User login
Now is the time for pulmonary clinicians to become comfortable counseling patients about and treating obesity. By 2030, half of the US population will have obesity, a quarter of which will be severe (Ward et al. NEJM. 2019;2440-2450).
Many pulmonary diseases, including asthma, COPD, and interstitial pulmonary fibrosis (IPF) are linked to and made worse by obesity with increased exacerbations, patient-reported decreased quality of life, and resistance to therapy (Ray et al. Am Rev Respir Dis. 1983;501-6). Asthma is even recognized as an obesity-related comorbid condition by both the American Society Metabolic and Bariatric Surgery (ASMBS) and the American Association of Clinical Endocrinologists (AACE) when considering indications for early or more aggressive treatment of obesity (Eisenberg et al. Obesity Surg. 2023;3-14) (Garvey et al. Endocr Pract. 2016;1-203).
Obesity has multiple negative effects on pulmonary function due to the physical forces of extra weight on the lungs and inflammation related to adipose tissue (see Figure 1) (Zerah et al. Chest. 1993;1470-6).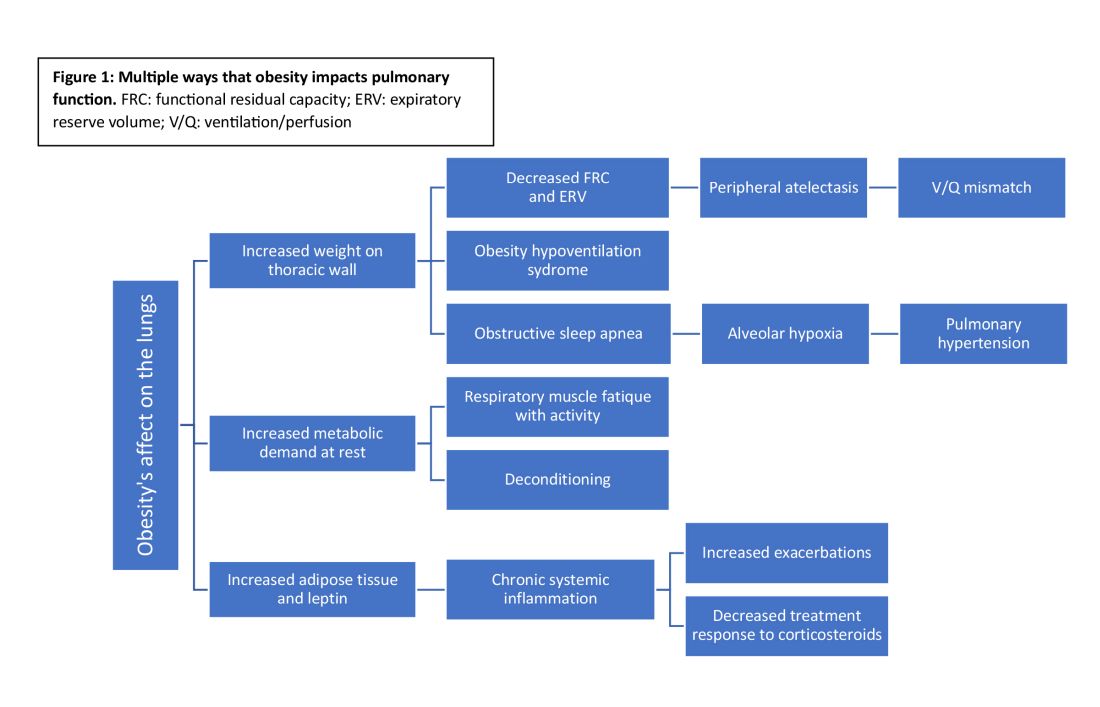
Obesity-related respiratory changes include reduced lung compliance, functional residual capacity (FRC), and expiratory reserve volume (ERV). These changes lead to peripheral atelectasis and V/Q mismatch and increased metabolic demands placed on the respiratory system (Parameswaran et al. Can Respir J. 2006;203-10). The increased weight supported by the thoracic cage alters the equilibrium between the chest wall and lung tissue decreasing FRC and ERV. This reduces lung compliance and increases stiffness by promoting areas of atelectasis and increased alveolar surface tension (Dixon et al. Expert Rev Respir Med. 2018;755-67).
