User login
CE/CME No: CR-1502
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Describe the presenting signs and symptoms of atrial fibrillation (A-fib).
• Define the differential and tests for diagnosing A-fib.
• Identify the risk factors associated with A-fib.
• Discuss the anticoagulant treatment options for nonvalvular A-fib.
• List aspects of A-fib management about which patients benefit from clinician instruction.
FACULTY
Deedra Harrington, Janis R. Guilbeau, and Christy McDonald Lenahan are Assistant Professors in the College of Nursing and Allied Health Professions at the University of Louisiana at Lafayette. Dr. Harrington is also a cardiology intensivist nurse practitioner at the Heart Hospital of Lafayette. The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of February 2015.
Article begins on next page >>
Patients with nonvalvular atrial fibrillation (A-fib) have a fivefold greater risk for ischemic stroke than those without. Newer oral anticoagulants reduce this risk—but also increase risk for serious bleeding, including intracranial hemorrhage. Here are the evidence-based guidelines to help you make the choice that’s best for your patient.
Atrial fibrillation (A-fib) is a supraventricular tachyarrhythmia characterized by uncoordinated atrial activation that results in ineffective atrial contraction; this causes inadequate ventricular rate control and variable ventricular filling.1 A common cardiac arrhythmia that is estimated to affect between 2.7 and 6.1 million Americans,2 A-fib is projected to affect as many as 12.1 million people by the year 2030.3 Incidence increases with age; while less than 1% of patients with A-fib are younger than 60, more than a third are 80 or older.1
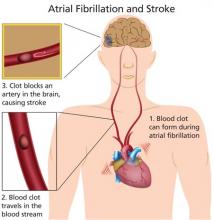
Morbidity and mortality associated with A-fib are significant. The risk for an embolic event is particularly profound—five times that of persons without A-fib; again, this risk increases with age. In patients ages 50 to 59, 1.5% of strokes are attributed to A-fib; this percentage increases to 23.5% for those ages 80 to 89.2
Treatment of A-fib is aimed at rate control and rhythm conversion, generally through the use of drugs or ablation procedures, and stroke risk reduction, using oral anticoagulants to prevent thrombus formation. This review will focus on the use of newer oral anticoagulants for reduction of stroke risk associated with nonvalvular A-fib.
PATIENT PRESENTATION
Patients with new-onset A-fib may present with a variety of symptoms, including palpitations, chest pain, pressure or discomfort, shortness of breath, lightheadedness, fatigue, or exercise intolerance.4 Patients with chest pain, palpitations, and shortness of breath in particular should be assessed immediately for myocardial infarction before evaluating for A-fib. Poor perfusion may cause a decreased level of patient consciousness; therefore, hypotension or even Alzheimer disease should be ruled out.
Continue for diagnostic evaluation >>
DIAGNOSTIC EVALUATION
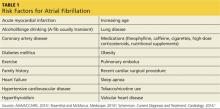
A complete patient history and thorough review of systems will enable the clinician to identify the risk factors for A-fib and establish a diagnosis (see Table 1).1,4,5 Evaluation should also include a detailed physical examination. Upon initial cardiovascular assessment, the patient’s apical pulse may be rapid, irregular, or disorganized during auscultation. If underlying A-fib is related to a valvular abnormality, an audible murmur may be auscultated.5
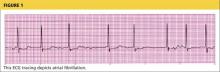
Workup for A-fib includes the standard 12-lead ECG, chest radiograph, thyroid function test, and echocardiogram. The 12-lead ECG is definitive for making the diagnosis of A-fib (see Figure 1). A-fib is characterized by irregular R-R intervals when atrioventricular conduction is present, absence of distinct repeating P waves, and irregular atrial activity.1
If the patient describes episodes consistent with A-fib that is not detectable at the office visit, 24- or 48-hour ambulatory Holter monitoring may be revealing. Event monitors can be used to determine the frequency with which the patient experiences A-fib over an extended period of time (up to 30 days).6
As part of the differential diagnosis of A-fib, clinicians need to consider other possible atrial conduction abnormalities, including atrial flutter, atrial tachycardia, atrioventricular nodal reentry tachycardia, multifocal atrial tachycardia, and Wolff-Parkinson-White syndrome.5
To rule out other etiologies, consider performing the following examinations and tests4
• A chest x-ray can rule out undiagnosed lung disease (eg, chronic obstructive pulmonary disease).
• To exclude hyperthyroidism as a cause of the patient’s symptoms, thyroid function testing and a physical examination for exophthalmos, carotid bruits, and thyromegaly are needed.
• Echocardiography is useful to exclude valvular abnormalities and/or heart failure.
• A complete blood cell count will rule out any infectious process or anemic state.
• Renal function studies and a comprehensive metabolic panel will detect signs of renal failure or electrolyte imbalance.
• Cardiac enzyme measurement can help rule out the occurrence of a myocardial event.
• A brain natriuretic peptide test can identify if heart failure is a contributing factor.
Continue for A-fib classification >>
A-FIB CLASSIFICATION
For purposes of choosing appropriate therapy, it is necessary to determine whether the cause of A-fib is valvular or nonvalvular. Valvular A-fib is described as A-fib that occurs in the presence of valvular heart disease or defect, such as rheumatic mitral stenosis, a mechanical or bioprosthetic heart valve, or mitral valve repair.1 In the absence of these types of conditions, A-fib is considered nonvalvular. The vast majority of patients have nonvalvular A-fib; in the ATRIA (AnTicoagulation and Risk Factors In Atrial Fibrillation) study, researchers found that, among 17,974 adults with A-fib who were members of a large California health maintenance organization, only 4.9% had valvular heart disease.8
A-fib is commonly classified into four subcategories, based on its duration: paroxysmal, persistent, longstanding persistent, and permanent.
Paroxysmal. The occurrence of at least two episodes that have terminated in less than seven days without treatment.
Persistent. An episode lasting more than seven days or less than seven days after electric or pharmacologic conversion.
Longstanding persistent. Continuous A-fib for more than one year.
Permanent. A category for patients in whom rhythm control is no longer being pursued.
This simplified classification is often used to choose between ablative or medication therapies. To ensure accuracy, however, underlying causes, risk factors, and mechanisms should be determined.9
Stroke risk calculation
Once nonvalvular A-fib is confirmed, the next step is to control the ventricular rate and attempt to convert the A-fib rhythm. To accomplish this, the patient’s risk for stroke must be estimated and the need for oral anticoagulation determined.
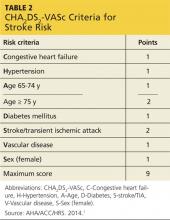
The CHADS2 risk stratification system for calculating an individual’s risk for ischemic stroke in A-fib was developed in 2001. The risk criteria used in the calculation are Congestive heart failure, Hypertension, Age ≥ 75 years, Diabetes mellitus, prior Stroke, transient ischemic attack, or thromboembolism.10
Recent additions to the criteria account for advanced age, gender, and known vascular disease.1,5 Known as the CHA2DS2-VASc, this scoring system is outlined in Table 2. If the patient’s score is 0, risk for stroke is low and anticoagulation therapy is not recommended. If the score is 1, the risk is intermediate, and the patient may be treated with aspirin therapy or anticoagulation. With a CHA2DS2-VASc score of 2 or greater, anticoagulation treatment is recommended to reduce the risk for stroke.1
While the expanded CHA2DS2-VASc criteria more clearly define the basis for an anticoagulation recommendation—particularly in older patients, women, and those with a vascular history—the superiority of one over the other is undetermined.11 However, the 2014 American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) guidelines for the management of patients with A-fib recommend use of the CHA2DS2-VASc.1
Continue for anticoagulation therapy >>
ANTICOAGULATION THERAPY
The choice of anticoagulation treatment requires weighing the risks and benefits of oral anticoagulation therapy. Stroke and bleeding risks, cost, tolerability, potential for drug interactions, likelihood of patient adherence to the anticoagulation regimen, and patient preferences should be considered.1
The three oral anticoagulants recently approved by the FDA for the reduction of stroke and systemic embolism risks in nonvalvular A-fib are dabigatran, a direct thrombin inhibitor, and rivaroxaban and apixaban, both factor Xa inhibitors.12-14
The clinical trials upon which the FDA’s approval of these anticoagulants was based included only patients with nonvalvular A-fib. For patients with valvular disease, warfarin, a vitamin-K–dependent inhibitor, is currently recommended.1,15 It is also recommended for patients with both end-stage renal disease (ESRD) and either nonvalvular or valvular A-fib.1
A-fib and chronic kidney disease
It is estimated that one-third of patients with A-fib are also diagnosed with chronic kidney disease (CKD).16 Because patients with CKD have a greater risk for bleeding, anticoagulant therapy for these patients requires reduced dosing and close monitoring for bleeding.
