User login
Gynecologic Oncology Consult: Update on Endometrial Cancer Treatment
While rates of most other cancers have declined or plateaued, the incidence and mortality rate of endometrial cancer continue to rise.1 The landscape of endometrial cancer treatment has evolved quickly over the past 2-3 years. While surgery and radiation therapy remain the mainstay of treatment for early-stage disease, the development of multiple targeted therapeutics has led to additional treatment options in advanced-stage disease and more aggressive tumor types, which are both associated with a poorer prognosis.
In this update, we highlight the recent advances in these targeted therapies in endometrial cancer. We review the landmark NRG-GY018 and RUBY trials, which demonstrated that checkpoint inhibitors improve outcomes in women with advanced endometrial cancer. We discuss an immunotherapy and antivascular endothelial growth factor (VEGF) combination useful in certain tumors lacking biomarker expression. We also highlight progress against endometrial cancers that overexpress human epidermal growth factor receptor 2 (HER2), demonstrated in the DESTINY PanTumor-02 trial.
PD-1 inhibitors
Programmed cell death protein 1 (PD-1) is an inhibitory receptor expressed on T cells that binds to programmed cell death ligand 1 (PD-L1). PD-L1 is expressed on many immune cells but can also be expressed on tumor cells. The interaction of PD-L1 expressed on the surface of endometrial cancer cells with the PD-1 receptor on T cells results in diminished T-cell function, eliminating the immune system’s ability to attack the tumor cells. Treatment with a PD-1 inhibitor prevents this ligand-receptor interaction and restores cancer-fighting function to T cells.
Pembrolizumab, an antibody against the PD-1 receptor, has been used as single-agent treatment for recurrent endometrial cancer since the KEYNOTE-158 study demonstrated clinical benefit in patients with mismatch repair deficient (dMMR) tumors.2
Additionally, in 2022, Makker et al. published results from a phase 3 trial3 evaluating immunotherapy in the treatment of recurrent endometrial cancer, specifically in patients with mismatch repair proficient (pMMR) tumors. They compared the combination of pembrolizumab and lenvatinib, an oral inhibitor of VEGF, to physician’s choice next-line chemotherapy in over 800 patients with advanced or recurrent endometrial cancer. They found that progression-free survival (PFS) was significantly improved from a median of 3.8 months with chemotherapy to a median of 6.6 months with pembrolizumab and lenvatinib in the pMMR population. Overall survival was also improved from 12 months to 17.4 months in the pMMR population.
With the clear benefit of immunotherapy in the recurrent setting established, Eskander and colleagues were the first to evaluate treatment with pembrolizumab as upfront treatment in the NRG-GY018 trial,4 comparing standard first-line chemotherapy (carboplatin and paclitaxel) with or without the addition of pembrolizumab. This randomized, international, phase 3 trial included over 800 patients with advanced or recurrent endometrial cancer of any histology except carcinosarcoma. Patients received carboplatin and paclitaxel with either pembrolizumab or placebo, followed by maintenance pembrolizumab or placebo. The results showed an improvement in PFS with the addition of immunotherapy, with a risk of disease progression or death with pembrolizumab 70% lower than with placebo in patients with dMMR tumors and 46% lower than with placebo in patients with pMMR tumors.
In the similar randomized, international, phase 3 RUBY trial,5 Mirza and colleagues compared standard chemotherapy with or without the addition of another PD-1 inhibitor, dostarlimab, in almost 500 patients with advanced or recurrent endometrial cancer of any histology. They found that the addition of dostarlimab to standard chemotherapy significantly improved PFS. Unpublished data presented at the Society of Gynecologic Oncology annual meeting in March also demonstrated an improvement in overall survival.6 As in NRG-GY018, they found a substantial benefit again in the dMMR population.
The results of these three landmark trials demonstrate the increasing role for immunotherapy in endometrial cancer, especially at the time of initial treatment, and how biomarkers can help direct treatment options.
Takeaway
Use of PD-1 inhibitors improves clinical outcomes in both the upfront and recurrent treatment settings. The magnitude of benefit of treatment with PD-1 inhibitors is greater in patients with dMMR tumors.
Anti-HER2 therapies
HER2 is a cell surface protein that can become overexpressed and promote tumorigenesis. It is used as a prognostic biomarker and a therapeutic target in breast, stomach, and colon cancer, but it has also been identified at high rates (20%-30%) in the most aggressive histologic subtypes in endometrial cancer (serous, clear cell, and carcinosarcoma). Trastuzumab is a monoclonal antibody directed against HER2, most commonly used in HER2-positive breast cancer. In 2018, a phase 2 trial demonstrated that trastuzumab combined with standard chemotherapy improved PFS in serous endometrial cancers that overexpress HER2.7 These results were important and promising given both the poor prognosis associated with the aggressive serous histology and the relative lack of effective therapies at the time of recurrence.
Recently, antibody-drug conjugates (ADCs) have come to the forefront of cancer-directed therapies. ADCs deliver chemotherapy agents directly to cancer cells via a monoclonal antibody that binds to a specific target on the cancer cells. Trastuzumab-deruxtecan (T-DXd) is an ADC consisting of an anti-HER2 monoclonal antibody, a topoisomerase I inhibitor payload, and a cleavable linker. T-DXd was evaluated in the tumor-agnostic phase 2 DESTINY-PanTumor02 trial,8 which included endometrial, ovarian, and cervical cancer cohorts, in addition to four other nongynecologic malignancies. In this study, 40 patients with advanced or recurrent malignancies overexpressing HER2 in each cohort were treated with T-DXd.
The results within the endometrial cancer cohort were particularly promising. The overall response rate in endometrial cancer was an astounding 57.5% with a median PFS of over 11 months. Even higher response rates were seen in endometrial cancer patients whose tumors demonstrated higher rates of HER2 overexpression. These results are unprecedented in a cohort in which most patients had seen at least 2 prior lines of therapy. Ocular and pulmonary toxicities are of particular interest with use of ADCs, but they were mostly low grade and manageable in this study.
Takeaway
Anti-HER2 therapies, including antibody-drug conjugates, are effective in treating patients with some of the highest-risk endometrial cancers when they overexpress this protein.
Dr. Haag is a gynecologic oncology fellow in the Department of Obstetrics and Gynecology, University of North Carolina Hospitals, Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill. They have no conflicts of interest.
References
1. Siegel R et al. CA Cancer J. 2024;74(1):12-49.
2. Marabelle A et al. J Clin Oncol. 2020;38(1):1-10.
3. Makker V et al. N Engl J Med. 2022;386(5):437-48.
4. Eskander RN et al. N Engl J Med. 2023;388(23):2159-70.
5. Mirza MR et al. N Engl J Med. 2023;388(23):2145-58.
6. Powell MA et al, editors. Society of Gynecologic Oncology Annual Meeting on Women’s Cancer, 2024; San Diego, CA.
7. Fader AN et al. J Clin Oncol. 2018;36(20):2044-51.
8. Meric-Bernstam F et al. J Clin Oncol. 2024;42(1):47-58.
While rates of most other cancers have declined or plateaued, the incidence and mortality rate of endometrial cancer continue to rise.1 The landscape of endometrial cancer treatment has evolved quickly over the past 2-3 years. While surgery and radiation therapy remain the mainstay of treatment for early-stage disease, the development of multiple targeted therapeutics has led to additional treatment options in advanced-stage disease and more aggressive tumor types, which are both associated with a poorer prognosis.
In this update, we highlight the recent advances in these targeted therapies in endometrial cancer. We review the landmark NRG-GY018 and RUBY trials, which demonstrated that checkpoint inhibitors improve outcomes in women with advanced endometrial cancer. We discuss an immunotherapy and antivascular endothelial growth factor (VEGF) combination useful in certain tumors lacking biomarker expression. We also highlight progress against endometrial cancers that overexpress human epidermal growth factor receptor 2 (HER2), demonstrated in the DESTINY PanTumor-02 trial.
PD-1 inhibitors
Programmed cell death protein 1 (PD-1) is an inhibitory receptor expressed on T cells that binds to programmed cell death ligand 1 (PD-L1). PD-L1 is expressed on many immune cells but can also be expressed on tumor cells. The interaction of PD-L1 expressed on the surface of endometrial cancer cells with the PD-1 receptor on T cells results in diminished T-cell function, eliminating the immune system’s ability to attack the tumor cells. Treatment with a PD-1 inhibitor prevents this ligand-receptor interaction and restores cancer-fighting function to T cells.
Pembrolizumab, an antibody against the PD-1 receptor, has been used as single-agent treatment for recurrent endometrial cancer since the KEYNOTE-158 study demonstrated clinical benefit in patients with mismatch repair deficient (dMMR) tumors.2
Additionally, in 2022, Makker et al. published results from a phase 3 trial3 evaluating immunotherapy in the treatment of recurrent endometrial cancer, specifically in patients with mismatch repair proficient (pMMR) tumors. They compared the combination of pembrolizumab and lenvatinib, an oral inhibitor of VEGF, to physician’s choice next-line chemotherapy in over 800 patients with advanced or recurrent endometrial cancer. They found that progression-free survival (PFS) was significantly improved from a median of 3.8 months with chemotherapy to a median of 6.6 months with pembrolizumab and lenvatinib in the pMMR population. Overall survival was also improved from 12 months to 17.4 months in the pMMR population.
With the clear benefit of immunotherapy in the recurrent setting established, Eskander and colleagues were the first to evaluate treatment with pembrolizumab as upfront treatment in the NRG-GY018 trial,4 comparing standard first-line chemotherapy (carboplatin and paclitaxel) with or without the addition of pembrolizumab. This randomized, international, phase 3 trial included over 800 patients with advanced or recurrent endometrial cancer of any histology except carcinosarcoma. Patients received carboplatin and paclitaxel with either pembrolizumab or placebo, followed by maintenance pembrolizumab or placebo. The results showed an improvement in PFS with the addition of immunotherapy, with a risk of disease progression or death with pembrolizumab 70% lower than with placebo in patients with dMMR tumors and 46% lower than with placebo in patients with pMMR tumors.
In the similar randomized, international, phase 3 RUBY trial,5 Mirza and colleagues compared standard chemotherapy with or without the addition of another PD-1 inhibitor, dostarlimab, in almost 500 patients with advanced or recurrent endometrial cancer of any histology. They found that the addition of dostarlimab to standard chemotherapy significantly improved PFS. Unpublished data presented at the Society of Gynecologic Oncology annual meeting in March also demonstrated an improvement in overall survival.6 As in NRG-GY018, they found a substantial benefit again in the dMMR population.
The results of these three landmark trials demonstrate the increasing role for immunotherapy in endometrial cancer, especially at the time of initial treatment, and how biomarkers can help direct treatment options.
Takeaway
Use of PD-1 inhibitors improves clinical outcomes in both the upfront and recurrent treatment settings. The magnitude of benefit of treatment with PD-1 inhibitors is greater in patients with dMMR tumors.
Anti-HER2 therapies
HER2 is a cell surface protein that can become overexpressed and promote tumorigenesis. It is used as a prognostic biomarker and a therapeutic target in breast, stomach, and colon cancer, but it has also been identified at high rates (20%-30%) in the most aggressive histologic subtypes in endometrial cancer (serous, clear cell, and carcinosarcoma). Trastuzumab is a monoclonal antibody directed against HER2, most commonly used in HER2-positive breast cancer. In 2018, a phase 2 trial demonstrated that trastuzumab combined with standard chemotherapy improved PFS in serous endometrial cancers that overexpress HER2.7 These results were important and promising given both the poor prognosis associated with the aggressive serous histology and the relative lack of effective therapies at the time of recurrence.
Recently, antibody-drug conjugates (ADCs) have come to the forefront of cancer-directed therapies. ADCs deliver chemotherapy agents directly to cancer cells via a monoclonal antibody that binds to a specific target on the cancer cells. Trastuzumab-deruxtecan (T-DXd) is an ADC consisting of an anti-HER2 monoclonal antibody, a topoisomerase I inhibitor payload, and a cleavable linker. T-DXd was evaluated in the tumor-agnostic phase 2 DESTINY-PanTumor02 trial,8 which included endometrial, ovarian, and cervical cancer cohorts, in addition to four other nongynecologic malignancies. In this study, 40 patients with advanced or recurrent malignancies overexpressing HER2 in each cohort were treated with T-DXd.
The results within the endometrial cancer cohort were particularly promising. The overall response rate in endometrial cancer was an astounding 57.5% with a median PFS of over 11 months. Even higher response rates were seen in endometrial cancer patients whose tumors demonstrated higher rates of HER2 overexpression. These results are unprecedented in a cohort in which most patients had seen at least 2 prior lines of therapy. Ocular and pulmonary toxicities are of particular interest with use of ADCs, but they were mostly low grade and manageable in this study.
Takeaway
Anti-HER2 therapies, including antibody-drug conjugates, are effective in treating patients with some of the highest-risk endometrial cancers when they overexpress this protein.
Dr. Haag is a gynecologic oncology fellow in the Department of Obstetrics and Gynecology, University of North Carolina Hospitals, Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill. They have no conflicts of interest.
References
1. Siegel R et al. CA Cancer J. 2024;74(1):12-49.
2. Marabelle A et al. J Clin Oncol. 2020;38(1):1-10.
3. Makker V et al. N Engl J Med. 2022;386(5):437-48.
4. Eskander RN et al. N Engl J Med. 2023;388(23):2159-70.
5. Mirza MR et al. N Engl J Med. 2023;388(23):2145-58.
6. Powell MA et al, editors. Society of Gynecologic Oncology Annual Meeting on Women’s Cancer, 2024; San Diego, CA.
7. Fader AN et al. J Clin Oncol. 2018;36(20):2044-51.
8. Meric-Bernstam F et al. J Clin Oncol. 2024;42(1):47-58.
While rates of most other cancers have declined or plateaued, the incidence and mortality rate of endometrial cancer continue to rise.1 The landscape of endometrial cancer treatment has evolved quickly over the past 2-3 years. While surgery and radiation therapy remain the mainstay of treatment for early-stage disease, the development of multiple targeted therapeutics has led to additional treatment options in advanced-stage disease and more aggressive tumor types, which are both associated with a poorer prognosis.
In this update, we highlight the recent advances in these targeted therapies in endometrial cancer. We review the landmark NRG-GY018 and RUBY trials, which demonstrated that checkpoint inhibitors improve outcomes in women with advanced endometrial cancer. We discuss an immunotherapy and antivascular endothelial growth factor (VEGF) combination useful in certain tumors lacking biomarker expression. We also highlight progress against endometrial cancers that overexpress human epidermal growth factor receptor 2 (HER2), demonstrated in the DESTINY PanTumor-02 trial.
PD-1 inhibitors
Programmed cell death protein 1 (PD-1) is an inhibitory receptor expressed on T cells that binds to programmed cell death ligand 1 (PD-L1). PD-L1 is expressed on many immune cells but can also be expressed on tumor cells. The interaction of PD-L1 expressed on the surface of endometrial cancer cells with the PD-1 receptor on T cells results in diminished T-cell function, eliminating the immune system’s ability to attack the tumor cells. Treatment with a PD-1 inhibitor prevents this ligand-receptor interaction and restores cancer-fighting function to T cells.
Pembrolizumab, an antibody against the PD-1 receptor, has been used as single-agent treatment for recurrent endometrial cancer since the KEYNOTE-158 study demonstrated clinical benefit in patients with mismatch repair deficient (dMMR) tumors.2
Additionally, in 2022, Makker et al. published results from a phase 3 trial3 evaluating immunotherapy in the treatment of recurrent endometrial cancer, specifically in patients with mismatch repair proficient (pMMR) tumors. They compared the combination of pembrolizumab and lenvatinib, an oral inhibitor of VEGF, to physician’s choice next-line chemotherapy in over 800 patients with advanced or recurrent endometrial cancer. They found that progression-free survival (PFS) was significantly improved from a median of 3.8 months with chemotherapy to a median of 6.6 months with pembrolizumab and lenvatinib in the pMMR population. Overall survival was also improved from 12 months to 17.4 months in the pMMR population.
With the clear benefit of immunotherapy in the recurrent setting established, Eskander and colleagues were the first to evaluate treatment with pembrolizumab as upfront treatment in the NRG-GY018 trial,4 comparing standard first-line chemotherapy (carboplatin and paclitaxel) with or without the addition of pembrolizumab. This randomized, international, phase 3 trial included over 800 patients with advanced or recurrent endometrial cancer of any histology except carcinosarcoma. Patients received carboplatin and paclitaxel with either pembrolizumab or placebo, followed by maintenance pembrolizumab or placebo. The results showed an improvement in PFS with the addition of immunotherapy, with a risk of disease progression or death with pembrolizumab 70% lower than with placebo in patients with dMMR tumors and 46% lower than with placebo in patients with pMMR tumors.
In the similar randomized, international, phase 3 RUBY trial,5 Mirza and colleagues compared standard chemotherapy with or without the addition of another PD-1 inhibitor, dostarlimab, in almost 500 patients with advanced or recurrent endometrial cancer of any histology. They found that the addition of dostarlimab to standard chemotherapy significantly improved PFS. Unpublished data presented at the Society of Gynecologic Oncology annual meeting in March also demonstrated an improvement in overall survival.6 As in NRG-GY018, they found a substantial benefit again in the dMMR population.
The results of these three landmark trials demonstrate the increasing role for immunotherapy in endometrial cancer, especially at the time of initial treatment, and how biomarkers can help direct treatment options.
Takeaway
Use of PD-1 inhibitors improves clinical outcomes in both the upfront and recurrent treatment settings. The magnitude of benefit of treatment with PD-1 inhibitors is greater in patients with dMMR tumors.
Anti-HER2 therapies
HER2 is a cell surface protein that can become overexpressed and promote tumorigenesis. It is used as a prognostic biomarker and a therapeutic target in breast, stomach, and colon cancer, but it has also been identified at high rates (20%-30%) in the most aggressive histologic subtypes in endometrial cancer (serous, clear cell, and carcinosarcoma). Trastuzumab is a monoclonal antibody directed against HER2, most commonly used in HER2-positive breast cancer. In 2018, a phase 2 trial demonstrated that trastuzumab combined with standard chemotherapy improved PFS in serous endometrial cancers that overexpress HER2.7 These results were important and promising given both the poor prognosis associated with the aggressive serous histology and the relative lack of effective therapies at the time of recurrence.
Recently, antibody-drug conjugates (ADCs) have come to the forefront of cancer-directed therapies. ADCs deliver chemotherapy agents directly to cancer cells via a monoclonal antibody that binds to a specific target on the cancer cells. Trastuzumab-deruxtecan (T-DXd) is an ADC consisting of an anti-HER2 monoclonal antibody, a topoisomerase I inhibitor payload, and a cleavable linker. T-DXd was evaluated in the tumor-agnostic phase 2 DESTINY-PanTumor02 trial,8 which included endometrial, ovarian, and cervical cancer cohorts, in addition to four other nongynecologic malignancies. In this study, 40 patients with advanced or recurrent malignancies overexpressing HER2 in each cohort were treated with T-DXd.
The results within the endometrial cancer cohort were particularly promising. The overall response rate in endometrial cancer was an astounding 57.5% with a median PFS of over 11 months. Even higher response rates were seen in endometrial cancer patients whose tumors demonstrated higher rates of HER2 overexpression. These results are unprecedented in a cohort in which most patients had seen at least 2 prior lines of therapy. Ocular and pulmonary toxicities are of particular interest with use of ADCs, but they were mostly low grade and manageable in this study.
Takeaway
Anti-HER2 therapies, including antibody-drug conjugates, are effective in treating patients with some of the highest-risk endometrial cancers when they overexpress this protein.
Dr. Haag is a gynecologic oncology fellow in the Department of Obstetrics and Gynecology, University of North Carolina Hospitals, Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill. They have no conflicts of interest.
References
1. Siegel R et al. CA Cancer J. 2024;74(1):12-49.
2. Marabelle A et al. J Clin Oncol. 2020;38(1):1-10.
3. Makker V et al. N Engl J Med. 2022;386(5):437-48.
4. Eskander RN et al. N Engl J Med. 2023;388(23):2159-70.
5. Mirza MR et al. N Engl J Med. 2023;388(23):2145-58.
6. Powell MA et al, editors. Society of Gynecologic Oncology Annual Meeting on Women’s Cancer, 2024; San Diego, CA.
7. Fader AN et al. J Clin Oncol. 2018;36(20):2044-51.
8. Meric-Bernstam F et al. J Clin Oncol. 2024;42(1):47-58.
Molecular Classification of Endometrial Carcinomas
Historically, endometrial cancer has been classified as type I or type II. Type I endometrial cancers are typically estrogen driven, low grade, with endometrioid histology, and have a more favorable prognosis. In contrast, type II endometrial cancers are typically high grade, have more aggressive histologies (eg, serous or clear cell), and have a poorer prognosis.1
While this system provides a helpful schema for understanding endometrial cancers, it fails to represent the immense variation of biologic behavior and outcomes in endometrial cancers and oversimplifies what has come to be understood as a complex and molecularly diverse disease.
