User login
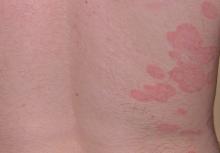
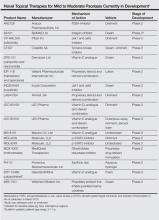
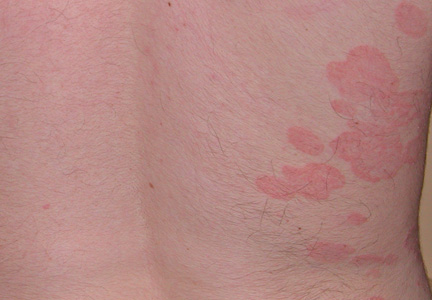
Topical therapies are a mainstay in the management of patients with mild to moderate psoriasis (Figure). Presently, US Food and Drug Administration–approved topical medications that are commercially available for use in patients with psoriasis include corticosteroids, vitamin D3 analogues, calcineurin inhibitors, retinoids, anthralin, and tar-based formulations.1 In recent years, research has furthered our understanding of the molecular mechanisms underlying the pathogenesis of psoriasis and has afforded the development of more targeted therapies. Novel topical medications currently in phase 2 and phase 3 clinical trials are discussed in this article, and a summary is provided in the Table.
AN2728 (Phosphodiesterase 4 Inhibitor)
AN2728 (Anacor Pharmaceuticals, Inc) is a phosphodiesterase 4 inhibitor that blocks the inactivation of cyclic adenosine monophosphate, resulting in decreased production of inflammatory cytokines (eg, IL-6, IL-12, IL-23, tumor necrosis factor α [TNF-α]).2,3 In a randomized, double-blind, phase 2 clinical trial (N=35), 40% of patients treated with AN2728 ointment 5% reported improvement of more than 2 points in overall target plaque severity score versus 6% of patients treated with vehicle. In another randomized, double-blind, dose-response trial of 145 patients, those treated with AN2728 ointment 2% twice daily reported a 60% improvement versus 40% improvement in those treated with AN2728 ointment 0.5% once daily.3 In total, 3 phase 1 trials (registered at www.clinicaltrials.gov with the identifiers NCT01258088, NCT00762658, NCT00763204) and 4 phase 2 trials (NCT01029405, NCT00755196, NCT00759161, NCT01300052) have been completed; results were not available at the time of publication.
AS101 (Integrin Inhibitor)
AS101 (BioMAS Ltd), or ammonium trichloro (dioxoethylene-o,o') tellurate, acts as stimulator of regulatory T cells and a redox modulator inhibiting the leukocyte integrins α4β1 and α4β7 that enable CD4+ T-cell and macrophage extravasation; it also limits expression of the inflammatory cytokines IL-6 and IL-17.4 A randomized, placebo-controlled, double-blind, phase 2 study evaluating the efficacy of AS101 cream 4% twice daily for 12 weeks was withdrawn prior to enrollment (NCT00788424).
Tofacitinib (Janus Kinase 1 and 3 Inhibitor)
Tofacitinib (formerly known as CP-690,550)(Pfizer Inc) is a selective Janus kinase (Jak) 1 and Jak3 inhibitor that limits expression of cytokines that promote inflammation (eg, IFN-γ) and inhibits helper T cells (TH17) by downregulating expression of the IL-23 receptor. Epidermal keratinocyte proliferation in psoriasis is activated by TH17 cells that release IL-17 as well as TH1 cells that release IFN-γ and tumor necrosis factor. A phase 2a trial showed statistically significant improvement from baseline in the target plaque severity score for tofacitinib ointment 2% (least squares mean, −54.4%) versus vehicle (least squares mean, −41.5%).5 Two other phase 2 trials (NCT01246583, NCT00678561) assessing the efficacy, safety, tolerability, and pharmacokinetics of tofacitinib ointment in patients with mild to moderate psoriasis have been completed; results were not available at the time of publication. A phase 2b study that compared 2 dose strengths of tofacitinib ointment—10 mg/g and 20 mg/g—versus placebo over a 12-week period also was completed (NCT01831466); results were not available at the time of publication.
CT327 (Tyrosine Kinase Inhibitor)
CT327 (Creabilis SA) is a tyrosine kinase A (TrkA) inhibitor that affords a novel perspective in the treatment of pruritus by shifting the focus to sensory neurons. In a phase 2b study of 160 patients, a 60% change in the visual analog scale was noted at 8 weeks in the treatment group versus 21% in the placebo group.6 Two other phase 2 studies have been completed, one with a cream formulation of pegylated K252a (NCT00995969) and another with an ointment formulation (NCT01465282); results were not available at the time of publication.
DPS-101 (Vitamin D Analogue)
DPS-101 (Dermipsor Ltd) is a combination of calcipotriol and niacinamide. Calcipotriol is a vitamin D3 analogue that increases IL-10 expression while decreasing IL-8 expression.7 It curbs epidermal keratinocyte proliferation by limiting the expression of polo-like kinase 2 and early growth response-1.8 It also may induce keratinocyte apoptosis.9 Niacinamide is the amide of vitamin B3 and inhibits proinflammatory cytokines such as TNF-α, IL-1β, IL-6, and IL-8.10 In a dose-response phase 2b trial of 168 patients, DPS-101 demonstrated better results than either calcipotriol or niacinamide alone.11
IDP-118 (Proprietary Steroid and Retinoid Combination)
IDP-118 (Valeant Pharmaceuticals International, Inc) is a combination of halobetasol propionate (HP) 0.01% (a topical corticosteroid) and tazar-otene 0.045% (a selective topical retinoid) in a lotion formulation. In isolation, tazarotene is as effective as a mid to highly potent corticosteroid, but irritation may limit its tolerability. The use of combination treatments of mid to highly potent corticosteroids and tazarotene has shown enhanced tolerability and therapeutic efficacy.12 Ongoing studies include a phase 1 trial and a phase 2 trial to evaluate low- and high-strength preparations of IDP-118, respectively (NCT01670513). Another phase 2 trial evaluating the efficacy and safety of IDP-118 lotion (HP 0.01% and tazarotene 0.045%) versus IDP-118 monad HP 0.01% lotion, IDP-118 monad tazar-otene 0.045% lotion, and placebo has been completed (NCT02045277); results were not available at the time of publication.
Ruxolitinib (Jak1 and Jak2 Inhibitor)
Ruxolitinib (formerly known as INCB18424)(Incyte Corporation) is a selective Jak1 and Jak2 inhibitor. A phase 2 trial of ruxolitinib showed a 53% decline in the score for mean total lesions in patients treated with ruxolitinib phosphate cream 1% (P=.033) versus 54% in those treated with ruxolitinib phosphate cream 1.5% (P=.056) and 32% in those treated with placebo.13 Three other phase 2 studies (NCT00617994, NCT00820950, NCT00778700) have been completed; results were not available at the time of publication.
