User login
NEW ORLEANS – Early data suggest that the second-generation oral BTK inhibitor ONO-4059 may give ibrutinib a run for its money in chronic lymphocytic leukemia.
The response rate to ONO-4059 monotherapy was 89% overall and 71% in those with the deleterious 17p deletion among 18 heavily pretreated patients with relapsed/refractory or high-risk CLL in a phase I, dose-escalation study.
Patients had already received a median of three prior therapies, including rituximab (84%) and fludarabine (95%), and had no higher priority therapy available to them, said Dr. Gilles Salles of Hospices Civils de Lyon (France), Universite Claude Bernard Lyon.
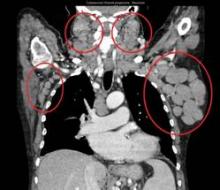
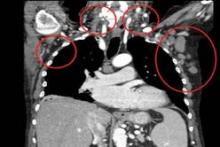
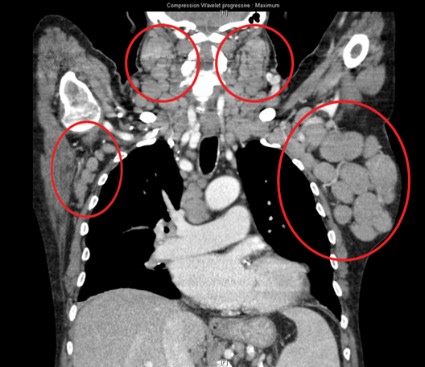
All patients had improved hemoglobin and platelet counts after 3 months on treatment and rapid reductions in lymph node size within the first 28-day cycle. Tumor burden was reduced by 50% for most patients, and all but one patient experienced a response that was detectable on a CT scan.
"This was true whatever their FISH status or 17p or 11q deletion status," Dr. Salles said at the annual meeting of the American Society of Hematology.
ONO-4059 is a highly selective Bruton’s tyrosine kinase (BTK) inhibitor with antitumor activity in several preclinical models.
No patients had received prior treatment with a P13 kinase or a BTK inhibitor, including ibrutinib (Imbruvica), which recently gained accelerated approval for previously treated mantle cell lymphoma.
ONO-4059 was given at daily doses ranging from 20 mg to 320 mg for up to 6 months, with the option of additional dosing up to 2 years. Sustained BTK inhibition was established at doses of 40 mg and higher.
Overall, the best response was a partial response in 14 patients, as well as two partial responses with lymphocytosis and one stable disease, he said. No complete responses occurred.
One patient progressed roughly 1 month after showing an initial response and complete disappearance of all palpable disease on physical exam. Richter’s syndrome was suspected.
"It’s very promising efficacy in this highly pretreated population," Dr. Salles said.
Patients with relapsed/refractory mantle cell lymphoma and diffuse large B-cell lymphoma, especially the ABC subtype, also appear sensitive to ONO-4059. Overall response rates were 43% and 75%, respectively, including three complete responses reported from the phase I study in a separate poster presentation at the meeting.
ONO-4059 had a favorable safety profile with a single dose-limiting toxicity observed in a patient who had Waldenstrom’s macroglobulinemia, was on the 320-mg dose, and was intolerant to all prior therapies. The maximum tolerated dose has not yet been reached.
The majority of adverse events in the CLL patients were grades 1 and 2. There were no clinically significant bleeding events or bruising, and there was a low incidence of diarrhea and rash, Dr. Salles said.
ONO-4059–related grade 3-4 events were independent of dose and included one grade 3 neutropenia at 20 mg and two grade 4 events at 20 mg and 320 mg. Four serious adverse events (febrile neutropenia, pyrexia, rash, and neutropenia) occurred in three patients, all of whom are still in the study and showing good clinical response, Dr. Salles said. Of the 30 patients dosed to date, 22 remain in the study.
No other trials are firmly planned, and pharmacokinetics/pharmacodynamics data continue to be explored in order to assess a phase II dosage, he said in an interview.
Dr. Salles reported consulting for and receiving honoraria from Roche. Several coauthors have financial ties, including employment with the study sponsor, Ono Pharmaceutical, which is developing ONO-4059.
NEW ORLEANS – Early data suggest that the second-generation oral BTK inhibitor ONO-4059 may give ibrutinib a run for its money in chronic lymphocytic leukemia.
The response rate to ONO-4059 monotherapy was 89% overall and 71% in those with the deleterious 17p deletion among 18 heavily pretreated patients with relapsed/refractory or high-risk CLL in a phase I, dose-escalation study.
Patients had already received a median of three prior therapies, including rituximab (84%) and fludarabine (95%), and had no higher priority therapy available to them, said Dr. Gilles Salles of Hospices Civils de Lyon (France), Universite Claude Bernard Lyon.
All patients had improved hemoglobin and platelet counts after 3 months on treatment and rapid reductions in lymph node size within the first 28-day cycle. Tumor burden was reduced by 50% for most patients, and all but one patient experienced a response that was detectable on a CT scan.
"This was true whatever their FISH status or 17p or 11q deletion status," Dr. Salles said at the annual meeting of the American Society of Hematology.
ONO-4059 is a highly selective Bruton’s tyrosine kinase (BTK) inhibitor with antitumor activity in several preclinical models.
No patients had received prior treatment with a P13 kinase or a BTK inhibitor, including ibrutinib (Imbruvica), which recently gained accelerated approval for previously treated mantle cell lymphoma.
ONO-4059 was given at daily doses ranging from 20 mg to 320 mg for up to 6 months, with the option of additional dosing up to 2 years. Sustained BTK inhibition was established at doses of 40 mg and higher.