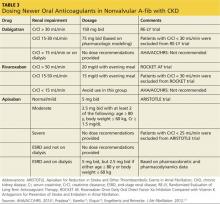
The 2014 ACC/AHA/HRS practice guidelines include guidance for selection of oral anticoagulants for patients with nonvalvular A-fib and CKD (see Table 3).1,12-14,17 Dosing of dabigatran and rivaroxaban require evaluation of creatinine clearance before treatment is initiated.
When warfarin is indicated, dose adjustments for renal impairment are based on the prothrombin time/international normalized ratio (INR) value.1 Current guidelines recommend maintaining a therapeutic INR between 2.0 and 3.0 for nonvalvular A-fib in patients with CKD.1 Patients with difficulty maintaining therapeutic INR levels may benefit from alternate therapy with Xa inhibitors or a direct thrombin inhibitor except in the presence of ESRD.1
Continue for patient adherence >>
PATIENT ADHERENCE
Recent studies have indicated that adherence to anticoagulation therapy among A-fib patients drops by as much as 50% after one year of therapy.18 Causes are multifactorial and include complexity of treatment regimen, missed doses, patient unawareness of stroke risk, and fear of bleeding.19 Educating both patients and caregivers has been associated with significant improvements in medication compliance in these patients.19
Complex regimens
Treatment requirements, such as the serial laboratory testing and dosage adjustments associated with warfarin therapy, can be a major contributing factor to anticoagulation nonadherence.18,20 In this regard, the newer once-daily medications that require limited follow-up may be good alternatives to warfarin.21
In patients for whom warfarin therapy is indicated, educational interventions may include
• Written information for patients and caregivers about medication regimens and dosage scheduling
• Reinforcement of treatment goals and outcomes
• Use of dosing aids such as dated and timed pill dispensers
• Incorporating caregiver support to help patients adhere to the medication regimen.
These interventions have been shown to improve adherence with complex treatment regimens.22
Missed doses
Missing anticoagulant doses is not an uncommon occurrence, and patients should be advised of appropriate catch-up strategies when this occurs.
For dabigatran, the missed dose should be taken as soon as the patient remembers, but only if the next scheduled dose is more than six hours away.12 For rivaroxaban, missed doses should be taken as soon as the patient remembers, and the next dose should resume as scheduled.13 For apixaban, a missed dose should be taken as soon as possible but not in combination with any other doses.14
For patients taking warfarin, a missed dose should be taken as soon as possible on the same day.23 If more than 24 hours have elapsed, the patient should contact his or her health care provider before taking any medication.23
Stroke risk
Adherence to anticoagulation therapy significantly reduces the risk for stroke among A-fib patients. Estimates suggest that anticoagulants can reduce stroke risk by as much as 68% in patients with A-fib.24
Even with optimal anticoagulation therapy, however, stroke remains a major complication.25 Through group sessions or patient education pamphlets, patients and caregivers should be informed about the high risk for stroke associated with A-fib and should know its early symptoms.26 These include sudden onset of one or more of the following: confusion or difficulty understanding speech; numbness or weakness of the face or extremities, limited to one side of the body; severe headache; dizziness, loss of balance, or difficulty ambulating; and/or visual disturbances in one or both eyes.26
Next page: Bleeding risk >>
Bleeding risk
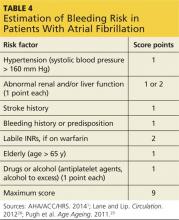
Patients should be advised of the major risk for bleeding associated with all anticoagulant therapies.27 Screening for bleeding includes assessment of Hypertension, Abnormal renal and/or liver function, previous Stroke, Bleeding history, Labile INR, being Elderly, and currently prescribed Drugs and/or excessive use of alcohol (known as HAS-BLED) (see Table 4).1,28 Use of a bleeding risk assessment tool such as HAS-BLED may help identify the patient’s risk but cannot be the basis for treatment decisions.1,29
Despite efforts to decrease bleeding risks, patients should understand that hemorrhagic complications can still occur. Patients taking anticoagulants should be familiar with early signs and symptoms of bleeding (eg, sudden, severe headache; melena; hematemesis; nosebleeds) and should notify their health care provider immediately if any of these symptoms occur.12-14,23
If bleeding occurs, it is recommended that anticoagulant treatment be stopped. In addition, depending on the severity of the bleeding, the clinician may elect to administer activated prothrombin complex concentrates, recombinant factor VIIa, or concentrates of factors II, IX, or X to reverse the effects of newer oral anticoagulants.1 Vitamin K, the antidote for warfarin, is not effective on direct thrombin inhibitors or factor Xa inhibitors. Currently, there is no established means of reversing the anticoagulant effects of the newer oral anticoagulants.12-14
FOLLOW-UP
Since optimal utilization of cardiovascular medication occurs in only 50% of the patient population, appropriate follow-up must be implemented to improve overall outcomes of pharmacologic therapy.30 Follow-up protocols depend on multiple factors, including type of anticoagulation therapy, patient response to therapy, and patient comorbidities.31 Monitoring warfarin use is time-consuming and resource-intensive; laboratory monitoring requirements for the newer oral anticoagulants have not been established.32
Patients taking warfarin should be vigilant in follow-up with serial laboratory measurements and dosage adjustments.23 Once therapy is initiated, INR is monitored every two to four days until two therapeutic INR levels are obtained.31,33 Monitoring can then be changed to once weekly until two more therapeutic levels are obtained.31 The INR monitoring interval can then be increased to every two to four weeks, with two weeks being a more conservative strategy.33 The practitioner may want to consider advancing to four-week monitoring intervals once four therapeutic INR levels have been obtained.31 It may be necessary to return to two-to-four day monitoring of INR if a nontherapeutic INR is obtained, the patient becomes ill, a medication is changed, or the patient makes a significant dietary change.31
When to refer
Primary care practitioners can manage anticoagulation therapy safely and efficiently, but cardiology referral may be warranted in certain situations. For example, patients with complex cardiac disease may benefit from cardiology referral.7 Considerations for referral to a cardiologist for further evaluation may include
• Abnormal exercise stress test results
• Abnormal echocardiogram results
• 12-lead ECG that reveals rapid, irregular wide pre-excited QRS complexes5
Patients who are drug intolerant or who remain symptomatic on pharmacologic rate control should also be referred to cardiology.7 In addition, patients who may require a pacemaker or defibrillator or who may be candidates for ablation should also be referred to an electrophysiology specialist.7
Continue for conclusion >>
CONCLUSION
Nonvalvular A-fib is a common arrhythmia that contributes significantly to morbidity among older adults. Use of the most current clinical practice guidelines coupled with patient education will improve overall patient outcomes.