In 2013, The Cancer Genome Atlas (TCGA) performed genomic, transcriptomic, and proteomic characterization of 373 endometrial carcinomas. They identified four categories with distinct genetic profiles corresponding to clinical outcomes: 1) DNA polymerase epsilon (POLE) mutated; 2) mismatch repair deficient; 3) copy number high/p53 abnormal; and 4) copy number low/no specific molecular profile.2 By providing both predictive and prognostic information, these molecular features may be incorporated into treatment planning decisions in the future.
The POLE-mutated subtype are endometrial cancers with recurrent mutations in the POLE gene, which is involved in DNA replication and repair. POLE mutations occur in about 5%-10% of endometrial cancers. Despite some more aggressive histopathologic findings (eg, higher grade, deeper myometrial invasion, positive lymphovascular space invasion), recurrences rarely occur, and patients with POLE mutations have the best prognosis of the four molecular subtypes, with a 5-year recurrence-free survival of 92%-100%.3
The mismatch repair–deficient (MMRd) subtype are endometrial cancers with abnormalities in any of the mismatch repair proteins (MLH1, PMS2, MSH2, MSH6). These alterations may result from hereditary or somatic mutations in any of the MMR genes or epigenetic changes in the MLH1 promoter. Germ-line mutations are associated with Lynch syndrome; thus, patients found to have a germ-line mutation in any of the MMR genes necessitate a genetics referral. The MMRd subtype accounts for about 20%-30% of endometrial cancers, and patients with MMRd tumors have an intermediate prognosis, with a 5-year recurrence-free survival of about 70%.3. These tumors are more responsive to the use of immunotherapy checkpoint inhibitors. Two recent landmark trials showed improved outcomes in patients with advanced MMRd endometrial cancer treated with immune checkpoint inhibitors in addition to standard chemotherapy.4,5
The worst prognosis belongs to the copy number high subgroup, which accounts for approximately 10% of endometrial cancers. Five-year recurrence-free survival is ~50%.3 These tumors often contain TP53 mutations and are composed of aggressive histologies, such as serous, clear cell, high-grade endometrioid, and carcinosarcomas. Recent data suggests that human epidermal growth factor receptor 2 (HER2) amplification may also be prevalent in this subgroup.6
Endometrial cancers that lack any of the above alterations fall into the no specific molecular profile (NSMP) or copy number low subgroup. Mutations in other genes, such as PTEN, PIK3CA, CTNNB1, KRAS, and ARID1A, are often present in these tumors. As the most common subtype, this heterogeneous group accounts for about 50% of all endometrial cancers. These tumors frequently comprise endometrioid histology with estrogen and progesterone receptor positivity, high rates of response to hormonal therapy, and an overall intermediate to favorable prognosis, with a 5-year recurrence-free survival of ~75%.3
The use of whole-genome sequencing in TCGA limits the clinical applicability of testing because of the cost and complex methodologies involved. Multiple algorithms have been developed in the interim that approximate TCGA subtypes using relatively less expensive and more widely available testing methods, such as immunohistochemistry and next-generation sequencing. In the ProMisE algorithm, immunohistochemistry for p53 and MMR proteins is used as a surrogate for copy number high and MMRd tumors, respectively, and targeted sequencing is used to identify POLE mutations.7
Full molecular classification of endometrial tumors provides important prognostic information and allows for incorporation into treatment planning. To this end, the new 2023 International Federation of Gynecology and Obstetrics (FIGO) endometrial cancer staging incorporates an option for the addition of molecular subtype, with the stance that it allows for better prognostic prediction.8 While complete molecular classification is not required, it is encouraged. Furthermore, several clinical trials are currently investigating different treatment regimens based on these distinct molecular profiles.
Dr. Haag is a gynecologic oncology fellow in the Department of Obstetrics and Gynecology, University of North Carolina Hospitals, Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill. They have no conflicts of interest.
References
1. Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecologic Oncology. 1983;15(1):10-17.
2. Kandoth C et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67-73.
3. León-Castillo A et al. Molecular classification of the PORTEC-3 trial for high-risk endometrial cancer: Impact on prognosis and benefit from adjuvant therapy. J Clin Oncology. 2020;38(29):3388-3397.
4. Mirza MR et al. Dostarlimab for primary advanced or recurrent endometrial cancer. N Engl J Med. 2023;388(23):2145-2158.
5. Eskander RN et al. Pembrolizumab plus chemotherapy in advanced endometrial cancer. N Engl J Med. 2023;388(23):2159-2170.
6. Talia KL et al. The role of HER2 as a therapeutic biomarker in gynaecological malignancy: Potential for use beyond uterine serous carcinoma. Pathology. 2023;55(1):8-18.
7. Kommoss S et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Annals Oncology. 2018;29(5):1180-1188.
8. Berek JS et al. FIGO staging of endometrial cancer: 2023. Int J Gynaecol Obstet. 2023;162(2):383-394.
Historically, endometrial cancer has been classified as type I or type II. Type I endometrial cancers are typically estrogen driven, low grade, with endometrioid histology, and have a more favorable prognosis. In contrast, type II endometrial cancers are typically high grade, have more aggressive histologies (eg, serous or clear cell), and have a poorer prognosis.1
While this system provides a helpful schema for understanding endometrial cancers, it fails to represent the immense variation of biologic behavior and outcomes in endometrial cancers and oversimplifies what has come to be understood as a complex and molecularly diverse disease.
In 2013, The Cancer Genome Atlas (TCGA) performed genomic, transcriptomic, and proteomic characterization of 373 endometrial carcinomas. They identified four categories with distinct genetic profiles corresponding to clinical outcomes: 1) DNA polymerase epsilon (POLE) mutated; 2) mismatch repair deficient; 3) copy number high/p53 abnormal; and 4) copy number low/no specific molecular profile.2 By providing both predictive and prognostic information, these molecular features may be incorporated into treatment planning decisions in the future.
The POLE-mutated subtype are endometrial cancers with recurrent mutations in the POLE gene, which is involved in DNA replication and repair. POLE mutations occur in about 5%-10% of endometrial cancers. Despite some more aggressive histopathologic findings (eg, higher grade, deeper myometrial invasion, positive lymphovascular space invasion), recurrences rarely occur, and patients with POLE mutations have the best prognosis of the four molecular subtypes, with a 5-year recurrence-free survival of 92%-100%.3
The mismatch repair–deficient (MMRd) subtype are endometrial cancers with abnormalities in any of the mismatch repair proteins (MLH1, PMS2, MSH2, MSH6). These alterations may result from hereditary or somatic mutations in any of the MMR genes or epigenetic changes in the MLH1 promoter. Germ-line mutations are associated with Lynch syndrome; thus, patients found to have a germ-line mutation in any of the MMR genes necessitate a genetics referral. The MMRd subtype accounts for about 20%-30% of endometrial cancers, and patients with MMRd tumors have an intermediate prognosis, with a 5-year recurrence-free survival of about 70%.3. These tumors are more responsive to the use of immunotherapy checkpoint inhibitors. Two recent landmark trials showed improved outcomes in patients with advanced MMRd endometrial cancer treated with immune checkpoint inhibitors in addition to standard chemotherapy.4,5
The worst prognosis belongs to the copy number high subgroup, which accounts for approximately 10% of endometrial cancers. Five-year recurrence-free survival is ~50%.3 These tumors often contain TP53 mutations and are composed of aggressive histologies, such as serous, clear cell, high-grade endometrioid, and carcinosarcomas. Recent data suggests that human epidermal growth factor receptor 2 (HER2) amplification may also be prevalent in this subgroup.6
Endometrial cancers that lack any of the above alterations fall into the no specific molecular profile (NSMP) or copy number low subgroup. Mutations in other genes, such as PTEN, PIK3CA, CTNNB1, KRAS, and ARID1A, are often present in these tumors. As the most common subtype, this heterogeneous group accounts for about 50% of all endometrial cancers. These tumors frequently comprise endometrioid histology with estrogen and progesterone receptor positivity, high rates of response to hormonal therapy, and an overall intermediate to favorable prognosis, with a 5-year recurrence-free survival of ~75%.3
The use of whole-genome sequencing in TCGA limits the clinical applicability of testing because of the cost and complex methodologies involved. Multiple algorithms have been developed in the interim that approximate TCGA subtypes using relatively less expensive and more widely available testing methods, such as immunohistochemistry and next-generation sequencing. In the ProMisE algorithm, immunohistochemistry for p53 and MMR proteins is used as a surrogate for copy number high and MMRd tumors, respectively, and targeted sequencing is used to identify POLE mutations.7
Full molecular classification of endometrial tumors provides important prognostic information and allows for incorporation into treatment planning. To this end, the new 2023 International Federation of Gynecology and Obstetrics (FIGO) endometrial cancer staging incorporates an option for the addition of molecular subtype, with the stance that it allows for better prognostic prediction.8 While complete molecular classification is not required, it is encouraged. Furthermore, several clinical trials are currently investigating different treatment regimens based on these distinct molecular profiles.
Dr. Haag is a gynecologic oncology fellow in the Department of Obstetrics and Gynecology, University of North Carolina Hospitals, Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill. They have no conflicts of interest.
References
1. Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecologic Oncology. 1983;15(1):10-17.
2. Kandoth C et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67-73.
3. León-Castillo A et al. Molecular classification of the PORTEC-3 trial for high-risk endometrial cancer: Impact on prognosis and benefit from adjuvant therapy. J Clin Oncology. 2020;38(29):3388-3397.
4. Mirza MR et al. Dostarlimab for primary advanced or recurrent endometrial cancer. N Engl J Med. 2023;388(23):2145-2158.
5. Eskander RN et al. Pembrolizumab plus chemotherapy in advanced endometrial cancer. N Engl J Med. 2023;388(23):2159-2170.
6. Talia KL et al. The role of HER2 as a therapeutic biomarker in gynaecological malignancy: Potential for use beyond uterine serous carcinoma. Pathology. 2023;55(1):8-18.
7. Kommoss S et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Annals Oncology. 2018;29(5):1180-1188.
8. Berek JS et al. FIGO staging of endometrial cancer: 2023. Int J Gynaecol Obstet. 2023;162(2):383-394.
Historically, endometrial cancer has been classified as type I or type II. Type I endometrial cancers are typically estrogen driven, low grade, with endometrioid histology, and have a more favorable prognosis. In contrast, type II endometrial cancers are typically high grade, have more aggressive histologies (eg, serous or clear cell), and have a poorer prognosis.1
While this system provides a helpful schema for understanding endometrial cancers, it fails to represent the immense variation of biologic behavior and outcomes in endometrial cancers and oversimplifies what has come to be understood as a complex and molecularly diverse disease.
In 2013, The Cancer Genome Atlas (TCGA) performed genomic, transcriptomic, and proteomic characterization of 373 endometrial carcinomas. They identified four categories with distinct genetic profiles corresponding to clinical outcomes: 1) DNA polymerase epsilon (POLE) mutated; 2) mismatch repair deficient; 3) copy number high/p53 abnormal; and 4) copy number low/no specific molecular profile.2 By providing both predictive and prognostic information, these molecular features may be incorporated into treatment planning decisions in the future.
The POLE-mutated subtype are endometrial cancers with recurrent mutations in the POLE gene, which is involved in DNA replication and repair. POLE mutations occur in about 5%-10% of endometrial cancers. Despite some more aggressive histopathologic findings (eg, higher grade, deeper myometrial invasion, positive lymphovascular space invasion), recurrences rarely occur, and patients with POLE mutations have the best prognosis of the four molecular subtypes, with a 5-year recurrence-free survival of 92%-100%.3
The mismatch repair–deficient (MMRd) subtype are endometrial cancers with abnormalities in any of the mismatch repair proteins (MLH1, PMS2, MSH2, MSH6). These alterations may result from hereditary or somatic mutations in any of the MMR genes or epigenetic changes in the MLH1 promoter. Germ-line mutations are associated with Lynch syndrome; thus, patients found to have a germ-line mutation in any of the MMR genes necessitate a genetics referral. The MMRd subtype accounts for about 20%-30% of endometrial cancers, and patients with MMRd tumors have an intermediate prognosis, with a 5-year recurrence-free survival of about 70%.3. These tumors are more responsive to the use of immunotherapy checkpoint inhibitors. Two recent landmark trials showed improved outcomes in patients with advanced MMRd endometrial cancer treated with immune checkpoint inhibitors in addition to standard chemotherapy.4,5
The worst prognosis belongs to the copy number high subgroup, which accounts for approximately 10% of endometrial cancers. Five-year recurrence-free survival is ~50%.3 These tumors often contain TP53 mutations and are composed of aggressive histologies, such as serous, clear cell, high-grade endometrioid, and carcinosarcomas. Recent data suggests that human epidermal growth factor receptor 2 (HER2) amplification may also be prevalent in this subgroup.6
Endometrial cancers that lack any of the above alterations fall into the no specific molecular profile (NSMP) or copy number low subgroup. Mutations in other genes, such as PTEN, PIK3CA, CTNNB1, KRAS, and ARID1A, are often present in these tumors. As the most common subtype, this heterogeneous group accounts for about 50% of all endometrial cancers. These tumors frequently comprise endometrioid histology with estrogen and progesterone receptor positivity, high rates of response to hormonal therapy, and an overall intermediate to favorable prognosis, with a 5-year recurrence-free survival of ~75%.3
The use of whole-genome sequencing in TCGA limits the clinical applicability of testing because of the cost and complex methodologies involved. Multiple algorithms have been developed in the interim that approximate TCGA subtypes using relatively less expensive and more widely available testing methods, such as immunohistochemistry and next-generation sequencing. In the ProMisE algorithm, immunohistochemistry for p53 and MMR proteins is used as a surrogate for copy number high and MMRd tumors, respectively, and targeted sequencing is used to identify POLE mutations.7
Full molecular classification of endometrial tumors provides important prognostic information and allows for incorporation into treatment planning. To this end, the new 2023 International Federation of Gynecology and Obstetrics (FIGO) endometrial cancer staging incorporates an option for the addition of molecular subtype, with the stance that it allows for better prognostic prediction.8 While complete molecular classification is not required, it is encouraged. Furthermore, several clinical trials are currently investigating different treatment regimens based on these distinct molecular profiles.
Dr. Haag is a gynecologic oncology fellow in the Department of Obstetrics and Gynecology, University of North Carolina Hospitals, Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill. They have no conflicts of interest.
References
1. Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecologic Oncology. 1983;15(1):10-17.
2. Kandoth C et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67-73.
3. León-Castillo A et al. Molecular classification of the PORTEC-3 trial for high-risk endometrial cancer: Impact on prognosis and benefit from adjuvant therapy. J Clin Oncology. 2020;38(29):3388-3397.
4. Mirza MR et al. Dostarlimab for primary advanced or recurrent endometrial cancer. N Engl J Med. 2023;388(23):2145-2158.
5. Eskander RN et al. Pembrolizumab plus chemotherapy in advanced endometrial cancer. N Engl J Med. 2023;388(23):2159-2170.
6. Talia KL et al. The role of HER2 as a therapeutic biomarker in gynaecological malignancy: Potential for use beyond uterine serous carcinoma. Pathology. 2023;55(1):8-18.
7. Kommoss S et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Annals Oncology. 2018;29(5):1180-1188.
8. Berek JS et al. FIGO staging of endometrial cancer: 2023. Int J Gynaecol Obstet. 2023;162(2):383-394.
Surgical management of borderline ovarian tumors, part 1
Borderline ovarian tumors (BOTs) are estimated to comprise 10%-15% of all epithelial tumors of the ovary. They are characterized by their behavior, which falls somewhere between benign ovarian masses and frank carcinomas. They have cytologic characteristics suggesting malignancy, such as higher cellular proliferation and more variable nuclear atypia, but, unlike carcinomas, they lack destructive stromal invasion. For decades after their recognition by the International Federation of Gynecology and Obstetrics in 1971, these tumors were classified as being of low malignant potential (and subsequently referred to as LMP tumors of the ovary). Beginning with the 2014 World Health Organization classification, the recommended terminology is now borderline tumor of the ovary.
The primary treatment for BOTs is surgery. With a mean age at diagnosis in the fifth decade, many patients with BOTs desire ovarian preservation to maintain fertility and/or prevent surgical menopause. This raises multiple questions regarding the use of fertility-sparing surgery for BOTs: What types of procedures are safe and should be offered? For those patients who undergo fertility-sparing surgery initially, is additional surgery indicated after completion of childbearing or at an age closer to natural menopause? What should this completion surgery include?
Ovarian-sparing surgery
The diagnosis of a BOT is frequently only confirmed after the decision for ovarian conservation has been made. What should be considered before electing to proceed with ovarian cystectomy instead of unilateral salpingo-oophorectomy (USO)?
Is the risk of recurrence higher with cystectomy versus oophorectomy?
Yes. The risk of recurrence of BOT appears to be higher after cystectomy than it is after oophorectomy. There is a large range reported in the literature, with the risk of recurrence after cystectomy described as between 12% and 58%. Most studies report recurrences between 25% and 35% of patients who undergo cystectomy. In contrast, the risk of recurrence after USO is often reported to be approximately 10%. Higher risk of recurrence after cystectomy is speculated to be due to leaving some BOT at the time of initial surgery.
Multiple meta-analyses have found an increased risk of recurrence after cystectomy. The risk of recurrence after unilateral cystectomy was 19.4%, compared with 9.1% after USO, in 2,145 patients included in a 2017 meta-analysis.1 Similarly, a 2021 meta-analysis found a significantly higher rate of BOT recurrence in patients who underwent unilateral or bilateral cystectomy compared with USO (odds ratio, 2.02; 95% confidence interval, 1.59-2.57).2
Does the higher recurrence risk translate into a difference in long-term outcomes?
No. Despite an increased risk of recurrence after cystectomy, ovarian-sparing surgery does not appear to alter patients’ survival. The pooled mortality estimate was 1.6% for those undergoing fertility-sparing surgery (95% CI, 0.011-0.023), compared with 2.0% for those undergoing radical surgery (95% CI, 0.014-0.029), in a 2015 meta-analysis of over 5,100 patients. The analysis included studies in which patients underwent unilateral cystectomy, bilateral cystectomy, USO, or USO plus contralateral cystectomy. The low mortality rate did not allow for comparison between the different types of fertility-sparing surgeries.3
Do we accept a higher risk of recurrence with ovarian sparing surgery to improve fertility?
Data are mixed. When we examine studies describing fertility rates after conservative surgery, there are significant limitations to interpreting the data available. Some studies do not differentiate among patients who underwent fertility-sparing surgery, or between those who had cystectomy versus USO. Other studies do not report the number of patients who tried to achieve pregnancy after surgery. Conception rates are reported to be as high as 88.2%, which was in 116 patients who were able to be reached after fertility-sparing surgery (retained at least one ovary). Of the 51 patients who tried to conceive, 45 were successful.4
Multiple studies and meta-analyses have shown no difference in postoperative pregnancy rates when comparing oophorectomy to cystectomy. For instance, in a 2021 meta-analysis, there was no significant difference noted in pregnancy rates between patients who underwent USO versus cystectomy (OR, 0.92; 95% CI, 0.60-1.42).
There are some data that support improved postoperative pregnancy rates in more conservative surgery, especially in the setting of bilateral BOT. In a small study of 32 patients who had laparoscopic staging for bilateral BOTs, patients were randomized to unilateral oophorectomy plus contralateral cystectomy or to bilateral cystectomy, which was referred to as ultraconservative surgery. The time to first recurrence was shorter in the ultraconservative group (although this lost significance when regression analysis was performed), but the time to first live birth was shorter and the relative chance of having a baby was higher in the bilateral cystectomy group.5
Ovarian-sparing procedures should be offered to patients in the setting of BOT. With ovarian-sparing surgery, it is important to counsel patients about the increased risk of recurrence and need for long-term follow-up. Pregnancy rates are generally good after fertility-sparing surgery. Surgery to conserve both ovaries does not seem to improve pregnancy rates in the setting of unilateral BOTs.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Jiao X et al. Int J Gynecol Cancer. 2017 Nov;27(9):1833-41.
