User login
Ovarian cancer remains the leading cause of death from gynecologic cancer worldwide and one of the five leading causes of death from cancer in women in the United States. In addition to surgery, treatment consists of combination platinum and taxane chemotherapy that offers a high response rate; however, a majority of women will develop persistent or recurrent disease.
A clinical practice statement released by the Society of Gynecologic Oncology in October 2014 states that “women diagnosed with epithelial ovarian, tubal, and peritoneal cancers should receive genetic counseling and be offered genetic testing, even in the absence of family history.” Patients should be informed that this genetic testing serves to prognosticate, inform about personal and familial cancer risk, but also aids in choices of novel therapeutic agents, specifically Poly (ADP-ribose) polymerase (PARP) inhibitors.
Genetic involvement of BRCA
A small proportion of ovarian cancers are attributable to genetic mutations, with approximately 10%-15% of cases caused by germline mutations of BRCA1 and BRCA2. BRCA1 deleterious mutations confer an ovarian cancer risk of approximately 39%-46%; and the risk of ovarian cancer is roughly 12%-20% for patients with BRCA2 deleterious mutations. As a tumor suppressor gene, BRCA is involved in the DNA repair process. Specifically, it is involved in homologous recombination (a form of double-stranded DNA repair mechanism). Thus, cells with defective BRCA proteins cannot repair double-stranded breaks (DSB) in DNA.
The homologous recombination pathway is complex and involves a number of genes. Deficiencies in this pathway confer a sensitivity to PARP inhibition. Tumors that share dysfunction in the homologous recombination pathway, but do not contain mutations in the BRCA gene, are classified as tumors with “BRCAness.”
Generally, the inheritance of a defective BRCA1 or BRCA2 allele (a germline mutation) alone is not enough to cause the development of cancer. Instead, once the second, functioning allele becomes nonfunctional, cancer can arise through an accumulation of mutations in the genetic code.
Furthermore, regardless of germline BRCA status, cancers have high rates of genetic mutation. As a result of the mutation rate, tumors can develop noninherited, noninheritable alterations in BRCA1 or BRCA2 genes (a somatic mutation).
Mechanism of PARP inhibitor activity
The PARP family of enzymes hold a vital role in the repair of DNA and the stabilization of the human genome through the repair of single-stranded breaks (SSB) in DNA. PARP inhibitors were originally developed as a chemosensitizing agent for other cytotoxic agents. It was only later discovered that ovarian cancer cells and mouse models that were deficient in BRCA proteins were especially sensitive to PARP inhibition. Eventually, the clinical development strategy became to employ PARP inhibitors in selected patients with BRCA mutations.
As previously mentioned, cells deficient in the tumor suppressor genes (BRCA1 and BRCA2) have an inability to repair DSBs. Inhibiting PARP enzymes will therefore cause an increase in SSB. During cell replication, these SSBs are converted to DSBs. Ultimately, the accumulation of DSBs leads to cell death. The concept that these two deficiencies – which alone are nonlethal – can be combined to induce cell death is described as synthetic lethality.
The exact mechanism through which PARP inhibitors function is not fully understood; however, four models currently exist to explain how PARP inhibitors instigate synthetic lethality. PARP inhibitors may block base excision repair mechanisms, trap PARP enzymes on damaged DNA, reduce the affinity of functioning BRCA enzymes to damaged DNA, and suppress nonhomologous end joining repair mechanisms.1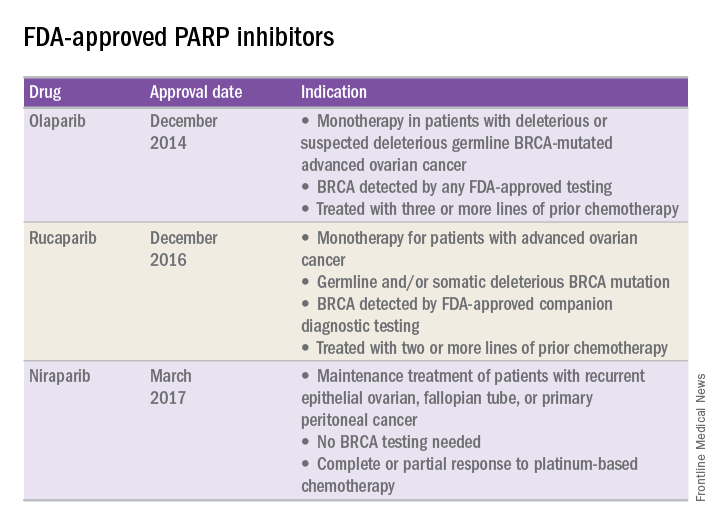
FDA approval of PARP inhibitors
In recent years, the Food and Drug Administration has approved three PARP inhibitors in the treatment of ovarian cancer in slightly different clinical scenarios.
