User login
Mr. B, age 27, has a 10-year history of schizophrenia. Last year, he was doing well and working 4 hours/day 3 days/week while taking oral risperidone, 6 mg, at bedtime. However, during the past 2 weeks Mr. B began to have a return of auditory hallucinations and reports that he stopped taking his medication again 6 weeks ago.
As a result, he is started on paliperidone palmitate following the product label’s initiation dosing recommendation. On the first day he is given the first dose of the initiation regimen, 234 mg IM. One week later, the second dose of the initiation regimen, 156 mg IM, is given. One month later, the first maintenance dose of 117 mg IM every 28 days is given. All injections are in his deltoid muscle at his request.
After 3 weeks on the first maintenance dose of 117 mg, the voices begin to bother him again. Subsequently, Mr. B’s maintenance dose is increased first to 156 mg, and for the same problem with breakthrough hallucinations the following month to 234 mg, the maximum dose in the product label. After 6 months of receiving 234 mg IM every 28 days, the auditory hallucinations continue to bother him, but only for a few days prior to his next injection. He misses work 1 or 2 times before each injection.
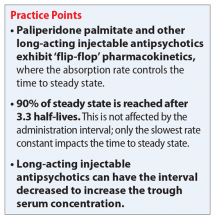
Can the injection frequency for Mr. B’s paliperidone palmitate, 234 mg IM, in the deltoid muscle be increased to every 21 days to prevent the monthly exacerbations? Yes, the injection frequency can be increased, and doing so will increase the concentrations of paliperidone. The use of long-acting injectable antipsychotics (LAIs) is complicated by the lengthy time needed to reach steady state. In the case of paliperidone palmitate, an initiation regimen was developed that achieves therapeutic concentrations that are close to steady state before the oral antipsychotic’s effects are lost. This initiation strategy avoids the need for oral supplementation to maintain clinical efficacy. However, even using an initiation regimen or a loading dose does not decrease the time to final steady state after a dose adjustment due to the slow absorption of the medication from the injection site. The time to steady state is controlled by “flip-flop” pharmacokinetics. In this kind of pharmacokinetics, which is observed with all LAIs, the absorption rate from the injection site is lower than the elimination rate.1
Cleton et al2 reported the pharmacokinetics of paliperidone palmitate for deltoid and gluteal injection sites. By combining the median data for the deltoid injection route from the article by Cleton et al2 and the dosing from Mr. B’s case, I created a model using superposition of a sixth-degree polynomial fitted to the single dose data. Gluteal injections were not included because their increased complexity is beyond the scope of this article, but the time to maximum concentration (gluteal > deltoid) and peak concentration (deltoid > gluteal) are different for each route. The polynomial was a good fit with the adjusted r2 = 0.976, P < .0001. This model illustrates the paliperidone serum concentrations for Mr. B and is shown in the Figure. As you can see, by Day 9, the serum concentrations had reached the lower limit of the expected range of 20 to 60 ng/mL, shown in the shaded region of the Figure.3
Steady state at the routine maintenance dose of 117 mg every 28 days was never reached as the medication was not sufficient to suppress Mr. B’s hallucinations, and his doses needed to be increased each month. First, Mr. B’s dose was increased to 156 mg and then to the maximum recommended dose of 234 mg every 28 days. Steady state can be considered to have been achieved when 90% of the final steady state is reached after 3.3 half-lives. Because of the flip-flop pharmacokinetics, the important half-life is the absorption half-life of approximately 40 days or 132 days at the same dose. In Mr. B’s case, this was Day 221, where the trough concentration was 35 ng/mL. However, this regimen was still inadequate because he had breakthrough symptoms prior to the next injection.
