User login
MILAN – Monotherapy with the oral BTK inhibitor ONO-4059 showed good efficacy over a range of doses in relapsed or refractory and high-risk chronic lymphocytic leukemia, with a best overall response rate of 84% in a phase I trial.
Among 25 evaluable patients, 2 achieved complete responses with incomplete blood count recovery, 12 had partial responses, and 7 had partial responses with lymphocytosis.
One patient had stable disease and another progressive disease. Two patients withdrew due to adverse events.
ONO-4059 showed particularly good efficacy in patients with the deleterious 17p deletion (89%, or 8/9 patients) and in those refractory to their last therapy (91.6% or 11/12 patients), Dr. Franck Morschhauser, reported at the annual congress of the European Hematology Association.
ONO-4059 is a selective Bruton’s tyrosine kinase (BTK) inhibitor that has demonstrated greater than 90% inhibition of BTK at 12 hours in chronic lymphocytic leukemia (CLL) cells in vivo and antitumor activity in non-Hodgkin’s lymphoma.
In relapsed or refractory B-cell lymphoma, the same investigators reported a best overall response rate of 42% with ONO-4059 at doses of 40, 80, and 160 mg among 12 patients in a phase I study.
The current dose-escalation study evaluated the safety and tolerability of once-daily ONO-4059 at doses ranging from 20 to 600 mg for up to 2 years in patients with relapsed/refractory or high-risk CLL for whom no therapy of higher priority was available. Dose escalation was permitted upon completion of 6 months of treatment. The median duration of treatment was 11.5 cycles.
The 25 evaluable patients (median age, 67 years) had received a median of four prior therapies (range 2-9); 92% had prior exposure to rituximab (Rituxan) and 92% to fludarabine (Fludara); and 48% were refractory to their last therapy.
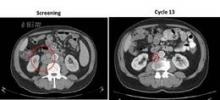
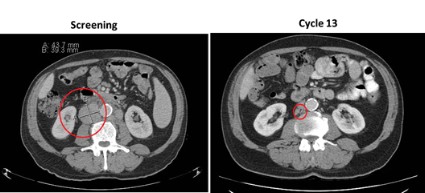
Lymph node reduction occurred early, between day 1 and 28 of the first cycle, and was accompanied by an improvement in hemoglobin and platelet counts within 3-4 months, said Dr. Morschhauser, head of the lymphoma unit, Centre Hospitalier Régional Universitaire–Claude Huriez Hospital, Lille, France.
"So far we do not have evidence that there is a dose-efficacy relationship, although there is quicker tumor shrinkage when you increase the dose," he said.
The majority of adverse events were grade 1 and 2, with a low incidence of diarrhea, fatigue, bruising, and rash and no dose-limiting toxicities, Dr. Morschhauser said. Five patients experienced grade 3/4 neutropenia: two grade 3 events at 20 and 40 mg and three grade 4 events at 20, 80, and 320 mg.
Eight ONO-4059–related serious adverse events were reported, including febrile neutropenia in the patient with grade 4 neutropenia at the 20-mg dose, pyrexia at 20 mg, rash at 80 mg, hepatitis E reactivation and neutropenia at 320 mg, lymphocytic infiltration of the right dorsal muscle and purpura at 400 mg, and spontaneous psoas hematoma at 500 mg. All events resolved, and four patients remain on study, he reported on behalf of lead author Dr. Christopher Fegan, clinical director of CLL, University Hospital of Wales, Cardiff.
The study was supported by Ono Pharmaceutical. The study authors reported no relevant financial disclosures.
MILAN – Monotherapy with the oral BTK inhibitor ONO-4059 showed good efficacy over a range of doses in relapsed or refractory and high-risk chronic lymphocytic leukemia, with a best overall response rate of 84% in a phase I trial.
Among 25 evaluable patients, 2 achieved complete responses with incomplete blood count recovery, 12 had partial responses, and 7 had partial responses with lymphocytosis.
One patient had stable disease and another progressive disease. Two patients withdrew due to adverse events.
ONO-4059 showed particularly good efficacy in patients with the deleterious 17p deletion (89%, or 8/9 patients) and in those refractory to their last therapy (91.6% or 11/12 patients), Dr. Franck Morschhauser, reported at the annual congress of the European Hematology Association.
ONO-4059 is a selective Bruton’s tyrosine kinase (BTK) inhibitor that has demonstrated greater than 90% inhibition of BTK at 12 hours in chronic lymphocytic leukemia (CLL) cells in vivo and antitumor activity in non-Hodgkin’s lymphoma.
In relapsed or refractory B-cell lymphoma, the same investigators reported a best overall response rate of 42% with ONO-4059 at doses of 40, 80, and 160 mg among 12 patients in a phase I study.
The current dose-escalation study evaluated the safety and tolerability of once-daily ONO-4059 at doses ranging from 20 to 600 mg for up to 2 years in patients with relapsed/refractory or high-risk CLL for whom no therapy of higher priority was available. Dose escalation was permitted upon completion of 6 months of treatment. The median duration of treatment was 11.5 cycles.
