In one study, the mean duration of clinically significant VMS was 5 years, and one-third of participants continued to have bothersome hot flashes 10 or more years after the onset of menopause.2 VMS may contribute to disrupted sleep patterns and depressed mood.3
All obstetrician-gynecologists know that estradiol and other estrogens are highly effective in the treatment of bothersome VMS. A meta-analysis reported that the frequency of VMS was reduced by 60% to 80% with oral estradiol (1 mg/day), transdermal estradiol(0.05 mg/day), and conjugated estrogen (0.625 mg).4 Breast tenderness and irregular uterine bleeding are common side effects of estrogen treatment of VMS. Estrogen treatment is contraindicated in patients with estrogen-responsive cancers, coronary heart disease, myocardial infarction, stroke, venous thromboembolism, and some cases of inherited thrombophilia. For these patients, an important option is the nonhormonal treatment of VMS, and several nonhormonal medications have been demonstrated to be effective therapy (TABLE 1). In this editorial I will review the medication treatment of VMS with escitalopram, paroxetine, gabapentin, and fezolinetant.

Escitalopram and paroxetine
Escitalopram and paroxetine have been shown to reduce VMS more than placebo in multiple clinical trials.5-10 In addition, escitalopram and paroxetine, at the doses tested, may be more effective for the treatment of VMS than sertraline, citalopram, or fluoxetine.11 In one trial assessing the efficacy of escitalopram to treat VMS, 205 patients with VMS were randomly assigned to 8 weeks of treatment with placebo or escitalopram.5 The initial escitalopram dose was 10 mg daily. At week 4:
- if VMS frequency was reduced by ≥ 50%, the patient remained on the 10-mg dose
- if VMS frequency was reduced by < 50%, the escitalopram dose was increased to 20 mg daily.
Following 8 weeks of treatment, the frequency of VMS decreased for patients in the placebo and escitalopram groups by 33% and 47%, respectively. Similar results have been reported in other studies.6
Paroxetine at a dose of 7.5 mg/day administered at bedtime is approved by the US Food and Drug Administration (FDA) for the treatment of VMS. In a pivotal study, 1,112 patients with VMS were randomly assigned to receive a placebo or paroxetine 7.5 mg at bedtime.9 In the 12-week study the reported decrease in mean weekly frequency of VMS for patients in the placebo and paroxetine groups were -37 and -44, respectively.9 Paroxetine 7.5 mg also reduced awakenings per night attributed to VMS and increased nighttime sleep duration.10
Depressed mood is prevalent among perimenopausal and postmenopausal patients.12 Prescribing escitalopram or paroxetine for VMS also may improve mood. Venlafaxine and desvenlafaxine are effective for the treatment of VMS;13,14 however, I seldom prescribe these medications for VMS because in my experience they are associated with more bothersome side effects, including dry mouth, decreased appetite, nausea, and insomnia than escitalopram or low-dose paroxetine.
Gabapentin
Numerous randomized clinical trials have reported that gabapentin is superior to placebo for the treatment of VMS.15 In one trial, 420 patients with breast cancer and VMS were randomly assigned to 8 weeks of treatment with placebo, gabapentin 300 mg/day (G300), or gabapentin 900 mg/day (G900) in 3 divided doses.16 Following 8 weeks of treatment, reduction in hot-flash severity score among patients receiving placebo, G300, or G900 was 15%, 31%, and 46%, respectively. Fatigue and somnolence were reported more frequently among patients taking gabapentin 900 mg/day. In a small trial, 60 patients with VMS were randomized to receive placebo, conjugated estrogen (0.2625 mg/day),or gabapentin (target dose of 2,400 mg/day in 3 divided doses).17 Following 12 weeks of treatment, the patient-reported decrease in VMS for those taking placebo, estrogen, or gabapentin was 54%, 72%, and 71%, respectively.
High-dose gabapentin treatment was associated with side effects of headache and dizziness more often than placebo or estrogen. Although gabapentin is not a treatment for insomnia, in my practice if a menopausal patient has prominent and bothersome symptoms of sleep disturbance and mild VMS symptoms, I will consider a trial of low-dose gabapentin. Some experts recommend initiating gabapentin at a dose of 100 mgdaily before bedtime to assess the effectiveness of a low dose that seldom causes significant side effects.