Another biomechanical cost of obesity on respiratory function is the increased consumption of oxygen to sustain ventilation at rest (Koenig SM, Am J Med Sci. 2001;249-79). This can lead to early respiratory muscle fatigue when respiratory rate and tidal volume increase with activity. Patients with obesity are more likely to develop obstructive sleep apnea and obesity hypoventilation syndrome. The resulting alveolar hypoxemia is thought to contribute to the increase in pulmonary hypertension observed in patients with obesity (Shah et al. Breathe. 2023;19[1]). In addition to the biomechanical consequences of obesity, increased adipose tissue can lead to chronic, systemic inflammation that can exacerbate or unmask underlying respiratory disease. Increased leptin and downregulation of adiponectin have been shown to increase systemic cytokine production (Ray et al. Am Rev Respir Dis. 1983;501-6). This inflammatory process contributes to increased airway resistance and an altered response to corticosteroids (inhaled or systemic) in obese patients treated for bronchial hyperresponsiveness. This perhaps reflects the Th2-low phenotype seen in patients with obesity and metabolic syndrome-related asthma (Shah et al. Breathe. 2023;19[1]) (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812).
Multiple studies have demonstrated weight loss through lifestyle changes, medical therapy, and obesity surgery result benefits pulmonary disease (Forno et al. PloS One. 2019;14[4]) (Ardila-Gatas et al. Surg Endosc. 2019;1952-8). Benefits include decreased exacerbation frequency, improved functional testing, and improved patient-reported quality of life. Pulmonary clinicians should be empowered to address obesity as a comorbid condition and treat with appropriate referrals for obesity surgery and initiation of medications when indicated.
GLP-1 receptor agonists
In the past year, glucagon-like peptide receptor agonists (GLP-1RAs) have garnered attention in the medical literature and popular news outlets. GLP-1RAs, including semaglutide, liraglutide, and tirzepatide, are currently FDA approved for the treatment of obesity in patients with a body mass index (BMI) greater than or equal to 30 or a BMI greater than or equal to 27 in the setting of an obesity-related comorbidity, including asthma.
This class of medications acts by increasing the physiologic insulin response to a glucose load, delaying gastric emptying, and reducing production of glucagon. In a phase III study, semaglutide resulted in greater than 15% weight reduction from baseline (Wadden et al. JAMA. 2021;1403-13). In clinical trials, these medications have not only resulted in significant, sustained weight loss but also improved lipid profiles, decreased A1c, and reduced major cardiovascular events (Lincoff et al. N Engl J Med. 2023;389[23]:2221-32) (Verma et al. Circulation. 2018;138[25]:2884-94).
GLP-1RAs and lung disease
GLP-1RAs are associated with ranges of weight loss that lead to symptom improvement. Beyond the anticipated benefits for pulmonary health, there is interest in whether GLP-1RAs may improve specific lung diseases. GLP-1 receptors are found throughout the body (eg, gastrointestinal tract, kidneys, and heart) with the largest proportion located in the lungs (Wu AY and Peebles RS. Expert Rev Clin Immunol. 2021;1053-7). In addition to their known effect on insulin response, GLP-1RAs are hypothesized to reduce proinflammatory cytokine signaling and alter surfactant production potentially improving both airway resistance and lung compliance (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812). Animal models suggest an antifibrotic effect with delay in the endothelial-mesenchymal transition. If further substantiated, this could impact both acute and chronic lung injury.
Early clinical studies of GLP-1RAs in patients with respiratory diseases have demonstrated improved symptoms and pulmonary function (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812). Even modest weight loss (2.5 kg in a year) with GLP-1RAs leads to improved symptoms and a reduction in asthma exacerbations. Other asthma literature shows GLP-1RAs improve symptoms and reduce exacerbations independent of changes in weight, supporting the hypothesis that the benefit of GLP-1RAs may be more than biomechanical improvement from weight loss alone (Foer et al. Am J Respir Crit Care Med. 2021;831-40).
GLP-1RAs reduce the proinflammatory cytokine signaling in both TH2-high and TH2-low asthma phenotypes and alter surfactant production, airway resistance, and perhaps even pulmonary vascular resistance (Altintas Dogan et al. Int J Chron Obstruct Pulmon Dis. 2022,405-14). GATA-3 is an ongoing clinical trial examining whether GLP-1RAs reduce airway inflammation via direct effects on of the respiratory tract (NCT05254314).