Olaparib was tested in a trial of 193 patients who harbored a deleterious or suspected deleterious germline BRCA-associated ovarian cancer who had received prior therapies.2 Overall, the response rate in this population was 41% (95% confidence interval, 28-54) with a median duration of response of 8.0 months. These results led to the FDA approval of olaparib for ovarian cancer treatment as fourth-line therapy in patients with BRCA mutations.
Two separate trials using rucaparib showed an overall response rate of 54% and a duration of response of 9.2 months.3,4 These early results allowed the FDA to grant accelerated approval to another PARP inhibitor for use in ovarian cancer.
More recently, a phase III trial of niraparib maintenance therapy versus placebo enrolled 553 women with recurrent epithelial ovarian cancer.5 Women with germline BRCA mutations had recurrence-free intervals of 21 months on niraparib, compared with 5.5 months for those on placebo. Even without germline BRCA mutations, women benefited from a recurrence-free interval of 9.3 months, compared with 3.9 months for placebo.
PARP inhibitors represent a novel targeted therapy for ovarian cancer, particularly those with deleterious germline or somatic BRCA mutations. When combined with genetic testing for BRCA mutations, PARP inhibitors represent an example of a predictive biomarker paired with a tailored therapeutic. Maturing data from ongoing trials will likely expand the opportunity to use PARP inhibitors for the treatment of ovarian cancer.
References
1. Br J Cancer. 2016 Nov 8;115(10):1157-73.
2. J Clin Oncol. 2015 Jan 20;33(3):244-50.
3. Clin Cancer Res. 2017 Mar 6. pii: clincanres.2796.2016. doi: 10.1158/1078-0432.CCR-16-2796.
4. Lancet Oncol. 2017 Jan;18(1):75-87.
5. N Engl J Med 2016; 375:2154-64.
Dr. Tran is a gynecologic oncology fellow in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC-Chapel Hill. They reported having no relevant financial disclosures.
Ovarian cancer remains the leading cause of death from gynecologic cancer worldwide and one of the five leading causes of death from cancer in women in the United States. In addition to surgery, treatment consists of combination platinum and taxane chemotherapy that offers a high response rate; however, a majority of women will develop persistent or recurrent disease.
A clinical practice statement released by the Society of Gynecologic Oncology in October 2014 states that “women diagnosed with epithelial ovarian, tubal, and peritoneal cancers should receive genetic counseling and be offered genetic testing, even in the absence of family history.” Patients should be informed that this genetic testing serves to prognosticate, inform about personal and familial cancer risk, but also aids in choices of novel therapeutic agents, specifically Poly (ADP-ribose) polymerase (PARP) inhibitors.
Genetic involvement of BRCA
A small proportion of ovarian cancers are attributable to genetic mutations, with approximately 10%-15% of cases caused by germline mutations of BRCA1 and BRCA2. BRCA1 deleterious mutations confer an ovarian cancer risk of approximately 39%-46%; and the risk of ovarian cancer is roughly 12%-20% for patients with BRCA2 deleterious mutations. As a tumor suppressor gene, BRCA is involved in the DNA repair process. Specifically, it is involved in homologous recombination (a form of double-stranded DNA repair mechanism). Thus, cells with defective BRCA proteins cannot repair double-stranded breaks (DSB) in DNA.
The homologous recombination pathway is complex and involves a number of genes. Deficiencies in this pathway confer a sensitivity to PARP inhibition. Tumors that share dysfunction in the homologous recombination pathway, but do not contain mutations in the BRCA gene, are classified as tumors with “BRCAness.”
Generally, the inheritance of a defective BRCA1 or BRCA2 allele (a germline mutation) alone is not enough to cause the development of cancer. Instead, once the second, functioning allele becomes nonfunctional, cancer can arise through an accumulation of mutations in the genetic code.
Furthermore, regardless of germline BRCA status, cancers have high rates of genetic mutation. As a result of the mutation rate, tumors can develop noninherited, noninheritable alterations in BRCA1 or BRCA2 genes (a somatic mutation).