Fezolinetant
In a study of genetic variation associated with VMS, investigators discovered that nucleic acid variation in the neurokinin 3 (NK3) receptor was strongly associated with the prevalence of VMS, suggesting that this receptor is in the causal pathway to menopausal VMS.18 Additional research demonstrated that the kisspeptin/neurokinin B/dynorphin (KNDy) neurons, which are involved in the control of hypothalamic thermoregulation, are stimulated by neurokinin B, acting through the NK3 receptor, and suppressed by estradiol. A reduction in hypothalamic estrogen results in unopposed neurokinin B activity, which stimulates KNDy neurons, destabilizing the hypothalamic thermoregulatory center, causing vasodilation, which is perceived as hot flashes and sweating followed by chills.19
Fezolinetant is a high-affinity NK3 receptor antagonist that blocks the activity of neurokinin B, stabilizing the hypothalamic thermoregulatory center, thereby suppressing hot flashes. It is approved by the FDA for the treatment of moderate to severe VMS due to menopause using a fixed dose of 45 mg daily.20 In one clinical trial, 500 menopausal patients with bothersome VMS were randomly assigned to 12 weeks of treatment with placebo, fezolinetant 30 mg/day, or fezolinetant 45 mg/day. Following 12 weeks of treatment, the reported frequency rates of VMS among patients in the placebo, F30, and F45 groups were reduced by 43%, 61%, and 64%, respectively.21 In addition, following 12 weeks of treatment, the severity of VMS rates among patients in the placebo, F30, and F45 groups were reduced by 20%, 26%, and 32%, respectively.
Fezolinetant improved the quality of sleep and was associated with an improvement in patient-reported quality of life. Following 12 weeks of treatment, sleep quality among patients in the placebo, F30, and F45 groups was reported to be “much or moderately better” in 34%, 45%, and 54% of the patients, respectively.21 Similar results were reported in a companion study.22
Fezolinetant is contraindicated for patients with liver cirrhosis or severe renal impairment (estimated glomerular filtration rate of < 30 mL/min/1.73 m2). Before initiating treatment, serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and bilirubin (total and direct). Fezolinetant should not be prescribed if any of these tests are greater than twice the upper limit of normal. These tests should be repeated at 3, 6, and 9 months, and if the patient reports symptoms or signs of liver injury (nausea, vomiting, jaundice). Fezolinetant is metabolized by CYP1A2 and should not be prescribed to patients taking strong CYP1A2 inhibitors. The most common side effects associated with fezolinetant treatment are abdominal pain (4.3%), diarrhea (3.9%), insomnia (3.9%), back pain (3.0%), and hepatic transaminase elevation (2.3%). Fezolinetant has not been thoroughly evaluated in patients older than age 65. Following an oral dose of the medication, the median maximum concentration is reached in 1.5 hours, and the half-life is estimated to be 10 hours.20 Of all the medications discussed in this editorial, fezolinetant is the most expensive.
Effective VMS treatment improves overall health
Estrogen therapy is the gold standard treatment of VMS. However, many menopausal patients with bothersome VMS prefer not to take estrogen, and some have a medical condition that is a contraindication to estrogen treatment. The nonhormonal medication options for the treatment of VMS include escitalopram, paroxetine, gabapentin, and fezolinetant. Patients value the ability to choose the treatment they prefer, among all available hormonal and nonhormonal medication options. For mid-life women, effectively treating bothersome VMS is only one of many interventions that improves health. Optimal health is best achieved with23:
- high-quality diet
- daily physical activity
- appropriate body mass index
- nicotine avoidance
- a healthy sleep schedule
- normal blood pressure, lipid, and glucose levels.