The 25 evaluable patients (median age, 67 years) had received a median of four prior therapies (range 2-9); 92% had prior exposure to rituximab (Rituxan) and 92% to fludarabine (Fludara); and 48% were refractory to their last therapy.
Lymph node reduction occurred early, between day 1 and 28 of the first cycle, and was accompanied by an improvement in hemoglobin and platelet counts within 3-4 months, said Dr. Morschhauser, head of the lymphoma unit, Centre Hospitalier Régional Universitaire–Claude Huriez Hospital, Lille, France.
"So far we do not have evidence that there is a dose-efficacy relationship, although there is quicker tumor shrinkage when you increase the dose," he said.
The majority of adverse events were grade 1 and 2, with a low incidence of diarrhea, fatigue, bruising, and rash and no dose-limiting toxicities, Dr. Morschhauser said. Five patients experienced grade 3/4 neutropenia: two grade 3 events at 20 and 40 mg and three grade 4 events at 20, 80, and 320 mg.
Eight ONO-4059–related serious adverse events were reported, including febrile neutropenia in the patient with grade 4 neutropenia at the 20-mg dose, pyrexia at 20 mg, rash at 80 mg, hepatitis E reactivation and neutropenia at 320 mg, lymphocytic infiltration of the right dorsal muscle and purpura at 400 mg, and spontaneous psoas hematoma at 500 mg. All events resolved, and four patients remain on study, he reported on behalf of lead author Dr. Christopher Fegan, clinical director of CLL, University Hospital of Wales, Cardiff.
The study was supported by Ono Pharmaceutical. The study authors reported no relevant financial disclosures.
MILAN – Monotherapy with the oral BTK inhibitor ONO-4059 showed good efficacy over a range of doses in relapsed or refractory and high-risk chronic lymphocytic leukemia, with a best overall response rate of 84% in a phase I trial.
Among 25 evaluable patients, 2 achieved complete responses with incomplete blood count recovery, 12 had partial responses, and 7 had partial responses with lymphocytosis.
One patient had stable disease and another progressive disease. Two patients withdrew due to adverse events.
ONO-4059 showed particularly good efficacy in patients with the deleterious 17p deletion (89%, or 8/9 patients) and in those refractory to their last therapy (91.6% or 11/12 patients), Dr. Franck Morschhauser, reported at the annual congress of the European Hematology Association.
ONO-4059 is a selective Bruton’s tyrosine kinase (BTK) inhibitor that has demonstrated greater than 90% inhibition of BTK at 12 hours in chronic lymphocytic leukemia (CLL) cells in vivo and antitumor activity in non-Hodgkin’s lymphoma.
In relapsed or refractory B-cell lymphoma, the same investigators reported a best overall response rate of 42% with ONO-4059 at doses of 40, 80, and 160 mg among 12 patients in a phase I study.
The current dose-escalation study evaluated the safety and tolerability of once-daily ONO-4059 at doses ranging from 20 to 600 mg for up to 2 years in patients with relapsed/refractory or high-risk CLL for whom no therapy of higher priority was available. Dose escalation was permitted upon completion of 6 months of treatment. The median duration of treatment was 11.5 cycles.
The 25 evaluable patients (median age, 67 years) had received a median of four prior therapies (range 2-9); 92% had prior exposure to rituximab (Rituxan) and 92% to fludarabine (Fludara); and 48% were refractory to their last therapy.
Lymph node reduction occurred early, between day 1 and 28 of the first cycle, and was accompanied by an improvement in hemoglobin and platelet counts within 3-4 months, said Dr. Morschhauser, head of the lymphoma unit, Centre Hospitalier Régional Universitaire–Claude Huriez Hospital, Lille, France.
"So far we do not have evidence that there is a dose-efficacy relationship, although there is quicker tumor shrinkage when you increase the dose," he said.
The majority of adverse events were grade 1 and 2, with a low incidence of diarrhea, fatigue, bruising, and rash and no dose-limiting toxicities, Dr. Morschhauser said. Five patients experienced grade 3/4 neutropenia: two grade 3 events at 20 and 40 mg and three grade 4 events at 20, 80, and 320 mg.
Eight ONO-4059–related serious adverse events were reported, including febrile neutropenia in the patient with grade 4 neutropenia at the 20-mg dose, pyrexia at 20 mg, rash at 80 mg, hepatitis E reactivation and neutropenia at 320 mg, lymphocytic infiltration of the right dorsal muscle and purpura at 400 mg, and spontaneous psoas hematoma at 500 mg. All events resolved, and four patients remain on study, he reported on behalf of lead author Dr. Christopher Fegan, clinical director of CLL, University Hospital of Wales, Cardiff.
The study was supported by Ono Pharmaceutical. The study authors reported no relevant financial disclosures.
AT THE EHA CONGRESS
Key clinical point: ONO-4059 induced responses in most patients with relapsed/refractory CLL, including those with a 17p deletion.
Major finding: The best response rate was 84% overall, 89% in 17p deletion patients, and 91.6% in refractory disease.
Data source: A phase I trial in 25 patients with relapsed/refractory or high-risk chronic lymphocytic leukemia.
Disclosures: The study was supported by Ono Pharmaceutical. The study authors reported no relevant financial disclosures.