User login
Jaundice is an important clinical entity associated with a wide variety of differential diagnoses for which the prognosis differs depending on etiology. Recently, the etiological spectrum of unselected patients with jaundice has been reported.12 Among patients with cholestatic jaundice, abdominal ultrasound remains the primary investigation in order to distinguish extrahepatic biliary obstruction from intrahepatic disease.
Recent studies of patients with jaundice have included a limited number of patients with jaundice due to biliary obstruction and provided no analysis of the clinical characteristics and prognosis of these patients.12 Moreover, the prognosis of unselected patients with severe obstructive jaundice is unclear nowadays. The most common clinical presentation of malignant obstructive jaundice traditionally has been considered silent jaundice.35 However, the clinical features of malignant versus benign causes of jaundice have not been a focus of interest during the last decades, and it is not clear from the literature what proportion of patients with choledocholithiasis and jaundice present with silent jaundice. Studies on the prognosis of patients with jaundice were published more than 20 years ago, prior to major advances in imaging modalities and endoscopic treatment.614 Thus, we aimed to study the clinical features, etiology, and prognosis of patients presenting with obstructive jaundice in the current era of improved imaging and noninvasive treatment.
MATERIAL AND METHODS
Over a 2‐year period from the beginning of 2003 to the end of 2004, all adult patients with s‐bilirubin of 5.85 mg/dL (100 mol/l; reference value < 25 mol/L) were identified at the clinical laboratory serving the Sahlgrenska University Hospital (Gothenburg, Sweden), which analyzed the serum bilirubin of all patients in Gothenburg. Sahlgrenska University Hospital provides hospital services for all inhabitants of the city and comprises 3 hospitals (Sahlgrenska Hospital, stra Hospital, and Mlndal Hospital) that serve as community hospitals as well as a university hospital. The Gothenburg metropolitan area has 600,000 inhabitants.
The inclusion criteria for the study cohort were s‐bilirubin 100 mol/L at any point during the 2‐year period with evidence of dilated biliary ducts on abdominal ultrasound. A retrospective review of medical records was performed to retrieve information on the presence of abdominal pain associated with jaundice, computerized tomography (CT) and/or magnetic resonance cholangio‐pancreatography (MRCP) testing, and s‐AST, s‐ALT, s‐ALP, and bilirubin levels. The liver tests performed at the time that s‐bilirubin peaked during hospitalization were analyzed. Information about whether abdominal pain was associated with jaundice at the time of admission to the hospital, was obtained from medical records. The etiology of and treatment for the biliary obstruction were noted. Furthermore, the prognosis of the patients was analyzed. If a patient was discharged from the hospital, information about whether the patient was alive at the time of follow‐up was obtained from the Swedish National Registration of Inhabitants; if the patient had died outside the hospital, a death certificate was requested from the Cause of Death Register of the Swedish National Board of Health and Welfare. Patients were followed up in July 2005, providing a follow‐up period ranging from 6 months to 2.5 years.
Statistics
To test differences between groups, the Fisher exact test was used for dichotomous variables and the Mann‐Whitney test for continuous variables. All tests were 2‐tailed and were conducted at a 5% significance level. The results are presented as medians and interquartile ranges (IQRs).
RESULTS
Patients
During the study period 749 patients were consecutively admitted to our hospital for severe jaundice with bilirubin 5.85 mg/dL (100 mol/L). Among these patients, a total of 241 (32%) had ultrasound evidence of obstructive jaundice at various levels of the biliary tree. In the total study group, the median age was 71 years (IQR 5981 years), with 129 women and 112 men. The oldest patient was 94 years old and the youngest 18 years. No patient was lost to follow‐up.
Causes
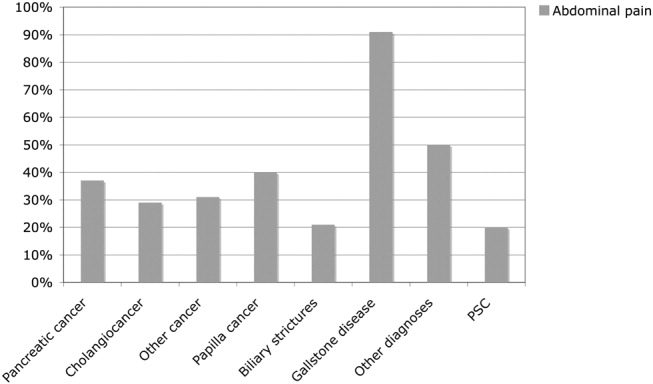
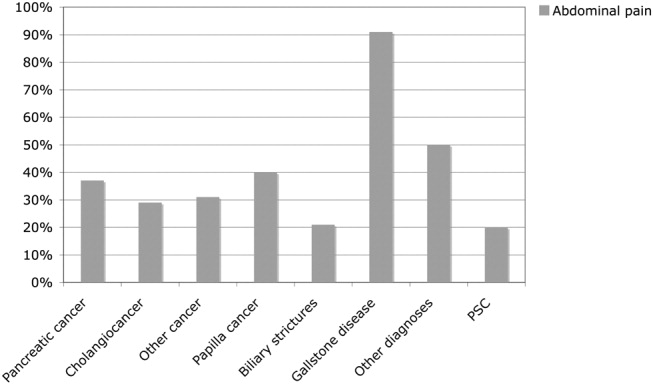
The causes of the obstructive jaundice are shown in Table 1. Among the different types of malignancy causing obstructive jaundice, pancreatic cancer and cholangiocarcinoma were the most common, followed by the other malignancies category (Table 1). As shown in Table 2, a wide variety of other malignancies caused cases of biliary obstruction, although most were a result of metastases from gastrointestinal malignancies. Gallstone disease and biliary stricture were the most common benign causes. Less common causes are shown in Table 2. Patients with malignant versus benign obstructive jaundice were similar in age (Table 3). However, only 10 patients (6.5%) with malignant obstructive jaundice (OJ) were less than 50 years old, whereas 23 patients (26%) with benignly caused OJ were under the age of 50. Among the patients with malignancy, patients with pancreatic cancer were significantly older than those with cholangiocarcinoma (P = .04, Table 1). No other major age differences were observed in the etiological groups. Most patients with gallstone disease presenting with jaundice had experienced abdominal pain in association with jaundice, but the jaundice of 9% of the patients was painless (Fig. 1). This was in contrast with patients whose OJ was caused by different types of malignancies, a minority of whom experienced pain at presentation (Fig. 1). Table 3 shows a comparison of patients with a malignant obstruction and those with a benign obstruction. Abdominal pain associated with jaundice was less prevalent at presentation in patients with malignant obstructive jaundice compared with those with nonmalignant obstructive jaundice (34% vs. 71%; P < .0001; Table 3). In 4 patients, the cause of jaundice could not be determined from chart review. Three of these patients presented late in a generally bad condition; ultrasound showed dilated ducts, but these patients died in a few days, before further investigations had been performed (autopsies were not performed), and 1 patient refused further investigations. Thus, an etiological explanation was found for the obstruction of almost all patients.