By decreasing the injection interval from 28 days to 21 days, the concentrations will increase to a new steady state. This will take the same 132 days. With the reduced injection frequency of 21 days, 7 injections will have been given prior to reaching the new steady state. Steady state is not dependent on the number of injections, but only on the absorption half-life. This new steady state trough is substantially higher at 52 ng/mL, but still in the expected range for commonly used doses. Because Mr. B’s hallucinations only appeared at the end of the dosing interval, it is reasonable to expect that his new regimen would be successful in suppressing his hallucinations. However, monitoring for peak-related adverse effects is essential. Based upon controlled clinical trials, the potential dose-related adverse effects of paliperidone include akathisia, other extrapyramidal symptoms, weight gain, and QTc prolongation.
Continue to: Would monitoring a patient's paliperidone serum concentrations be useful?
Would monitoring a patient’s paliperidone serum concentrations be useful? Currently, measuring an individual’s paliperidone serum concentration is generally considered unwarranted.3,4 One of the major reasons is a lack of appropriately designed studies to determine a therapeutic range.5 Flexible dose designs, commonly used in registration studies, cloud the relationships between concentration, time, response, and adverse effects. There are additional problems that are the result of diagnostic heterogeneity and placebo responders. A well-designed study to determine the therapeutic range would have ≥1 fixed dose groups and be diagnostically homogeneous. There are currently only a limited number of clinical laboratories that have implemented suitable assays.
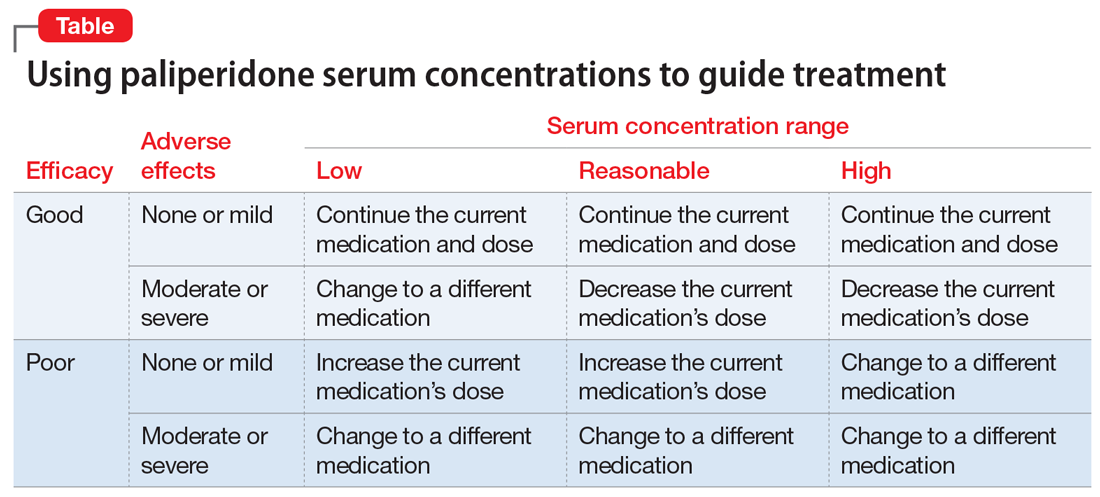
Given the lack of knowledge of a therapeutic range, assured knowledge of nonadherence to LAIs, and the absence of significant drug interactions for paliperidone, there remain a few reasonable justifications for obtaining a patient’s paliperidone serum concentration (Table). If the patient had a good response with mild adverse effects, there is no reason to obtain a paliperidone serum concentration or make any change in the medication or dose. However, if the patient had a good response accompanied by moderate or severe adverse effects, or the patient has a poor response, then obtaining the paliperidone serum concentration could help determine an appropriate course of action.
CASE CONTINUED
After the second dose at the increased frequency on Day 252, the paliperidone serum concentration was maintained above 40 ng/mL. Mr. B continued to tolerate the LAI well and no longer reported any breakthrough hallucinations.
Related Resources
- ARUP Laboratories. Paliperidone, serum or plasma. http://ltd.aruplab.com/tests/pub/2007949.