Drugs developed to treat one condition are often found to impact others during validation studies or postmarketing observation. Some examples are aspirin, sildenafil, minoxidil, hydroxychloroquine, and SGLT-2 inhibitors. Will GLP-1RAs be the latest medication to affect a broad array of physiologic process and end up improving not just metabolic but also lung health?
Now is the time for pulmonary clinicians to become comfortable counseling patients about and treating obesity. By 2030, half of the US population will have obesity, a quarter of which will be severe (Ward et al. NEJM. 2019;2440-2450).
Many pulmonary diseases, including asthma, COPD, and interstitial pulmonary fibrosis (IPF) are linked to and made worse by obesity with increased exacerbations, patient-reported decreased quality of life, and resistance to therapy (Ray et al. Am Rev Respir Dis. 1983;501-6). Asthma is even recognized as an obesity-related comorbid condition by both the American Society Metabolic and Bariatric Surgery (ASMBS) and the American Association of Clinical Endocrinologists (AACE) when considering indications for early or more aggressive treatment of obesity (Eisenberg et al. Obesity Surg. 2023;3-14) (Garvey et al. Endocr Pract. 2016;1-203).
Obesity has multiple negative effects on pulmonary function due to the physical forces of extra weight on the lungs and inflammation related to adipose tissue (see Figure 1) (Zerah et al. Chest. 1993;1470-6).
Obesity-related respiratory changes include reduced lung compliance, functional residual capacity (FRC), and expiratory reserve volume (ERV). These changes lead to peripheral atelectasis and V/Q mismatch and increased metabolic demands placed on the respiratory system (Parameswaran et al. Can Respir J. 2006;203-10). The increased weight supported by the thoracic cage alters the equilibrium between the chest wall and lung tissue decreasing FRC and ERV. This reduces lung compliance and increases stiffness by promoting areas of atelectasis and increased alveolar surface tension (Dixon et al. Expert Rev Respir Med. 2018;755-67).
Another biomechanical cost of obesity on respiratory function is the increased consumption of oxygen to sustain ventilation at rest (Koenig SM, Am J Med Sci. 2001;249-79). This can lead to early respiratory muscle fatigue when respiratory rate and tidal volume increase with activity. Patients with obesity are more likely to develop obstructive sleep apnea and obesity hypoventilation syndrome. The resulting alveolar hypoxemia is thought to contribute to the increase in pulmonary hypertension observed in patients with obesity (Shah et al. Breathe. 2023;19[1]). In addition to the biomechanical consequences of obesity, increased adipose tissue can lead to chronic, systemic inflammation that can exacerbate or unmask underlying respiratory disease. Increased leptin and downregulation of adiponectin have been shown to increase systemic cytokine production (Ray et al. Am Rev Respir Dis. 1983;501-6). This inflammatory process contributes to increased airway resistance and an altered response to corticosteroids (inhaled or systemic) in obese patients treated for bronchial hyperresponsiveness. This perhaps reflects the Th2-low phenotype seen in patients with obesity and metabolic syndrome-related asthma (Shah et al. Breathe. 2023;19[1]) (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812).
Multiple studies have demonstrated weight loss through lifestyle changes, medical therapy, and obesity surgery result benefits pulmonary disease (Forno et al. PloS One. 2019;14[4]) (Ardila-Gatas et al. Surg Endosc. 2019;1952-8). Benefits include decreased exacerbation frequency, improved functional testing, and improved patient-reported quality of life. Pulmonary clinicians should be empowered to address obesity as a comorbid condition and treat with appropriate referrals for obesity surgery and initiation of medications when indicated.
GLP-1 receptor agonists
In the past year, glucagon-like peptide receptor agonists (GLP-1RAs) have garnered attention in the medical literature and popular news outlets. GLP-1RAs, including semaglutide, liraglutide, and tirzepatide, are currently FDA approved for the treatment of obesity in patients with a body mass index (BMI) greater than or equal to 30 or a BMI greater than or equal to 27 in the setting of an obesity-related comorbidity, including asthma.
This class of medications acts by increasing the physiologic insulin response to a glucose load, delaying gastric emptying, and reducing production of glucagon. In a phase III study, semaglutide resulted in greater than 15% weight reduction from baseline (Wadden et al. JAMA. 2021;1403-13). In clinical trials, these medications have not only resulted in significant, sustained weight loss but also improved lipid profiles, decreased A1c, and reduced major cardiovascular events (Lincoff et al. N Engl J Med. 2023;389[23]:2221-32) (Verma et al. Circulation. 2018;138[25]:2884-94).