* Editor's note: At press time, the FDA had announced approval of another oral anticoagulant, edoxaban, for the reduction of stroke and systemic embolism risks in nonvalvular A-fib.
1. January CT, Wann S, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):2246-2280.
2. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics: 2014 update. Circulation. 2014;129(3):e28-e292.
3. Colilla S, Crow A, Petkun W, et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the US adult population. Am J Cardiol. 2013:112:1142-1147.
4. Rosenthal L, McManus DD. Atrial fibrillation workup. http://emedicine.medscape.com/article/151066-workup. Accessed January 19, 2015.
5. Scheinman MM. Atrial fibrillation. In: Crawford MH, ed. Current Diagnosis and Treatment: Cardiology. Fourth Edition. New York: McGraw-Hill Education; 2014: 141-149.
6. Berry E, Padgett H. Management of patients with atrial fibrillation: diagnosis and treatment. Nurs Stand. 2012;26(22):47-56.
7. Gutierrez C, Blanchard DG. Atrial fibrillation: diagnosis and treatment. Am Fam Physician. 2011;83(1):61-68.
8. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370-2375.
9. Corradi D. Atrial fibrillation from the pathologist’s perspective. Cardiovasc Pathol. 2014;23(2):71-84.
10. Rietbrock S, Heeley E, Plumb J, van Staa T. Chronic atrial fibrillation: incidence, prevalence, and prediction of stroke using the Congestive heart failure, Hypertension Age > 75, Diabetes mellitus, and prior Stroke or transient ischemic attack (CHADS2) risk stratification scheme. Am Heart J. 2008;156(1):57-64.
11. Mason PK, Lake DE, DiMarco JP, et al. Impact of the CHA2DS2-VASc score on anticoagulation recommendations for atrial fibrillation. Am J Med. 2012; 125:603.e1-603.e6.
12. Pradaxa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.; 2010.
13. Xarelto [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2011.
14. Eliquis [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2012.
15. Verma A, Cairns JA, Mitchell LB, et al, for the CCC Atrial Fibrillation Guidelines Committee. 2014 focused update of the Canadian cardiovascular society guidelines for the management of atrial fibrillation. Can J Cardiol. 2014;30(10):1114-1130.
16. Hart RG, Eielboom JW, Brimble KS, et al. Stroke prevention in atrial fibrillation patients with chronic kidney disease. Can J Cardiol. 2013;29(7 suppl):S71-S78.
17. Engelbertz C, Reinecke H. Atrial fibrillation and oral anticoagulation in chronic kidney disease. J Atr Fibrillation. 2012;4(6):89-100.
18. Nelson WW, Song X, Coleman CI, et al. Medication persistence and discontinuation of rivaroxaban vs. warfarin among patients with nonvalvular atrial fibrillation. Curr Med Res Opin. 2014;30(12):2461-2469.
19. Clarkesmith DE, Pattison HM, Lane DA. Educational and behavioural interventions for anticoagulant therapy in patients with atrial fibrillation. Cochrane Database Syst Rev. 2013;6:CD008600.
20. Albert NM. Use of novel oral anticoagulants for patients with atrial fibrillation: systematic review and clinical implications. Heart Lung. 2014;43:48-59.
21. Kneeland PP, Fang MC. Current issues in patient adherence and persistence: focus on anticoagulants for the treatment and prevention of thromboembolism. Patient Preference and Adherence. 2010;4:52-60.
22. National Heart Foundation of Australia. Improving adherence in cardiovascular care. A toolkit for health professionals. www.heartfoundation.org.au/SiteCollectionDocuments/Improving-adherence-in-cardiovascular-care-toolkit.pdf. Accessed January 19, 2015.
23. Coumadin [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 1954.
24. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials [published correction appears in Arch Intern Med. 1994;154(19):2254]. Arch Intern Med. 1994;154(13):1449-1457.
25. Kirchhof P, Breithardt G, Camm AJ, et al. Improving outcomes in patients with atrial fibrillation: rationale and design of the Early treatment of Atrial fibrillation for Stroke prevention Trial. Am Heart J. 2013;166(3),442-448.
26. Morimoto A, Miyamatsu, M, Okamura T, et al. Effects of intensive and moderate public education on knowledge of early stroke symptoms among a Japanese population: the Acquisition of Stroke Knowledge study. Stroke. 2013;44(10):2829-2834.
27. Nutescu EA. Oral anticoagulant therapies: balancing the risks. Am J Health Syst Pharm. 2013;70(10 suppl 1):S3-S11.
28. Lane DA, Lip GY. Use of the CHA(2)DS(2)-VASc and HAS-BLED scores to aid decision making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation. 2012;126(7):860-865.
29. Pugh D, Pugh J, Mead GE. Attitudes of physicians regarding anticoagulation for atrial fibrillation: a systematic review. Age Ageing. 2011;40(6):675–683.
30. ten Cate H. New oral anticoagulants: discussion on monitoring and adherence should start now! Thrombosis J. 2013;11(8):1-5.
31. Ivers N, Dorian P. Applying the atrial fibrillation guidelines update to manage your patients with atrial fibrillation. Can J Cardiol. 2014;30:1241-1244.
32. Wigle P, Bloomfield HE, Tubb M, Doherty M. Updated guidelines on outpatient anticoagulation. Am Fam Physician. 2013;87(8):556-566.
33. Horton JD, Bushwick BM. Warfarin therapy: evolving strategies in anticoagulation. Am Fam Physician. 1999;59(3):635-646.
CE/CME No: CR-1502
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Describe the presenting signs and symptoms of atrial fibrillation (A-fib).
• Define the differential and tests for diagnosing A-fib.
• Identify the risk factors associated with A-fib.
• Discuss the anticoagulant treatment options for nonvalvular A-fib.
• List aspects of A-fib management about which patients benefit from clinician instruction.
FACULTY
Deedra Harrington, Janis R. Guilbeau, and Christy McDonald Lenahan are Assistant Professors in the College of Nursing and Allied Health Professions at the University of Louisiana at Lafayette. Dr. Harrington is also a cardiology intensivist nurse practitioner at the Heart Hospital of Lafayette. The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of February 2015.
Article begins on next page >>
Patients with nonvalvular atrial fibrillation (A-fib) have a fivefold greater risk for ischemic stroke than those without. Newer oral anticoagulants reduce this risk—but also increase risk for serious bleeding, including intracranial hemorrhage. Here are the evidence-based guidelines to help you make the choice that’s best for your patient.
Atrial fibrillation (A-fib) is a supraventricular tachyarrhythmia characterized by uncoordinated atrial activation that results in ineffective atrial contraction; this causes inadequate ventricular rate control and variable ventricular filling.1 A common cardiac arrhythmia that is estimated to affect between 2.7 and 6.1 million Americans,2 A-fib is projected to affect as many as 12.1 million people by the year 2030.3 Incidence increases with age; while less than 1% of patients with A-fib are younger than 60, more than a third are 80 or older.1
Morbidity and mortality associated with A-fib are significant. The risk for an embolic event is particularly profound—five times that of persons without A-fib; again, this risk increases with age. In patients ages 50 to 59, 1.5% of strokes are attributed to A-fib; this percentage increases to 23.5% for those ages 80 to 89.2
Treatment of A-fib is aimed at rate control and rhythm conversion, generally through the use of drugs or ablation procedures, and stroke risk reduction, using oral anticoagulants to prevent thrombus formation. This review will focus on the use of newer oral anticoagulants for reduction of stroke risk associated with nonvalvular A-fib.
