User login
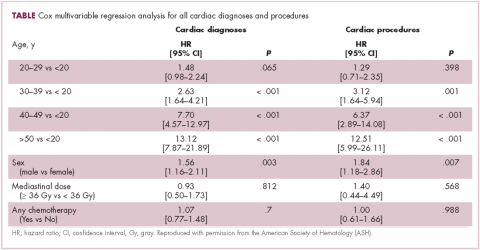
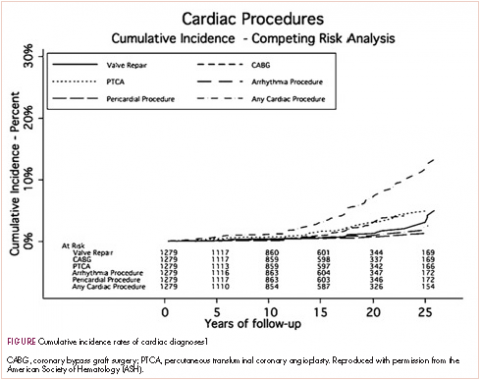
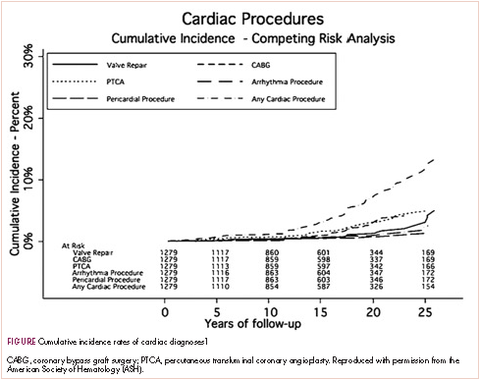
A typical example is a patient who had Hodgkin disease in his teens and received mediastinal mantle radiation. Fifteen to 25 years later, the patient has a pacemaker for heart block, coronary artery disease that requires a stent, and most recently has two valves replaced—so aortic and mitral valve replacement because of late radiation effects. This scenario is typical for the “old” days. The 20-year cumulative incidence of radiation-induced cardiac toxicity is 15%-20% (Table, Figure).1 Sitting with a patient about to begin chest radiation, the absolute risks are unknown but presumed to be less as treatment is delivered according to the modern techniques that you described in the question.
DH They’re so much better now, so this is less common.
JC With the shielding and breath-holding techniques and position changes, doing upright radiation rather than supine, and because the technology has improved both in the delivery of radiation and the technology in understanding where all the radiation is going, in today’s world, we can calculate pretty precisely how much radiation the heart actually receives. Ultimately, with the protective mechanisms that are in place going forward, the risks that I described for that survivor are probably exponentially less than what’s reported in the literature and what we see clinically. Radiation has become much, much safer. There is still probably some small risk of development of late changes, but I don’t think we know what that risk is today because the shielding and things we do to protect the heart have not yet been studied in the long term.
DH Of course, the patient is breathing and there’ll be some movement of the target. Some of the radiation techniques can follow the target despite the breathing?
JC Yes, definitely true. Radiation delivery is much more precise today. Not only has the delivery changed, but so has what we know about the location of potential arterial disease. For example, if you read any textbook, it says that for the coronaries, that it’s ostial and proximal disease of the left main, or the left anterior descending, or the right coronary artery. Today, somebody who gets chest/mediastinal radiation, for either breast cancer, lymphoma, or for a mediastinal tumor, the location of potential disease is more likely to mimic the location of classic coronary disease in the mid-portion of the left anterior descending artery rather than at the ostium. It’s going to be a different disease going forward.2,3
DH Let’s switch from radiation to chemotherapy. Of course, all of us worry about and are very familiar with the toxicity potential of doxorubicin and trastuzumab. I remember an American Society of Clinical Oncology meeting a few years ago, one of the speakers was a cardiologist and was advising us that perhaps the ejection fraction, albeit readily available and reproducible, was probably too simple and we should watch more closely with other techniques. My final question and then I’ll let you comment – I thought I recalled 5-fluorouracil (5-FU) infusions, which we do in some of our colorectal cancers, for example, can cause a vasospasm, Prinzmetal-type angina from time to time, and is that true in capecitabine? What are your thoughts on how to follow the doxorubicin, trastuzumab analogs, and anything about 5-FU and its analogs?
JC Okay, this is a giant question. I’ll take them in order. First, doxorubicin. Cumulative dose-related cardiotoxicity was first described by Von Hoff in 1979.4 That is, the more you get, the higher likelihood of developing cardiotoxicity. Up to a total of 400 mg/m2, the risk is <1%, with a sharp rise as the dose increases beyond this level.4 That being said, there is a clear large and individual variation: I’ve seen sarcoma patients who’ve gotten close to 1,000 mg/m2 without cardiac dysfunction, and some people with minimal exposure have full-blown cardiomyopathy. One of the protective strategies that we developed over the years is to give less of the drug, and with that get the same cancer treatment efficacy. There is definitely a risk for anthracyclines. Full-blown heart failure is probably in the 4%-8% range – and that’s cumulative lifetime – it’s not as high as we once thought it was. That doesn’t mean that it isn’t there, but, relatively speaking, from the standpoint of benefit of anthracyclines, the benefit certainly clearly outweighs the cardiac risk.