GLP-1RAs and lung disease
GLP-1RAs are associated with ranges of weight loss that lead to symptom improvement. Beyond the anticipated benefits for pulmonary health, there is interest in whether GLP-1RAs may improve specific lung diseases. GLP-1 receptors are found throughout the body (eg, gastrointestinal tract, kidneys, and heart) with the largest proportion located in the lungs (Wu AY and Peebles RS. Expert Rev Clin Immunol. 2021;1053-7). In addition to their known effect on insulin response, GLP-1RAs are hypothesized to reduce proinflammatory cytokine signaling and alter surfactant production potentially improving both airway resistance and lung compliance (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812). Animal models suggest an antifibrotic effect with delay in the endothelial-mesenchymal transition. If further substantiated, this could impact both acute and chronic lung injury.
Early clinical studies of GLP-1RAs in patients with respiratory diseases have demonstrated improved symptoms and pulmonary function (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812). Even modest weight loss (2.5 kg in a year) with GLP-1RAs leads to improved symptoms and a reduction in asthma exacerbations. Other asthma literature shows GLP-1RAs improve symptoms and reduce exacerbations independent of changes in weight, supporting the hypothesis that the benefit of GLP-1RAs may be more than biomechanical improvement from weight loss alone (Foer et al. Am J Respir Crit Care Med. 2021;831-40).
GLP-1RAs reduce the proinflammatory cytokine signaling in both TH2-high and TH2-low asthma phenotypes and alter surfactant production, airway resistance, and perhaps even pulmonary vascular resistance (Altintas Dogan et al. Int J Chron Obstruct Pulmon Dis. 2022,405-14). GATA-3 is an ongoing clinical trial examining whether GLP-1RAs reduce airway inflammation via direct effects on of the respiratory tract (NCT05254314).
Drugs developed to treat one condition are often found to impact others during validation studies or postmarketing observation. Some examples are aspirin, sildenafil, minoxidil, hydroxychloroquine, and SGLT-2 inhibitors. Will GLP-1RAs be the latest medication to affect a broad array of physiologic process and end up improving not just metabolic but also lung health?
Now is the time for pulmonary clinicians to become comfortable counseling patients about and treating obesity. By 2030, half of the US population will have obesity, a quarter of which will be severe (Ward et al. NEJM. 2019;2440-2450).
Many pulmonary diseases, including asthma, COPD, and interstitial pulmonary fibrosis (IPF) are linked to and made worse by obesity with increased exacerbations, patient-reported decreased quality of life, and resistance to therapy (Ray et al. Am Rev Respir Dis. 1983;501-6). Asthma is even recognized as an obesity-related comorbid condition by both the American Society Metabolic and Bariatric Surgery (ASMBS) and the American Association of Clinical Endocrinologists (AACE) when considering indications for early or more aggressive treatment of obesity (Eisenberg et al. Obesity Surg. 2023;3-14) (Garvey et al. Endocr Pract. 2016;1-203).
Obesity has multiple negative effects on pulmonary function due to the physical forces of extra weight on the lungs and inflammation related to adipose tissue (see Figure 1) (Zerah et al. Chest. 1993;1470-6).
Obesity-related respiratory changes include reduced lung compliance, functional residual capacity (FRC), and expiratory reserve volume (ERV). These changes lead to peripheral atelectasis and V/Q mismatch and increased metabolic demands placed on the respiratory system (Parameswaran et al. Can Respir J. 2006;203-10). The increased weight supported by the thoracic cage alters the equilibrium between the chest wall and lung tissue decreasing FRC and ERV. This reduces lung compliance and increases stiffness by promoting areas of atelectasis and increased alveolar surface tension (Dixon et al. Expert Rev Respir Med. 2018;755-67).
