User login
LAS VEGAS – Treatment with ferric citrate coordination complex conferred multiple benefits with cardioprotective implications in non–dialysis-dependent chronic kidney disease patients with elevated serum phosphate and iron-deficiency anemia in a randomized trial.
Ferric citrate coordination complex (FCCC), an investigational oral product also known as Zerenex, effectively lowered patients’ elevated serum phosphate into normal range while repleting iron stores, boosting hemoglobin, and reducing levels of the cardiotoxic protein fibroblast growth factor 23 (FGF23), Dr. Geoffrey A. Block reported at a meeting sponsored by the National Kidney Foundation.
"We are quite happy with these results and the implications they may have in trying to address cardiovascular risk at multiple levels in chronic kidney disease," declared Dr. Block of Denver Nephrology, who was principal investigator in the phase II study.
FCCC is currently under Food and Drug Administration review for potential marketing approval as a treatment in patients with end-stage renal disease complicated by iron-deficiency anemia and elevated serum phosphate. However, the phase II study led by Dr. Block focused on the much larger patient population with non–dialysis-dependent chronic kidney disease (CKD) with elevated serum phosphate and iron-deficiency anemia. Experience has shown it is far more difficult to lower serum phosphate in such patients than in those with end-stage renal disease, the nephrologist noted.
The 12-week, double-blind clinical trial included 141 subjects with an estimated glomerular filtration rate below 60 mL/min per 1.73 m2, a serum phosphate in excess of 4.0 mg/dL, a transferrin saturation below 30%, a hemoglobin level of 9-12 g/dL, and a serum ferritin below 300 ng/mL. Participants were not permitted to use intravenous iron or an erythropoietin-stimulating agent in the months prior to or during the trial. They were randomized to FCCC titrated to achieve a serum phosphate below 3.5 mg/dL or to placebo.
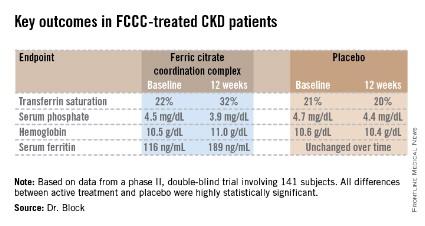
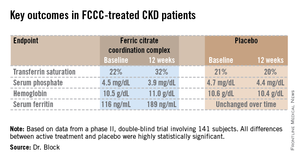
The coprimary endpoints were changes from baseline through 12 weeks in serum phosphate and transferrin saturation. The FCCC-treated patients fared significantly better than controls on those endpoints as well as the secondary outcomes (see chart).
• FGF23: The FCCC group’s 40% drop in C-terminal FGF23 over the course of the 12-week study, while levels remained static in the control group, was particularly noteworthy, according to Dr. Block. In several observational studies, including the Heart and Soul Study (Ann. Intern. Med. 2010;152:640-8), elevated FGF23 levels have been associated with significantly increased risk of cardiovascular events and all-cause mortality.
Elevated FGF23 levels appear to promote left-ventricular hypertrophy, while hyperphosphatemia promotes vascular calcification. Through these different mechanisms, both abnormalities increase the risk of cardiovascular events. Both abnormalities are common in patients with CKD. And FCCC resulted in significant reductions in both FGF23 and serum phosphate, he noted.
A prespecified safety feature of the phase II trial was that any participant whose hemoglobin fell below 9.0 g/dL or whose serum phosphate climbed above 6.0 mg/dL would have to be taken out of the study. One patient on FCCC and nine controls were withdrawn from the study for low hemoglobin. No patients in the FCCC group and two controls were removed due to an excessive phosphate level.
The safety profile of FCCC was essentially the same as for placebo with two exceptions: 20% of the FCCC group reported diarrhea, compared with 6% of controls; and one-third of the FCCC group reported discolored feces as a consequence of iron utilization.
• Serum phosphate: Dr. Block said that "reasonably solid evidence" from observational studies, clinical trials, and animal studies indicates that a serum phosphate level greater than 4.0 mg/dL leads to faster progression of kidney disease and an increase in cardiovascular events. For example, in a fully adjusted reanalysis of data from the randomized prospective REIN (Ramipril Efficacy in Nephropathy) trial, patients with a baseline serum phosphate above 4.0 mg/dL were at greatly increased risk for the combined endpoint of a doubling of serum creatinine or progression to end-stage renal disease (J. Am. Soc. Nephrol. 2011;22:1923-30).