LAS41004 (Proprietary Steroid and Retinoid Combination)
LAS41004 (Almirall, SA) is an ointment containing the corticosteroid betamethasone dipropionate and the retinoid bexarotene that is being evaluated for treatment of mild to moderate psoriasis. Five phase 2 studies (NCT01119339, NCT01283698, NCT01360944, NCT02111499, NCT01462643) have been completed; results were not available at the time of publication. A randomized, double-blind, phase 2a study (NCT02180464) with a left-right design assessing clinical response to LAS41004 versus control in patients with mild to moderate psoriasis was actively recruiting at the time of publication.
LEO 80190 (Vitamin D3 Analogue and Steroid Combination)
LEO 80190 (LEO Pharma) is a combination of the vitamin D3 analogue calcipotriol and the corticosteroid hydrocortisone. It was developed as a treatment for sensitive areas such as the face and intertriginous regions. A randomized, investigator-blind, phase 3 trial (NCT00640822) of LEO 80190 ointment versus tacalcitol ointment and placebo once daily for 8 weeks demonstrated controlled disease of the face in 56.8% (183/322) of patients in the LEO 80190 group, 46.4% (147/317) in the tacalcitol group, and 36.3% (37/102) in the placebo group.14 Another phase 2 study (NCT00704262) and 2 phase 3 studies (NCT00691002, NCT01007591) have been completed; results were not available at the time of publication.
LEO 90100 (Vitamin D Analogue and Steroid Combination)
LEO 90100 (LEO Pharma) contains the vitamin D3 analogue calcipotriol and the corticosteroid betamethasone. Three phase 2 studies (NCT01347255, NCT01536886, NCT01536938) and a phase 3 study (NCT01866163) examining the efficacy and safety of various vehicles and formulations of LEO 90100 have been completed; results were not available at the time of publication. Another phase 3 study (NCT02132936) is ongoing but not recruiting participants. Other completed studies whose results were not yet available include a phase 1 pharmacodynamic study (NCT01946386), a phase 1 study that used patch testing to assess the degree of skin irritation and sensitization associated with LEO 90100 (NCT01935869), and a phase 2 study examining the impact of LEO 90100 on calcium metabolism and the hypothalamic-pituitary-adrenal axis (NCT01600222).
M518101 (Vitamin D Analogue)
M518101 (Maruho Co, Ltd) is a novel topical vitamin D3 analogue. Phase 1 (NCT01844973) and phase 2 (NCT01301157, NCT00884169) trials evaluating the safety, pharmacokinetics, and efficacy of M518101 have been completed; results were not available at the time of publication. A phase 3 study (NCT01989429) assessing the safety and therapeutic efficacy of M518101 according to changes in the modified psoriasis area and severity index over an 8-week treatment period also has been completed; results were not yet available. Three phase 3 studies assessing the safety and therapeutic efficacy of M518101 are ongoing: one is currently closed to recruitment (NCT01908595) and 2 are actively recruiting participants at the time of publication (NCT01878461, NCT01873677).
MOL4239 and MOL4249 (Phosphorylated Signal Transducer and Activator of Transcription 3 Inhibitors)
MOL4239 (Moleculin, LLC) is a novel topical agent for use in mild to moderate psoriasis that acts via phosphorylated signal transducer and activator of transcription 3 (p-STAT3) inhibition.15 The p-STAT3 protein has increased expression in psoriasis.16 A phase 2 trial of MOL4239 ointment (NCT01826201) has been completed, showing a greater mean (standard deviation) change in the psoriasis severity score in lesions treated at 28 days with MOL4239 ointment 10% (−1.9 [1.45]) versus lesions treated with placebo ointment (−1.5 [1.87]).17
MOL4249 (Moleculin, LLC) is more potent than MOL4239 with better lipid solubility. In the MOL4249 subset of a placebo-controlled, double-blind, phase 2a study of 16 patients with mild to moderate psoriasis, 10% (1/10) of patients experienced complete clearance of psoriatic plaques, 30% (3/10) of patients experienced 75% or greater improvement, and 50% (5/10) of patients experienced 50% or greater improvement compared to 17% (1/6) in the placebo group. Currently, a phase 2a contralateral study, a phase 2b psoriasis area and severity index trial, and a phase 3 pivotal trial are planned, according to the manufacturer.18
MQX-5902 (Dihydrofolate Reductase Inhibitor)
MQX-5902 (MediQuest Therapeutics) is a topical preparation of methotrexate for the treatment of fingernail psoriasis. Methotrexate is a dihydrofolate reductase inhibitor and antimetabolite that inhibits folic acid metabolism, thereby disrupting DNA synthesis.19 A phase 2b dose-ranging trial (NCT00666354) was designed to assess the therapeutic efficacy and safety of MQX-5902 delivered via a proprietary drug delivery formulation in fingernail psoriasis; the outcome of this trial was not available at the time of publication.
PH-10 (Xanthine Dye)
PH-10 (Provectus Biopharmaceuticals, Inc) is a topical aqueous hydrogel derived from rose bengal disodium that may be beneficial in treating skin conditions such as atopic dermatitis and mild to moderate psoriasis. Rose bengal disodium is a hydrophilic xanthine dye with diagnostic utility in ophthalmology and gastroenterology as well as projected use as a melanoma treatment as demonstrated in phase 1 and phase 2 clinical trials of PV-10 (Provectus Biopharmaceuticals, Inc).20 Two phase 2 studies assessing the safety and therapeutic efficacy of PH-10 in psoriasis (NCT01247818, NCT00941278) have been completed; results were not available at the time of publication.
STF115469 (Vitamin D Analogue)
STF115469 (GlaxoSmithKline) is a calcipotriene foam. At the time of publication, a randomized, placebo-controlled, double-blind, phase 3 trial (NCT01582932) of this vitamin D3 analogue with a projected enrollment of 180 participants was actively recruiting patients aged 2 to 11 years with mild to moderate plaque psoriasis to study the efficacy, safety, and tolerability of STF115469, as well as its pharmacokinetics and pharmacodynamics.
WBI-1001 (Proprietary Product)
WBI-1001 (Welichem Biotech Inc), or 2-isopropyl-5-[(E)-2-phenylethenyl] benzene-1, 3-diol, is a novel proprietary agent that inhibits proinflammatory cytokines (eg, IFN-γ, TNF-α). A randomized, placebo-controlled, double-blind, phase 1 trial (NCT00830817) assessing the efficacy, safety, tolerability, and pharmacokinetics of WBI-1001 has been completed; results were not available at the time of publication. Another randomized, placebo-controlled, double-blind, phase 2 trial (NCT01098721) evaluating its efficacy and safety according to the physician’s global assessment demonstrated a therapeutic benefit of 62.8% in patients treated with WBI-1001 cream 1% versus 13.0% in those treated with a placebo after a 12-week treatment period (P<.0001).21 WBI-1001 may offer a novel approach in the treatment of mild to moderate psoriasis.
