User login
Tumor markers are serum measures that are valuable in the discrimination of an adnexal mass. However, given the long list from which to choose, it can be confusing to know exactly which might best serve your diagnostic needs. I am commonly asked by obstetrician/gynecologists and primary care doctors for guidance on this subject. In this column I will explore some of the decision making that I use when determining which markers might be most helpful for individual patients.
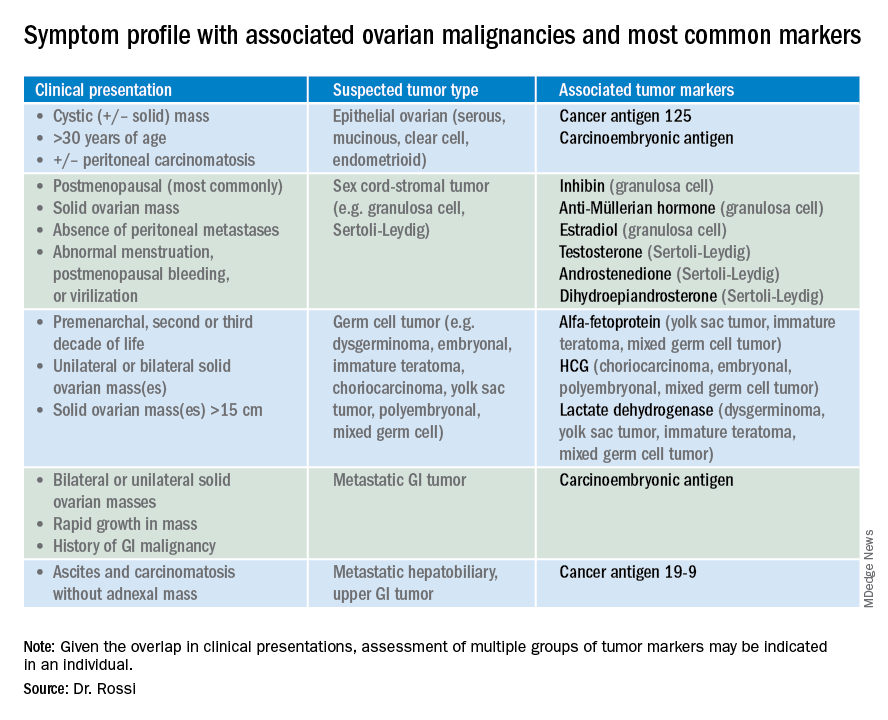
So which tumor markers should you order when you have diagnosed an adnexal mass? Because tumor marker profiles can differ dramatically based on the cell type of the neoplasm, perhaps the first question to ask is what is the most likely category of neoplasm based on other clinical data? Ovarian neoplasms fit into the following subgroups: epithelial (including the most common cell type, serous ovarian cancer, but also the less common mucinous and low malignant potential tumors), sex cord-stromal tumors, germ cell tumors, and metastatic tumors. Table 1 summarizes which tumor markers should be considered based on the clinical setting.
You should suspect an epithelial tumor if there is an adnexal mass with significant cystic components in older, postmenopausal patients, or the presence of peritoneal carcinomatosis on imaging. The tumor markers most commonly elevated in this clinical setting are cancer antigen 125 (CA 125), carcinoembryonic antigen (CEA), and possibly CA 19-9. The CA 125 antigen is a glycoprotein derived from the epithelium of peritoneum, pleura, pericardium, and Müllerian tissues. The multiple sites of origin of this glycoprotein speaks to the poor specificity associated with its elevation, as it is well known to be elevated in both benign conditions such as endometriosis, fibroids, pregnancy, ovulation, cirrhosis, and pericarditis as well as in nongynecologic malignancies, particularly those metastatic to the peritoneal cavity. Multiple different assays are available to measure CA 125, and each is associated with a slightly different reference range. Therefore, if measuring serial values, it is best to have these assessed by the same laboratory. Similarly, as it can be physiologically elevated during the menstrual cycle, premenopausal women should have serial assessments at the same point in their menstrual cycle or ideally within the first 2 weeks of their cycle.