PATIENT PRESENTATION
Patients with new-onset A-fib may present with a variety of symptoms, including palpitations, chest pain, pressure or discomfort, shortness of breath, lightheadedness, fatigue, or exercise intolerance.4 Patients with chest pain, palpitations, and shortness of breath in particular should be assessed immediately for myocardial infarction before evaluating for A-fib. Poor perfusion may cause a decreased level of patient consciousness; therefore, hypotension or even Alzheimer disease should be ruled out.
Continue for diagnostic evaluation >>
DIAGNOSTIC EVALUATION
A complete patient history and thorough review of systems will enable the clinician to identify the risk factors for A-fib and establish a diagnosis (see Table 1).1,4,5 Evaluation should also include a detailed physical examination. Upon initial cardiovascular assessment, the patient’s apical pulse may be rapid, irregular, or disorganized during auscultation. If underlying A-fib is related to a valvular abnormality, an audible murmur may be auscultated.5
Workup for A-fib includes the standard 12-lead ECG, chest radiograph, thyroid function test, and echocardiogram. The 12-lead ECG is definitive for making the diagnosis of A-fib (see Figure 1). A-fib is characterized by irregular R-R intervals when atrioventricular conduction is present, absence of distinct repeating P waves, and irregular atrial activity.1
If the patient describes episodes consistent with A-fib that is not detectable at the office visit, 24- or 48-hour ambulatory Holter monitoring may be revealing. Event monitors can be used to determine the frequency with which the patient experiences A-fib over an extended period of time (up to 30 days).6
As part of the differential diagnosis of A-fib, clinicians need to consider other possible atrial conduction abnormalities, including atrial flutter, atrial tachycardia, atrioventricular nodal reentry tachycardia, multifocal atrial tachycardia, and Wolff-Parkinson-White syndrome.5
To rule out other etiologies, consider performing the following examinations and tests4
• A chest x-ray can rule out undiagnosed lung disease (eg, chronic obstructive pulmonary disease).
• To exclude hyperthyroidism as a cause of the patient’s symptoms, thyroid function testing and a physical examination for exophthalmos, carotid bruits, and thyromegaly are needed.
• Echocardiography is useful to exclude valvular abnormalities and/or heart failure.
• A complete blood cell count will rule out any infectious process or anemic state.
• Renal function studies and a comprehensive metabolic panel will detect signs of renal failure or electrolyte imbalance.
• Cardiac enzyme measurement can help rule out the occurrence of a myocardial event.
• A brain natriuretic peptide test can identify if heart failure is a contributing factor.
Continue for A-fib classification >>
A-FIB CLASSIFICATION
For purposes of choosing appropriate therapy, it is necessary to determine whether the cause of A-fib is valvular or nonvalvular. Valvular A-fib is described as A-fib that occurs in the presence of valvular heart disease or defect, such as rheumatic mitral stenosis, a mechanical or bioprosthetic heart valve, or mitral valve repair.1 In the absence of these types of conditions, A-fib is considered nonvalvular. The vast majority of patients have nonvalvular A-fib; in the ATRIA (AnTicoagulation and Risk Factors In Atrial Fibrillation) study, researchers found that, among 17,974 adults with A-fib who were members of a large California health maintenance organization, only 4.9% had valvular heart disease.8
A-fib is commonly classified into four subcategories, based on its duration: paroxysmal, persistent, longstanding persistent, and permanent.
Paroxysmal. The occurrence of at least two episodes that have terminated in less than seven days without treatment.
Persistent. An episode lasting more than seven days or less than seven days after electric or pharmacologic conversion.
Longstanding persistent. Continuous A-fib for more than one year.
Permanent. A category for patients in whom rhythm control is no longer being pursued.
This simplified classification is often used to choose between ablative or medication therapies. To ensure accuracy, however, underlying causes, risk factors, and mechanisms should be determined.9
Stroke risk calculation
Once nonvalvular A-fib is confirmed, the next step is to control the ventricular rate and attempt to convert the A-fib rhythm. To accomplish this, the patient’s risk for stroke must be estimated and the need for oral anticoagulation determined.
The CHADS2 risk stratification system for calculating an individual’s risk for ischemic stroke in A-fib was developed in 2001. The risk criteria used in the calculation are Congestive heart failure, Hypertension, Age ≥ 75 years, Diabetes mellitus, prior Stroke, transient ischemic attack, or thromboembolism.10
Recent additions to the criteria account for advanced age, gender, and known vascular disease.1,5 Known as the CHA2DS2-VASc, this scoring system is outlined in Table 2. If the patient’s score is 0, risk for stroke is low and anticoagulation therapy is not recommended. If the score is 1, the risk is intermediate, and the patient may be treated with aspirin therapy or anticoagulation. With a CHA2DS2-VASc score of 2 or greater, anticoagulation treatment is recommended to reduce the risk for stroke.1
While the expanded CHA2DS2-VASc criteria more clearly define the basis for an anticoagulation recommendation—particularly in older patients, women, and those with a vascular history—the superiority of one over the other is undetermined.11 However, the 2014 American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) guidelines for the management of patients with A-fib recommend use of the CHA2DS2-VASc.1
Continue for anticoagulation therapy >>
ANTICOAGULATION THERAPY
The choice of anticoagulation treatment requires weighing the risks and benefits of oral anticoagulation therapy. Stroke and bleeding risks, cost, tolerability, potential for drug interactions, likelihood of patient adherence to the anticoagulation regimen, and patient preferences should be considered.1
The three oral anticoagulants recently approved by the FDA for the reduction of stroke and systemic embolism risks in nonvalvular A-fib are dabigatran, a direct thrombin inhibitor, and rivaroxaban and apixaban, both factor Xa inhibitors.12-14
The clinical trials upon which the FDA’s approval of these anticoagulants was based included only patients with nonvalvular A-fib. For patients with valvular disease, warfarin, a vitamin-K–dependent inhibitor, is currently recommended.1,15 It is also recommended for patients with both end-stage renal disease (ESRD) and either nonvalvular or valvular A-fib.1
A-fib and chronic kidney disease
It is estimated that one-third of patients with A-fib are also diagnosed with chronic kidney disease (CKD).16 Because patients with CKD have a greater risk for bleeding, anticoagulant therapy for these patients requires reduced dosing and close monitoring for bleeding.
The 2014 ACC/AHA/HRS practice guidelines include guidance for selection of oral anticoagulants for patients with nonvalvular A-fib and CKD (see Table 3).1,12-14,17 Dosing of dabigatran and rivaroxaban require evaluation of creatinine clearance before treatment is initiated.
When warfarin is indicated, dose adjustments for renal impairment are based on the prothrombin time/international normalized ratio (INR) value.1 Current guidelines recommend maintaining a therapeutic INR between 2.0 and 3.0 for nonvalvular A-fib in patients with CKD.1 Patients with difficulty maintaining therapeutic INR levels may benefit from alternate therapy with Xa inhibitors or a direct thrombin inhibitor except in the presence of ESRD.1
Continue for patient adherence >>
PATIENT ADHERENCE
Recent studies have indicated that adherence to anticoagulation therapy among A-fib patients drops by as much as 50% after one year of therapy.18 Causes are multifactorial and include complexity of treatment regimen, missed doses, patient unawareness of stroke risk, and fear of bleeding.19 Educating both patients and caregivers has been associated with significant improvements in medication compliance in these patients.19
Complex regimens
Treatment requirements, such as the serial laboratory testing and dosage adjustments associated with warfarin therapy, can be a major contributing factor to anticoagulation nonadherence.18,20 In this regard, the newer once-daily medications that require limited follow-up may be good alternatives to warfarin.21
In patients for whom warfarin therapy is indicated, educational interventions may include
• Written information for patients and caregivers about medication regimens and dosage scheduling
• Reinforcement of treatment goals and outcomes
• Use of dosing aids such as dated and timed pill dispensers
• Incorporating caregiver support to help patients adhere to the medication regimen.