Conclusion
Enhanced knowledge of the underlying pathogeneses of psoriasis and psoriatic arthritis has identified new therapeutic targets and enabled the development of exciting novel treatments for these conditions. The topical agents currently in phase 2 and phase 3 clinical trials show promise in enhancing the way physicians treat psoriasis. There is hope for more individualized treatment regimens with improved tolerability and better safety profiles with increased therapeutic efficacy. As our understanding of the molecular underpinnings of psoriasis continues to deepen, it will afford the development of even more innovative therapeutics for use in the management of psoriasis.
1. Mason A, Mason J, Cork M, et al. Topical treatments for chronic plaque psoriasis: an abridged Cochrane systematic review. J Am Acad Dermatol. 2013;69:799-807.
2. Nazarian R, Weinberg JM. AN-2728, a PDE4 inhibitor for the potential topical treatment of psoriasis and atopic dermatitis. Curr Opin Investig Drugs. 2009;10:1236-1242.
3. Moustafa F, Feldman SR. A review of phosphodiesterase-inhibition and the potential role for phosphodiesterase 4-inhibitors in clinical dermatology. Dermatol Online J. 2014;20:22608.
4. Halpert G, Sredni B. The effect of the novel tellurium compound AS101 on autoimmune diseases. Autoimmun Rev. 2014;13:1230-1235.
5. Ports WC, Khan S, Lan S, et al. A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br J Dermatol. 2013;169:137-145.
6. Yosipovitch G, Roblin D, Traversa S, et al. A novel topical targeted anti-pruritic treatment in phase 2b development for chronic pruritus. Paper presented at: 72nd Annual Meeting of the American Academy of Dermatology; March 21-25, 2014; Denver, CO.
7. Kang S, Yi S, Griffiths CE, et al. Calcipotriene-induced improvement in psoriasis is associated with reduced interleukin-8 and increased interleukin-10 levels within lesions. Br J Dermatol. 1998;138:77-83.
8. Kristl J, Slanc P, Krasna M, et al. Calcipotriol affects keratinocyte proliferation by decreasing expression of early growth response-1 and polo-like kinase-2. Pharm Res. 2008;25:521-529.
9. Tiberio R, Bozzo C, Pertusi G, et al. Calcipotriol induces apoptosis in psoriatic keratinocytes. Clin Exp Dermatol. 2009;34:e972-e974.
10. Luger T, Seite S, Humbert P, et al. Recommendations for adjunctive basic skin care in patients with psoriasis. Eur J Dermatol. 2014;24:194-200.
11. Dermipsor reports good results in DPS-101 Phase IIb study for plaque psoriasis [press release]. Evaluate Web site. http://www.evaluategroup.com/Universal/View.aspx?type=Story&id=250042. Published October 15, 2007. Accessed February 13, 2015.
12. Rivera AM, Hsu S. Topical halobetasol propionate in the treatment of plaque psoriasis: a review. Am J Clin Dermatol. 2005;6:311-316.
13. Punwani N, Scherle P, Flores R, et al. Preliminary clinical activity of a topical JAK1/2 inhibitor in the treatment of psoriasis. J Am Acad Dermatol. 2012;67:658-664.
14. Efficacy and safety of calcipotriol plus hydrocortisone ointment compared with tacalcitol ointment in patients with psoriasis on the face and skin folds (NCT00640822). https://clinicaltrials.gov/ct2/show/results/NCT00640822?term=NCT00640822&rank=1. Updated October 21, 2013. Accessed May 30, 2014.
15. Product candidates: targeting p-STAT3 for improved psoriasis treatment. Moleculin Web site. http://moleculin.com/product-candidates/mol4239. Accessed February 13, 2015.
16. Chowdhari S, Saini N. hsa-miR-4516 mediated downregulation of STAT3/CDK6/UBE2N plays a role in PUVA induced apoptosis in keratinocytes. J Cell Physiol. 2014;229:1630-1638.
17. Paired psoriasis lesion, comparative, study to evaluate MOL4239 in psoriasis (NCT01826201). https://clinicaltrials.gov/ct2/show/results/NCT01826201?term=NCT01826201&rank=1§=X01256#all. Updated December 22, 2014. Accessed February 25, 2015.
18. Clinical development pipeline. Moleculin Web site. http://moleculin.com/clinical-trials/psoriasis-trials. Accessed February 13, 2015.
19. de la Brassinne M, Nikkels A. Psoriasis: state of the art 2013. Acta Clin Belg. 2013;68:433-441.
20. Ross MI. Intralesional therapy with PV-10 (Rose Bengal) for in-transit melanoma. J Surg Oncol. 2014;109:314-319.
21. Bissonnette R, Bolduc C, Maari C, et al. Efficacy and safety of topical WBI-1001 in patients with mild to moderate psoriasis: results from a randomized, double-blind placebo-controlled, phase II trial. J Eur Acad Dermatol Venereol. 2012;26:1516-1521.
Topical therapies are a mainstay in the management of patients with mild to moderate psoriasis (Figure). Presently, US Food and Drug Administration–approved topical medications that are commercially available for use in patients with psoriasis include corticosteroids, vitamin D3 analogues, calcineurin inhibitors, retinoids, anthralin, and tar-based formulations.1 In recent years, research has furthered our understanding of the molecular mechanisms underlying the pathogenesis of psoriasis and has afforded the development of more targeted therapies. Novel topical medications currently in phase 2 and phase 3 clinical trials are discussed in this article, and a summary is provided in the Table.
AN2728 (Phosphodiesterase 4 Inhibitor)
AN2728 (Anacor Pharmaceuticals, Inc) is a phosphodiesterase 4 inhibitor that blocks the inactivation of cyclic adenosine monophosphate, resulting in decreased production of inflammatory cytokines (eg, IL-6, IL-12, IL-23, tumor necrosis factor α [TNF-α]).2,3 In a randomized, double-blind, phase 2 clinical trial (N=35), 40% of patients treated with AN2728 ointment 5% reported improvement of more than 2 points in overall target plaque severity score versus 6% of patients treated with vehicle. In another randomized, double-blind, dose-response trial of 145 patients, those treated with AN2728 ointment 2% twice daily reported a 60% improvement versus 40% improvement in those treated with AN2728 ointment 0.5% once daily.3 In total, 3 phase 1 trials (registered at www.clinicaltrials.gov with the identifiers NCT01258088, NCT00762658, NCT00763204) and 4 phase 2 trials (NCT01029405, NCT00755196, NCT00759161, NCT01300052) have been completed; results were not available at the time of publication.
AS101 (Integrin Inhibitor)
AS101 (BioMAS Ltd), or ammonium trichloro (dioxoethylene-o,o') tellurate, acts as stimulator of regulatory T cells and a redox modulator inhibiting the leukocyte integrins α4β1 and α4β7 that enable CD4+ T-cell and macrophage extravasation; it also limits expression of the inflammatory cytokines IL-6 and IL-17.4 A randomized, placebo-controlled, double-blind, phase 2 study evaluating the efficacy of AS101 cream 4% twice daily for 12 weeks was withdrawn prior to enrollment (NCT00788424).