| Pancreatic cancer | Cholangio‐cancer | Other cancers | Papilla cancers | Biliary strictures | Gallstone disease | Other diagnoses | PSC | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Total number | 69 | 44 | 36 | 5 | 7 | 57 | 18 | 5 |
| Age | 73 (6782) | 67 (5678) | 67 (5975) | 79 (7081) | 80 (5184) | 69 (4983) | 72 (5285) | 61 (3468) |
| Sex‐F/M | 36/33 | 27/17 | 19/17 | 3/2 | 4/3 | 29/28 | 9/9 | 2/3 |
| Surgery | 10/69 | 10/44 | 1/36 | 3/5 | 0/7 | 21/57 | 4/18 | 1/5 |
| Alive at follow‐up | 3/69 (4.3%) | 2/44 (4.5%) | 2/36 (5.6%) | 1/5 (20%) | 6/7 (86%) | 46/57 (80.1%) | 12/18 (66%) | 4/5 (80%) |
| Survival (days) | 142 (58267) | 166 (80300) | 31 (1873) | 257 (130380) | 510 (455900) | 558 (424665) | 412 (103558) | 570 (319676) |
| Type of malignancy | Number of patients | Other cause of OJ not classified elsewhere | Number of patients |
|---|---|---|---|
| Colorectal cancer with liver metastases | 9 | Cholangitis | 4 |
| Liver metastases with unknown primary tumor | 9 | Papillary adenoma | 4 |
| Gastric cancer with liver metastases | 5 | Unknown cause | 4 |
| Small bowel cancer with liver metastases | 2 | Choledochal injury after cholecystectomy | 2 |
| Neuroendocrine tumor with liver metastases | 2 | Retroperitoneal fibrosis | 1 |
| Primary hepatocellular cancer | 2 | Mirizzis syndrome | 1 |
| Esophageal cancer with liver metastases | 1 | Chronic pancreatitis | 1 |
| Renal cancer with liver metastases | 1 | Duodenal diverticula | 1 |
| Lung cancer with liver metastases | 1 | ||
| Tuba uteri cancer with liver metastases | 1 | ||
| Prostate cancer with liver metastases | 1 | ||
| Chronic lymphatic leukemia with liver infiltrates | 1 | ||
| Liver cancer of unknown source | 1 |
| Malignant obstruction | Benign obstruction | |
|---|---|---|
| ||
| Total number of patients | 154 | 87 |
| Female/male | 85/69 | 44/43 |
| Age | 72 (6181) | 69 (4983) |
| Abdominal pain at presentation | 53/154 (34%) | 62/87 (71%) |
| AST | 3.3 (2.35.1) | 3.3 (2.16.4) |
| ALT | 3.1 (26) | 4.3 (2.69.1) |
| ALP | 9.9 (4.813.3) | 5.9 (3.78.5) |
| Bilirubin | 13.8 (1020) | 7.1 (5.79.0) |
| CT | 125/154 (81%) | 47/87 (54%) |
| MRCP | 47/154 (30%) | 27/87 (31%) |
| ERCP | 108/154 (70%) | 67/87 (77%) |
| PTC | 59/154 (38%) | 7/87 (8%) |
| Surgery | 24/154 (15.6%)* | 26/87 (29.9%) |
| Alive at follow‐up | 8/154 (5.2%) | 68/87 (78%) |
Investigations
Investigations carried out to verify the diagnoses of all 241 patients are listed in Table 3. All patients underwent abdominal ultrasound, which was a prerequisite for inclusion in the analysis of patients with dilated biliary ducts. Other diagnostic tools were MRCP, endoscopic retrograde cholangio‐pancreatography (ERCP; as well as therapeutic), and percutaneous transhepatic choangiography (PTC); 12 patients received a diagnostic abdominal laparoscopy, and 1 patient had a laparotomy for diagnostic purposes. CT was more commonly utilized in patients with malignancies, whereas the use of MRCP was similar for patients with malignancies and those without malignancies (Table 3). Median s‐bilirubin level of the patients with malignancies was higher than that of patients without malignancies (Table 3; P < .0001). Among the major etiological groups, s‐bilirubin level was higher in the cholangiocarcinoma group than in the group with pancreatic cancer (Table 4; P < .05), but otherwise no significant differences were observed within the malignant group. However, patients with gallstone disease had significantly lower levels of bilirubin compared with those in the different etiological groups with malignant obstruction (P < .05 for all comparisons; Table 4). Patients with liver metastases had the highest levels of alkaline phosphatase (ALP), followed by patients with cholangiocarcinoma (Table 4). In general, patients with a malignant cause of obstructive jaundice had higher ALP values than those whose OJ had a nonmalignant cause (Table 4). The groups did not differ in aspartate aminotransferase level. However, alanine aminotransferase level of patients with gallstone disease was higher than that of patients with cholangiocarcinoma, P = .009, and of patients with other cancers, P = .02 (Table 4).
| Pancreatic cancer | Cholangio‐carcinoma | Other cancers | Papilla cancer | Biliary strictures | Gallstone disease | Other diagnoses | PSC | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| AST | 3.3 (2.46) | 3.1 (2.15) | 3.7 (2.45.1) | 3.7 (2.64.7) | 5.6 (2.77.3) | 3.1 (1.76.7) | 3.9 (2.46.1) | 3.3 (3.16.1) |
| ALT | 4.4 (2.16.3) | 2.7 (2.14.1) | 2.7 (1.43.7) | 2.6 (2.33.4) | 4.4 (2.96.9) | 4.3 (2.610.9) | 4.4 (2.96.3) | 2.6 (1.45.7) |
| ALP | 8.3 (4.815.6) | 10.1 (516.6) | 12.4 (4.419.4) | 8.1 (4.410.1) | 7.2 (3.38.9) | 5.4 (3.37.8) | 7.1 (4.49.4) | 10 (513.3) |
| Bilirubin | 12.9 (9.517.3) | 16.7 (11.921.4) | 13.1 (8.821.2) | 14.8 (13.416.8) | 8.6 (7.611.3) | 6.7 (5.68.1) | 7.9 (5.211.4) | 13.8 (12.816.7) |
Treatment
Of all 241 patients with obstructive jaundice, 56 (23%) had been operated on, with 1 patient with a cholangiocarcinoma (klatskin tumor) receiving a transplanted liver. Of patients with malignant obstruction, 24 of 154 (15%) underwent an operation, whereas 30% of those with benign obstructions were operated on (Tables 1 and 2). Therapeutic ERCP was used similarly in the malignant and the nonmalignant cases (Table 3).
PTC was used in the vast majority of patients whose obstruction was caused by a malignancy and in only a few of the patients whose obstruction had a benign cause (Table 3).
Prognosis
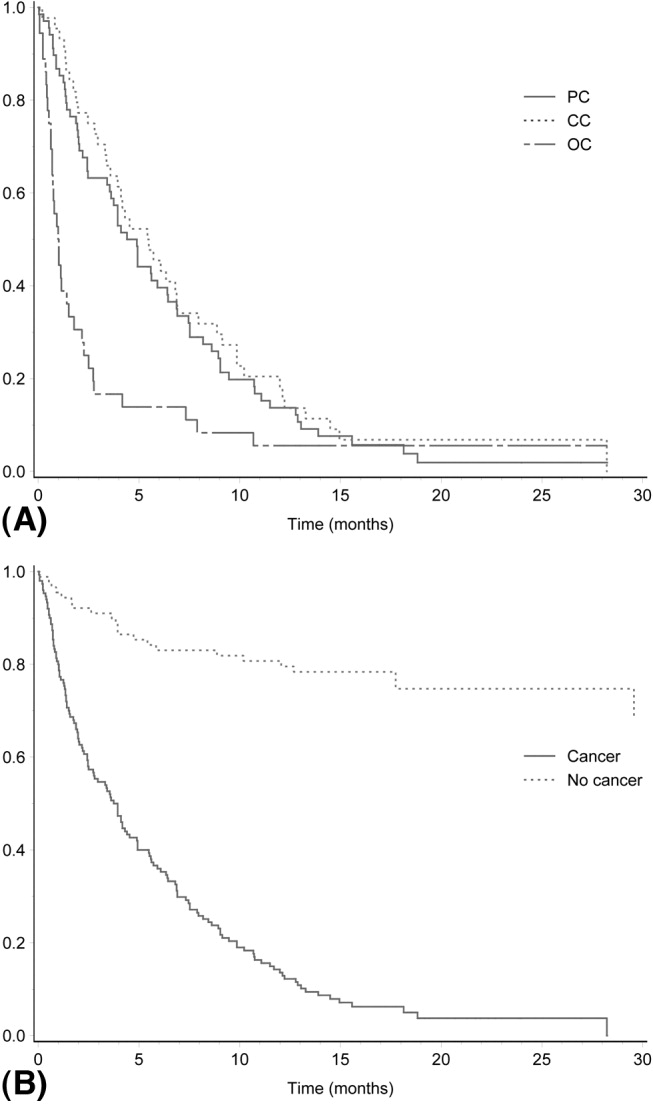
A total of 165 of the 241 patients (68.5%) died during follow‐up. Mortality was very high among patients with malignant obstructive jaundice, with only 8 of 154 patients (5.2%) alive at the end of follow‐up (Table 3). All these patients had been followed for at least 1 year (mean duration of follow‐up, 535 days). Among those who had survived at least 1 year, 1 patient with cholangiocarcinoma had undergone a liver transplantation, and another 5 patients had been operated on, 3 with pancreatic cancer, 1 with cholangiocarcinoma, 1 with a papilla Vateri cancer, and 1 with carcinoid syndrome with liver metastases. One patient with tuba uteri cancer who had received chemotherapy was also alive. The survival rates of the different etiological groups are shown in Table 1. Of 24 patients undergoing surgery for a malignant condition leading to obstructive jaundice, only 9 (37.5%) survived 1 year. The mortality in the total study group within 3 months of diagnosis was 32% (Table 5). The 3‐month, 6‐month, and 1‐year survival rates for the different etiological groups are given in Table 5. Generally, the patients with benign obstructive jaundice had a good prognosis. A total of 46 of 57 patients (80%) with gallstone disease were alive at the end of follow‐up. Only 3 patients with gallstone disease did not survive for 3 months after jaundice occurred. All these patients were very old, none died while hospitalized for jaundice, and only 1 death could be attributed to gallstone disease (cholangitis and sepsis) 1 month after the initial hospitalization. Figure 2A,B shows the mortality over time among the major etiological groups.