Mechanism of PARP inhibitor activity
The PARP family of enzymes hold a vital role in the repair of DNA and the stabilization of the human genome through the repair of single-stranded breaks (SSB) in DNA. PARP inhibitors were originally developed as a chemosensitizing agent for other cytotoxic agents. It was only later discovered that ovarian cancer cells and mouse models that were deficient in BRCA proteins were especially sensitive to PARP inhibition. Eventually, the clinical development strategy became to employ PARP inhibitors in selected patients with BRCA mutations.
As previously mentioned, cells deficient in the tumor suppressor genes (BRCA1 and BRCA2) have an inability to repair DSBs. Inhibiting PARP enzymes will therefore cause an increase in SSB. During cell replication, these SSBs are converted to DSBs. Ultimately, the accumulation of DSBs leads to cell death. The concept that these two deficiencies – which alone are nonlethal – can be combined to induce cell death is described as synthetic lethality.
The exact mechanism through which PARP inhibitors function is not fully understood; however, four models currently exist to explain how PARP inhibitors instigate synthetic lethality. PARP inhibitors may block base excision repair mechanisms, trap PARP enzymes on damaged DNA, reduce the affinity of functioning BRCA enzymes to damaged DNA, and suppress nonhomologous end joining repair mechanisms.1
FDA approval of PARP inhibitors
In recent years, the Food and Drug Administration has approved three PARP inhibitors in the treatment of ovarian cancer in slightly different clinical scenarios.
Olaparib was tested in a trial of 193 patients who harbored a deleterious or suspected deleterious germline BRCA-associated ovarian cancer who had received prior therapies.2 Overall, the response rate in this population was 41% (95% confidence interval, 28-54) with a median duration of response of 8.0 months. These results led to the FDA approval of olaparib for ovarian cancer treatment as fourth-line therapy in patients with BRCA mutations.
Two separate trials using rucaparib showed an overall response rate of 54% and a duration of response of 9.2 months.3,4 These early results allowed the FDA to grant accelerated approval to another PARP inhibitor for use in ovarian cancer.
More recently, a phase III trial of niraparib maintenance therapy versus placebo enrolled 553 women with recurrent epithelial ovarian cancer.5 Women with germline BRCA mutations had recurrence-free intervals of 21 months on niraparib, compared with 5.5 months for those on placebo. Even without germline BRCA mutations, women benefited from a recurrence-free interval of 9.3 months, compared with 3.9 months for placebo.
PARP inhibitors represent a novel targeted therapy for ovarian cancer, particularly those with deleterious germline or somatic BRCA mutations. When combined with genetic testing for BRCA mutations, PARP inhibitors represent an example of a predictive biomarker paired with a tailored therapeutic. Maturing data from ongoing trials will likely expand the opportunity to use PARP inhibitors for the treatment of ovarian cancer.
References
1. Br J Cancer. 2016 Nov 8;115(10):1157-73.
2. J Clin Oncol. 2015 Jan 20;33(3):244-50.
3. Clin Cancer Res. 2017 Mar 6. pii: clincanres.2796.2016. doi: 10.1158/1078-0432.CCR-16-2796.
4. Lancet Oncol. 2017 Jan;18(1):75-87.
5. N Engl J Med 2016; 375:2154-64.
Dr. Tran is a gynecologic oncology fellow in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC-Chapel Hill. They reported having no relevant financial disclosures.
Ovarian cancer remains the leading cause of death from gynecologic cancer worldwide and one of the five leading causes of death from cancer in women in the United States. In addition to surgery, treatment consists of combination platinum and taxane chemotherapy that offers a high response rate; however, a majority of women will develop persistent or recurrent disease.
A clinical practice statement released by the Society of Gynecologic Oncology in October 2014 states that “women diagnosed with epithelial ovarian, tubal, and peritoneal cancers should receive genetic counseling and be offered genetic testing, even in the absence of family history.” Patients should be informed that this genetic testing serves to prognosticate, inform about personal and familial cancer risk, but also aids in choices of novel therapeutic agents, specifically Poly (ADP-ribose) polymerase (PARP) inhibitors.
Genetic involvement of BRCA
A small proportion of ovarian cancers are attributable to genetic mutations, with approximately 10%-15% of cases caused by germline mutations of BRCA1 and BRCA2. BRCA1 deleterious mutations confer an ovarian cancer risk of approximately 39%-46%; and the risk of ovarian cancer is roughly 12%-20% for patients with BRCA2 deleterious mutations. As a tumor suppressor gene, BRCA is involved in the DNA repair process. Specifically, it is involved in homologous recombination (a form of double-stranded DNA repair mechanism). Thus, cells with defective BRCA proteins cannot repair double-stranded breaks (DSB) in DNA.