Tofacitinib (Janus Kinase 1 and 3 Inhibitor)
Tofacitinib (formerly known as CP-690,550)(Pfizer Inc) is a selective Janus kinase (Jak) 1 and Jak3 inhibitor that limits expression of cytokines that promote inflammation (eg, IFN-γ) and inhibits helper T cells (TH17) by downregulating expression of the IL-23 receptor. Epidermal keratinocyte proliferation in psoriasis is activated by TH17 cells that release IL-17 as well as TH1 cells that release IFN-γ and tumor necrosis factor. A phase 2a trial showed statistically significant improvement from baseline in the target plaque severity score for tofacitinib ointment 2% (least squares mean, −54.4%) versus vehicle (least squares mean, −41.5%).5 Two other phase 2 trials (NCT01246583, NCT00678561) assessing the efficacy, safety, tolerability, and pharmacokinetics of tofacitinib ointment in patients with mild to moderate psoriasis have been completed; results were not available at the time of publication. A phase 2b study that compared 2 dose strengths of tofacitinib ointment—10 mg/g and 20 mg/g—versus placebo over a 12-week period also was completed (NCT01831466); results were not available at the time of publication.
CT327 (Tyrosine Kinase Inhibitor)
CT327 (Creabilis SA) is a tyrosine kinase A (TrkA) inhibitor that affords a novel perspective in the treatment of pruritus by shifting the focus to sensory neurons. In a phase 2b study of 160 patients, a 60% change in the visual analog scale was noted at 8 weeks in the treatment group versus 21% in the placebo group.6 Two other phase 2 studies have been completed, one with a cream formulation of pegylated K252a (NCT00995969) and another with an ointment formulation (NCT01465282); results were not available at the time of publication.
DPS-101 (Vitamin D Analogue)
DPS-101 (Dermipsor Ltd) is a combination of calcipotriol and niacinamide. Calcipotriol is a vitamin D3 analogue that increases IL-10 expression while decreasing IL-8 expression.7 It curbs epidermal keratinocyte proliferation by limiting the expression of polo-like kinase 2 and early growth response-1.8 It also may induce keratinocyte apoptosis.9 Niacinamide is the amide of vitamin B3 and inhibits proinflammatory cytokines such as TNF-α, IL-1β, IL-6, and IL-8.10 In a dose-response phase 2b trial of 168 patients, DPS-101 demonstrated better results than either calcipotriol or niacinamide alone.11
IDP-118 (Proprietary Steroid and Retinoid Combination)
IDP-118 (Valeant Pharmaceuticals International, Inc) is a combination of halobetasol propionate (HP) 0.01% (a topical corticosteroid) and tazar-otene 0.045% (a selective topical retinoid) in a lotion formulation. In isolation, tazarotene is as effective as a mid to highly potent corticosteroid, but irritation may limit its tolerability. The use of combination treatments of mid to highly potent corticosteroids and tazarotene has shown enhanced tolerability and therapeutic efficacy.12 Ongoing studies include a phase 1 trial and a phase 2 trial to evaluate low- and high-strength preparations of IDP-118, respectively (NCT01670513). Another phase 2 trial evaluating the efficacy and safety of IDP-118 lotion (HP 0.01% and tazarotene 0.045%) versus IDP-118 monad HP 0.01% lotion, IDP-118 monad tazar-otene 0.045% lotion, and placebo has been completed (NCT02045277); results were not available at the time of publication.
Ruxolitinib (Jak1 and Jak2 Inhibitor)
Ruxolitinib (formerly known as INCB18424)(Incyte Corporation) is a selective Jak1 and Jak2 inhibitor. A phase 2 trial of ruxolitinib showed a 53% decline in the score for mean total lesions in patients treated with ruxolitinib phosphate cream 1% (P=.033) versus 54% in those treated with ruxolitinib phosphate cream 1.5% (P=.056) and 32% in those treated with placebo.13 Three other phase 2 studies (NCT00617994, NCT00820950, NCT00778700) have been completed; results were not available at the time of publication.
LAS41004 (Proprietary Steroid and Retinoid Combination)
LAS41004 (Almirall, SA) is an ointment containing the corticosteroid betamethasone dipropionate and the retinoid bexarotene that is being evaluated for treatment of mild to moderate psoriasis. Five phase 2 studies (NCT01119339, NCT01283698, NCT01360944, NCT02111499, NCT01462643) have been completed; results were not available at the time of publication. A randomized, double-blind, phase 2a study (NCT02180464) with a left-right design assessing clinical response to LAS41004 versus control in patients with mild to moderate psoriasis was actively recruiting at the time of publication.
LEO 80190 (Vitamin D3 Analogue and Steroid Combination)
LEO 80190 (LEO Pharma) is a combination of the vitamin D3 analogue calcipotriol and the corticosteroid hydrocortisone. It was developed as a treatment for sensitive areas such as the face and intertriginous regions. A randomized, investigator-blind, phase 3 trial (NCT00640822) of LEO 80190 ointment versus tacalcitol ointment and placebo once daily for 8 weeks demonstrated controlled disease of the face in 56.8% (183/322) of patients in the LEO 80190 group, 46.4% (147/317) in the tacalcitol group, and 36.3% (37/102) in the placebo group.14 Another phase 2 study (NCT00704262) and 2 phase 3 studies (NCT00691002, NCT01007591) have been completed; results were not available at the time of publication.
LEO 90100 (Vitamin D Analogue and Steroid Combination)
LEO 90100 (LEO Pharma) contains the vitamin D3 analogue calcipotriol and the corticosteroid betamethasone. Three phase 2 studies (NCT01347255, NCT01536886, NCT01536938) and a phase 3 study (NCT01866163) examining the efficacy and safety of various vehicles and formulations of LEO 90100 have been completed; results were not available at the time of publication. Another phase 3 study (NCT02132936) is ongoing but not recruiting participants. Other completed studies whose results were not yet available include a phase 1 pharmacodynamic study (NCT01946386), a phase 1 study that used patch testing to assess the degree of skin irritation and sensitization associated with LEO 90100 (NCT01935869), and a phase 2 study examining the impact of LEO 90100 on calcium metabolism and the hypothalamic-pituitary-adrenal axis (NCT01600222).
M518101 (Vitamin D Analogue)
M518101 (Maruho Co, Ltd) is a novel topical vitamin D3 analogue. Phase 1 (NCT01844973) and phase 2 (NCT01301157, NCT00884169) trials evaluating the safety, pharmacokinetics, and efficacy of M518101 have been completed; results were not available at the time of publication. A phase 3 study (NCT01989429) assessing the safety and therapeutic efficacy of M518101 according to changes in the modified psoriasis area and severity index over an 8-week treatment period also has been completed; results were not yet available. Three phase 3 studies assessing the safety and therapeutic efficacy of M518101 are ongoing: one is currently closed to recruitment (NCT01908595) and 2 are actively recruiting participants at the time of publication (NCT01878461, NCT01873677).