| Pancreatic cancer | Cholangio cancer | Other cancers | Papilla cancer | Biliary strictures | Gallstone disease | Other diagnoses | PSC | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| 3 months' survival | 44/69 (63.8%) | 32/44 (72.7%) | 6/36 (16.7%) | 4/5 (80%) | 7/7 (100%) | 54/57 (95%) | 13/16 (81%) | 5/5 (100%) |
| 6 months' survival | 28/69 (40.6%) | 20/44 (45.5%) | 5/36 (13.9%) | 3/5 (60%) | 7/7 (100%) | 50/57 (88%) | 11/16 (68%) | 4/5 (80%) |
| 12 months' survival | 10/69 (14.5%) | 9/44 (20.5%) | 2/36 (5.6%) | 2/5 (40%) | 7/7 (100%) | 47/57 (82%) | 14/18 (77%) | 4/5 (80%) |
DISCUSSION
Our analysis of the causes of jaundice among unselected patients with a bilirubin level 5.85 mg/dL (100 mol/L) revealed that approximately one third of the cases were a result of obstructive jaundice. Studies published more than 20 years ago are available on the etiology and prognosis of these patients, reflecting the diagnostic techniques and hospital practice at that time.69 Furthermore, more recently published studies report the etiological spectrum of patients with obstructive jaundice in Africa and India.1012 In our study population, 154 of 241 patients (64%) had a malignancy, which is remarkably similar to the 61% and 65% of cases of obstructive jaundice due to malignancy reported in studies published more than 25 years ago from Denmark and Spain, respectively.67, 14
The results of the current study demonstrate the poor prognosis of patients with hepatobiliary malignancy that causes obstructive jaundice. The patients whose OJ was caused by a malignancy had a mortality rate of approximately 95% during the study's rather short follow‐up. Previous studies, mostly from the 1980s, found similarly dismal outcomes,1213 suggesting that the prognosis of these patients has not improved during the last 3 decades. Among patients hospitalized for malignant obstructive jaundice in Denmark in the 1970s and the beginning of the 1980s, 1‐year survival was reported to be 11%.14 Thus, unfortunately, the prognosis of patients presenting with jaundice caused by hepatobiliary cancer obstructing the biliary ducts (or due to liver metastases) does not seem to have improved during the last 3 decades. The most common cause of malignant obstructive jaundice in the current study was pancreatic cancer, which is in agreement what was reported in earlier studies.3, 1115 The poor prognosis of patients with pancreatic cancer is well known, but operative mortality is very low nowadays, and some series have reported 5‐year survival rates in the range of 10%30%.1620 The results of the current study suggest that the prognosis for patients with pancreatic cancer presenting with bilirubin 5.85 (100 mL/L) is worse than the 5‐year survival rates reported in recent studies.1620
In the current study patients with cholangiocarcinoma were almost one third of those with malignant obstructive jaundice, which is a higher proportion than that previously reported in series from Australia (9%), India (14%), and Denmark (17%).13, 11, 14 The increased proportion of patients with cholangiocarcinoma might reflect the recent observations of an increased incidence of cholangiocarcinoma in many countries.2122 Similar to the situation of patients with pancreatic cancer, very few patients with cholangiocancer will survive long term,2325 and as in the current study, the prognosis of these patients presenting with severe jaundice seems even worse. Mortality among our patients with malignancies other than pancreatic cancer and cholangiocancer was also very high, with only 5% surviving, mostly because of liver metastases. This is identical to the 1‐year survival of patients with liver metastases presenting with jaundice in Denmark more than 25 years ago.14 Although our patients might not be directly comparable to those seen in Denmark at that time, the results of the current study do suggest that the prognosis of patients with jaundice resulting from liver metastases has not improved over time, despite the considerable advances in diagnostic procedures during the last 2 or 3 decades. One of the limitations of the study besides its retrospective design is potentially excluding patients who were less sick (those with bilirubin levels below that used as an inclusion criterion), affecting survival rates. Other limitations might be defining biliary obstruction based on the results of ultrasound. However, we found very good correlation between ultrasound evidence of dilated biliary ducts and those who had evidence of biliary obstruction on MRCP and ERCP (data not shown).
However, one seemingly large difference between the current study and the Danish study14 is in the prognosis of patients with gallstone disease. The overall 1‐year survival was similar in the 2 studies, but whereas in the Danish study the deaths of 9 of 105 patients (8.6%) was attributed to their gallstone disease, the death of only 1 of the 57 patients (1.8%) in the current study could be attributed to gallstone disease. The reason for this difference is not easily explained but might have been a result of better diagnostic instruments and/or more commonly used ERCP procedures than were previously available.
Although jaundice resulting from a malignancy in the hepatobiliary tract is said to be painless,35 in our study approximately one third of patients with a malignancy experienced pain at presentation. However, our study confirmed that abdominal pain was significantly more often associated with benign conditions. The limitation of the current study was its retrospective nature. However, information about the occurrence of abdominal pain was available in medical records of all patients. Although we could not analyze the character, location, or nature of the abdominal pain, the attending doctor always asked a patient about whether he or she was experiencing abdominal pain.
We can conclude that the severe jaundice of one third of patients was a result of obstructive jaundice. Most of these cases were also a result of a malignancy, with high bilirubin levels indicating prolonged biliary obstruction. Obstructive jaundice caused by a malignancy carried a very poor prognosis, with approximately 95% mortality during a 1‐ to 2‐year follow‐up period. In the absence of methods to cure a significant number of these patients, good methods of palliation are important challenges in the near future.
- ,,.The causes of obvious jaundice in South West Wales: perceptions versus reality.Gut.2001;48:409–413.
- ,,,.Severe jaundice in Sweden in the new millennium: causes, investigations, treatment and prognosis.Scand J Gastroenterol.2003;38:86–94.
- .Jaundice. In:O'Grady JG,Lake JR,Howdle PD, eds.Comprehensive Clinical Hepatology.London UK:Mosby;2000:5.1–5.17.
- .Examination of an adolescent or an adult patient with jaundice. Hamilton Baileys Demonstrations of Clinical Skills in Clinical Surgery.Bristol, UK:John Wright 1967:271–273.
- ,.Jaundice. In:Sherlock S,Dooley J, eds.Diseases of the Liver and Biliary Tract.London, UK:Blackwell;1993:1999–2013.
- ,,,,.Percutaneous transhepatic cholangiography in diagnostic evaluation of 160 jaundiced patients. Results of an improved technic.Am J Surg.1977;133:559–561.
- ,,,.Computed tomography in obstructive jaundice. Part II: The cause of obstruction.Radiology.1981;139:635–645.
- ,,.Usefulness of diagnostic tests for biliary obstruction.Am J Surg.1982;144:102–108.
- ,,, et al.Ultrasound in obstructive jaundice: prospective evaluation of site and cause.Radiology.1983;147:511–515.
- ,,,.Obstructive jaundice in the South African black population.J Clin Gastroenterol.1986;8:538–541.
- ,.Aetiological spectrum of obstructive jaundice and diagnostic ability of ultrasonography: a clinician's perspective.Trop Gastroenterol.1999;20:167–169.
- ,.Failure to improve survival by improved diagnostic techniques in patients with malignant jaundice.Br J Surg.1986;73:631–633.
- ,.Obstructive jaundice in a referral unit: surgical practice and risk factors.Aust N Z J Surg.1985;55:427–432.
- ,,, et al.Causes and characteristics of 500 consecutive cases of jaundice.Scand J Gastroenterol.1981;16:1–6.
- .Management of malignant bile duct obstruction.J Gastroenterol Hepatol.1990;5(Suppl 1):63–77.
- ,,, et al.A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer.N Engl J Med.2004;350:1200–1210.
- ,,.Prognostic factors following curative resection for pancreatic adenocarcinoma: a population‐based, linked database analysis of 396 patients.Ann Surg.2003;237:74–85.
- .Critical look at resection for pancreatic cancer.Lancet.1996;348:1676.
- ,,,.Survival of patients with primary liver cancer, pancreatic cancer and biliary tract cancer in Europe.EUROCARE Working Group.Eur J Cancer.1998;34:2184–2190.
- ,,,.Pancreatic cancer.Lancet.2004;363:1049–1057.
- ,,, et al.Increase in mortality rates from intrahepatic cholangiocarcinoma in England and Wales 1968–1998.Gut.2001;48:816–820.
- ,,,.Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase?J Hepatol.2004;40:472–477.
- ,.Surgical management of cholangiocarcinoma.Semin Liver Dis.2004;24:189–199.
- ,,, et al.Improved survival in resected biliary malignancies.Surgery.2002;132:555–563.
- ,,,,.Intrahepatic biliary enteric bypass provides effective palliation in selected patients with malignant obstruction at the hepatic duct confluence.Am J Surg.1998;175:453–460.
Jaundice is an important clinical entity associated with a wide variety of differential diagnoses for which the prognosis differs depending on etiology. Recently, the etiological spectrum of unselected patients with jaundice has been reported.12 Among patients with cholestatic jaundice, abdominal ultrasound remains the primary investigation in order to distinguish extrahepatic biliary obstruction from intrahepatic disease.