With administration of the anthracyclines, we try to do whatever protective things we can do. There are some people who believe that continuous infusion is safer for the heart than bolus injection. It’s pretty controversial. Dexrazoxane, which is a chelating agent, has been shown to reduce cardiotoxicity, and using a lipophilic anthracycline preparation may also have less cardiac toxicity.
DH I have a population in which a lot of liposomal doxorubicin is used and I’ve given a lot and rarely if ever get cardiac toxicity. You see that as well?
JC Yes. There’s a significant financial difference between doxorubicin and liposomal doxorubicin; the latter is more expensive. From the standpoint of safety, and from the standpoint of if I ever needed doxorubicin, I would probably jump on that and ask for the liposomal preparation and/or dexrazoxane.
DH For trastuzumab, we are getting echo- cardiograms every 9 weeks. That seems awfully simple, but there’s a whole algorithm we follow for particular change in ejection fraction and watch the drug or stop the drug. Are we doing that correctly?
JC The first statement I would make about that is that there are too many women who need trastuzumab whose therapy has been prematurely stopped because of just looking at the ejection fractions. So, there has to be more to decision-making other than just the number of the ejection fraction. We’re pretty aggressive and tend to try to get women to get the full dose and whatever dose-effective dose they need, especially with curative intent in the adjuvant setting that we make decisions based not only on the ejection fraction.
We also have, I would say, a handful of our medical breast oncologists who do not follow the package insert. We don’t get ejection fractions every 3 cycles. We have substituted a little bit by following biomarkers so that we use N-terminal pro b-type natriuretic peptide (NT-proBNP) to monitor people, either with each cycle or every third cycle. The benefit of BNP is its negative predictive value. If it’s normal, it’s hard to have any clinically significant myocardial dysfunction.
What we’re going to see over – I would hope – the next year or two is that the recommendations about getting echocardiograms frequently will go away.
DH That would be welcome because in our electronic medical records, it’s 9 weeks, stop, do this, etc. How about a comment on infusional 5-FU and possibly its cousins, such as capecitabine, and any coronary issues?
JC Let me come back, just one more thing about trastuzumab. For metastatic disease, we do whatever is necessary to continue effective cancer therapy and in the absence of any cardiac symptoms or abnormal physical findings, we continue cancer treatment without any serial echocardiographic monitoring.
DH You think the NT-proBNP might be useful? I know that’s excreted by the kidneys, so that might rise in renal failure, but we can adjust for that.
JC The negative predictive value of having a normal BNP is helpful. I think what I wanted to say was that screening echocardiograms and looking at ejection fraction in low-risk populations probably is clearly not cost-effective. It probably never alters decision making. If you have a 30-year-old person with no cardiac risk factors and no past cardiac history who develops B-cell lymphoma and is going to get anthracycline-based chemotherapy, the likelihood of finding a reason not to give that therapy based on an echocardiogram is quite small. I would even go further and say close to zero. We’ve begun to look at this. There is literature that supports the concept. Also, that in low-risk people – if you can define the low-risk population in an accurate way – for lymphoma patients or women with breast cancer getting either anthracyclines, trastuzumab, or the other human epidermal growth factor receptor-2 (HER2)-directed therapies, there’s probably little yield to even getting a baseline study.
DH Very interesting. I would agree with you.
JC We’re going to talk about 5-FU, of course. The 5-FU thing has become a passion of mine. Over the last two to two-and-a- half years we have gotten very aggressive with treating coronary spasm that’s induced by the fluoropyrimidines. That’s 5-FU and capecitabine, the oral version.
There is an incidence that the literature says is less than 1%. It probably is somewhere between 3% and 5%. It’s a little bit more common than has been reported. The reason is the way that it presents has classically been described in the literature as different than what occurs in real life. It is a phenomenon. It’s the most common cardiac side effect. Sometimes it is large epicardial coronary artery spasm. Sometimes it’s small vessel spasm. You can have chest pain with no electrocardiographic changes or ECG changes without chest pain (so-called silent ischemia). The description doesn’t always sound like classic angina but symptoms are temporally related to getting the drug.
So, we’ve developed a protocol to treat documented spasm as an outpatient to be able to continue those drugs to their logical conclusion from an oncologic standpoint. In fact, we just submitted a manuscript to the
DH Finally, it occurred to me that we cause problems with radiation. We cause problems with chemotherapy and other infusions. Are there particular cancers that you think of or you’re called in to see that you worry about cardiac involvement by their location? What comes to mind are cases I’ve had in which there is pericardial involvement and tamponade or restrictive pericarditis.