MOL4239 and MOL4249 (Phosphorylated Signal Transducer and Activator of Transcription 3 Inhibitors)
MOL4239 (Moleculin, LLC) is a novel topical agent for use in mild to moderate psoriasis that acts via phosphorylated signal transducer and activator of transcription 3 (p-STAT3) inhibition.15 The p-STAT3 protein has increased expression in psoriasis.16 A phase 2 trial of MOL4239 ointment (NCT01826201) has been completed, showing a greater mean (standard deviation) change in the psoriasis severity score in lesions treated at 28 days with MOL4239 ointment 10% (−1.9 [1.45]) versus lesions treated with placebo ointment (−1.5 [1.87]).17
MOL4249 (Moleculin, LLC) is more potent than MOL4239 with better lipid solubility. In the MOL4249 subset of a placebo-controlled, double-blind, phase 2a study of 16 patients with mild to moderate psoriasis, 10% (1/10) of patients experienced complete clearance of psoriatic plaques, 30% (3/10) of patients experienced 75% or greater improvement, and 50% (5/10) of patients experienced 50% or greater improvement compared to 17% (1/6) in the placebo group. Currently, a phase 2a contralateral study, a phase 2b psoriasis area and severity index trial, and a phase 3 pivotal trial are planned, according to the manufacturer.18
MQX-5902 (Dihydrofolate Reductase Inhibitor)
MQX-5902 (MediQuest Therapeutics) is a topical preparation of methotrexate for the treatment of fingernail psoriasis. Methotrexate is a dihydrofolate reductase inhibitor and antimetabolite that inhibits folic acid metabolism, thereby disrupting DNA synthesis.19 A phase 2b dose-ranging trial (NCT00666354) was designed to assess the therapeutic efficacy and safety of MQX-5902 delivered via a proprietary drug delivery formulation in fingernail psoriasis; the outcome of this trial was not available at the time of publication.
PH-10 (Xanthine Dye)
PH-10 (Provectus Biopharmaceuticals, Inc) is a topical aqueous hydrogel derived from rose bengal disodium that may be beneficial in treating skin conditions such as atopic dermatitis and mild to moderate psoriasis. Rose bengal disodium is a hydrophilic xanthine dye with diagnostic utility in ophthalmology and gastroenterology as well as projected use as a melanoma treatment as demonstrated in phase 1 and phase 2 clinical trials of PV-10 (Provectus Biopharmaceuticals, Inc).20 Two phase 2 studies assessing the safety and therapeutic efficacy of PH-10 in psoriasis (NCT01247818, NCT00941278) have been completed; results were not available at the time of publication.
STF115469 (Vitamin D Analogue)
STF115469 (GlaxoSmithKline) is a calcipotriene foam. At the time of publication, a randomized, placebo-controlled, double-blind, phase 3 trial (NCT01582932) of this vitamin D3 analogue with a projected enrollment of 180 participants was actively recruiting patients aged 2 to 11 years with mild to moderate plaque psoriasis to study the efficacy, safety, and tolerability of STF115469, as well as its pharmacokinetics and pharmacodynamics.
WBI-1001 (Proprietary Product)
WBI-1001 (Welichem Biotech Inc), or 2-isopropyl-5-[(E)-2-phenylethenyl] benzene-1, 3-diol, is a novel proprietary agent that inhibits proinflammatory cytokines (eg, IFN-γ, TNF-α). A randomized, placebo-controlled, double-blind, phase 1 trial (NCT00830817) assessing the efficacy, safety, tolerability, and pharmacokinetics of WBI-1001 has been completed; results were not available at the time of publication. Another randomized, placebo-controlled, double-blind, phase 2 trial (NCT01098721) evaluating its efficacy and safety according to the physician’s global assessment demonstrated a therapeutic benefit of 62.8% in patients treated with WBI-1001 cream 1% versus 13.0% in those treated with a placebo after a 12-week treatment period (P<.0001).21 WBI-1001 may offer a novel approach in the treatment of mild to moderate psoriasis.
Conclusion
Enhanced knowledge of the underlying pathogeneses of psoriasis and psoriatic arthritis has identified new therapeutic targets and enabled the development of exciting novel treatments for these conditions. The topical agents currently in phase 2 and phase 3 clinical trials show promise in enhancing the way physicians treat psoriasis. There is hope for more individualized treatment regimens with improved tolerability and better safety profiles with increased therapeutic efficacy. As our understanding of the molecular underpinnings of psoriasis continues to deepen, it will afford the development of even more innovative therapeutics for use in the management of psoriasis.
Topical therapies are a mainstay in the management of patients with mild to moderate psoriasis (Figure). Presently, US Food and Drug Administration–approved topical medications that are commercially available for use in patients with psoriasis include corticosteroids, vitamin D3 analogues, calcineurin inhibitors, retinoids, anthralin, and tar-based formulations.1 In recent years, research has furthered our understanding of the molecular mechanisms underlying the pathogenesis of psoriasis and has afforded the development of more targeted therapies. Novel topical medications currently in phase 2 and phase 3 clinical trials are discussed in this article, and a summary is provided in the Table.
AN2728 (Phosphodiesterase 4 Inhibitor)
AN2728 (Anacor Pharmaceuticals, Inc) is a phosphodiesterase 4 inhibitor that blocks the inactivation of cyclic adenosine monophosphate, resulting in decreased production of inflammatory cytokines (eg, IL-6, IL-12, IL-23, tumor necrosis factor α [TNF-α]).2,3 In a randomized, double-blind, phase 2 clinical trial (N=35), 40% of patients treated with AN2728 ointment 5% reported improvement of more than 2 points in overall target plaque severity score versus 6% of patients treated with vehicle. In another randomized, double-blind, dose-response trial of 145 patients, those treated with AN2728 ointment 2% twice daily reported a 60% improvement versus 40% improvement in those treated with AN2728 ointment 0.5% once daily.3 In total, 3 phase 1 trials (registered at www.clinicaltrials.gov with the identifiers NCT01258088, NCT00762658, NCT00763204) and 4 phase 2 trials (NCT01029405, NCT00755196, NCT00759161, NCT01300052) have been completed; results were not available at the time of publication.
AS101 (Integrin Inhibitor)
AS101 (BioMAS Ltd), or ammonium trichloro (dioxoethylene-o,o') tellurate, acts as stimulator of regulatory T cells and a redox modulator inhibiting the leukocyte integrins α4β1 and α4β7 that enable CD4+ T-cell and macrophage extravasation; it also limits expression of the inflammatory cytokines IL-6 and IL-17.4 A randomized, placebo-controlled, double-blind, phase 2 study evaluating the efficacy of AS101 cream 4% twice daily for 12 weeks was withdrawn prior to enrollment (NCT00788424).