2. Wang P and Fang L. World J Surg Oncol. 2021 Apr 21;19(1):132.
3. Vasconcelos I and de Sousa Mendes M. Eur J Cancer. 2015 Mar;51(5):620-31.
4. Song T et al. Int J Gynecol Cancer. 2011 May;21(4):640-6.
5. Palomba S et al. Hum Reprod. 2010 Aug;25(8):1966-72.
Borderline ovarian tumors (BOTs) are estimated to comprise 10%-15% of all epithelial tumors of the ovary. They are characterized by their behavior, which falls somewhere between benign ovarian masses and frank carcinomas. They have cytologic characteristics suggesting malignancy, such as higher cellular proliferation and more variable nuclear atypia, but, unlike carcinomas, they lack destructive stromal invasion. For decades after their recognition by the International Federation of Gynecology and Obstetrics in 1971, these tumors were classified as being of low malignant potential (and subsequently referred to as LMP tumors of the ovary). Beginning with the 2014 World Health Organization classification, the recommended terminology is now borderline tumor of the ovary.
The primary treatment for BOTs is surgery. With a mean age at diagnosis in the fifth decade, many patients with BOTs desire ovarian preservation to maintain fertility and/or prevent surgical menopause. This raises multiple questions regarding the use of fertility-sparing surgery for BOTs: What types of procedures are safe and should be offered? For those patients who undergo fertility-sparing surgery initially, is additional surgery indicated after completion of childbearing or at an age closer to natural menopause? What should this completion surgery include?
Ovarian-sparing surgery
The diagnosis of a BOT is frequently only confirmed after the decision for ovarian conservation has been made. What should be considered before electing to proceed with ovarian cystectomy instead of unilateral salpingo-oophorectomy (USO)?
Is the risk of recurrence higher with cystectomy versus oophorectomy?
Yes. The risk of recurrence of BOT appears to be higher after cystectomy than it is after oophorectomy. There is a large range reported in the literature, with the risk of recurrence after cystectomy described as between 12% and 58%. Most studies report recurrences between 25% and 35% of patients who undergo cystectomy. In contrast, the risk of recurrence after USO is often reported to be approximately 10%. Higher risk of recurrence after cystectomy is speculated to be due to leaving some BOT at the time of initial surgery.
Multiple meta-analyses have found an increased risk of recurrence after cystectomy. The risk of recurrence after unilateral cystectomy was 19.4%, compared with 9.1% after USO, in 2,145 patients included in a 2017 meta-analysis.1 Similarly, a 2021 meta-analysis found a significantly higher rate of BOT recurrence in patients who underwent unilateral or bilateral cystectomy compared with USO (odds ratio, 2.02; 95% confidence interval, 1.59-2.57).2
Does the higher recurrence risk translate into a difference in long-term outcomes?
No. Despite an increased risk of recurrence after cystectomy, ovarian-sparing surgery does not appear to alter patients’ survival. The pooled mortality estimate was 1.6% for those undergoing fertility-sparing surgery (95% CI, 0.011-0.023), compared with 2.0% for those undergoing radical surgery (95% CI, 0.014-0.029), in a 2015 meta-analysis of over 5,100 patients. The analysis included studies in which patients underwent unilateral cystectomy, bilateral cystectomy, USO, or USO plus contralateral cystectomy. The low mortality rate did not allow for comparison between the different types of fertility-sparing surgeries.3
Do we accept a higher risk of recurrence with ovarian sparing surgery to improve fertility?
Data are mixed. When we examine studies describing fertility rates after conservative surgery, there are significant limitations to interpreting the data available. Some studies do not differentiate among patients who underwent fertility-sparing surgery, or between those who had cystectomy versus USO. Other studies do not report the number of patients who tried to achieve pregnancy after surgery. Conception rates are reported to be as high as 88.2%, which was in 116 patients who were able to be reached after fertility-sparing surgery (retained at least one ovary). Of the 51 patients who tried to conceive, 45 were successful.4
Multiple studies and meta-analyses have shown no difference in postoperative pregnancy rates when comparing oophorectomy to cystectomy. For instance, in a 2021 meta-analysis, there was no significant difference noted in pregnancy rates between patients who underwent USO versus cystectomy (OR, 0.92; 95% CI, 0.60-1.42).
There are some data that support improved postoperative pregnancy rates in more conservative surgery, especially in the setting of bilateral BOT. In a small study of 32 patients who had laparoscopic staging for bilateral BOTs, patients were randomized to unilateral oophorectomy plus contralateral cystectomy or to bilateral cystectomy, which was referred to as ultraconservative surgery. The time to first recurrence was shorter in the ultraconservative group (although this lost significance when regression analysis was performed), but the time to first live birth was shorter and the relative chance of having a baby was higher in the bilateral cystectomy group.5
Ovarian-sparing procedures should be offered to patients in the setting of BOT. With ovarian-sparing surgery, it is important to counsel patients about the increased risk of recurrence and need for long-term follow-up. Pregnancy rates are generally good after fertility-sparing surgery. Surgery to conserve both ovaries does not seem to improve pregnancy rates in the setting of unilateral BOTs.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Jiao X et al. Int J Gynecol Cancer. 2017 Nov;27(9):1833-41.
2. Wang P and Fang L. World J Surg Oncol. 2021 Apr 21;19(1):132.
3. Vasconcelos I and de Sousa Mendes M. Eur J Cancer. 2015 Mar;51(5):620-31.
4. Song T et al. Int J Gynecol Cancer. 2011 May;21(4):640-6.
5. Palomba S et al. Hum Reprod. 2010 Aug;25(8):1966-72.
Borderline ovarian tumors (BOTs) are estimated to comprise 10%-15% of all epithelial tumors of the ovary. They are characterized by their behavior, which falls somewhere between benign ovarian masses and frank carcinomas. They have cytologic characteristics suggesting malignancy, such as higher cellular proliferation and more variable nuclear atypia, but, unlike carcinomas, they lack destructive stromal invasion. For decades after their recognition by the International Federation of Gynecology and Obstetrics in 1971, these tumors were classified as being of low malignant potential (and subsequently referred to as LMP tumors of the ovary). Beginning with the 2014 World Health Organization classification, the recommended terminology is now borderline tumor of the ovary.
The primary treatment for BOTs is surgery. With a mean age at diagnosis in the fifth decade, many patients with BOTs desire ovarian preservation to maintain fertility and/or prevent surgical menopause. This raises multiple questions regarding the use of fertility-sparing surgery for BOTs: What types of procedures are safe and should be offered? For those patients who undergo fertility-sparing surgery initially, is additional surgery indicated after completion of childbearing or at an age closer to natural menopause? What should this completion surgery include?
Ovarian-sparing surgery
The diagnosis of a BOT is frequently only confirmed after the decision for ovarian conservation has been made. What should be considered before electing to proceed with ovarian cystectomy instead of unilateral salpingo-oophorectomy (USO)?
Is the risk of recurrence higher with cystectomy versus oophorectomy?
Yes. The risk of recurrence of BOT appears to be higher after cystectomy than it is after oophorectomy. There is a large range reported in the literature, with the risk of recurrence after cystectomy described as between 12% and 58%. Most studies report recurrences between 25% and 35% of patients who undergo cystectomy. In contrast, the risk of recurrence after USO is often reported to be approximately 10%. Higher risk of recurrence after cystectomy is speculated to be due to leaving some BOT at the time of initial surgery.
Multiple meta-analyses have found an increased risk of recurrence after cystectomy. The risk of recurrence after unilateral cystectomy was 19.4%, compared with 9.1% after USO, in 2,145 patients included in a 2017 meta-analysis.1 Similarly, a 2021 meta-analysis found a significantly higher rate of BOT recurrence in patients who underwent unilateral or bilateral cystectomy compared with USO (odds ratio, 2.02; 95% confidence interval, 1.59-2.57).2
Does the higher recurrence risk translate into a difference in long-term outcomes?
No. Despite an increased risk of recurrence after cystectomy, ovarian-sparing surgery does not appear to alter patients’ survival. The pooled mortality estimate was 1.6% for those undergoing fertility-sparing surgery (95% CI, 0.011-0.023), compared with 2.0% for those undergoing radical surgery (95% CI, 0.014-0.029), in a 2015 meta-analysis of over 5,100 patients. The analysis included studies in which patients underwent unilateral cystectomy, bilateral cystectomy, USO, or USO plus contralateral cystectomy. The low mortality rate did not allow for comparison between the different types of fertility-sparing surgeries.3
Do we accept a higher risk of recurrence with ovarian sparing surgery to improve fertility?
Data are mixed. When we examine studies describing fertility rates after conservative surgery, there are significant limitations to interpreting the data available. Some studies do not differentiate among patients who underwent fertility-sparing surgery, or between those who had cystectomy versus USO. Other studies do not report the number of patients who tried to achieve pregnancy after surgery. Conception rates are reported to be as high as 88.2%, which was in 116 patients who were able to be reached after fertility-sparing surgery (retained at least one ovary). Of the 51 patients who tried to conceive, 45 were successful.4
Multiple studies and meta-analyses have shown no difference in postoperative pregnancy rates when comparing oophorectomy to cystectomy. For instance, in a 2021 meta-analysis, there was no significant difference noted in pregnancy rates between patients who underwent USO versus cystectomy (OR, 0.92; 95% CI, 0.60-1.42).
There are some data that support improved postoperative pregnancy rates in more conservative surgery, especially in the setting of bilateral BOT. In a small study of 32 patients who had laparoscopic staging for bilateral BOTs, patients were randomized to unilateral oophorectomy plus contralateral cystectomy or to bilateral cystectomy, which was referred to as ultraconservative surgery. The time to first recurrence was shorter in the ultraconservative group (although this lost significance when regression analysis was performed), but the time to first live birth was shorter and the relative chance of having a baby was higher in the bilateral cystectomy group.5
Ovarian-sparing procedures should be offered to patients in the setting of BOT. With ovarian-sparing surgery, it is important to counsel patients about the increased risk of recurrence and need for long-term follow-up. Pregnancy rates are generally good after fertility-sparing surgery. Surgery to conserve both ovaries does not seem to improve pregnancy rates in the setting of unilateral BOTs.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Jiao X et al. Int J Gynecol Cancer. 2017 Nov;27(9):1833-41.
2. Wang P and Fang L. World J Surg Oncol. 2021 Apr 21;19(1):132.
3. Vasconcelos I and de Sousa Mendes M. Eur J Cancer. 2015 Mar;51(5):620-31.
4. Song T et al. Int J Gynecol Cancer. 2011 May;21(4):640-6.
5. Palomba S et al. Hum Reprod. 2010 Aug;25(8):1966-72.
Update on secondary cytoreduction in recurrent ovarian cancer
Recurrent ovarian cancer is difficult to treat; it has high recurrence rates and poor targeted treatment options. Between 60% and 75% of patients initially diagnosed with advanced-stage ovarian cancer will relapse within 2-3 years.1 Survival for these patients is poor, with an average overall survival (OS) of 30-40 months from the time of recurrence.2 Historically, immunotherapy has shown poor efficacy for recurrent ovarian malignancy, leaving few options for patients and their providers. Given the lack of effective treatment options, secondary cytoreductive surgery (surgery at the time of recurrence) has been heavily studied as a potential therapeutic option.
The initial rationale for cytoreductive surgery (CRS) in patients with advanced ovarian cancer focused on palliation of symptoms from large, bulky disease that frequently caused obstructive symptoms and pain. Now, cytoreduction is a critical part of therapy. It decreases chemotherapy-resistant tumor cells, improves the immune response, and is thought to optimize perfusion of the residual cancer for systemic therapy. The survival benefit of surgery in the frontline setting, either with primary or interval debulking, is well established, and much of the data now demonstrate that complete resection of all macroscopic disease (also known as an R0 resection) has the greatest survival benefit.3 Given the benefits of an initial debulking surgery, secondary cytoreduction has been studied since the 1980s with mixed results. These data have demonstrated that the largest barrier to care has been appropriate patient selection for this often complex surgical procedure.
The 2020 National Comprehensive Cancer Network guidelines list secondary CRS as a treatment option; however, the procedure should only be considered in patients who have platinum sensitive disease, a performance status of 0-1, no ascites, and an isolated focus or limited focus of disease that is amenable to complete resection. Numerous retrospective studies have suggested that secondary CRS is beneficial to patients with recurrent ovarian cancer, especially if complete cytoreduction can be accomplished. Many of these studies have similarly concluded that there are benefits, such as less ascites at the time of recurrence, smaller disease burden, and a longer disease-free interval. From that foundation, multiple groups used retrospective data to investigate prognostic models to determine who would benefit most from secondary cytoreduction.
The DESKTOP Group initially published their retrospective study in 2006 and created a scoring system assessing who would benefit from secondary CRS.4 Data demonstrated that a performance status of 0, FIGO stage of I/II at the time of initial diagnosis, no residual tumor after primary surgery, and ascites less than 500 mL were associated with improved survival after secondary cytoreduction. They created the AGO score out of these data, which is positive only if three criteria are met: a performance status of 0, R0 after primary debulk, and ascites less than 500 mL at the time of recurrence.
They prospectively tested this score in DESKTOP II, which validated their findings and showed that complete secondary CRS could be achieved in 76% of those with a positive AGO score.5 Many believed that the AGO score was too restrictive, and a second retrospective study performed by a group at Memorial Sloan Kettering showed that optimal secondary cytoreduction could be achieved to prolong survival by a median of 30 months in patients with a longer disease-free interval, a single site of recurrence, and residual disease measuring less than 5 mm at time of initial/first-line surgery.6 Many individuals now use this scoring system to determine candidacy for secondary debulking: disease-free interval, number of sites of recurrence (ideally oligometastatic disease), and residual disease less than 5 mm at the time of primary debulking.
Finally, the iMODEL was developed by a group from China and found that complete R0 secondary CRS was associated with a low initial FIGO stage, no residual disease after primary surgery, longer platinum-free interval, better Eastern Cooperative Oncology Group performance status, lower CA-125 levels, as well as no ascites at the time of recurrence. Based on these criteria, individuals received either high or low iMODEL scores, and those with a low score were said to be candidates for secondary CRS. Overall, these models demonstrate that the strongest predictive factor that suggests a survival benefit from secondary CRS is the ability to achieve a complete R0 resection at the time of surgery.
Secondary debulking surgery has been tested in three large randomized controlled trials. The DESKTOP investigators and the SOC-1 trial have been the most successful groups to publish on this topic with positive results. Both groups use prognostic models for their inclusion criteria to select candidates in whom an R0 resection is believed to be most feasible. The first randomized controlled trial to publish on this topic was GOG-213,7 which did not use prognostic modeling for their inclusion criteria. Patients were randomized to secondary cytoreduction followed by platinum-based chemotherapy with or without bevacizumab versus chemotherapy alone. The median OS was 50.6 months in the surgery group and 64.7 months in the no-surgery group (P = .08), suggesting no survival benefit to secondary cytoreduction; however, an ad hoc exploratory analysis of the surgery arm showed that both overall and progression-free survival were significantly improved in the complete cytoreduction group, compared with those with residual disease at time of surgery.
The results from the GOG-213 group suggested that improved survival from secondary debulking might be achieved when prognostic modeling is used to select optimal surgical candidates. The SOC-1 trial, published in 2021, was a phase 3, randomized, controlled trial that used the iMODEL scoring system combined with PET/CT imaging for patient selection.8 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Complete cytoreduction was achieved in 73% of patients with a low iMODEL score, and these data showed improved OS in the surgery group of 58.1 months versus 53.9 months (P < .05) in the no-surgery group. Lastly, the DESKTOP group most recently published results on this topic in a large randomized, controlled trial.9 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Inclusion criteria were only met in patients with a positive AGO score. An improved OS of 7.7 months (53.7 vs. 46 months; P < .05) was demonstrated in patients that underwent surgery versus those exposed to only chemotherapy. Again, this group showed that overall survival was further improved when complete cytoreduction was achieved.
Given the results of these three trials, the Society for Gynecologic Oncology has released a statement on secondary cytoreduction in recurrent ovarian cancer (see Table).10 While it is important to use caution when comparing the three studies as study populations differed substantially, the most important takeaway the difference in survival outcomes in patients in whom complete gross resection was achieved versus no complete gross resection versus no surgery. This comparison highlights the benefit of complete cytoreduction as well as the potential harms of secondary debulking when an R0 resection cannot be achieved. Although not yet evaluated in this clinical setting, laparoscopic exploration may be useful to augment assessment of disease extent and possibility of disease resection, just as it is in frontline ovarian cancer surgery.
The importance of bevacizumab use in recurrent ovarian cancer is also highlighted in the SGO statement. In GOG-213, 84% of the total study population (in both the surgery and no surgery cohort) were treated with concurrent followed by maintenance bevacizumab with an improved survival outcome, which may suggest that this trial generalizes better than the others to contemporary management of platinum-sensitive recurrent ovarian cancer.
Overall, given the mixed data, the recommendation is for surgeons to consider all available data to guide them in treatment planning with a strong emphasis on using all available technology to assess whether complete cytoreduction can be achieved in the setting of recurrence so as to not delay the patient’s ability to receive chemotherapy.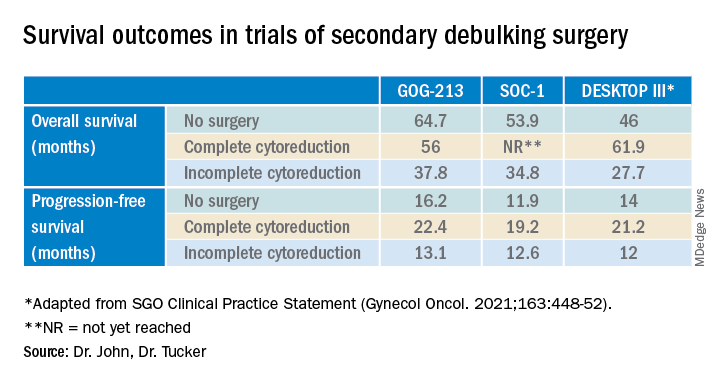
Dr. John is a gynecologic oncology fellow at the University of North Carolina at Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the university.
References
1. du Bois A et al. J Natl Cancer Inst. 2003;95:1320-9.
2. Wagner U et al. Br J Cancer. 2012;107:588-91.
3. Vergote I et al. N Engl J Med. 2010;363:943-53.
4. Harter P et al. Ann Surg Oncol. 2006;13:1702-10.
5. Harter P et al. Int J Gynecol Cancer. 2011;21:289-95.
6. Chi DS et al. Cancer. 2006 106:1933-9.
7. Coleman RL et al. Lancet Oncol. 2017;18:779-1.
8. Shi T et al. Lancet Oncol. 2021;22:439-49.
9. Harter P et al. N Engl J Med 2021;385:2123-31.
10. Harrison R, et al. Gynecol Oncol. 2021;163:448-52.
Recurrent ovarian cancer is difficult to treat; it has high recurrence rates and poor targeted treatment options. Between 60% and 75% of patients initially diagnosed with advanced-stage ovarian cancer will relapse within 2-3 years.1 Survival for these patients is poor, with an average overall survival (OS) of 30-40 months from the time of recurrence.2 Historically, immunotherapy has shown poor efficacy for recurrent ovarian malignancy, leaving few options for patients and their providers. Given the lack of effective treatment options, secondary cytoreductive surgery (surgery at the time of recurrence) has been heavily studied as a potential therapeutic option.
The initial rationale for cytoreductive surgery (CRS) in patients with advanced ovarian cancer focused on palliation of symptoms from large, bulky disease that frequently caused obstructive symptoms and pain. Now, cytoreduction is a critical part of therapy. It decreases chemotherapy-resistant tumor cells, improves the immune response, and is thought to optimize perfusion of the residual cancer for systemic therapy. The survival benefit of surgery in the frontline setting, either with primary or interval debulking, is well established, and much of the data now demonstrate that complete resection of all macroscopic disease (also known as an R0 resection) has the greatest survival benefit.3 Given the benefits of an initial debulking surgery, secondary cytoreduction has been studied since the 1980s with mixed results. These data have demonstrated that the largest barrier to care has been appropriate patient selection for this often complex surgical procedure.
The 2020 National Comprehensive Cancer Network guidelines list secondary CRS as a treatment option; however, the procedure should only be considered in patients who have platinum sensitive disease, a performance status of 0-1, no ascites, and an isolated focus or limited focus of disease that is amenable to complete resection. Numerous retrospective studies have suggested that secondary CRS is beneficial to patients with recurrent ovarian cancer, especially if complete cytoreduction can be accomplished. Many of these studies have similarly concluded that there are benefits, such as less ascites at the time of recurrence, smaller disease burden, and a longer disease-free interval. From that foundation, multiple groups used retrospective data to investigate prognostic models to determine who would benefit most from secondary cytoreduction.