JC We see metastatic disease to the pericardium with breast cancer, lung cancer, and lymphoma. Renal cell has an interesting predilection to go to the pericardium. We’ve seen in the last probably 6 months 2 cases of bladder cancer with pericardial metastases. When we reviewed the literature, we were only able to find 9 or 10 case reports. It’s rare, but it occurs.
Fluid in the pericardium with and without tamponade is increasingly common, and because we do a better job in treating complicated cancer, people successively can receive cycles of sequential chemotherapeutic regimens – they are living longer, their cancer can get more complicated and/or resistant and with it, there’s more time for metastatic disease to occur. Tamponade is a common phenomenon. We always say that at 4 o’clock on Friday we always see somebody who has tamponade. We see a lot of pericardial disease.
Then, another area of a concern is the tyrosine kinase inhibitors that can cause hypertension, which is very common. We’ve become pretty aggressive. The oncologists recognize the importance of being able to follow and treat blood pressures to allow patients to get these treatments. I guess we couldn’t end without talking about checkpoint inhibitors and the recent lay press flurry about reporting myocarditis.
DH I haven’t personally experienced that. How common is that, and how do we watch for it?
JC Personally, I’ve seen probably four or five people who were referred because of heart failure on checkpoint inhibitors. For each of them, there was historically something as a preexisting problem before the checkpoint inhibitor. It was coincident that with either fluid changes or blood pressure changes associated with the treatment that they had a flare-up of heart failure.
We have not seen, fortunately, the dynamics that were reported in the
DH Well, certainly with the proliferation of the checkpoint inhibitors, and so many different tumors, and so much widespread use, it looks like there is a small safety signal there but still yet to be defined. How common is that, and what should we watch for?
JC Actually, it’s serendipitous that yesterday I was walking to the parking lot with one of the nurse practitioners who takes care of the melanoma population. She said to me, “Now, do you think that we should be getting BNP levels on everybody who is getting a checkpoint inhibitor?”
I don’t think that we’re there. Just the awareness to ask the right questions when you see a patient and before starting ask, is this somebody who, in the absence of a checkpoint inhibitor, could be at risk for myocardial disease? Recognize that and use the cardiology and oncology community to work together and try to make sure that you do whatever cardioprotective things you can do and to monitor them a little bit more closely. I’m not sure that everybody who is going to start a checkpoint inhibitor needs a cardiac evaluation, doesn’t need an echocardiogram, and doesn’t need baseline biomarkers to decide if there’s a potential cardiotoxicity problem.
DH Well certainly, you’ve raised my awareness. It was not something that I had been thinking of with checkpoint inhibitors. Now, I certainly would if the patient has some comorbid illness that involves the heart, maybe think about it, wait to see how these reports develop, and what you and the registry do.
JC You’ve seen people who get this sort of immunologic reaction that they require steroids for fluid accumulation, rash, or other things that are in this constellation. I wouldn’t be surprised if that group might have some subclinical myocarditis that just gets better when they get treated for the other things.
We have actually been trying to get a quick look at the left ventricle when patients on checkpoint inhibitors present with systemic, noncardiac symptoms to see if there is a cardiac signal we are missing. We have a handheld portable echocardiogram device called a Vscan (General Electric Company, Fairfield, CT). It’s not much bigger than the larger cellphones that are available. We’ve been going to the bedside when people have the reaction and sticking the transducer on to get a feeling of what the ventricle looks like. There’s a lot that we don’t know. It’s a fertile ground for investigation.
DH Well, I couldn’t ask you to end on a higher note than covering the checkpoint inhibitors, which are so popular and so interesting and used everywhere. We’re still managing that whole concept. I want to thank you very much.
JC It was a great pleasure. Thank you.
1. Galper SL, Yu JB, Mauch PM, et al. Clinically significant cardiac disease in patients with Hodgkin lymphoma treated with mediastinal irradiation. Blood. 2011;117(2):412-418.
2. Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987-998.
3. Specht L, Yahalom J, Illidge T, et al. Modern radiation therapy for Hodgkin lymphoma: field and dose guidelines from the International Lymphoma Radiation Oncology Group (ILROG). Int J Radiat Oncol Biol Phys. 2014;89(4):854-862.
4. Von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91(5):710-717.
5. Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749-1755.
A typical example is a patient who had Hodgkin disease in his teens and received mediastinal mantle radiation. Fifteen to 25 years later, the patient has a pacemaker for heart block, coronary artery disease that requires a stent, and most recently has two valves replaced—so aortic and mitral valve replacement because of late radiation effects. This scenario is typical for the “old” days. The 20-year cumulative incidence of radiation-induced cardiac toxicity is 15%-20% (Table, Figure).1 Sitting with a patient about to begin chest radiation, the absolute risks are unknown but presumed to be less as treatment is delivered according to the modern techniques that you described in the question.