Tofacitinib (Janus Kinase 1 and 3 Inhibitor)
Tofacitinib (formerly known as CP-690,550)(Pfizer Inc) is a selective Janus kinase (Jak) 1 and Jak3 inhibitor that limits expression of cytokines that promote inflammation (eg, IFN-γ) and inhibits helper T cells (TH17) by downregulating expression of the IL-23 receptor. Epidermal keratinocyte proliferation in psoriasis is activated by TH17 cells that release IL-17 as well as TH1 cells that release IFN-γ and tumor necrosis factor. A phase 2a trial showed statistically significant improvement from baseline in the target plaque severity score for tofacitinib ointment 2% (least squares mean, −54.4%) versus vehicle (least squares mean, −41.5%).5 Two other phase 2 trials (NCT01246583, NCT00678561) assessing the efficacy, safety, tolerability, and pharmacokinetics of tofacitinib ointment in patients with mild to moderate psoriasis have been completed; results were not available at the time of publication. A phase 2b study that compared 2 dose strengths of tofacitinib ointment—10 mg/g and 20 mg/g—versus placebo over a 12-week period also was completed (NCT01831466); results were not available at the time of publication.
CT327 (Tyrosine Kinase Inhibitor)
CT327 (Creabilis SA) is a tyrosine kinase A (TrkA) inhibitor that affords a novel perspective in the treatment of pruritus by shifting the focus to sensory neurons. In a phase 2b study of 160 patients, a 60% change in the visual analog scale was noted at 8 weeks in the treatment group versus 21% in the placebo group.6 Two other phase 2 studies have been completed, one with a cream formulation of pegylated K252a (NCT00995969) and another with an ointment formulation (NCT01465282); results were not available at the time of publication.
DPS-101 (Vitamin D Analogue)
DPS-101 (Dermipsor Ltd) is a combination of calcipotriol and niacinamide. Calcipotriol is a vitamin D3 analogue that increases IL-10 expression while decreasing IL-8 expression.7 It curbs epidermal keratinocyte proliferation by limiting the expression of polo-like kinase 2 and early growth response-1.8 It also may induce keratinocyte apoptosis.9 Niacinamide is the amide of vitamin B3 and inhibits proinflammatory cytokines such as TNF-α, IL-1β, IL-6, and IL-8.10 In a dose-response phase 2b trial of 168 patients, DPS-101 demonstrated better results than either calcipotriol or niacinamide alone.11
IDP-118 (Proprietary Steroid and Retinoid Combination)
IDP-118 (Valeant Pharmaceuticals International, Inc) is a combination of halobetasol propionate (HP) 0.01% (a topical corticosteroid) and tazar-otene 0.045% (a selective topical retinoid) in a lotion formulation. In isolation, tazarotene is as effective as a mid to highly potent corticosteroid, but irritation may limit its tolerability. The use of combination treatments of mid to highly potent corticosteroids and tazarotene has shown enhanced tolerability and therapeutic efficacy.12 Ongoing studies include a phase 1 trial and a phase 2 trial to evaluate low- and high-strength preparations of IDP-118, respectively (NCT01670513). Another phase 2 trial evaluating the efficacy and safety of IDP-118 lotion (HP 0.01% and tazarotene 0.045%) versus IDP-118 monad HP 0.01% lotion, IDP-118 monad tazar-otene 0.045% lotion, and placebo has been completed (NCT02045277); results were not available at the time of publication.
Ruxolitinib (Jak1 and Jak2 Inhibitor)
Ruxolitinib (formerly known as INCB18424)(Incyte Corporation) is a selective Jak1 and Jak2 inhibitor. A phase 2 trial of ruxolitinib showed a 53% decline in the score for mean total lesions in patients treated with ruxolitinib phosphate cream 1% (P=.033) versus 54% in those treated with ruxolitinib phosphate cream 1.5% (P=.056) and 32% in those treated with placebo.13 Three other phase 2 studies (NCT00617994, NCT00820950, NCT00778700) have been completed; results were not available at the time of publication.
LAS41004 (Proprietary Steroid and Retinoid Combination)
LAS41004 (Almirall, SA) is an ointment containing the corticosteroid betamethasone dipropionate and the retinoid bexarotene that is being evaluated for treatment of mild to moderate psoriasis. Five phase 2 studies (NCT01119339, NCT01283698, NCT01360944, NCT02111499, NCT01462643) have been completed; results were not available at the time of publication. A randomized, double-blind, phase 2a study (NCT02180464) with a left-right design assessing clinical response to LAS41004 versus control in patients with mild to moderate psoriasis was actively recruiting at the time of publication.
LEO 80190 (Vitamin D3 Analogue and Steroid Combination)
LEO 80190 (LEO Pharma) is a combination of the vitamin D3 analogue calcipotriol and the corticosteroid hydrocortisone. It was developed as a treatment for sensitive areas such as the face and intertriginous regions. A randomized, investigator-blind, phase 3 trial (NCT00640822) of LEO 80190 ointment versus tacalcitol ointment and placebo once daily for 8 weeks demonstrated controlled disease of the face in 56.8% (183/322) of patients in the LEO 80190 group, 46.4% (147/317) in the tacalcitol group, and 36.3% (37/102) in the placebo group.14 Another phase 2 study (NCT00704262) and 2 phase 3 studies (NCT00691002, NCT01007591) have been completed; results were not available at the time of publication.
LEO 90100 (Vitamin D Analogue and Steroid Combination)
LEO 90100 (LEO Pharma) contains the vitamin D3 analogue calcipotriol and the corticosteroid betamethasone. Three phase 2 studies (NCT01347255, NCT01536886, NCT01536938) and a phase 3 study (NCT01866163) examining the efficacy and safety of various vehicles and formulations of LEO 90100 have been completed; results were not available at the time of publication. Another phase 3 study (NCT02132936) is ongoing but not recruiting participants. Other completed studies whose results were not yet available include a phase 1 pharmacodynamic study (NCT01946386), a phase 1 study that used patch testing to assess the degree of skin irritation and sensitization associated with LEO 90100 (NCT01935869), and a phase 2 study examining the impact of LEO 90100 on calcium metabolism and the hypothalamic-pituitary-adrenal axis (NCT01600222).
M518101 (Vitamin D Analogue)
M518101 (Maruho Co, Ltd) is a novel topical vitamin D3 analogue. Phase 1 (NCT01844973) and phase 2 (NCT01301157, NCT00884169) trials evaluating the safety, pharmacokinetics, and efficacy of M518101 have been completed; results were not available at the time of publication. A phase 3 study (NCT01989429) assessing the safety and therapeutic efficacy of M518101 according to changes in the modified psoriasis area and severity index over an 8-week treatment period also has been completed; results were not yet available. Three phase 3 studies assessing the safety and therapeutic efficacy of M518101 are ongoing: one is currently closed to recruitment (NCT01908595) and 2 are actively recruiting participants at the time of publication (NCT01878461, NCT01873677).