These interventions have been shown to improve adherence with complex treatment regimens.22
Missed doses
Missing anticoagulant doses is not an uncommon occurrence, and patients should be advised of appropriate catch-up strategies when this occurs.
For dabigatran, the missed dose should be taken as soon as the patient remembers, but only if the next scheduled dose is more than six hours away.12 For rivaroxaban, missed doses should be taken as soon as the patient remembers, and the next dose should resume as scheduled.13 For apixaban, a missed dose should be taken as soon as possible but not in combination with any other doses.14
For patients taking warfarin, a missed dose should be taken as soon as possible on the same day.23 If more than 24 hours have elapsed, the patient should contact his or her health care provider before taking any medication.23
Stroke risk
Adherence to anticoagulation therapy significantly reduces the risk for stroke among A-fib patients. Estimates suggest that anticoagulants can reduce stroke risk by as much as 68% in patients with A-fib.24
Even with optimal anticoagulation therapy, however, stroke remains a major complication.25 Through group sessions or patient education pamphlets, patients and caregivers should be informed about the high risk for stroke associated with A-fib and should know its early symptoms.26 These include sudden onset of one or more of the following: confusion or difficulty understanding speech; numbness or weakness of the face or extremities, limited to one side of the body; severe headache; dizziness, loss of balance, or difficulty ambulating; and/or visual disturbances in one or both eyes.26
Next page: Bleeding risk >>
Bleeding risk
Patients should be advised of the major risk for bleeding associated with all anticoagulant therapies.27 Screening for bleeding includes assessment of Hypertension, Abnormal renal and/or liver function, previous Stroke, Bleeding history, Labile INR, being Elderly, and currently prescribed Drugs and/or excessive use of alcohol (known as HAS-BLED) (see Table 4).1,28 Use of a bleeding risk assessment tool such as HAS-BLED may help identify the patient’s risk but cannot be the basis for treatment decisions.1,29
Despite efforts to decrease bleeding risks, patients should understand that hemorrhagic complications can still occur. Patients taking anticoagulants should be familiar with early signs and symptoms of bleeding (eg, sudden, severe headache; melena; hematemesis; nosebleeds) and should notify their health care provider immediately if any of these symptoms occur.12-14,23
If bleeding occurs, it is recommended that anticoagulant treatment be stopped. In addition, depending on the severity of the bleeding, the clinician may elect to administer activated prothrombin complex concentrates, recombinant factor VIIa, or concentrates of factors II, IX, or X to reverse the effects of newer oral anticoagulants.1 Vitamin K, the antidote for warfarin, is not effective on direct thrombin inhibitors or factor Xa inhibitors. Currently, there is no established means of reversing the anticoagulant effects of the newer oral anticoagulants.12-14
FOLLOW-UP
Since optimal utilization of cardiovascular medication occurs in only 50% of the patient population, appropriate follow-up must be implemented to improve overall outcomes of pharmacologic therapy.30 Follow-up protocols depend on multiple factors, including type of anticoagulation therapy, patient response to therapy, and patient comorbidities.31 Monitoring warfarin use is time-consuming and resource-intensive; laboratory monitoring requirements for the newer oral anticoagulants have not been established.32
Patients taking warfarin should be vigilant in follow-up with serial laboratory measurements and dosage adjustments.23 Once therapy is initiated, INR is monitored every two to four days until two therapeutic INR levels are obtained.31,33 Monitoring can then be changed to once weekly until two more therapeutic levels are obtained.31 The INR monitoring interval can then be increased to every two to four weeks, with two weeks being a more conservative strategy.33 The practitioner may want to consider advancing to four-week monitoring intervals once four therapeutic INR levels have been obtained.31 It may be necessary to return to two-to-four day monitoring of INR if a nontherapeutic INR is obtained, the patient becomes ill, a medication is changed, or the patient makes a significant dietary change.31
When to refer
Primary care practitioners can manage anticoagulation therapy safely and efficiently, but cardiology referral may be warranted in certain situations. For example, patients with complex cardiac disease may benefit from cardiology referral.7 Considerations for referral to a cardiologist for further evaluation may include
• Abnormal exercise stress test results
• Abnormal echocardiogram results
• 12-lead ECG that reveals rapid, irregular wide pre-excited QRS complexes5
Patients who are drug intolerant or who remain symptomatic on pharmacologic rate control should also be referred to cardiology.7 In addition, patients who may require a pacemaker or defibrillator or who may be candidates for ablation should also be referred to an electrophysiology specialist.7
Continue for conclusion >>
CONCLUSION
Nonvalvular A-fib is a common arrhythmia that contributes significantly to morbidity among older adults. Use of the most current clinical practice guidelines coupled with patient education will improve overall patient outcomes.
* Editor's note: At press time, the FDA had announced approval of another oral anticoagulant, edoxaban, for the reduction of stroke and systemic embolism risks in nonvalvular A-fib.
CE/CME No: CR-1502
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Describe the presenting signs and symptoms of atrial fibrillation (A-fib).
• Define the differential and tests for diagnosing A-fib.
• Identify the risk factors associated with A-fib.
• Discuss the anticoagulant treatment options for nonvalvular A-fib.
• List aspects of A-fib management about which patients benefit from clinician instruction.
FACULTY
Deedra Harrington, Janis R. Guilbeau, and Christy McDonald Lenahan are Assistant Professors in the College of Nursing and Allied Health Professions at the University of Louisiana at Lafayette. Dr. Harrington is also a cardiology intensivist nurse practitioner at the Heart Hospital of Lafayette. The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of February 2015.
Article begins on next page >>
Patients with nonvalvular atrial fibrillation (A-fib) have a fivefold greater risk for ischemic stroke than those without. Newer oral anticoagulants reduce this risk—but also increase risk for serious bleeding, including intracranial hemorrhage. Here are the evidence-based guidelines to help you make the choice that’s best for your patient.
Atrial fibrillation (A-fib) is a supraventricular tachyarrhythmia characterized by uncoordinated atrial activation that results in ineffective atrial contraction; this causes inadequate ventricular rate control and variable ventricular filling.1 A common cardiac arrhythmia that is estimated to affect between 2.7 and 6.1 million Americans,2 A-fib is projected to affect as many as 12.1 million people by the year 2030.3 Incidence increases with age; while less than 1% of patients with A-fib are younger than 60, more than a third are 80 or older.1
Morbidity and mortality associated with A-fib are significant. The risk for an embolic event is particularly profound—five times that of persons without A-fib; again, this risk increases with age. In patients ages 50 to 59, 1.5% of strokes are attributed to A-fib; this percentage increases to 23.5% for those ages 80 to 89.2
Treatment of A-fib is aimed at rate control and rhythm conversion, generally through the use of drugs or ablation procedures, and stroke risk reduction, using oral anticoagulants to prevent thrombus formation. This review will focus on the use of newer oral anticoagulants for reduction of stroke risk associated with nonvalvular A-fib.
PATIENT PRESENTATION
Patients with new-onset A-fib may present with a variety of symptoms, including palpitations, chest pain, pressure or discomfort, shortness of breath, lightheadedness, fatigue, or exercise intolerance.4 Patients with chest pain, palpitations, and shortness of breath in particular should be assessed immediately for myocardial infarction before evaluating for A-fib. Poor perfusion may cause a decreased level of patient consciousness; therefore, hypotension or even Alzheimer disease should be ruled out.