- LabCorp. Paliperidone Paliperidone (as 9-hydroxyrisperidone), serum or plasma. https://www.labcorp.com/test-menu/38351/paliperidone-as-9-hydroxyrisperidone-serum-or-plasma.
- Janssen Scientific Affairs. Educational dose illustrator. http://www.educationaldoseillustrator.com.
Drug Brand Names
Paliperidone palmitate • Invega Sustenna
Risperidone • Risperdal
1. Jann MW, Ereshefsky L, Saklad SR. Clinical pharmacokinetics of the depot antipsychotics. Clin Pharmacokinet. 1985;10(4):315-333.
2. Cleton A, Rossenu S, Crauwels H, et al. A single-dose, open-label, parallel, randomized, dose-proportionality study of paliperidone after intramuscular injections of paliperidone palmitate in the deltoid or gluteal muscle in patients with schizophrenia. J Clin Pharmacol. 2014;54(9):1048-1057.
3. Taylor D, Paton C, Kapur S. The Maudsley prescribing guidelines in psychiatry. 12th ed. Oxford, UK: John Wiley & Sons, Ltd.; 2015:1-10.
4. Hiemke C, Baumann P, Bergemann N, et al. AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry. 2011;44(6):195-235.
5. Lopez LV, Kane JM. Plasma levels of second-generation antipsychotics and clinical response in acute psychosis: a review of the literature. Schizophr Res. 2013;147(2-3):368-374.
Mr. B, age 27, has a 10-year history of schizophrenia. Last year, he was doing well and working 4 hours/day 3 days/week while taking oral risperidone, 6 mg, at bedtime. However, during the past 2 weeks Mr. B began to have a return of auditory hallucinations and reports that he stopped taking his medication again 6 weeks ago.
As a result, he is started on paliperidone palmitate following the product label’s initiation dosing recommendation. On the first day he is given the first dose of the initiation regimen, 234 mg IM. One week later, the second dose of the initiation regimen, 156 mg IM, is given. One month later, the first maintenance dose of 117 mg IM every 28 days is given. All injections are in his deltoid muscle at his request.
After 3 weeks on the first maintenance dose of 117 mg, the voices begin to bother him again. Subsequently, Mr. B’s maintenance dose is increased first to 156 mg, and for the same problem with breakthrough hallucinations the following month to 234 mg, the maximum dose in the product label. After 6 months of receiving 234 mg IM every 28 days, the auditory hallucinations continue to bother him, but only for a few days prior to his next injection. He misses work 1 or 2 times before each injection.
Can the injection frequency for Mr. B’s paliperidone palmitate, 234 mg IM, in the deltoid muscle be increased to every 21 days to prevent the monthly exacerbations? Yes, the injection frequency can be increased, and doing so will increase the concentrations of paliperidone. The use of long-acting injectable antipsychotics (LAIs) is complicated by the lengthy time needed to reach steady state. In the case of paliperidone palmitate, an initiation regimen was developed that achieves therapeutic concentrations that are close to steady state before the oral antipsychotic’s effects are lost. This initiation strategy avoids the need for oral supplementation to maintain clinical efficacy. However, even using an initiation regimen or a loading dose does not decrease the time to final steady state after a dose adjustment due to the slow absorption of the medication from the injection site. The time to steady state is controlled by “flip-flop” pharmacokinetics. In this kind of pharmacokinetics, which is observed with all LAIs, the absorption rate from the injection site is lower than the elimination rate.1
Cleton et al2 reported the pharmacokinetics of paliperidone palmitate for deltoid and gluteal injection sites. By combining the median data for the deltoid injection route from the article by Cleton et al2 and the dosing from Mr. B’s case, I created a model using superposition of a sixth-degree polynomial fitted to the single dose data. Gluteal injections were not included because their increased complexity is beyond the scope of this article, but the time to maximum concentration (gluteal > deltoid) and peak concentration (deltoid > gluteal) are different for each route. The polynomial was a good fit with the adjusted r2 = 0.976, P < .0001. This model illustrates the paliperidone serum concentrations for Mr. B and is shown in the Figure. As you can see, by Day 9, the serum concentrations had reached the lower limit of the expected range of 20 to 60 ng/mL, shown in the shaded region of the Figure.3
Steady state at the routine maintenance dose of 117 mg every 28 days was never reached as the medication was not sufficient to suppress Mr. B’s hallucinations, and his doses needed to be increased each month. First, Mr. B’s dose was increased to 156 mg and then to the maximum recommended dose of 234 mg every 28 days. Steady state can be considered to have been achieved when 90% of the final steady state is reached after 3.3 half-lives. Because of the flip-flop pharmacokinetics, the important half-life is the absorption half-life of approximately 40 days or 132 days at the same dose. In Mr. B’s case, this was Day 221, where the trough concentration was 35 ng/mL. However, this regimen was still inadequate because he had breakthrough symptoms prior to the next injection.