Moreover, the renoprotective effect of ramipril decreased as baseline serum phosphate increased: While the ACE inhibitor reduced the risk of the combined endpoint by 85%, compared with placebo, in subjects with a serum phosphate less than 3.45 mg/dL and by 63% in those with a level of 3.45-4.0 mg/dL, ramipril wasn’t significantly more effective than placebo in those with a serum phosphate above 4.0 mg/dL.
Dietary phosphorus intake, which is high in the United States, has also come under scrutiny as a public health concern. A recent prospective cohort study in 9,686 healthy U.S. adults concluded that consumption of more than 1,400 mg daily – the median intake in this representative study population was 1,166 mg per day – was independently associated with higher all-cause mortality. Those with a phosphorus density greater than 0.35 mg/kcal, a statistic derived by dividing phosphorus intake by energy intake – had a significantly increased risk of cardiovascular mortality (Am. J. Clin. Nutr. 2014;99:320-7). And in an analysis of 4,494 participants in MESA (Multi-Ethnic Study of Atherosclerosis), dietary phosphorus intake was independently associated with greater left-ventricular mass such that subjects in the top dietary phosphorus quintile had a 6.1-g greater left-ventricular mass than those in the lowest quintile (Kidney Intl. 2013;83:707-14).
The three phosphate binders currently on the market for reduction of elevated serum phosphate in CKD patients are "really marginal treatments," according to Dr. Block. He was first author on a study in which 148 patients with moderate CKD were randomized to 9 months of calcium acetate, sevelamer carbonate, lanthanum carbonate, or placebo. Serum phosphorus inched lower over the 9 months from a baseline of 4.2 mg/dL to 3.9 mg/dL with active therapy, which was only 0.2 mg/dL better than with placebo. In contrast, serum phosphate fell by an average of 0.6 mg/dL during 3 months on FCCC in the phase II study. Moreover, active therapy with the commercially available phosphate binders had no effect upon FGF23 levels, and it significantly increased coronary artery and abdominal aorta calcification by a median of 18% and 15%, respectively (J. Am. Soc. Nephrol. 2012;23:1407-15).
• Iron-deficiency anemia: The Kidney Dialysis International Guideline Organization defines iron deficiency warranting iron supplementation in CKD patients as a transferrin saturation of 30% or less and a serum ferritin of 50 ng/mL or less. By those criteria, it is estimated that nearly 70% of CKD patients are iron deficient. So there is a large unmet need for iron repletion therapies that avoid the use of erythropoietin-stimulating agents and intravenous iron, Dr. Block noted.
The FCCC trial was sponsored by Keryx Biopharmaceuticals. Dr. Block serves as a consultant to the company and was principal investigator in the study.
LAS VEGAS – Treatment with ferric citrate coordination complex conferred multiple benefits with cardioprotective implications in non–dialysis-dependent chronic kidney disease patients with elevated serum phosphate and iron-deficiency anemia in a randomized trial.
Ferric citrate coordination complex (FCCC), an investigational oral product also known as Zerenex, effectively lowered patients’ elevated serum phosphate into normal range while repleting iron stores, boosting hemoglobin, and reducing levels of the cardiotoxic protein fibroblast growth factor 23 (FGF23), Dr. Geoffrey A. Block reported at a meeting sponsored by the National Kidney Foundation.

"We are quite happy with these results and the implications they may have in trying to address cardiovascular risk at multiple levels in chronic kidney disease," declared Dr. Block of Denver Nephrology, who was principal investigator in the phase II study.
FCCC is currently under Food and Drug Administration review for potential marketing approval as a treatment in patients with end-stage renal disease complicated by iron-deficiency anemia and elevated serum phosphate. However, the phase II study led by Dr. Block focused on the much larger patient population with non–dialysis-dependent chronic kidney disease (CKD) with elevated serum phosphate and iron-deficiency anemia. Experience has shown it is far more difficult to lower serum phosphate in such patients than in those with end-stage renal disease, the nephrologist noted.