Recent studies of patients with jaundice have included a limited number of patients with jaundice due to biliary obstruction and provided no analysis of the clinical characteristics and prognosis of these patients.12 Moreover, the prognosis of unselected patients with severe obstructive jaundice is unclear nowadays. The most common clinical presentation of malignant obstructive jaundice traditionally has been considered silent jaundice.35 However, the clinical features of malignant versus benign causes of jaundice have not been a focus of interest during the last decades, and it is not clear from the literature what proportion of patients with choledocholithiasis and jaundice present with silent jaundice. Studies on the prognosis of patients with jaundice were published more than 20 years ago, prior to major advances in imaging modalities and endoscopic treatment.614 Thus, we aimed to study the clinical features, etiology, and prognosis of patients presenting with obstructive jaundice in the current era of improved imaging and noninvasive treatment.
MATERIAL AND METHODS
Over a 2‐year period from the beginning of 2003 to the end of 2004, all adult patients with s‐bilirubin of 5.85 mg/dL (100 mol/l; reference value < 25 mol/L) were identified at the clinical laboratory serving the Sahlgrenska University Hospital (Gothenburg, Sweden), which analyzed the serum bilirubin of all patients in Gothenburg. Sahlgrenska University Hospital provides hospital services for all inhabitants of the city and comprises 3 hospitals (Sahlgrenska Hospital, stra Hospital, and Mlndal Hospital) that serve as community hospitals as well as a university hospital. The Gothenburg metropolitan area has 600,000 inhabitants.
The inclusion criteria for the study cohort were s‐bilirubin 100 mol/L at any point during the 2‐year period with evidence of dilated biliary ducts on abdominal ultrasound. A retrospective review of medical records was performed to retrieve information on the presence of abdominal pain associated with jaundice, computerized tomography (CT) and/or magnetic resonance cholangio‐pancreatography (MRCP) testing, and s‐AST, s‐ALT, s‐ALP, and bilirubin levels. The liver tests performed at the time that s‐bilirubin peaked during hospitalization were analyzed. Information about whether abdominal pain was associated with jaundice at the time of admission to the hospital, was obtained from medical records. The etiology of and treatment for the biliary obstruction were noted. Furthermore, the prognosis of the patients was analyzed. If a patient was discharged from the hospital, information about whether the patient was alive at the time of follow‐up was obtained from the Swedish National Registration of Inhabitants; if the patient had died outside the hospital, a death certificate was requested from the Cause of Death Register of the Swedish National Board of Health and Welfare. Patients were followed up in July 2005, providing a follow‐up period ranging from 6 months to 2.5 years.
Statistics
To test differences between groups, the Fisher exact test was used for dichotomous variables and the Mann‐Whitney test for continuous variables. All tests were 2‐tailed and were conducted at a 5% significance level. The results are presented as medians and interquartile ranges (IQRs).
RESULTS
Patients
During the study period 749 patients were consecutively admitted to our hospital for severe jaundice with bilirubin 5.85 mg/dL (100 mol/L). Among these patients, a total of 241 (32%) had ultrasound evidence of obstructive jaundice at various levels of the biliary tree. In the total study group, the median age was 71 years (IQR 5981 years), with 129 women and 112 men. The oldest patient was 94 years old and the youngest 18 years. No patient was lost to follow‐up.
Causes
The causes of the obstructive jaundice are shown in Table 1. Among the different types of malignancy causing obstructive jaundice, pancreatic cancer and cholangiocarcinoma were the most common, followed by the other malignancies category (Table 1). As shown in Table 2, a wide variety of other malignancies caused cases of biliary obstruction, although most were a result of metastases from gastrointestinal malignancies. Gallstone disease and biliary stricture were the most common benign causes. Less common causes are shown in Table 2. Patients with malignant versus benign obstructive jaundice were similar in age (Table 3). However, only 10 patients (6.5%) with malignant obstructive jaundice (OJ) were less than 50 years old, whereas 23 patients (26%) with benignly caused OJ were under the age of 50. Among the patients with malignancy, patients with pancreatic cancer were significantly older than those with cholangiocarcinoma (P = .04, Table 1). No other major age differences were observed in the etiological groups. Most patients with gallstone disease presenting with jaundice had experienced abdominal pain in association with jaundice, but the jaundice of 9% of the patients was painless (Fig. 1). This was in contrast with patients whose OJ was caused by different types of malignancies, a minority of whom experienced pain at presentation (Fig. 1). Table 3 shows a comparison of patients with a malignant obstruction and those with a benign obstruction. Abdominal pain associated with jaundice was less prevalent at presentation in patients with malignant obstructive jaundice compared with those with nonmalignant obstructive jaundice (34% vs. 71%; P < .0001; Table 3). In 4 patients, the cause of jaundice could not be determined from chart review. Three of these patients presented late in a generally bad condition; ultrasound showed dilated ducts, but these patients died in a few days, before further investigations had been performed (autopsies were not performed), and 1 patient refused further investigations. Thus, an etiological explanation was found for the obstruction of almost all patients.
| Pancreatic cancer | Cholangio‐cancer | Other cancers | Papilla cancers | Biliary strictures | Gallstone disease | Other diagnoses | PSC | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Total number | 69 | 44 | 36 | 5 | 7 | 57 | 18 | 5 |
| Age | 73 (6782) | 67 (5678) | 67 (5975) | 79 (7081) | 80 (5184) | 69 (4983) | 72 (5285) | 61 (3468) |
| Sex‐F/M | 36/33 | 27/17 | 19/17 | 3/2 | 4/3 | 29/28 | 9/9 | 2/3 |
| Surgery | 10/69 | 10/44 | 1/36 | 3/5 | 0/7 | 21/57 | 4/18 | 1/5 |
| Alive at follow‐up | 3/69 (4.3%) | 2/44 (4.5%) | 2/36 (5.6%) | 1/5 (20%) | 6/7 (86%) | 46/57 (80.1%) | 12/18 (66%) | 4/5 (80%) |
| Survival (days) | 142 (58267) | 166 (80300) | 31 (1873) | 257 (130380) | 510 (455900) | 558 (424665) | 412 (103558) | 570 (319676) |
| Type of malignancy | Number of patients | Other cause of OJ not classified elsewhere | Number of patients |
|---|---|---|---|
| Colorectal cancer with liver metastases | 9 | Cholangitis | 4 |
| Liver metastases with unknown primary tumor | 9 | Papillary adenoma | 4 |
| Gastric cancer with liver metastases | 5 | Unknown cause | 4 |
| Small bowel cancer with liver metastases | 2 | Choledochal injury after cholecystectomy | 2 |
| Neuroendocrine tumor with liver metastases | 2 | Retroperitoneal fibrosis | 1 |
| Primary hepatocellular cancer | 2 | Mirizzis syndrome | 1 |
| Esophageal cancer with liver metastases | 1 | Chronic pancreatitis | 1 |
| Renal cancer with liver metastases | 1 | Duodenal diverticula | 1 |
| Lung cancer with liver metastases | 1 | ||
| Tuba uteri cancer with liver metastases | 1 | ||
| Prostate cancer with liver metastases | 1 | ||
| Chronic lymphatic leukemia with liver infiltrates | 1 | ||
| Liver cancer of unknown source | 1 |
| Malignant obstruction | Benign obstruction | |
|---|---|---|
| ||
| Total number of patients | 154 | 87 |
| Female/male | 85/69 | 44/43 |
| Age | 72 (6181) | 69 (4983) |
| Abdominal pain at presentation | 53/154 (34%) | 62/87 (71%) |
| AST | 3.3 (2.35.1) | 3.3 (2.16.4) |
| ALT | 3.1 (26) | 4.3 (2.69.1) |
| ALP | 9.9 (4.813.3) | 5.9 (3.78.5) |
| Bilirubin | 13.8 (1020) | 7.1 (5.79.0) |
| CT | 125/154 (81%) | 47/87 (54%) |
| MRCP | 47/154 (30%) | 27/87 (31%) |
| ERCP | 108/154 (70%) | 67/87 (77%) |
| PTC | 59/154 (38%) | 7/87 (8%) |
| Surgery | 24/154 (15.6%)* | 26/87 (29.9%) |
| Alive at follow‐up | 8/154 (5.2%) | 68/87 (78%) |

Investigations
Investigations carried out to verify the diagnoses of all 241 patients are listed in Table 3. All patients underwent abdominal ultrasound, which was a prerequisite for inclusion in the analysis of patients with dilated biliary ducts. Other diagnostic tools were MRCP, endoscopic retrograde cholangio‐pancreatography (ERCP; as well as therapeutic), and percutaneous transhepatic choangiography (PTC); 12 patients received a diagnostic abdominal laparoscopy, and 1 patient had a laparotomy for diagnostic purposes. CT was more commonly utilized in patients with malignancies, whereas the use of MRCP was similar for patients with malignancies and those without malignancies (Table 3). Median s‐bilirubin level of the patients with malignancies was higher than that of patients without malignancies (Table 3; P < .0001). Among the major etiological groups, s‐bilirubin level was higher in the cholangiocarcinoma group than in the group with pancreatic cancer (Table 4; P < .05), but otherwise no significant differences were observed within the malignant group. However, patients with gallstone disease had significantly lower levels of bilirubin compared with those in the different etiological groups with malignant obstruction (P < .05 for all comparisons; Table 4). Patients with liver metastases had the highest levels of alkaline phosphatase (ALP), followed by patients with cholangiocarcinoma (Table 4). In general, patients with a malignant cause of obstructive jaundice had higher ALP values than those whose OJ had a nonmalignant cause (Table 4). The groups did not differ in aspartate aminotransferase level. However, alanine aminotransferase level of patients with gallstone disease was higher than that of patients with cholangiocarcinoma, P = .009, and of patients with other cancers, P = .02 (Table 4).