DH They’re so much better now, so this is less common.
JC With the shielding and breath-holding techniques and position changes, doing upright radiation rather than supine, and because the technology has improved both in the delivery of radiation and the technology in understanding where all the radiation is going, in today’s world, we can calculate pretty precisely how much radiation the heart actually receives. Ultimately, with the protective mechanisms that are in place going forward, the risks that I described for that survivor are probably exponentially less than what’s reported in the literature and what we see clinically. Radiation has become much, much safer. There is still probably some small risk of development of late changes, but I don’t think we know what that risk is today because the shielding and things we do to protect the heart have not yet been studied in the long term.
DH Of course, the patient is breathing and there’ll be some movement of the target. Some of the radiation techniques can follow the target despite the breathing?
JC Yes, definitely true. Radiation delivery is much more precise today. Not only has the delivery changed, but so has what we know about the location of potential arterial disease. For example, if you read any textbook, it says that for the coronaries, that it’s ostial and proximal disease of the left main, or the left anterior descending, or the right coronary artery. Today, somebody who gets chest/mediastinal radiation, for either breast cancer, lymphoma, or for a mediastinal tumor, the location of potential disease is more likely to mimic the location of classic coronary disease in the mid-portion of the left anterior descending artery rather than at the ostium. It’s going to be a different disease going forward.2,3
DH Let’s switch from radiation to chemotherapy. Of course, all of us worry about and are very familiar with the toxicity potential of doxorubicin and trastuzumab. I remember an American Society of Clinical Oncology meeting a few years ago, one of the speakers was a cardiologist and was advising us that perhaps the ejection fraction, albeit readily available and reproducible, was probably too simple and we should watch more closely with other techniques. My final question and then I’ll let you comment – I thought I recalled 5-fluorouracil (5-FU) infusions, which we do in some of our colorectal cancers, for example, can cause a vasospasm, Prinzmetal-type angina from time to time, and is that true in capecitabine? What are your thoughts on how to follow the doxorubicin, trastuzumab analogs, and anything about 5-FU and its analogs?
JC Okay, this is a giant question. I’ll take them in order. First, doxorubicin. Cumulative dose-related cardiotoxicity was first described by Von Hoff in 1979.4 That is, the more you get, the higher likelihood of developing cardiotoxicity. Up to a total of 400 mg/m2, the risk is <1%, with a sharp rise as the dose increases beyond this level.4 That being said, there is a clear large and individual variation: I’ve seen sarcoma patients who’ve gotten close to 1,000 mg/m2 without cardiac dysfunction, and some people with minimal exposure have full-blown cardiomyopathy. One of the protective strategies that we developed over the years is to give less of the drug, and with that get the same cancer treatment efficacy. There is definitely a risk for anthracyclines. Full-blown heart failure is probably in the 4%-8% range – and that’s cumulative lifetime – it’s not as high as we once thought it was. That doesn’t mean that it isn’t there, but, relatively speaking, from the standpoint of benefit of anthracyclines, the benefit certainly clearly outweighs the cardiac risk.
With administration of the anthracyclines, we try to do whatever protective things we can do. There are some people who believe that continuous infusion is safer for the heart than bolus injection. It’s pretty controversial. Dexrazoxane, which is a chelating agent, has been shown to reduce cardiotoxicity, and using a lipophilic anthracycline preparation may also have less cardiac toxicity.
DH I have a population in which a lot of liposomal doxorubicin is used and I’ve given a lot and rarely if ever get cardiac toxicity. You see that as well?
JC Yes. There’s a significant financial difference between doxorubicin and liposomal doxorubicin; the latter is more expensive. From the standpoint of safety, and from the standpoint of if I ever needed doxorubicin, I would probably jump on that and ask for the liposomal preparation and/or dexrazoxane.
DH For trastuzumab, we are getting echo- cardiograms every 9 weeks. That seems awfully simple, but there’s a whole algorithm we follow for particular change in ejection fraction and watch the drug or stop the drug. Are we doing that correctly?
JC The first statement I would make about that is that there are too many women who need trastuzumab whose therapy has been prematurely stopped because of just looking at the ejection fractions. So, there has to be more to decision-making other than just the number of the ejection fraction. We’re pretty aggressive and tend to try to get women to get the full dose and whatever dose-effective dose they need, especially with curative intent in the adjuvant setting that we make decisions based not only on the ejection fraction.
We also have, I would say, a handful of our medical breast oncologists who do not follow the package insert. We don’t get ejection fractions every 3 cycles. We have substituted a little bit by following biomarkers so that we use N-terminal pro b-type natriuretic peptide (NT-proBNP) to monitor people, either with each cycle or every third cycle. The benefit of BNP is its negative predictive value. If it’s normal, it’s hard to have any clinically significant myocardial dysfunction.