By decreasing the injection interval from 28 days to 21 days, the concentrations will increase to a new steady state. This will take the same 132 days. With the reduced injection frequency of 21 days, 7 injections will have been given prior to reaching the new steady state. Steady state is not dependent on the number of injections, but only on the absorption half-life. This new steady state trough is substantially higher at 52 ng/mL, but still in the expected range for commonly used doses. Because Mr. B’s hallucinations only appeared at the end of the dosing interval, it is reasonable to expect that his new regimen would be successful in suppressing his hallucinations. However, monitoring for peak-related adverse effects is essential. Based upon controlled clinical trials, the potential dose-related adverse effects of paliperidone include akathisia, other extrapyramidal symptoms, weight gain, and QTc prolongation.
Continue to: Would monitoring a patient's paliperidone serum concentrations be useful?
Would monitoring a patient’s paliperidone serum concentrations be useful? Currently, measuring an individual’s paliperidone serum concentration is generally considered unwarranted.3,4 One of the major reasons is a lack of appropriately designed studies to determine a therapeutic range.5 Flexible dose designs, commonly used in registration studies, cloud the relationships between concentration, time, response, and adverse effects. There are additional problems that are the result of diagnostic heterogeneity and placebo responders. A well-designed study to determine the therapeutic range would have ≥1 fixed dose groups and be diagnostically homogeneous. There are currently only a limited number of clinical laboratories that have implemented suitable assays.
Given the lack of knowledge of a therapeutic range, assured knowledge of nonadherence to LAIs, and the absence of significant drug interactions for paliperidone, there remain a few reasonable justifications for obtaining a patient’s paliperidone serum concentration (Table). If the patient had a good response with mild adverse effects, there is no reason to obtain a paliperidone serum concentration or make any change in the medication or dose. However, if the patient had a good response accompanied by moderate or severe adverse effects, or the patient has a poor response, then obtaining the paliperidone serum concentration could help determine an appropriate course of action.
CASE CONTINUED
After the second dose at the increased frequency on Day 252, the paliperidone serum concentration was maintained above 40 ng/mL. Mr. B continued to tolerate the LAI well and no longer reported any breakthrough hallucinations.
Related Resources
- ARUP Laboratories. Paliperidone, serum or plasma. http://ltd.aruplab.com/tests/pub/2007949.
- LabCorp. Paliperidone Paliperidone (as 9-hydroxyrisperidone), serum or plasma. https://www.labcorp.com/test-menu/38351/paliperidone-as-9-hydroxyrisperidone-serum-or-plasma.
- Janssen Scientific Affairs. Educational dose illustrator. http://www.educationaldoseillustrator.com.
Drug Brand Names
Paliperidone palmitate • Invega Sustenna
Risperidone • Risperdal
Mr. B, age 27, has a 10-year history of schizophrenia. Last year, he was doing well and working 4 hours/day 3 days/week while taking oral risperidone, 6 mg, at bedtime. However, during the past 2 weeks Mr. B began to have a return of auditory hallucinations and reports that he stopped taking his medication again 6 weeks ago.