| Pancreatic cancer | Cholangio‐carcinoma | Other cancers | Papilla cancer | Biliary strictures | Gallstone disease | Other diagnoses | PSC | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| AST | 3.3 (2.46) | 3.1 (2.15) | 3.7 (2.45.1) | 3.7 (2.64.7) | 5.6 (2.77.3) | 3.1 (1.76.7) | 3.9 (2.46.1) | 3.3 (3.16.1) |
| ALT | 4.4 (2.16.3) | 2.7 (2.14.1) | 2.7 (1.43.7) | 2.6 (2.33.4) | 4.4 (2.96.9) | 4.3 (2.610.9) | 4.4 (2.96.3) | 2.6 (1.45.7) |
| ALP | 8.3 (4.815.6) | 10.1 (516.6) | 12.4 (4.419.4) | 8.1 (4.410.1) | 7.2 (3.38.9) | 5.4 (3.37.8) | 7.1 (4.49.4) | 10 (513.3) |
| Bilirubin | 12.9 (9.517.3) | 16.7 (11.921.4) | 13.1 (8.821.2) | 14.8 (13.416.8) | 8.6 (7.611.3) | 6.7 (5.68.1) | 7.9 (5.211.4) | 13.8 (12.816.7) |
Treatment
Of all 241 patients with obstructive jaundice, 56 (23%) had been operated on, with 1 patient with a cholangiocarcinoma (klatskin tumor) receiving a transplanted liver. Of patients with malignant obstruction, 24 of 154 (15%) underwent an operation, whereas 30% of those with benign obstructions were operated on (Tables 1 and 2). Therapeutic ERCP was used similarly in the malignant and the nonmalignant cases (Table 3).
PTC was used in the vast majority of patients whose obstruction was caused by a malignancy and in only a few of the patients whose obstruction had a benign cause (Table 3).
Prognosis
A total of 165 of the 241 patients (68.5%) died during follow‐up. Mortality was very high among patients with malignant obstructive jaundice, with only 8 of 154 patients (5.2%) alive at the end of follow‐up (Table 3). All these patients had been followed for at least 1 year (mean duration of follow‐up, 535 days). Among those who had survived at least 1 year, 1 patient with cholangiocarcinoma had undergone a liver transplantation, and another 5 patients had been operated on, 3 with pancreatic cancer, 1 with cholangiocarcinoma, 1 with a papilla Vateri cancer, and 1 with carcinoid syndrome with liver metastases. One patient with tuba uteri cancer who had received chemotherapy was also alive. The survival rates of the different etiological groups are shown in Table 1. Of 24 patients undergoing surgery for a malignant condition leading to obstructive jaundice, only 9 (37.5%) survived 1 year. The mortality in the total study group within 3 months of diagnosis was 32% (Table 5). The 3‐month, 6‐month, and 1‐year survival rates for the different etiological groups are given in Table 5. Generally, the patients with benign obstructive jaundice had a good prognosis. A total of 46 of 57 patients (80%) with gallstone disease were alive at the end of follow‐up. Only 3 patients with gallstone disease did not survive for 3 months after jaundice occurred. All these patients were very old, none died while hospitalized for jaundice, and only 1 death could be attributed to gallstone disease (cholangitis and sepsis) 1 month after the initial hospitalization. Figure 2A,B shows the mortality over time among the major etiological groups.
| Pancreatic cancer | Cholangio cancer | Other cancers | Papilla cancer | Biliary strictures | Gallstone disease | Other diagnoses | PSC | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| 3 months' survival | 44/69 (63.8%) | 32/44 (72.7%) | 6/36 (16.7%) | 4/5 (80%) | 7/7 (100%) | 54/57 (95%) | 13/16 (81%) | 5/5 (100%) |
| 6 months' survival | 28/69 (40.6%) | 20/44 (45.5%) | 5/36 (13.9%) | 3/5 (60%) | 7/7 (100%) | 50/57 (88%) | 11/16 (68%) | 4/5 (80%) |
| 12 months' survival | 10/69 (14.5%) | 9/44 (20.5%) | 2/36 (5.6%) | 2/5 (40%) | 7/7 (100%) | 47/57 (82%) | 14/18 (77%) | 4/5 (80%) |

DISCUSSION
Our analysis of the causes of jaundice among unselected patients with a bilirubin level 5.85 mg/dL (100 mol/L) revealed that approximately one third of the cases were a result of obstructive jaundice. Studies published more than 20 years ago are available on the etiology and prognosis of these patients, reflecting the diagnostic techniques and hospital practice at that time.69 Furthermore, more recently published studies report the etiological spectrum of patients with obstructive jaundice in Africa and India.1012 In our study population, 154 of 241 patients (64%) had a malignancy, which is remarkably similar to the 61% and 65% of cases of obstructive jaundice due to malignancy reported in studies published more than 25 years ago from Denmark and Spain, respectively.67, 14
The results of the current study demonstrate the poor prognosis of patients with hepatobiliary malignancy that causes obstructive jaundice. The patients whose OJ was caused by a malignancy had a mortality rate of approximately 95% during the study's rather short follow‐up. Previous studies, mostly from the 1980s, found similarly dismal outcomes,1213 suggesting that the prognosis of these patients has not improved during the last 3 decades. Among patients hospitalized for malignant obstructive jaundice in Denmark in the 1970s and the beginning of the 1980s, 1‐year survival was reported to be 11%.14 Thus, unfortunately, the prognosis of patients presenting with jaundice caused by hepatobiliary cancer obstructing the biliary ducts (or due to liver metastases) does not seem to have improved during the last 3 decades. The most common cause of malignant obstructive jaundice in the current study was pancreatic cancer, which is in agreement what was reported in earlier studies.3, 1115 The poor prognosis of patients with pancreatic cancer is well known, but operative mortality is very low nowadays, and some series have reported 5‐year survival rates in the range of 10%30%.1620 The results of the current study suggest that the prognosis for patients with pancreatic cancer presenting with bilirubin 5.85 (100 mL/L) is worse than the 5‐year survival rates reported in recent studies.1620
In the current study patients with cholangiocarcinoma were almost one third of those with malignant obstructive jaundice, which is a higher proportion than that previously reported in series from Australia (9%), India (14%), and Denmark (17%).13, 11, 14 The increased proportion of patients with cholangiocarcinoma might reflect the recent observations of an increased incidence of cholangiocarcinoma in many countries.2122 Similar to the situation of patients with pancreatic cancer, very few patients with cholangiocancer will survive long term,2325 and as in the current study, the prognosis of these patients presenting with severe jaundice seems even worse. Mortality among our patients with malignancies other than pancreatic cancer and cholangiocancer was also very high, with only 5% surviving, mostly because of liver metastases. This is identical to the 1‐year survival of patients with liver metastases presenting with jaundice in Denmark more than 25 years ago.14 Although our patients might not be directly comparable to those seen in Denmark at that time, the results of the current study do suggest that the prognosis of patients with jaundice resulting from liver metastases has not improved over time, despite the considerable advances in diagnostic procedures during the last 2 or 3 decades. One of the limitations of the study besides its retrospective design is potentially excluding patients who were less sick (those with bilirubin levels below that used as an inclusion criterion), affecting survival rates. Other limitations might be defining biliary obstruction based on the results of ultrasound. However, we found very good correlation between ultrasound evidence of dilated biliary ducts and those who had evidence of biliary obstruction on MRCP and ERCP (data not shown).
However, one seemingly large difference between the current study and the Danish study14 is in the prognosis of patients with gallstone disease. The overall 1‐year survival was similar in the 2 studies, but whereas in the Danish study the deaths of 9 of 105 patients (8.6%) was attributed to their gallstone disease, the death of only 1 of the 57 patients (1.8%) in the current study could be attributed to gallstone disease. The reason for this difference is not easily explained but might have been a result of better diagnostic instruments and/or more commonly used ERCP procedures than were previously available.