The sensitivity of CA 125 in detecting ovarian cancer is only 78%, which is limited by the fact that not all epithelial ovarian cancer cell types (including some clear cell, carcinosarcoma, and mucinous) express elevations in this tumor marker, and because CA 125 is elevated in less than half of stage I ovarian cancers.1 Therefore, given the lack of sensitivity and specificity for this tumor marker, you should integrate other clinical data, such as imaging findings, age of the patient, and associated benign medical conditions, when evaluating the likelihood of cancer. The American College of Obstetricians and Gynecologists (ACOG) recommends that in the setting of an adnexal mass, referral to gynecologic oncology is recommended when the CA 125 value is greater than 200 U/mL in premenopausal women, or greater than 35U/mL in postmenopausal women.2
CEA is a protein that can be expressed in the colon but not in other normal tissues after birth, and therefore its elevation is commonly associated with metastatic GI tumors to the ovary and peritoneum, or mucinous ovarian tumors, including borderline tumors. Metastatic GI tumors typically are suspected when there are bilateral ovarian solid masses. Right-sided ovarian cysts also can be associated with appendiceal pathology and checking a CEA level can be considered in these cases. I will commonly draw both CA 125 and CEA tumor markers in the setting of cystic +/– solid ovarian masses. This allows the recognition of CA 125-negative/CEA-positive ovarian cancers, such as mucinous tumors, which aids in later surveillance or increases my suspicion for an occult GI tumor (particularly if there is a disproportionately higher elevation in CEA than CA 125).3 If tumor marker profiles are suggestive of an occult GI tumor, I often will consider a preoperative colonoscopy and upper GI endoscopic assessment.
CA 19-9 is a much less specific tumor marker which can be elevated in a variety of solid organ tumors including pancreatic, hepatobiliary, gastric and ovarian tumors. I typically reserve adding this marker for atypical clinical presentations of ovarian cancer, such as carcinomatosis in the absence of pelvic masses.
Ovarian sex cord-stromal neoplasms most commonly present as solid tumors in the ovary. The ovarian stroma includes the bland fibroblasts and the hormone-producing sex-cord granulosa, Sertoli and Leydig cells. Therefore the sex cord-stromal tumors commonly are associated with elevations in serum inhibin, anti-Müllerian hormone, and potentially androstenedione and dehydroepiandrosterone.4 These tumors rarely have advanced disease at diagnosis. Granulosa cell tumors should be suspected in women with a solid ovarian mass and abnormal uterine bleeding (including postmenopausal bleeding), and the appropriate tumor markers (inhibin and anti-Müllerian hormone) can guide this diagnosis preoperatively.4 Androgen-secreting stromal tumors such as Sertoli-Leydig tumors often present with virilization or menstrual irregularities. Interestingly, these patients may have dramatic clinical symptoms with corresponding nonvisible or very small solid adnexal lesions seen on imaging. In the case of fibromas, these solid tumors have normal hormonal tumor markers but may present with ascites and pleural effusions as part of Meigs syndrome, which can confuse the clinician who may suspect advanced-stage epithelial cancer especially as this condition may be associated with elevated CA 125.
Germ cell tumors make up the other main group of primary ovarian tumors, and typically strongly express tumor markers. These tumors typically are solid and highly vascularized on imaging, can be bilateral, and may be very large at the time of diagnosis.5 They most commonly are unilateral and arise among younger women (including usually in the second and third decades of life). Table 1 demonstrates the different tumor markers associated with different germ cell tumors. It is my practice to order a panel of all of these germ cell markers in young women with solid adnexal masses in whom germ cell tumors are suspected, but I will not routinely draw this expansive panel for older women with cystic lesions.