What we’re going to see over – I would hope – the next year or two is that the recommendations about getting echocardiograms frequently will go away.
DH That would be welcome because in our electronic medical records, it’s 9 weeks, stop, do this, etc. How about a comment on infusional 5-FU and possibly its cousins, such as capecitabine, and any coronary issues?
JC Let me come back, just one more thing about trastuzumab. For metastatic disease, we do whatever is necessary to continue effective cancer therapy and in the absence of any cardiac symptoms or abnormal physical findings, we continue cancer treatment without any serial echocardiographic monitoring.
DH You think the NT-proBNP might be useful? I know that’s excreted by the kidneys, so that might rise in renal failure, but we can adjust for that.
JC The negative predictive value of having a normal BNP is helpful. I think what I wanted to say was that screening echocardiograms and looking at ejection fraction in low-risk populations probably is clearly not cost-effective. It probably never alters decision making. If you have a 30-year-old person with no cardiac risk factors and no past cardiac history who develops B-cell lymphoma and is going to get anthracycline-based chemotherapy, the likelihood of finding a reason not to give that therapy based on an echocardiogram is quite small. I would even go further and say close to zero. We’ve begun to look at this. There is literature that supports the concept. Also, that in low-risk people – if you can define the low-risk population in an accurate way – for lymphoma patients or women with breast cancer getting either anthracyclines, trastuzumab, or the other human epidermal growth factor receptor-2 (HER2)-directed therapies, there’s probably little yield to even getting a baseline study.
DH Very interesting. I would agree with you.
JC We’re going to talk about 5-FU, of course. The 5-FU thing has become a passion of mine. Over the last two to two-and-a- half years we have gotten very aggressive with treating coronary spasm that’s induced by the fluoropyrimidines. That’s 5-FU and capecitabine, the oral version.
There is an incidence that the literature says is less than 1%. It probably is somewhere between 3% and 5%. It’s a little bit more common than has been reported. The reason is the way that it presents has classically been described in the literature as different than what occurs in real life. It is a phenomenon. It’s the most common cardiac side effect. Sometimes it is large epicardial coronary artery spasm. Sometimes it’s small vessel spasm. You can have chest pain with no electrocardiographic changes or ECG changes without chest pain (so-called silent ischemia). The description doesn’t always sound like classic angina but symptoms are temporally related to getting the drug.
So, we’ve developed a protocol to treat documented spasm as an outpatient to be able to continue those drugs to their logical conclusion from an oncologic standpoint. In fact, we just submitted a manuscript to the
DH Finally, it occurred to me that we cause problems with radiation. We cause problems with chemotherapy and other infusions. Are there particular cancers that you think of or you’re called in to see that you worry about cardiac involvement by their location? What comes to mind are cases I’ve had in which there is pericardial involvement and tamponade or restrictive pericarditis.
JC We see metastatic disease to the pericardium with breast cancer, lung cancer, and lymphoma. Renal cell has an interesting predilection to go to the pericardium. We’ve seen in the last probably 6 months 2 cases of bladder cancer with pericardial metastases. When we reviewed the literature, we were only able to find 9 or 10 case reports. It’s rare, but it occurs.
Fluid in the pericardium with and without tamponade is increasingly common, and because we do a better job in treating complicated cancer, people successively can receive cycles of sequential chemotherapeutic regimens – they are living longer, their cancer can get more complicated and/or resistant and with it, there’s more time for metastatic disease to occur. Tamponade is a common phenomenon. We always say that at 4 o’clock on Friday we always see somebody who has tamponade. We see a lot of pericardial disease.
Then, another area of a concern is the tyrosine kinase inhibitors that can cause hypertension, which is very common. We’ve become pretty aggressive. The oncologists recognize the importance of being able to follow and treat blood pressures to allow patients to get these treatments. I guess we couldn’t end without talking about checkpoint inhibitors and the recent lay press flurry about reporting myocarditis.
DH I haven’t personally experienced that. How common is that, and how do we watch for it?
JC Personally, I’ve seen probably four or five people who were referred because of heart failure on checkpoint inhibitors. For each of them, there was historically something as a preexisting problem before the checkpoint inhibitor. It was coincident that with either fluid changes or blood pressure changes associated with the treatment that they had a flare-up of heart failure.
We have not seen, fortunately, the dynamics that were reported in the
DH Well, certainly with the proliferation of the checkpoint inhibitors, and so many different tumors, and so much widespread use, it looks like there is a small safety signal there but still yet to be defined. How common is that, and what should we watch for?
JC Actually, it’s serendipitous that yesterday I was walking to the parking lot with one of the nurse practitioners who takes care of the melanoma population. She said to me, “Now, do you think that we should be getting BNP levels on everybody who is getting a checkpoint inhibitor?”