The DESKTOP Group initially published their retrospective study in 2006 and created a scoring system assessing who would benefit from secondary CRS.4 Data demonstrated that a performance status of 0, FIGO stage of I/II at the time of initial diagnosis, no residual tumor after primary surgery, and ascites less than 500 mL were associated with improved survival after secondary cytoreduction. They created the AGO score out of these data, which is positive only if three criteria are met: a performance status of 0, R0 after primary debulk, and ascites less than 500 mL at the time of recurrence.
They prospectively tested this score in DESKTOP II, which validated their findings and showed that complete secondary CRS could be achieved in 76% of those with a positive AGO score.5 Many believed that the AGO score was too restrictive, and a second retrospective study performed by a group at Memorial Sloan Kettering showed that optimal secondary cytoreduction could be achieved to prolong survival by a median of 30 months in patients with a longer disease-free interval, a single site of recurrence, and residual disease measuring less than 5 mm at time of initial/first-line surgery.6 Many individuals now use this scoring system to determine candidacy for secondary debulking: disease-free interval, number of sites of recurrence (ideally oligometastatic disease), and residual disease less than 5 mm at the time of primary debulking.
Finally, the iMODEL was developed by a group from China and found that complete R0 secondary CRS was associated with a low initial FIGO stage, no residual disease after primary surgery, longer platinum-free interval, better Eastern Cooperative Oncology Group performance status, lower CA-125 levels, as well as no ascites at the time of recurrence. Based on these criteria, individuals received either high or low iMODEL scores, and those with a low score were said to be candidates for secondary CRS. Overall, these models demonstrate that the strongest predictive factor that suggests a survival benefit from secondary CRS is the ability to achieve a complete R0 resection at the time of surgery.
Secondary debulking surgery has been tested in three large randomized controlled trials. The DESKTOP investigators and the SOC-1 trial have been the most successful groups to publish on this topic with positive results. Both groups use prognostic models for their inclusion criteria to select candidates in whom an R0 resection is believed to be most feasible. The first randomized controlled trial to publish on this topic was GOG-213,7 which did not use prognostic modeling for their inclusion criteria. Patients were randomized to secondary cytoreduction followed by platinum-based chemotherapy with or without bevacizumab versus chemotherapy alone. The median OS was 50.6 months in the surgery group and 64.7 months in the no-surgery group (P = .08), suggesting no survival benefit to secondary cytoreduction; however, an ad hoc exploratory analysis of the surgery arm showed that both overall and progression-free survival were significantly improved in the complete cytoreduction group, compared with those with residual disease at time of surgery.
The results from the GOG-213 group suggested that improved survival from secondary debulking might be achieved when prognostic modeling is used to select optimal surgical candidates. The SOC-1 trial, published in 2021, was a phase 3, randomized, controlled trial that used the iMODEL scoring system combined with PET/CT imaging for patient selection.8 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Complete cytoreduction was achieved in 73% of patients with a low iMODEL score, and these data showed improved OS in the surgery group of 58.1 months versus 53.9 months (P < .05) in the no-surgery group. Lastly, the DESKTOP group most recently published results on this topic in a large randomized, controlled trial.9 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Inclusion criteria were only met in patients with a positive AGO score. An improved OS of 7.7 months (53.7 vs. 46 months; P < .05) was demonstrated in patients that underwent surgery versus those exposed to only chemotherapy. Again, this group showed that overall survival was further improved when complete cytoreduction was achieved.
Given the results of these three trials, the Society for Gynecologic Oncology has released a statement on secondary cytoreduction in recurrent ovarian cancer (see Table).10 While it is important to use caution when comparing the three studies as study populations differed substantially, the most important takeaway the difference in survival outcomes in patients in whom complete gross resection was achieved versus no complete gross resection versus no surgery. This comparison highlights the benefit of complete cytoreduction as well as the potential harms of secondary debulking when an R0 resection cannot be achieved. Although not yet evaluated in this clinical setting, laparoscopic exploration may be useful to augment assessment of disease extent and possibility of disease resection, just as it is in frontline ovarian cancer surgery.
The importance of bevacizumab use in recurrent ovarian cancer is also highlighted in the SGO statement. In GOG-213, 84% of the total study population (in both the surgery and no surgery cohort) were treated with concurrent followed by maintenance bevacizumab with an improved survival outcome, which may suggest that this trial generalizes better than the others to contemporary management of platinum-sensitive recurrent ovarian cancer.
Overall, given the mixed data, the recommendation is for surgeons to consider all available data to guide them in treatment planning with a strong emphasis on using all available technology to assess whether complete cytoreduction can be achieved in the setting of recurrence so as to not delay the patient’s ability to receive chemotherapy.
Dr. John is a gynecologic oncology fellow at the University of North Carolina at Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the university.
References
1. du Bois A et al. J Natl Cancer Inst. 2003;95:1320-9.
2. Wagner U et al. Br J Cancer. 2012;107:588-91.
3. Vergote I et al. N Engl J Med. 2010;363:943-53.
4. Harter P et al. Ann Surg Oncol. 2006;13:1702-10.
5. Harter P et al. Int J Gynecol Cancer. 2011;21:289-95.
6. Chi DS et al. Cancer. 2006 106:1933-9.
7. Coleman RL et al. Lancet Oncol. 2017;18:779-1.
8. Shi T et al. Lancet Oncol. 2021;22:439-49.
9. Harter P et al. N Engl J Med 2021;385:2123-31.
10. Harrison R, et al. Gynecol Oncol. 2021;163:448-52.
Recurrent ovarian cancer is difficult to treat; it has high recurrence rates and poor targeted treatment options. Between 60% and 75% of patients initially diagnosed with advanced-stage ovarian cancer will relapse within 2-3 years.1 Survival for these patients is poor, with an average overall survival (OS) of 30-40 months from the time of recurrence.2 Historically, immunotherapy has shown poor efficacy for recurrent ovarian malignancy, leaving few options for patients and their providers. Given the lack of effective treatment options, secondary cytoreductive surgery (surgery at the time of recurrence) has been heavily studied as a potential therapeutic option.
The initial rationale for cytoreductive surgery (CRS) in patients with advanced ovarian cancer focused on palliation of symptoms from large, bulky disease that frequently caused obstructive symptoms and pain. Now, cytoreduction is a critical part of therapy. It decreases chemotherapy-resistant tumor cells, improves the immune response, and is thought to optimize perfusion of the residual cancer for systemic therapy. The survival benefit of surgery in the frontline setting, either with primary or interval debulking, is well established, and much of the data now demonstrate that complete resection of all macroscopic disease (also known as an R0 resection) has the greatest survival benefit.3 Given the benefits of an initial debulking surgery, secondary cytoreduction has been studied since the 1980s with mixed results. These data have demonstrated that the largest barrier to care has been appropriate patient selection for this often complex surgical procedure.
The 2020 National Comprehensive Cancer Network guidelines list secondary CRS as a treatment option; however, the procedure should only be considered in patients who have platinum sensitive disease, a performance status of 0-1, no ascites, and an isolated focus or limited focus of disease that is amenable to complete resection. Numerous retrospective studies have suggested that secondary CRS is beneficial to patients with recurrent ovarian cancer, especially if complete cytoreduction can be accomplished. Many of these studies have similarly concluded that there are benefits, such as less ascites at the time of recurrence, smaller disease burden, and a longer disease-free interval. From that foundation, multiple groups used retrospective data to investigate prognostic models to determine who would benefit most from secondary cytoreduction.
The DESKTOP Group initially published their retrospective study in 2006 and created a scoring system assessing who would benefit from secondary CRS.4 Data demonstrated that a performance status of 0, FIGO stage of I/II at the time of initial diagnosis, no residual tumor after primary surgery, and ascites less than 500 mL were associated with improved survival after secondary cytoreduction. They created the AGO score out of these data, which is positive only if three criteria are met: a performance status of 0, R0 after primary debulk, and ascites less than 500 mL at the time of recurrence.
They prospectively tested this score in DESKTOP II, which validated their findings and showed that complete secondary CRS could be achieved in 76% of those with a positive AGO score.5 Many believed that the AGO score was too restrictive, and a second retrospective study performed by a group at Memorial Sloan Kettering showed that optimal secondary cytoreduction could be achieved to prolong survival by a median of 30 months in patients with a longer disease-free interval, a single site of recurrence, and residual disease measuring less than 5 mm at time of initial/first-line surgery.6 Many individuals now use this scoring system to determine candidacy for secondary debulking: disease-free interval, number of sites of recurrence (ideally oligometastatic disease), and residual disease less than 5 mm at the time of primary debulking.
Finally, the iMODEL was developed by a group from China and found that complete R0 secondary CRS was associated with a low initial FIGO stage, no residual disease after primary surgery, longer platinum-free interval, better Eastern Cooperative Oncology Group performance status, lower CA-125 levels, as well as no ascites at the time of recurrence. Based on these criteria, individuals received either high or low iMODEL scores, and those with a low score were said to be candidates for secondary CRS. Overall, these models demonstrate that the strongest predictive factor that suggests a survival benefit from secondary CRS is the ability to achieve a complete R0 resection at the time of surgery.
Secondary debulking surgery has been tested in three large randomized controlled trials. The DESKTOP investigators and the SOC-1 trial have been the most successful groups to publish on this topic with positive results. Both groups use prognostic models for their inclusion criteria to select candidates in whom an R0 resection is believed to be most feasible. The first randomized controlled trial to publish on this topic was GOG-213,7 which did not use prognostic modeling for their inclusion criteria. Patients were randomized to secondary cytoreduction followed by platinum-based chemotherapy with or without bevacizumab versus chemotherapy alone. The median OS was 50.6 months in the surgery group and 64.7 months in the no-surgery group (P = .08), suggesting no survival benefit to secondary cytoreduction; however, an ad hoc exploratory analysis of the surgery arm showed that both overall and progression-free survival were significantly improved in the complete cytoreduction group, compared with those with residual disease at time of surgery.
The results from the GOG-213 group suggested that improved survival from secondary debulking might be achieved when prognostic modeling is used to select optimal surgical candidates. The SOC-1 trial, published in 2021, was a phase 3, randomized, controlled trial that used the iMODEL scoring system combined with PET/CT imaging for patient selection.8 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Complete cytoreduction was achieved in 73% of patients with a low iMODEL score, and these data showed improved OS in the surgery group of 58.1 months versus 53.9 months (P < .05) in the no-surgery group. Lastly, the DESKTOP group most recently published results on this topic in a large randomized, controlled trial.9 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Inclusion criteria were only met in patients with a positive AGO score. An improved OS of 7.7 months (53.7 vs. 46 months; P < .05) was demonstrated in patients that underwent surgery versus those exposed to only chemotherapy. Again, this group showed that overall survival was further improved when complete cytoreduction was achieved.
Given the results of these three trials, the Society for Gynecologic Oncology has released a statement on secondary cytoreduction in recurrent ovarian cancer (see Table).10 While it is important to use caution when comparing the three studies as study populations differed substantially, the most important takeaway the difference in survival outcomes in patients in whom complete gross resection was achieved versus no complete gross resection versus no surgery. This comparison highlights the benefit of complete cytoreduction as well as the potential harms of secondary debulking when an R0 resection cannot be achieved. Although not yet evaluated in this clinical setting, laparoscopic exploration may be useful to augment assessment of disease extent and possibility of disease resection, just as it is in frontline ovarian cancer surgery.
The importance of bevacizumab use in recurrent ovarian cancer is also highlighted in the SGO statement. In GOG-213, 84% of the total study population (in both the surgery and no surgery cohort) were treated with concurrent followed by maintenance bevacizumab with an improved survival outcome, which may suggest that this trial generalizes better than the others to contemporary management of platinum-sensitive recurrent ovarian cancer.
Overall, given the mixed data, the recommendation is for surgeons to consider all available data to guide them in treatment planning with a strong emphasis on using all available technology to assess whether complete cytoreduction can be achieved in the setting of recurrence so as to not delay the patient’s ability to receive chemotherapy.
Dr. John is a gynecologic oncology fellow at the University of North Carolina at Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the university.
References
1. du Bois A et al. J Natl Cancer Inst. 2003;95:1320-9.
2. Wagner U et al. Br J Cancer. 2012;107:588-91.
3. Vergote I et al. N Engl J Med. 2010;363:943-53.
4. Harter P et al. Ann Surg Oncol. 2006;13:1702-10.
5. Harter P et al. Int J Gynecol Cancer. 2011;21:289-95.
6. Chi DS et al. Cancer. 2006 106:1933-9.
7. Coleman RL et al. Lancet Oncol. 2017;18:779-1.
8. Shi T et al. Lancet Oncol. 2021;22:439-49.
9. Harter P et al. N Engl J Med 2021;385:2123-31.
10. Harrison R, et al. Gynecol Oncol. 2021;163:448-52.
Update on high-grade vulvar interepithelial neoplasia
Vulvar squamous cell carcinomas (VSCC) comprise approximately 90% of all vulvar malignancies. Unlike cervical SCC, which are predominantly human papilloma virus (HPV) positive, only a minority of VSCC are HPV positive – on the order of 15%-25% of cases. Most cases occur in the setting of lichen sclerosus and are HPV negative.
Lichen sclerosus is a chronic inflammatory dermatitis typically involving the anogenital area, which in some cases can become seriously distorted (e.g. atrophy of the labia minora, clitoral phimosis, and introital stenosis). Although most cases are diagnosed in postmenopausal women, LS can affect women of any age. The true prevalence of lichen sclerosus is unknown. Recent studies have shown a prevalence of 1 in 60; among older women, it can even be as high as 1 in 30. While lichen sclerosus is a pruriginous condition, it is often asymptomatic. It is not considered a premalignant condition. The diagnosis is clinical; however, suspicious lesions (erosions/ulcerations, hyperkeratosis, pigmented areas, ecchymosis, warty or papular lesions), particularly when recalcitrant to adequate first-line therapy, should be biopsied.
VSCC arises from precursor lesions or high-grade vulvar intraepithelial neoplasia (VIN). The 2015 International Society for the Study of Vulvovaginal Disease nomenclature classifies high-grade VIN into high-grade squamous intraepithelial lesion (HSIL) and differentiated VIN (dVIN). Most patients with high-grade VIN are diagnosed with HSIL or usual type VIN. A preponderance of these lesions (75%-85%) are HPV positive, predominantly HPV 16. Vulvar HSIL (vHSIL) lesions affect younger women. The lesions tend to be multifocal and extensive. On the other hand, dVIN typically affects older women and commonly develops as a solitary lesion. While dVIN accounts for only a small subset of patients with high-grade VIN, these lesions are HPV negative and associated with lichen sclerosus.
Both disease entities, vHSIL and dVIN, are increasing in incidence. There is a higher risk and shortened period of progression to cancer in patients with dVIN compared to HSIL. The cancer risk of vHSIL is relatively low. The 10-year cumulative VSCC risk reported in the literature is 10.3%; 9.7% for vHSIL and 50% for dVIN. Patients with vHSIL could benefit from less aggressive treatment modalities.
Patients present with a constellation of signs such as itching, pain, burning, bleeding, and discharge. Chronic symptoms portend HPV-independent lesions associated with lichen sclerosus while episodic signs are suggestive of HPV-positive lesions.
The recurrence risk of high-grade VIN is 46%-70%. Risk factors for recurrence include age greater than 50, immunosuppression, metasynchronous HSIL, and multifocal lesions. Recurrences occur in up to 50% of women who have undergone surgery. For those who undergo surgical treatment for high-grade VIN, recurrence is more common in the setting of positive margins, underlying lichen sclerosis, persistent HPV infection, and immunosuppression.
Management of high-grade VIN is determined by the lesion characteristics, patient characteristics, and medical expertise. Given the risk of progression of high-grade VIN to cancer and risk of underlying cancer, surgical therapy is typically recommended. The treatment of choice is surgical excision in cases of dVIN. Surgical treatments include CO2 laser ablation, wide local excision, and vulvectomy. Women who undergo surgical treatment for vHSIL have about a 50% chance of the condition recurring 1 year later, irrespective of whether treatment is by surgical excision or laser vaporization.
Since surgery can be associated with disfigurement and sexual dysfunction, alternatives to surgery should be considered in cases of vHSIL. The potential for effect on sexual function should be part of preoperative counseling and treatment. Women treated for VIN often experience increased inhibition of sexual excitement and increased inhibition of orgasm. One study found that in women undergoing vulvar excision for VIN, the impairment was found to be psychological in nature. Overall, the studies of sexual effect from treatment of VIN have found that women do not return to their pretreatment sexual function. However, the optimal management of vHSIL has not been determined. Nonsurgical options include topical therapies (imiquimod, 5-fluorouracil, cidofovir, and interferon) and nonpharmacologic treatments, such as photodynamic therapy.
Imiquimod, a topical immune modulator, is the most studied pharmacologic treatment of vHSIL. The drug induces secretion of cytokines, creating an immune response that clears the HPV infection. Imiquimod is safe and well tolerated. The clinical response rate varies between 35% and 81%. A recent study demonstrated the efficacy of imiquimod and the treatment was found to be noninferior to surgery. Adverse events differed, with local pain following surgical treatment and local pruritus and erythema associated with imiquimod use. Some patients did not respond to imiquimod; it was thought by the authors of the study that specific immunological factors affect the clinical response. 
In conclusion, high-grade VIN is a heterogeneous disease made up of two distinct disease entities with rising incidence. In contrast to dVIN, the cancer risk is low for patients with vHSIL. Treatment should be driven by the clinical characteristics of the vulvar lesions, patients’ preferences, sexual activity, and compliance. Future directions include risk stratification of patients with vHSIL who are most likely to benefit from topical treatments, thus reducing overtreatment. Molecular biomarkers that could identify dVIN at an early stage are needed.
Dr. Jackson-Moore is associate professor in gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the university.
References
Cendejas BR et al. Am J Obstet Gynecol. 2015 Mar;212(3):291-7.
Lebreton M et al. J Gynecol Obstet Hum Reprod. 2020 Nov;49(9):101801.
Thuijs NB et al. Int J Cancer. 2021 Jan 1;148(1):90-8. doi: 10.1002/ijc.33198. .
Trutnovsky G et al. Lancet. 2022 May 7;399(10337):1790-8. Erratum in: Lancet. 2022 Oct 8;400(10359):1194.
Vulvar squamous cell carcinomas (VSCC) comprise approximately 90% of all vulvar malignancies. Unlike cervical SCC, which are predominantly human papilloma virus (HPV) positive, only a minority of VSCC are HPV positive – on the order of 15%-25% of cases. Most cases occur in the setting of lichen sclerosus and are HPV negative.
Lichen sclerosus is a chronic inflammatory dermatitis typically involving the anogenital area, which in some cases can become seriously distorted (e.g. atrophy of the labia minora, clitoral phimosis, and introital stenosis). Although most cases are diagnosed in postmenopausal women, LS can affect women of any age. The true prevalence of lichen sclerosus is unknown. Recent studies have shown a prevalence of 1 in 60; among older women, it can even be as high as 1 in 30. While lichen sclerosus is a pruriginous condition, it is often asymptomatic. It is not considered a premalignant condition. The diagnosis is clinical; however, suspicious lesions (erosions/ulcerations, hyperkeratosis, pigmented areas, ecchymosis, warty or papular lesions), particularly when recalcitrant to adequate first-line therapy, should be biopsied.
VSCC arises from precursor lesions or high-grade vulvar intraepithelial neoplasia (VIN). The 2015 International Society for the Study of Vulvovaginal Disease nomenclature classifies high-grade VIN into high-grade squamous intraepithelial lesion (HSIL) and differentiated VIN (dVIN). Most patients with high-grade VIN are diagnosed with HSIL or usual type VIN. A preponderance of these lesions (75%-85%) are HPV positive, predominantly HPV 16. Vulvar HSIL (vHSIL) lesions affect younger women. The lesions tend to be multifocal and extensive. On the other hand, dVIN typically affects older women and commonly develops as a solitary lesion. While dVIN accounts for only a small subset of patients with high-grade VIN, these lesions are HPV negative and associated with lichen sclerosus.
Both disease entities, vHSIL and dVIN, are increasing in incidence. There is a higher risk and shortened period of progression to cancer in patients with dVIN compared to HSIL. The cancer risk of vHSIL is relatively low. The 10-year cumulative VSCC risk reported in the literature is 10.3%; 9.7% for vHSIL and 50% for dVIN. Patients with vHSIL could benefit from less aggressive treatment modalities.
Patients present with a constellation of signs such as itching, pain, burning, bleeding, and discharge. Chronic symptoms portend HPV-independent lesions associated with lichen sclerosus while episodic signs are suggestive of HPV-positive lesions.
The recurrence risk of high-grade VIN is 46%-70%. Risk factors for recurrence include age greater than 50, immunosuppression, metasynchronous HSIL, and multifocal lesions. Recurrences occur in up to 50% of women who have undergone surgery. For those who undergo surgical treatment for high-grade VIN, recurrence is more common in the setting of positive margins, underlying lichen sclerosis, persistent HPV infection, and immunosuppression.