Tumor marker panels (such as OVA 1, Overa, Risk of Malignancy Algorithm or ROMA) have become popular in recent years. These panels include multiple serum markers (such as CA 125, beta-2 microglobulin, human epididymis secretory protein 4, transferrin, etc.) evaluated in concert with the goal being a more nuanced assessment of likelihood for malignancy.6,7 These assays typically are stratified by age or menopausal status given the physiologic differences in normal reference ranges that occur between these groups. While these studies do improve upon the sensitivity and specificity for identifying malignancy, compared with single-assay tests, they are not definitively diagnostic for this purpose. Therefore, I typically recommend these assays if a referring doctor needs additional risk stratification to guide whether or not to refer to an oncologist for surgery.
Not all tumor markers are of equal value in all patients with an adnexal mass. I recommend careful consideration of other clinical factors such as age, menopausal status, ultrasonographic features, and associated findings such as GI symptoms or manifestations of hormonal alterations when considering which markers to assess.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Hum Reprod. 1989 Jan;4(1):1-12.
2. Obstet Gynecol. 2016 Nov;128(5):e210-e26.
3. Dan Med Bull. 2011 Nov;58(11):A4331.
4. Int J Cancer. 2015 Oct 1;137(7):1661-71.
5. Obstet Gynecol. 2000 Jan;95(1):128-33.
6. Obstet Gynecol. 2011 Jun;117(6):1289-97.
7. Obstet Gynecol. 2011 Aug;118(2 Pt 1):280-8.
Tumor markers are serum measures that are valuable in the discrimination of an adnexal mass. However, given the long list from which to choose, it can be confusing to know exactly which might best serve your diagnostic needs. I am commonly asked by obstetrician/gynecologists and primary care doctors for guidance on this subject. In this column I will explore some of the decision making that I use when determining which markers might be most helpful for individual patients.

So which tumor markers should you order when you have diagnosed an adnexal mass? Because tumor marker profiles can differ dramatically based on the cell type of the neoplasm, perhaps the first question to ask is what is the most likely category of neoplasm based on other clinical data? Ovarian neoplasms fit into the following subgroups: epithelial (including the most common cell type, serous ovarian cancer, but also the less common mucinous and low malignant potential tumors), sex cord-stromal tumors, germ cell tumors, and metastatic tumors. Table 1 summarizes which tumor markers should be considered based on the clinical setting.
You should suspect an epithelial tumor if there is an adnexal mass with significant cystic components in older, postmenopausal patients, or the presence of peritoneal carcinomatosis on imaging. The tumor markers most commonly elevated in this clinical setting are cancer antigen 125 (CA 125), carcinoembryonic antigen (CEA), and possibly CA 19-9. The CA 125 antigen is a glycoprotein derived from the epithelium of peritoneum, pleura, pericardium, and Müllerian tissues. The multiple sites of origin of this glycoprotein speaks to the poor specificity associated with its elevation, as it is well known to be elevated in both benign conditions such as endometriosis, fibroids, pregnancy, ovulation, cirrhosis, and pericarditis as well as in nongynecologic malignancies, particularly those metastatic to the peritoneal cavity. Multiple different assays are available to measure CA 125, and each is associated with a slightly different reference range. Therefore, if measuring serial values, it is best to have these assessed by the same laboratory. Similarly, as it can be physiologically elevated during the menstrual cycle, premenopausal women should have serial assessments at the same point in their menstrual cycle or ideally within the first 2 weeks of their cycle.