I don’t think that we’re there. Just the awareness to ask the right questions when you see a patient and before starting ask, is this somebody who, in the absence of a checkpoint inhibitor, could be at risk for myocardial disease? Recognize that and use the cardiology and oncology community to work together and try to make sure that you do whatever cardioprotective things you can do and to monitor them a little bit more closely. I’m not sure that everybody who is going to start a checkpoint inhibitor needs a cardiac evaluation, doesn’t need an echocardiogram, and doesn’t need baseline biomarkers to decide if there’s a potential cardiotoxicity problem.
DH Well certainly, you’ve raised my awareness. It was not something that I had been thinking of with checkpoint inhibitors. Now, I certainly would if the patient has some comorbid illness that involves the heart, maybe think about it, wait to see how these reports develop, and what you and the registry do.
JC You’ve seen people who get this sort of immunologic reaction that they require steroids for fluid accumulation, rash, or other things that are in this constellation. I wouldn’t be surprised if that group might have some subclinical myocarditis that just gets better when they get treated for the other things.
We have actually been trying to get a quick look at the left ventricle when patients on checkpoint inhibitors present with systemic, noncardiac symptoms to see if there is a cardiac signal we are missing. We have a handheld portable echocardiogram device called a Vscan (General Electric Company, Fairfield, CT). It’s not much bigger than the larger cellphones that are available. We’ve been going to the bedside when people have the reaction and sticking the transducer on to get a feeling of what the ventricle looks like. There’s a lot that we don’t know. It’s a fertile ground for investigation.
DH Well, I couldn’t ask you to end on a higher note than covering the checkpoint inhibitors, which are so popular and so interesting and used everywhere. We’re still managing that whole concept. I want to thank you very much.
JC It was a great pleasure. Thank you.
A typical example is a patient who had Hodgkin disease in his teens and received mediastinal mantle radiation. Fifteen to 25 years later, the patient has a pacemaker for heart block, coronary artery disease that requires a stent, and most recently has two valves replaced—so aortic and mitral valve replacement because of late radiation effects. This scenario is typical for the “old” days. The 20-year cumulative incidence of radiation-induced cardiac toxicity is 15%-20% (Table, Figure).1 Sitting with a patient about to begin chest radiation, the absolute risks are unknown but presumed to be less as treatment is delivered according to the modern techniques that you described in the question.
DH They’re so much better now, so this is less common.
JC With the shielding and breath-holding techniques and position changes, doing upright radiation rather than supine, and because the technology has improved both in the delivery of radiation and the technology in understanding where all the radiation is going, in today’s world, we can calculate pretty precisely how much radiation the heart actually receives. Ultimately, with the protective mechanisms that are in place going forward, the risks that I described for that survivor are probably exponentially less than what’s reported in the literature and what we see clinically. Radiation has become much, much safer. There is still probably some small risk of development of late changes, but I don’t think we know what that risk is today because the shielding and things we do to protect the heart have not yet been studied in the long term.
DH Of course, the patient is breathing and there’ll be some movement of the target. Some of the radiation techniques can follow the target despite the breathing?
JC Yes, definitely true. Radiation delivery is much more precise today. Not only has the delivery changed, but so has what we know about the location of potential arterial disease. For example, if you read any textbook, it says that for the coronaries, that it’s ostial and proximal disease of the left main, or the left anterior descending, or the right coronary artery. Today, somebody who gets chest/mediastinal radiation, for either breast cancer, lymphoma, or for a mediastinal tumor, the location of potential disease is more likely to mimic the location of classic coronary disease in the mid-portion of the left anterior descending artery rather than at the ostium. It’s going to be a different disease going forward.2,3
DH Let’s switch from radiation to chemotherapy. Of course, all of us worry about and are very familiar with the toxicity potential of doxorubicin and trastuzumab. I remember an American Society of Clinical Oncology meeting a few years ago, one of the speakers was a cardiologist and was advising us that perhaps the ejection fraction, albeit readily available and reproducible, was probably too simple and we should watch more closely with other techniques. My final question and then I’ll let you comment – I thought I recalled 5-fluorouracil (5-FU) infusions, which we do in some of our colorectal cancers, for example, can cause a vasospasm, Prinzmetal-type angina from time to time, and is that true in capecitabine? What are your thoughts on how to follow the doxorubicin, trastuzumab analogs, and anything about 5-FU and its analogs?
JC Okay, this is a giant question. I’ll take them in order. First, doxorubicin. Cumulative dose-related cardiotoxicity was first described by Von Hoff in 1979.4 That is, the more you get, the higher likelihood of developing cardiotoxicity. Up to a total of 400 mg/m2, the risk is <1%, with a sharp rise as the dose increases beyond this level.4 That being said, there is a clear large and individual variation: I’ve seen sarcoma patients who’ve gotten close to 1,000 mg/m2 without cardiac dysfunction, and some people with minimal exposure have full-blown cardiomyopathy. One of the protective strategies that we developed over the years is to give less of the drug, and with that get the same cancer treatment efficacy. There is definitely a risk for anthracyclines. Full-blown heart failure is probably in the 4%-8% range – and that’s cumulative lifetime – it’s not as high as we once thought it was. That doesn’t mean that it isn’t there, but, relatively speaking, from the standpoint of benefit of anthracyclines, the benefit certainly clearly outweighs the cardiac risk.
