News

Secukinumab brings high PASI 75 results in 6- to 17-year-olds with psoriasis
- Author:
- Bruce Jancin
The week-24 PASI 75 rate was 95% in a phase 2 study.
News

Enzymatic injections show durable improvement in buttock cellulite
- Author:
- Bruce Jancin
Expert says success with collagenase clostridium histolyticum–aaes injections will be all about managing expectations.
News
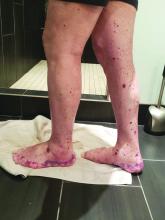
VEXAS: A novel rheumatologic, hematologic syndrome that’s making waves
- Author:
- Jeff Evans
- Bruce Jancin
VEXAS is a novel adult-onset autoinflammatory syndrome in older men and is often associated with hematologic...
News
Bimekizumab superior to adalimumab in head-to-head psoriasis study
- Author:
- Bruce Jancin
The response to bimekizumab was notably fast.
News
Hedgehog inhibitor alternative dosing advantageous for BCC
- Author:
- Bruce Jancin
The goal of step-down therapy is lifelong tolerability.
News

Coffee could be the secret weapon against NAFLD
- Author:
- Bruce Jancin
Two or more cups daily slashes risk of hepatic cirrhosis and hepatocellular carcinoma.
News

AI system beats endoscopists for detecting early neoplasia in Barrett’s
- Author:
- Bruce Jancin
“I think this will be a really helpful addition, the equivalent of a second endoscopist raising a yellow flag to take a closer look at a...
News

Treatment paradigm for chronic HBV in flux
- Author:
- Bruce Jancin
Some patients achieve functional cure after treatment withdrawal, while others face flares or other challenges.
News

Oral sarecycline promising for papulopustular rosacea
- Author:
- Bruce Jancin
“Always aim for clear skin.”
News

National Psoriasis Foundation recommends some stop methotrexate for 2 weeks after J&J vaccine
- Author:
- Bruce Jancin
The rationale is to optimize the antibody response to the killed-adenovirus COVID-19 vaccine.
News

AI system beats endoscopists for detecting early neoplasia in Barrett’s
- Author:
- Bruce Jancin
“I think this will be a really helpful addition, the equivalent of a second endoscopist raising a yellow flag to take a closer look at a...
News
Experts highlight recent breakthroughs in psoriatic arthritis
- Author:
- Bruce Jancin
Signal of possible gender disparity in PsA response to biologics sparks questions.
News

Will psoriasis patients embrace proactive topical therapy?
- Author:
- Bruce Jancin
Results of year-long PSO-LONG trial show superior efficacy, compared with conventional reactive management.
News
Baricitinib hits mark for severe alopecia areata
- Author:
- Bruce Jancin
Clinically meaningful hair regrowth occurred in the BRAVE-AA1 trial despite 16-year disease duration.
News

Ruxolitinib cream for atopic dermatitis is in regulatory home stretch
- Author:
- Bruce Jancin
Topical JAK inhibitor displayed dual mechanisms of benefit in pivotal trials.