The sensitivity of CA 125 in detecting ovarian cancer is only 78%, which is limited by the fact that not all epithelial ovarian cancer cell types (including some clear cell, carcinosarcoma, and mucinous) express elevations in this tumor marker, and because CA 125 is elevated in less than half of stage I ovarian cancers.1 Therefore, given the lack of sensitivity and specificity for this tumor marker, you should integrate other clinical data, such as imaging findings, age of the patient, and associated benign medical conditions, when evaluating the likelihood of cancer. The American College of Obstetricians and Gynecologists (ACOG) recommends that in the setting of an adnexal mass, referral to gynecologic oncology is recommended when the CA 125 value is greater than 200 U/mL in premenopausal women, or greater than 35U/mL in postmenopausal women.2
CEA is a protein that can be expressed in the colon but not in other normal tissues after birth, and therefore its elevation is commonly associated with metastatic GI tumors to the ovary and peritoneum, or mucinous ovarian tumors, including borderline tumors. Metastatic GI tumors typically are suspected when there are bilateral ovarian solid masses. Right-sided ovarian cysts also can be associated with appendiceal pathology and checking a CEA level can be considered in these cases. I will commonly draw both CA 125 and CEA tumor markers in the setting of cystic +/– solid ovarian masses. This allows the recognition of CA 125-negative/CEA-positive ovarian cancers, such as mucinous tumors, which aids in later surveillance or increases my suspicion for an occult GI tumor (particularly if there is a disproportionately higher elevation in CEA than CA 125).3 If tumor marker profiles are suggestive of an occult GI tumor, I often will consider a preoperative colonoscopy and upper GI endoscopic assessment.
CA 19-9 is a much less specific tumor marker which can be elevated in a variety of solid organ tumors including pancreatic, hepatobiliary, gastric and ovarian tumors. I typically reserve adding this marker for atypical clinical presentations of ovarian cancer, such as carcinomatosis in the absence of pelvic masses.
Ovarian sex cord-stromal neoplasms most commonly present as solid tumors in the ovary. The ovarian stroma includes the bland fibroblasts and the hormone-producing sex-cord granulosa, Sertoli and Leydig cells. Therefore the sex cord-stromal tumors commonly are associated with elevations in serum inhibin, anti-Müllerian hormone, and potentially androstenedione and dehydroepiandrosterone.4 These tumors rarely have advanced disease at diagnosis. Granulosa cell tumors should be suspected in women with a solid ovarian mass and abnormal uterine bleeding (including postmenopausal bleeding), and the appropriate tumor markers (inhibin and anti-Müllerian hormone) can guide this diagnosis preoperatively.4 Androgen-secreting stromal tumors such as Sertoli-Leydig tumors often present with virilization or menstrual irregularities. Interestingly, these patients may have dramatic clinical symptoms with corresponding nonvisible or very small solid adnexal lesions seen on imaging. In the case of fibromas, these solid tumors have normal hormonal tumor markers but may present with ascites and pleural effusions as part of Meigs syndrome, which can confuse the clinician who may suspect advanced-stage epithelial cancer especially as this condition may be associated with elevated CA 125.
Germ cell tumors make up the other main group of primary ovarian tumors, and typically strongly express tumor markers. These tumors typically are solid and highly vascularized on imaging, can be bilateral, and may be very large at the time of diagnosis.5 They most commonly are unilateral and arise among younger women (including usually in the second and third decades of life). Table 1 demonstrates the different tumor markers associated with different germ cell tumors. It is my practice to order a panel of all of these germ cell markers in young women with solid adnexal masses in whom germ cell tumors are suspected, but I will not routinely draw this expansive panel for older women with cystic lesions.
Tumor marker panels (such as OVA 1, Overa, Risk of Malignancy Algorithm or ROMA) have become popular in recent years. These panels include multiple serum markers (such as CA 125, beta-2 microglobulin, human epididymis secretory protein 4, transferrin, etc.) evaluated in concert with the goal being a more nuanced assessment of likelihood for malignancy.6,7 These assays typically are stratified by age or menopausal status given the physiologic differences in normal reference ranges that occur between these groups. While these studies do improve upon the sensitivity and specificity for identifying malignancy, compared with single-assay tests, they are not definitively diagnostic for this purpose. Therefore, I typically recommend these assays if a referring doctor needs additional risk stratification to guide whether or not to refer to an oncologist for surgery.