As a result, he is started on paliperidone palmitate following the product label’s initiation dosing recommendation. On the first day he is given the first dose of the initiation regimen, 234 mg IM. One week later, the second dose of the initiation regimen, 156 mg IM, is given. One month later, the first maintenance dose of 117 mg IM every 28 days is given. All injections are in his deltoid muscle at his request.
After 3 weeks on the first maintenance dose of 117 mg, the voices begin to bother him again. Subsequently, Mr. B’s maintenance dose is increased first to 156 mg, and for the same problem with breakthrough hallucinations the following month to 234 mg, the maximum dose in the product label. After 6 months of receiving 234 mg IM every 28 days, the auditory hallucinations continue to bother him, but only for a few days prior to his next injection. He misses work 1 or 2 times before each injection.
Can the injection frequency for Mr. B’s paliperidone palmitate, 234 mg IM, in the deltoid muscle be increased to every 21 days to prevent the monthly exacerbations? Yes, the injection frequency can be increased, and doing so will increase the concentrations of paliperidone. The use of long-acting injectable antipsychotics (LAIs) is complicated by the lengthy time needed to reach steady state. In the case of paliperidone palmitate, an initiation regimen was developed that achieves therapeutic concentrations that are close to steady state before the oral antipsychotic’s effects are lost. This initiation strategy avoids the need for oral supplementation to maintain clinical efficacy. However, even using an initiation regimen or a loading dose does not decrease the time to final steady state after a dose adjustment due to the slow absorption of the medication from the injection site. The time to steady state is controlled by “flip-flop” pharmacokinetics. In this kind of pharmacokinetics, which is observed with all LAIs, the absorption rate from the injection site is lower than the elimination rate.1
Cleton et al2 reported the pharmacokinetics of paliperidone palmitate for deltoid and gluteal injection sites. By combining the median data for the deltoid injection route from the article by Cleton et al2 and the dosing from Mr. B’s case, I created a model using superposition of a sixth-degree polynomial fitted to the single dose data. Gluteal injections were not included because their increased complexity is beyond the scope of this article, but the time to maximum concentration (gluteal > deltoid) and peak concentration (deltoid > gluteal) are different for each route. The polynomial was a good fit with the adjusted r2 = 0.976, P < .0001. This model illustrates the paliperidone serum concentrations for Mr. B and is shown in the Figure. As you can see, by Day 9, the serum concentrations had reached the lower limit of the expected range of 20 to 60 ng/mL, shown in the shaded region of the Figure.3
Steady state at the routine maintenance dose of 117 mg every 28 days was never reached as the medication was not sufficient to suppress Mr. B’s hallucinations, and his doses needed to be increased each month. First, Mr. B’s dose was increased to 156 mg and then to the maximum recommended dose of 234 mg every 28 days. Steady state can be considered to have been achieved when 90% of the final steady state is reached after 3.3 half-lives. Because of the flip-flop pharmacokinetics, the important half-life is the absorption half-life of approximately 40 days or 132 days at the same dose. In Mr. B’s case, this was Day 221, where the trough concentration was 35 ng/mL. However, this regimen was still inadequate because he had breakthrough symptoms prior to the next injection.
By decreasing the injection interval from 28 days to 21 days, the concentrations will increase to a new steady state. This will take the same 132 days. With the reduced injection frequency of 21 days, 7 injections will have been given prior to reaching the new steady state. Steady state is not dependent on the number of injections, but only on the absorption half-life. This new steady state trough is substantially higher at 52 ng/mL, but still in the expected range for commonly used doses. Because Mr. B’s hallucinations only appeared at the end of the dosing interval, it is reasonable to expect that his new regimen would be successful in suppressing his hallucinations. However, monitoring for peak-related adverse effects is essential. Based upon controlled clinical trials, the potential dose-related adverse effects of paliperidone include akathisia, other extrapyramidal symptoms, weight gain, and QTc prolongation.
Continue to: Would monitoring a patient's paliperidone serum concentrations be useful?