The homologous recombination pathway is complex and involves a number of genes. Deficiencies in this pathway confer a sensitivity to PARP inhibition. Tumors that share dysfunction in the homologous recombination pathway, but do not contain mutations in the BRCA gene, are classified as tumors with “BRCAness.”
Generally, the inheritance of a defective BRCA1 or BRCA2 allele (a germline mutation) alone is not enough to cause the development of cancer. Instead, once the second, functioning allele becomes nonfunctional, cancer can arise through an accumulation of mutations in the genetic code.
Furthermore, regardless of germline BRCA status, cancers have high rates of genetic mutation. As a result of the mutation rate, tumors can develop noninherited, noninheritable alterations in BRCA1 or BRCA2 genes (a somatic mutation).
Mechanism of PARP inhibitor activity
The PARP family of enzymes hold a vital role in the repair of DNA and the stabilization of the human genome through the repair of single-stranded breaks (SSB) in DNA. PARP inhibitors were originally developed as a chemosensitizing agent for other cytotoxic agents. It was only later discovered that ovarian cancer cells and mouse models that were deficient in BRCA proteins were especially sensitive to PARP inhibition. Eventually, the clinical development strategy became to employ PARP inhibitors in selected patients with BRCA mutations.
As previously mentioned, cells deficient in the tumor suppressor genes (BRCA1 and BRCA2) have an inability to repair DSBs. Inhibiting PARP enzymes will therefore cause an increase in SSB. During cell replication, these SSBs are converted to DSBs. Ultimately, the accumulation of DSBs leads to cell death. The concept that these two deficiencies – which alone are nonlethal – can be combined to induce cell death is described as synthetic lethality.
The exact mechanism through which PARP inhibitors function is not fully understood; however, four models currently exist to explain how PARP inhibitors instigate synthetic lethality. PARP inhibitors may block base excision repair mechanisms, trap PARP enzymes on damaged DNA, reduce the affinity of functioning BRCA enzymes to damaged DNA, and suppress nonhomologous end joining repair mechanisms.1
FDA approval of PARP inhibitors
In recent years, the Food and Drug Administration has approved three PARP inhibitors in the treatment of ovarian cancer in slightly different clinical scenarios.
Olaparib was tested in a trial of 193 patients who harbored a deleterious or suspected deleterious germline BRCA-associated ovarian cancer who had received prior therapies.2 Overall, the response rate in this population was 41% (95% confidence interval, 28-54) with a median duration of response of 8.0 months. These results led to the FDA approval of olaparib for ovarian cancer treatment as fourth-line therapy in patients with BRCA mutations.
Two separate trials using rucaparib showed an overall response rate of 54% and a duration of response of 9.2 months.3,4 These early results allowed the FDA to grant accelerated approval to another PARP inhibitor for use in ovarian cancer.
More recently, a phase III trial of niraparib maintenance therapy versus placebo enrolled 553 women with recurrent epithelial ovarian cancer.5 Women with germline BRCA mutations had recurrence-free intervals of 21 months on niraparib, compared with 5.5 months for those on placebo. Even without germline BRCA mutations, women benefited from a recurrence-free interval of 9.3 months, compared with 3.9 months for placebo.
PARP inhibitors represent a novel targeted therapy for ovarian cancer, particularly those with deleterious germline or somatic BRCA mutations. When combined with genetic testing for BRCA mutations, PARP inhibitors represent an example of a predictive biomarker paired with a tailored therapeutic. Maturing data from ongoing trials will likely expand the opportunity to use PARP inhibitors for the treatment of ovarian cancer.
References
1. Br J Cancer. 2016 Nov 8;115(10):1157-73.
2. J Clin Oncol. 2015 Jan 20;33(3):244-50.
3. Clin Cancer Res. 2017 Mar 6. pii: clincanres.2796.2016. doi: 10.1158/1078-0432.CCR-16-2796.
4. Lancet Oncol. 2017 Jan;18(1):75-87.
5. N Engl J Med 2016; 375:2154-64.
Dr. Tran is a gynecologic oncology fellow in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC-Chapel Hill. They reported having no relevant financial disclosures.