Not all tumor markers are of equal value in all patients with an adnexal mass. I recommend careful consideration of other clinical factors such as age, menopausal status, ultrasonographic features, and associated findings such as GI symptoms or manifestations of hormonal alterations when considering which markers to assess.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Hum Reprod. 1989 Jan;4(1):1-12.
2. Obstet Gynecol. 2016 Nov;128(5):e210-e26.
3. Dan Med Bull. 2011 Nov;58(11):A4331.
4. Int J Cancer. 2015 Oct 1;137(7):1661-71.
5. Obstet Gynecol. 2000 Jan;95(1):128-33.
6. Obstet Gynecol. 2011 Jun;117(6):1289-97.
7. Obstet Gynecol. 2011 Aug;118(2 Pt 1):280-8.
Tumor markers are serum measures that are valuable in the discrimination of an adnexal mass. However, given the long list from which to choose, it can be confusing to know exactly which might best serve your diagnostic needs. I am commonly asked by obstetrician/gynecologists and primary care doctors for guidance on this subject. In this column I will explore some of the decision making that I use when determining which markers might be most helpful for individual patients.

So which tumor markers should you order when you have diagnosed an adnexal mass? Because tumor marker profiles can differ dramatically based on the cell type of the neoplasm, perhaps the first question to ask is what is the most likely category of neoplasm based on other clinical data? Ovarian neoplasms fit into the following subgroups: epithelial (including the most common cell type, serous ovarian cancer, but also the less common mucinous and low malignant potential tumors), sex cord-stromal tumors, germ cell tumors, and metastatic tumors. Table 1 summarizes which tumor markers should be considered based on the clinical setting.
You should suspect an epithelial tumor if there is an adnexal mass with significant cystic components in older, postmenopausal patients, or the presence of peritoneal carcinomatosis on imaging. The tumor markers most commonly elevated in this clinical setting are cancer antigen 125 (CA 125), carcinoembryonic antigen (CEA), and possibly CA 19-9. The CA 125 antigen is a glycoprotein derived from the epithelium of peritoneum, pleura, pericardium, and Müllerian tissues. The multiple sites of origin of this glycoprotein speaks to the poor specificity associated with its elevation, as it is well known to be elevated in both benign conditions such as endometriosis, fibroids, pregnancy, ovulation, cirrhosis, and pericarditis as well as in nongynecologic malignancies, particularly those metastatic to the peritoneal cavity. Multiple different assays are available to measure CA 125, and each is associated with a slightly different reference range. Therefore, if measuring serial values, it is best to have these assessed by the same laboratory. Similarly, as it can be physiologically elevated during the menstrual cycle, premenopausal women should have serial assessments at the same point in their menstrual cycle or ideally within the first 2 weeks of their cycle.
The sensitivity of CA 125 in detecting ovarian cancer is only 78%, which is limited by the fact that not all epithelial ovarian cancer cell types (including some clear cell, carcinosarcoma, and mucinous) express elevations in this tumor marker, and because CA 125 is elevated in less than half of stage I ovarian cancers.1 Therefore, given the lack of sensitivity and specificity for this tumor marker, you should integrate other clinical data, such as imaging findings, age of the patient, and associated benign medical conditions, when evaluating the likelihood of cancer. The American College of Obstetricians and Gynecologists (ACOG) recommends that in the setting of an adnexal mass, referral to gynecologic oncology is recommended when the CA 125 value is greater than 200 U/mL in premenopausal women, or greater than 35U/mL in postmenopausal women.2
CEA is a protein that can be expressed in the colon but not in other normal tissues after birth, and therefore its elevation is commonly associated with metastatic GI tumors to the ovary and peritoneum, or mucinous ovarian tumors, including borderline tumors. Metastatic GI tumors typically are suspected when there are bilateral ovarian solid masses. Right-sided ovarian cysts also can be associated with appendiceal pathology and checking a CEA level can be considered in these cases. I will commonly draw both CA 125 and CEA tumor markers in the setting of cystic +/– solid ovarian masses. This allows the recognition of CA 125-negative/CEA-positive ovarian cancers, such as mucinous tumors, which aids in later surveillance or increases my suspicion for an occult GI tumor (particularly if there is a disproportionately higher elevation in CEA than CA 125).3 If tumor marker profiles are suggestive of an occult GI tumor, I often will consider a preoperative colonoscopy and upper GI endoscopic assessment.