MOL4239 and MOL4249 (Phosphorylated Signal Transducer and Activator of Transcription 3 Inhibitors)
MOL4239 (Moleculin, LLC) is a novel topical agent for use in mild to moderate psoriasis that acts via phosphorylated signal transducer and activator of transcription 3 (p-STAT3) inhibition.15 The p-STAT3 protein has increased expression in psoriasis.16 A phase 2 trial of MOL4239 ointment (NCT01826201) has been completed, showing a greater mean (standard deviation) change in the psoriasis severity score in lesions treated at 28 days with MOL4239 ointment 10% (−1.9 [1.45]) versus lesions treated with placebo ointment (−1.5 [1.87]).17
MOL4249 (Moleculin, LLC) is more potent than MOL4239 with better lipid solubility. In the MOL4249 subset of a placebo-controlled, double-blind, phase 2a study of 16 patients with mild to moderate psoriasis, 10% (1/10) of patients experienced complete clearance of psoriatic plaques, 30% (3/10) of patients experienced 75% or greater improvement, and 50% (5/10) of patients experienced 50% or greater improvement compared to 17% (1/6) in the placebo group. Currently, a phase 2a contralateral study, a phase 2b psoriasis area and severity index trial, and a phase 3 pivotal trial are planned, according to the manufacturer.18
MQX-5902 (Dihydrofolate Reductase Inhibitor)
MQX-5902 (MediQuest Therapeutics) is a topical preparation of methotrexate for the treatment of fingernail psoriasis. Methotrexate is a dihydrofolate reductase inhibitor and antimetabolite that inhibits folic acid metabolism, thereby disrupting DNA synthesis.19 A phase 2b dose-ranging trial (NCT00666354) was designed to assess the therapeutic efficacy and safety of MQX-5902 delivered via a proprietary drug delivery formulation in fingernail psoriasis; the outcome of this trial was not available at the time of publication.
PH-10 (Xanthine Dye)
PH-10 (Provectus Biopharmaceuticals, Inc) is a topical aqueous hydrogel derived from rose bengal disodium that may be beneficial in treating skin conditions such as atopic dermatitis and mild to moderate psoriasis. Rose bengal disodium is a hydrophilic xanthine dye with diagnostic utility in ophthalmology and gastroenterology as well as projected use as a melanoma treatment as demonstrated in phase 1 and phase 2 clinical trials of PV-10 (Provectus Biopharmaceuticals, Inc).20 Two phase 2 studies assessing the safety and therapeutic efficacy of PH-10 in psoriasis (NCT01247818, NCT00941278) have been completed; results were not available at the time of publication.
STF115469 (Vitamin D Analogue)
STF115469 (GlaxoSmithKline) is a calcipotriene foam. At the time of publication, a randomized, placebo-controlled, double-blind, phase 3 trial (NCT01582932) of this vitamin D3 analogue with a projected enrollment of 180 participants was actively recruiting patients aged 2 to 11 years with mild to moderate plaque psoriasis to study the efficacy, safety, and tolerability of STF115469, as well as its pharmacokinetics and pharmacodynamics.
WBI-1001 (Proprietary Product)
WBI-1001 (Welichem Biotech Inc), or 2-isopropyl-5-[(E)-2-phenylethenyl] benzene-1, 3-diol, is a novel proprietary agent that inhibits proinflammatory cytokines (eg, IFN-γ, TNF-α). A randomized, placebo-controlled, double-blind, phase 1 trial (NCT00830817) assessing the efficacy, safety, tolerability, and pharmacokinetics of WBI-1001 has been completed; results were not available at the time of publication. Another randomized, placebo-controlled, double-blind, phase 2 trial (NCT01098721) evaluating its efficacy and safety according to the physician’s global assessment demonstrated a therapeutic benefit of 62.8% in patients treated with WBI-1001 cream 1% versus 13.0% in those treated with a placebo after a 12-week treatment period (P<.0001).21 WBI-1001 may offer a novel approach in the treatment of mild to moderate psoriasis.
Conclusion
Enhanced knowledge of the underlying pathogeneses of psoriasis and psoriatic arthritis has identified new therapeutic targets and enabled the development of exciting novel treatments for these conditions. The topical agents currently in phase 2 and phase 3 clinical trials show promise in enhancing the way physicians treat psoriasis. There is hope for more individualized treatment regimens with improved tolerability and better safety profiles with increased therapeutic efficacy. As our understanding of the molecular underpinnings of psoriasis continues to deepen, it will afford the development of even more innovative therapeutics for use in the management of psoriasis.
1. Mason A, Mason J, Cork M, et al. Topical treatments for chronic plaque psoriasis: an abridged Cochrane systematic review. J Am Acad Dermatol. 2013;69:799-807.
2. Nazarian R, Weinberg JM. AN-2728, a PDE4 inhibitor for the potential topical treatment of psoriasis and atopic dermatitis. Curr Opin Investig Drugs. 2009;10:1236-1242.
3. Moustafa F, Feldman SR. A review of phosphodiesterase-inhibition and the potential role for phosphodiesterase 4-inhibitors in clinical dermatology. Dermatol Online J. 2014;20:22608.
4. Halpert G, Sredni B. The effect of the novel tellurium compound AS101 on autoimmune diseases. Autoimmun Rev. 2014;13:1230-1235.
5. Ports WC, Khan S, Lan S, et al. A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br J Dermatol. 2013;169:137-145.
6. Yosipovitch G, Roblin D, Traversa S, et al. A novel topical targeted anti-pruritic treatment in phase 2b development for chronic pruritus. Paper presented at: 72nd Annual Meeting of the American Academy of Dermatology; March 21-25, 2014; Denver, CO.
7. Kang S, Yi S, Griffiths CE, et al. Calcipotriene-induced improvement in psoriasis is associated with reduced interleukin-8 and increased interleukin-10 levels within lesions. Br J Dermatol. 1998;138:77-83.
8. Kristl J, Slanc P, Krasna M, et al. Calcipotriol affects keratinocyte proliferation by decreasing expression of early growth response-1 and polo-like kinase-2. Pharm Res. 2008;25:521-529.
9. Tiberio R, Bozzo C, Pertusi G, et al. Calcipotriol induces apoptosis in psoriatic keratinocytes. Clin Exp Dermatol. 2009;34:e972-e974.
10. Luger T, Seite S, Humbert P, et al. Recommendations for adjunctive basic skin care in patients with psoriasis. Eur J Dermatol. 2014;24:194-200.
11. Dermipsor reports good results in DPS-101 Phase IIb study for plaque psoriasis [press release]. Evaluate Web site. http://www.evaluategroup.com/Universal/View.aspx?type=Story&id=250042. Published October 15, 2007. Accessed February 13, 2015.
12. Rivera AM, Hsu S. Topical halobetasol propionate in the treatment of plaque psoriasis: a review. Am J Clin Dermatol. 2005;6:311-316.