Another biomechanical cost of obesity on respiratory function is the increased consumption of oxygen to sustain ventilation at rest (Koenig SM, Am J Med Sci. 2001;249-79). This can lead to early respiratory muscle fatigue when respiratory rate and tidal volume increase with activity. Patients with obesity are more likely to develop obstructive sleep apnea and obesity hypoventilation syndrome. The resulting alveolar hypoxemia is thought to contribute to the increase in pulmonary hypertension observed in patients with obesity (Shah et al. Breathe. 2023;19[1]). In addition to the biomechanical consequences of obesity, increased adipose tissue can lead to chronic, systemic inflammation that can exacerbate or unmask underlying respiratory disease. Increased leptin and downregulation of adiponectin have been shown to increase systemic cytokine production (Ray et al. Am Rev Respir Dis. 1983;501-6). This inflammatory process contributes to increased airway resistance and an altered response to corticosteroids (inhaled or systemic) in obese patients treated for bronchial hyperresponsiveness. This perhaps reflects the Th2-low phenotype seen in patients with obesity and metabolic syndrome-related asthma (Shah et al. Breathe. 2023;19[1]) (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812).
Multiple studies have demonstrated weight loss through lifestyle changes, medical therapy, and obesity surgery result benefits pulmonary disease (Forno et al. PloS One. 2019;14[4]) (Ardila-Gatas et al. Surg Endosc. 2019;1952-8). Benefits include decreased exacerbation frequency, improved functional testing, and improved patient-reported quality of life. Pulmonary clinicians should be empowered to address obesity as a comorbid condition and treat with appropriate referrals for obesity surgery and initiation of medications when indicated.
GLP-1 receptor agonists
In the past year, glucagon-like peptide receptor agonists (GLP-1RAs) have garnered attention in the medical literature and popular news outlets. GLP-1RAs, including semaglutide, liraglutide, and tirzepatide, are currently FDA approved for the treatment of obesity in patients with a body mass index (BMI) greater than or equal to 30 or a BMI greater than or equal to 27 in the setting of an obesity-related comorbidity, including asthma.
This class of medications acts by increasing the physiologic insulin response to a glucose load, delaying gastric emptying, and reducing production of glucagon. In a phase III study, semaglutide resulted in greater than 15% weight reduction from baseline (Wadden et al. JAMA. 2021;1403-13). In clinical trials, these medications have not only resulted in significant, sustained weight loss but also improved lipid profiles, decreased A1c, and reduced major cardiovascular events (Lincoff et al. N Engl J Med. 2023;389[23]:2221-32) (Verma et al. Circulation. 2018;138[25]:2884-94).
GLP-1RAs and lung disease
GLP-1RAs are associated with ranges of weight loss that lead to symptom improvement. Beyond the anticipated benefits for pulmonary health, there is interest in whether GLP-1RAs may improve specific lung diseases. GLP-1 receptors are found throughout the body (eg, gastrointestinal tract, kidneys, and heart) with the largest proportion located in the lungs (Wu AY and Peebles RS. Expert Rev Clin Immunol. 2021;1053-7). In addition to their known effect on insulin response, GLP-1RAs are hypothesized to reduce proinflammatory cytokine signaling and alter surfactant production potentially improving both airway resistance and lung compliance (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812). Animal models suggest an antifibrotic effect with delay in the endothelial-mesenchymal transition. If further substantiated, this could impact both acute and chronic lung injury.
Early clinical studies of GLP-1RAs in patients with respiratory diseases have demonstrated improved symptoms and pulmonary function (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812). Even modest weight loss (2.5 kg in a year) with GLP-1RAs leads to improved symptoms and a reduction in asthma exacerbations. Other asthma literature shows GLP-1RAs improve symptoms and reduce exacerbations independent of changes in weight, supporting the hypothesis that the benefit of GLP-1RAs may be more than biomechanical improvement from weight loss alone (Foer et al. Am J Respir Crit Care Med. 2021;831-40).
GLP-1RAs reduce the proinflammatory cytokine signaling in both TH2-high and TH2-low asthma phenotypes and alter surfactant production, airway resistance, and perhaps even pulmonary vascular resistance (Altintas Dogan et al. Int J Chron Obstruct Pulmon Dis. 2022,405-14). GATA-3 is an ongoing clinical trial examining whether GLP-1RAs reduce airway inflammation via direct effects on of the respiratory tract (NCT05254314).
Drugs developed to treat one condition are often found to impact others during validation studies or postmarketing observation. Some examples are aspirin, sildenafil, minoxidil, hydroxychloroquine, and SGLT-2 inhibitors. Will GLP-1RAs be the latest medication to affect a broad array of physiologic process and end up improving not just metabolic but also lung health?