The 12-week, double-blind clinical trial included 141 subjects with an estimated glomerular filtration rate below 60 mL/min per 1.73 m2, a serum phosphate in excess of 4.0 mg/dL, a transferrin saturation below 30%, a hemoglobin level of 9-12 g/dL, and a serum ferritin below 300 ng/mL. Participants were not permitted to use intravenous iron or an erythropoietin-stimulating agent in the months prior to or during the trial. They were randomized to FCCC titrated to achieve a serum phosphate below 3.5 mg/dL or to placebo.
The coprimary endpoints were changes from baseline through 12 weeks in serum phosphate and transferrin saturation. The FCCC-treated patients fared significantly better than controls on those endpoints as well as the secondary outcomes (see chart).
• FGF23: The FCCC group’s 40% drop in C-terminal FGF23 over the course of the 12-week study, while levels remained static in the control group, was particularly noteworthy, according to Dr. Block. In several observational studies, including the Heart and Soul Study (Ann. Intern. Med. 2010;152:640-8), elevated FGF23 levels have been associated with significantly increased risk of cardiovascular events and all-cause mortality.
Elevated FGF23 levels appear to promote left-ventricular hypertrophy, while hyperphosphatemia promotes vascular calcification. Through these different mechanisms, both abnormalities increase the risk of cardiovascular events. Both abnormalities are common in patients with CKD. And FCCC resulted in significant reductions in both FGF23 and serum phosphate, he noted.
A prespecified safety feature of the phase II trial was that any participant whose hemoglobin fell below 9.0 g/dL or whose serum phosphate climbed above 6.0 mg/dL would have to be taken out of the study. One patient on FCCC and nine controls were withdrawn from the study for low hemoglobin. No patients in the FCCC group and two controls were removed due to an excessive phosphate level.
The safety profile of FCCC was essentially the same as for placebo with two exceptions: 20% of the FCCC group reported diarrhea, compared with 6% of controls; and one-third of the FCCC group reported discolored feces as a consequence of iron utilization.
• Serum phosphate: Dr. Block said that "reasonably solid evidence" from observational studies, clinical trials, and animal studies indicates that a serum phosphate level greater than 4.0 mg/dL leads to faster progression of kidney disease and an increase in cardiovascular events. For example, in a fully adjusted reanalysis of data from the randomized prospective REIN (Ramipril Efficacy in Nephropathy) trial, patients with a baseline serum phosphate above 4.0 mg/dL were at greatly increased risk for the combined endpoint of a doubling of serum creatinine or progression to end-stage renal disease (J. Am. Soc. Nephrol. 2011;22:1923-30).
Moreover, the renoprotective effect of ramipril decreased as baseline serum phosphate increased: While the ACE inhibitor reduced the risk of the combined endpoint by 85%, compared with placebo, in subjects with a serum phosphate less than 3.45 mg/dL and by 63% in those with a level of 3.45-4.0 mg/dL, ramipril wasn’t significantly more effective than placebo in those with a serum phosphate above 4.0 mg/dL.
Dietary phosphorus intake, which is high in the United States, has also come under scrutiny as a public health concern. A recent prospective cohort study in 9,686 healthy U.S. adults concluded that consumption of more than 1,400 mg daily – the median intake in this representative study population was 1,166 mg per day – was independently associated with higher all-cause mortality. Those with a phosphorus density greater than 0.35 mg/kcal, a statistic derived by dividing phosphorus intake by energy intake – had a significantly increased risk of cardiovascular mortality (Am. J. Clin. Nutr. 2014;99:320-7). And in an analysis of 4,494 participants in MESA (Multi-Ethnic Study of Atherosclerosis), dietary phosphorus intake was independently associated with greater left-ventricular mass such that subjects in the top dietary phosphorus quintile had a 6.1-g greater left-ventricular mass than those in the lowest quintile (Kidney Intl. 2013;83:707-14).