Management of high-grade VIN is determined by the lesion characteristics, patient characteristics, and medical expertise. Given the risk of progression of high-grade VIN to cancer and risk of underlying cancer, surgical therapy is typically recommended. The treatment of choice is surgical excision in cases of dVIN. Surgical treatments include CO2 laser ablation, wide local excision, and vulvectomy. Women who undergo surgical treatment for vHSIL have about a 50% chance of the condition recurring 1 year later, irrespective of whether treatment is by surgical excision or laser vaporization.
Since surgery can be associated with disfigurement and sexual dysfunction, alternatives to surgery should be considered in cases of vHSIL. The potential for effect on sexual function should be part of preoperative counseling and treatment. Women treated for VIN often experience increased inhibition of sexual excitement and increased inhibition of orgasm. One study found that in women undergoing vulvar excision for VIN, the impairment was found to be psychological in nature. Overall, the studies of sexual effect from treatment of VIN have found that women do not return to their pretreatment sexual function. However, the optimal management of vHSIL has not been determined. Nonsurgical options include topical therapies (imiquimod, 5-fluorouracil, cidofovir, and interferon) and nonpharmacologic treatments, such as photodynamic therapy.
Imiquimod, a topical immune modulator, is the most studied pharmacologic treatment of vHSIL. The drug induces secretion of cytokines, creating an immune response that clears the HPV infection. Imiquimod is safe and well tolerated. The clinical response rate varies between 35% and 81%. A recent study demonstrated the efficacy of imiquimod and the treatment was found to be noninferior to surgery. Adverse events differed, with local pain following surgical treatment and local pruritus and erythema associated with imiquimod use. Some patients did not respond to imiquimod; it was thought by the authors of the study that specific immunological factors affect the clinical response. 
In conclusion, high-grade VIN is a heterogeneous disease made up of two distinct disease entities with rising incidence. In contrast to dVIN, the cancer risk is low for patients with vHSIL. Treatment should be driven by the clinical characteristics of the vulvar lesions, patients’ preferences, sexual activity, and compliance. Future directions include risk stratification of patients with vHSIL who are most likely to benefit from topical treatments, thus reducing overtreatment. Molecular biomarkers that could identify dVIN at an early stage are needed.
Dr. Jackson-Moore is associate professor in gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the university.
References
Cendejas BR et al. Am J Obstet Gynecol. 2015 Mar;212(3):291-7.
Lebreton M et al. J Gynecol Obstet Hum Reprod. 2020 Nov;49(9):101801.
Thuijs NB et al. Int J Cancer. 2021 Jan 1;148(1):90-8. doi: 10.1002/ijc.33198. .
Trutnovsky G et al. Lancet. 2022 May 7;399(10337):1790-8. Erratum in: Lancet. 2022 Oct 8;400(10359):1194.
Vulvar squamous cell carcinomas (VSCC) comprise approximately 90% of all vulvar malignancies. Unlike cervical SCC, which are predominantly human papilloma virus (HPV) positive, only a minority of VSCC are HPV positive – on the order of 15%-25% of cases. Most cases occur in the setting of lichen sclerosus and are HPV negative.
Lichen sclerosus is a chronic inflammatory dermatitis typically involving the anogenital area, which in some cases can become seriously distorted (e.g. atrophy of the labia minora, clitoral phimosis, and introital stenosis). Although most cases are diagnosed in postmenopausal women, LS can affect women of any age. The true prevalence of lichen sclerosus is unknown. Recent studies have shown a prevalence of 1 in 60; among older women, it can even be as high as 1 in 30. While lichen sclerosus is a pruriginous condition, it is often asymptomatic. It is not considered a premalignant condition. The diagnosis is clinical; however, suspicious lesions (erosions/ulcerations, hyperkeratosis, pigmented areas, ecchymosis, warty or papular lesions), particularly when recalcitrant to adequate first-line therapy, should be biopsied.
VSCC arises from precursor lesions or high-grade vulvar intraepithelial neoplasia (VIN). The 2015 International Society for the Study of Vulvovaginal Disease nomenclature classifies high-grade VIN into high-grade squamous intraepithelial lesion (HSIL) and differentiated VIN (dVIN). Most patients with high-grade VIN are diagnosed with HSIL or usual type VIN. A preponderance of these lesions (75%-85%) are HPV positive, predominantly HPV 16. Vulvar HSIL (vHSIL) lesions affect younger women. The lesions tend to be multifocal and extensive. On the other hand, dVIN typically affects older women and commonly develops as a solitary lesion. While dVIN accounts for only a small subset of patients with high-grade VIN, these lesions are HPV negative and associated with lichen sclerosus.
Both disease entities, vHSIL and dVIN, are increasing in incidence. There is a higher risk and shortened period of progression to cancer in patients with dVIN compared to HSIL. The cancer risk of vHSIL is relatively low. The 10-year cumulative VSCC risk reported in the literature is 10.3%; 9.7% for vHSIL and 50% for dVIN. Patients with vHSIL could benefit from less aggressive treatment modalities.
Patients present with a constellation of signs such as itching, pain, burning, bleeding, and discharge. Chronic symptoms portend HPV-independent lesions associated with lichen sclerosus while episodic signs are suggestive of HPV-positive lesions.
The recurrence risk of high-grade VIN is 46%-70%. Risk factors for recurrence include age greater than 50, immunosuppression, metasynchronous HSIL, and multifocal lesions. Recurrences occur in up to 50% of women who have undergone surgery. For those who undergo surgical treatment for high-grade VIN, recurrence is more common in the setting of positive margins, underlying lichen sclerosis, persistent HPV infection, and immunosuppression.
Management of high-grade VIN is determined by the lesion characteristics, patient characteristics, and medical expertise. Given the risk of progression of high-grade VIN to cancer and risk of underlying cancer, surgical therapy is typically recommended. The treatment of choice is surgical excision in cases of dVIN. Surgical treatments include CO2 laser ablation, wide local excision, and vulvectomy. Women who undergo surgical treatment for vHSIL have about a 50% chance of the condition recurring 1 year later, irrespective of whether treatment is by surgical excision or laser vaporization.
Since surgery can be associated with disfigurement and sexual dysfunction, alternatives to surgery should be considered in cases of vHSIL. The potential for effect on sexual function should be part of preoperative counseling and treatment. Women treated for VIN often experience increased inhibition of sexual excitement and increased inhibition of orgasm. One study found that in women undergoing vulvar excision for VIN, the impairment was found to be psychological in nature. Overall, the studies of sexual effect from treatment of VIN have found that women do not return to their pretreatment sexual function. However, the optimal management of vHSIL has not been determined. Nonsurgical options include topical therapies (imiquimod, 5-fluorouracil, cidofovir, and interferon) and nonpharmacologic treatments, such as photodynamic therapy.
Imiquimod, a topical immune modulator, is the most studied pharmacologic treatment of vHSIL. The drug induces secretion of cytokines, creating an immune response that clears the HPV infection. Imiquimod is safe and well tolerated. The clinical response rate varies between 35% and 81%. A recent study demonstrated the efficacy of imiquimod and the treatment was found to be noninferior to surgery. Adverse events differed, with local pain following surgical treatment and local pruritus and erythema associated with imiquimod use. Some patients did not respond to imiquimod; it was thought by the authors of the study that specific immunological factors affect the clinical response. 
In conclusion, high-grade VIN is a heterogeneous disease made up of two distinct disease entities with rising incidence. In contrast to dVIN, the cancer risk is low for patients with vHSIL. Treatment should be driven by the clinical characteristics of the vulvar lesions, patients’ preferences, sexual activity, and compliance. Future directions include risk stratification of patients with vHSIL who are most likely to benefit from topical treatments, thus reducing overtreatment. Molecular biomarkers that could identify dVIN at an early stage are needed.
Dr. Jackson-Moore is associate professor in gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the university.
References
Cendejas BR et al. Am J Obstet Gynecol. 2015 Mar;212(3):291-7.
Lebreton M et al. J Gynecol Obstet Hum Reprod. 2020 Nov;49(9):101801.
Thuijs NB et al. Int J Cancer. 2021 Jan 1;148(1):90-8. doi: 10.1002/ijc.33198. .
Trutnovsky G et al. Lancet. 2022 May 7;399(10337):1790-8. Erratum in: Lancet. 2022 Oct 8;400(10359):1194.
Germline genetic testing: Why it matters and where we are failing
Historically, the role of genetic testing has been to identify familial cancer syndromes and initiate cascade testing. If a germline pathogenic variant is found in an individual, cascade testing involves genetic counseling and testing of blood relatives, starting with those closest in relation to the proband, to identify other family members at high hereditary cancer risk. Once testing identifies those family members at higher cancer risk, these individuals can be referred for risk-reducing procedures. They can undergo screening tests starting at an earlier age and/or increased frequency to help prevent invasive cancer or diagnose it at an earlier stage.
Genetic testing can also inform prognosis. While women with a BRCA1 or BRCA2 mutation are at higher risk of developing ovarian cancer compared with the baseline population, the presence of a germline BRCA mutation has been shown to confer improved survival compared with no BRCA mutation (BRCA wild type). However, more recent data have shown that when long-term survival was analyzed, the prognostic benefit seen in patients with a germline BRCA mutation was lost. The initial survival advantage seen in this population may be related to increased sensitivity to treatment. There appears to be improved response to platinum therapy, which is the standard of care for upfront treatment, in germline BRCA mutation carriers.
Most recently, genetic testing has been used to guide treatment decisions in gynecologic cancers. In 2014, the first poly ADP-ribose polymerase (PARP) inhibitor, olaparib, received Food and Drug Administration approval for the treatment of recurrent ovarian cancer in the presence of a germline BRCA mutation. Now there are multiple PARP inhibitors that have FDA approval for ovarian cancer treatment, some as frontline treatment.
Previous data indicate that 13%-18% of women with ovarian cancer have a germline BRCA mutation that places them at increased risk of hereditary ovarian cancer.1 Current guidelines from the American Society of Clinical Oncology, the U.S. Preventive Services Task Force, the National Comprehensive Cancer Network, the Society of Gynecologic Oncology (SGO), and the American College of Obstetricians and Gynecologists recommend universal genetic counseling and testing for patients diagnosed with epithelial ovarian cancer. Despite these guidelines, rates of referral for genetic counseling and completion of genetic testing are low.
There has been improvement for both referrals and testing since the publication of the 2014 SGO clinical practice statement on genetic testing for ovarian cancer patients, which recommended that all women, even those without any significant family history, should receive genetic counseling and be offered genetic testing.2 When including only studies that collected data after the publication of the 2014 SGO clinical practice statement on genetic testing, a recent systematic review found that 64% of patients were referred for genetic counseling and 63% underwent testing.3
Clinical interventions to target genetic evaluation appear to improve uptake of both counseling and testing. These interventions include using telemedicine to deliver genetic counseling services, mainstreaming (counseling and testing are provided in an oncology clinic by nongenetics specialists), having a genetic counselor within the clinic, and performing reflex testing. With limited numbers of genetic counselors (and even further limited numbers of cancer-specific genetic counselors),4 referral for genetic counseling before testing is often challenging and may not be feasible. There is continued need for strategies to help overcome the barrier to accessing genetic counseling.
While the data are limited, there appear to be significant disparities in rates of genetic testing. Genetic counseling and testing were completed by White (43% and 40%) patients more frequently than by either Black (24% and 26%) or Asian (23% and 14%) patients.4 Uninsured patients were about half as likely (23% vs. 47%) to complete genetic testing as were those with private insurance.4
Genetic testing is an important tool to help identify individuals and families at risk of having hereditary cancer syndromes. This identification allows us to prevent many cancers and identify others while still early stage, significantly decreasing the health care and financial burden on our society and improving outcomes for patients. While we have seen improvement in rates of referral for genetic counseling and testing, we are still falling short. Given the shortage of genetic counselors, it is imperative that we find solutions to ensure continued and improved access to genetic testing for our patients.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Norquist BM et al. JAMA Oncol. 2016;2(4):482-90.
2. SGO Clinical Practice Statement. 2014 Oct 1.
3. Lin J et al. Gynecol Oncol. 2021;162(2):506-16.
4. American Society of Clinical Oncology. J Oncol Pract. 2016 Apr;12(4):339-83.
Historically, the role of genetic testing has been to identify familial cancer syndromes and initiate cascade testing. If a germline pathogenic variant is found in an individual, cascade testing involves genetic counseling and testing of blood relatives, starting with those closest in relation to the proband, to identify other family members at high hereditary cancer risk. Once testing identifies those family members at higher cancer risk, these individuals can be referred for risk-reducing procedures. They can undergo screening tests starting at an earlier age and/or increased frequency to help prevent invasive cancer or diagnose it at an earlier stage.
Genetic testing can also inform prognosis. While women with a BRCA1 or BRCA2 mutation are at higher risk of developing ovarian cancer compared with the baseline population, the presence of a germline BRCA mutation has been shown to confer improved survival compared with no BRCA mutation (BRCA wild type). However, more recent data have shown that when long-term survival was analyzed, the prognostic benefit seen in patients with a germline BRCA mutation was lost. The initial survival advantage seen in this population may be related to increased sensitivity to treatment. There appears to be improved response to platinum therapy, which is the standard of care for upfront treatment, in germline BRCA mutation carriers.
Most recently, genetic testing has been used to guide treatment decisions in gynecologic cancers. In 2014, the first poly ADP-ribose polymerase (PARP) inhibitor, olaparib, received Food and Drug Administration approval for the treatment of recurrent ovarian cancer in the presence of a germline BRCA mutation. Now there are multiple PARP inhibitors that have FDA approval for ovarian cancer treatment, some as frontline treatment.
Previous data indicate that 13%-18% of women with ovarian cancer have a germline BRCA mutation that places them at increased risk of hereditary ovarian cancer.1 Current guidelines from the American Society of Clinical Oncology, the U.S. Preventive Services Task Force, the National Comprehensive Cancer Network, the Society of Gynecologic Oncology (SGO), and the American College of Obstetricians and Gynecologists recommend universal genetic counseling and testing for patients diagnosed with epithelial ovarian cancer. Despite these guidelines, rates of referral for genetic counseling and completion of genetic testing are low.
There has been improvement for both referrals and testing since the publication of the 2014 SGO clinical practice statement on genetic testing for ovarian cancer patients, which recommended that all women, even those without any significant family history, should receive genetic counseling and be offered genetic testing.2 When including only studies that collected data after the publication of the 2014 SGO clinical practice statement on genetic testing, a recent systematic review found that 64% of patients were referred for genetic counseling and 63% underwent testing.3
Clinical interventions to target genetic evaluation appear to improve uptake of both counseling and testing. These interventions include using telemedicine to deliver genetic counseling services, mainstreaming (counseling and testing are provided in an oncology clinic by nongenetics specialists), having a genetic counselor within the clinic, and performing reflex testing. With limited numbers of genetic counselors (and even further limited numbers of cancer-specific genetic counselors),4 referral for genetic counseling before testing is often challenging and may not be feasible. There is continued need for strategies to help overcome the barrier to accessing genetic counseling.
While the data are limited, there appear to be significant disparities in rates of genetic testing. Genetic counseling and testing were completed by White (43% and 40%) patients more frequently than by either Black (24% and 26%) or Asian (23% and 14%) patients.4 Uninsured patients were about half as likely (23% vs. 47%) to complete genetic testing as were those with private insurance.4
Genetic testing is an important tool to help identify individuals and families at risk of having hereditary cancer syndromes. This identification allows us to prevent many cancers and identify others while still early stage, significantly decreasing the health care and financial burden on our society and improving outcomes for patients. While we have seen improvement in rates of referral for genetic counseling and testing, we are still falling short. Given the shortage of genetic counselors, it is imperative that we find solutions to ensure continued and improved access to genetic testing for our patients.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Norquist BM et al. JAMA Oncol. 2016;2(4):482-90.
2. SGO Clinical Practice Statement. 2014 Oct 1.
3. Lin J et al. Gynecol Oncol. 2021;162(2):506-16.
4. American Society of Clinical Oncology. J Oncol Pract. 2016 Apr;12(4):339-83.
Historically, the role of genetic testing has been to identify familial cancer syndromes and initiate cascade testing. If a germline pathogenic variant is found in an individual, cascade testing involves genetic counseling and testing of blood relatives, starting with those closest in relation to the proband, to identify other family members at high hereditary cancer risk. Once testing identifies those family members at higher cancer risk, these individuals can be referred for risk-reducing procedures. They can undergo screening tests starting at an earlier age and/or increased frequency to help prevent invasive cancer or diagnose it at an earlier stage.
Genetic testing can also inform prognosis. While women with a BRCA1 or BRCA2 mutation are at higher risk of developing ovarian cancer compared with the baseline population, the presence of a germline BRCA mutation has been shown to confer improved survival compared with no BRCA mutation (BRCA wild type). However, more recent data have shown that when long-term survival was analyzed, the prognostic benefit seen in patients with a germline BRCA mutation was lost. The initial survival advantage seen in this population may be related to increased sensitivity to treatment. There appears to be improved response to platinum therapy, which is the standard of care for upfront treatment, in germline BRCA mutation carriers.
Most recently, genetic testing has been used to guide treatment decisions in gynecologic cancers. In 2014, the first poly ADP-ribose polymerase (PARP) inhibitor, olaparib, received Food and Drug Administration approval for the treatment of recurrent ovarian cancer in the presence of a germline BRCA mutation. Now there are multiple PARP inhibitors that have FDA approval for ovarian cancer treatment, some as frontline treatment.
Previous data indicate that 13%-18% of women with ovarian cancer have a germline BRCA mutation that places them at increased risk of hereditary ovarian cancer.1 Current guidelines from the American Society of Clinical Oncology, the U.S. Preventive Services Task Force, the National Comprehensive Cancer Network, the Society of Gynecologic Oncology (SGO), and the American College of Obstetricians and Gynecologists recommend universal genetic counseling and testing for patients diagnosed with epithelial ovarian cancer. Despite these guidelines, rates of referral for genetic counseling and completion of genetic testing are low.
There has been improvement for both referrals and testing since the publication of the 2014 SGO clinical practice statement on genetic testing for ovarian cancer patients, which recommended that all women, even those without any significant family history, should receive genetic counseling and be offered genetic testing.2 When including only studies that collected data after the publication of the 2014 SGO clinical practice statement on genetic testing, a recent systematic review found that 64% of patients were referred for genetic counseling and 63% underwent testing.3
Clinical interventions to target genetic evaluation appear to improve uptake of both counseling and testing. These interventions include using telemedicine to deliver genetic counseling services, mainstreaming (counseling and testing are provided in an oncology clinic by nongenetics specialists), having a genetic counselor within the clinic, and performing reflex testing. With limited numbers of genetic counselors (and even further limited numbers of cancer-specific genetic counselors),4 referral for genetic counseling before testing is often challenging and may not be feasible. There is continued need for strategies to help overcome the barrier to accessing genetic counseling.
While the data are limited, there appear to be significant disparities in rates of genetic testing. Genetic counseling and testing were completed by White (43% and 40%) patients more frequently than by either Black (24% and 26%) or Asian (23% and 14%) patients.4 Uninsured patients were about half as likely (23% vs. 47%) to complete genetic testing as were those with private insurance.4
Genetic testing is an important tool to help identify individuals and families at risk of having hereditary cancer syndromes. This identification allows us to prevent many cancers and identify others while still early stage, significantly decreasing the health care and financial burden on our society and improving outcomes for patients. While we have seen improvement in rates of referral for genetic counseling and testing, we are still falling short. Given the shortage of genetic counselors, it is imperative that we find solutions to ensure continued and improved access to genetic testing for our patients.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Norquist BM et al. JAMA Oncol. 2016;2(4):482-90.
2. SGO Clinical Practice Statement. 2014 Oct 1.
3. Lin J et al. Gynecol Oncol. 2021;162(2):506-16.
4. American Society of Clinical Oncology. J Oncol Pract. 2016 Apr;12(4):339-83.
The role of repeat uterine curettage in postmolar gestational trophoblastic neoplasia
Trophoblastic tissue is responsible for formation of the placenta during pregnancy. Gestational trophoblastic disease (GTD), a group comprising benign (hydatidiform moles) and malignant tumors, occurs when gestational trophoblastic tissue behaves in an abnormal manner. Hydatidiform moles, which are thought to be caused by errors in fertilization, occur in approximately 1 in 1,200 pregnancies in the United States. Gestational trophoblastic neoplasia (GTN) refers to the subgroup of these trophoblastic or placental tumors with malignant behavior and includes postmolar GTN, invasive mole, gestational choriocarcinoma, placental-site trophoblastic tumor (PSTT), and epithelioid trophoblastic tumor. Postmolar GTN arises after evacuation of a molar pregnancy and is most frequently diagnosed by a plateau or increase in human chorionic gonadotropin (hCG).1 The risk of postmolar GTN is much higher after a complete mole (7%-30%) compared with a partial mole (2.5%-7.5%).2 Once postmolar GTN is diagnosed, a World Health Organization score is assigned to determine if patients have low- or high-risk disease.3 The primary treatment for most GTN is chemotherapy. A patient’s WHO score helps determine whether they would benefit from single-agent or multiagent chemotherapy. The standard of care for low-risk disease is single-agent chemotherapy with either methotrexate or actinomycin D.