Continue for diagnostic evaluation >>
DIAGNOSTIC EVALUATION
A complete patient history and thorough review of systems will enable the clinician to identify the risk factors for A-fib and establish a diagnosis (see Table 1).1,4,5 Evaluation should also include a detailed physical examination. Upon initial cardiovascular assessment, the patient’s apical pulse may be rapid, irregular, or disorganized during auscultation. If underlying A-fib is related to a valvular abnormality, an audible murmur may be auscultated.5
Workup for A-fib includes the standard 12-lead ECG, chest radiograph, thyroid function test, and echocardiogram. The 12-lead ECG is definitive for making the diagnosis of A-fib (see Figure 1). A-fib is characterized by irregular R-R intervals when atrioventricular conduction is present, absence of distinct repeating P waves, and irregular atrial activity.1
If the patient describes episodes consistent with A-fib that is not detectable at the office visit, 24- or 48-hour ambulatory Holter monitoring may be revealing. Event monitors can be used to determine the frequency with which the patient experiences A-fib over an extended period of time (up to 30 days).6
As part of the differential diagnosis of A-fib, clinicians need to consider other possible atrial conduction abnormalities, including atrial flutter, atrial tachycardia, atrioventricular nodal reentry tachycardia, multifocal atrial tachycardia, and Wolff-Parkinson-White syndrome.5
To rule out other etiologies, consider performing the following examinations and tests4
• A chest x-ray can rule out undiagnosed lung disease (eg, chronic obstructive pulmonary disease).
• To exclude hyperthyroidism as a cause of the patient’s symptoms, thyroid function testing and a physical examination for exophthalmos, carotid bruits, and thyromegaly are needed.
• Echocardiography is useful to exclude valvular abnormalities and/or heart failure.
• A complete blood cell count will rule out any infectious process or anemic state.
• Renal function studies and a comprehensive metabolic panel will detect signs of renal failure or electrolyte imbalance.
• Cardiac enzyme measurement can help rule out the occurrence of a myocardial event.
• A brain natriuretic peptide test can identify if heart failure is a contributing factor.
Continue for A-fib classification >>
A-FIB CLASSIFICATION
For purposes of choosing appropriate therapy, it is necessary to determine whether the cause of A-fib is valvular or nonvalvular. Valvular A-fib is described as A-fib that occurs in the presence of valvular heart disease or defect, such as rheumatic mitral stenosis, a mechanical or bioprosthetic heart valve, or mitral valve repair.1 In the absence of these types of conditions, A-fib is considered nonvalvular. The vast majority of patients have nonvalvular A-fib; in the ATRIA (AnTicoagulation and Risk Factors In Atrial Fibrillation) study, researchers found that, among 17,974 adults with A-fib who were members of a large California health maintenance organization, only 4.9% had valvular heart disease.8
A-fib is commonly classified into four subcategories, based on its duration: paroxysmal, persistent, longstanding persistent, and permanent.
Paroxysmal. The occurrence of at least two episodes that have terminated in less than seven days without treatment.
Persistent. An episode lasting more than seven days or less than seven days after electric or pharmacologic conversion.
Longstanding persistent. Continuous A-fib for more than one year.
Permanent. A category for patients in whom rhythm control is no longer being pursued.
This simplified classification is often used to choose between ablative or medication therapies. To ensure accuracy, however, underlying causes, risk factors, and mechanisms should be determined.9
Stroke risk calculation
Once nonvalvular A-fib is confirmed, the next step is to control the ventricular rate and attempt to convert the A-fib rhythm. To accomplish this, the patient’s risk for stroke must be estimated and the need for oral anticoagulation determined.
The CHADS2 risk stratification system for calculating an individual’s risk for ischemic stroke in A-fib was developed in 2001. The risk criteria used in the calculation are Congestive heart failure, Hypertension, Age ≥ 75 years, Diabetes mellitus, prior Stroke, transient ischemic attack, or thromboembolism.10
Recent additions to the criteria account for advanced age, gender, and known vascular disease.1,5 Known as the CHA2DS2-VASc, this scoring system is outlined in Table 2. If the patient’s score is 0, risk for stroke is low and anticoagulation therapy is not recommended. If the score is 1, the risk is intermediate, and the patient may be treated with aspirin therapy or anticoagulation. With a CHA2DS2-VASc score of 2 or greater, anticoagulation treatment is recommended to reduce the risk for stroke.1
While the expanded CHA2DS2-VASc criteria more clearly define the basis for an anticoagulation recommendation—particularly in older patients, women, and those with a vascular history—the superiority of one over the other is undetermined.11 However, the 2014 American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) guidelines for the management of patients with A-fib recommend use of the CHA2DS2-VASc.1
Continue for anticoagulation therapy >>
ANTICOAGULATION THERAPY
The choice of anticoagulation treatment requires weighing the risks and benefits of oral anticoagulation therapy. Stroke and bleeding risks, cost, tolerability, potential for drug interactions, likelihood of patient adherence to the anticoagulation regimen, and patient preferences should be considered.1
The three oral anticoagulants recently approved by the FDA for the reduction of stroke and systemic embolism risks in nonvalvular A-fib are dabigatran, a direct thrombin inhibitor, and rivaroxaban and apixaban, both factor Xa inhibitors.12-14
The clinical trials upon which the FDA’s approval of these anticoagulants was based included only patients with nonvalvular A-fib. For patients with valvular disease, warfarin, a vitamin-K–dependent inhibitor, is currently recommended.1,15 It is also recommended for patients with both end-stage renal disease (ESRD) and either nonvalvular or valvular A-fib.1
A-fib and chronic kidney disease
It is estimated that one-third of patients with A-fib are also diagnosed with chronic kidney disease (CKD).16 Because patients with CKD have a greater risk for bleeding, anticoagulant therapy for these patients requires reduced dosing and close monitoring for bleeding.
The 2014 ACC/AHA/HRS practice guidelines include guidance for selection of oral anticoagulants for patients with nonvalvular A-fib and CKD (see Table 3).1,12-14,17 Dosing of dabigatran and rivaroxaban require evaluation of creatinine clearance before treatment is initiated.
When warfarin is indicated, dose adjustments for renal impairment are based on the prothrombin time/international normalized ratio (INR) value.1 Current guidelines recommend maintaining a therapeutic INR between 2.0 and 3.0 for nonvalvular A-fib in patients with CKD.1 Patients with difficulty maintaining therapeutic INR levels may benefit from alternate therapy with Xa inhibitors or a direct thrombin inhibitor except in the presence of ESRD.1
Continue for patient adherence >>
PATIENT ADHERENCE
Recent studies have indicated that adherence to anticoagulation therapy among A-fib patients drops by as much as 50% after one year of therapy.18 Causes are multifactorial and include complexity of treatment regimen, missed doses, patient unawareness of stroke risk, and fear of bleeding.19 Educating both patients and caregivers has been associated with significant improvements in medication compliance in these patients.19
Complex regimens
Treatment requirements, such as the serial laboratory testing and dosage adjustments associated with warfarin therapy, can be a major contributing factor to anticoagulation nonadherence.18,20 In this regard, the newer once-daily medications that require limited follow-up may be good alternatives to warfarin.21
In patients for whom warfarin therapy is indicated, educational interventions may include
• Written information for patients and caregivers about medication regimens and dosage scheduling
• Reinforcement of treatment goals and outcomes
• Use of dosing aids such as dated and timed pill dispensers
• Incorporating caregiver support to help patients adhere to the medication regimen.
These interventions have been shown to improve adherence with complex treatment regimens.22
Missed doses
Missing anticoagulant doses is not an uncommon occurrence, and patients should be advised of appropriate catch-up strategies when this occurs.