CA 19-9 is a much less specific tumor marker which can be elevated in a variety of solid organ tumors including pancreatic, hepatobiliary, gastric and ovarian tumors. I typically reserve adding this marker for atypical clinical presentations of ovarian cancer, such as carcinomatosis in the absence of pelvic masses.
Ovarian sex cord-stromal neoplasms most commonly present as solid tumors in the ovary. The ovarian stroma includes the bland fibroblasts and the hormone-producing sex-cord granulosa, Sertoli and Leydig cells. Therefore the sex cord-stromal tumors commonly are associated with elevations in serum inhibin, anti-Müllerian hormone, and potentially androstenedione and dehydroepiandrosterone.4 These tumors rarely have advanced disease at diagnosis. Granulosa cell tumors should be suspected in women with a solid ovarian mass and abnormal uterine bleeding (including postmenopausal bleeding), and the appropriate tumor markers (inhibin and anti-Müllerian hormone) can guide this diagnosis preoperatively.4 Androgen-secreting stromal tumors such as Sertoli-Leydig tumors often present with virilization or menstrual irregularities. Interestingly, these patients may have dramatic clinical symptoms with corresponding nonvisible or very small solid adnexal lesions seen on imaging. In the case of fibromas, these solid tumors have normal hormonal tumor markers but may present with ascites and pleural effusions as part of Meigs syndrome, which can confuse the clinician who may suspect advanced-stage epithelial cancer especially as this condition may be associated with elevated CA 125.
Germ cell tumors make up the other main group of primary ovarian tumors, and typically strongly express tumor markers. These tumors typically are solid and highly vascularized on imaging, can be bilateral, and may be very large at the time of diagnosis.5 They most commonly are unilateral and arise among younger women (including usually in the second and third decades of life). Table 1 demonstrates the different tumor markers associated with different germ cell tumors. It is my practice to order a panel of all of these germ cell markers in young women with solid adnexal masses in whom germ cell tumors are suspected, but I will not routinely draw this expansive panel for older women with cystic lesions.
Tumor marker panels (such as OVA 1, Overa, Risk of Malignancy Algorithm or ROMA) have become popular in recent years. These panels include multiple serum markers (such as CA 125, beta-2 microglobulin, human epididymis secretory protein 4, transferrin, etc.) evaluated in concert with the goal being a more nuanced assessment of likelihood for malignancy.6,7 These assays typically are stratified by age or menopausal status given the physiologic differences in normal reference ranges that occur between these groups. While these studies do improve upon the sensitivity and specificity for identifying malignancy, compared with single-assay tests, they are not definitively diagnostic for this purpose. Therefore, I typically recommend these assays if a referring doctor needs additional risk stratification to guide whether or not to refer to an oncologist for surgery.
Not all tumor markers are of equal value in all patients with an adnexal mass. I recommend careful consideration of other clinical factors such as age, menopausal status, ultrasonographic features, and associated findings such as GI symptoms or manifestations of hormonal alterations when considering which markers to assess.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Hum Reprod. 1989 Jan;4(1):1-12.
2. Obstet Gynecol. 2016 Nov;128(5):e210-e26.
3. Dan Med Bull. 2011 Nov;58(11):A4331.
4. Int J Cancer. 2015 Oct 1;137(7):1661-71.
5. Obstet Gynecol. 2000 Jan;95(1):128-33.
6. Obstet Gynecol. 2011 Jun;117(6):1289-97.
7. Obstet Gynecol. 2011 Aug;118(2 Pt 1):280-8.