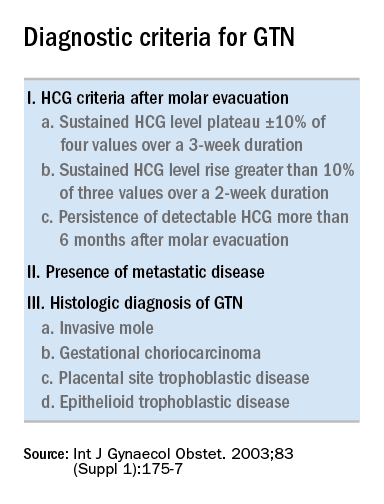
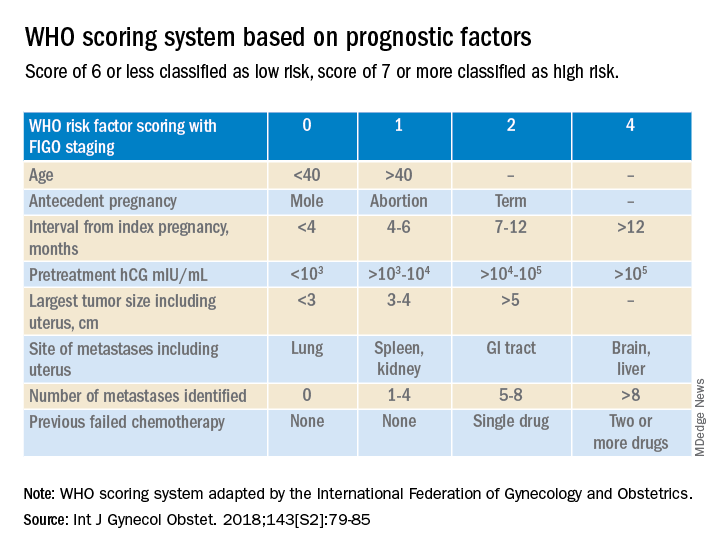
The role of a second uterine curettage, after the diagnosis of low-risk postmolar GTN, has been controversial because of the limited data and disparate outcomes reported. In older retrospective series, a second curettage affected treatment or produced remission in only 9%-20% of patients and caused uterine perforation or major hemorrhage in 5%-8% of patients.4,5 Given relatively high rates of major complications compared with surgical cure or decreased chemotherapy cycles needed, only a limited number of patients seemed to benefit from a second procedure. On the other hand, an observational study of 544 patients who underwent second uterine evacuation after a presumed diagnosis of persistent GTD found that up to 60% of patients did not require chemotherapy afterward.6 Those with hCG levels greater than 1,500 IU/L or histologic evidence of GTD were less likely to have a surgical cure after second curettage. The indications for uterine evacuations were varied across these studies and make it nearly impossible to compare their results.
More recently, there have been two prospective trials that have tackled the question of the utility of second uterine evacuation in low-risk, nonmetastatic GTN. The Gynecologic Oncology Group performed a single-arm prospective study in the United States that enrolled patients with postmolar GTN to undergo second curettage as initial treatment of their disease.7 Of 60 eligible patients, 40% had a surgical cure (defined as normalization of hCG followed by at least 6 months of subsequent normal hCG values). Overall, 47% of patients were able to avoid chemotherapy. All surgical cures were seen in patients with WHO scores between 0 and 4. Importantly, three women were diagnosed with PSTT, which tends to be resistant to methotrexate and actinomycin D (treatment for nonmetastatic PSTT is definitive surgery with hysterectomy). The study found that hCG was a poor discriminator for achieving surgical cure. While age appeared to have an association with surgical cure (cure less likely for younger and older ages, younger than 19 and older than 40), patient numbers were too small to make a statistical conclusion. There were no uterine perforations and one patient had a grade 3 hemorrhage (requiring transfusion).
In the second prospective trial, performed in Iran, 62 patients were randomized to either second uterine evacuation or standard treatment after diagnosis of postmolar GTN.8 All patients in the surgical arm received a cervical ripening agent prior to their procedure, had their procedure under ultrasound guidance, and received misoprostol afterward to prevent uterine bleeding. Among those undergoing second uterine evacuation, 50% were cured (no need for chemotherapy). Among those needing chemotherapy after surgery, the mean number of cycles of chemotherapy needed (3.07 vs. 6.69) and the time it took to achieve negative hCG (3.23 vs. 9.19 weeks) were significantly less compared with patients who did not undergo surgery. hCG prior to second uterine evacuation could distinguish response to surgery compared with those needing chemotherapy (hCG of 1,983 IU/L or less was the level determined to best predict response). No complications related to surgery were reported.
Given prospective data available, second uterine evacuation for treatment of nonmetastatic, low-risk postmolar GTN is a reasonable treatment option and one that should be considered and discussed with patients given the potential to avoid chemotherapy or decrease the number of cycles needed. It may be prudent to limit the procedure to patients with an hCG less than 1,500-2,000 IU/L and to those between the ages of 20 and 40. While uterine hemorrhage and perforation have been reported in the literature, more recent data suggest low rates of these complications. Unfortunately, given the rarity of the disease and the historically controversial use of second curettage, little is known about the effects on future fertility that this procedure may have, including the development of uterine synechiae.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Ngan HY et al, FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2003 Oct;83 Suppl 1:175-7. Erratum in: Int J Gynaecol Obstet. 2021 Dec;155(3):563.
2. Soper JT. Obstet Gynecol. 2021 Feb.;137(2):355-70.
3. Ngan HY et al. Int J Gynecol Obstet. 2018;143:79-85.
4. Schlaerth JB et al. Am J Obstet Gynecol. 1990 Jun;162(6):1465-70.
5. van Trommel NE et al. Gynecol Oncol. 2005 Oct;99(1):6-13.
6. Pezeshki M et al. Gynecol Oncol. 2004 Dec;95(3):423-9.
7. Osborne RJ et al. Obstet Gynecol. 2016 Sep;128(3):535-42.
8. Ayatollahi H et al. Int J Womens Health. 2017 Sep 21;9:665-71.
Trophoblastic tissue is responsible for formation of the placenta during pregnancy. Gestational trophoblastic disease (GTD), a group comprising benign (hydatidiform moles) and malignant tumors, occurs when gestational trophoblastic tissue behaves in an abnormal manner. Hydatidiform moles, which are thought to be caused by errors in fertilization, occur in approximately 1 in 1,200 pregnancies in the United States. Gestational trophoblastic neoplasia (GTN) refers to the subgroup of these trophoblastic or placental tumors with malignant behavior and includes postmolar GTN, invasive mole, gestational choriocarcinoma, placental-site trophoblastic tumor (PSTT), and epithelioid trophoblastic tumor. Postmolar GTN arises after evacuation of a molar pregnancy and is most frequently diagnosed by a plateau or increase in human chorionic gonadotropin (hCG).1 The risk of postmolar GTN is much higher after a complete mole (7%-30%) compared with a partial mole (2.5%-7.5%).2 Once postmolar GTN is diagnosed, a World Health Organization score is assigned to determine if patients have low- or high-risk disease.3 The primary treatment for most GTN is chemotherapy. A patient’s WHO score helps determine whether they would benefit from single-agent or multiagent chemotherapy. The standard of care for low-risk disease is single-agent chemotherapy with either methotrexate or actinomycin D.


The role of a second uterine curettage, after the diagnosis of low-risk postmolar GTN, has been controversial because of the limited data and disparate outcomes reported. In older retrospective series, a second curettage affected treatment or produced remission in only 9%-20% of patients and caused uterine perforation or major hemorrhage in 5%-8% of patients.4,5 Given relatively high rates of major complications compared with surgical cure or decreased chemotherapy cycles needed, only a limited number of patients seemed to benefit from a second procedure. On the other hand, an observational study of 544 patients who underwent second uterine evacuation after a presumed diagnosis of persistent GTD found that up to 60% of patients did not require chemotherapy afterward.6 Those with hCG levels greater than 1,500 IU/L or histologic evidence of GTD were less likely to have a surgical cure after second curettage. The indications for uterine evacuations were varied across these studies and make it nearly impossible to compare their results.
More recently, there have been two prospective trials that have tackled the question of the utility of second uterine evacuation in low-risk, nonmetastatic GTN. The Gynecologic Oncology Group performed a single-arm prospective study in the United States that enrolled patients with postmolar GTN to undergo second curettage as initial treatment of their disease.7 Of 60 eligible patients, 40% had a surgical cure (defined as normalization of hCG followed by at least 6 months of subsequent normal hCG values). Overall, 47% of patients were able to avoid chemotherapy. All surgical cures were seen in patients with WHO scores between 0 and 4. Importantly, three women were diagnosed with PSTT, which tends to be resistant to methotrexate and actinomycin D (treatment for nonmetastatic PSTT is definitive surgery with hysterectomy). The study found that hCG was a poor discriminator for achieving surgical cure. While age appeared to have an association with surgical cure (cure less likely for younger and older ages, younger than 19 and older than 40), patient numbers were too small to make a statistical conclusion. There were no uterine perforations and one patient had a grade 3 hemorrhage (requiring transfusion).
In the second prospective trial, performed in Iran, 62 patients were randomized to either second uterine evacuation or standard treatment after diagnosis of postmolar GTN.8 All patients in the surgical arm received a cervical ripening agent prior to their procedure, had their procedure under ultrasound guidance, and received misoprostol afterward to prevent uterine bleeding. Among those undergoing second uterine evacuation, 50% were cured (no need for chemotherapy). Among those needing chemotherapy after surgery, the mean number of cycles of chemotherapy needed (3.07 vs. 6.69) and the time it took to achieve negative hCG (3.23 vs. 9.19 weeks) were significantly less compared with patients who did not undergo surgery. hCG prior to second uterine evacuation could distinguish response to surgery compared with those needing chemotherapy (hCG of 1,983 IU/L or less was the level determined to best predict response). No complications related to surgery were reported.
Given prospective data available, second uterine evacuation for treatment of nonmetastatic, low-risk postmolar GTN is a reasonable treatment option and one that should be considered and discussed with patients given the potential to avoid chemotherapy or decrease the number of cycles needed. It may be prudent to limit the procedure to patients with an hCG less than 1,500-2,000 IU/L and to those between the ages of 20 and 40. While uterine hemorrhage and perforation have been reported in the literature, more recent data suggest low rates of these complications. Unfortunately, given the rarity of the disease and the historically controversial use of second curettage, little is known about the effects on future fertility that this procedure may have, including the development of uterine synechiae.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Ngan HY et al, FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2003 Oct;83 Suppl 1:175-7. Erratum in: Int J Gynaecol Obstet. 2021 Dec;155(3):563.
2. Soper JT. Obstet Gynecol. 2021 Feb.;137(2):355-70.
3. Ngan HY et al. Int J Gynecol Obstet. 2018;143:79-85.
4. Schlaerth JB et al. Am J Obstet Gynecol. 1990 Jun;162(6):1465-70.
5. van Trommel NE et al. Gynecol Oncol. 2005 Oct;99(1):6-13.
6. Pezeshki M et al. Gynecol Oncol. 2004 Dec;95(3):423-9.
7. Osborne RJ et al. Obstet Gynecol. 2016 Sep;128(3):535-42.
8. Ayatollahi H et al. Int J Womens Health. 2017 Sep 21;9:665-71.
Trophoblastic tissue is responsible for formation of the placenta during pregnancy. Gestational trophoblastic disease (GTD), a group comprising benign (hydatidiform moles) and malignant tumors, occurs when gestational trophoblastic tissue behaves in an abnormal manner. Hydatidiform moles, which are thought to be caused by errors in fertilization, occur in approximately 1 in 1,200 pregnancies in the United States. Gestational trophoblastic neoplasia (GTN) refers to the subgroup of these trophoblastic or placental tumors with malignant behavior and includes postmolar GTN, invasive mole, gestational choriocarcinoma, placental-site trophoblastic tumor (PSTT), and epithelioid trophoblastic tumor. Postmolar GTN arises after evacuation of a molar pregnancy and is most frequently diagnosed by a plateau or increase in human chorionic gonadotropin (hCG).1 The risk of postmolar GTN is much higher after a complete mole (7%-30%) compared with a partial mole (2.5%-7.5%).2 Once postmolar GTN is diagnosed, a World Health Organization score is assigned to determine if patients have low- or high-risk disease.3 The primary treatment for most GTN is chemotherapy. A patient’s WHO score helps determine whether they would benefit from single-agent or multiagent chemotherapy. The standard of care for low-risk disease is single-agent chemotherapy with either methotrexate or actinomycin D.


The role of a second uterine curettage, after the diagnosis of low-risk postmolar GTN, has been controversial because of the limited data and disparate outcomes reported. In older retrospective series, a second curettage affected treatment or produced remission in only 9%-20% of patients and caused uterine perforation or major hemorrhage in 5%-8% of patients.4,5 Given relatively high rates of major complications compared with surgical cure or decreased chemotherapy cycles needed, only a limited number of patients seemed to benefit from a second procedure. On the other hand, an observational study of 544 patients who underwent second uterine evacuation after a presumed diagnosis of persistent GTD found that up to 60% of patients did not require chemotherapy afterward.6 Those with hCG levels greater than 1,500 IU/L or histologic evidence of GTD were less likely to have a surgical cure after second curettage. The indications for uterine evacuations were varied across these studies and make it nearly impossible to compare their results.
More recently, there have been two prospective trials that have tackled the question of the utility of second uterine evacuation in low-risk, nonmetastatic GTN. The Gynecologic Oncology Group performed a single-arm prospective study in the United States that enrolled patients with postmolar GTN to undergo second curettage as initial treatment of their disease.7 Of 60 eligible patients, 40% had a surgical cure (defined as normalization of hCG followed by at least 6 months of subsequent normal hCG values). Overall, 47% of patients were able to avoid chemotherapy. All surgical cures were seen in patients with WHO scores between 0 and 4. Importantly, three women were diagnosed with PSTT, which tends to be resistant to methotrexate and actinomycin D (treatment for nonmetastatic PSTT is definitive surgery with hysterectomy). The study found that hCG was a poor discriminator for achieving surgical cure. While age appeared to have an association with surgical cure (cure less likely for younger and older ages, younger than 19 and older than 40), patient numbers were too small to make a statistical conclusion. There were no uterine perforations and one patient had a grade 3 hemorrhage (requiring transfusion).
In the second prospective trial, performed in Iran, 62 patients were randomized to either second uterine evacuation or standard treatment after diagnosis of postmolar GTN.8 All patients in the surgical arm received a cervical ripening agent prior to their procedure, had their procedure under ultrasound guidance, and received misoprostol afterward to prevent uterine bleeding. Among those undergoing second uterine evacuation, 50% were cured (no need for chemotherapy). Among those needing chemotherapy after surgery, the mean number of cycles of chemotherapy needed (3.07 vs. 6.69) and the time it took to achieve negative hCG (3.23 vs. 9.19 weeks) were significantly less compared with patients who did not undergo surgery. hCG prior to second uterine evacuation could distinguish response to surgery compared with those needing chemotherapy (hCG of 1,983 IU/L or less was the level determined to best predict response). No complications related to surgery were reported.
Given prospective data available, second uterine evacuation for treatment of nonmetastatic, low-risk postmolar GTN is a reasonable treatment option and one that should be considered and discussed with patients given the potential to avoid chemotherapy or decrease the number of cycles needed. It may be prudent to limit the procedure to patients with an hCG less than 1,500-2,000 IU/L and to those between the ages of 20 and 40. While uterine hemorrhage and perforation have been reported in the literature, more recent data suggest low rates of these complications. Unfortunately, given the rarity of the disease and the historically controversial use of second curettage, little is known about the effects on future fertility that this procedure may have, including the development of uterine synechiae.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Ngan HY et al, FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2003 Oct;83 Suppl 1:175-7. Erratum in: Int J Gynaecol Obstet. 2021 Dec;155(3):563.
2. Soper JT. Obstet Gynecol. 2021 Feb.;137(2):355-70.
3. Ngan HY et al. Int J Gynecol Obstet. 2018;143:79-85.
4. Schlaerth JB et al. Am J Obstet Gynecol. 1990 Jun;162(6):1465-70.
5. van Trommel NE et al. Gynecol Oncol. 2005 Oct;99(1):6-13.
6. Pezeshki M et al. Gynecol Oncol. 2004 Dec;95(3):423-9.
7. Osborne RJ et al. Obstet Gynecol. 2016 Sep;128(3):535-42.
8. Ayatollahi H et al. Int J Womens Health. 2017 Sep 21;9:665-71.
Estrogen replacement therapy in endometrial cancer survivors
In the United States, uterine cancer is the fourth most common cancer among women, behind breast, lung/bronchus, and colorectal cancer. There are expected to be almost 66,000 new cases of uterine cancer in 2022.1 The majority of uterine cancers are endometrioid in histology and tend to be low grade, diagnosed at an early stage, and have a good prognosis. While our molecular understanding of endometrial cancers (EC) has changed significantly in recent years, low-grade endometrioid adenocarcinomas have historically been described as type 1 ECs. Type 1 ECs are typically caused by excess estrogen exposure (often unopposed or lacking progesterone protection) and are preceded by endometrial hyperplasia. Excess estrogen can come from exogenous sources (such as unopposed estrogen replacement therapy or tamoxifen, a commonly used treatment in estrogen receptor–positive breast cancer that acts as an estrogen agonist in the endometrium in postmenopausal patients) or endogenous ones (such as obesity).
Peripheral adipose tissue converts androgens into estrogens; paired with the decreased levels of sex hormone–binding globulin seen in obesity, there is more unbound or free serum estrogen (specifically estradiol) in obese women. Estrogen acts on the endometrium to cause proliferation and, if unopposed or imbalanced in relation to progesterone exposure, can ultimately lead to hyperplasia and malignancy.
If excess and unopposed estrogen exposure are major risk factors for the development of EC, is it safe to consider estrogen replacement therapy (ERT) in patients after EC treatment?
The short answer is the data are limited, but in a patient with a history of low-risk early-stage EC who undergoes appropriate counseling, it is likely safe to consider ERT.
Among EC survivors, there has been only one prospective randomized controlled trial that assessed the effect of recurrence rate and survival in women on ERT after EC treatment.2 Patients with stage I or occult stage II endometrial adenocarcinoma treated with at least a total hysterectomy and bilateral salpingo-oophorectomy were randomized to ERT versus placebo for 3 years of treatment, with therapy starting once recovered and within 20 weeks after surgery. Trial participation required an indication for ERT, such as vasomotor symptoms, vaginal atrophy, or increased risk of cardiovascular disease or osteoporosis.
The trial accrued 1,236 patients, falling short of its goal of 2,108 patients after enrollment decreased following the publication of the Women’s Health Initiative results in 2002. This publication prompted a review of the ERT study protocol that found that between decreased accrual and lower than expected recurrence rate, goal accrual would be impossible. Of those enrolled, participants were overwhelmingly white (84%-85%), 41-70 years old (80%-82%), and had stage IA or IB disease (88%). Median follow-up was almost 3 years.
Twenty-six (2.1%) patients experienced cancer recurrence, with similar rates in both groups. Three-year progression-free and overall survival were high overall among all study participants (94.8% and 96.5%). Unfortunately, because the study was closed early, definitive conclusions about the noninferiority of ERT versus placebo regarding oncologic outcomes in early-stage endometrial adenocarcinoma could not be made.
A subsequent meta-analysis looked at the effect of hormone therapy (HT) on recurrence rate in EC survivors.3 Five observational studies were included along with the previously discussed randomized controlled trial. Among 1,975 participants across six studies, there were cancer recurrences in 19 of 896 (2.1%) HT users and 64 of 1,079 (5.9%) controls. HT did not negatively affect cancer recurrence or overall survival. There was significant heterogeneity between studies as to dosing, duration, and type of HT given (some used estrogen-only replacement, others used estrogen and progesterone replacement, and some used both estrogen only and the combination of estrogen and progesterone replacement). Among the five nonrandomized studies included, a protective effect of combined HT on EC recurrence was noted. One study included patients with stage III disease, but only four patients received HT in this cohort.