Women who have a high-quality diet; daily physical activity; an appropriate body mass index; and normal blood pressure, cholesterol, and glucose levels are estimated to live 9 disease-free years longer than other women.24 ●
VMS, also known as hot flashes, night sweats, or cold sweats, occur for the majority of perimenopausal and menopausal women.1 In one study, the mean duration of clinically significant VMS was 5 years, and one-third of participants continued to have bothersome hot flashes 10 or more years after the onset of menopause.2 VMS may contribute to disrupted sleep patterns and depressed mood.3
All obstetrician-gynecologists know that estradiol and other estrogens are highly effective in the treatment of bothersome VMS. A meta-analysis reported that the frequency of VMS was reduced by 60% to 80% with oral estradiol (1 mg/day), transdermal estradiol(0.05 mg/day), and conjugated estrogen (0.625 mg).4 Breast tenderness and irregular uterine bleeding are common side effects of estrogen treatment of VMS. Estrogen treatment is contraindicated in patients with estrogen-responsive cancers, coronary heart disease, myocardial infarction, stroke, venous thromboembolism, and some cases of inherited thrombophilia. For these patients, an important option is the nonhormonal treatment of VMS, and several nonhormonal medications have been demonstrated to be effective therapy (TABLE 1). In this editorial I will review the medication treatment of VMS with escitalopram, paroxetine, gabapentin, and fezolinetant.

Escitalopram and paroxetine
Escitalopram and paroxetine have been shown to reduce VMS more than placebo in multiple clinical trials.5-10 In addition, escitalopram and paroxetine, at the doses tested, may be more effective for the treatment of VMS than sertraline, citalopram, or fluoxetine.11 In one trial assessing the efficacy of escitalopram to treat VMS, 205 patients with VMS were randomly assigned to 8 weeks of treatment with placebo or escitalopram.5 The initial escitalopram dose was 10 mg daily. At week 4:
- if VMS frequency was reduced by ≥ 50%, the patient remained on the 10-mg dose
- if VMS frequency was reduced by < 50%, the escitalopram dose was increased to 20 mg daily.
Following 8 weeks of treatment, the frequency of VMS decreased for patients in the placebo and escitalopram groups by 33% and 47%, respectively. Similar results have been reported in other studies.6
Paroxetine at a dose of 7.5 mg/day administered at bedtime is approved by the US Food and Drug Administration (FDA) for the treatment of VMS. In a pivotal study, 1,112 patients with VMS were randomly assigned to receive a placebo or paroxetine 7.5 mg at bedtime.9 In the 12-week study the reported decrease in mean weekly frequency of VMS for patients in the placebo and paroxetine groups were -37 and -44, respectively.9 Paroxetine 7.5 mg also reduced awakenings per night attributed to VMS and increased nighttime sleep duration.10
Depressed mood is prevalent among perimenopausal and postmenopausal patients.12 Prescribing escitalopram or paroxetine for VMS also may improve mood. Venlafaxine and desvenlafaxine are effective for the treatment of VMS;13,14 however, I seldom prescribe these medications for VMS because in my experience they are associated with more bothersome side effects, including dry mouth, decreased appetite, nausea, and insomnia than escitalopram or low-dose paroxetine.
Gabapentin
Numerous randomized clinical trials have reported that gabapentin is superior to placebo for the treatment of VMS.15 In one trial, 420 patients with breast cancer and VMS were randomly assigned to 8 weeks of treatment with placebo, gabapentin 300 mg/day (G300), or gabapentin 900 mg/day (G900) in 3 divided doses.16 Following 8 weeks of treatment, reduction in hot-flash severity score among patients receiving placebo, G300, or G900 was 15%, 31%, and 46%, respectively. Fatigue and somnolence were reported more frequently among patients taking gabapentin 900 mg/day. In a small trial, 60 patients with VMS were randomized to receive placebo, conjugated estrogen (0.2625 mg/day),or gabapentin (target dose of 2,400 mg/day in 3 divided doses).17 Following 12 weeks of treatment, the patient-reported decrease in VMS for those taking placebo, estrogen, or gabapentin was 54%, 72%, and 71%, respectively.
High-dose gabapentin treatment was associated with side effects of headache and dizziness more often than placebo or estrogen. Although gabapentin is not a treatment for insomnia, in my practice if a menopausal patient has prominent and bothersome symptoms of sleep disturbance and mild VMS symptoms, I will consider a trial of low-dose gabapentin. Some experts recommend initiating gabapentin at a dose of 100 mgdaily before bedtime to assess the effectiveness of a low dose that seldom causes significant side effects.