Would monitoring a patient’s paliperidone serum concentrations be useful? Currently, measuring an individual’s paliperidone serum concentration is generally considered unwarranted.3,4 One of the major reasons is a lack of appropriately designed studies to determine a therapeutic range.5 Flexible dose designs, commonly used in registration studies, cloud the relationships between concentration, time, response, and adverse effects. There are additional problems that are the result of diagnostic heterogeneity and placebo responders. A well-designed study to determine the therapeutic range would have ≥1 fixed dose groups and be diagnostically homogeneous. There are currently only a limited number of clinical laboratories that have implemented suitable assays.
Given the lack of knowledge of a therapeutic range, assured knowledge of nonadherence to LAIs, and the absence of significant drug interactions for paliperidone, there remain a few reasonable justifications for obtaining a patient’s paliperidone serum concentration (Table). If the patient had a good response with mild adverse effects, there is no reason to obtain a paliperidone serum concentration or make any change in the medication or dose. However, if the patient had a good response accompanied by moderate or severe adverse effects, or the patient has a poor response, then obtaining the paliperidone serum concentration could help determine an appropriate course of action.
CASE CONTINUED
After the second dose at the increased frequency on Day 252, the paliperidone serum concentration was maintained above 40 ng/mL. Mr. B continued to tolerate the LAI well and no longer reported any breakthrough hallucinations.
Related Resources
- ARUP Laboratories. Paliperidone, serum or plasma. http://ltd.aruplab.com/tests/pub/2007949.
- LabCorp. Paliperidone Paliperidone (as 9-hydroxyrisperidone), serum or plasma. https://www.labcorp.com/test-menu/38351/paliperidone-as-9-hydroxyrisperidone-serum-or-plasma.
- Janssen Scientific Affairs. Educational dose illustrator. http://www.educationaldoseillustrator.com.
Drug Brand Names
Paliperidone palmitate • Invega Sustenna
Risperidone • Risperdal
1. Jann MW, Ereshefsky L, Saklad SR. Clinical pharmacokinetics of the depot antipsychotics. Clin Pharmacokinet. 1985;10(4):315-333.
2. Cleton A, Rossenu S, Crauwels H, et al. A single-dose, open-label, parallel, randomized, dose-proportionality study of paliperidone after intramuscular injections of paliperidone palmitate in the deltoid or gluteal muscle in patients with schizophrenia. J Clin Pharmacol. 2014;54(9):1048-1057.
3. Taylor D, Paton C, Kapur S. The Maudsley prescribing guidelines in psychiatry. 12th ed. Oxford, UK: John Wiley & Sons, Ltd.; 2015:1-10.
4. Hiemke C, Baumann P, Bergemann N, et al. AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry. 2011;44(6):195-235.
5. Lopez LV, Kane JM. Plasma levels of second-generation antipsychotics and clinical response in acute psychosis: a review of the literature. Schizophr Res. 2013;147(2-3):368-374.
1. Jann MW, Ereshefsky L, Saklad SR. Clinical pharmacokinetics of the depot antipsychotics. Clin Pharmacokinet. 1985;10(4):315-333.
2. Cleton A, Rossenu S, Crauwels H, et al. A single-dose, open-label, parallel, randomized, dose-proportionality study of paliperidone after intramuscular injections of paliperidone palmitate in the deltoid or gluteal muscle in patients with schizophrenia. J Clin Pharmacol. 2014;54(9):1048-1057.
3. Taylor D, Paton C, Kapur S. The Maudsley prescribing guidelines in psychiatry. 12th ed. Oxford, UK: John Wiley & Sons, Ltd.; 2015:1-10.
4. Hiemke C, Baumann P, Bergemann N, et al. AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry. 2011;44(6):195-235.
5. Lopez LV, Kane JM. Plasma levels of second-generation antipsychotics and clinical response in acute psychosis: a review of the literature. Schizophr Res. 2013;147(2-3):368-374.