For dabigatran, the missed dose should be taken as soon as the patient remembers, but only if the next scheduled dose is more than six hours away.12 For rivaroxaban, missed doses should be taken as soon as the patient remembers, and the next dose should resume as scheduled.13 For apixaban, a missed dose should be taken as soon as possible but not in combination with any other doses.14
For patients taking warfarin, a missed dose should be taken as soon as possible on the same day.23 If more than 24 hours have elapsed, the patient should contact his or her health care provider before taking any medication.23
Stroke risk
Adherence to anticoagulation therapy significantly reduces the risk for stroke among A-fib patients. Estimates suggest that anticoagulants can reduce stroke risk by as much as 68% in patients with A-fib.24
Even with optimal anticoagulation therapy, however, stroke remains a major complication.25 Through group sessions or patient education pamphlets, patients and caregivers should be informed about the high risk for stroke associated with A-fib and should know its early symptoms.26 These include sudden onset of one or more of the following: confusion or difficulty understanding speech; numbness or weakness of the face or extremities, limited to one side of the body; severe headache; dizziness, loss of balance, or difficulty ambulating; and/or visual disturbances in one or both eyes.26
Next page: Bleeding risk >>
Bleeding risk
Patients should be advised of the major risk for bleeding associated with all anticoagulant therapies.27 Screening for bleeding includes assessment of Hypertension, Abnormal renal and/or liver function, previous Stroke, Bleeding history, Labile INR, being Elderly, and currently prescribed Drugs and/or excessive use of alcohol (known as HAS-BLED) (see Table 4).1,28 Use of a bleeding risk assessment tool such as HAS-BLED may help identify the patient’s risk but cannot be the basis for treatment decisions.1,29
Despite efforts to decrease bleeding risks, patients should understand that hemorrhagic complications can still occur. Patients taking anticoagulants should be familiar with early signs and symptoms of bleeding (eg, sudden, severe headache; melena; hematemesis; nosebleeds) and should notify their health care provider immediately if any of these symptoms occur.12-14,23
If bleeding occurs, it is recommended that anticoagulant treatment be stopped. In addition, depending on the severity of the bleeding, the clinician may elect to administer activated prothrombin complex concentrates, recombinant factor VIIa, or concentrates of factors II, IX, or X to reverse the effects of newer oral anticoagulants.1 Vitamin K, the antidote for warfarin, is not effective on direct thrombin inhibitors or factor Xa inhibitors. Currently, there is no established means of reversing the anticoagulant effects of the newer oral anticoagulants.12-14
FOLLOW-UP
Since optimal utilization of cardiovascular medication occurs in only 50% of the patient population, appropriate follow-up must be implemented to improve overall outcomes of pharmacologic therapy.30 Follow-up protocols depend on multiple factors, including type of anticoagulation therapy, patient response to therapy, and patient comorbidities.31 Monitoring warfarin use is time-consuming and resource-intensive; laboratory monitoring requirements for the newer oral anticoagulants have not been established.32
Patients taking warfarin should be vigilant in follow-up with serial laboratory measurements and dosage adjustments.23 Once therapy is initiated, INR is monitored every two to four days until two therapeutic INR levels are obtained.31,33 Monitoring can then be changed to once weekly until two more therapeutic levels are obtained.31 The INR monitoring interval can then be increased to every two to four weeks, with two weeks being a more conservative strategy.33 The practitioner may want to consider advancing to four-week monitoring intervals once four therapeutic INR levels have been obtained.31 It may be necessary to return to two-to-four day monitoring of INR if a nontherapeutic INR is obtained, the patient becomes ill, a medication is changed, or the patient makes a significant dietary change.31
When to refer
Primary care practitioners can manage anticoagulation therapy safely and efficiently, but cardiology referral may be warranted in certain situations. For example, patients with complex cardiac disease may benefit from cardiology referral.7 Considerations for referral to a cardiologist for further evaluation may include
• Abnormal exercise stress test results
• Abnormal echocardiogram results
• 12-lead ECG that reveals rapid, irregular wide pre-excited QRS complexes5
Patients who are drug intolerant or who remain symptomatic on pharmacologic rate control should also be referred to cardiology.7 In addition, patients who may require a pacemaker or defibrillator or who may be candidates for ablation should also be referred to an electrophysiology specialist.7
Continue for conclusion >>
CONCLUSION
Nonvalvular A-fib is a common arrhythmia that contributes significantly to morbidity among older adults. Use of the most current clinical practice guidelines coupled with patient education will improve overall patient outcomes.
* Editor's note: At press time, the FDA had announced approval of another oral anticoagulant, edoxaban, for the reduction of stroke and systemic embolism risks in nonvalvular A-fib.
1. January CT, Wann S, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):2246-2280.
2. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics: 2014 update. Circulation. 2014;129(3):e28-e292.
3. Colilla S, Crow A, Petkun W, et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the US adult population. Am J Cardiol. 2013:112:1142-1147.
4. Rosenthal L, McManus DD. Atrial fibrillation workup. http://emedicine.medscape.com/article/151066-workup. Accessed January 19, 2015.
5. Scheinman MM. Atrial fibrillation. In: Crawford MH, ed. Current Diagnosis and Treatment: Cardiology. Fourth Edition. New York: McGraw-Hill Education; 2014: 141-149.
6. Berry E, Padgett H. Management of patients with atrial fibrillation: diagnosis and treatment. Nurs Stand. 2012;26(22):47-56.
7. Gutierrez C, Blanchard DG. Atrial fibrillation: diagnosis and treatment. Am Fam Physician. 2011;83(1):61-68.
8. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370-2375.
9. Corradi D. Atrial fibrillation from the pathologist’s perspective. Cardiovasc Pathol. 2014;23(2):71-84.
10. Rietbrock S, Heeley E, Plumb J, van Staa T. Chronic atrial fibrillation: incidence, prevalence, and prediction of stroke using the Congestive heart failure, Hypertension Age > 75, Diabetes mellitus, and prior Stroke or transient ischemic attack (CHADS2) risk stratification scheme. Am Heart J. 2008;156(1):57-64.
11. Mason PK, Lake DE, DiMarco JP, et al. Impact of the CHA2DS2-VASc score on anticoagulation recommendations for atrial fibrillation. Am J Med. 2012; 125:603.e1-603.e6.
12. Pradaxa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.; 2010.
13. Xarelto [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2011.
14. Eliquis [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2012.
15. Verma A, Cairns JA, Mitchell LB, et al, for the CCC Atrial Fibrillation Guidelines Committee. 2014 focused update of the Canadian cardiovascular society guidelines for the management of atrial fibrillation. Can J Cardiol. 2014;30(10):1114-1130.
16. Hart RG, Eielboom JW, Brimble KS, et al. Stroke prevention in atrial fibrillation patients with chronic kidney disease. Can J Cardiol. 2013;29(7 suppl):S71-S78.
17. Engelbertz C, Reinecke H. Atrial fibrillation and oral anticoagulation in chronic kidney disease. J Atr Fibrillation. 2012;4(6):89-100.
18. Nelson WW, Song X, Coleman CI, et al. Medication persistence and discontinuation of rivaroxaban vs. warfarin among patients with nonvalvular atrial fibrillation. Curr Med Res Opin. 2014;30(12):2461-2469.
19. Clarkesmith DE, Pattison HM, Lane DA. Educational and behavioural interventions for anticoagulant therapy in patients with atrial fibrillation. Cochrane Database Syst Rev. 2013;6:CD008600.
20. Albert NM. Use of novel oral anticoagulants for patients with atrial fibrillation: systematic review and clinical implications. Heart Lung. 2014;43:48-59.