Fezolinetant
In a study of genetic variation associated with VMS, investigators discovered that nucleic acid variation in the neurokinin 3 (NK3) receptor was strongly associated with the prevalence of VMS, suggesting that this receptor is in the causal pathway to menopausal VMS.18 Additional research demonstrated that the kisspeptin/neurokinin B/dynorphin (KNDy) neurons, which are involved in the control of hypothalamic thermoregulation, are stimulated by neurokinin B, acting through the NK3 receptor, and suppressed by estradiol. A reduction in hypothalamic estrogen results in unopposed neurokinin B activity, which stimulates KNDy neurons, destabilizing the hypothalamic thermoregulatory center, causing vasodilation, which is perceived as hot flashes and sweating followed by chills.19
Fezolinetant is a high-affinity NK3 receptor antagonist that blocks the activity of neurokinin B, stabilizing the hypothalamic thermoregulatory center, thereby suppressing hot flashes. It is approved by the FDA for the treatment of moderate to severe VMS due to menopause using a fixed dose of 45 mg daily.20 In one clinical trial, 500 menopausal patients with bothersome VMS were randomly assigned to 12 weeks of treatment with placebo, fezolinetant 30 mg/day, or fezolinetant 45 mg/day. Following 12 weeks of treatment, the reported frequency rates of VMS among patients in the placebo, F30, and F45 groups were reduced by 43%, 61%, and 64%, respectively.21 In addition, following 12 weeks of treatment, the severity of VMS rates among patients in the placebo, F30, and F45 groups were reduced by 20%, 26%, and 32%, respectively.
Fezolinetant improved the quality of sleep and was associated with an improvement in patient-reported quality of life. Following 12 weeks of treatment, sleep quality among patients in the placebo, F30, and F45 groups was reported to be “much or moderately better” in 34%, 45%, and 54% of the patients, respectively.21 Similar results were reported in a companion study.22
Fezolinetant is contraindicated for patients with liver cirrhosis or severe renal impairment (estimated glomerular filtration rate of < 30 mL/min/1.73 m2). Before initiating treatment, serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and bilirubin (total and direct). Fezolinetant should not be prescribed if any of these tests are greater than twice the upper limit of normal. These tests should be repeated at 3, 6, and 9 months, and if the patient reports symptoms or signs of liver injury (nausea, vomiting, jaundice). Fezolinetant is metabolized by CYP1A2 and should not be prescribed to patients taking strong CYP1A2 inhibitors. The most common side effects associated with fezolinetant treatment are abdominal pain (4.3%), diarrhea (3.9%), insomnia (3.9%), back pain (3.0%), and hepatic transaminase elevation (2.3%). Fezolinetant has not been thoroughly evaluated in patients older than age 65. Following an oral dose of the medication, the median maximum concentration is reached in 1.5 hours, and the half-life is estimated to be 10 hours.20 Of all the medications discussed in this editorial, fezolinetant is the most expensive.
Effective VMS treatment improves overall health
Estrogen therapy is the gold standard treatment of VMS. However, many menopausal patients with bothersome VMS prefer not to take estrogen, and some have a medical condition that is a contraindication to estrogen treatment. The nonhormonal medication options for the treatment of VMS include escitalopram, paroxetine, gabapentin, and fezolinetant. Patients value the ability to choose the treatment they prefer, among all available hormonal and nonhormonal medication options. For mid-life women, effectively treating bothersome VMS is only one of many interventions that improves health. Optimal health is best achieved with23:
- high-quality diet
- daily physical activity
- appropriate body mass index
- nicotine avoidance
- a healthy sleep schedule
- normal blood pressure, lipid, and glucose levels.
Women who have a high-quality diet; daily physical activity; an appropriate body mass index; and normal blood pressure, cholesterol, and glucose levels are estimated to live 9 disease-free years longer than other women.24 ●
- Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopause transition: study of women’s health across the nation. Am J Pub Health. 2006;1226-1235.