Although jaundice resulting from a malignancy in the hepatobiliary tract is said to be painless,35 in our study approximately one third of patients with a malignancy experienced pain at presentation. However, our study confirmed that abdominal pain was significantly more often associated with benign conditions. The limitation of the current study was its retrospective nature. However, information about the occurrence of abdominal pain was available in medical records of all patients. Although we could not analyze the character, location, or nature of the abdominal pain, the attending doctor always asked a patient about whether he or she was experiencing abdominal pain.
We can conclude that the severe jaundice of one third of patients was a result of obstructive jaundice. Most of these cases were also a result of a malignancy, with high bilirubin levels indicating prolonged biliary obstruction. Obstructive jaundice caused by a malignancy carried a very poor prognosis, with approximately 95% mortality during a 1‐ to 2‐year follow‐up period. In the absence of methods to cure a significant number of these patients, good methods of palliation are important challenges in the near future.
Jaundice is an important clinical entity associated with a wide variety of differential diagnoses for which the prognosis differs depending on etiology. Recently, the etiological spectrum of unselected patients with jaundice has been reported.12 Among patients with cholestatic jaundice, abdominal ultrasound remains the primary investigation in order to distinguish extrahepatic biliary obstruction from intrahepatic disease.
Recent studies of patients with jaundice have included a limited number of patients with jaundice due to biliary obstruction and provided no analysis of the clinical characteristics and prognosis of these patients.12 Moreover, the prognosis of unselected patients with severe obstructive jaundice is unclear nowadays. The most common clinical presentation of malignant obstructive jaundice traditionally has been considered silent jaundice.35 However, the clinical features of malignant versus benign causes of jaundice have not been a focus of interest during the last decades, and it is not clear from the literature what proportion of patients with choledocholithiasis and jaundice present with silent jaundice. Studies on the prognosis of patients with jaundice were published more than 20 years ago, prior to major advances in imaging modalities and endoscopic treatment.614 Thus, we aimed to study the clinical features, etiology, and prognosis of patients presenting with obstructive jaundice in the current era of improved imaging and noninvasive treatment.
MATERIAL AND METHODS
Over a 2‐year period from the beginning of 2003 to the end of 2004, all adult patients with s‐bilirubin of 5.85 mg/dL (100 mol/l; reference value < 25 mol/L) were identified at the clinical laboratory serving the Sahlgrenska University Hospital (Gothenburg, Sweden), which analyzed the serum bilirubin of all patients in Gothenburg. Sahlgrenska University Hospital provides hospital services for all inhabitants of the city and comprises 3 hospitals (Sahlgrenska Hospital, stra Hospital, and Mlndal Hospital) that serve as community hospitals as well as a university hospital. The Gothenburg metropolitan area has 600,000 inhabitants.
The inclusion criteria for the study cohort were s‐bilirubin 100 mol/L at any point during the 2‐year period with evidence of dilated biliary ducts on abdominal ultrasound. A retrospective review of medical records was performed to retrieve information on the presence of abdominal pain associated with jaundice, computerized tomography (CT) and/or magnetic resonance cholangio‐pancreatography (MRCP) testing, and s‐AST, s‐ALT, s‐ALP, and bilirubin levels. The liver tests performed at the time that s‐bilirubin peaked during hospitalization were analyzed. Information about whether abdominal pain was associated with jaundice at the time of admission to the hospital, was obtained from medical records. The etiology of and treatment for the biliary obstruction were noted. Furthermore, the prognosis of the patients was analyzed. If a patient was discharged from the hospital, information about whether the patient was alive at the time of follow‐up was obtained from the Swedish National Registration of Inhabitants; if the patient had died outside the hospital, a death certificate was requested from the Cause of Death Register of the Swedish National Board of Health and Welfare. Patients were followed up in July 2005, providing a follow‐up period ranging from 6 months to 2.5 years.
Statistics
To test differences between groups, the Fisher exact test was used for dichotomous variables and the Mann‐Whitney test for continuous variables. All tests were 2‐tailed and were conducted at a 5% significance level. The results are presented as medians and interquartile ranges (IQRs).
RESULTS
Patients
During the study period 749 patients were consecutively admitted to our hospital for severe jaundice with bilirubin 5.85 mg/dL (100 mol/L). Among these patients, a total of 241 (32%) had ultrasound evidence of obstructive jaundice at various levels of the biliary tree. In the total study group, the median age was 71 years (IQR 5981 years), with 129 women and 112 men. The oldest patient was 94 years old and the youngest 18 years. No patient was lost to follow‐up.
Causes
The causes of the obstructive jaundice are shown in Table 1. Among the different types of malignancy causing obstructive jaundice, pancreatic cancer and cholangiocarcinoma were the most common, followed by the other malignancies category (Table 1). As shown in Table 2, a wide variety of other malignancies caused cases of biliary obstruction, although most were a result of metastases from gastrointestinal malignancies. Gallstone disease and biliary stricture were the most common benign causes. Less common causes are shown in Table 2. Patients with malignant versus benign obstructive jaundice were similar in age (Table 3). However, only 10 patients (6.5%) with malignant obstructive jaundice (OJ) were less than 50 years old, whereas 23 patients (26%) with benignly caused OJ were under the age of 50. Among the patients with malignancy, patients with pancreatic cancer were significantly older than those with cholangiocarcinoma (P = .04, Table 1). No other major age differences were observed in the etiological groups. Most patients with gallstone disease presenting with jaundice had experienced abdominal pain in association with jaundice, but the jaundice of 9% of the patients was painless (Fig. 1). This was in contrast with patients whose OJ was caused by different types of malignancies, a minority of whom experienced pain at presentation (Fig. 1). Table 3 shows a comparison of patients with a malignant obstruction and those with a benign obstruction. Abdominal pain associated with jaundice was less prevalent at presentation in patients with malignant obstructive jaundice compared with those with nonmalignant obstructive jaundice (34% vs. 71%; P < .0001; Table 3). In 4 patients, the cause of jaundice could not be determined from chart review. Three of these patients presented late in a generally bad condition; ultrasound showed dilated ducts, but these patients died in a few days, before further investigations had been performed (autopsies were not performed), and 1 patient refused further investigations. Thus, an etiological explanation was found for the obstruction of almost all patients.
| Pancreatic cancer | Cholangio‐cancer | Other cancers | Papilla cancers | Biliary strictures | Gallstone disease | Other diagnoses | PSC | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Total number | 69 | 44 | 36 | 5 | 7 | 57 | 18 | 5 |
| Age | 73 (6782) | 67 (5678) | 67 (5975) | 79 (7081) | 80 (5184) | 69 (4983) | 72 (5285) | 61 (3468) |
| Sex‐F/M | 36/33 | 27/17 | 19/17 | 3/2 | 4/3 | 29/28 | 9/9 | 2/3 |
| Surgery | 10/69 | 10/44 | 1/36 | 3/5 | 0/7 | 21/57 | 4/18 | 1/5 |
| Alive at follow‐up | 3/69 (4.3%) | 2/44 (4.5%) | 2/36 (5.6%) | 1/5 (20%) | 6/7 (86%) | 46/57 (80.1%) | 12/18 (66%) | 4/5 (80%) |
| Survival (days) | 142 (58267) | 166 (80300) | 31 (1873) | 257 (130380) | 510 (455900) | 558 (424665) | 412 (103558) | 570 (319676) |
| Type of malignancy | Number of patients | Other cause of OJ not classified elsewhere | Number of patients |
|---|---|---|---|
| Colorectal cancer with liver metastases | 9 | Cholangitis | 4 |
| Liver metastases with unknown primary tumor | 9 | Papillary adenoma | 4 |
| Gastric cancer with liver metastases | 5 | Unknown cause | 4 |
| Small bowel cancer with liver metastases | 2 | Choledochal injury after cholecystectomy | 2 |
| Neuroendocrine tumor with liver metastases | 2 | Retroperitoneal fibrosis | 1 |
| Primary hepatocellular cancer | 2 | Mirizzis syndrome | 1 |
| Esophageal cancer with liver metastases | 1 | Chronic pancreatitis | 1 |
| Renal cancer with liver metastases | 1 | Duodenal diverticula | 1 |
| Lung cancer with liver metastases | 1 | ||
| Tuba uteri cancer with liver metastases | 1 | ||
| Prostate cancer with liver metastases | 1 | ||
| Chronic lymphatic leukemia with liver infiltrates | 1 | ||
| Liver cancer of unknown source | 1 |
| Malignant obstruction | Benign obstruction | |
|---|---|---|
| ||
| Total number of patients | 154 | 87 |
| Female/male | 85/69 | 44/43 |
| Age | 72 (6181) | 69 (4983) |
| Abdominal pain at presentation | 53/154 (34%) | 62/87 (71%) |
| AST | 3.3 (2.35.1) | 3.3 (2.16.4) |
| ALT | 3.1 (26) | 4.3 (2.69.1) |
| ALP | 9.9 (4.813.3) | 5.9 (3.78.5) |
| Bilirubin | 13.8 (1020) | 7.1 (5.79.0) |
| CT | 125/154 (81%) | 47/87 (54%) |
| MRCP | 47/154 (30%) | 27/87 (31%) |
| ERCP | 108/154 (70%) | 67/87 (77%) |
| PTC | 59/154 (38%) | 7/87 (8%) |
| Surgery | 24/154 (15.6%)* | 26/87 (29.9%) |
| Alive at follow‐up | 8/154 (5.2%) | 68/87 (78%) |

Investigations
Investigations carried out to verify the diagnoses of all 241 patients are listed in Table 3. All patients underwent abdominal ultrasound, which was a prerequisite for inclusion in the analysis of patients with dilated biliary ducts. Other diagnostic tools were MRCP, endoscopic retrograde cholangio‐pancreatography (ERCP; as well as therapeutic), and percutaneous transhepatic choangiography (PTC); 12 patients received a diagnostic abdominal laparoscopy, and 1 patient had a laparotomy for diagnostic purposes. CT was more commonly utilized in patients with malignancies, whereas the use of MRCP was similar for patients with malignancies and those without malignancies (Table 3). Median s‐bilirubin level of the patients with malignancies was higher than that of patients without malignancies (Table 3; P < .0001). Among the major etiological groups, s‐bilirubin level was higher in the cholangiocarcinoma group than in the group with pancreatic cancer (Table 4; P < .05), but otherwise no significant differences were observed within the malignant group. However, patients with gallstone disease had significantly lower levels of bilirubin compared with those in the different etiological groups with malignant obstruction (P < .05 for all comparisons; Table 4). Patients with liver metastases had the highest levels of alkaline phosphatase (ALP), followed by patients with cholangiocarcinoma (Table 4). In general, patients with a malignant cause of obstructive jaundice had higher ALP values than those whose OJ had a nonmalignant cause (Table 4). The groups did not differ in aspartate aminotransferase level. However, alanine aminotransferase level of patients with gallstone disease was higher than that of patients with cholangiocarcinoma, P = .009, and of patients with other cancers, P = .02 (Table 4).