Overall, the best response was a partial response in 14 patients, as well as two partial responses with lymphocytosis and one stable disease, he said. No complete responses occurred.
One patient progressed roughly 1 month after showing an initial response and complete disappearance of all palpable disease on physical exam. Richter’s syndrome was suspected.
"It’s very promising efficacy in this highly pretreated population," Dr. Salles said.
Patients with relapsed/refractory mantle cell lymphoma and diffuse large B-cell lymphoma, especially the ABC subtype, also appear sensitive to ONO-4059. Overall response rates were 43% and 75%, respectively, including three complete responses reported from the phase I study in a separate poster presentation at the meeting.
ONO-4059 had a favorable safety profile with a single dose-limiting toxicity observed in a patient who had Waldenstrom’s macroglobulinemia, was on the 320-mg dose, and was intolerant to all prior therapies. The maximum tolerated dose has not yet been reached.
The majority of adverse events in the CLL patients were grades 1 and 2. There were no clinically significant bleeding events or bruising, and there was a low incidence of diarrhea and rash, Dr. Salles said.
ONO-4059–related grade 3-4 events were independent of dose and included one grade 3 neutropenia at 20 mg and two grade 4 events at 20 mg and 320 mg. Four serious adverse events (febrile neutropenia, pyrexia, rash, and neutropenia) occurred in three patients, all of whom are still in the study and showing good clinical response, Dr. Salles said. Of the 30 patients dosed to date, 22 remain in the study.
No other trials are firmly planned, and pharmacokinetics/pharmacodynamics data continue to be explored in order to assess a phase II dosage, he said in an interview.
Dr. Salles reported consulting for and receiving honoraria from Roche. Several coauthors have financial ties, including employment with the study sponsor, Ono Pharmaceutical, which is developing ONO-4059.
NEW ORLEANS – Early data suggest that the second-generation oral BTK inhibitor ONO-4059 may give ibrutinib a run for its money in chronic lymphocytic leukemia.
The response rate to ONO-4059 monotherapy was 89% overall and 71% in those with the deleterious 17p deletion among 18 heavily pretreated patients with relapsed/refractory or high-risk CLL in a phase I, dose-escalation study.
Patients had already received a median of three prior therapies, including rituximab (84%) and fludarabine (95%), and had no higher priority therapy available to them, said Dr. Gilles Salles of Hospices Civils de Lyon (France), Universite Claude Bernard Lyon.
All patients had improved hemoglobin and platelet counts after 3 months on treatment and rapid reductions in lymph node size within the first 28-day cycle. Tumor burden was reduced by 50% for most patients, and all but one patient experienced a response that was detectable on a CT scan.
"This was true whatever their FISH status or 17p or 11q deletion status," Dr. Salles said at the annual meeting of the American Society of Hematology.
ONO-4059 is a highly selective Bruton’s tyrosine kinase (BTK) inhibitor with antitumor activity in several preclinical models.
No patients had received prior treatment with a P13 kinase or a BTK inhibitor, including ibrutinib (Imbruvica), which recently gained accelerated approval for previously treated mantle cell lymphoma.
ONO-4059 was given at daily doses ranging from 20 mg to 320 mg for up to 6 months, with the option of additional dosing up to 2 years. Sustained BTK inhibition was established at doses of 40 mg and higher.
Overall, the best response was a partial response in 14 patients, as well as two partial responses with lymphocytosis and one stable disease, he said. No complete responses occurred.
One patient progressed roughly 1 month after showing an initial response and complete disappearance of all palpable disease on physical exam. Richter’s syndrome was suspected.
"It’s very promising efficacy in this highly pretreated population," Dr. Salles said.
Patients with relapsed/refractory mantle cell lymphoma and diffuse large B-cell lymphoma, especially the ABC subtype, also appear sensitive to ONO-4059. Overall response rates were 43% and 75%, respectively, including three complete responses reported from the phase I study in a separate poster presentation at the meeting.
ONO-4059 had a favorable safety profile with a single dose-limiting toxicity observed in a patient who had Waldenstrom’s macroglobulinemia, was on the 320-mg dose, and was intolerant to all prior therapies. The maximum tolerated dose has not yet been reached.
The majority of adverse events in the CLL patients were grades 1 and 2. There were no clinically significant bleeding events or bruising, and there was a low incidence of diarrhea and rash, Dr. Salles said.
ONO-4059–related grade 3-4 events were independent of dose and included one grade 3 neutropenia at 20 mg and two grade 4 events at 20 mg and 320 mg. Four serious adverse events (febrile neutropenia, pyrexia, rash, and neutropenia) occurred in three patients, all of whom are still in the study and showing good clinical response, Dr. Salles said. Of the 30 patients dosed to date, 22 remain in the study.
No other trials are firmly planned, and pharmacokinetics/pharmacodynamics data continue to be explored in order to assess a phase II dosage, he said in an interview.
Dr. Salles reported consulting for and receiving honoraria from Roche. Several coauthors have financial ties, including employment with the study sponsor, Ono Pharmaceutical, which is developing ONO-4059.
AT ASH 2013
Major finding: The response rate was 89% overall and 71% for patients with 17p deletion.
Data source: A prospective, phase I dose-escalation study in 18 patients with relapsed/refractory or high-risk CLL.
Disclosures: Dr. Salles reported honoraria from Janssen, Gilead, and Celgene. Several coauthors have financial ties, including employment with the study sponsor, Ono Pharmaceutical, which is developing ONO-4059.