The three phosphate binders currently on the market for reduction of elevated serum phosphate in CKD patients are "really marginal treatments," according to Dr. Block. He was first author on a study in which 148 patients with moderate CKD were randomized to 9 months of calcium acetate, sevelamer carbonate, lanthanum carbonate, or placebo. Serum phosphorus inched lower over the 9 months from a baseline of 4.2 mg/dL to 3.9 mg/dL with active therapy, which was only 0.2 mg/dL better than with placebo. In contrast, serum phosphate fell by an average of 0.6 mg/dL during 3 months on FCCC in the phase II study. Moreover, active therapy with the commercially available phosphate binders had no effect upon FGF23 levels, and it significantly increased coronary artery and abdominal aorta calcification by a median of 18% and 15%, respectively (J. Am. Soc. Nephrol. 2012;23:1407-15).
• Iron-deficiency anemia: The Kidney Dialysis International Guideline Organization defines iron deficiency warranting iron supplementation in CKD patients as a transferrin saturation of 30% or less and a serum ferritin of 50 ng/mL or less. By those criteria, it is estimated that nearly 70% of CKD patients are iron deficient. So there is a large unmet need for iron repletion therapies that avoid the use of erythropoietin-stimulating agents and intravenous iron, Dr. Block noted.
The FCCC trial was sponsored by Keryx Biopharmaceuticals. Dr. Block serves as a consultant to the company and was principal investigator in the study.
LAS VEGAS – Treatment with ferric citrate coordination complex conferred multiple benefits with cardioprotective implications in non–dialysis-dependent chronic kidney disease patients with elevated serum phosphate and iron-deficiency anemia in a randomized trial.
Ferric citrate coordination complex (FCCC), an investigational oral product also known as Zerenex, effectively lowered patients’ elevated serum phosphate into normal range while repleting iron stores, boosting hemoglobin, and reducing levels of the cardiotoxic protein fibroblast growth factor 23 (FGF23), Dr. Geoffrey A. Block reported at a meeting sponsored by the National Kidney Foundation.

"We are quite happy with these results and the implications they may have in trying to address cardiovascular risk at multiple levels in chronic kidney disease," declared Dr. Block of Denver Nephrology, who was principal investigator in the phase II study.
FCCC is currently under Food and Drug Administration review for potential marketing approval as a treatment in patients with end-stage renal disease complicated by iron-deficiency anemia and elevated serum phosphate. However, the phase II study led by Dr. Block focused on the much larger patient population with non–dialysis-dependent chronic kidney disease (CKD) with elevated serum phosphate and iron-deficiency anemia. Experience has shown it is far more difficult to lower serum phosphate in such patients than in those with end-stage renal disease, the nephrologist noted.
The 12-week, double-blind clinical trial included 141 subjects with an estimated glomerular filtration rate below 60 mL/min per 1.73 m2, a serum phosphate in excess of 4.0 mg/dL, a transferrin saturation below 30%, a hemoglobin level of 9-12 g/dL, and a serum ferritin below 300 ng/mL. Participants were not permitted to use intravenous iron or an erythropoietin-stimulating agent in the months prior to or during the trial. They were randomized to FCCC titrated to achieve a serum phosphate below 3.5 mg/dL or to placebo.
The coprimary endpoints were changes from baseline through 12 weeks in serum phosphate and transferrin saturation. The FCCC-treated patients fared significantly better than controls on those endpoints as well as the secondary outcomes (see chart).
• FGF23: The FCCC group’s 40% drop in C-terminal FGF23 over the course of the 12-week study, while levels remained static in the control group, was particularly noteworthy, according to Dr. Block. In several observational studies, including the Heart and Soul Study (Ann. Intern. Med. 2010;152:640-8), elevated FGF23 levels have been associated with significantly increased risk of cardiovascular events and all-cause mortality.
Elevated FGF23 levels appear to promote left-ventricular hypertrophy, while hyperphosphatemia promotes vascular calcification. Through these different mechanisms, both abnormalities increase the risk of cardiovascular events. Both abnormalities are common in patients with CKD. And FCCC resulted in significant reductions in both FGF23 and serum phosphate, he noted.
A prespecified safety feature of the phase II trial was that any participant whose hemoglobin fell below 9.0 g/dL or whose serum phosphate climbed above 6.0 mg/dL would have to be taken out of the study. One patient on FCCC and nine controls were withdrawn from the study for low hemoglobin. No patients in the FCCC group and two controls were removed due to an excessive phosphate level.