| Pancreatic cancer | Cholangio‐carcinoma | Other cancers | Papilla cancer | Biliary strictures | Gallstone disease | Other diagnoses | PSC | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| AST | 3.3 (2.46) | 3.1 (2.15) | 3.7 (2.45.1) | 3.7 (2.64.7) | 5.6 (2.77.3) | 3.1 (1.76.7) | 3.9 (2.46.1) | 3.3 (3.16.1) |
| ALT | 4.4 (2.16.3) | 2.7 (2.14.1) | 2.7 (1.43.7) | 2.6 (2.33.4) | 4.4 (2.96.9) | 4.3 (2.610.9) | 4.4 (2.96.3) | 2.6 (1.45.7) |
| ALP | 8.3 (4.815.6) | 10.1 (516.6) | 12.4 (4.419.4) | 8.1 (4.410.1) | 7.2 (3.38.9) | 5.4 (3.37.8) | 7.1 (4.49.4) | 10 (513.3) |
| Bilirubin | 12.9 (9.517.3) | 16.7 (11.921.4) | 13.1 (8.821.2) | 14.8 (13.416.8) | 8.6 (7.611.3) | 6.7 (5.68.1) | 7.9 (5.211.4) | 13.8 (12.816.7) |
Treatment
Of all 241 patients with obstructive jaundice, 56 (23%) had been operated on, with 1 patient with a cholangiocarcinoma (klatskin tumor) receiving a transplanted liver. Of patients with malignant obstruction, 24 of 154 (15%) underwent an operation, whereas 30% of those with benign obstructions were operated on (Tables 1 and 2). Therapeutic ERCP was used similarly in the malignant and the nonmalignant cases (Table 3).
PTC was used in the vast majority of patients whose obstruction was caused by a malignancy and in only a few of the patients whose obstruction had a benign cause (Table 3).
Prognosis
A total of 165 of the 241 patients (68.5%) died during follow‐up. Mortality was very high among patients with malignant obstructive jaundice, with only 8 of 154 patients (5.2%) alive at the end of follow‐up (Table 3). All these patients had been followed for at least 1 year (mean duration of follow‐up, 535 days). Among those who had survived at least 1 year, 1 patient with cholangiocarcinoma had undergone a liver transplantation, and another 5 patients had been operated on, 3 with pancreatic cancer, 1 with cholangiocarcinoma, 1 with a papilla Vateri cancer, and 1 with carcinoid syndrome with liver metastases. One patient with tuba uteri cancer who had received chemotherapy was also alive. The survival rates of the different etiological groups are shown in Table 1. Of 24 patients undergoing surgery for a malignant condition leading to obstructive jaundice, only 9 (37.5%) survived 1 year. The mortality in the total study group within 3 months of diagnosis was 32% (Table 5). The 3‐month, 6‐month, and 1‐year survival rates for the different etiological groups are given in Table 5. Generally, the patients with benign obstructive jaundice had a good prognosis. A total of 46 of 57 patients (80%) with gallstone disease were alive at the end of follow‐up. Only 3 patients with gallstone disease did not survive for 3 months after jaundice occurred. All these patients were very old, none died while hospitalized for jaundice, and only 1 death could be attributed to gallstone disease (cholangitis and sepsis) 1 month after the initial hospitalization. Figure 2A,B shows the mortality over time among the major etiological groups.
| Pancreatic cancer | Cholangio cancer | Other cancers | Papilla cancer | Biliary strictures | Gallstone disease | Other diagnoses | PSC | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| 3 months' survival | 44/69 (63.8%) | 32/44 (72.7%) | 6/36 (16.7%) | 4/5 (80%) | 7/7 (100%) | 54/57 (95%) | 13/16 (81%) | 5/5 (100%) |
| 6 months' survival | 28/69 (40.6%) | 20/44 (45.5%) | 5/36 (13.9%) | 3/5 (60%) | 7/7 (100%) | 50/57 (88%) | 11/16 (68%) | 4/5 (80%) |
| 12 months' survival | 10/69 (14.5%) | 9/44 (20.5%) | 2/36 (5.6%) | 2/5 (40%) | 7/7 (100%) | 47/57 (82%) | 14/18 (77%) | 4/5 (80%) |

DISCUSSION
Our analysis of the causes of jaundice among unselected patients with a bilirubin level 5.85 mg/dL (100 mol/L) revealed that approximately one third of the cases were a result of obstructive jaundice. Studies published more than 20 years ago are available on the etiology and prognosis of these patients, reflecting the diagnostic techniques and hospital practice at that time.69 Furthermore, more recently published studies report the etiological spectrum of patients with obstructive jaundice in Africa and India.1012 In our study population, 154 of 241 patients (64%) had a malignancy, which is remarkably similar to the 61% and 65% of cases of obstructive jaundice due to malignancy reported in studies published more than 25 years ago from Denmark and Spain, respectively.67, 14
The results of the current study demonstrate the poor prognosis of patients with hepatobiliary malignancy that causes obstructive jaundice. The patients whose OJ was caused by a malignancy had a mortality rate of approximately 95% during the study's rather short follow‐up. Previous studies, mostly from the 1980s, found similarly dismal outcomes,1213 suggesting that the prognosis of these patients has not improved during the last 3 decades. Among patients hospitalized for malignant obstructive jaundice in Denmark in the 1970s and the beginning of the 1980s, 1‐year survival was reported to be 11%.14 Thus, unfortunately, the prognosis of patients presenting with jaundice caused by hepatobiliary cancer obstructing the biliary ducts (or due to liver metastases) does not seem to have improved during the last 3 decades. The most common cause of malignant obstructive jaundice in the current study was pancreatic cancer, which is in agreement what was reported in earlier studies.3, 1115 The poor prognosis of patients with pancreatic cancer is well known, but operative mortality is very low nowadays, and some series have reported 5‐year survival rates in the range of 10%30%.1620 The results of the current study suggest that the prognosis for patients with pancreatic cancer presenting with bilirubin 5.85 (100 mL/L) is worse than the 5‐year survival rates reported in recent studies.1620
In the current study patients with cholangiocarcinoma were almost one third of those with malignant obstructive jaundice, which is a higher proportion than that previously reported in series from Australia (9%), India (14%), and Denmark (17%).13, 11, 14 The increased proportion of patients with cholangiocarcinoma might reflect the recent observations of an increased incidence of cholangiocarcinoma in many countries.2122 Similar to the situation of patients with pancreatic cancer, very few patients with cholangiocancer will survive long term,2325 and as in the current study, the prognosis of these patients presenting with severe jaundice seems even worse. Mortality among our patients with malignancies other than pancreatic cancer and cholangiocancer was also very high, with only 5% surviving, mostly because of liver metastases. This is identical to the 1‐year survival of patients with liver metastases presenting with jaundice in Denmark more than 25 years ago.14 Although our patients might not be directly comparable to those seen in Denmark at that time, the results of the current study do suggest that the prognosis of patients with jaundice resulting from liver metastases has not improved over time, despite the considerable advances in diagnostic procedures during the last 2 or 3 decades. One of the limitations of the study besides its retrospective design is potentially excluding patients who were less sick (those with bilirubin levels below that used as an inclusion criterion), affecting survival rates. Other limitations might be defining biliary obstruction based on the results of ultrasound. However, we found very good correlation between ultrasound evidence of dilated biliary ducts and those who had evidence of biliary obstruction on MRCP and ERCP (data not shown).