13. Punwani N, Scherle P, Flores R, et al. Preliminary clinical activity of a topical JAK1/2 inhibitor in the treatment of psoriasis. J Am Acad Dermatol. 2012;67:658-664.
14. Efficacy and safety of calcipotriol plus hydrocortisone ointment compared with tacalcitol ointment in patients with psoriasis on the face and skin folds (NCT00640822). https://clinicaltrials.gov/ct2/show/results/NCT00640822?term=NCT00640822&rank=1. Updated October 21, 2013. Accessed May 30, 2014.
15. Product candidates: targeting p-STAT3 for improved psoriasis treatment. Moleculin Web site. http://moleculin.com/product-candidates/mol4239. Accessed February 13, 2015.
16. Chowdhari S, Saini N. hsa-miR-4516 mediated downregulation of STAT3/CDK6/UBE2N plays a role in PUVA induced apoptosis in keratinocytes. J Cell Physiol. 2014;229:1630-1638.
17. Paired psoriasis lesion, comparative, study to evaluate MOL4239 in psoriasis (NCT01826201). https://clinicaltrials.gov/ct2/show/results/NCT01826201?term=NCT01826201&rank=1§=X01256#all. Updated December 22, 2014. Accessed February 25, 2015.
18. Clinical development pipeline. Moleculin Web site. http://moleculin.com/clinical-trials/psoriasis-trials. Accessed February 13, 2015.
19. de la Brassinne M, Nikkels A. Psoriasis: state of the art 2013. Acta Clin Belg. 2013;68:433-441.
20. Ross MI. Intralesional therapy with PV-10 (Rose Bengal) for in-transit melanoma. J Surg Oncol. 2014;109:314-319.
21. Bissonnette R, Bolduc C, Maari C, et al. Efficacy and safety of topical WBI-1001 in patients with mild to moderate psoriasis: results from a randomized, double-blind placebo-controlled, phase II trial. J Eur Acad Dermatol Venereol. 2012;26:1516-1521.
1. Mason A, Mason J, Cork M, et al. Topical treatments for chronic plaque psoriasis: an abridged Cochrane systematic review. J Am Acad Dermatol. 2013;69:799-807.
2. Nazarian R, Weinberg JM. AN-2728, a PDE4 inhibitor for the potential topical treatment of psoriasis and atopic dermatitis. Curr Opin Investig Drugs. 2009;10:1236-1242.
3. Moustafa F, Feldman SR. A review of phosphodiesterase-inhibition and the potential role for phosphodiesterase 4-inhibitors in clinical dermatology. Dermatol Online J. 2014;20:22608.
4. Halpert G, Sredni B. The effect of the novel tellurium compound AS101 on autoimmune diseases. Autoimmun Rev. 2014;13:1230-1235.
5. Ports WC, Khan S, Lan S, et al. A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br J Dermatol. 2013;169:137-145.
6. Yosipovitch G, Roblin D, Traversa S, et al. A novel topical targeted anti-pruritic treatment in phase 2b development for chronic pruritus. Paper presented at: 72nd Annual Meeting of the American Academy of Dermatology; March 21-25, 2014; Denver, CO.
7. Kang S, Yi S, Griffiths CE, et al. Calcipotriene-induced improvement in psoriasis is associated with reduced interleukin-8 and increased interleukin-10 levels within lesions. Br J Dermatol. 1998;138:77-83.
8. Kristl J, Slanc P, Krasna M, et al. Calcipotriol affects keratinocyte proliferation by decreasing expression of early growth response-1 and polo-like kinase-2. Pharm Res. 2008;25:521-529.
9. Tiberio R, Bozzo C, Pertusi G, et al. Calcipotriol induces apoptosis in psoriatic keratinocytes. Clin Exp Dermatol. 2009;34:e972-e974.
10. Luger T, Seite S, Humbert P, et al. Recommendations for adjunctive basic skin care in patients with psoriasis. Eur J Dermatol. 2014;24:194-200.
11. Dermipsor reports good results in DPS-101 Phase IIb study for plaque psoriasis [press release]. Evaluate Web site. http://www.evaluategroup.com/Universal/View.aspx?type=Story&id=250042. Published October 15, 2007. Accessed February 13, 2015.
12. Rivera AM, Hsu S. Topical halobetasol propionate in the treatment of plaque psoriasis: a review. Am J Clin Dermatol. 2005;6:311-316.
13. Punwani N, Scherle P, Flores R, et al. Preliminary clinical activity of a topical JAK1/2 inhibitor in the treatment of psoriasis. J Am Acad Dermatol. 2012;67:658-664.
14. Efficacy and safety of calcipotriol plus hydrocortisone ointment compared with tacalcitol ointment in patients with psoriasis on the face and skin folds (NCT00640822). https://clinicaltrials.gov/ct2/show/results/NCT00640822?term=NCT00640822&rank=1. Updated October 21, 2013. Accessed May 30, 2014.
15. Product candidates: targeting p-STAT3 for improved psoriasis treatment. Moleculin Web site. http://moleculin.com/product-candidates/mol4239. Accessed February 13, 2015.
16. Chowdhari S, Saini N. hsa-miR-4516 mediated downregulation of STAT3/CDK6/UBE2N plays a role in PUVA induced apoptosis in keratinocytes. J Cell Physiol. 2014;229:1630-1638.
17. Paired psoriasis lesion, comparative, study to evaluate MOL4239 in psoriasis (NCT01826201). https://clinicaltrials.gov/ct2/show/results/NCT01826201?term=NCT01826201&rank=1§=X01256#all. Updated December 22, 2014. Accessed February 25, 2015.
18. Clinical development pipeline. Moleculin Web site. http://moleculin.com/clinical-trials/psoriasis-trials. Accessed February 13, 2015.
19. de la Brassinne M, Nikkels A. Psoriasis: state of the art 2013. Acta Clin Belg. 2013;68:433-441.
20. Ross MI. Intralesional therapy with PV-10 (Rose Bengal) for in-transit melanoma. J Surg Oncol. 2014;109:314-319.
21. Bissonnette R, Bolduc C, Maari C, et al. Efficacy and safety of topical WBI-1001 in patients with mild to moderate psoriasis: results from a randomized, double-blind placebo-controlled, phase II trial. J Eur Acad Dermatol Venereol. 2012;26:1516-1521.
Practice Points
- Topical therapies are the cornerstone of treating patients with mild to moderate psoriasis. Commercially available medications approved by the US Food and Drug Administration for use in patients with psoriasis include corticosteroids, vitamin D3 analogues, calcineurin inhibitors, retinoids, anthralin, and tar-based formulations.
- Recent developments in our understanding of inflammatory mediators and the underlying pathogenesis of psoriasis have revealed new potential therapeutic targets, leading to the discovery of many promising treatments.
- Novel topical therapies currently in phase 2 and phase 3 clinical trials for patients with mild to moderate psoriasis may offer hope to patients who have reported a suboptimal therapeutic response to conventional treatments.