- Freeman EW, Sammel MD, Sanders RJ. Risk of long-term hot flashes after natural menopause: evidence from the Penn Ovarian Aging Study cohort. Menopause. 2014;21:924-932.
- Hatcher KM, Smith RL, Chiang C, et al. Nocturnal hot flashes, but not serum hormone concentrations as a predictor of insomnia in menopausal women: results from the Midlife Women’s Health Study. J Women’s Health. 2023;32:94-101.
- Nelson HD. Commonly used types of postmenopausal estrogen for treatment of hot flashes: scientific review. JAMA. 2004;291:1610.
- Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011;305:267-227.
- Carpenter JS, Guthrie KA, Larson JC, et al. Effect of escitalopram on hot flash interference: a randomized, controlled trial. Fertil Steril. 2012;97:1399-1404.e1.
- Slaton RM, Champion MN, Palmore KB. A review of paroxetine for the treatment of vasomotor symptoms. J Pharm Pract. 2015;28:266-274.
- Stearns V, Slack R, Greep N, et al. Paroxetine is an effective treatment for hot flashes: results from a prospective randomized clinical trial. J Clin Oncol. 2005;23:6919-6930.
- Simon JA, Portman DJ, Kaunitz AM, et al. Lowdose paroxetine 7.5 mg for menopausal vasomotor symptoms: two randomized controlled trials. Menopause. 2013;20:1027-1035.
- Pinkerton JV, Joffe H, Kazempour K, et al. Lowdose paroxetine (7.5 mg) improves sleep in women with vasomotor symptoms associated with menopause. Menopause. 2015;22:50-58.
- Shams T, Firwana B, Habib F, et al. SSRIs for hot flashes: a systematic review and metaanalysis of randomized trials. J Gen Intern Med. 2014;29:204-213.
- Freeman EW. Depression in the menopause transition: risks in the changing hormone milieu as observed in the general population. Womens Midlife Health. 2015;1:2.
- Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet. 2000;356:2059-2063.
- Sun Z, Hao Y, Zhang M. Efficacy and safety of desvenlafaxine treatment for hot flashes associated with menopause: a meta-analysis of randomized controlled trials. Gynecol Obstet Invest. 2013;75:255-262.
- Toulis KA, Tzellos T, Kouvelas D, et al. Gabapentin for the treatment of hot flashes in women with natural or tamoxifen-induced menopause: a systematic review and meta-analysis. Clin Ther. 2009;31:221-235.
- Pandya KJ, Morrow GR, Roscoe JA, et al. Gabapentin for hot flashes in 420 women with breast cancer: a randomized double-blind placebocontrolled trial. Lancet. 2005;366:818-824.
- Reddy SY, Warner H, Guttuso T Jr, et al. Gabapentin, estrogen, and placebo for treating hot flushes: a randomized controlled trial. Obstet Gynecol. 2006;108:41-48.
- Crandall CJ, Manson JE, Hohensee C, et al. Association of genetic variation in the tachykinin receptor 3 locus with hot flashes and night sweats in the Women’s Health Initiative Study. Menopause. 2017;24:252.
- Rance NE, Dacks PA, Mittelman-Smith MA, et al. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neurendocrinol. 2013;34:211-227.
- Veozah (package insert). Astellas Pharma; Northbrook, Illinois. May 2023.
- Johnson KA, Martin N, Nappi RE, et al. Efficacy and safety of fezolinetant in moderate-to-severe vasomotor symptoms associated with menopause: a Phase 3 RCT. J Clin Endocrinol Metab. 2023;108:1981-1997.
- Lederman S, Ottery FD, Cano A, et al. Fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause (SKYLIGHT 1): a phase 3 randomised controlled study. Lancet. 2023;401:1091-1102.
- Lloyd-Jones DM, Allen NB, Anderson CAM, et al. Life’s essential 8: updating and enhancing the American Heart Association’s construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. 2022;146:e18-43.
- Wang X, Ma H, Li X, et al. Association of cardiovascular health with life expectancy free of cardiovascular disease, diabetes, cancer, and dementia in U.K. adults. JAMA Int Med. 2023;183:340-349.
- Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopause transition: study of women’s health across the nation. Am J Pub Health. 2006;1226-1235.