However, one seemingly large difference between the current study and the Danish study14 is in the prognosis of patients with gallstone disease. The overall 1‐year survival was similar in the 2 studies, but whereas in the Danish study the deaths of 9 of 105 patients (8.6%) was attributed to their gallstone disease, the death of only 1 of the 57 patients (1.8%) in the current study could be attributed to gallstone disease. The reason for this difference is not easily explained but might have been a result of better diagnostic instruments and/or more commonly used ERCP procedures than were previously available.
Although jaundice resulting from a malignancy in the hepatobiliary tract is said to be painless,35 in our study approximately one third of patients with a malignancy experienced pain at presentation. However, our study confirmed that abdominal pain was significantly more often associated with benign conditions. The limitation of the current study was its retrospective nature. However, information about the occurrence of abdominal pain was available in medical records of all patients. Although we could not analyze the character, location, or nature of the abdominal pain, the attending doctor always asked a patient about whether he or she was experiencing abdominal pain.
We can conclude that the severe jaundice of one third of patients was a result of obstructive jaundice. Most of these cases were also a result of a malignancy, with high bilirubin levels indicating prolonged biliary obstruction. Obstructive jaundice caused by a malignancy carried a very poor prognosis, with approximately 95% mortality during a 1‐ to 2‐year follow‐up period. In the absence of methods to cure a significant number of these patients, good methods of palliation are important challenges in the near future.
- ,,.The causes of obvious jaundice in South West Wales: perceptions versus reality.Gut.2001;48:409–413.
- ,,,.Severe jaundice in Sweden in the new millennium: causes, investigations, treatment and prognosis.Scand J Gastroenterol.2003;38:86–94.
- .Jaundice. In:O'Grady JG,Lake JR,Howdle PD, eds.Comprehensive Clinical Hepatology.London UK:Mosby;2000:5.1–5.17.
- .Examination of an adolescent or an adult patient with jaundice. Hamilton Baileys Demonstrations of Clinical Skills in Clinical Surgery.Bristol, UK:John Wright 1967:271–273.
- ,.Jaundice. In:Sherlock S,Dooley J, eds.Diseases of the Liver and Biliary Tract.London, UK:Blackwell;1993:1999–2013.
- ,,,,.Percutaneous transhepatic cholangiography in diagnostic evaluation of 160 jaundiced patients. Results of an improved technic.Am J Surg.1977;133:559–561.
- ,,,.Computed tomography in obstructive jaundice. Part II: The cause of obstruction.Radiology.1981;139:635–645.
- ,,.Usefulness of diagnostic tests for biliary obstruction.Am J Surg.1982;144:102–108.
- ,,, et al.Ultrasound in obstructive jaundice: prospective evaluation of site and cause.Radiology.1983;147:511–515.
- ,,,.Obstructive jaundice in the South African black population.J Clin Gastroenterol.1986;8:538–541.
- ,.Aetiological spectrum of obstructive jaundice and diagnostic ability of ultrasonography: a clinician's perspective.Trop Gastroenterol.1999;20:167–169.
- ,.Failure to improve survival by improved diagnostic techniques in patients with malignant jaundice.Br J Surg.1986;73:631–633.
- ,.Obstructive jaundice in a referral unit: surgical practice and risk factors.Aust N Z J Surg.1985;55:427–432.
- ,,, et al.Causes and characteristics of 500 consecutive cases of jaundice.Scand J Gastroenterol.1981;16:1–6.
- .Management of malignant bile duct obstruction.J Gastroenterol Hepatol.1990;5(Suppl 1):63–77.
- ,,, et al.A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer.N Engl J Med.2004;350:1200–1210.
- ,,.Prognostic factors following curative resection for pancreatic adenocarcinoma: a population‐based, linked database analysis of 396 patients.Ann Surg.2003;237:74–85.
- .Critical look at resection for pancreatic cancer.Lancet.1996;348:1676.
- ,,,.Survival of patients with primary liver cancer, pancreatic cancer and biliary tract cancer in Europe.EUROCARE Working Group.Eur J Cancer.1998;34:2184–2190.
- ,,,.Pancreatic cancer.Lancet.2004;363:1049–1057.
- ,,, et al.Increase in mortality rates from intrahepatic cholangiocarcinoma in England and Wales 1968–1998.Gut.2001;48:816–820.
- ,,,.Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase?J Hepatol.2004;40:472–477.
- ,.Surgical management of cholangiocarcinoma.Semin Liver Dis.2004;24:189–199.
- ,,, et al.Improved survival in resected biliary malignancies.Surgery.2002;132:555–563.
- ,,,,.Intrahepatic biliary enteric bypass provides effective palliation in selected patients with malignant obstruction at the hepatic duct confluence.Am J Surg.1998;175:453–460.
- ,,.The causes of obvious jaundice in South West Wales: perceptions versus reality.Gut.2001;48:409–413.
- ,,,.Severe jaundice in Sweden in the new millennium: causes, investigations, treatment and prognosis.Scand J Gastroenterol.2003;38:86–94.
- .Jaundice. In:O'Grady JG,Lake JR,Howdle PD, eds.Comprehensive Clinical Hepatology.London UK:Mosby;2000:5.1–5.17.
- .Examination of an adolescent or an adult patient with jaundice. Hamilton Baileys Demonstrations of Clinical Skills in Clinical Surgery.Bristol, UK:John Wright 1967:271–273.
- ,.Jaundice. In:Sherlock S,Dooley J, eds.Diseases of the Liver and Biliary Tract.London, UK:Blackwell;1993:1999–2013.
- ,,,,.Percutaneous transhepatic cholangiography in diagnostic evaluation of 160 jaundiced patients. Results of an improved technic.Am J Surg.1977;133:559–561.
- ,,,.Computed tomography in obstructive jaundice. Part II: The cause of obstruction.Radiology.1981;139:635–645.
- ,,.Usefulness of diagnostic tests for biliary obstruction.Am J Surg.1982;144:102–108.
- ,,, et al.Ultrasound in obstructive jaundice: prospective evaluation of site and cause.Radiology.1983;147:511–515.
- ,,,.Obstructive jaundice in the South African black population.J Clin Gastroenterol.1986;8:538–541.
- ,.Aetiological spectrum of obstructive jaundice and diagnostic ability of ultrasonography: a clinician's perspective.Trop Gastroenterol.1999;20:167–169.
- ,.Failure to improve survival by improved diagnostic techniques in patients with malignant jaundice.Br J Surg.1986;73:631–633.
- ,.Obstructive jaundice in a referral unit: surgical practice and risk factors.Aust N Z J Surg.1985;55:427–432.
- ,,, et al.Causes and characteristics of 500 consecutive cases of jaundice.Scand J Gastroenterol.1981;16:1–6.
- .Management of malignant bile duct obstruction.J Gastroenterol Hepatol.1990;5(Suppl 1):63–77.
- ,,, et al.A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer.N Engl J Med.2004;350:1200–1210.
- ,,.Prognostic factors following curative resection for pancreatic adenocarcinoma: a population‐based, linked database analysis of 396 patients.Ann Surg.2003;237:74–85.
- .Critical look at resection for pancreatic cancer.Lancet.1996;348:1676.
- ,,,.Survival of patients with primary liver cancer, pancreatic cancer and biliary tract cancer in Europe.EUROCARE Working Group.Eur J Cancer.1998;34:2184–2190.
- ,,,.Pancreatic cancer.Lancet.2004;363:1049–1057.
- ,,, et al.Increase in mortality rates from intrahepatic cholangiocarcinoma in England and Wales 1968–1998.Gut.2001;48:816–820.
- ,,,.Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase?J Hepatol.2004;40:472–477.
- ,.Surgical management of cholangiocarcinoma.Semin Liver Dis.2004;24:189–199.
- ,,, et al.Improved survival in resected biliary malignancies.Surgery.2002;132:555–563.
- ,,,,.Intrahepatic biliary enteric bypass provides effective palliation in selected patients with malignant obstruction at the hepatic duct confluence.Am J Surg.1998;175:453–460.
Copyright © 2008 Society of Hospital Medicine