21. Kneeland PP, Fang MC. Current issues in patient adherence and persistence: focus on anticoagulants for the treatment and prevention of thromboembolism. Patient Preference and Adherence. 2010;4:52-60.
22. National Heart Foundation of Australia. Improving adherence in cardiovascular care. A toolkit for health professionals. www.heartfoundation.org.au/SiteCollectionDocuments/Improving-adherence-in-cardiovascular-care-toolkit.pdf. Accessed January 19, 2015.
23. Coumadin [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 1954.
24. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials [published correction appears in Arch Intern Med. 1994;154(19):2254]. Arch Intern Med. 1994;154(13):1449-1457.
25. Kirchhof P, Breithardt G, Camm AJ, et al. Improving outcomes in patients with atrial fibrillation: rationale and design of the Early treatment of Atrial fibrillation for Stroke prevention Trial. Am Heart J. 2013;166(3),442-448.
26. Morimoto A, Miyamatsu, M, Okamura T, et al. Effects of intensive and moderate public education on knowledge of early stroke symptoms among a Japanese population: the Acquisition of Stroke Knowledge study. Stroke. 2013;44(10):2829-2834.
27. Nutescu EA. Oral anticoagulant therapies: balancing the risks. Am J Health Syst Pharm. 2013;70(10 suppl 1):S3-S11.
28. Lane DA, Lip GY. Use of the CHA(2)DS(2)-VASc and HAS-BLED scores to aid decision making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation. 2012;126(7):860-865.
29. Pugh D, Pugh J, Mead GE. Attitudes of physicians regarding anticoagulation for atrial fibrillation: a systematic review. Age Ageing. 2011;40(6):675–683.
30. ten Cate H. New oral anticoagulants: discussion on monitoring and adherence should start now! Thrombosis J. 2013;11(8):1-5.
31. Ivers N, Dorian P. Applying the atrial fibrillation guidelines update to manage your patients with atrial fibrillation. Can J Cardiol. 2014;30:1241-1244.
32. Wigle P, Bloomfield HE, Tubb M, Doherty M. Updated guidelines on outpatient anticoagulation. Am Fam Physician. 2013;87(8):556-566.
33. Horton JD, Bushwick BM. Warfarin therapy: evolving strategies in anticoagulation. Am Fam Physician. 1999;59(3):635-646.
1. January CT, Wann S, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):2246-2280.
2. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics: 2014 update. Circulation. 2014;129(3):e28-e292.
3. Colilla S, Crow A, Petkun W, et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the US adult population. Am J Cardiol. 2013:112:1142-1147.
4. Rosenthal L, McManus DD. Atrial fibrillation workup. http://emedicine.medscape.com/article/151066-workup. Accessed January 19, 2015.
5. Scheinman MM. Atrial fibrillation. In: Crawford MH, ed. Current Diagnosis and Treatment: Cardiology. Fourth Edition. New York: McGraw-Hill Education; 2014: 141-149.
6. Berry E, Padgett H. Management of patients with atrial fibrillation: diagnosis and treatment. Nurs Stand. 2012;26(22):47-56.
7. Gutierrez C, Blanchard DG. Atrial fibrillation: diagnosis and treatment. Am Fam Physician. 2011;83(1):61-68.
8. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370-2375.
9. Corradi D. Atrial fibrillation from the pathologist’s perspective. Cardiovasc Pathol. 2014;23(2):71-84.
10. Rietbrock S, Heeley E, Plumb J, van Staa T. Chronic atrial fibrillation: incidence, prevalence, and prediction of stroke using the Congestive heart failure, Hypertension Age > 75, Diabetes mellitus, and prior Stroke or transient ischemic attack (CHADS2) risk stratification scheme. Am Heart J. 2008;156(1):57-64.
11. Mason PK, Lake DE, DiMarco JP, et al. Impact of the CHA2DS2-VASc score on anticoagulation recommendations for atrial fibrillation. Am J Med. 2012; 125:603.e1-603.e6.
12. Pradaxa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.; 2010.
13. Xarelto [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2011.
14. Eliquis [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2012.
15. Verma A, Cairns JA, Mitchell LB, et al, for the CCC Atrial Fibrillation Guidelines Committee. 2014 focused update of the Canadian cardiovascular society guidelines for the management of atrial fibrillation. Can J Cardiol. 2014;30(10):1114-1130.
16. Hart RG, Eielboom JW, Brimble KS, et al. Stroke prevention in atrial fibrillation patients with chronic kidney disease. Can J Cardiol. 2013;29(7 suppl):S71-S78.
17. Engelbertz C, Reinecke H. Atrial fibrillation and oral anticoagulation in chronic kidney disease. J Atr Fibrillation. 2012;4(6):89-100.
18. Nelson WW, Song X, Coleman CI, et al. Medication persistence and discontinuation of rivaroxaban vs. warfarin among patients with nonvalvular atrial fibrillation. Curr Med Res Opin. 2014;30(12):2461-2469.
19. Clarkesmith DE, Pattison HM, Lane DA. Educational and behavioural interventions for anticoagulant therapy in patients with atrial fibrillation. Cochrane Database Syst Rev. 2013;6:CD008600.
20. Albert NM. Use of novel oral anticoagulants for patients with atrial fibrillation: systematic review and clinical implications. Heart Lung. 2014;43:48-59.
21. Kneeland PP, Fang MC. Current issues in patient adherence and persistence: focus on anticoagulants for the treatment and prevention of thromboembolism. Patient Preference and Adherence. 2010;4:52-60.
22. National Heart Foundation of Australia. Improving adherence in cardiovascular care. A toolkit for health professionals. www.heartfoundation.org.au/SiteCollectionDocuments/Improving-adherence-in-cardiovascular-care-toolkit.pdf. Accessed January 19, 2015.
23. Coumadin [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 1954.
24. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials [published correction appears in Arch Intern Med. 1994;154(19):2254]. Arch Intern Med. 1994;154(13):1449-1457.
25. Kirchhof P, Breithardt G, Camm AJ, et al. Improving outcomes in patients with atrial fibrillation: rationale and design of the Early treatment of Atrial fibrillation for Stroke prevention Trial. Am Heart J. 2013;166(3),442-448.
26. Morimoto A, Miyamatsu, M, Okamura T, et al. Effects of intensive and moderate public education on knowledge of early stroke symptoms among a Japanese population: the Acquisition of Stroke Knowledge study. Stroke. 2013;44(10):2829-2834.
27. Nutescu EA. Oral anticoagulant therapies: balancing the risks. Am J Health Syst Pharm. 2013;70(10 suppl 1):S3-S11.
28. Lane DA, Lip GY. Use of the CHA(2)DS(2)-VASc and HAS-BLED scores to aid decision making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation. 2012;126(7):860-865.
29. Pugh D, Pugh J, Mead GE. Attitudes of physicians regarding anticoagulation for atrial fibrillation: a systematic review. Age Ageing. 2011;40(6):675–683.
30. ten Cate H. New oral anticoagulants: discussion on monitoring and adherence should start now! Thrombosis J. 2013;11(8):1-5.
31. Ivers N, Dorian P. Applying the atrial fibrillation guidelines update to manage your patients with atrial fibrillation. Can J Cardiol. 2014;30:1241-1244.
32. Wigle P, Bloomfield HE, Tubb M, Doherty M. Updated guidelines on outpatient anticoagulation. Am Fam Physician. 2013;87(8):556-566.
33. Horton JD, Bushwick BM. Warfarin therapy: evolving strategies in anticoagulation. Am Fam Physician. 1999;59(3):635-646.