- Freeman EW, Sammel MD, Sanders RJ. Risk of long-term hot flashes after natural menopause: evidence from the Penn Ovarian Aging Study cohort. Menopause. 2014;21:924-932.
- Hatcher KM, Smith RL, Chiang C, et al. Nocturnal hot flashes, but not serum hormone concentrations as a predictor of insomnia in menopausal women: results from the Midlife Women’s Health Study. J Women’s Health. 2023;32:94-101.
- Nelson HD. Commonly used types of postmenopausal estrogen for treatment of hot flashes: scientific review. JAMA. 2004;291:1610.
- Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011;305:267-227.
- Carpenter JS, Guthrie KA, Larson JC, et al. Effect of escitalopram on hot flash interference: a randomized, controlled trial. Fertil Steril. 2012;97:1399-1404.e1.
- Slaton RM, Champion MN, Palmore KB. A review of paroxetine for the treatment of vasomotor symptoms. J Pharm Pract. 2015;28:266-274.
- Stearns V, Slack R, Greep N, et al. Paroxetine is an effective treatment for hot flashes: results from a prospective randomized clinical trial. J Clin Oncol. 2005;23:6919-6930.
- Simon JA, Portman DJ, Kaunitz AM, et al. Lowdose paroxetine 7.5 mg for menopausal vasomotor symptoms: two randomized controlled trials. Menopause. 2013;20:1027-1035.
- Pinkerton JV, Joffe H, Kazempour K, et al. Lowdose paroxetine (7.5 mg) improves sleep in women with vasomotor symptoms associated with menopause. Menopause. 2015;22:50-58.
- Shams T, Firwana B, Habib F, et al. SSRIs for hot flashes: a systematic review and metaanalysis of randomized trials. J Gen Intern Med. 2014;29:204-213.
- Freeman EW. Depression in the menopause transition: risks in the changing hormone milieu as observed in the general population. Womens Midlife Health. 2015;1:2.
- Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet. 2000;356:2059-2063.
- Sun Z, Hao Y, Zhang M. Efficacy and safety of desvenlafaxine treatment for hot flashes associated with menopause: a meta-analysis of randomized controlled trials. Gynecol Obstet Invest. 2013;75:255-262.
- Toulis KA, Tzellos T, Kouvelas D, et al. Gabapentin for the treatment of hot flashes in women with natural or tamoxifen-induced menopause: a systematic review and meta-analysis. Clin Ther. 2009;31:221-235.
- Pandya KJ, Morrow GR, Roscoe JA, et al. Gabapentin for hot flashes in 420 women with breast cancer: a randomized double-blind placebocontrolled trial. Lancet. 2005;366:818-824.
- Reddy SY, Warner H, Guttuso T Jr, et al. Gabapentin, estrogen, and placebo for treating hot flushes: a randomized controlled trial. Obstet Gynecol. 2006;108:41-48.
- Crandall CJ, Manson JE, Hohensee C, et al. Association of genetic variation in the tachykinin receptor 3 locus with hot flashes and night sweats in the Women’s Health Initiative Study. Menopause. 2017;24:252.
- Rance NE, Dacks PA, Mittelman-Smith MA, et al. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neurendocrinol. 2013;34:211-227.
- Veozah (package insert). Astellas Pharma; Northbrook, Illinois. May 2023.
- Johnson KA, Martin N, Nappi RE, et al. Efficacy and safety of fezolinetant in moderate-to-severe vasomotor symptoms associated with menopause: a Phase 3 RCT. J Clin Endocrinol Metab. 2023;108:1981-1997.
- Lederman S, Ottery FD, Cano A, et al. Fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause (SKYLIGHT 1): a phase 3 randomised controlled study. Lancet. 2023;401:1091-1102.
- Lloyd-Jones DM, Allen NB, Anderson CAM, et al. Life’s essential 8: updating and enhancing the American Heart Association’s construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. 2022;146:e18-43.
- Wang X, Ma H, Li X, et al. Association of cardiovascular health with life expectancy free of cardiovascular disease, diabetes, cancer, and dementia in U.K. adults. JAMA Int Med. 2023;183:340-349.