The safety profile of FCCC was essentially the same as for placebo with two exceptions: 20% of the FCCC group reported diarrhea, compared with 6% of controls; and one-third of the FCCC group reported discolored feces as a consequence of iron utilization.
• Serum phosphate: Dr. Block said that "reasonably solid evidence" from observational studies, clinical trials, and animal studies indicates that a serum phosphate level greater than 4.0 mg/dL leads to faster progression of kidney disease and an increase in cardiovascular events. For example, in a fully adjusted reanalysis of data from the randomized prospective REIN (Ramipril Efficacy in Nephropathy) trial, patients with a baseline serum phosphate above 4.0 mg/dL were at greatly increased risk for the combined endpoint of a doubling of serum creatinine or progression to end-stage renal disease (J. Am. Soc. Nephrol. 2011;22:1923-30).
Moreover, the renoprotective effect of ramipril decreased as baseline serum phosphate increased: While the ACE inhibitor reduced the risk of the combined endpoint by 85%, compared with placebo, in subjects with a serum phosphate less than 3.45 mg/dL and by 63% in those with a level of 3.45-4.0 mg/dL, ramipril wasn’t significantly more effective than placebo in those with a serum phosphate above 4.0 mg/dL.
Dietary phosphorus intake, which is high in the United States, has also come under scrutiny as a public health concern. A recent prospective cohort study in 9,686 healthy U.S. adults concluded that consumption of more than 1,400 mg daily – the median intake in this representative study population was 1,166 mg per day – was independently associated with higher all-cause mortality. Those with a phosphorus density greater than 0.35 mg/kcal, a statistic derived by dividing phosphorus intake by energy intake – had a significantly increased risk of cardiovascular mortality (Am. J. Clin. Nutr. 2014;99:320-7). And in an analysis of 4,494 participants in MESA (Multi-Ethnic Study of Atherosclerosis), dietary phosphorus intake was independently associated with greater left-ventricular mass such that subjects in the top dietary phosphorus quintile had a 6.1-g greater left-ventricular mass than those in the lowest quintile (Kidney Intl. 2013;83:707-14).
The three phosphate binders currently on the market for reduction of elevated serum phosphate in CKD patients are "really marginal treatments," according to Dr. Block. He was first author on a study in which 148 patients with moderate CKD were randomized to 9 months of calcium acetate, sevelamer carbonate, lanthanum carbonate, or placebo. Serum phosphorus inched lower over the 9 months from a baseline of 4.2 mg/dL to 3.9 mg/dL with active therapy, which was only 0.2 mg/dL better than with placebo. In contrast, serum phosphate fell by an average of 0.6 mg/dL during 3 months on FCCC in the phase II study. Moreover, active therapy with the commercially available phosphate binders had no effect upon FGF23 levels, and it significantly increased coronary artery and abdominal aorta calcification by a median of 18% and 15%, respectively (J. Am. Soc. Nephrol. 2012;23:1407-15).
• Iron-deficiency anemia: The Kidney Dialysis International Guideline Organization defines iron deficiency warranting iron supplementation in CKD patients as a transferrin saturation of 30% or less and a serum ferritin of 50 ng/mL or less. By those criteria, it is estimated that nearly 70% of CKD patients are iron deficient. So there is a large unmet need for iron repletion therapies that avoid the use of erythropoietin-stimulating agents and intravenous iron, Dr. Block noted.
The FCCC trial was sponsored by Keryx Biopharmaceuticals. Dr. Block serves as a consultant to the company and was principal investigator in the study.
AT SCM 14
Major finding: FCCC safely repleted low iron stores, reduced elevated serum phosphate, raised hemoglobin, and slashed FGF23 levels, compared with placebo.
Data source: A prospective, double-blind, placebo-controlled, 12-week trial in which 141 patients with non–dialysis-dependent CKD, iron-deficiency anemia, and elevated serum phosphate were assigned to FCCC or placebo.
Disclosures: The study was sponsored by Keryx Biopharmaceuticals. Dr. Block is a consultant to the company and was principal investigator in the trial.