With administration of the anthracyclines, we try to do whatever protective things we can do. There are some people who believe that continuous infusion is safer for the heart than bolus injection. It’s pretty controversial. Dexrazoxane, which is a chelating agent, has been shown to reduce cardiotoxicity, and using a lipophilic anthracycline preparation may also have less cardiac toxicity.
DH I have a population in which a lot of liposomal doxorubicin is used and I’ve given a lot and rarely if ever get cardiac toxicity. You see that as well?
JC Yes. There’s a significant financial difference between doxorubicin and liposomal doxorubicin; the latter is more expensive. From the standpoint of safety, and from the standpoint of if I ever needed doxorubicin, I would probably jump on that and ask for the liposomal preparation and/or dexrazoxane.
DH For trastuzumab, we are getting echo- cardiograms every 9 weeks. That seems awfully simple, but there’s a whole algorithm we follow for particular change in ejection fraction and watch the drug or stop the drug. Are we doing that correctly?
JC The first statement I would make about that is that there are too many women who need trastuzumab whose therapy has been prematurely stopped because of just looking at the ejection fractions. So, there has to be more to decision-making other than just the number of the ejection fraction. We’re pretty aggressive and tend to try to get women to get the full dose and whatever dose-effective dose they need, especially with curative intent in the adjuvant setting that we make decisions based not only on the ejection fraction.
We also have, I would say, a handful of our medical breast oncologists who do not follow the package insert. We don’t get ejection fractions every 3 cycles. We have substituted a little bit by following biomarkers so that we use N-terminal pro b-type natriuretic peptide (NT-proBNP) to monitor people, either with each cycle or every third cycle. The benefit of BNP is its negative predictive value. If it’s normal, it’s hard to have any clinically significant myocardial dysfunction.
What we’re going to see over – I would hope – the next year or two is that the recommendations about getting echocardiograms frequently will go away.
DH That would be welcome because in our electronic medical records, it’s 9 weeks, stop, do this, etc. How about a comment on infusional 5-FU and possibly its cousins, such as capecitabine, and any coronary issues?
JC Let me come back, just one more thing about trastuzumab. For metastatic disease, we do whatever is necessary to continue effective cancer therapy and in the absence of any cardiac symptoms or abnormal physical findings, we continue cancer treatment without any serial echocardiographic monitoring.
DH You think the NT-proBNP might be useful? I know that’s excreted by the kidneys, so that might rise in renal failure, but we can adjust for that.
JC The negative predictive value of having a normal BNP is helpful. I think what I wanted to say was that screening echocardiograms and looking at ejection fraction in low-risk populations probably is clearly not cost-effective. It probably never alters decision making. If you have a 30-year-old person with no cardiac risk factors and no past cardiac history who develops B-cell lymphoma and is going to get anthracycline-based chemotherapy, the likelihood of finding a reason not to give that therapy based on an echocardiogram is quite small. I would even go further and say close to zero. We’ve begun to look at this. There is literature that supports the concept. Also, that in low-risk people – if you can define the low-risk population in an accurate way – for lymphoma patients or women with breast cancer getting either anthracyclines, trastuzumab, or the other human epidermal growth factor receptor-2 (HER2)-directed therapies, there’s probably little yield to even getting a baseline study.
DH Very interesting. I would agree with you.
JC We’re going to talk about 5-FU, of course. The 5-FU thing has become a passion of mine. Over the last two to two-and-a- half years we have gotten very aggressive with treating coronary spasm that’s induced by the fluoropyrimidines. That’s 5-FU and capecitabine, the oral version.
There is an incidence that the literature says is less than 1%. It probably is somewhere between 3% and 5%. It’s a little bit more common than has been reported. The reason is the way that it presents has classically been described in the literature as different than what occurs in real life. It is a phenomenon. It’s the most common cardiac side effect. Sometimes it is large epicardial coronary artery spasm. Sometimes it’s small vessel spasm. You can have chest pain with no electrocardiographic changes or ECG changes without chest pain (so-called silent ischemia). The description doesn’t always sound like classic angina but symptoms are temporally related to getting the drug.
So, we’ve developed a protocol to treat documented spasm as an outpatient to be able to continue those drugs to their logical conclusion from an oncologic standpoint. In fact, we just submitted a manuscript to the
DH Finally, it occurred to me that we cause problems with radiation. We cause problems with chemotherapy and other infusions. Are there particular cancers that you think of or you’re called in to see that you worry about cardiac involvement by their location? What comes to mind are cases I’ve had in which there is pericardial involvement and tamponade or restrictive pericarditis.