Given the data we have, ERT does not appear to significantly affect oncologic outcomes in low-risk, early-stage EC survivors. We do not have data to support this same assertion in more advanced, high-risk disease. Before initiation of any ERT in an EC survivor, there should be a detailed discussion to weigh the risks and benefits of starting therapy. The goal of treatment should be to use the lowest dose of ERT possible to treat symptoms, with planned surveillance visits for symptom check-in and assessment of readiness to start tapering treatment.
Footnote: vaginal estrogen therapy
There are no randomized trials assessing the safety of vaginal estrogen preparations or their effect on oncologic outcomes in EC survivors. Observational data from the Women’s Health Initiative showed no increased risk of endometrial cancer in patients who used vaginal estrogen with an intact uterus.4 A recently published retrospective study among 244 gynecologic cancer survivors found low rates of disease recurrence and adverse outcomes among women who used vaginal estrogen for genitourinary symptoms.5 Among EC survivors, the incidence of recurrence was 2.4% for patients with stage I/II disease and 4.3% for stage III/IV disease, with a median follow-up of 80.2 months. While there appears to be some systemic absorption with vaginal estrogen use, this can be quite challenging to measure because of the current sensitivity of serum estradiol and estrone assays. Given the significantly lower serum levels with vaginal estrogen preparations compared with ERT, vaginal estrogen use appears to be safe in EC survivors.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Cancer Stat Facts: Uterine Cancer. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Accessed 12 Aug. 2022. https://seer.cancer.gov/statfacts/html/corp.html.
2. Barakat RR et al. J Clin Oncol. 2006;24(4):587-92.
3. Shim SH et al. Eur J Cancer. 2014;50(9):1628-37.
4. Crandall CJ et al. Menopause. 2018 Jan;25(1):11-20.
5. Chambers LM et al. Int J Gynecol Cancer. 2020 Apr;30(4):515-24.
In the United States, uterine cancer is the fourth most common cancer among women, behind breast, lung/bronchus, and colorectal cancer. There are expected to be almost 66,000 new cases of uterine cancer in 2022.1 The majority of uterine cancers are endometrioid in histology and tend to be low grade, diagnosed at an early stage, and have a good prognosis. While our molecular understanding of endometrial cancers (EC) has changed significantly in recent years, low-grade endometrioid adenocarcinomas have historically been described as type 1 ECs. Type 1 ECs are typically caused by excess estrogen exposure (often unopposed or lacking progesterone protection) and are preceded by endometrial hyperplasia. Excess estrogen can come from exogenous sources (such as unopposed estrogen replacement therapy or tamoxifen, a commonly used treatment in estrogen receptor–positive breast cancer that acts as an estrogen agonist in the endometrium in postmenopausal patients) or endogenous ones (such as obesity).
Peripheral adipose tissue converts androgens into estrogens; paired with the decreased levels of sex hormone–binding globulin seen in obesity, there is more unbound or free serum estrogen (specifically estradiol) in obese women. Estrogen acts on the endometrium to cause proliferation and, if unopposed or imbalanced in relation to progesterone exposure, can ultimately lead to hyperplasia and malignancy.
If excess and unopposed estrogen exposure are major risk factors for the development of EC, is it safe to consider estrogen replacement therapy (ERT) in patients after EC treatment?
The short answer is the data are limited, but in a patient with a history of low-risk early-stage EC who undergoes appropriate counseling, it is likely safe to consider ERT.
Among EC survivors, there has been only one prospective randomized controlled trial that assessed the effect of recurrence rate and survival in women on ERT after EC treatment.2 Patients with stage I or occult stage II endometrial adenocarcinoma treated with at least a total hysterectomy and bilateral salpingo-oophorectomy were randomized to ERT versus placebo for 3 years of treatment, with therapy starting once recovered and within 20 weeks after surgery. Trial participation required an indication for ERT, such as vasomotor symptoms, vaginal atrophy, or increased risk of cardiovascular disease or osteoporosis.
The trial accrued 1,236 patients, falling short of its goal of 2,108 patients after enrollment decreased following the publication of the Women’s Health Initiative results in 2002. This publication prompted a review of the ERT study protocol that found that between decreased accrual and lower than expected recurrence rate, goal accrual would be impossible. Of those enrolled, participants were overwhelmingly white (84%-85%), 41-70 years old (80%-82%), and had stage IA or IB disease (88%). Median follow-up was almost 3 years.
Twenty-six (2.1%) patients experienced cancer recurrence, with similar rates in both groups. Three-year progression-free and overall survival were high overall among all study participants (94.8% and 96.5%). Unfortunately, because the study was closed early, definitive conclusions about the noninferiority of ERT versus placebo regarding oncologic outcomes in early-stage endometrial adenocarcinoma could not be made.
A subsequent meta-analysis looked at the effect of hormone therapy (HT) on recurrence rate in EC survivors.3 Five observational studies were included along with the previously discussed randomized controlled trial. Among 1,975 participants across six studies, there were cancer recurrences in 19 of 896 (2.1%) HT users and 64 of 1,079 (5.9%) controls. HT did not negatively affect cancer recurrence or overall survival. There was significant heterogeneity between studies as to dosing, duration, and type of HT given (some used estrogen-only replacement, others used estrogen and progesterone replacement, and some used both estrogen only and the combination of estrogen and progesterone replacement). Among the five nonrandomized studies included, a protective effect of combined HT on EC recurrence was noted. One study included patients with stage III disease, but only four patients received HT in this cohort.
Given the data we have, ERT does not appear to significantly affect oncologic outcomes in low-risk, early-stage EC survivors. We do not have data to support this same assertion in more advanced, high-risk disease. Before initiation of any ERT in an EC survivor, there should be a detailed discussion to weigh the risks and benefits of starting therapy. The goal of treatment should be to use the lowest dose of ERT possible to treat symptoms, with planned surveillance visits for symptom check-in and assessment of readiness to start tapering treatment.
Footnote: vaginal estrogen therapy
There are no randomized trials assessing the safety of vaginal estrogen preparations or their effect on oncologic outcomes in EC survivors. Observational data from the Women’s Health Initiative showed no increased risk of endometrial cancer in patients who used vaginal estrogen with an intact uterus.4 A recently published retrospective study among 244 gynecologic cancer survivors found low rates of disease recurrence and adverse outcomes among women who used vaginal estrogen for genitourinary symptoms.5 Among EC survivors, the incidence of recurrence was 2.4% for patients with stage I/II disease and 4.3% for stage III/IV disease, with a median follow-up of 80.2 months. While there appears to be some systemic absorption with vaginal estrogen use, this can be quite challenging to measure because of the current sensitivity of serum estradiol and estrone assays. Given the significantly lower serum levels with vaginal estrogen preparations compared with ERT, vaginal estrogen use appears to be safe in EC survivors.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Cancer Stat Facts: Uterine Cancer. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Accessed 12 Aug. 2022. https://seer.cancer.gov/statfacts/html/corp.html.
2. Barakat RR et al. J Clin Oncol. 2006;24(4):587-92.
3. Shim SH et al. Eur J Cancer. 2014;50(9):1628-37.
4. Crandall CJ et al. Menopause. 2018 Jan;25(1):11-20.
5. Chambers LM et al. Int J Gynecol Cancer. 2020 Apr;30(4):515-24.
In the United States, uterine cancer is the fourth most common cancer among women, behind breast, lung/bronchus, and colorectal cancer. There are expected to be almost 66,000 new cases of uterine cancer in 2022.1 The majority of uterine cancers are endometrioid in histology and tend to be low grade, diagnosed at an early stage, and have a good prognosis. While our molecular understanding of endometrial cancers (EC) has changed significantly in recent years, low-grade endometrioid adenocarcinomas have historically been described as type 1 ECs. Type 1 ECs are typically caused by excess estrogen exposure (often unopposed or lacking progesterone protection) and are preceded by endometrial hyperplasia. Excess estrogen can come from exogenous sources (such as unopposed estrogen replacement therapy or tamoxifen, a commonly used treatment in estrogen receptor–positive breast cancer that acts as an estrogen agonist in the endometrium in postmenopausal patients) or endogenous ones (such as obesity).
Peripheral adipose tissue converts androgens into estrogens; paired with the decreased levels of sex hormone–binding globulin seen in obesity, there is more unbound or free serum estrogen (specifically estradiol) in obese women. Estrogen acts on the endometrium to cause proliferation and, if unopposed or imbalanced in relation to progesterone exposure, can ultimately lead to hyperplasia and malignancy.
If excess and unopposed estrogen exposure are major risk factors for the development of EC, is it safe to consider estrogen replacement therapy (ERT) in patients after EC treatment?
The short answer is the data are limited, but in a patient with a history of low-risk early-stage EC who undergoes appropriate counseling, it is likely safe to consider ERT.
Among EC survivors, there has been only one prospective randomized controlled trial that assessed the effect of recurrence rate and survival in women on ERT after EC treatment.2 Patients with stage I or occult stage II endometrial adenocarcinoma treated with at least a total hysterectomy and bilateral salpingo-oophorectomy were randomized to ERT versus placebo for 3 years of treatment, with therapy starting once recovered and within 20 weeks after surgery. Trial participation required an indication for ERT, such as vasomotor symptoms, vaginal atrophy, or increased risk of cardiovascular disease or osteoporosis.
The trial accrued 1,236 patients, falling short of its goal of 2,108 patients after enrollment decreased following the publication of the Women’s Health Initiative results in 2002. This publication prompted a review of the ERT study protocol that found that between decreased accrual and lower than expected recurrence rate, goal accrual would be impossible. Of those enrolled, participants were overwhelmingly white (84%-85%), 41-70 years old (80%-82%), and had stage IA or IB disease (88%). Median follow-up was almost 3 years.
Twenty-six (2.1%) patients experienced cancer recurrence, with similar rates in both groups. Three-year progression-free and overall survival were high overall among all study participants (94.8% and 96.5%). Unfortunately, because the study was closed early, definitive conclusions about the noninferiority of ERT versus placebo regarding oncologic outcomes in early-stage endometrial adenocarcinoma could not be made.
A subsequent meta-analysis looked at the effect of hormone therapy (HT) on recurrence rate in EC survivors.3 Five observational studies were included along with the previously discussed randomized controlled trial. Among 1,975 participants across six studies, there were cancer recurrences in 19 of 896 (2.1%) HT users and 64 of 1,079 (5.9%) controls. HT did not negatively affect cancer recurrence or overall survival. There was significant heterogeneity between studies as to dosing, duration, and type of HT given (some used estrogen-only replacement, others used estrogen and progesterone replacement, and some used both estrogen only and the combination of estrogen and progesterone replacement). Among the five nonrandomized studies included, a protective effect of combined HT on EC recurrence was noted. One study included patients with stage III disease, but only four patients received HT in this cohort.
Given the data we have, ERT does not appear to significantly affect oncologic outcomes in low-risk, early-stage EC survivors. We do not have data to support this same assertion in more advanced, high-risk disease. Before initiation of any ERT in an EC survivor, there should be a detailed discussion to weigh the risks and benefits of starting therapy. The goal of treatment should be to use the lowest dose of ERT possible to treat symptoms, with planned surveillance visits for symptom check-in and assessment of readiness to start tapering treatment.
Footnote: vaginal estrogen therapy
There are no randomized trials assessing the safety of vaginal estrogen preparations or their effect on oncologic outcomes in EC survivors. Observational data from the Women’s Health Initiative showed no increased risk of endometrial cancer in patients who used vaginal estrogen with an intact uterus.4 A recently published retrospective study among 244 gynecologic cancer survivors found low rates of disease recurrence and adverse outcomes among women who used vaginal estrogen for genitourinary symptoms.5 Among EC survivors, the incidence of recurrence was 2.4% for patients with stage I/II disease and 4.3% for stage III/IV disease, with a median follow-up of 80.2 months. While there appears to be some systemic absorption with vaginal estrogen use, this can be quite challenging to measure because of the current sensitivity of serum estradiol and estrone assays. Given the significantly lower serum levels with vaginal estrogen preparations compared with ERT, vaginal estrogen use appears to be safe in EC survivors.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Cancer Stat Facts: Uterine Cancer. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Accessed 12 Aug. 2022. https://seer.cancer.gov/statfacts/html/corp.html.
2. Barakat RR et al. J Clin Oncol. 2006;24(4):587-92.
3. Shim SH et al. Eur J Cancer. 2014;50(9):1628-37.
4. Crandall CJ et al. Menopause. 2018 Jan;25(1):11-20.
5. Chambers LM et al. Int J Gynecol Cancer. 2020 Apr;30(4):515-24.
Racial disparities in endometrial cancer
Endometrial cancer (EC) is the most common gynecologic malignancy and is the fourth most common cancer seen in U.S. women. It is the only major cancer that has continued to see a rise in incidence and mortality for the past 2 decades, and it is anticipated that nearly 66,000 new cases of EC will be diagnosed this year with 12,550 deaths.1 Given that the well-established risk factors for developing EC including obesity, diabetes, and insulin resistance, the obesity epidemic is indisputably playing a significant role in the increasing incidence.
Historically, White women were thought to have the highest incidence of EC; however, this incidence rate did not account for hysterectomy prevalence, which can vary widely by numerous factors including age, race, ethnicity, and geographic region. When correcting EC incidence rates for prevalence of hysterectomy, Black women have had the highest incidence of EC since 2007, and rates continue to climb.2 In fact, the average annual percent change (APC) in EC incidence from 2000 to 2015 was stable for White women at 0.2% while Black women had a near order of magnitude greater APC at 2.1%.2
Differing incidence rates of EC can also be seen by histologic subtype. Endometrioid EC is the more common and less lethal histology of EC that often coincides with the type I classification of EC. These tumors are estrogen driven; therefore, they are associated with conditions resulting in excess estrogen (for example, anovulation, obesity, and hyperlipidemia). Nonendometrioid histologies, primarily composed of serous tumors, are more rare, are typically more aggressive, are not estrogen driven, and are commonly classified as type II tumors. Racial differences between type I and type II tumors are seen with White women more commonly being diagnosed with type I tumors while Black women more typically have type II tumors. White women have the greatest incidence rate of endometrioid EC with an APC that remained relatively unchanged from 2000 to 2015. Black women’s APC in incidence rate of endometrioid EC has increased during this same period at 1.3%. For nonendometrioid tumors, an increasing incidence is seen in all races and ethnicities; however, Black women have a much higher incidence of these tumors, with a rate that continues to increase at an APC of 3.2%.2
EC incidence is increasing with a particularly concerning rise in those who report Black race, but are these same disparities being seen in EC mortality? Unfortunately, drastic disparities are seen in survival data for Black women afflicted with EC. Black patients are more likely to be diagnosed with advanced or metastatic EC and less likely to be diagnosed with localized tumors. While being diagnosed with a more advanced stage of disease does affect survival in EC, Black patients have worse survival regardless of stage of disease at the time of diagnosis.1 As discussed earlier, the more aggressive type II tumors are composed of nonendometrioid histologies and are more common in Black women. This could lead to the false assumption that these higher-risk tumors are why Black women are disproportionately dying from EC; however, when examining survival by histologic subtype, Black women are more frequently dying from the lower-risk endometrioid EC regardless of stage of disease. The same disparate survival outcomes are also seen in nonendometrioid histologies.2 Thus, Black patients have the lowest survival rates irrespective of stage at diagnosis or histologic subtype.
The disparities seen in EC mortality are not new. They can be seen in data for over 30 years and are only widening. While there has been an increase in mortality rates from EC across all races and ethnicities from 2015 to 2019 compared with 1990 to 1994, the mortality rate ratio for Black women compared with White women has increased from 1.83 in 1990-1994 to 1.98 in 2015-2019.3 In the early 1990s, the risk of death from ovarian cancer was twice that of EC. The mortality of EC is now similar to that of ovarian cancer. This threshold in mortality ratio of EC to ovarian cancer has already been seen in Black women, who have experienced greater mortality in EC compared with ovarian cancer since 2005. In fact, the EC mortality of Black women in 2019 was similar to the mortality of White women with ovarian cancer nearly 30 years ago.3
Decades of data have demonstrated the glaring racial disparities seen in EC, and yet, no significant progress has been made in addressing this inequity. Oncology research is now beginning to move beyond describing these differences to a strategy of achieving equitable cancer care. While the study frameworks and novel investigations aimed at addressing the disparities in EC is outside the scope of this article, disparities in clinical trial enrollment continue to exist.
A recent example can be seen in the practice-changing KEYNOTE-775 trial, which led to the Food and Drug Administration approval of lenvatinib plus pembrolizumab in EC treatment.4 A total of 827 patients with EC that progressed or recurred following treatment with platinum-based chemotherapy were enrolled in this multinational, multicenter trial. Thirty-one (3.7%) of the patients enrolled were Black. Of those who were enrolled in the United States, 14% were Black. The authors report that this proportion of Black patients in the United States is consistent with 2020 census data, which reported 13.4% of people identified as Black. However, using census data as a benchmark for equitable enrollment is inappropriate. Certain demographic groups are historically more difficult to count, and the COVID-19 pandemic exacerbated the challenge in obtaining an accurate count through job loss, government distrust, and access restrictions resulting in an estimated net undercount of 2.45% in those who report Black race.5 Composition of trial enrollment should mirror the population that will be affected by the study results. As advanced EC disproportionately affects Black patients, their enrollment must be higher in these pivotal trials. How else are we to know if these novel therapeutics will work in the population that is most afflicted by EC?
Future studies must account for socioeconomic factors while acknowledging the role of social determinants of health. It is imperative that we use the knowledge that race is a social construct created to control access to power and that there are biologic responses to environmental stresses, including that of racism, affecting health and disease. Changes at every level, from individual practitioners up to federal policies, will need to be enacted or else the unacceptable status quo will continue.
Dr. Burkett is a clinical fellow in the division of gynecologic oncology, department of obstetrics and gynecology, at the University of North Carolina at Chapel Hill.
References
1. Siegel RL et al. CA Cancer J Clin. 2022;72:7-33.
2. Clarke MA et al. J Clin Oncol. 2019;37:1895-908.
3. Giaquinto AN et al. Obstet Gynecol. 2022;139:440-2.
4. Makker V et al. N Engl J Med. 2022;386:437-48.
5. Elliott D et al. Simulating the 2020 Census: Miscounts and the fairness of outcomes. Urban Institute; 2021.
Endometrial cancer (EC) is the most common gynecologic malignancy and is the fourth most common cancer seen in U.S. women. It is the only major cancer that has continued to see a rise in incidence and mortality for the past 2 decades, and it is anticipated that nearly 66,000 new cases of EC will be diagnosed this year with 12,550 deaths.1 Given that the well-established risk factors for developing EC including obesity, diabetes, and insulin resistance, the obesity epidemic is indisputably playing a significant role in the increasing incidence.
Historically, White women were thought to have the highest incidence of EC; however, this incidence rate did not account for hysterectomy prevalence, which can vary widely by numerous factors including age, race, ethnicity, and geographic region. When correcting EC incidence rates for prevalence of hysterectomy, Black women have had the highest incidence of EC since 2007, and rates continue to climb.2 In fact, the average annual percent change (APC) in EC incidence from 2000 to 2015 was stable for White women at 0.2% while Black women had a near order of magnitude greater APC at 2.1%.2
Differing incidence rates of EC can also be seen by histologic subtype. Endometrioid EC is the more common and less lethal histology of EC that often coincides with the type I classification of EC. These tumors are estrogen driven; therefore, they are associated with conditions resulting in excess estrogen (for example, anovulation, obesity, and hyperlipidemia). Nonendometrioid histologies, primarily composed of serous tumors, are more rare, are typically more aggressive, are not estrogen driven, and are commonly classified as type II tumors. Racial differences between type I and type II tumors are seen with White women more commonly being diagnosed with type I tumors while Black women more typically have type II tumors. White women have the greatest incidence rate of endometrioid EC with an APC that remained relatively unchanged from 2000 to 2015. Black women’s APC in incidence rate of endometrioid EC has increased during this same period at 1.3%. For nonendometrioid tumors, an increasing incidence is seen in all races and ethnicities; however, Black women have a much higher incidence of these tumors, with a rate that continues to increase at an APC of 3.2%.2
EC incidence is increasing with a particularly concerning rise in those who report Black race, but are these same disparities being seen in EC mortality? Unfortunately, drastic disparities are seen in survival data for Black women afflicted with EC. Black patients are more likely to be diagnosed with advanced or metastatic EC and less likely to be diagnosed with localized tumors. While being diagnosed with a more advanced stage of disease does affect survival in EC, Black patients have worse survival regardless of stage of disease at the time of diagnosis.1 As discussed earlier, the more aggressive type II tumors are composed of nonendometrioid histologies and are more common in Black women. This could lead to the false assumption that these higher-risk tumors are why Black women are disproportionately dying from EC; however, when examining survival by histologic subtype, Black women are more frequently dying from the lower-risk endometrioid EC regardless of stage of disease. The same disparate survival outcomes are also seen in nonendometrioid histologies.2 Thus, Black patients have the lowest survival rates irrespective of stage at diagnosis or histologic subtype.