JC We see metastatic disease to the pericardium with breast cancer, lung cancer, and lymphoma. Renal cell has an interesting predilection to go to the pericardium. We’ve seen in the last probably 6 months 2 cases of bladder cancer with pericardial metastases. When we reviewed the literature, we were only able to find 9 or 10 case reports. It’s rare, but it occurs.
Fluid in the pericardium with and without tamponade is increasingly common, and because we do a better job in treating complicated cancer, people successively can receive cycles of sequential chemotherapeutic regimens – they are living longer, their cancer can get more complicated and/or resistant and with it, there’s more time for metastatic disease to occur. Tamponade is a common phenomenon. We always say that at 4 o’clock on Friday we always see somebody who has tamponade. We see a lot of pericardial disease.
Then, another area of a concern is the tyrosine kinase inhibitors that can cause hypertension, which is very common. We’ve become pretty aggressive. The oncologists recognize the importance of being able to follow and treat blood pressures to allow patients to get these treatments. I guess we couldn’t end without talking about checkpoint inhibitors and the recent lay press flurry about reporting myocarditis.
DH I haven’t personally experienced that. How common is that, and how do we watch for it?
JC Personally, I’ve seen probably four or five people who were referred because of heart failure on checkpoint inhibitors. For each of them, there was historically something as a preexisting problem before the checkpoint inhibitor. It was coincident that with either fluid changes or blood pressure changes associated with the treatment that they had a flare-up of heart failure.
We have not seen, fortunately, the dynamics that were reported in the
DH Well, certainly with the proliferation of the checkpoint inhibitors, and so many different tumors, and so much widespread use, it looks like there is a small safety signal there but still yet to be defined. How common is that, and what should we watch for?
JC Actually, it’s serendipitous that yesterday I was walking to the parking lot with one of the nurse practitioners who takes care of the melanoma population. She said to me, “Now, do you think that we should be getting BNP levels on everybody who is getting a checkpoint inhibitor?”
I don’t think that we’re there. Just the awareness to ask the right questions when you see a patient and before starting ask, is this somebody who, in the absence of a checkpoint inhibitor, could be at risk for myocardial disease? Recognize that and use the cardiology and oncology community to work together and try to make sure that you do whatever cardioprotective things you can do and to monitor them a little bit more closely. I’m not sure that everybody who is going to start a checkpoint inhibitor needs a cardiac evaluation, doesn’t need an echocardiogram, and doesn’t need baseline biomarkers to decide if there’s a potential cardiotoxicity problem.
DH Well certainly, you’ve raised my awareness. It was not something that I had been thinking of with checkpoint inhibitors. Now, I certainly would if the patient has some comorbid illness that involves the heart, maybe think about it, wait to see how these reports develop, and what you and the registry do.
JC You’ve seen people who get this sort of immunologic reaction that they require steroids for fluid accumulation, rash, or other things that are in this constellation. I wouldn’t be surprised if that group might have some subclinical myocarditis that just gets better when they get treated for the other things.
We have actually been trying to get a quick look at the left ventricle when patients on checkpoint inhibitors present with systemic, noncardiac symptoms to see if there is a cardiac signal we are missing. We have a handheld portable echocardiogram device called a Vscan (General Electric Company, Fairfield, CT). It’s not much bigger than the larger cellphones that are available. We’ve been going to the bedside when people have the reaction and sticking the transducer on to get a feeling of what the ventricle looks like. There’s a lot that we don’t know. It’s a fertile ground for investigation.
DH Well, I couldn’t ask you to end on a higher note than covering the checkpoint inhibitors, which are so popular and so interesting and used everywhere. We’re still managing that whole concept. I want to thank you very much.
JC It was a great pleasure. Thank you.
1. Galper SL, Yu JB, Mauch PM, et al. Clinically significant cardiac disease in patients with Hodgkin lymphoma treated with mediastinal irradiation. Blood. 2011;117(2):412-418.
2. Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987-998.
3. Specht L, Yahalom J, Illidge T, et al. Modern radiation therapy for Hodgkin lymphoma: field and dose guidelines from the International Lymphoma Radiation Oncology Group (ILROG). Int J Radiat Oncol Biol Phys. 2014;89(4):854-862.
4. Von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91(5):710-717.
5. Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749-1755.
1. Galper SL, Yu JB, Mauch PM, et al. Clinically significant cardiac disease in patients with Hodgkin lymphoma treated with mediastinal irradiation. Blood. 2011;117(2):412-418.
2. Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987-998.
3. Specht L, Yahalom J, Illidge T, et al. Modern radiation therapy for Hodgkin lymphoma: field and dose guidelines from the International Lymphoma Radiation Oncology Group (ILROG). Int J Radiat Oncol Biol Phys. 2014;89(4):854-862.
4. Von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91(5):710-717.
5. Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749-1755.