The disparities seen in EC mortality are not new. They can be seen in data for over 30 years and are only widening. While there has been an increase in mortality rates from EC across all races and ethnicities from 2015 to 2019 compared with 1990 to 1994, the mortality rate ratio for Black women compared with White women has increased from 1.83 in 1990-1994 to 1.98 in 2015-2019.3 In the early 1990s, the risk of death from ovarian cancer was twice that of EC. The mortality of EC is now similar to that of ovarian cancer. This threshold in mortality ratio of EC to ovarian cancer has already been seen in Black women, who have experienced greater mortality in EC compared with ovarian cancer since 2005. In fact, the EC mortality of Black women in 2019 was similar to the mortality of White women with ovarian cancer nearly 30 years ago.3
Decades of data have demonstrated the glaring racial disparities seen in EC, and yet, no significant progress has been made in addressing this inequity. Oncology research is now beginning to move beyond describing these differences to a strategy of achieving equitable cancer care. While the study frameworks and novel investigations aimed at addressing the disparities in EC is outside the scope of this article, disparities in clinical trial enrollment continue to exist.
A recent example can be seen in the practice-changing KEYNOTE-775 trial, which led to the Food and Drug Administration approval of lenvatinib plus pembrolizumab in EC treatment.4 A total of 827 patients with EC that progressed or recurred following treatment with platinum-based chemotherapy were enrolled in this multinational, multicenter trial. Thirty-one (3.7%) of the patients enrolled were Black. Of those who were enrolled in the United States, 14% were Black. The authors report that this proportion of Black patients in the United States is consistent with 2020 census data, which reported 13.4% of people identified as Black. However, using census data as a benchmark for equitable enrollment is inappropriate. Certain demographic groups are historically more difficult to count, and the COVID-19 pandemic exacerbated the challenge in obtaining an accurate count through job loss, government distrust, and access restrictions resulting in an estimated net undercount of 2.45% in those who report Black race.5 Composition of trial enrollment should mirror the population that will be affected by the study results. As advanced EC disproportionately affects Black patients, their enrollment must be higher in these pivotal trials. How else are we to know if these novel therapeutics will work in the population that is most afflicted by EC?
Future studies must account for socioeconomic factors while acknowledging the role of social determinants of health. It is imperative that we use the knowledge that race is a social construct created to control access to power and that there are biologic responses to environmental stresses, including that of racism, affecting health and disease. Changes at every level, from individual practitioners up to federal policies, will need to be enacted or else the unacceptable status quo will continue.
Dr. Burkett is a clinical fellow in the division of gynecologic oncology, department of obstetrics and gynecology, at the University of North Carolina at Chapel Hill.
References
1. Siegel RL et al. CA Cancer J Clin. 2022;72:7-33.
2. Clarke MA et al. J Clin Oncol. 2019;37:1895-908.
3. Giaquinto AN et al. Obstet Gynecol. 2022;139:440-2.
4. Makker V et al. N Engl J Med. 2022;386:437-48.
5. Elliott D et al. Simulating the 2020 Census: Miscounts and the fairness of outcomes. Urban Institute; 2021.
Endometrial cancer (EC) is the most common gynecologic malignancy and is the fourth most common cancer seen in U.S. women. It is the only major cancer that has continued to see a rise in incidence and mortality for the past 2 decades, and it is anticipated that nearly 66,000 new cases of EC will be diagnosed this year with 12,550 deaths.1 Given that the well-established risk factors for developing EC including obesity, diabetes, and insulin resistance, the obesity epidemic is indisputably playing a significant role in the increasing incidence.
Historically, White women were thought to have the highest incidence of EC; however, this incidence rate did not account for hysterectomy prevalence, which can vary widely by numerous factors including age, race, ethnicity, and geographic region. When correcting EC incidence rates for prevalence of hysterectomy, Black women have had the highest incidence of EC since 2007, and rates continue to climb.2 In fact, the average annual percent change (APC) in EC incidence from 2000 to 2015 was stable for White women at 0.2% while Black women had a near order of magnitude greater APC at 2.1%.2
Differing incidence rates of EC can also be seen by histologic subtype. Endometrioid EC is the more common and less lethal histology of EC that often coincides with the type I classification of EC. These tumors are estrogen driven; therefore, they are associated with conditions resulting in excess estrogen (for example, anovulation, obesity, and hyperlipidemia). Nonendometrioid histologies, primarily composed of serous tumors, are more rare, are typically more aggressive, are not estrogen driven, and are commonly classified as type II tumors. Racial differences between type I and type II tumors are seen with White women more commonly being diagnosed with type I tumors while Black women more typically have type II tumors. White women have the greatest incidence rate of endometrioid EC with an APC that remained relatively unchanged from 2000 to 2015. Black women’s APC in incidence rate of endometrioid EC has increased during this same period at 1.3%. For nonendometrioid tumors, an increasing incidence is seen in all races and ethnicities; however, Black women have a much higher incidence of these tumors, with a rate that continues to increase at an APC of 3.2%.2
EC incidence is increasing with a particularly concerning rise in those who report Black race, but are these same disparities being seen in EC mortality? Unfortunately, drastic disparities are seen in survival data for Black women afflicted with EC. Black patients are more likely to be diagnosed with advanced or metastatic EC and less likely to be diagnosed with localized tumors. While being diagnosed with a more advanced stage of disease does affect survival in EC, Black patients have worse survival regardless of stage of disease at the time of diagnosis.1 As discussed earlier, the more aggressive type II tumors are composed of nonendometrioid histologies and are more common in Black women. This could lead to the false assumption that these higher-risk tumors are why Black women are disproportionately dying from EC; however, when examining survival by histologic subtype, Black women are more frequently dying from the lower-risk endometrioid EC regardless of stage of disease. The same disparate survival outcomes are also seen in nonendometrioid histologies.2 Thus, Black patients have the lowest survival rates irrespective of stage at diagnosis or histologic subtype.
The disparities seen in EC mortality are not new. They can be seen in data for over 30 years and are only widening. While there has been an increase in mortality rates from EC across all races and ethnicities from 2015 to 2019 compared with 1990 to 1994, the mortality rate ratio for Black women compared with White women has increased from 1.83 in 1990-1994 to 1.98 in 2015-2019.3 In the early 1990s, the risk of death from ovarian cancer was twice that of EC. The mortality of EC is now similar to that of ovarian cancer. This threshold in mortality ratio of EC to ovarian cancer has already been seen in Black women, who have experienced greater mortality in EC compared with ovarian cancer since 2005. In fact, the EC mortality of Black women in 2019 was similar to the mortality of White women with ovarian cancer nearly 30 years ago.3
Decades of data have demonstrated the glaring racial disparities seen in EC, and yet, no significant progress has been made in addressing this inequity. Oncology research is now beginning to move beyond describing these differences to a strategy of achieving equitable cancer care. While the study frameworks and novel investigations aimed at addressing the disparities in EC is outside the scope of this article, disparities in clinical trial enrollment continue to exist.
A recent example can be seen in the practice-changing KEYNOTE-775 trial, which led to the Food and Drug Administration approval of lenvatinib plus pembrolizumab in EC treatment.4 A total of 827 patients with EC that progressed or recurred following treatment with platinum-based chemotherapy were enrolled in this multinational, multicenter trial. Thirty-one (3.7%) of the patients enrolled were Black. Of those who were enrolled in the United States, 14% were Black. The authors report that this proportion of Black patients in the United States is consistent with 2020 census data, which reported 13.4% of people identified as Black. However, using census data as a benchmark for equitable enrollment is inappropriate. Certain demographic groups are historically more difficult to count, and the COVID-19 pandemic exacerbated the challenge in obtaining an accurate count through job loss, government distrust, and access restrictions resulting in an estimated net undercount of 2.45% in those who report Black race.5 Composition of trial enrollment should mirror the population that will be affected by the study results. As advanced EC disproportionately affects Black patients, their enrollment must be higher in these pivotal trials. How else are we to know if these novel therapeutics will work in the population that is most afflicted by EC?
Future studies must account for socioeconomic factors while acknowledging the role of social determinants of health. It is imperative that we use the knowledge that race is a social construct created to control access to power and that there are biologic responses to environmental stresses, including that of racism, affecting health and disease. Changes at every level, from individual practitioners up to federal policies, will need to be enacted or else the unacceptable status quo will continue.
Dr. Burkett is a clinical fellow in the division of gynecologic oncology, department of obstetrics and gynecology, at the University of North Carolina at Chapel Hill.
References
1. Siegel RL et al. CA Cancer J Clin. 2022;72:7-33.
2. Clarke MA et al. J Clin Oncol. 2019;37:1895-908.
3. Giaquinto AN et al. Obstet Gynecol. 2022;139:440-2.
4. Makker V et al. N Engl J Med. 2022;386:437-48.
5. Elliott D et al. Simulating the 2020 Census: Miscounts and the fairness of outcomes. Urban Institute; 2021.
The perils of CA-125 as a diagnostic tool in patients with adnexal masses
CA-125, or cancer antigen 125, is an epitope (antigen) on the transmembrane glycoprotein MUC16, or mucin 16. This protein is expressed on the surface of tissue derived from embryonic coelomic and Müllerian epithelium including the reproductive tract. CA-125 is also expressed in other tissue such as the pleura, lungs, pericardium, intestines, and kidneys. MUC16 plays an important role in tumor proliferation, invasiveness, and cell motility.1 In patients with epithelial ovarian cancer (EOC), CA-125 may be found on the surface of ovarian cancer cells. It is shed in the bloodstream and can be quantified using a serum test.
There are a number of CA-125 assays in commercial use, and although none have been deemed to be clinically superior, there can be some differences between assays. It is important, if possible, to use the same assay when following serial CA-125 values. Most frequently, this will mean getting the test through the same laboratory.
CA-125 has Food and Drug Administration approval for use in patients with a current or prior diagnosis of ovarian cancer to monitor treatment response, progression of disease, or disease recurrence.
It is frequently used off label in the workup of adnexal masses, while not FDA approved. CA-125 and other serum biomarkers may help determine the etiology of an adnexal mass; however, they are not diagnostic and should be used thoughtfully. It is important to have conversations with patients before ordering a CA-125 (or other serum biomarkers) about potential results and their effect on diagnosis and treatment. This will lessen some patient anxiety when tests results become available, especially in the setting of autoreleasing results under the Cures Act.
One of the reasons that CA-125 can be difficult to interpret when used as a diagnostic tool is the number of nonmalignant disease processes that can result in CA-125 elevations. CA-125 can be elevated in inflammatory and infectious disease states, including but not limited to, chronic obstructive pulmonary disease, pelvic inflammatory disease, diverticulitis, and pneumonia. Severe/critical COVID-19 infection has recently been found to be associated with increased levels of CA-125.2 It is important to obtain a complete medical history and to take specific note of any current or recent flares in inflammatory or infectious processes that could contribute to CA-125 elevations.
Special caution should be taken in premenopausal patients. The sensitivity and specificity of CA-125 are lower in this cohort of patients compared to postmenopausal women. This is multifactorial but in part due to gynecologic conditions that can increase CA-125, such as uterine fibroids and endometriosis, and the higher incidence of nonepithelial ovarian cancers (which frequently have different serum biomarkers) in younger patients. A patient’s gynecologic history, her age, and ultrasound or other imaging findings should help determine what, if any, serum biomarkers are appropriate for workup of an adnexal mass rather than the default ordering of CA-125 to determine need for referral to gynecologic oncology. If the decision has been made to take the patient to the operating room, CA-125 is not approved as a triage tool to guide who best to perform the surgery. In this case, one of two serum tumor marker panel tests that has received FDA approval for triage after the decision for surgery has been made (the multivariate index assay or the risk of ovarian malignancy algorithm) should be used.
When considering its ability to serve as a diagnostic test for ovarian cancer, the sensitivity of CA-125 is affected by the number of patients with epithelial ovarian cancer who have a test result that falls within the normal range (up to 50% of patients with stage I disease).3 The specificity of CA-125 is affected by the large number of nonmalignant conditions that can cause its elevation. Depending on the age of the patient, her menopausal status, comorbid conditions, and reason for obtaining serum biomarkers (e.g., decision for surgery has already been made), CA-125 (or CA-125 alone) may not be the best tool to use in the workup of an adnexal mass and can cause significant patient anxiety. In the setting of acute disease, such as COVID-19 infection, it may be better to delay obtaining serum biomarkers for the work-up of an adnexal mass. If delay is not feasible, then repeat serum biomarkers once the acute phase of illness has passed.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Thériault C et al. Gynecol Oncol. 2011 Jun 1;121(3):434-43.
2. Wei X et al. J Med Virol. 2020;92(10):2036-41.
3. Zurawski VR Jr et al. Int J Cancer. 1988;42:677-80.
CA-125, or cancer antigen 125, is an epitope (antigen) on the transmembrane glycoprotein MUC16, or mucin 16. This protein is expressed on the surface of tissue derived from embryonic coelomic and Müllerian epithelium including the reproductive tract. CA-125 is also expressed in other tissue such as the pleura, lungs, pericardium, intestines, and kidneys. MUC16 plays an important role in tumor proliferation, invasiveness, and cell motility.1 In patients with epithelial ovarian cancer (EOC), CA-125 may be found on the surface of ovarian cancer cells. It is shed in the bloodstream and can be quantified using a serum test.
There are a number of CA-125 assays in commercial use, and although none have been deemed to be clinically superior, there can be some differences between assays. It is important, if possible, to use the same assay when following serial CA-125 values. Most frequently, this will mean getting the test through the same laboratory.
CA-125 has Food and Drug Administration approval for use in patients with a current or prior diagnosis of ovarian cancer to monitor treatment response, progression of disease, or disease recurrence.
It is frequently used off label in the workup of adnexal masses, while not FDA approved. CA-125 and other serum biomarkers may help determine the etiology of an adnexal mass; however, they are not diagnostic and should be used thoughtfully. It is important to have conversations with patients before ordering a CA-125 (or other serum biomarkers) about potential results and their effect on diagnosis and treatment. This will lessen some patient anxiety when tests results become available, especially in the setting of autoreleasing results under the Cures Act.
One of the reasons that CA-125 can be difficult to interpret when used as a diagnostic tool is the number of nonmalignant disease processes that can result in CA-125 elevations. CA-125 can be elevated in inflammatory and infectious disease states, including but not limited to, chronic obstructive pulmonary disease, pelvic inflammatory disease, diverticulitis, and pneumonia. Severe/critical COVID-19 infection has recently been found to be associated with increased levels of CA-125.2 It is important to obtain a complete medical history and to take specific note of any current or recent flares in inflammatory or infectious processes that could contribute to CA-125 elevations.
Special caution should be taken in premenopausal patients. The sensitivity and specificity of CA-125 are lower in this cohort of patients compared to postmenopausal women. This is multifactorial but in part due to gynecologic conditions that can increase CA-125, such as uterine fibroids and endometriosis, and the higher incidence of nonepithelial ovarian cancers (which frequently have different serum biomarkers) in younger patients. A patient’s gynecologic history, her age, and ultrasound or other imaging findings should help determine what, if any, serum biomarkers are appropriate for workup of an adnexal mass rather than the default ordering of CA-125 to determine need for referral to gynecologic oncology. If the decision has been made to take the patient to the operating room, CA-125 is not approved as a triage tool to guide who best to perform the surgery. In this case, one of two serum tumor marker panel tests that has received FDA approval for triage after the decision for surgery has been made (the multivariate index assay or the risk of ovarian malignancy algorithm) should be used.
When considering its ability to serve as a diagnostic test for ovarian cancer, the sensitivity of CA-125 is affected by the number of patients with epithelial ovarian cancer who have a test result that falls within the normal range (up to 50% of patients with stage I disease).3 The specificity of CA-125 is affected by the large number of nonmalignant conditions that can cause its elevation. Depending on the age of the patient, her menopausal status, comorbid conditions, and reason for obtaining serum biomarkers (e.g., decision for surgery has already been made), CA-125 (or CA-125 alone) may not be the best tool to use in the workup of an adnexal mass and can cause significant patient anxiety. In the setting of acute disease, such as COVID-19 infection, it may be better to delay obtaining serum biomarkers for the work-up of an adnexal mass. If delay is not feasible, then repeat serum biomarkers once the acute phase of illness has passed.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Thériault C et al. Gynecol Oncol. 2011 Jun 1;121(3):434-43.
2. Wei X et al. J Med Virol. 2020;92(10):2036-41.
3. Zurawski VR Jr et al. Int J Cancer. 1988;42:677-80.
CA-125, or cancer antigen 125, is an epitope (antigen) on the transmembrane glycoprotein MUC16, or mucin 16. This protein is expressed on the surface of tissue derived from embryonic coelomic and Müllerian epithelium including the reproductive tract. CA-125 is also expressed in other tissue such as the pleura, lungs, pericardium, intestines, and kidneys. MUC16 plays an important role in tumor proliferation, invasiveness, and cell motility.1 In patients with epithelial ovarian cancer (EOC), CA-125 may be found on the surface of ovarian cancer cells. It is shed in the bloodstream and can be quantified using a serum test.
There are a number of CA-125 assays in commercial use, and although none have been deemed to be clinically superior, there can be some differences between assays. It is important, if possible, to use the same assay when following serial CA-125 values. Most frequently, this will mean getting the test through the same laboratory.
CA-125 has Food and Drug Administration approval for use in patients with a current or prior diagnosis of ovarian cancer to monitor treatment response, progression of disease, or disease recurrence.
It is frequently used off label in the workup of adnexal masses, while not FDA approved. CA-125 and other serum biomarkers may help determine the etiology of an adnexal mass; however, they are not diagnostic and should be used thoughtfully. It is important to have conversations with patients before ordering a CA-125 (or other serum biomarkers) about potential results and their effect on diagnosis and treatment. This will lessen some patient anxiety when tests results become available, especially in the setting of autoreleasing results under the Cures Act.
One of the reasons that CA-125 can be difficult to interpret when used as a diagnostic tool is the number of nonmalignant disease processes that can result in CA-125 elevations. CA-125 can be elevated in inflammatory and infectious disease states, including but not limited to, chronic obstructive pulmonary disease, pelvic inflammatory disease, diverticulitis, and pneumonia. Severe/critical COVID-19 infection has recently been found to be associated with increased levels of CA-125.2 It is important to obtain a complete medical history and to take specific note of any current or recent flares in inflammatory or infectious processes that could contribute to CA-125 elevations.
Special caution should be taken in premenopausal patients. The sensitivity and specificity of CA-125 are lower in this cohort of patients compared to postmenopausal women. This is multifactorial but in part due to gynecologic conditions that can increase CA-125, such as uterine fibroids and endometriosis, and the higher incidence of nonepithelial ovarian cancers (which frequently have different serum biomarkers) in younger patients. A patient’s gynecologic history, her age, and ultrasound or other imaging findings should help determine what, if any, serum biomarkers are appropriate for workup of an adnexal mass rather than the default ordering of CA-125 to determine need for referral to gynecologic oncology. If the decision has been made to take the patient to the operating room, CA-125 is not approved as a triage tool to guide who best to perform the surgery. In this case, one of two serum tumor marker panel tests that has received FDA approval for triage after the decision for surgery has been made (the multivariate index assay or the risk of ovarian malignancy algorithm) should be used.
When considering its ability to serve as a diagnostic test for ovarian cancer, the sensitivity of CA-125 is affected by the number of patients with epithelial ovarian cancer who have a test result that falls within the normal range (up to 50% of patients with stage I disease).3 The specificity of CA-125 is affected by the large number of nonmalignant conditions that can cause its elevation. Depending on the age of the patient, her menopausal status, comorbid conditions, and reason for obtaining serum biomarkers (e.g., decision for surgery has already been made), CA-125 (or CA-125 alone) may not be the best tool to use in the workup of an adnexal mass and can cause significant patient anxiety. In the setting of acute disease, such as COVID-19 infection, it may be better to delay obtaining serum biomarkers for the work-up of an adnexal mass. If delay is not feasible, then repeat serum biomarkers once the acute phase of illness has passed.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Thériault C et al. Gynecol Oncol. 2011 Jun 1;121(3):434-43.
2. Wei X et al. J Med Virol. 2020;92(10):2036-41.
3. Zurawski VR Jr et al. Int J Cancer. 1988;42:677-80.









